Abstract
Throughout its 40-year history, the field of gene therapy has been marked by many transitions. It has seen great strides in combating human disease, has given hope to patients and families with limited treatment options, but has also been subject to many setbacks. Treatment of patients with this class of investigational drugs has resulted in severe adverse effects and, even in rare cases, death. At the heart of this dichotomous field are the viral-based vectors, the delivery vehicles that have allowed researchers and clinicians to develop powerful drug platforms, and have radically changed the face of medicine. Within the past 5 years, the gene therapy field has seen a wave of drugs based on viral vectors that have gained regulatory approval that come in a variety of designs and purposes. These modalities range from vector-based cancer therapies, to treating monogenic diseases with life-altering outcomes. At present, the three key vector strategies are based on adenoviruses, adeno-associated viruses, and lentiviruses. They have led the way in preclinical and clinical successes in the past two decades. However, despite these successes, many challenges still limit these approaches from attaining their full potential. To review the viral vector-based gene therapy landscape, we focus on these three highly regarded vector platforms and describe mechanisms of action and their roles in treating human disease.
Subject terms: Molecular medicine, Diseases
Introduction
Gene therapy is the treatment of a genetic disease by the introduction of specific cell function-altering genetic material into a patient. The key step in gene therapy is efficient gene delivery to the target tissue/cells, which is carried out by gene delivery vehicles called vectors. There are two types of vectors: viral and non-viral. Non-viral vectors will not be discussed in this review. Contemporary viral vector-based gene therapy is achieved by in vivo delivery of the therapeutic gene into the patient by vectors based on retroviruses, adenoviruses (Ads) or adeno-associated viruses (AAVs) (Fig. 1). Alternatively, a therapeutic transgene can be delivered ex vivo, whereby cells of a patient are extracted and cultured outside of the body. Cells are then genetically modified by introduction of a therapeutic transgene and are then re-introduced back into the patient. There are four basic gene therapy approaches as follows: gene replacement, the delivery of a functional gene to replace a non-working gene; gene silencing, inactivation of a mutated gene that has become toxic to cells; gene addition, over expression of a “foreign” or exogenous gene to impact cellular function; and gene editing, a permanent manipulation of a gene in a patient’s genome.
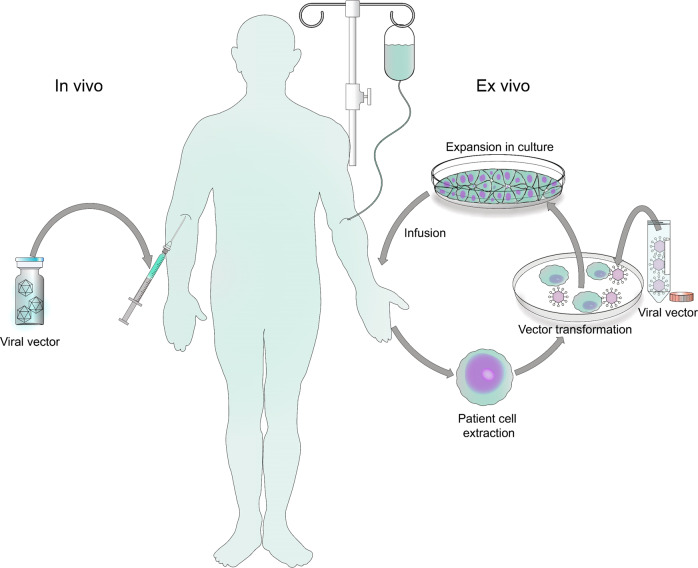
Fig. 1. Summary of viral gene therapy modalities.
