Abstract
For trees in forests, striving for light is matter of life and death, either by growing taller toward brighter conditions or by expanding the crown to capture more of the available light. Here, we present a mechanistic model for the development path of stem height and crown size, accounting for light capture and growth, as well as mortality risk. We determine the optimal growth path among all possible trajectories using dynamic programming. The optimal growth path follows a sequence of distinct phases: (i) initial crown size expansion, (ii) stem height growth toward the canopy, (iii) final expansion of the crown in the canopy and (iv) seed production without further increase in size. The transition points between these phases can be optimized by maximizing fitness, defined as expected lifetime reproductive production. The results imply that to reach the canopy in an optimal way, trees must consider the full profile of expected increasing light levels toward the canopy. A shortsighted maximization of growth based on initial light conditions can result in arrested height growth, preventing the tree from reaching the canopy. The previous result can explain canopy stratification, and why canopy species often get stuck at a certain size under a shading canopy. The model explains why trees with lower wood density have a larger diameter at a given tree height and grow taller than trees with higher wood density. The model can be used to implement plasticity in height versus diameter growth in individual-based vegetation and forestry models.
Keywords: allocation, growth strategy, life history, optimal control, tree
Introduction
In forests, a tree’s growth strategy is to a large extent determined by a struggle for light, either by growing taller toward brighter conditions or by expanding leaf area or crown size to capture more of the available light. In addition, the tree’s growth strategy is not only important for the tree itself, but due to the dominant ecological role of trees it has consequences for the whole forest ecosystem. However, despite the critical importance of this height versus crown size strategy for trees, to our knowledge, a general model for the optimal growth strategy under a shading canopy has not yet been presented.
A plethora of growth and allocation models exists (Franklin et al. 2012) ranging from the most simple fixed allometric relationships to game-theoretic optimality models of even-aged stands (Mäkelä 1985, King 1993), and sophisticated mechanistic models of tree evolution (Falster et al. 2017). However, while the optimality models only apply to even-aged stands of identical trees, the evolutionary models do not account for plasticity of the growth path in response to a variable light environment. Thus, none of the existing models addresses the problem that trees growing under established canopies face: how can the growth path of an individual tree be optimized in terms of height, crown size and reproduction under the prevailing light environment?
This problem is a specific case of the more general problem of optimal growth allocation, which has long been an intense area of research in plant ecophysiology, and which has been addressed using many different approaches (Franklin et al. 2012). Perrin (1992) investigated, for organisms in general, how resources should be optimally allocated when accounting for both growth and mortality, showing that the ratio between production and mortality (hereafter we denote this ratio as P:m) plays an important role for optimal allocation. Plants should invest all resources into the organ that gives the highest return in terms of this ratio. Only when two or more organs give the same increase in the ratio, should simultaneous allocation be carried out. However, in this study, plants were assumed to grow in constant full light, which contrasts with conditions in many forests, where the light availability increases with height. Thus, it is unclear whether the local-growth optimization strategy to maximize P:m under current conditions (Perrin 1992) is still valid. Instead it may pay to overinvest in height growth initially, in order to reach better light conditions more quickly. To address this problem, it is necessary to optimize the allocation with respect not only to current conditions but also to the expected future conditions.
In this study we model optimal growth paths in terms of height, crown size and seed production, based on maximization of lifetime fitness using a mechanistic model of tree growth and mortality. We address the following questions: can optimal growth paths be determined based on optimization with respect to the local environment only (as in previous optimal allocation models), and if not, what does the optimal path look like? Can the model provide an explanation for observed tree height growth strategies in forests, and differences between fast strategies with low wood density and slow strategies with high wood density?
Theory and model
Light environment
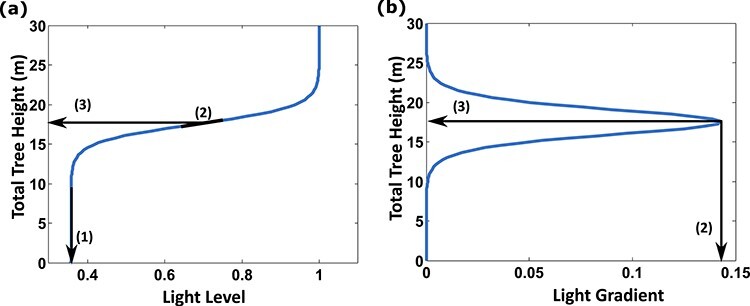
We assume that the light environment that a tree experiences varies only with its total height Htot, which is the sum of the stem height Hs and the crown height Hb. The light level is measured relative to the above canopy level, assuming values between 0 and 1. The light profile (Q) is described by a sigmoid function of tree height Htot as illustrated in Figure 1 (equation A. 2 in Appendix A available as Supplementary Data at Tree Physiology Online). The choice of functional form is motivated by the shape of observed height-light level curves in a rain forest (Poorter et al. 2005). The shape of the light profile is determined by three properties: (1) the light level at initial tree height Q0, (2) the maximum light gradient, dQmax and (3) the height at maximum light gradient, HdQmax. For a detailed description of the light profile see Appendix A available as Supplementary Data at Tree Physiology Online. The different light environments used in the simulations were created by assigning different values for 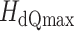 ,
, 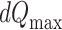 and
and 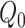 in the ranges 5 to 30 m, 0.07 to 0.36 m−1 and 0.23 to 0.40, respectively.
in the ranges 5 to 30 m, 0.07 to 0.36 m−1 and 0.23 to 0.40, respectively.
Figure 1.
The light environment (a) is defined by the three properties: (1) the light level at initial stem height Q0, (2) the maximum light gradient dQmax and (3) the height at maximum light gradient HdQmax. The light level increases with the height of the tree (which is determined by the stem height and crown size). (b) The corresponding light gradient (derivative).
