This editorial refers to ‘Therapeutic inhibition of microRNA-34a ameliorates aortic valve calcification via modulation of Notch1-Runx2 signalling’, by T. Toshima et al., pp. 983–994.
Calcific aortic valve disease (CAVD) affects one in four people over 65 years of age, but decades of mechanistic investigation of CAVD have yielded little change in management.1 One reason for this is that translational research has largely been limited to ex vivo analysis of diseased tissues or end-stage phenotypes of the valve interstitial cells (VICs) in vitro.2 At these endpoints, the eponymous calcification of CAVD is classified by the transition of VICs into either osteoblast-like or myofibroblast-like cells, but early signals that instigate the typically quiescent VICs to transition from fibroblasts to one of these disease-associated phenotypes are unknown.3 This lack of a unifying pathogenic theory has stymied work to find either a biomarker for the disease or an effective pharmacological target. However, one signalling pathway continually resurfaces as researchers look for early molecular drivers of CAVD: Notch1.
Notch1, a transmembrane receptor involved in intercellular signalling and cellular differentiation, rose to prominence in the valve field when it was discovered that mutations in the human gene (i.e. NOTCH1) caused CAVD.4 Notch1 signalling normally represses Runx2 expression, but when NOTCH1 is mutated, Runx2 increases, leading to osteoblast-like differentiation of VICs. Suppression of Notch1 expression and the ensuing signalling pathways has been found in nearly all in vitro and in vivo models of CAVD, and is sufficient to cause calcification.5 However, while CAVD is highly prevalent, we have learned in the years since that NOTCH1 mutations are very rare. In 2016, a pathophysiological mechanism for Notch1 suppression was described when Hadji et al.6 found that the long non-coding RNA, H19, was increased in CAVD and able to suppress Notch1 expression by outcompeting p53 for NOTCH1’s promoter region. These findings highlighted the possibility of biologically relevant pathogenic mechanisms upstream of NOTCH1.
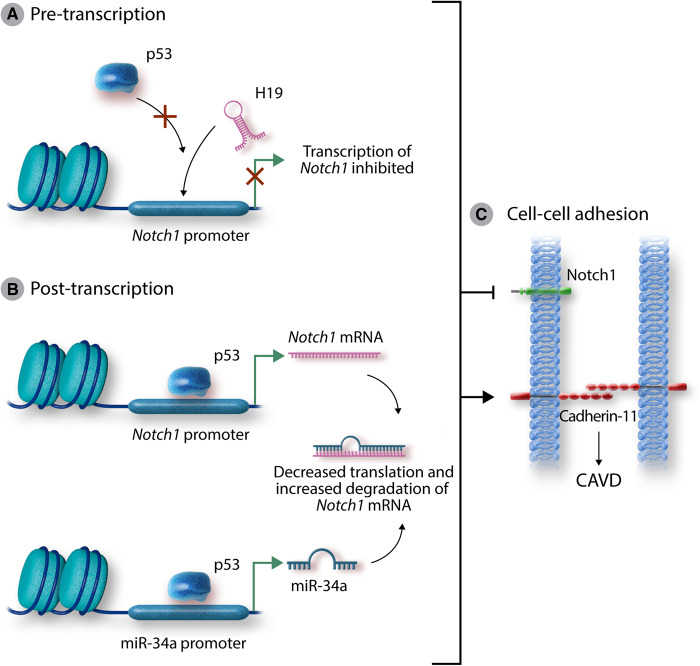
In the current paper by Toshima et al.,7 microRNA-34a (miR-34a) appears to similarly suppress Notch1 expression in VICs by binding its mRNA. Like H19, miR-34a is increased in human CAVD specimens, and in porcine VICs treated with osteogenic medium. Administration of miR-34a mimic additionally replicates the CAVD phenotype associated with Notch1 suppression, while a locked nucleic acid (LNA) miR-34a inhibitor rescues a murine wire-injury model of CAVD, mitigating haemodynamic (peak jet velocity), histological (valve thickening), and molecular changes. These new findings provide a more complete picture of Notch1-related calcification in which H19 and miR-34a mimic the genetic mutation of Notch1 through pre- and post-transcriptional mechanisms (Figure 1).
Figure 1.