In vivo gene therapy entails the direct administration of vector carrying a therapeutic transgene into the patient. Ex vivo gene therapy involves the extraction of a patient’s cells or from an allogenic source, genetic modification by a vector carrying a therapeutic transgene, selection and expansion in culture, and infusion to re-introduce the engineered cells back into the patient
The first gene therapy trial, at least conceptually, was performed by Dr. Stanfield Rogers, who treated two sisters who had hyperargininemia.1 The treatment was based on his observations that patients with Shope papilloma virus had decreased serum arginine levels. Unfortunately, the trial failed to reverse the disease, as the Shope papilloma genome did not encode for arginase production. In a 1980 study that was not formally published, Dr. Martin Cline attempted to insert recombinant β-globin, a factor needed in the production of hemoglobin, into bone marrow cells of two patients with β-thalassemia.2 The transfected cells were then re-introduced back into the patients. Although groundbreaking at the time, this first attempt at an ex vivo gene therapy was ultimately a failure. Nonetheless, these two efforts were among several others that endeavored to deliver genetic material to patients, in the hopes of treating disease. It was not until the early 90s that viral vector gene therapies found clinical success. A trial led by French Anderson, Michael Blaese, and Steven Rosenburg also employed an ex vivo strategy in treating a patient named Ashanthi DeSilva, who had adenosine deaminase deficiency severe combined immunodeficiency disease (ADA-SCID). Several infusions of T cells transformed by a recombinant retrovirus carrying the ADA gene were administered, resulting in what is regarded as the first successful gene therapy in humans.3,4
The use of viruses for therapy has long been practiced and actually belongs to a class of viral-based treatments known as virotherapies. Perhaps, the main reason why earlier viral-based therapies failed to achieve efficacy was due to a lack of full understanding of the viral biology. Now with 40 years of accumulated knowledge on viruses, promising viral vector-based strategies to treat genetic diseases are numerous. Some human diseases even have several effective treatment options to choose from. Typically, a viral vector is defined by three components as follows: (1) the protein capsid and/or envelope that encapsidates the genetic payload, and defines the vector’s tissue or cell tropism and antigen recognition; (2) the transgene of interest, which when expressed in cells, serves to confer a desired effect; and (3) the “regulatory cassette,” the combined enhancer/promoter/auxiliary elements that controls stable or transient somatic expression of the transgene as an episome or as a chromosomal integrant. Design aspects for these three components are different for each viral vector platform, have unique considerations, and harbor their own strengths and weaknesses. In this review, we will describe three viral vector platforms that have gained wide use for efficacious gene therapy and regulatory approval. These three strategies are based on Ads, AAVs, and lentiviruses (retroviruses), the viruses that a majority of gene therapy vectors are based upon (Fig. 2). For each of these vector platforms, we will review their general compositions and their mode of infection, highlight key vector platforms and their biological properties, describe current production strategies in use, feature their uses as commercialized drugs and in clinical trials, and finally discuss challenges and future outlooks.
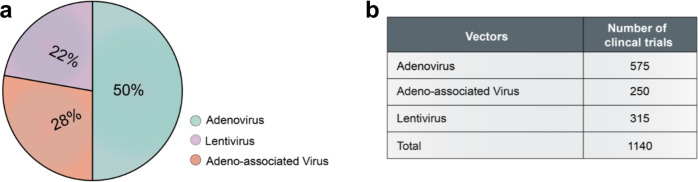
Fig. 2. Summary of viral vectors used in clinical trials.