Physiological model of an individual tree
The physiological model used in our simulations is based on the work of Mäkelä (1986) (Eqs (1) and (2)) and considers five biomass pools: stem, branches, foliage, coarse roots and fine roots. The net production 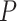 (Eq. (1)) is determined by subtracting from the gross primary production,
(Eq. (1)) is determined by subtracting from the gross primary production, 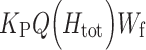 , the maintenance respiration and tissue turnover (adjusted for growth respiration),
, the maintenance respiration and tissue turnover (adjusted for growth respiration), 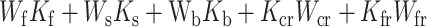 :
:
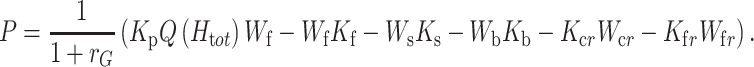 |
(1) |
Here, Wf, Ws, Wb, Wcr and Wfr are the total dry biomass pool for foliage, sapwood in stem, branches, coarse roots and fine roots, respectively; Kf, Ks, Kb Kcr and Kfr are the combined maintenance and turnover costs (adjusted for growth respiration), rG is the growth respiration constant (growth respiration, rGP) and Kp is the maximum gross production rate; parameter values are listed in Table 1. Q is the light level relative to that above the canopy at the crown top and Htot denotes the total height of the tree. We use the light-use efficiency concept (net production is linearly proportional to absorbed photosynthetically active radiation), which is appropriate for modeling seasonal or longer term productivity (Medlyn 1998). Based on the pipe model (Shinozaki et al. 1964), we assume that the stem sapwood area (As) is proportional to the total dry weight of foliage (Wf) and similarly for branch sapwood area. Further, we assume that the ratio of the leaf area to crown surface area, i.e., the surface area of the polygon that encloses the tree crown, and the specific leaf area are constant. With these assumptions gross primary production is proportional to the foliage dry weight, i.e., P 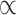 crown surface area
crown surface area 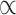 leaf area
leaf area 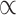 leaf biomass. We also assume that the coarse root mass is proportional to stem sapwood biomass and that fine root biomass is proportional to foliage mass (i.e., functional balance), which is reasonable as we do not consider variability in soil resources (Mäkelä 2002). The crown height is assumed to relate to sapwood area (sapwood cross-sectional area) through a power law relation (Mäkelä 1997, Valentine and Mäkelä 2005),
leaf biomass. We also assume that the coarse root mass is proportional to stem sapwood biomass and that fine root biomass is proportional to foliage mass (i.e., functional balance), which is reasonable as we do not consider variability in soil resources (Mäkelä 2002). The crown height is assumed to relate to sapwood area (sapwood cross-sectional area) through a power law relation (Mäkelä 1997, Valentine and Mäkelä 2005), 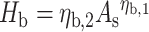 , where
, where 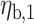 and
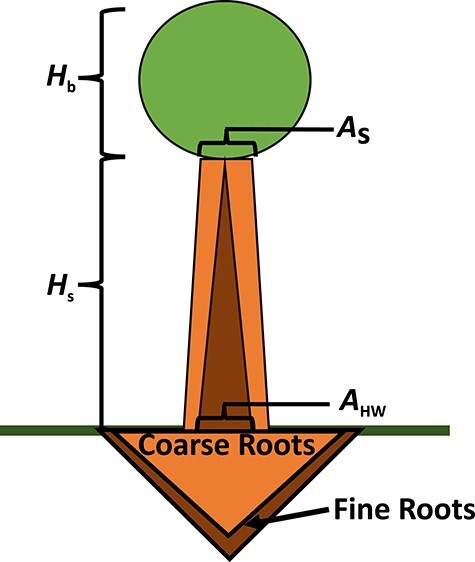
and 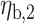 are parameters. This means that crown surface area, crown height and sapwood area are linked in a fixed relationship. We motivate this with findings that while stem to crown biomass ratio changes over the course of a tree’s life span, crown shape is relatively stable and crown size remains strongly correlated to stem girth (Harja et al. 2012, Antin et al. 2013), and that Mäkelä and Sievänen (1992) found a power law relation as a result from an optimization study of height growth in open-growth trees. With these assumptions, all the biomass pools can be expressed in terms of two quantities: the crown size and the stem height (Hs; Figure 2). Because the crown size is determined by As, we will use As as a proxy for crown size. The relationships between the biomass pools, As and Hs are described by Eq. 2.
are parameters. This means that crown surface area, crown height and sapwood area are linked in a fixed relationship. We motivate this with findings that while stem to crown biomass ratio changes over the course of a tree’s life span, crown shape is relatively stable and crown size remains strongly correlated to stem girth (Harja et al. 2012, Antin et al. 2013), and that Mäkelä and Sievänen (1992) found a power law relation as a result from an optimization study of height growth in open-growth trees. With these assumptions, all the biomass pools can be expressed in terms of two quantities: the crown size and the stem height (Hs; Figure 2). Because the crown size is determined by As, we will use As as a proxy for crown size. The relationships between the biomass pools, As and Hs are described by Eq. 2.
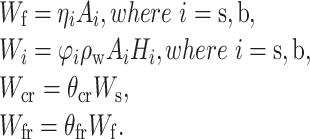 |
(2) |
Table 1.
List of symbol definitions, units and default parameter values used in the main text.