Mechanisms of Notch1-associated calcification. H19 has previously been shown to suppress p53-driven transcription of Notch1 by preventing the binding of p53 to the Notch1 promoter (A). Toshima et al. showed that miR-34a, itself a p53-dependent transcript, directly binds to the Notch1 transcript to suppress Notch1 signalling (B). Both pathways of Notch1 suppression lead to increased expression of cadherin-11 and calcification of the aortic valve (C). These findings reaffirm the role of Notch1 suppression in CAVD, describe a novel pathogenic molecule, and highlight the need for further work targeting this pathway in CAVD. CAVD, calcific aortic valve disease.
In addition to new mechanistic understanding of non-genetic Notch1 dysfunction in VICs leading to CAVD, the finding that miR-34a binds Notch1 mRNA in the valve and prevents translation of the receptor provides a host of new areas of exploration for preventing CAVD. First, these findings add another potential biomarker for CAVD. Unfortunately, miR-34a is implicated in a variety of cardiovascular diseases and is therefore not specific enough for diagnosis in isolation, but in combination with currently available markers of CAVD, this new target may be of use.8 Additionally, as the field of nucleic acid therapies expands, miR-34a may be a potential target in those with progressive aortic stenosis (AS) due to CAVD. Results in the current work demonstrated the utility of LNA-based inhibition of miR-34a, and several other studies show early signs of efficacy in utilizing LNAs, antagomirs, or other anti-sense-based strategies to target miRNA species in cardiovascular disease.9 As with concerns about diagnostic specificity, therapeutic targeting may necessitate tissue- or cell type-specific drug delivery, and earlier results targeting miR-34a in moderate dilated cardiomyopathy indicated sex-based differences in therapeutic efficacy.10 Nevertheless, RNA-based therapeutics seem poised to enter into clinical use, and targets such as these are attractive candidates for previously untreatable diseases.
Second, this set of findings regarding miR-34a highlights the role of p53 signalling in CAVD. These two molecules have a well-established interaction in early oncogenic events where they collaboratively influence apoptosis and inflammation. P53 is a master regulator of the cell cycle and prevents cellular proliferation, while miR-34a expression is promoted by p53 and serves an auto-regulatory function by modulating p53-associated transcripts.11 This relationship between p53 and miR-34a coincides nicely with the previously reported findings regarding H19 which outcompetes p53 for the promoter region of the NOTCH1 gene. Although it is well-established that p53 acts downstream to promote NOTCH1 transcription which suppresses calcification, these ‘two hits’ on the p53 pathway (H19 and miR-34a) highlight the possible role of other molecules in the p53 pathway in CAVD, a disease in which apoptosis and cell cycle checkpoints have previously been implicated.
Finally, and perhaps most promisingly, this finding of an alternative mechanism underlying Notch1-associated calcification reinforces the potential of current work targeting proteins downstream of suppressed Notch1 signalling. Currently, there are no pharmacological therapies for patients with AS due to CAVD, and the only trials in humans have attempted unsuccessfully to repurpose antihypertensive and lipid-lowering agents. However, promising work has outlined the potential for alternative targets downstream of suppressed Notch1 like that seen by Toshima et al. Among these is cadherin-11, a cell–cell junction protein that is upregulated with Notch1 suppression and is necessary for calcification in vitro.12,13 Administration of a monoclonal antibody against cadherin-11 has been shown to mitigate the CAVD phenotype in mice with Notch1 haploinsufficiency,14 and the small molecule celecoxib analogue dimethyl celecoxib, which binds cadherin-11, has been shown to prevent calcification in vitro.15 The findings by Toshima et al. suggest that this therapeutic strategy may have promise in instances of CAVD not caused by direct NOTCH1 mutation, and encourage future work to identify additional targets associated with Notch1 suppression.
Although the management of AS has changed rapidly, the need remains for pharmacological approaches to CAVD as an alternative to surgical intervention. This work by Toshima et al. provides a novel approach for targeting aortic valve calcification and provides insight for an abundance of new studies to understand the progression and treatment of CAVD.
Conflict of interest: none declared.
Funding
This work is funded by the National Institutes of Health (R35 HL135970 and T32 GM007347) and Fondation Leducq.
The opinions expressed in this article are not necessarily those of the Editors of Cardiovascular Research or of the European Society of Cardiology.