a Pie chart showing the percentage of adenovirus, adeno-associated virus, or lentivirus vectors in use. b A table of the current number of clinical trials employing the different viral vectors. Data source: Wiley database on Gene Therapy Trials Worldwide. http://www.abedia.com/wiley/vectors.php
Ad vectors: large cargo capacities for transient targeted gene delivery
Structure and genome
Ad is a non-enveloped virus that is known to mostly cause infections of the upper respiratory tract but can also infect other organs such as the brain and bladder. It possesses an icosahedral protein capsid that accommodates a 26- to 45-kb linear, double-stranded DNA genome. The Ad genome is flanked by hairpin-like inverted terminal repeats (ITRs) that vary in length (30–371 bp at its termini).5,6 The ITRs serve as self-priming structures that promote primase-independent DNA replication. A packaging signal located at the left arm of the genome is required for viral genome packaging. The Ad genome encodes ~35 proteins that are expressed in the early and late phases of viral gene transcription. The Ad genome comprises five so-called “early-phase” genes, E1A, E1B, E2, E3, and E4.7 The early-phase genes are transcribed before the initiation of viral DNA replication (about 7 h post infection). The “immediate-early” E1A gene is essential for transcription of other viral genes (e.g., E1B, E2, E3, and E4), which are responsible for viral DNA synthesis and play roles in modulating expression of host genes. E1B plays roles in counteracting the cell’s activation of apoptosis by binding and inactivating p53, permitting viral replication to progress.8 The “late-phase” genes (L1–L5) are generally required for virus assembly, release, and lysis of the host cell.9 These gene products are derived from the five late transcriptional units that are produced by alternative splicing and polyadenylation of the major late messenger RNAs (Fig. 3a).10
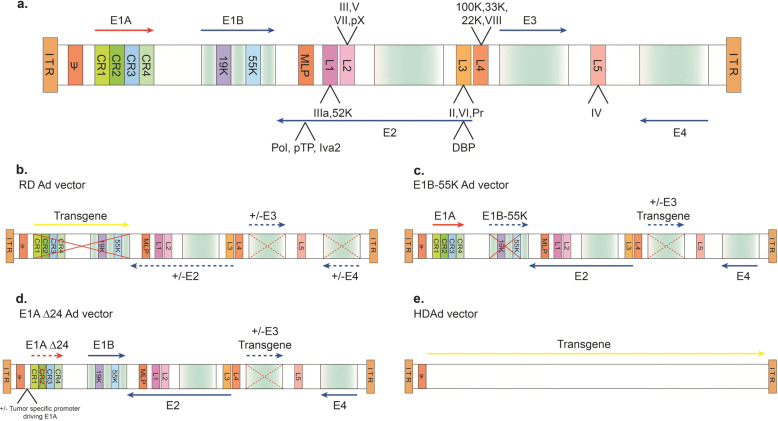
Fig. 3. Schematic of the wild-type adenovirus type 5 (Ad5) genome and the genetic modifications of common Ad5-based vectors.
a Diagram of the wild-type Ad5 genome structure. The 36 kb genome consists of four early transcription elements (E1, E2, E3, and E4), five late expression genes (L1–L5), cis-packaging elements (ψ) and two inverted terminal repeat sequences (ITR). The E1A (red arrow) gene contains four conserved domains (CR1-4), each of which interacts with special cellular proteins. The E1B gene encodes for two distinct tumor antigens, the 19 kDa (19K) and 55 kDa (55K) proteins. The E2 gene encodes DNA-binding protein (DBP), terminal protein (pTP), IVa2, and DNA polymerase (Pol). The E4 gene encodes 1–7 open reading frames. The major late messenger RNAs (L1–L5) mainly encode for virion structural proteins and are derived from a pre-mRNA, which is driven by a major late promoter (MLP) via alternative splicing and polyadenylation. L1 encodes for IIIa and 52K. L2 encodes for the penton base gene (capsid protein III) and the core proteins V, pVII, and pX. L3 encodes for the hexon (capsid protein II), capsid protein precursor (pVI), and protease (Pr) genes. L4 encodes for 100K, 33K, 22K, and pVIII. L5 encodes for the fiber gene (capsid protein IV). b–e Diagrams of rAd vectors. b Replication-defective (RD) vector. The E1A and E1B regions are deleted and replaced with an expression cassette containing an exogenous promoter and a transgene of interest (indicated by the solid red X and yellow arrow). The E3 and E4 regions are usually deleted to accommodate larger insertions and eliminates leaky expression of other viral genes. c E1B-55K replication-competent vector. The E1B-55K region is deleted (solid red X and dashed blue arrow), whereas in some vectors, the E3 region is deleted and replaced with an expression cassette (dashed red X and dashed blue arrow). d E1A-Δ24 (Δ24) replication-competent vector. The CR2 region in E1A is deleted (solid red X and dashed red arrow) and the E1A promoter can be replaced with various tumor-specific promoters to drive CR2-deleted E1A expression. In some vector designs, the E3 region is deleted and replaced with an expression cassette (dashed red X and dashed blue arrow). e Helper-dependent Ad vectors (HDAds). Most or all of the Ad genomic elements are replaced with a therapeutic expressing cassette (yellow arrow), save for the cis-packaging sequences (ψ) and ITRs. These vectors are propagated in the presence of an Ad helper vector