| Symbol | Unit | Value | Description | Source(s) and motivation |
|---|---|---|---|---|
| Tree variables | ||||
| P | kg year−1 | Net production (dry matter) | ||
| W f, Ws, Wb, Wcr | kg | Dry matter weight* | ||
| A s, Ab | m2 | Sapwood area* | ||
| A HW | m2 | Heartwood area | ||
| H s, Hb | m | Height* | ||
| Tree parameters | ||||
| r G | − | 0.25 | Growth respiration constant | Mäkelä (1986) |
| K p | Year−1 | 4 | Maximum gross production rate | Mäkelä (1986) |
| K f | Year−1 | 0.5 | Cost rate for foliage | Mäkelä and Valentine (2001) |
| K s/Kb/Kcr | Year−1 | 0.03 | Cost rate for sapwood | Mäkelä and Valentine (2001) |
| K fr | Year−1 | 0.5 | Cost rate for fine roots | Mäkelä and Valentine (2001) |
| ηb,1 | m−2ηb,2 + 1 | 0.5 | Power law constant relating crown height to sapwood area | Based on a ratio between foliage mass and stem sapwood area = 600 kg m−2 (Mäkelä 1986) specific leaf area = 4 m2 kg−1, ratio between total leaf area and crown surface area = 1 (Duursma and Mäkelä 2007), and spherical crown geometry |
| ηb,2 | − | 13 | ||
| m 1/m2/m3 | − | 0.02/5/0.7 | Constants used in mortality model | Selected to give reasonable width−height relationships |
| η s/ηb | kg m−2 | 600/450 | Foliage mass to sapwood area ratio | Mäkelä (1986) |
| φ s/φb | − | 0.75/0.7 | Form factor | Mäkelä (1986) |
| ρw | kg m−3 | 400 | Wood density | Mäkelä (1986) |
| θcr/θfr | − | 0.2/0.7 | Root mass to biomass ratios | Guess/Mäkelä and Valentine (2001) |
| θ HW,1 | m−2θHW,2 + 1 | 0.0724 | Heartwood parameters | Chosen such that when Hs increases by HB all sapwood has been exchanged |
| θ HW,2 | − | 2 | ||
| Light-environment parameters | ||||
| Q 0 | − | 0.23–0.40 | Light level at initial tree height | |
| dQ max | − | 0.071–0.36 | Maximum light gradient | |
| H dQmax | m | 5–30 | Height at maximum light gradient | |
| a | − | 0.6–0.95 | Parameters used in the light environment model | |
| b | m | 5–30 | ||
| c | m−1 | 0.3–2.4 | ||
| d | − | 0.05–0.4 | ||
*Index indicate organs: f = foliage, s = stem, b = branches, cr = crown.
Figure 2.
The whole tree is described by the quantities: sapwood area below the main branches As, stem height Hs. Heartwood area at ground level AHW is determined by the accumulated As and Hs growth. The tree crown height Hb is determined by As.
The subscripts in Eq. (2) are abbreviations for branches (b), coarse roots (cr), fine roots (fr) and stem (s). Ab is defined as sapwood in primary branches. 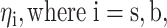 are foliage mass to sapwood area ratios,
are foliage mass to sapwood area ratios, 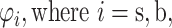 are stem and branch form factors,
are stem and branch form factors, 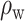 is the wood density and
is the wood density and 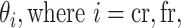 are root mass to biomass ratios.
are root mass to biomass ratios.
Heartwood dynamics
Aside from the five aforementioned biomass pools we also consider the development of heartwood, defined by heartwood area at the base of the tree trunk (AHW). The pipe model assumes that every leaf is connected to the roots through a so-called pipe. Because a leaf cannot change its vertical position, when the crown rises, the lower leaves and branches will die off and the associated pipes will disconnect (Shinozaki et al. 1964). We assume that these disconnected pipes constitute the heartwood. Thus, heartwood increases when the stem height increases, i.e., we consider heartwood development to be a growth process as suggested by Bamber (1976). The dynamics of AHW are given by differential equation Eq. (3).
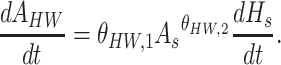 |
(3) |
In Eq. (3), 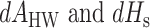 are the infinitesimal increments of heartwood and stem height, respectively, and
are the infinitesimal increments of heartwood and stem height, respectively, and 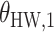 and
and 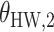 are power law parameters.
are power law parameters.
Growth dynamics
In our model, allocation is determined by the portions of the net production that is invested in each of stem-height growth and crown size growth (and associated sapwood growth), while the remainder goes to reproduction. We denote by uH and uA the portion of net production invested in stem height and crown size, respectively, thus uH, uA ≥ 0 and uH + uA ≤ 1. Importantly, we allow the allocation to the different compartments to change over time and hence both proportions are functions of time, uH = uH(t) and uA = uA(t), and each such pair of functions corresponds to unique growth trajectory. The part of the net production not invested into stem height or crown size is invested into reproductive output (seed production), i.e., the proportion equal to 1 uH uA. The dynamics of the biomass pools is given by Eq. (4).
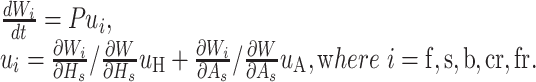 |
(4) |
In Eq. (4), 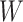 is the total dry mass of living tissue, i.e.,
is the total dry mass of living tissue, i.e., 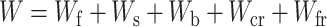 .
.
Definition of optimal growth
We assume that the tree allocates biomass to stem height and crown size growth optimally, i.e., in a way that maximizes fitness. Fitness (J) is modeled as the lifetime amount of production invested in reproduction, which is equal to the reproductive production at each time t and the probability of survival until that time, integrated over time. Mathematically, this is expressed as:
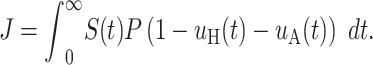 |
(5) |
The probability of the tree still being alive declines over time as:
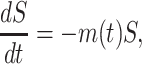 |
where m(t) denotes the mortality rate. For more information on 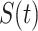 see Appendix C available as Supplementary Data at Tree Physiology Online.
see Appendix C available as Supplementary Data at Tree Physiology Online.
Mortality
In our model, we use a size-dependent mortality based on the model by Fridman and Ståhl (2001):
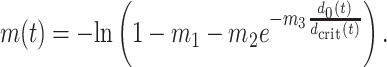 |
(6) |
In Eq. (6), m1, m2 and m3 are parameters (Table 1) and d0(t) is the stem diameter at ground level, calculated as the diameter of a circle with the area equal to the sum AHW(t) and AS(t). The parameter dcrit(t) is the minimum diameter to avoid mechanical failure (Landsberg and Sands 2010), where 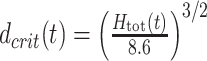 and Htot(t) is the height of the tree at time t. m1 represents the base mortality, i.e., the minimum mortality, which is independent of size, and m2 and m3 define the size-dependent mortality. While the mortality accounts specifically for mechanical failure, all other mortality risks, such as disease and herbivory, are subsumed in the base mortality.
and Htot(t) is the height of the tree at time t. m1 represents the base mortality, i.e., the minimum mortality, which is independent of size, and m2 and m3 define the size-dependent mortality. While the mortality accounts specifically for mechanical failure, all other mortality risks, such as disease and herbivory, are subsumed in the base mortality.
