Abstract
Aims
Cardiac fibrosis is a major cause of heart failure (HF), and mediated by the differentiation of cardiac fibroblasts into myofibroblasts. However, limited tools are available to block cardiac fibrosis. ADAMTS16 is a member of the ADAMTS superfamily of extracellular protease enzymes involved in extracellular matrix (ECM) degradation and remodelling. In this study, we aimed to establish ADAMTS16 as a key regulator of cardiac fibrosis.
Methods and results
Western blot and qRT–PCR analyses demonstrated that ADAMTS16 was significantly up-regulated in mice with transverse aortic constriction (TAC) associated with left ventricular hypertrophy and HF, which was correlated with increased expression of Mmp2, Mmp9, Col1a1, and Col3a1. Overexpression of ADAMTS16 accelerated the AngII-induced activation of cardiac fibroblasts into myofibroblasts. Protein structural analysis and co-immunoprecipitation revealed that ADAMTS16 interacted with the latency-associated peptide (LAP)-transforming growth factor (TGF)-β via a RRFR motif. Overexpression of ADAMTS16 induced the activation of TGF-β in cardiac fibroblasts; however, the effects were blocked by a mutation of the RRFR motif to IIFI, knockdown of Adamts16 expression, or a TGF-β-neutralizing antibody (ΝAb). The RRFR tetrapeptide, but not control IIFI peptide, blocked the interaction between ADAMTS16 and LAP-TGF-β, and accelerated the activation of TGF-β in cardiac fibroblasts. In TAC mice, the RRFR tetrapeptide aggravated cardiac fibrosis and hypertrophy by up-regulation of ECM proteins, activation of TGF-β, and increased SMAD2/SMAD3 signalling, however, the effects were blocked by TGF-β-NAb.
Conclusion
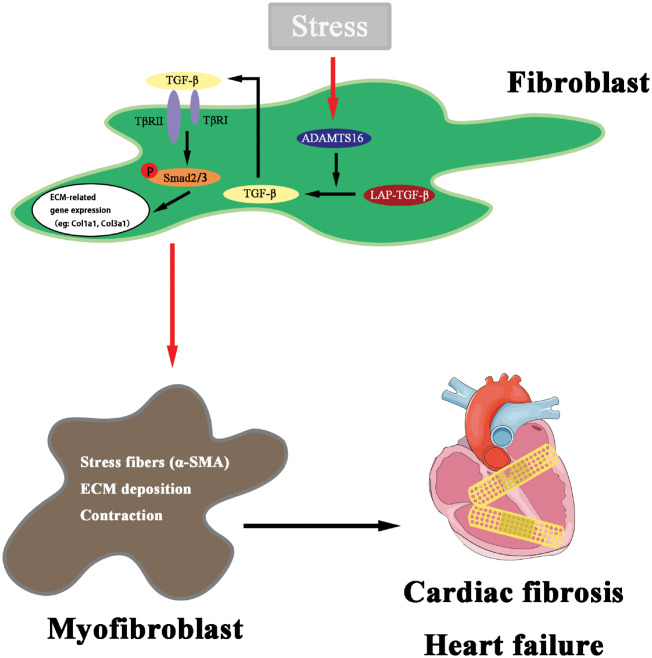
ADAMTS16 promotes cardiac fibrosis, cardiac hypertrophy, and HF by facilitating cardiac fibroblasts activation via interacting with and activating LAP-TGF-β signalling. The RRFR motif of ADAMTS16 disrupts the interaction between ADAMTS16 and LAP-TGF-β, activates TGF-β, and aggravated cardiac fibrosis and hypertrophy. This study identifies a novel regulator of TGF-β signalling and cardiac fibrosis, and provides a new target for the development of therapeutic treatment of cardiac fibrosis and HF.
Keywords: Cardiac fibrosis, Heart failure, ADAMTS16, TGF-β, SMAD2/SMAD3
Graphical Abstract
Graphical Abstract.
1. Introduction
Transforming growth factor (TGF)-β is a master cytokine/growth factor crucially involved in many biological processes such as embryogenesis, angiogenesis, and immune modulation and multiple fibrotic human diseases.1,2 TGF-β mediates its biological effects by binding to its receptors TGFβRI/TGFβRII, and phosphorylating SMAD2 and SMAD3 transcription factors, which are then translocated into the nucleus and activate transcription of target genes.3 However, the most important checkpoint for TGF-β signalling is at the stage of the activation of TGF-β, a process converting the latent precursor [latency associated peptide (LAP)-TGF-β] to the mature, biologically active TGF-β. The LAP domain is cleaved first from the mature TGF-β, however, the two regions remain associated with each other, preventing receptor binding and signalling and leaving only a small fraction of TGF-β biologically active. The molecular mechanisms for activation of latent TGF-β are not well-understood. Proteolysis by plasmin, binding to some integrins, oxidative modifications, and interaction with the extracellular matrix (ECM) protein thrombospondin 1 (TSP-1) were reported to activate latent TGF-β.2 Blockade of disease-associated TGF-β activation by targeting the latent TGF-β activation mechanism represents a selective approach to develop novel therapeutic strategies to treat related diseases.
Heart failure (HF) is a major public health problem, affecting 26 million people worldwide.4 However, the 5-year survival rate for HF is 25–52%, which is worse than most cancers.5 Cardiac fibrosis is the major factor in the development of HF. Anti-fibrotic therapy is considered to be beneficial in alleviating HF.6,7 Excessive accumulation of ECM components leads to fibrosis.8,9 Cardiac fibrosis is predominantly mediated by the activation of cardiac fibroblasts, which is a process of conversion from the tissue-resident fibroblasts to proliferating, fibrogenic, and contractile myofibroblasts with increased expression of genes such as ACTA2 (encoding α-smooth muscle actin, α−SMA).8,10 Moreover, myofibroblasts also drive ECM remodelling by producing and secreting matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs (TIMPs).8 The TGF-β expression level is elevated in response to injury,11–13 and TGF-β signalling is associated with repair and fibrosis of different tissues.11–13 In particular, TGF-β plays a pivotal role in fibroblast activation and ECM production.9,11 To date, no clinical therapies are available to effectively block cardiac fibrosis without side effects. Therefore, additional new regulators of cardiac fibrosis need to be identified to facilitate the development of novel therapeutic strategies targeting cardiac fibrosis.
ADAMTS16 is one of 19 members of the ADAMTS family of metalloproteinases involved in diverse biological processes, including spermatogenesis/fertilization, neurogenesis, inflammatory responses, and cancer.14,15 ADAMTS16 was shown to be involved in premature ovarian failure, male genitourinary system dysfunction, oesophageal squamous cell carcinoma, and blood pressure regulation.16–21 However, the role of ADAMTS16 in cardiac fibrosis and HF is unknown.
In this study, we identify ADAMTS16 as a novel regulator of cardiac fibrosis. ADAMTS16 is up-regulated in a mouse model for cardiac hypertrophy and HF, and its expression is strongly correlated with markers for cardiac fibrosis and the activation of cardiac fibroblasts. We show that ADAMTS16 promotes cardiac fibrosis by interaction with the latent form of TGF-β, which leads to TGF-β activation. Furthermore, we show that cardiac fibrosis in transverse aortic constriction (TAC) mice is accelerated by treatment with a tetrapeptide RRFR peptide derived from ADAMTS16. The RRFR peptide activates TGF-β, promotes the activation of cardiac fibroblasts and cardiac fibrosis, and exacerbates cardiac hypertrophy and HF. The effect of ADAMTS16 overexpression on activation of TGF-β and the effect of the RRFR peptide on cardiac fibrosis and hypertrophy were all blocked by a TGF-β-neutralizing antibody. Taken together, these data suggest that modulating ADAMTS16, in particular, the RRFR motif, may become a powerful new strategy to attenuate cardiac fibrosis and treat patients with HF.
2. Methods
2.1 Plasmids, antibodies and peptides
The expression plasmids for ADAMTS16, pcDNA-4 ADAMTS16-FLAG-C14, or pCDNA4-ADAMTS16-FLAG-C2, were described previously.22 The coding region of ADAMTS16 was PCR-amplified from pcDNA-4 ADAMTS16-FLAG-C14 and subcloned into pcDNA3.1, resulting in another expression plasmid for ADAMTS16, pcDNA3.1-ADAMTS16. A mutation of the RRFR motif to IIFI was created in pcDNA3.1-ADAMTS16 using the PCR-based mutagenesis, resulting in an expression plasmid for pcDNA3.1-ADAMTS16-IIFI.
An expression plasmid for TGFB1 was constructed by amplification of the coding by PCR using human cDNA samples and subcloning into the N-terminal p3*flag-cmv vector (pcDNA3.1-Flag-LAP-TGF-β).
A rabbit anti-ADAMTS16 antibody was purchased from Abcam (Cambridge, MA, USA) (ab45048, 1:1000 dilution for western blotting). A rabbit anti-β-Tubulin antibody was purchased from Proteintech (Wuhan, Hubei, China) (10068-1-AP, 1:1000 dilution for western blotting). A rabbit anti-phosphorylated SMAD3 (Ser423+Ser425) antibody was from Bioss (Beijing, China) (bs-3425R, 1:500 dilution for western blotting). An antibody for phosphorylated SMAD2 (Ser465/467) was from Cell Signaling Technology (Boston, MA, USA) (#3101, 1:1000 dilution for western blotting). A rabbit anti-Flag antibody was from MBL Life science (PM020, 1:1000 dilution for western blotting). A TGF-β-neutralizing antibody (TGF-β ΝAb) and control IgG were from R&D Systems (Minneapolis, MN, USA).
The RRFR tetrapeptide from ADAMTS16 and negative control peptide IIFI were synthesized by GENEWIZB (Wuhan, China).