The Ad viral capsid is 90–100 nm in diameter. It is composed of the structural proteins, hexon (capsid protein II), penton base (capsid protein III), fiber (capsid protein IV), capsid protein precursors pIIIa, pVI, and pVIII, and capsid protein IX, and the virion core proteins (V, VII, and X).11 Hexons are the most abundant structural components residing on the surface of the virus, comprising 240 trimers in the assembled capsid. There are 12 penton proteins located at the apex of the icosahedral vertices, giving rise to the protruding fibers. The V, VII, and X proteins mainly associate with the viral genome and play critical roles in genome replication, condensation, and assembly processes.11 The IIIa proteins are located at the inner surface of capsid, and drive the assembly of the packaged genome via binding with L1 52/55K and stabilization of the vertex regions via interaction with penton base, hexon, and VI proteins.12,13 The VI proteins link the core to the inner icosahedral shell, while also serving as lytic factors during endosomal disruption.14 The VIII proteins bind to the peripentonal hexons to stabilize the viral capsid.15 The terminal protein (pTP) covalently links to the 5′-ends of the Ad genome and enhances genome replication.16
Ad assembly is thought to proceed by the following sequential pathway steps: (1) empty capsids (procapsids) are assembled with capsomers (hexons, penton bases, and fibers) along with some minor capsid proteins and unstructured proteins; (2) the viral genome binds to packaging proteins (IVa2, L4 33K, L1 52/55K, and L4 22K) via the packaging signal (ψ) within the left ITR; (3) encapsidation of the viral genome into the procapsid through a hypothetical portal located at a unique vertex that is accompanied by the release of scaffolding and some of the packaging proteins; and (4) cleavage of the precursor proteins (pIIIa, L1 52/55K, pVI, pVII, pVIII, mu, and pTP) by the adenovirus protease (AVP), resulting in the final mature viral particle. Two to 3 days after entering the cell nucleus, new virions are assembled and cells lyse to release virions.17
Infection pathway
To date, more than a hundred human Ad genotypes have been identified, falling into seven subgroups classified A to G (http://hadvwg.gmu.edu). Knowledge on the Ad infection pathway is largely based on human Ad5 (HAd5). Infection initiates with interaction between the cell surface-localized coxsackievirus-Ad receptor (CAR) and the distal domain of the virus capsid fiber.18,19 In addition, many other receptors for entry of Ads have also been found, such as CD46, DSG2, and sialic acid.20–22 Binding of the virus to the cell surface is then followed by endocytosis, which is mediated by interaction between the tripeptide Arg-Gly-Asp (RGD) motif in the penton base and the αV integrins on the host cell surface.23 Following endocytosis, the capsid is disassembled and the V and VI proteins facilitate endosomal escape.14,24 The viral DNA subsequently enters the nucleus through the nuclear envelope pore complex.25 In addition, hexons from partially disrupted virions bind with dynein motors to help the virus traffic to the nuclear pore via the microtubular network.26 In the nucleus, the viral DNA predominantly remains epichromosomal and is not incorporated into the host cell genome.27
Ad natural infections and pathological association
Most people carry neutralizing antibodies (NAbs) against one or more of the prevalent human Ad serotypes, as a result of exposure to Ads from past infections.28,29 Ads typically result in asymptomatic responses or lead to mild or severe disease in immunocompetent individuals.30 Among the seven known species of human Ads, species B and C are usually acquired in early childhood and cause infections in the upper respiratory, gastrointestinal, and urinary tracts. Some serotypes in species D cause epidemic keratoconjunctivitis, whereas HAd4 from species E causes acute respiratory disease.31 Due in part to the epidemiology of Ads in the human population and Ad antigen-specific T cells, which result in the lifelong immunity, vectors based on Ad tend to have compromised potencies32,33 and trigger a stronger immunological response compared to other viral vectors, such as those based on AAV (discussed further below).