Perfect-information approximation
We assume that the tree possesses information on the whole light environment (from the ground to the top of the canopy) when it develops its allocation strategy. That is to say, the function Q can be evaluated for the whole light profile to determine the allocation to stem and crown in each time step. We refer to this assumption as the Perfect Information Approximation (PIA).
Dynamic programming
Dynamic programming is a method used for solving complex decision-making problems, by dividing the main problem into smaller problems and solve these in a recursive manner. A detailed explanation of this method is given in Appendix B available as Supplementary Data at Tree Physiology Online. In short, the method discretizes the problem and finds the optimal growth trajectory by trying all possible allocation decisions. In this study, we use dynamic programming for finding the allocation functions uH(t) and uA(t), which maximize the fitness proxy Eq. (5).
Heuristic allocation method
We propose a heuristic allocation method, based on Pontryagin’s maximum principle (see Appendix C available as Supplementary Data at Tree Physiology Online). The method is built on the PIA and consists of two sub-strategies to cope with the two different types of local environmental conditions faced during growth: one for the first phase of growth in shaded or understorey conditions (the shade strategy) and one for growth in high-light conditions just below the canopy (the canopy strategy). The shade strategy begins with crown size growth only up to a switching point (mark 1, Figure 4), where growth switches to stem-height growth only. The next switching point from shade to canopy strategy is triggered when the tree reaches a stem height where a switch to crown size growth (mark 2, Figure 4), or simultaneous investment in crown size and height (mark 2′, Figure 4), becomes optimal (i.e., gradual shift, see case 2 in Appendix C available as Supplementary Data at Tree Physiology Online). Growth stops when the tree reaches an optimal final size and seed production starts (mark 3, Figure 4). Based on the PIA, we determine the switching points (mark 1 and 2, in Figure 4) by optimizing them with respect to fitness, i.e., maximizing the fitness proxy (Eq. (5)) using the built-in MATLAB optimization method genetic algorithm.
Figure 4.
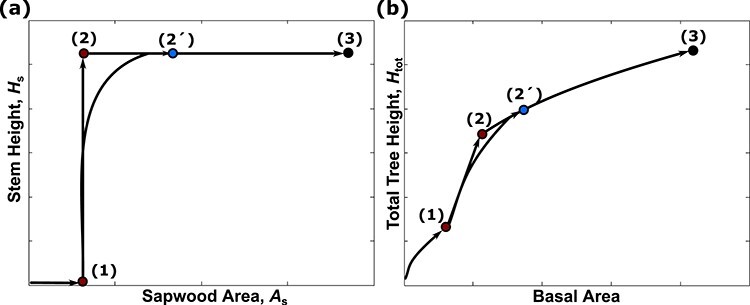
Growth as determined by our heuristic allocation model. (a) The growth in the stem height (Hs)−sapwood area (As) plane and (b) the corresponding growth trajectory in the total tree height (Htot)−basal area plane. Initial allocation is focused on crown size growth. Once the switching crown size (1) is reached, allocation switches to stem height growth. Once the switching stem height is reached (marked (2)), the allocation strategy again switches to crown size. However, if the condition for simultaneous growth is met, the optimal growth trajectory will follow along an arc and allocation gradually shifts from full stem height investment to full crown size investment (marked (2′)). From mark (2)/mark (2′) crown size growth recommences and lasts until an optimal size is reached (marked (3)), where size growth stops, and seed production starts.
The difference between dynamic programming and the heuristic method is that for dynamic programming we find the optimal growth trajectory by considering all possible trajectories, or more accurately all possible allocation functions uH(t) and uA(t). In the case of the heuristic method a functional form for the optimal growth trajectory is already given and we merely need to optimize the two strategy switching points.
Results
Tree height and diameter growth can be described in terms of three phases
In addition to the numerical optimizations of the growth trajectory, we performed a theoretical analysis of the generality of the findings (Appendix C available as Supplementary Data at Tree Physiology Online). The analysis reveals that the sequence of growth phases followed by a reproductive phase, described under Heuristic allocation method above is not only true for our choice of growth model and light function but will hold true for a wider range of growth models (see Appendix C available as Supplementary Data at Tree Physiology Online for more details). However, the specific growth trajectory, i.e., the numerical results, will depend on the particular model and parameters applied.
Heuristic method predicts near-optimal growth trajectories
To test if our heuristic method correctly predicts the optimal trajectory, we compared the growth trajectory predicted by the heuristic method to the trajectories derived by dynamic programming for a wide range of light environments. The close agreement between the results of the two methods indicates that the heuristic method provides a very good approximation of the true optimal growth trajectory (Figure 5).
Figure 5.
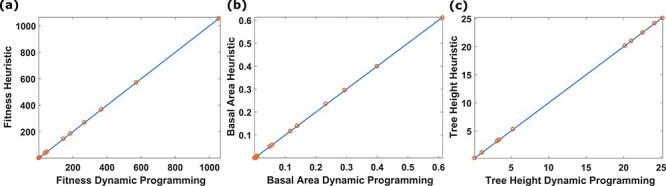
The heuristic model gives good approximation of the optimal growth strategy. We compare the resultants from our heuristic method and dynamic programming for different light environments. Our heuristic method produces results close to dynamic programming, when comparing fitness, the calculated fitness value (a), the tree basal area (b) and the height (c). Each circle represents a unique light environment. The different light environments were generated as described in theory and model.