2.2 Isolation of MCFs, cardiomyocytes culture and transfection
Neonatal mouse cardiac fibroblasts (MCFs) were prepared from hearts of 1- to 2-day-old C57BL/6 mice by digestion with 0.1% (w/v) collagenase type II (Worthington) for 30 min at 37°C as described previously.23 The cells were cultured in Iscove’s Modified Dulbecco’s Medium (IMDM, Gibco) with 10% (v/v) foetal bovine serum (FBS), 100 U/mL penicillin G, and 100 μg/mL streptomycin. After 4 h of culture, the non-adherent cells were washed off. The isolated MCFs were cultured in the Dulbecco’s Modified Eagle’s medium (DMEM) with high-glucose supplemented with 10% (v/v) FBS (Gibco Life Technologies, Gaitherburg, MD, USA) in a humidified incubator with 5% (v/v) CO2 at 37°C. Cardiomyocytes were isolated from mice as previously described.24
MCFs were transfected with plasmid DNA (1–2 μg) and siRNA (100 nM) using the FuGENE HD Transfection Reagent (Roche, Indianapolis, IN, USA) according to the manufacturer’s instruction. The siRNAs targeting the Adamts16 mRNA sequence and negative control siRNA (siNC) were chemically synthesized by RiboBio (Guangzhou, Guangdong, China). The siNC does not have homology to any known gene sequences from humans and mice. The sequences of siAdamts16 duplexes were 5′-GAAGACGCAAGAAAUACAUTT-3′ (sense).
2.3 Luciferase assays
HeLa cells were plated in a 24-well plate and transfected with a 3TP-Luc reporter construct and pRL-TK with or without an ADAMTS16 expression plasmid using Fugene HD as described.25–28 30 h later, cells were used for luciferase assays using the Dual Luciferase Reporter Assay System (Promega, Madison, WI, USA) as described by us previously.25–28 Luciferase activities were normalized to Renilla luciferase activity.
2.4 ELISA for quantification of TGF-β
To measure the amount of TGF-β in the supernatant of cell culture of MCFs, an ELISA was performed using the mouse TGF-β1 Quantikine ELISA Kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s recommendations. Standard curves were created using the CurveExpert 1.3 software programme.
2.5 Animal procedures
C57BL/6 mice (12-week-old) were used for all animal studies. Animal care and experimental procedures were approved by the Ethics Committee on Animal Research of Huazhong University of Science and Technology. All animal experiments conform to the guidelines from Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes.
Pressure overload of the heart was induced in 12-week-old male mice (20–25 g) by TAC as described.7 The mice were injected intraperitoneally with the RRFR peptide (0.25 mg/kg body weight) twice a week for 8 weeks. TGF-β ΝAb and control IgG were injected intraperitoneally into mice at the dose of 10 mg/kg body weight twice a week for 8 weeks. Echocardiography was performed by an operator who was blinded to treatments as described.29–31 After study, all animals were anaesthetized with an intraperitoneal injection of sodium pentobarbital (50 mg/kg) and then euthanized by cervical dislocation.
2.6 Quantitative real-time RT–PCR analysis
Total RNA was extracted from cultured cells or mouse hearts using Trizol (Invitrogen), and 2 μg of RNA samples were used for quantitative real-time PCR analysis with the FastStart Universal SYBR Green Master (Roche, Basel, BS, Switzerland) as described previously.32,33 Experiments were performed in triplicate and repeated at least three times.
2.7 Western blot analysis and co-immunoprecipitation (Co-IP)
Western blot analysis was carried out using protein extracts from transfected cells and mouse hearts as described. Western blot images were captured and quantified using 1-D Analysis Software and Quantity One (Bio-Rad, Hercules, CA, USA).7,29,31
For Co-IP, MCFs were transfected with pcDNA3.1-ADAMTS16 and pcDNA3.1-Flag-LAP-TGF-β for 48 h, and lysed with pre-cooled lysis buffer (50 mM Tris/HCl, pH 7.5, 150 mM NaCl, 2 mM EDTA, 1% (v/v) Nonidet P-40, proteinase inhibitor cocktail). The cell extracts were pre-adsorbed with 30 μL of Protein A/G PLUS-agarose (SantaCruz, TX, USA) for 4 h at 4°C and used for Co-IP with anti-FLAG recognizing LAP-TGF-β and anti-ADAMTS16 antibodies as described.34
2.8 Immunohistological analysis and immunostaining
Eight weeks after TAC, mice were euthanized, the hearts were excised, fixed overnight, paraffin-embedded, and sectioned. Heart sections were stained with Masson staining and Typical Sirius Red staining as described.29,30 Immunostaining was performed with MCFs for α-SMA staining as described.29,30
2.9 Cell migration assays
Migration assays with cardiac fibroblasts were carried out as previously described.24 In brief, cardiac fibroblasts were plated in six-well plates for 24 h and transfected with pcDNA3.1-ADAMTS16 or pcDNA3.1, and then a wound was made by mechanical scratch with a pipette tip. Cells were incubated with angiotensin II (Ang II, 1 μM) or vehicle for 24 h, and cell migration was quantified according to the ratio of cell coverage to the acellular area.
2.10 Collagen contraction assay
Cardiac fibroblasts were transfected with pcDNA3.1-ADAMTS16 or pcDNA3.1 for 24 h. Cells were then trypsinized, counted and added to a collagen solution (Shengyou Biotechnology, Hangzhou, China). The cell collagen mixture was grown in 96 well culture plates, and then incubated for 1 h under standard conditions for polymerization of collagen cell lattices. The culture medium containing Ang II (1 μM) or vehicle was added. After 8, 16, or 24 h, the collagen lattice area was measured to calculate the relative contraction ratio of gel contraction.35
2.11 Statistical analysis
All quantitative data were shown as mean ± standard deviation. The difference between two groups of variables was compared by the two-tailed, paired, or unpaired Student’s t-test. For comparisons of more than two groups, one-way analysis of variance was employed for normal distributions and the Kruskal–Wallis test for non-normal or small samples. A P-value of <0.05 was considered as significant.
3. Results
3.1 ADAMTS16 expression is up-regulated in a TAC model for cardiac hypertrophy and HF
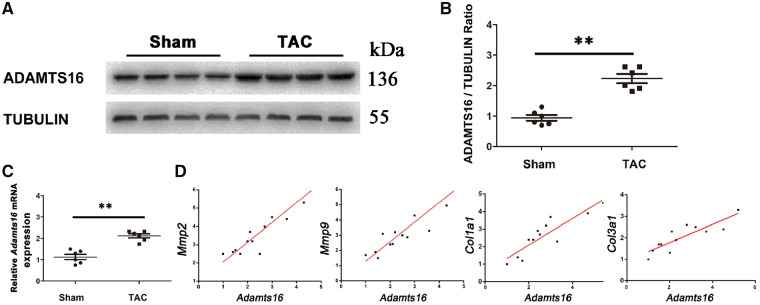
To characterize the role of ADAMTS16 in cardiac fibrosis and HF, we created the TAC model in 12-week-old C57BL/6 mice to induce left ventricular hypertrophy, which can progress to HF.7 Eight weeks after TAC, the mice were used for western blotting and real-time RT–PCR analyses. The expression levels of both the ADAMTS16 protein and the Adamts16 mRNA were significantly higher in the hearts from TAC mice than in control sham hearts (Figure 1A–C). To identify cell-type-specific expression changes of Adamts16 in normal and pressure-overload hearts, we isolated cardiomyocytes and cardiac fibroblasts from TAC and sham mice. Real-time RT–PCR analysis showed a significantly increased Adamts16 mRNA level in cardiac fibroblasts but not in cardiomyocytes from TAC mice compared with sham mice (Supplementary material online, Figure S1).
Figure 1.
The expression level of Adamts16 is up-regulated and correlated with the levels of Mmp2, Mmp9, Col1a1, and Col3a1 in the hearts from TAC mice with pressure-overload-induced cardiac hypertrophy and heart failure. (A) Western blot analysis for ADAMTS16 using heart protein extracts from TAC mice and control sham mice. Tubulin was used as loading control. Data are shown as mean ± SD. **P < 0.01 (n = 6). (B) Quantification of Western blot images as in (A). (C) Real-time RT–PCR analysis for Adamts16 using total cardiac RNA samples from 16-week-old TAC mice and control sham mice. Data are shown as mean ± SD. **P < 0.01 (n = 6). (D) Real-time RT–PCR analysis showed that the expression levels of Adamts16 significantly correlated with the levels of Mmp2 (r = 0.84), Mmp9 (r = 0.78), Col1a1 (r = 0.75), and Col3a1 (r = 0.79) in TAC mice. P < 0.05 (n = 12). Statistical analysis was carried out by a Student’s t-test.