Ad as a vector in gene therapy
Ad vectors have the following advantages: (1) high transduction efficiency, both in quiescent and dividing cells; (2) epichromosomal persistence in the host cell; (3) broad tropism for different tissue targets; and (4) and the availability of scalable production systems.34 Contemporary Ad vectors are derived from human serotypes HAd2 and HAd5. The major objectives in Ad vector development are to overcome the challenges associated with its widely pre-existing viral immunity among the general population, life-threatening strong innate immune responses to its capsid proteins, and robust adaptive immune responses to de novo synthesized viral and transgene products.35 Since the first generation of E1A-deleted Ad vectors were established, various strategies have been developed to improve their capacity, efficacy, gene transfer longevity, and safety.
First generation. The first generation of Ad vectors were engineered by replacing the E1A/E1B region with transgene cassettes that can be up to 4.5 kb in length (Fig. 3b). Removal of the E1A gene results in the inability of recombinant Ad (rAd) to replicate in the host cell.36 Therefore, complementary cell lines designed to express E1A and E1B, such as HEK293, are needed for production.37 As the E3 region is not required for viral propagation in cultured cells, the E1/E3 double-deletion frees up more space for the transgene cassettes (6.5 kb in length). The first generation of Ad vectors has two main disadvantages as follows: (1) de novo expression of Ad proteins can still activate the host immune response, causing clearance of vector-transduced cells;38 and (2) possible spontaneous homologous recombination between the vector and engineered E1 region from HEK293 during genome amplification can generate replication-competent adenovirus.
Second generation. Due to issues with first-generation Ad vectors, researchers developed improved versions by further deleting the other early gene regions (E2a, E2b, or E4), permitting additional space for larger transgene cassettes (10.5 kb) (Fig. 3b). These new vector designs include temperature-sensitive rAd vectors, generated by ablation of E2A-encoded DNA-binding protein,39,40 deletion of the E2b-encoded DNA polymerase (Pol) protein,41,42 and deletion of the E4 region.43 All of these Ad vectors were shown to have significantly decreased late gene expression and elicit less of a cytotoxic T-lymphocyte response when administered in vivo. As a result, transgene expression was substantially prolonged in mice compared to first-generation vectors.39–43 Again, the deleted genes are complemented by engineered production cell lines. Unfortunately, the deletion of E2 and/or E4 genes negatively affects viral vector amplification, resulting in lower titers.44 This reduction is a consequence of inefficient complementation of E2/4 with engineered cell lines. Despite the changes, the native Ad late genes that are still retained within the vector genome can trigger host immunogenicity and cellular toxicity.45
Third generation. Third-generation Ad vectors, referred to as “gutless” or “helper-dependent” Ad vectors, have all viral sequences deleted, except for the ITRs and the packaging signal (Fig. 3e). These vectors, also called “high-capacity” adenoviral vectors (HCAds),46 can accommodate ~36 kb of space for cargo gene(s). Production of HCAds in cell culture requires an additional adenoviral helper virus (HV) that is similar in composition to first-generation vectors, but with the distinction that they contain loxP sites inserted to flank the packaging signal. The HCAd genome is transfected into HEK293 producer cells that express Cre recombinase, along with the HV infection. Replication and packaging are permitted by the viral proteins provided by the helper genome. The engineered Cre in producer cells ensures that only the HCAd genome can be packaged, as the helper-virus genome-packaging signal is excised by Cre-mediated recombination at the loxP sites.47 Compared with the previous generations of Ad vectors, HCAds have reduced immunogenicity, prolonged transduction in the host cell, and a significantly larger cargo capacity, which can accommodate multiple transgene cassettes, or therapeutic genes that are driven by their larger native promoters and enhancers to mimic physiological levels of expression. However, the main challenge in HCAd production is ensuring that the helper adenovirus is eliminated from vector preparations, which may alter efficacy and safety of HCAd vectors in vivo.