The early growth strategy in shade depend on the whole light profile up to the canopy
The first strategic switching point from crown size expansion to stem height growth depends on the whole light profile, in particular, the maximum light gradient and its position (Figure 3a and b). In contrast, a tree that maximizes growth based on only the local light gradient would invest only in crown growth (with no stem height increase) due to the shallow light gradient near the ground.
Figure 3.
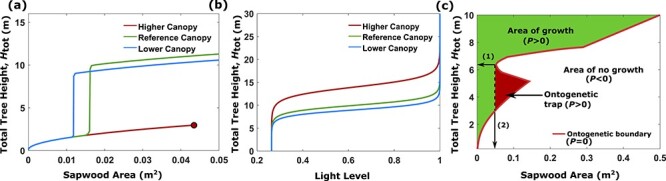
The optimal growth trajectory depends on the whole light environment and a critical threshold in sapwood area determines which trees can reach the canopy top. When the maximum light gradient, dQmax, is decreased and the height at maximum light gradient, HdQmax, is increased, the tree will invest more into crown size before switching to stem height and invest more in stem height before switching back to crown size growth. (a) The optimal growth paths and (b) the corresponding light environments for three different cases: reference canopy, lower canopy and higher canopy. For the reference canopy, the height at maximum light gradient is set to 10 m and the maximum light gradient is 0.25 m−1. For the lower canopy, the height at maximum light gradient is decreased to 9 m and for the higher canopy, it is increased to 14 m. The corresponding maximum light gradients are 0.27 and 0.17 m−1, respectively. The light condition (light level and gradient) at ground level is the same in all three cases. In the left figure a circle signals the end of a growth trajectory and when seed production starts. (c) The ontogenetic plane. The ontogenetic plane is divided into three areas, an area where the tree is able to grow (area of growth), an area where the tree cannot grow (area of no growth) and an area we call the ontogenetic trap (triangular area). Inside the ontogenetic trap which starts at a sapwood area (and associated crown size) indicated by (2), growth is possible, but the tree cannot reach a height larger than the critical tree height (marked (1)), and is unable to reach the canopy.
The height growth strategy (all the switching points) depends on the tree species, i.e., on the physiological parameters. This means that for a given light profile, some species can reach the canopy top according to the strategy described above, while others (with different parameters) cannot. For these species it is therefore optimal to remain at low height, i.e., a sub-canopy strategy (Figure 3a).
Early crown expansion can lead to an ‘ontogenetic trap’ that prevents the tree from reaching the canopy top
We explored how the canopy light profile, in terms of canopy height and density, affects the optimal growth path (Figure 3a). The steeper the maximum light gradient, and the lower down it occurs, the smaller is the crown size at which the tree switches to height growth and the lower the stem height at which crown expansion resumes again toward the top of the canopy. When the maximum light gradient (steepest increase in light) is too high up, the optimal growth path is to remain at ground level and only invest into crown size growth. A critical threshold in sapwood area determines which trees can reach the top of the canopy, and to illustrate this, we define an ontogenetic plane with sapwood area on the horizontal axis and total tree height on the vertical axis. Calculating the net production at every point, we found that the plane is divided into two regions. The first region is where the net production is negative (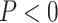 ), and the tree cannot sustain its body size, and consequently must reduce its size. The second region is where the net production is positive (
), and the tree cannot sustain its body size, and consequently must reduce its size. The second region is where the net production is positive (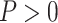 ), and the tree will grow. On the border between these two regions the net production is zero (
), and the tree will grow. On the border between these two regions the net production is zero (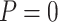 ) (Figure 3c). The size of these regions depends on the physiological model parameters, i.e., the size differs among tree species. Trees can only grow within the positive growth region with increasing height and increasing or constant sapwood area, toward a point near or at the top of the canopy where they stop growing and start reproductive production. However, they can also get stuck on a much lower height due to an ontogenetic trap created by the shape of the zero-growth border (Figure 3c). The trap is associated with a threshold crown size (marked (2) in Figure 3c); any growth trajectory crossing this threshold will be unable to grow taller than a critical tree height (marked (1) in Figure 3c).
) (Figure 3c). The size of these regions depends on the physiological model parameters, i.e., the size differs among tree species. Trees can only grow within the positive growth region with increasing height and increasing or constant sapwood area, toward a point near or at the top of the canopy where they stop growing and start reproductive production. However, they can also get stuck on a much lower height due to an ontogenetic trap created by the shape of the zero-growth border (Figure 3c). The trap is associated with a threshold crown size (marked (2) in Figure 3c); any growth trajectory crossing this threshold will be unable to grow taller than a critical tree height (marked (1) in Figure 3c).
The ontogenetic ‘trap’ is best explained by the relationship between light gradient (the light increase with tree height) and the associated photosynthetic gain. Because of the shape of the light profile, the change in light level is shallow at low tree height, leading to a small gain in gross production with height growth. The maintenance cost, however, always increases linearly with tree height and may exceed the photosynthetic gain and lead to a decrease in net production with height. This trend will continue as long as the light gradient is below the maintenance cost increment, i.e., until the light gradient gets steeper higher up toward the canopy. An optimal growth path must avoid growing into this trap, in order to reach canopy height.
The ontogenetic trap will become larger when the maximum light gradient is reduced, or is positioned higher up, i.e., when growing under taller trees with a higher canopy. If the maximum light gradient is very small, or it is positioned too high up, the ontogenetic trap will cut through the ontogenetic plane and divide it into two regions, making it impossible for the tree to reach the canopy, which means that the optimal strategy is not to grow up to the canopy.
The model predicts taller trees and increased investment into crown size when wood density decreases
Beside the light environment, the optimal growth path is determined by the values of the parameters in the physiological growth model. We assess the effect of wood density and mortality (m1) on the switching points (Figure 6). Decreasing wood density resulted in increased initial crown expansion before switching to height growth, earlier switching to final crown expansion in the canopy and increased final tree size (Figure 6a). We found that increasing mortality had a small effect on the above switching points, but there was a noticeable reduction of the final tree size (Figure 6a). When the wood density is decreased and mortality increased simultaneously according to a relationship observed across species (Iida et al. 2012), the effects are similar to the effect of only reducing wood density (Figure 6b).