3.2 Up-regulation of ADAMTS16 is associated with cardiac fibrosis and activation of cardiac fibroblasts
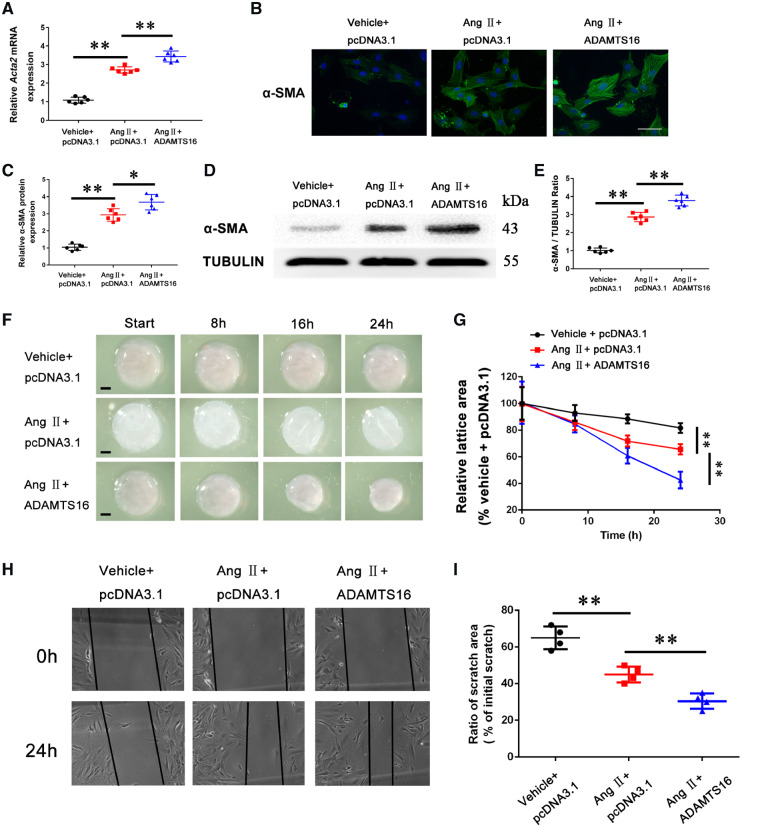
Mmp2 and Mmp9 are involved in pathogenesis of cardiac fibrosis and hypertrophy by regulating ECM homeostasis.36–38 Real-time RT–PCR analysis with cardiac RNA samples showed that up-regulation of Adamts16 was strongly correlated with the increased expression levels of Mmp2 (r = 0.8355, P < 0.05) and Mmp9 (r = 0.7817, P < 0.05) in TAC mice (Figure 1D). MMP2 and MMP9 are involved in processing several different collagen types.39 Similarly, up-regulation of Adamts16 was significantly correlated with the increased expression levels of Col1a1 (r = 0.7541, P < 0.05) and Col3a1 (r = 0.7945, P < 0.05) in TAC mice (Figure 1D). One key molecular mechanism for cardiac fibrosis is the phenotypic differentiation of fibroblasts into myofibroblasts, which is marked with increased expression of α-SMA encoded by the Acta2 gene.9 We overexpressed ADAMTS16 in isolated primary neonatal mice cardiac fibroblasts (MCFs) treated with Angiotensin II (Ang II). Consistent with the pro-fibrotic effects of AngII, real-time RT–PCR analysis showed that the expression level of the myofibroblast marker Acta2 was significantly increased by AngII (Figure 2A), and interestingly, overexpression of ADAMTS16 further increased Acta2 expression significantly at the mRNA level (Figure 2A). The interesting finding of up-regulation of the myofibroblast marker by overexpression of ADAMTS16 was confirmed at the protein level of α-SMA using immunostaining and western blot analysis (Figure 2B–E). We also characterized the functional effect of ADAMTS16 on contractile capability of MCFs. Overexpression of ADAMTS16 significantly increased the contraction of collagen matrices, an indicator for contraction capacity of MCFs (Figure 2F and G). A wound healing assay showed that overexpression of ADAMTS16 significantly increased the migration of MCFs (Figure 2H and I). Together, these data suggest that ADAMTS16 overexpression induces the activation of MCFs.
Figure 2.
Overexpression of ADAMTS16 accelerates the activation of cardiac fibroblasts (differentiation of cardiac fibroblasts into myofibroblasts with increased α-SMA). (A) Real-time RT–PCR analysis for Acta2 (encoding α-SMA) in mouse cardiac fibroblasts transfected with pcDNA3.1 or a mammalian expression plasmid for human ADAMTS16 and treated with Ang II (48 h) or negative control buffer. (B) Immunostaining analysis of α-SMA for mouse primary cardiac fibroblasts treated as in (A). Scale bar =12.5 μm. (C) Quantification of immunostaining images as in (B). (D) Western blot analysis for α-SMA using protein extracts from mouse cardiac fibroblasts treated as in (A). (E) Quantification of western blotting data as in (D). (F) Collagen lattice contraction analysis of mouse cardiac fibroblasts treated as in (A). (G) Quantification of collagen lattice contraction data as in (F). (H) Migration of cardiac fibroblasts treated as in (A) using scratch wound assays. (I) Quantification of scratch wound data as in (H). Data are shown as mean ± SD. *P < 0.05, **P < 0.01 (A–E, n = 6/group; F-I, n = 4/group). Statistical analysis was carried out by a one-way ANOVA test.
3.3 ADAMTS16 interacts with the latent form of TGFβ (LAP-TGF-β)
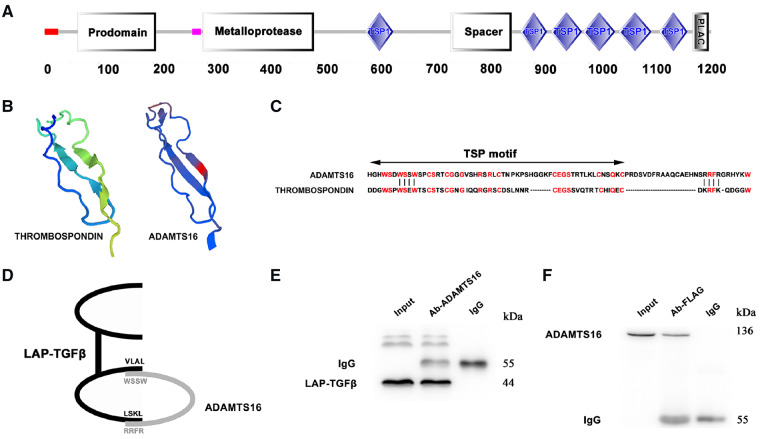
To identify a molecular mechanism by which ADAMTS16 induces fibroblast activation, we carefully analysed the protein structure of ADAMTS16, and found that it has multiple thrombospondin type 1 (TSP1) motifs (Figure 3A). Structural modelling analysis showed that the first TSP1 motif spanning amino acid residues 587-667 of ADAMTS16 (SWISS-MODEL: https://swissmodel.expasy.org/) resembles the TSP1 motif of TSP1 (Figure 3B and C). TSP1 was shown to interact with LAP-TGF-β through a KRFK motif and a WxxW motif (WSPW, WSHW, WGPW).40 A similar, although not identical, WxxW motif was identified in the TSP1 motif of ADAMTS16 (Figure 3C). We did not identify the KRFK motif in ADAMTS16, but found an RRFR motif which shares similar charged amino acid residues with KRFK (Figure 3C). We hypothesized that ADAMTS16 interacts with LAP-TGF-β through the WxxW and RRFR motifs (Figure 3D). We co-expressed ADAMTS16 and FLAG-tagged LAP-TGFβ in MCFs cells for Co-IP analysis. An anti-ADAMTS16 antibody successfully precipitated FLAG-tagged LAP-TGF-β from MCFs cell extracts (Figure 3E), whereas an anti-FLAG antibody precipitated ADAMTS16 (Figure 3F). These data demonstrate the interaction between ADAMTS16 and LAP-TGF-β.
Figure 3.
ADAMTS16 interacts with LAP-TGF-β. (A) Schematic diagram showing the major structural domains of ADAMTS16. (B) Structural modelling showing overall similarities between TSP1 and the TSP1-domain of ADAMTS16. (C) Alignment of sequences of the proximal TSP1-domain of ADAMTS16 and thrombospondin-1 (TSP1). The homologous amino acid sequences at the WxxW and KRFK/RRFR motifs responsible for binding to the LAP are marked. (D) Schematic diagram showing the predicted interaction between ADAMTS16 and LAP-TGF-β via the WxxW and RRFR motifs. (E) Co-IP analysis using cellular protein extracts from mouse primary cardiac fibroblasts with co-expression of ADAMTS16 and LAP-TGFβ. An anti-ADAMTS16 antibody (Ab-Adam) was used for immunoprecipitation and an anti-FLAG antibody recognizing LAP-TGFβ was used for immunoblotting. (F) Reciprocal Co-IP analysis. An anti-FLAG antibody recognizing LAP-TGFβ (Ab-FLAG) was used for immunoprecipitation and an anti-ADAMTS16 antibody was used for immunoblotting.
3.4 ADAMTS16 induces the activation of TGF-β
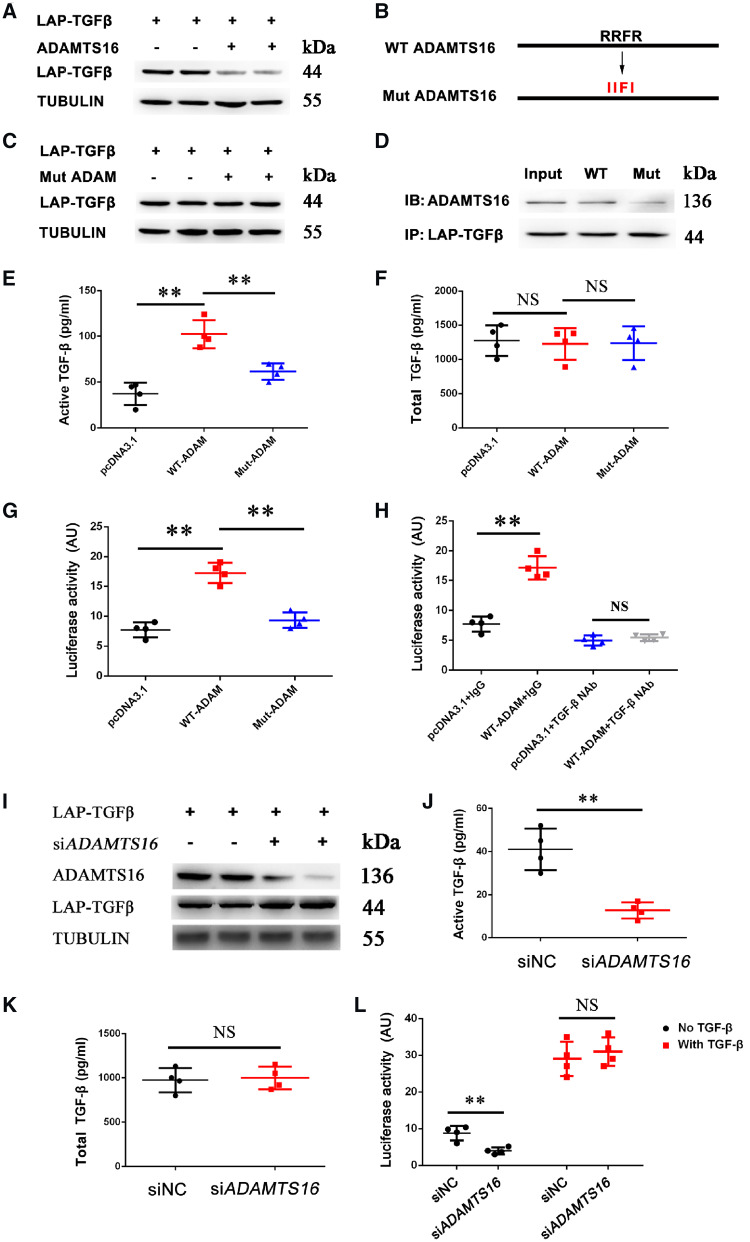
TGF-β activation is indicated by cleavage of the LAP domain from LAP-TGF-β and the release of mature TGF-β. To determine the effect of ADAMTS16 on the activation of LAP-TGF-β, MCFs were co-transfected with an expression plasmid for LAP-TGF-β with or without an ADAMTS16 expression plasmid and used for western blot analysis. Moreover, we tested the effect of the RRFR motif on ADAMTS16 activation of TGF-β because the KRFK motif, but not the WXXW motif, of TSP1 was found to activate LAP-TGF-β.41 Overexpression of ADAMTS16 decreased the level of LAP-TGF-β (Figure 4A), however, the effect was abolished by mutating the RRFR motif to IIFI motif (Figure 4B and C). Co-IP showed that the mutant ADAMTS16 with IIFI reduced the interaction between ADAMTS16 and LAP-TGF-β (Figure 4D). These data suggest that the RRFR motif can mediate the interaction between ADAMTS16 and LAP-TGF-β and their functional effects.