Conditionally replicating Ad vectors. The engineering of tumor-specific gene promoters into the Ad genome can be used to control the initiation of viral replication to create conditionally replicative adenoviral vectors (CRAds). The first CRAds were based on the partial deletion of the E1B sequence (E1B-55K) (Fig. 3c).48 As viral genome replication can be completed without E1B-55K in most tumor cells, as they lack p53, E1B-55K-deleted Ads only undergo genome amplification and subsequent lysis in a patient’s tumor cells, leaving non-tumor cells unaffected. In the next generation of CRAds, the 24-amino acid CR2 domain of E1A protein was deleted to generate AdD24 vectors (Fig. 3d).49 The CR2 domain is known to bind retinoblastoma protein to release E2F, the S-phase-activating transcription factor required for viral genome replication in normal cells. As cancer cells express excessive amount of E2F, Ad replication can proceed without E1A. The resulting dl922-947 and AdD24 vectors both showed high replication potency and selectivity in tumor cells.50 These oncolytic Ad vectors are unique from other natural oncolytic viruses such as reoviruses, senecaviruses, and the α-virus M1 virus.51,52 Improvements to tumor specificity through these engineering efforts have inherent advantages over natural viruses, as natural viruses carry wild-type viral genomes that may result in unknown consequences.
Therapeutic Ad vectors and commercial availability
In the early 1990s, Ad vectors for in vivo therapy were first used to deliver and express the α-1 antitrypsin (A1AT) gene in rat hepatocytes and lung tissues.53,54 Following this demonstration, many Ad-based gene delivery trials for treating human monogenic disease were conducted, including the expression of cystic fibrosis transmembrane conductance regulator (CFTR) in lung tissues of patients with cystic fibrosis, and vascular endothelial growth factor (VEGF) in patients with peripheral vascular disease.55,56 Unfortunately, a series of studies revealed that Ad is strongly immunogenic, which not only restricted the delivery and expression of exogenous genes but also caused undesired immune responses in treated subjects.57 Soon after in 1999, the death of Jesse Gelsinger, who was enrolled in a clinical trial for OTC (ornithine transcarbamylase) gene therapy with an Ad vector,58 raised safety concerns for human gene therapies and caused a significant decline in related studies for the following decade. It was discovered that innate response to the capsid protein triggered cytokine storm.58 These unfortunate results and backup evidence in animal models indicated that even with gutless rAd designs, the capsid may still trigger a strong immune response towards the therapy,59,60 thus limiting the use of Ad vectors in human gene therapy.
It is now well-accepted that Ad can trigger strong immune responses in humans, reinforcing safety concerns for their application.61 However, for therapies that are not impacted by an immunological response, but may even rely on them to kill the cells they transduce, Ad vectors have seen significant utility. For example in 2003, Gendicine, an Ad vector harboring a Rous sarcoma virus promoter-driven p53 gene, was approved in China as the world’s first commercialized gene therapy drug for cancer.62 ONYX-105 (dl1520) was the first CRAd to enter clinical trials,63,64 followed by a similar replication-selective Ad vector called H101 (Oncorine), which also gained commercial approval in China.65 Although numerous clinical results have confirmed the safety of ONYX-105 and H101, the drugs were ultimately not very effective. The major reason for the lowered efficacies is that deletion of E1B-55K also causes attenuated viral replication, even in tumor cells in vivo.
Ad vectors in clinical trials
Ad vectors have seen a rebirth in human gene therapy research. They maintain many practical advantages, including their broad tropism profiles, lack of host genome integration, and large packaging capacities (~36 kb). Currently, Ad-based gene therapy clinical trials account for 50% of total worldwide trials (Fig. 2a). They have been mainly applied towards novel vaccines and cancer therapies.
Ad-mediated genetic vaccines. Immunogenicity is a critical hurdle for viral vector efficacy, but has been exploited in the development of Ad-based vaccines.66 rAd vectors can deliver foreign epitopes to enhance the host immune response to pathogens by boosting the production of pro-inflammatory cytokines and effective adaptive humoral and cellular immune responses.67 These innovative approaches have made Ad vectors an ideal vaccine carrier.