Figure 6.
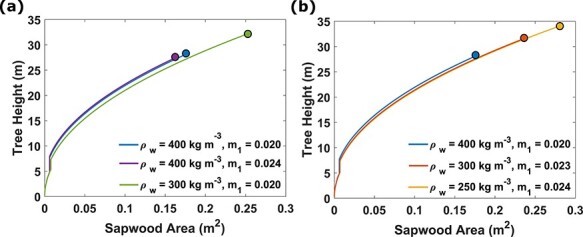
The model predicts that a tree with lower wood density (ρw) and higher mortality (m1) grows to a larger size. We compare growth trajectories for trees with lower wood density or higher mortality (a) and examine the change in growth trajectory when wood density and mortality are changed simultaneously (b). The relationship between the wood change in density and mortality was calculated 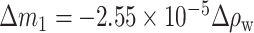 , and the number
, and the number 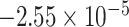 m3 kg−1 was determined as the mean slope from Figure 1 in Iida et al. (2012). The circle at the end of each growth trajectory signals the final size, and when seed production starts. All trees were subjected to the same light condition (height at maximum light gradient, HdQmax = 10 m, maximum light gradient, dQmax = 0.25 m−1 and initial light level, Q0 = 0.27).
m3 kg−1 was determined as the mean slope from Figure 1 in Iida et al. (2012). The circle at the end of each growth trajectory signals the final size, and when seed production starts. All trees were subjected to the same light condition (height at maximum light gradient, HdQmax = 10 m, maximum light gradient, dQmax = 0.25 m−1 and initial light level, Q0 = 0.27).
Discussion and conclusion
Simple heuristic strategies predict optimal growth to the top of the canopy
Growing the crown size beyond a certain critical threshold at low height will render the tree unable to reach the canopy, i.e., it will be trapped at a sub-canopy height. This critical threshold is defined by a bottleneck in terms of a growth rate minimum in the ontogenetic plane defined by stem height and crown size. These findings imply that existing optimization approaches based on local information (Perrin 1992, Dewar et al. 2009, Franklin et al. 2012) are not sufficient to predict variation in stem and crown diameter in individual-based forest models. Instead, the two-stage optimization model presented here provides a solution. Alternatively, dynamic programming could be used, which, however, suffers from heavy computation load and does not provide insights into the determinants of the optimal strategy. In comparison with the most commonly used allocation model—static allometric relationships—our method has two decisive advantages: the optimization eliminates the need for species-specific allometric parameters, and it automatically adjusts allocation behavior to changing environmental conditions.
Optimal height growth explains key aspects of tree-height distributions in forests
The tree growth literature comprises countless studies of tree growth in even-aged stands (in full light) (Bergh et al. 1999, Binkley et al. 2010), some short-term studies of seedlings in controlled light conditions (Kubiske and Pregitzer 1996, Poorter et al. 2005), and a few long-term studies in forests under unknown light conditions (Pretzsch 2005). Remarkably, however, we did not find any published observations of growth paths in terms of height and crown size (or stem diameter) for trees grown toward increasing light under measured light conditions.
From the simulations we observe that if the canopy density is below, or the canopy height is above, a certain critical threshold then it will no longer be profitable (in terms of fitness) to grow up to canopy height, but rather to develop only crown size, creating an understory layer in the forest, see Figure 3. The critical threshold is sensitive to the physiological parameters, which vary with species. Thus, the threshold varies among species, which implies that in a given light environment some species may follow a sub-canopy strategy, whereas others should strive to reach the canopy, resulting in height-stratified forests as often observed (e.g., Cermák 1998, Batista et al. 2014). The model also offers an explanation for why canopy species do not grow crowns as large as sub-canopy species of the same height (e.g., Bohlman and O’Brien 2006), and why crown size stops growing if their height growth has stalled in the shade (Kubota et al. 1994)—increased crown size would impair continued height growth when light conditions improve.
In contrast to some other studies of height growth strategies (e.g., Mäkelä 1985, King 1990, Falster and Westoby 2003), we do not account for the feedback of the trees’ strategy on their own light environment, which explains the evolution of stems and light competition in forests as an evolutionarily stable strategy (ESS). This, however, does not mean that our model is inconsistent with ESS models, instead it addresses a different question: although ESS models usually converge to an equilibrium strategy and an associated generated light environment, in reality a tree species or genotype will face unpredictable and variable environments. Our model predicts how a tree responds by plasticity to such variation, although it does not explain what the tree’s evolved mean ESS environment is. However, although the model does not derive the full forest ESS environment, it provides an explanation why sub-canopy species have evolved in the presence of taller canopy species due to their diverging height versus crown size growth strategies.
Model predicts similar trend between wood density and tree diameter as observed in empirical data
When mortality was increased and wood density remained constant, the switching points remained largely unchanged. However, the switch from growth to reproduction occurred earlier, which results in a decrease in the final tree size. This trend is reasonable as increasing mortality (m1) will decrease the probability of survival, which has a negative effect on fitness (see Appendix D available as Supplementary Data at Tree Physiology Online).
When the wood density decreased, and mortality remained constant, we observed an increase in sapwood investment and an increase in the final tree size. Looking at the model we see that decreased wood density will lead to an increase in net production, which in return will increase growth rate. A decrease in wood density will also cause an increase in the region of positive net productivity (see Figure 3c) and the point in the ontogenetic plane of maximum net productivity will appear at a larger crown size. Thus, it is reasonable that a decrease in wood density would result in an increase in crown size as this would increase fitness (see Appendix D available as Supplementary Data at Tree Physiology Online).
A number of empirical studies have observed a negative correlation between mortality and wood density (Poorter 2008, Kraft et al. 2010) and if we are using a linear relation between wood density and base mortality (m1), the model predicts an increased investment into crown size, both at an early stage before stem height growth starts and later, during the final crown expansion in the canopy. We also observe that at a given tree height, a decrease in wood density leads to an increase in the total sapwood area and stem diameter. This is consistent with empirical observations made by Iida et al. (2012), who found a negative correlation between wood density and stem diameter when different tree species are compared. However, the study also observed a positive correlation between wood density and crown width, which is not the case in our model because we assume that the crown geometry is the same among all species.