Figure 4.
ADAMTS16 induces the activation of TGF-β. (A) Western blot analysis showed that overexpression of ADAMTS16 decreased the level of LAP-TGF-β in mouse cardiac fibroblasts transfected with LAP-TGF-β. The experiment was independently repeated for three times (n = 6). (B) Schematic diagram showing wild-type (WT) ADAMTS16 and mutant ADAMTS16 with the RRFR motif mutated into IIFI. (C) Western blot analysis showed that overexpression of mutant ADAMTS16 did not affect the level of LAP-TGF-β in mouse cardiac fibroblasts transfected with LAP-TGF-β (n = 6). (D) Co-IP analysis showed that the interaction between LAP-TGF-β and ADAMTS16 was reduced in mouse cardiac fibroblasts by mutation IIFI (n = 6). (E) ELISA for measurements of active TGF-β (pg/mL) in the supernatant of mouse cardiac fibroblasts co-transfected with LAP-TGF-β and WT-ADAMTS16 or Mut-ADAMTS16. Data are shown as mean ± SD. **P < 0.01 (n = 4). Statistical analysis was carried out by a one-way ANOVA test. (F) ELISA for measurements of total TGF-β (acid treated) in the supernatant of mouse cardiac fibroblasts treated as in (E). (G) Luciferase assays for TGF-β-mediated transcription activation in HeLa cells. Data are shown as mean ± SD. **P < 0.01 (n = 4/group). Statistical analysis was carried out by a one-way ANOVA test. (H) Effects of TGF-β NAb on TGF-β-mediated transcription activation in HeLa cells. Data are shown as mean ± SD. **P < 0.01, NS, not significant (n = 4/group). Statistical analysis was carried out by a one-way ANOVA test. (I) Western blot analysis showed that knockdown of Adamts16 expression by siRNA significantly increased the level of LAP-TGF-β in mouse cardiac fibroblast (n = 6/group). (J) ELISA for measurements of the level of active TGF-β in the supernatant of mouse cardiac fibroblasts transfected with Adamts16 siRNA or siNC. Data are shown as mean ± SD. **P < 0.01 (n = 4). Statistical analysis was carried out by a Student’s t-test. (K) ELISA for measurements of the level of total TGF-β (acid treated) treated as in (J). (L) Luciferase assays showing reduced TGF-β-mediated transcriptional activation by Adamts16 siRNA. Data are shown as mean ± SD. **P < 0.01 (n = 4). Statistical analysis was carried out by a Student’s t-test.
The activation of TGF-β was directly measured by an ELISA in the supernatant of MCF culture. Overexpression of WT ADAMTS16 markedly increased the amount of active TGF-β released in the culture, however, the effect was abolished by the mutant ADAMTS16 with the IIFI mutation (Figure 4E). No significant difference was observed for total TGF-β (acid treated) (Figure 4F). A sensitive luciferase assay was also used to measure the level of TGF-β activation by measuring TGF-β-mediated promoter activation. A TGF-β-responsive TPE-luciferase reporter gene was co-transfected in HeLa cells with WT-ADAMTS16 or Mut-ADAMTS16, and used for luciferase assays. TGF-β treatment (positive control) and overexpression of WT ADAMTS16 significantly increased TGF-β-dependent transcription activation (Figure 4G and Supplementary material online, Figure S2), however, the effect was abolished by overexpression of mutant ADAMTS16 or TGF-β NAb (Figure 4G and H and Supplementary material online, Figure S2).
To further confirm the important role of ADAMTS16 in TGF-β activation, we knocked Adamts16 expression down in MCFs using siRNA (Figure 4I and Supplementary material online, Figure S3). Knockdown of Adamts16 increased the level of LAP-TGFβ (Figure 4I), dramatically decreased the level of active and secreted TGF-β (Figure 4J) and significantly reduced TGF-β-dependent transcription activation (Figure 4L). No significant difference was observed for total TGF-β (acid treated) (Figure 4K).
3.5 An RRFR tetrapeptide induces TGF-β activation
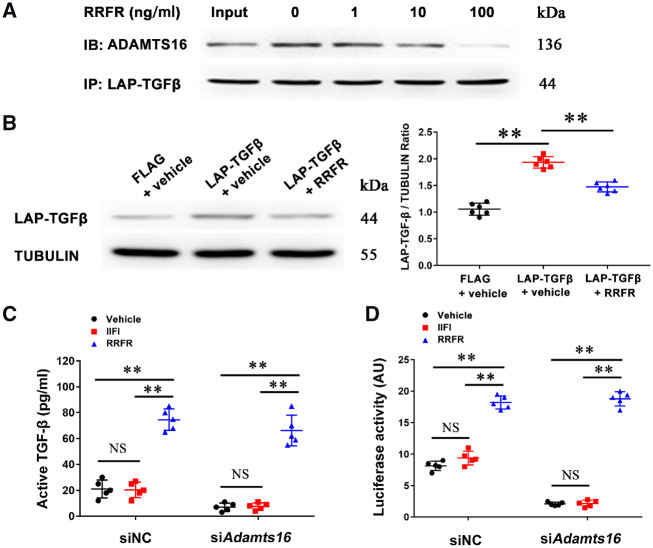
Because the RRFR motif of ADAMTS16 is required for binding to and activation of TGF-β in MCFs, we hypothesized that a RRFR tetrapeptide mimicked the function of ADAMTS16 and plays a critical role in TGF-β activation. MCFs were co-expressed with ADAMTS16 and LAP-TGF-β and incubated with different doses of the RRFR peptide. Co-IP showed that the RRFR peptide reduced the interaction between ADAMTS16 and LAP-TGF-β (Figure 5A), which further suggests that the RRFR motif of ADAMTS16 is responsible for the interaction between ADAMTS16 and LAP-TGF-β. Western blot analysis showed that the RRFR peptide significantly reduced the level of LAP-TGF-β, an indication for the activation of TGF-β (Figure 5B). An ELISA showed that the RRFR peptide, but not the IIFI peptide, significantly increased the release of mature TGF-β in MCF culture media (Figure 5C), and induced TGF-β-dependent transcription activation (Figure 5D). In addition, the effect of the RRFR peptide was independent of ADAMTS16 as similar results were obtained in MCFs treated with Adamts16 specific siRNA or negative control siRNA (siNC) (Figure 5C and D).
Figure 5.
The RRFR peptide inhibits the interaction between ADAMTS16 and LAP-TGF-β and induces the activation of TGF-β. (A) Co-IP analysis for the interaction between Adamts16 and LAP-TGF-β in mouse cardiac fibroblasts treated with different concentrations of the RRFR peptide. An anti-FLAG antibody was used for immunoprecipitation and an anti-ADAMTS16 antibody was used for immunoblotting (IB). (B) Western blot analysis showing the effect of the RRFR peptide on the level of LAP-TGF-β in mouse cardiac fibroblasts. Data are shown as mean ± SD. **P < 0.01 (n = 6). Statistical analysis was carried out by a one-way ANOVA test. (C) ELISA showing the effect of the RRFR peptide on the level of active TGF-β in the supernatant of mouse cardiac fibroblasts. Data are shown as mean ± SD. **P < 0.01 (n = 5). Statistical analysis was carried out by a two-way ANOVA analysis. NS, not significant. (D) Luciferase assays for TGF-β-mediated transcriptional activation in HeLa cells transfected with the TGF-β-responsive 3TPE luciferase reporter gene and treated with Adamts16 siRNA or negative control siRNA (siNC) for 18 h. Cells were then incubated with the RRFR peptide or IIFI peptide for 18 h, and used for luciferase assays. Data are shown as mean ± SD. **P < 0.01 (n = 5). Statistical analysis was carried out by a two-way ANOVA analysis. NS, not significant.