For example, Ebola vaccines based on Ad vectors showed induction of specific antibody and T-cell responses in clinical trial subjects.68–70 Notably, HAd26-ZEBOV/MVA-BN-Filo (ClinicalTrials.gov identifier: NCT04028349) has been shown to be well-tolerated and produced durable humoral immune responses for more than a year post vaccination. A vector based on a non-human primate-derived Ad (ChAd3-ZEBOV) was also well-tolerated in both adults and children.71,72 In addition, human Ad-based influenza vaccines that can confer expression of major influenza viral antigens, such as HA, NP, and M2, have been tested in clinical trials including the H1N1 and H5N1 (NCT03232567 and NCT00755703).73 A chimpanzee Ad vector, ChAdOx1, expressing NP and M1 antigens were also designed and used in two phase I trials (NCT01818362 and NCT01623518).74,75 Ad-mediated human immunodeficiency virus (HIV) vaccines that individually confer expression of the HIV-1 genes gal, pol, and env (HAd5-gag, HAd5-pol, and HAd5-Nef, respectively) have also been developed and tested.76–79 However, these trials did not reveal efficacious vaccination. At the time of this review, no Ad-based HIV vaccine has been proven successful.
The global COVID-19 pandemic, which is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is currently the most threatening public health emergency. At the time that this review was written, SARS-CoV-2 has resulted in more than 100 million infection cases with more than 2 million deaths worldwide. In response, multiple vaccine strategies have been under development. An HAd5-based vaccine that delivers the SARS-CoV-2 spike glycoprotein was launched in a phase I clinical trial with 108 participants. Thus far, outcomes showed rapid humoral and T-cell responses after 14 days post vaccination in most participants, with no serious adverse events.80 A phase II clinical trial with a group of 508 participants to evaluate its efficacy has recently begun. In addition, a phase I/II clinical trial to evaluate the efficacy of a SARS-CoV-2 vaccine based on the chimp-derived ChAdOx1 capsid is also underway (NCT04324606).81 Preliminary data demonstrated that ChAdOx1-nCoV-19 treatment induced both humoral and cellular immune responses against SARS-CoV-2, with NAbs in 91% of participants after a single dose and 100% response after a booster dose.
rAds have also been used to deliver vaccines for cancer prevention. The overall strategy for immunizing against tumor cells is to induce the expression of tumor-associated antigens (TAA) and/or oncolytic processes that promote antitumor immune responses via Ad-mediated gene delivery. Currently tested antigens include prostate-specific antigen for prostate cancer (NCT00583752 and NCT00583024), MAGE-A3 for solid tumor, human papilloma virus (HPV) E6/E7 for HPV-associated cancers (NCT02285816, NCT02879760, and NCT03773744), and carcinoembryonic antigen (CEA) for colorectal and pancreatic cancers (NCT03329248 and NCT03387098). A non-human-derived AdOx1 was employed to deliver the oncofetal TAA, 5T4, to immunize against prostate cancers (NCT03815942 and NCT02390063). Using HAd5 vectors to express a combination of TAAs, such as CEA + brachyury + MUC1 and CEA + brachyury + mucin 1 (MUC1) + Her2 are also used to promote cancer vaccination.82
Anticancer therapy. To date, various approaches using Ad vectors to specifically kill tumor cells have been developed and tested in clinical trials (Table 1). The early generation of Ad vector-based anticancer therapies mainly relied on replication-defective vectors for their immunogenic properties to deliver tumor repressor, cytotoxic, or immune-regulating genes to induce tumor cell death and antitumor immune responses.
Table 1.