How much can a tree know—the PIA
Regarding a tree’s ability to sense its environment, one can imagine a range of different levels, with two hypotheses serving as the extremes. As one extreme, we can assume that the tree possesses no knowledge about its environment. This has the consequence that no matter the environment, the tree morphology will always follow a genetically preprogrammed allometric trajectory. The other extreme is our PIA assumption, that the tree possesses absolute knowledge of its surroundings (the light environment in our case). This assumption allows the tree to change morphology and be plastic in response to environmental variation. Out of the two extreme hypotheses it could be argued that the latter is closer to reality (e.g., Trewavas 2005, Gagliano et al. 2014). This would also be expected given the advantages shown here of being able to sense of the full light environment, which should result in a strong selection pressure for this trait.
Outlook
The key benefit of our allocation model, compared with traditional allocation methods such as static allometric functions, lies in its ability to explain and predict changes of growth allocation in response to variations in the surrounding environment. This aspect is important for a variety of fields, from natural vegetation modeling to forestry. For example, in forestry applications, it is of great importance to realistically simulate the impact of changes in the light environment on productivity and diameter growth in response to planting density or after a thinning operation. Thus, the model can provide a vital component for individual-based forest models, and model-aided decision tools for optimizing thinning in forestry planning.
In our current model we do not consider any plasticity in the crown shape and we do not consider the light variation within the tree’s crown. In a more realistic setting, one would include crown area as an independent variable to account for crown plasticity where the tree can optimize the leaf area directly exposed to sunlight and self-shading inside of the crown architecture. However, the model can readily be extended, e.g., to include biomass investment in additional organs or optimal adjustment of additional traits (see Appendix C available as Supplementary Data at Tree Physiology Online). In our current model we focused on two traits, stem height and sapwood area, and a natural extension would be to include crown height and crown width.
While our model describes growth strategies in newly formed gaps, it does not apply to growth under gap formation, i.e., it does not account for dynamic competition between the focal tree and its neighbors. However, although it is beyond the scope of this paper, in principle our model can be applied to gaps if the optimal strategy is re-evaluated in each time step according to the increasing canopy height. On the other hand, some prominent forest models assume that canopy gaps are always filled by growth and stem bending of surrounding trees, i.e., the perfect plasticity approximation (e.g., Purves et al. 2008, Strigul et al. 2008). Such a representation of forest structures could therefore readily be combined with our model of optimal height and diameter growth.
Our model can also be of use to complement other general forest growth models (e.g., Pacala et al. 1996, Falster et al. 2011) to provide further insight into the role of height and crown plasticity in the development and structure of forests. However, the importance of height growth strategy in response to light environment is in stark contrast to the lack of relevant observations, which suggests an urgent need for more observational studies of tree growth patterns under variable light conditions.
Supplementary Material
Acknowledgments
We would like to thank Ola Lindroos and Johan Holmgren for their valuable input during writing of this paper.
Contributor Information
Peter Fransson, Department of Mathematics and Mathematical Statistics, Umeå University, Linneaus väg 49, Umeå, SE-901 87, Sweden; Department of Forest Ecology and Management, Swedish University of Agricultural Sciences, Skogsmarksgränd 17, SE-901 83 Umeå, Sweden.
Åke Brännström, Department of Mathematics and Mathematical Statistics, Umeå University, Linneaus väg 49, Umeå, SE-901 87, Sweden; International Institute for Applied Systems Analysis, Schlossplatz 1, Laxenburg, A-2361, Austria.
Oskar Franklin, International Institute for Applied Systems Analysis, Schlossplatz 1, Laxenburg, A-2361, Austria; Department of Forest Ecology and Management, Swedish University of Agricultural Sciences, Skogsmarksgränd 17, SE-901 83 Umeå, Sweden.
Conflict of interest
None declared.
Funding
The work presented in this paper was funded by the Swedish Research Council Formas. Additional funding for OF and PF was provided by the Knut and Alice Wallenberg Foundation.
Authors' contributions
All authors conceived the study, discussed the results and implications and revised the manuscript. P.F. analyzed the model and wrote the manuscript.