3.6 The RRFR tetrapeptide accelerates cardiac hypertrophy
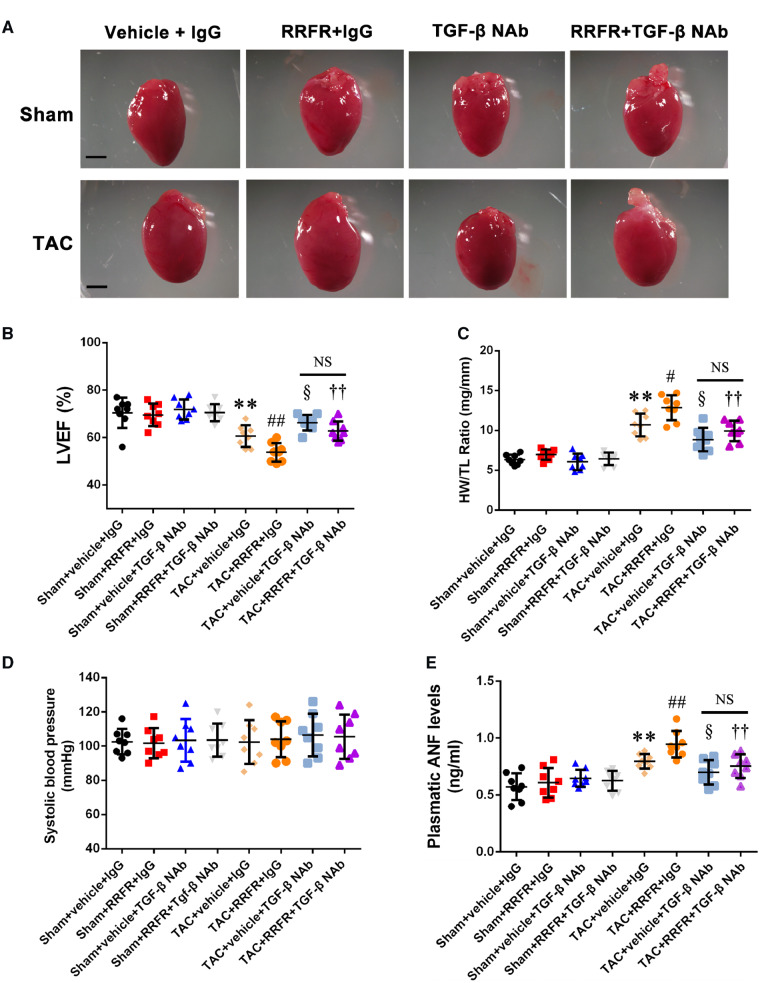
As the RRFR peptide significantly increased the activation of TGF-β in MCFs (Figure 5), we hypothesized that the RRFR peptide would promote cardiac hypertrophy in mice. Twelve-week-old male TAC mice were treated with intraperitoneal injection of the RRFR peptide (0.25 mg/kg; PBS as negative control) twice a week for 8 weeks. Treatment with the RRFR peptide, aggravated cardiac hypertrophy in TAC mice compared with PBS control, however, the effect was significantly attenuated by TGF-β NAb (Figure 6A). Echocardiography showed that the RRFR peptide aggravated cardiac dysfunction in TAC mice by decreasing left ventricular ejection fraction compared with PBS control, however, the effect was significantly attenuated by TGF-β NAb (Figure 6B). Similarly, the RRFR peptide significantly increased the ratio of HW/TL (Heart Weight/Tibial Length) compared with PBS control, however, the effect was significantly attenuated by TGF-β NAb (Figure 6C). On the other hand, RRFR treatment did not have significant effect on blood pressure (Figure 6D). The plasma level of atrial natriuretic factor (ANF) was also significantly higher with the RRFR peptide treatment than PBS control, however, the effect was significantly attenuated by TGF-β NAb (Figure 6E). To determine whether there is a sex difference, similar studies as above were performed for female mice. Interestingly, similar results as found in male mice were obtained in female mice (Supplementary material online, Figure S4A–E). All together, these data suggest that the RRFR peptide derived from ADAMTS16 promotes cardiac hypertrophy and HF, but the effects were abolished by TGF-β NAb, suggesting that the RRFR peptide promotes cardiac hypertrophy and HF by activating the TGF-β signalling pathway.
Figure 6.
The RRFR peptide accelerates cardiac hypertrophy. (A) Morphology of the hearts from sham mice and TAC mice injected with the RRFR peptide in combination with or without TGF-β NAb for 8 weeks. Scale bar = 1 mm. (B) Echocardiographic data showing left ventricular ejection fractions (LVEF, n = 8/group). (C) Ratio of heart weight to tibia length (HW/TL, n = 8/group). (D) Systolic blood pressure (mmHg, n = 8/group). (E) Plasma ANF levels (n = 8/group). Data are shown as mean ± SD. **P < 0.01 vs. Sham+vehicle+IgG group; #P < 0.05, ##P < 0.01 vs. TAC+vehicle+IgG group; §P < 0.05, vs. TAC+vehicle+IgG group; ††P < 0.01 vs. TAC+RRFR+IgG group. NS, not significant. Statistical analysis was carried out by a one-way ANOVA test.
3.7 The RRFR tetrapeptide accelerates cardiac fibrosis
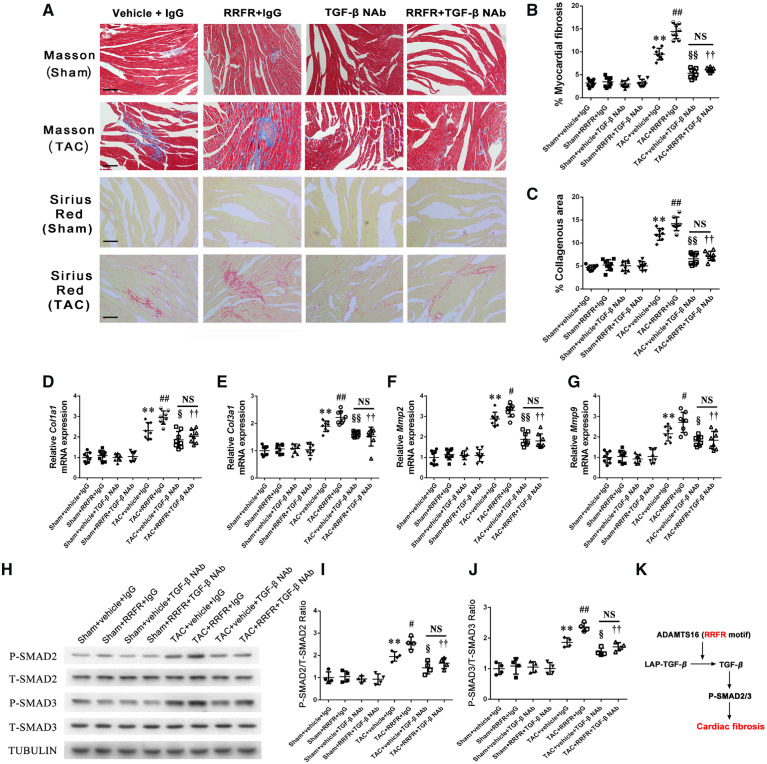
To identify the molecular mechanism by which the RRFR tetrapeptide promotes cardiac hypertrophy and HF, we analysed its effects on cardiac fibrosis because TGF-β plays a key role in cardiac fibrosis. In TAC mice 8 weeks after the TAC surgery, Masson staining showed that the RRFR peptide significantly aggravated TAC-induced myocardial fibrosis (collagen content) compared with PBS control, however, the effect was significantly attenuated by TGF-β NAb (Figure7A and B). Similar findings were obtained with Sirius red staining (Figure7A and C). Moreover, the RRFR peptide significantly increased the expression levels of Col1a1, Col3a1, Mmp2, and Mmp9 mRNA after TAC compared with PBS control, however, the effect was significantly attenuated by TGF-β NAb (Figure 7D–G).
Figure 7.
The RRFR peptide accelerates cardiac fibrosis. (A) Masson staining (upper panel) and typical Sirius Red staining (lower panel) of the hearts in sham mice and TAC mice with pressure overloading for 8 weeks. The myocardial fibrosis and collagenous area of the hearts are graphed for different groups of mice (n = 8). Scale bar = 100 μm, and the quantified data are shown at the right (B) and (C). (D–G) represent the effects of the RRFR peptide treatment combined with TGF-β NAb or IgG on the mRNA expression levels for Col1a1, Col3a1, Mmp2, and Mmp9 in mice after 8 weeks of TAC (n = 8). (H) Western blot analysis showing the effects of the combined treatment of the RRFR peptide with TGF-β NAb or IgG on levels of SMAD2/3 phosphorylation in mice after 8 weeks of TAC operation (n = 8). The quantified data are shown at the right (I) and (J). (K) Diagram showing the molecular signalling pathway for ADAMTS16 as a central activator of TGF-β to accelerate cardiac fibrosis, cardiac hypertrophy, and heart failure. Data are shown as mean ± SD. **P < 0.01 vs. Sham+vehicle+IgG group; #P < 0.05, ##P < 0.01 vs. TAC+vehicle+IgG group; §P < 0.05, §§P < 0.01, vs. TAC+vehicle+IgG group; ††P < 0.01 vs. TAC+RRFR+IgG group. NS, not significant. Statistical analysis was carried out by a one-way ANOVA test.
TGF-β activation was shown to activate SMAD2/3 (increased phosphorylation), which accelerates cardiac fibrosis and causes cardiac hypertrophy.42 Interestingly, we found that the RRFR peptide increased SMAD2/3 phosphorylation in hearts from male TAC mice compared with the PBS control, however, the effect was significantly attenuated by TGF-β NAb (Figure 7H–J). Similar results as found in male mice were obtained in female mice (Supplementary material online, Figure S5A–G). Taken together, these results support the notion that the RRFR motif of ADAMTS16 plays a pivotal role in cardiac fibrosis by activating TGF-β signalling (Figure 7K).