References
- 1. Lindman BR, Clavel MA, Mathieu P, Iung B, Lancellotti P, Otto CM, Pibarot P.. Calcific aortic stenosis. Nat Rev Dis Primers 2016;2:16006.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Taylor PM, Batten P, Brand NJ, Thomas PS, Yacoub MH.. The cardiac valve interstitial cell. Int J Biochem Cell Biol 2003;35:113–118. [DOI] [PubMed] [Google Scholar]
- 3. Merryman WD, Schoen FJ.. Mechanisms of calcification in aortic valve disease: role of mechanokinetics and mechanodynamics. Curr Cardiol Rep 2013;15:355.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, Grossfeld PD, Srivastava D.. Mutations in NOTCH1 cause aortic valve disease. Nature 2005;437:270–274. [DOI] [PubMed] [Google Scholar]
- 5. Nigam V, Srivastava D.. Notch1 represses osteogenic pathways in aortic valve cells. J Mol Cell Cardiol 2009;47:828–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hadji F, Boulanger M-C, Guay S-P, Gaudreault N, Amellah S, Mkannez G, Bouchareb R, Marchand JT, Nsaibia MJ, Guauque-Olarte S, Pibarot P, Bouchard L, Bossé Y, Mathieu P.. Altered DNA methylation of long noncoding RNA H19 in calcific aortic valve disease promotes mineralization by silencing NOTCH1. Circulation 2016;134:1848–1862. [DOI] [PubMed] [Google Scholar]
- 7. Toshima T, Watanabe T, Narumi T, Otaki Y, Shishido T, Aono T, Goto J, Watanabe K, Sugai T, Takahashi T, Yokoyama M, Kinoshita D, Tamura H, Kato S, Nishiyama S, Arimoto T, Takahashi H, Miyamoto T, Sadahiro M, Watanabe M.. Therapeutic inhibition of microRNA-34a ameliorates aortic valve calcification via modulation of Notch1-Runx2 signalling. Cardiovasc Res 2020;116:983–994. [DOI] [PubMed] [Google Scholar]
- 8. Zhou SS, Jin JP, Wang JQ, Zhang ZG, Freedman JH, Zheng Y, Cai L.. miRNAS in cardiovascular diseases: potential biomarkers, therapeutic targets and challenges. Acta Pharmacol Sin 2018;39:1073–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chakraborty C, Sharma AR, Sharma G, Doss CGP, Lee SS.. Therapeutic miRNA and siRNA: moving from bench to clinic as next generation medicine. Mol Ther Nucleic Acids 2017;8:132–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bernardo BC, Ooi JY, Matsumoto A, Tham YK, Singla S, Kiriazis H, Patterson NL, Sadoshima J, Obad S, Lin RC, McMullen JR.. Sex differences in response to miRNA-34a therapy in mouse models of cardiac disease: identification of sex-, disease- and treatment-regulated miRNAs. J Physiol 2016;594:5959–5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chang TC, Wentzel EA, Kent OA, Ramachandran K, Mullendore M, Lee KH, Feldmann G, Yamakuchi M, Ferlito M, Lowenstein CJ, Arking DE, Beer MA, Maitra A, Mendell JT.. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell 2007;26:745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hutcheson JD, Chen J, Sewell-Loftin MK, Ryzhova LM, Fisher CI, Su YR, Merryman WD.. Cadherin-11 regulates cell-cell tension necessary for calcific nodule formation by valvular myofibroblasts. Arterioscler Thromb Vasc Biol 2013;33:114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen J, Ryzhova LM, Sewell-Loftin MK, Brown CB, Huppert SS, Baldwin HS, Merryman WD.. Notch1 mutation leads to valvular calcification through enhanced myofibroblast mechanotransduction. Arterioscler Thromb Vasc Biol 2015;35:1597–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clark CR, Bowler MA, Snider JC, Merryman WD.. Targeting cadherin-11 prevents notch1-mediated calcific aortic valve disease. Circulation 2017;135:2448–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bowler MA, Raddatz MA, Johnson CL, Lindman BR, Merryman WD.. Celecoxib is associated with dystrophic calcification and aortic valve stenosis. JACC Basic Transl Sci 2019;4:135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]