References
- Antin C, Pélissier R, Vincent G, Couteron P (2013) Crown allometries are less responsive than stem allometry to tree size and habitat variations in an Indian monsoon forest. Trees 27:1485–1495. [Google Scholar]
- Bamber RK (1976) Heartwood, its function and formation. Wood Sci Technol 10:1–8. [Google Scholar]
- Batista NDA, Bianchini E, Carvalho EDS, Pimenta JA (2014) Architecture of tree species of different strata developing in environments with the same light intensity in a semideciduous forest in southern Brazil. Acta Bot Brasilica 28:34–45. [Google Scholar]
- Bergh J, Linder S, Lundmark T, Elfving B (1999) The effect of water and nutrient availability on the productivity of Norway spruce in northern and southern Sweden. For Ecol Manage 119:51–62. [Google Scholar]
- Binkley D, Stape JL, Bauerle WL, Ryan MG (2010) Explaining growth of individual trees: light interception and efficiency of light use by eucalyptus at four sites in Brazil. For Ecol Manage 259:1704–1713. [Google Scholar]
- Bohlman S, O’Brien S (2006) Allometry, adult stature and regeneration requirement of 65 tree species on Barro Colorado Island, Panama. J Trop Ecol 22:123–136. [Google Scholar]
- Cermák J (1998) Leaf distribution in large trees and stands of the floodplain forest in southern Moravia. Tree Physiol 18:727–737. [DOI] [PubMed] [Google Scholar]
- Dewar RC, Franklin O, Mäkelä A, McMurtrie RE, Valentine HT (2009) Optimal function explains forest responses to global change. Bioscience 59:127–139. [Google Scholar]
- Duursma RA, Mäkelä A (2007) Summary models for light interception and light-use efficiency of non-homogeneous canopies. Physiol 27:859–870. [DOI] [PubMed] [Google Scholar]
- Falster DS, Westoby M (2003) Plant height and evolutionary games. Trends Ecol Evol 18:337–343. [Google Scholar]
- Falster DS, Brännström Å, Dieckmann U, Westoby M (2011) Influence of four major plant traits on average height, leaf-area cover, net primary productivity, and biomass density in single-species forests: a theoretical investigation. J Ecol 99:148–164. [Google Scholar]
- Falster DS, Brännström Å, Westoby M, Dieckmann U (2017) Multitrait successional forest dynamics enable diverse competitive coexistence. Proc Natl Acad Sci USA 114:E2719–E2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin O, Johansson J, Dewar RC, Dieckmann U, McMurtrie RE, Brännström Å, Dybzinski R (2012) Modeling carbon allocation in trees: a search for principles. Tree Physiol 32:648–666. [DOI] [PubMed] [Google Scholar]
- Fridman J, Ståhl G (2001) A three-step approach for modelling tree mortality in Swedish forests. Scand J For Res 16:455–466. [Google Scholar]
- Gagliano M, Renton M, Depczynski M, Mancuso S (2014) Experience teaches plants to learn faster and forget slower in environments where it matters. Oecologia 175:63–72. [DOI] [PubMed] [Google Scholar]
- Harja D, Vincent G, Mulia R, van Noordwijk M (2012) Tree shape plasticity in relation to crown exposure. Trees 26:1275–1285. [Google Scholar]
- Iida Y, Poorter L, Sterck FJ, Kassim AR, Kubo T, Potts MD, Kohyama TS (2012) Wood density explains architectural differentiation across 145 co-occurring tropical tree species. Funct Ecol 26:274–282. [DOI] [PubMed] [Google Scholar]
- King DA (1990) The adaptive significance of tree height. Am Nat 135:809–828. [Google Scholar]
- King DA (1993) A model analysis of the influence of root and foliage allocation on forest production and competition between trees. Tree Physiol 12:119–135. [DOI] [PubMed] [Google Scholar]
- Kraft NJB, Metz MR, Condit RS, Chave J (2010) The relationship between wood density and mortality in a global tropical forest data set. New Phytol 188:1124–1136. [DOI] [PubMed] [Google Scholar]
- Kubiske ME, Pregitzer KS (1996) Effects of elevated CO2 and light availability on the photosynthetic light response of trees of contrasting shade tolerance. Tree Physiol 16:351–358. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Konno Y, Hiura T (1994) Stand structure and growth patterns of understorey trees in a coniferous forest, Taisetsuzan National Park, northern Japan. Ecol Res 9:333–341. [Google Scholar]
- Landsberg JJ, Sands P (2010) Physiological ecology of forest production: principles, processes and models. Amsterdam, the Netherlands: Academic Press. [Google Scholar]
- Mäkelä A (1985) Differential games in evolutionary theory: height growth strategies of trees. Theor Popul Biol 27:239–267. [Google Scholar]
- Mäkelä A (1986) Implications of the pipe model theory on dry matter partitioning. J Theor Biol 123:103–120. [Google Scholar]
- Mäkelä A (1997) A carbon blance model of growth and self-pruning in trees based on structural relationships. For Sci 43:7–23. amakela@silvia.helsinki.fi [Google Scholar]
- Mäkelä A, Valentine HT (2001) The ratio of NPP to GPP: evidence of change over the course of stand development. Tree Physiol 21:1015–1030. [DOI] [PubMed] [Google Scholar]
- Mäkelä A (2002) Derivation of stem taper from the pipe theory in a carbon balance framework. Tree Physiol 22:891–905. [DOI] [PubMed] [Google Scholar]
- Mäkelä A, Sievänen R (1992) Height growth strategies in open-grown trees. J Theor Biol 159:443–467. [Google Scholar]
- Medlyn BE (1998) Physiological basis of the light use efficiency model. Tree Physiol 18:167–176. [DOI] [PubMed] [Google Scholar]
- Pacala SW, Canham CD, Saponara J, Silander JA, Kobe RK, Ribbens E (1996) Forest models defined by field measurements: estimation, error analysis and dynamics. Ecol Monogr 66:1–43. [Google Scholar]
- Perrin N (1992) Optimal resource allocation and the marginal value of organs. Am Nat 139:1344–1369. [Google Scholar]
- Poorter L (2008) The relationships of wood-, gas- and water fractions of tree stems to performance and life history variation in tropical trees. Ann Bot 102:367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorter L, Bongers F, Sterck FJ, Wöll H (2005) Beyond the regeneration phase: differentiation of height-light trajectories among tropical tree species. J Ecol 93:256–267. [Google Scholar]
- Pretzsch H (2005) Stand density and growth of Norway spruce (Picea abies (L.) Karst.) and European beech (Fagus sylvatica L.): evidence from long-term experimental plots. Eur J For Res 124:193–205. [Google Scholar]
- Purves DW, Lichstein JW, Strigul N, Pacala SW (2008) Predicting and understanding forest dynamics using a simple tractable model. Proc Natl Acad Sci USA 105:17018–17022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K, Yoda K, Hozumi K, Kira T (1964) A quantitative analysis of plant form. The pipe model theory: II. Further evidence of the theory and its application in forest ecology. Jap J Ecol 14:133–139. [Google Scholar]
- Strigul N, Pristinski D, Purves D, Dushoff J, Pacala S (2008) Scaling from trees to forests: tractable macroscopic equations for forest dynamics. Ecol Monogr 78:523–545. [Google Scholar]
- Trewavas A (2005) Plant intelligence. Naturwissenschaften 92:401–413. [DOI] [PubMed] [Google Scholar]
- Valentine HT, Mäkelä A (2005) Bridging process-based and empirical approaches to modeling tree growth. Tree Physiol 25:769–779. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