3.8 TAC affects the expression levels of other members of the ADAMTS family
There are 19 members in the ADAMTS family. We hypothesized that as with Adamts16, other Adamts genes may show expression differences between TAC mice and sham mice. Real-time RT–PCR analysis showed that the expression level of Adamts7 was significantly up-regulated in TAC hearts when compared with control sham hearts to the same level as Adamts16 (Supplementary material online, Figure S6). Adamts13 was also significantly up-regulated in TAC hearts compared with sham hearts, but not to the scale of Adamts7 or Adamts16 (Supplementary material online, Figure S6). On the other hands, the expression levels of Adamts2, Adamts6, Adamts15, and Adamts18 were significantly down-regulated in TAC hearts when compared with control sham hearts (Supplementary material online, Figure S6). Adamts18 showed the highest down-regulation in TAC mice.
4. Discussion
Cardiac fibrosis contributes critically to cardiac dysfunction in patients with a hypertensive heart and HF by causing structural and functional remodelling.43 Therefore, the reduction of cardiac fibrosis by targeting novel regulators of cardiac fibrosis is considered to be an effective strategy for clinical treatment of HF.9 In this study, we showed that ADAMTS16 is a novel regulator of activation of cardiac fibroblast, TGF-β signalling, and cardiac fibrosis. The expression level of ADAMTS16 was significantly up-regulated in mouse hearts with pressure-overload-induced cardiac hypertrophy and HF associated with cardiac fibrosis (Figure 1A and B). In addition, we found significant and strong correlation of the expression level of Adamts16 with that of Mmp2, Mmp9, Col1a1, and Col3a1 in the hearts of TAC mice (Figure 1D). Overexpression of ADAMTS16 triggered MCF activation, proliferation, contraction, and migration induced by AngII (Figure 2). Under pressure-overload, TGF-β is the central player in cardiac fibrosis accompanied by the activation of fibroblasts, leading to the remodelling and deposits of ECM proteins, such as different types of collagen and fibronectin.9,44 Recent clinical studies have demonstrated an increased myocardial TGF-β expression level during cardiac hypertrophy and fibrosis.45 Our results demonstrate that ADAMTS16 interacts with LAP-TGF-β via an interesting RRFR motif, which promotes the release of the LAP from LAP-TGF-β, converting the latent form of LAP-TGF-β to the active form of TGF-β (Figure 3). Moreover, increased ADAMTS16 expression contributes to TGF-β-dependent transcriptional activities (Figure 4). The effect was inhibited by TGF-β-NAb (Figure 4H and Supplementary material online, Figure S2), indicating an important regulatory role of TGF-β signalling in functions of ADAMTS16. We showed that intraperitoneal injection of the RRFR peptide derived from ADAMTS16 aggravated cardiac fibrosis, hypertrophy, and dysfunction in TAC mice, however, the effects were inhibited by TGF-β-NAb (Figures 6 and 7). The in vivo data again suggest that TGF-β signalling mediates functions of ADAMTS16. A schematic diagram showing the regulation and function of ADAMTS16 in cardiac fibrosis/hypertrophy and HF is shown in Figure 7E. TGF-β was previously found to stimulate the expression of endogenous ADAMTS16,22 whereas we found here that ADAMTS16 activates TGF-β. The two proteins may form an interesting feed forward loop which exacerbates cardiac hypertrophy and HF.
ADAMTS16 was previously shown to control blood pressure by regulating the aortic pulse wave velocity, vascular media thickness, glomerular filtration rate, renal haemodynamics, and renal handling of sodium.20,21 However, the detailed molecular mechanism is not clear. Our finding that ADATMTS16 activates TGF-β provides a novel potential molecular mechanism for the regulation of blood pressure by ADAMTS16. Zacchigna et al.46 found that knockout of Emilin1 increased blood pressure and peripheral vascular resistance, and reduced vessel size in mice. Emilin1 was found to inhibit TGF-β signalling, and inactivation of a single TGFB1 allele rescued the high blood pressure phenotype in Emilin1 knockout mice.46 Therefore, it appears that Emilin1 knockout mice developed hypertension because of activated TGF-β. Similarly, knockout of Adamts16 inhibits TGF-β activation, thereby reducing blood pressure in mutant rats with a 17 bp deletion in exon 1 of Adamts16.21 ADAMTS16 was also shown to be involved in the development of the testis, premature ovarian failure, male genitourinary system dysfunction, oesophageal squamous cell carcinoma, and optic fissure (OF) closure.16–21,47 The results in this study identify a novel function for ADAMTS16 in cardiac hypertrophy and HF.
ADAMTS16 is one of 19 members of the ADAMTS family of metalloproteinases.21 Typical functions of the ADAMTS proteases include processing of procollagens, von Willebrand factor, aggrecan, versican, brevican, and neurocan, resulting in the turnover and remodelling of ECM.48,49 The data in this study indicate a non-canonical function of ADAMTS16 in promoting the activation of TGF-β and related activities, resulting in the differentiation of cardiac fibroblasts to myofibroblasts and increased proliferation. Several ADAMTS metalloproteinases have been shown to play an important role in cardiovascular diseases. We found that the expression levels of Adamts7, Adamts13, and Adamts16 were significantly up-regulated in TAC hearts, whereas Adamts2, Adamts6, Adamts15, and Adamts18 were down-regulated by TAC (Supplementary material online, Figure S6). Interestingly, Adamts7 and Adamts16 were up-regulated by TAC to the similar level (Supplementary material online, Figure S6), and only the ADAMTS7 and ADAMTS16 proteins share the homologous amino acid sequences at the WxxW and KRFK/RRFR motifs. Genomic variants in ADAMTS7 were significantly associated with risk of coronary artery disease and acute myocardial infarction in humans, and knockout of Adamts7 reduces atherosclerosis in mice.50 Wang et al.51 recently showed that knockout of Adamts2 exacerbated cardiac hypertrophy in TAC mice, and cardiac-specific overexpression of Adamts2 in mice attenuated cardiac hypertrophy. The proposed mechanism is the inhibition of PI3K/AKT signalling in cardiomyocytes by ADAMTS2. Although both ADAMTS16 and ADAMTS2 are involved in regulation of cardiac hypertrophy and HF, they act in completely different manners. First, the two metalloproteinases confer opposite effects on cardiac hypertrophy, with ADAMTS16 as a risk factor and ADAMTS2 as a protective factor. This is consistent with our expression data showing that Adamts16 was up-regulated in TAC hearts, whereas Adamts2 was down-regulated (Supplementary material online, Figure S6). Second, ADAMTS2 regulates cardiac hypertrophy by inhibiting the PI3K/AKT signalling in cardiomyocytes, whereas ADAMTS16 activates TGF-β signalling in cardiac fibroblasts and promotes cardiac fibroblast differentiation into myofibroblasts and proliferation. It should be interesting to investigate the role of other members of the Adamts gene family showing either up-regulation or down-regulation in TAC hearts in cardiac fibrosis, hypertrophy, and HF in the future. It is particularly interesting to characterize Adamts18 for its role in cardiac fibrosis, hypertrophy and HF as it showed the highest down-regulation in TAC mice.
Gunes et al.19 studied the expression levels of ADAMTS16 in thoracic aorta tissue samples from human patients with thoracic aortic aneurysms (TAA) and thoracic aortic dissection (TAD) vs. age-matched controls using western blot analysis. Mutations in multiple gene/protein components of the TGF-β signalling pathway were found to cause TAA and TAD.52 In this study, we found that ADAMTS16 interacts with LAP-TGF-β, which leads to the activation of TGF-β (Figure 3), increased TGF-β signalling, and increased TGF-β-dependent transcriptional activities (Figure 4). Therefore, it should be interesting to further investigate whether up-regulation of ADAMTS16 in TAA and TAD aortic tissue samples may cause TAA and TAD by regulating TGF-β signalling.
TGF-β is a strong inducer of cardiac hypertrophy.53 Some studies suggested that TGF-β was secreted from cardiac fibroblasts, and induces the secretion of connecting tissue growth factor (CTGF) from cardiomyocytes; CTGF from cardiomyocytes induces cardiomyocyte hypertrophy following pressure overload via the AKT pathway in a cell-autonomous manner.53,54 Another study showed that beta-adrenergic signalling was activated in TGF-β-transgenic mice, leading to increased cardiac hypertrophy.55 Therefore, ADAMTS16 may also regulate cardiac hypertrophy and HF by regulating TGF-β, CTGF, and AKT functions in cardiomyocytes.
TSP1 was found to interact with and activates LAP-TGF-β.2 Subsequent studies showed that the KRFK motif of TSP1 was critical for interaction with the LSKL motif of the LAP-TGF-β, disrupting LAP-mature domain interactions so that the receptor binding sequences are exposed for TGF-β signalling.2 ADAMTS16 does not have the exact KRFK motif of TSP1, however, a motif of RRFR was found and shown to mediate the interaction between ADAMTS16 and LAP-TGF-β (Figures 3 and 4). In vivo mouse studies showed that intraperitoneal injection of the RRFR peptide promoted cardiac fibrosis and exacerbates cardiac hypertrophy in TAC mice (Figures 6 and 7). These data suggest that the ADAMTS16 RRFR motif is a new therapeutic target for treatment of cardiac fibrosis, cardiac hypertrophy, and HF. Future studies may develop therapeutic monoclonal antibodies against the RRFR motif of ADAMTS16 to block the interaction between ADAMTS16 and LAP-TGF-β, which may inhibit the activation of TGF-β signalling and block cardiac fibrosis. Other types of inhibitors against ADAMTS16 function, e.g. small chemical inhibitors, may also inhibit cardiac fibrosis and treat cardiac hypertrophy and HF. In addition, TGF-β is known as the most potent pro-fibrogenic cytokine and involved in many other fibrotic diseases, including renal fibrosis, pulmonary fibrosis, liver fibrosis, and subepithelial fibrosis.56–59 Therefore, the ADAMTS16 or its RRFR motif therapeutics may become a novel treatment of many other fibrotic diseases.
Our study has several limitations. First, although studies in cultured cardiac fibroblasts suggest an important role of ADAMTS16 in activation of cardiac fibroblasts and TGF-β activation, and exogenous infusion of the RRFR peptide from ADAMTS16 enhanced cardiac hypertrophy and fibrosis, the in vivo role of endogenous Adamts16 remains to be established. Global knockout (KO) mice deficient in Adamts16, and cardiomyocyte-specific or cardiac fibroblasts-specific KO mice can be developed to demonstrate the critical role of Adamts16 in cardiac fibrosis and hypertrophy, and distinguish the specific cell type(s) involved. Second, the interaction between endogenous ADAMTS16 and LAP-TGF-β in primary cardiac fibroblasts cells needs to be further analysed. Third, it may be interesting to determine whether the expression level of ADAMTS16 is affected in human hearts with HF.
In conclusion, we show that ADAMTS16 is a central activator of TGF-β to accelerate activation of cardiac fibroblasts. Furthermore, the RRFR motif in ADAMTS16 plays a key role in the activation of TGF-β signalling. Our data in mouse models showed that the RRFR peptide from ADAMTS16 treatment aggravated TAC-induced cardiac fibrosis, hypertrophy, and HF, also by regulating TGF-β signalling. Together, we demonstrated that the RRFR motif of ADAMTS16 is a key player in the dynamic interplay that regulates TGF-β activation. As TGF-β signalling is involved in numerous fibrotic diseases, the development of inhibitors, or neutralizing antibodies of ADAMTS16 or the RRFR motif may serve as a new strategy to treat not only cardiovascular diseases but also other diseases caused by abnormal TGF-β signalling.
Authors’ contributions
Experimental design: Y.Y., Q.C., and Q.K.W.; experiments and data collection: Y.Y., C.H., Q.S., Y.L., Z.H., X.D., Y.Y., H.L., and I.M.C.; data analysis: Q.K.W., Q.C., Y.Y., C.H., Q.S., Y.L., Z.H., X.D., Y.Y., H.L., and I.M.C.; draft of manuscript: Y.Y., C.H., Q.S., Q.C., and Q.K.W.; critical revision of manuscript: Y.Y., C.H., Q.C., I.M.C., and Q.K.W.; project supervision: Q.C., C.X., and Q.K.W.
Supplementary Material
Acknowledgements
We thank Sarah M. Schumacher Bass for reading and comments of the manuscript.
Conflict of interest: none declared.
Funding
This study was supported by the National Natural Science Foundation of China (81630002, 31430047, and 91439129); Hubei Province’s Innovative Team (2017CFA014); China Postdoctoral Science Foundation funded project (2017M622409); Certificate of China Postdoctoral Science Foundation funded (2018T110754).
Time for primary review: 37 days
References
- 1. Gyorfi AH, Matei AE, Distler J.. Targeting TGF-beta signaling for the treatment of fibrosis. Matrix Biol 2018;68–69:8–27. [DOI] [PubMed] [Google Scholar]
- 2. Murphy-Ullrich JE, Suto MJ.. Thrombospondin-1 regulation of latent TGF-beta activation: a therapeutic target for fibrotic disease. Matrix Biol 2018;68–69:28–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ruiz-Ortega M, Rodriguez-Vita J, Sanchez-Lopez E, Carvajal G, Egido J.. TGF-beta signaling in vascular fibrosis. Cardiovasc Res 2007;74:196–206. [DOI] [PubMed] [Google Scholar]
- 4. Savarese G, Lund LH.. Global public health burden of heart failure. Card Fail Rev 2017;3:7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Askoxylakis V, Thieke C, Pleger ST, Most P, Tanner J, Lindel K, Katus HA, Debus J, Bischof M.. Long-term survival of cancer patients compared to heart failure and stroke: a systematic review. BMC Cancer 2010;10:105.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ho CY, López B, Coelho-Filho OR, Lakdawala NK, Cirino AL, Jarolim P, Kwong R, González A, Colan SD, Seidman JG, Díez J, Seidman CE.. Myocardial fibrosis as an early manifestation of hypertrophic cardiomyopathy. N Engl J Med 2010;363:552–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yao Y, Lu Q, Hu Z, Yu Y, Chen Q, Wang QK.. A non-canonical pathway regulates ER stress signaling and blocks ER stress-induced apoptosis and heart failure. Nat Commun 2017;8:133.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fan D, Takawale A, Lee J, Kassiri Z.. Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenesis Tissue Repair 2012;5:15.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Okayama K, Azuma J, Dosaka N, Iekushi K, Sanada F, Kusunoki H, Iwabayashi M, Rakugi H, Taniyama Y, Morishita R.. Hepatocyte growth factor reduces cardiac fibrosis by inhibiting endothelial-mesenchymal transition. Hypertension 2012;59:958–965. [DOI] [PubMed] [Google Scholar]
- 10. Zhou HT, Yu XF, Zhou GM.. Diosgenin inhibits angiotensin II-induced extracellular matrix remodeling in cardiac fibroblasts through regulating the TGFbeta1/Smad3 signaling pathway. Mol Med Rep 2017;15:2823–2828. [DOI] [PubMed] [Google Scholar]
- 11. Dobaczewski M, Chen W, Frangogiannis NG.. Transforming growth factor (TGF)-beta signaling in cardiac remodeling. J Mol Cell Cardiol 2011;51:600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Frangogiannis NG. The role of transforming growth factor (TGF)-beta in the infarcted myocardium. J Thorac Dis 2017;9:S52–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bujak M, Frangogiannis NG.. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res 2007;74:184–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reiss K, Saftig P.. The “a disintegrin and metalloprotease” (ADAM) family of sheddases: physiological and cellular functions. Semin Cell Dev Biol 2009;20:126–137. [DOI] [PubMed] [Google Scholar]
- 15. Sun Y, Huang J, Yang Z.. The roles of ADAMTS in angiogenesis and cancer. Tumour Biol 2015;36:4039–4051. [DOI] [PubMed] [Google Scholar]
- 16. Pyun JA, Kim S, Kwack K.. Interaction between thyroglobulin and ADAMTS16 in premature ovarian failure. Clin Exp Reprod Med 2014;41:120–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abdul-Majeed S, Mell B, Nauli SM, Joe B.. Cryptorchidism and infertility in rats with targeted disruption of the Adamts16 locus. PLoS One 2014;9:e100967.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sakamoto N, Oue N, Noguchi T, Sentani K, Anami K, Sanada Y, Yoshida K, Yasui W.. Serial analysis of gene expression of esophageal squamous cell carcinoma: aDAMTS16 is upregulated in esophageal squamous cell carcinoma. Cancer Sci 2010;101:1038–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gunes MF, Akpinar MB, Comertoglu I, Akyol S, Demircelik B, Gurel OM, Aynekin B, Erdemli HK, Ates M, Eryonucu B, Demircan K.. The investigation of a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) 1, 5 and 16 in thoracic aortic aneurysms and dissections. Clin Lab 2016;62:425–433. [PubMed] [Google Scholar]
- 20. Joe B, Saad Y, Dhindaw S, Lee NH, Frank BC, Achinike OH, Luu TV, Gopalakrishnan K, Toland EJ, Farms P, Yerga-Woolwine S, Manickavasagam E, Rapp JP, Garrett MR, Coe D, Apte SS, Rankinen T, Perusse L, Ehret GB, Ganesh SK, Cooper RS, O'Connor A, Rice T, Weder AB, Chakravarti A, Rao DC, Bouchard C.. Positional identification of variants of Adamts16 linked to inherited hypertension. Hum Mol Genet 2009;18:2825–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gopalakrishnan K, Kumarasamy S, Abdul-Majeed S, Kalinoski AL, Morgan EE, Gohara AF, Nauli SM, Filipiak WE, Saunders TL, Joe B.. Targeted disruption of Adamts16 gene in a rat genetic model of hypertension. Proc Natl Acad Sci USA 2012;109:20555–20559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Surridge AK, Rodgers UR, Swingler TE, Davidson RK, Kevorkian L, Norton R, Waters JG, Goldring MB, Parker AE, Clark IM.. Characterization and regulation of ADAMTS-16. Matrix Biol 2009;28:416–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang W, St Clair D, Butterfield A, Vore M.. Loss of Mrp1 potentiates doxorubicin-induced cytotoxicity in neonatal mouse cardiomyocytes and cardiac fibroblasts. Toxicol Sci 2016;151:44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cosme J, Guo H, Hadipour-Lakmehsari S, Emili A, Gramolini AO.. Hypoxia-induced changes in the fibroblast secretome, exosome, and whole-cell proteome using cultured, cardiac-derived cells isolated from neonatal mice. J Proteome Res 2017;16:2836–2847. [DOI] [PubMed] [Google Scholar]
- 25. Luo C, Pook E, Tang B, Zhang W, Li S, Leineweber K, Cheung SH, Chen Q, Bechem M, Hu JS, Laux V, Wang QK.. Androgen inhibits key atherosclerotic processes by directly activating ADTRP transcription. Biochim Biophys Acta Mol Basis Dis 2017;1863:2319–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Luo C, Wang F, Ren X, Ke T, Xu C, Tang B, Qin S, Yao Y, Chen Q, Wang QK.. Identification of a molecular signaling gene-gene regulatory network between GWAS susceptibility genes ADTRP and MIA3/TANGO1 for coronary artery disease. Biochim Biophys Acta Mol Basis Dis 2017;1863:1640–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu Y, Zhou M, Wang J, Zhao Y, Li S, Zhou B, Su Z, Xu C, Xia Y, Qian H, Tu X, Xiao W, Chen X, Chen Q, Wang QK.. Role of microRNA-27a in down-regulation of angiogenic factor AGGF1 under hypoxia associated with high-grade bladder urothelial carcinoma. Biochim Biophys Acta 2014;1842:712–725. [DOI] [PubMed] [Google Scholar]
- 28. Zhou B, Si W, Su Z, Deng W, Tu X, Wang Q.. Transcriptional activation of the Prox1 gene by HIF-1alpha and HIF-2alpha in response to hypoxia. FEBS Lett 2013;587:724–731. [DOI] [PubMed] [Google Scholar]
- 29. Lu Q, Yao Y, Hu Z, Hu C, Song Q, Ye J, Xu C, Wang AZ, Chen Q, Wang QK.. Angiogenic factor AGGF1 activates autophagy with an essential role in therapeutic angiogenesis for heart disease. PLoS Biol 2016;14:e1002529.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yao Y, Hu Z, Ye J, Hu C, Song Q, Da X, Yu Y, Li H, Xu C, Chen Q, Wang QK.. Targeting AGGF1 (angiogenic factor with G patch and FHA domains 1) for blocking neointimal formation after vascular injury. J Am Heart Assoc 2017;6:e005889.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang T, Yao Y, Wang J, Li Y, He P, Pasupuleti V, Hu Z, Jia X, Song Q, Tian X, Hu C, Chen Q, Wang QK.. Haploinsufficiency of Klippel-Trenaunay syndrome gene Aggf1 inhibits developmental and pathological angiogenesis by inactivating PI3K and AKT and disrupts vascular integrity by activating VE-cadherin. Hum Mol Genet 2016;25:5094–5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen S, Wang X, Wang J, Zhao Y, Wang D, Tan C, Fa J, Zhang R, Wang F, Xu C, Huang Y, Li S, Yin D, Xiong X, Li X, Chen Q, Tu X, Yang Y, Xia Y, Xu C, Wang QK.. Genomic variant in CAV1 increases susceptibility to coronary artery disease and myocardial infarction. Atherosclerosis 2016;246:148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xiong X, Xu C, Zhang Y, Li X, Wang B, Wang F, Yang Q, Wang D, Wang X, Li S, Chen S, Zhao Y, Yin D, Huang Y, Zhu X, Wang L, Wang L, Chang L, Xu C, Li H, Ke T, Ren X, Wu Y, Zhang R, Wu T, Xia Y, Yang Y, Ma X, Tu X, Wang QK.. BRG1 variant rs1122608 on chromosome 19p13.2 confers protection against stroke and regulates expression of pre-mRNA-splicing factor SFRS3. Hum Genet 2014;133:499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huang Y, Wang Z, Liu Y, Xiong H, Zhao Y, Wu L, Yuan C, Wang L, Hou Y, Yu G, Huang Z, Xu C, Chen Q, Wang QK.. alphaB-Crystallin interacts with Nav1.5 and regulates ubiquitination and internalization of cell surface Nav1.5. J Biol Chem 2016;291:11030–11041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kopp J, Preis E, Said H, Hafemann B, Wickert L, Gressner AM, Pallua N, Dooley S.. Abrogation of transforming growth factor-beta signaling by SMAD7 inhibits collagen gel contraction of human dermal fibroblasts. J Biol Chem 2005;280:21570–21576. [DOI] [PubMed] [Google Scholar]
- 36. Duarte S, Baber J, Fujii T, Coito AJ.. Matrix metalloproteinases in liver injury, repair and fibrosis. Matrix Biol 2015;44–46:147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Piccoli MT, Gupta SK, Viereck J, Foinquinos A, Samolovac S, Kramer FL, Garg A, Remke J, Zimmer K, Batkai S, Thum T.. Inhibition of the cardiac fibroblast-enriched lncRNA Meg3 prevents cardiac fibrosis and diastolic dysfunction. Circ Res 2017;121:575–583. [DOI] [PubMed] [Google Scholar]
- 38. Toba H, Cannon PL, Yabluchanskiy A, Iyer RP, D’Armiento J, Lindsey ML.. Transgenic overexpression of macrophage matrix metalloproteinase-9 exacerbates age-related cardiac hypertrophy, vessel rarefaction, inflammation, and fibrosis. Am J Physiol Heart Circ Physiol 2017;312:H375–H383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Steffensen B, Wallon UM, Overall CM.. Extracellular matrix binding properties of recombinant fibronectin type II-like modules of human 72-kDa gelatinase/type IV collagenase. High affinity binding to native type I collagen but not native type IV collagen. J Biol Chem 1995;270:11555–11566. [DOI] [PubMed] [Google Scholar]
- 40. Murphy-Ullrich JE, Poczatek M.. Activation of latent TGF-beta by thrombospondin-1: mechanisms and physiology. Cytokine Growth Factor Rev 2000;11:59–69. [DOI] [PubMed] [Google Scholar]
- 41. Ribeiro SM, Poczatek M, Schultz-Cherry S, Villain M, Murphy-Ullrich JE.. The activation sequence of thrombospondin-1 interacts with the latency-associated peptide to regulate activation of latent transforming growth factor-beta. J Biol Chem 1999;274:13586–13593. [DOI] [PubMed] [Google Scholar]
- 42. Kong P, Christia P, Frangogiannis NG.. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci 2014;71:549–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bangalore S, Wild D, Parkar S, Kukin M, Messerli FH.. Beta-blockers for primary prevention of heart failure in patients with hypertension insights from a meta-analysis. J Am Coll Cardiol 2008;52:1062–1072. [DOI] [PubMed] [Google Scholar]
- 44. Koitabashi N, Danner T, Zaiman AL, Pinto YM, Rowell J, Mankowski J, Zhang D, Nakamura T, Takimoto E, Kass DA.. Pivotal role of cardiomyocyte TGF-beta signaling in the murine pathological response to sustained pressure overload. J Clin Invest 2011;121:2301–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hein S, Arnon E, Kostin S, Schonburg M, Elsasser A, Polyakova V, Bauer EP, Klovekorn WP, Schaper J.. Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: structural deterioration and compensatory mechanisms. Circulation 2003;107:984–991. [DOI] [PubMed] [Google Scholar]
- 46. Zacchigna L, Vecchione C, Notte A, Cordenonsi M, Dupont S, Maretto S, Cifelli G, Ferrari A, Maffei A, Fabbro C, Braghetta P, Marino G, Selvetella G, Aretini A, Colonnese C, Bettarini U, Russo G, Soligo S, Adorno M, Bonaldo P, Volpin D, Piccolo S, Lembo G, Bressan GM.. Emilin1 links TGF-beta maturation to blood pressure homeostasis. Cell 2006;124:929–942. [DOI] [PubMed] [Google Scholar]
- 47. Cao M, Ouyang J, Guo J, Lin S, Chen S.. Metalloproteinase Adamts16 is required for proper closure of the optic fissure. Invest Ophthalmol Vis Sci 2018;59:1167–1177. [DOI] [PubMed] [Google Scholar]
- 48. Stanton H, Melrose J, Little CB, Fosang AJ.. Proteoglycan degradation by the ADAMTS family of proteinases. Biochim Biophys Acta 2011;1812:1616–1629. [DOI] [PubMed] [Google Scholar]
- 49. Gao W, Zhu J, Westfield LA, Tuley EA, Anderson PJ, Sadler JE.. Rearranging exosites in noncatalytic domains can redirect the substrate specificity of ADAMTS proteases. J Biol Chem 2012;287:26944–26952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bauer RC, Tohyama J, Cui J, Cheng L, Yang J, Zhang X, Ou K, Paschos GK, Zheng XL, Parmacek MS, Rader DJ, Reilly MP.. Knockout of Adamts7, a novel coronary artery disease locus in humans, reduces atherosclerosis in mice. Circulation 2015;131:1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang X, Chen W, Zhang J, Khan A, Li L, Huang F, Qiu Z, Wang L, Chen X.. Critical role of ADAMTS2 (A Disintegrin and Metalloproteinase With Thrombospondin Motifs 2) in cardiac hypertrophy induced by pressure overload. Hypertension 2017;69:1060–1069. [DOI] [PubMed] [Google Scholar]
- 52. Giusti B, Nistri S, Sticchi E, De Cario R, Abbate R, Gensini GF, Pepe G.. A case based approach to clinical genetics of thoracic aortic aneurysm/dissection. Biomed Res Int 2016;2016:9579654.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fujiu K, Nagai R.. Fibroblast-mediated pathways in cardiac hypertrophy. J Mol Cell Cardiol 2014;70:64–73. [DOI] [PubMed] [Google Scholar]
- 54. Hayata N, Fujio Y, Yamamoto Y, Iwakura T, Obana M, Takai M, Mohri T, Nonen S, Maeda M, Azuma J.. Connective tissue growth factor induces cardiac hypertrophy through Akt signaling. Biochem Biophys Res Commun 2008;370:274–278. [DOI] [PubMed] [Google Scholar]
- 55. Rosenkranz S, Flesch M, Amann K, Haeuseler C, Kilter H, Seeland U, Schluter KD, Bohm M.. Alterations of beta-adrenergic signaling and cardiac hypertrophy in transgenic mice overexpressing TGF-beta(1). Am J Physiol Heart Circ Physiol 2002;283:H1253–H1262. [DOI] [PubMed] [Google Scholar]
- 56. Ellmers LJ, Scott NJ, Medicherla S, Pilbrow AP, Bridgman PG, Yandle TG, Richards AM, Protter AA, Cameron VA.. Transforming growth factor-beta blockade down-regulates the renin-angiotensin system and modifies cardiac remodeling after myocardial infarction. Endocrinology 2008;149:5828–5834. [DOI] [PubMed] [Google Scholar]
- 57. Liu RM, Desai LP.. Reciprocal regulation of TGF-beta and reactive oxygen species: a perverse cycle for fibrosis. Redox Biol 2015;6:565–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bergeron A, Soler P, Kambouchner M, Loiseau P, Milleron B, Valeyre D, Hance AJ, Tazi A.. Cytokine profiles in idiopathic pulmonary fibrosis suggest an important role for TGF-beta and IL-10. Eur Respir J 2003;22:69–76. [DOI] [PubMed] [Google Scholar]
- 59. Kumar RK, Herbert C, Foster PS.. Expression of growth factors by airway epithelial cells in a model of chronic asthma: regulation and relationship to subepithelial fibrosis. Clin Exp Allergy 2004;34:567–575. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.