A selection of ongoing and completed clinical trials employing Ad vectors
| Conditions | Intervention | Transgene | Trial stage | Identifier |
|---|---|---|---|---|
| Prostate cancer | CG7870 combination with Docetaxel | E1A under control of the rat probasin promoter and E1B under control of the PSA promoter-enhancer | Phase I/II | NCT00103428 |
| Prostate cancer | CG7870 | E1A under control of the rat probasin promoter and E1B under control of the PSA promoter-enhancer | Phase I/II | NCT00116155 |
| Metastatic melanoma | OBP-301 (Telomelysin) | E1A/E1B under control of human telomerase reverse transcriptase gene hTERT promoter | Phase II | NCT03190824 |
| Esophagogastric adenocarcinoma | OBP-301 (Telomelysin) | E1A/E1B under control of human telomerase reverse -transcriptase gene hTERT promoter | Phase II | NCT03921021 |
| Solid tumors | Ad/PNP coupled with fludarabine phosphate | PNP | Phase I | NCT01310179 |
| Hormone-refractory metastatic prostate cancer | Ad-OC-TK + valacyclovir | HSV-TK gene under control of osteocalcin promoter in the area of the excised E1 region | Phase I/II | in Japan |
| Prostate cancer | AdV/RSC-TK + Brachytherapy | HSV-TK gene under control of Rous sarcoma virus long terminal repeat promoter RSV | Phase II | NCT01913106 |
| Prostate cancer | CTL102 + CB1954 | NTR under control of CMV promoter | Phase I/II | 40 Patient cohort in UK |
| Advanced pancreatic cancer | Ad5-yCD/mutTK(SR39)rep-ADP | CD/TK/ADP | Phase I | NCT02894944 |
| Prostate cancer | Ad5-yCD/mutTK(SR39)rep-ADP + IMRT intensity-modulated radiation therapy | CD/TK/ADP | Phase I/II | NCT00583492 |
| Prostate cancer | Ad5-yCD/mutTK(SR39)rep-hIL12 | CD/TK/hIL12 | Phase I | NCT02555397 |
| Malignant pleural mesothelioma | rAd-IFN administered with Celecoxib and Gemcitabine | IFN-α-2b | Phase III | NCT03710876 |
| Advanced peritoneal malignancies | Oncos-102 + Durvalumab | GM-CSF | Phase I/II | NCT02963831 |
| Metastatic castration-resistant prostate cancer | Oncos-102 + DCVAC/PCA | GM-CSF | Phase I/II | NCT03514836 |
| High-grade NMIBC after BCG failure | CG0070 | E1A under control of E2F-1 promoter, CM-CSF under control of E3 promoter | Phase II | NCT02365818 |
| Mutiple advanced cancer | LOAd703 | CD40L and 4-1BBL under control of CMV promoter | Phase I/II | NCT03225989 |
| Pancreatic cancer | LOAd703 | CD40L and 4-1BBL under control of CMV promoter | Phase I/II | NCT02705196 |
| Malignant melanoma | LOAd703 + Atezolizumab | CD40L and 4-1BBL under control of CMV promoter | Phase I/II | NCT04123470 |
| Recurrent glioblastoma | DNX-2401 | NA | Phase I | NCT00805376 |
| Recurrent glioblastoma | DNX-2401 + Temozolomide | NA | Phase I | NCT01956734 |
| Recurrent glioblastoma or gliosarcoma brain tumors | DNX-2401 + IFN-γ | NA | Phase I | NCT02197169 |
| Naive diffuse intrinsic pontine gliomas | DNX-2401 | NA | Phase I | NCT03178032 |
| Recurrent high-grade glioma | DNX-2401 | NA | Phase I | NCT03896568 |
| Advanced or metastatic melanoma | ICOVIR-5 (Ad-DM-E2F-K-Delta-24-RGD) | E1A under control of E2F-1 promoter and myotonic dystrophy locus insulator DM-1 | Phase I | NCT01864759 |
| Refractory solid tumors | ICOVIR-7 | E1A under control of E2F-1 promoter with four new E2F-responsive palindromes | 21 Patient cohort in Finland | |
| Advanced pancreatic cancer | VCN-01 + Gemcitabine and Abraxane® | E1A under control of E2F-1 promoter and myotonic dystrophy locus insulator DM-1, and Hyaluronidase | Phase I | NCT02045589 |
| R/M head and neck squamous cell carcinoma | VCN-01 + Durvalumab | E1A under control of E2F-1 promoter and myotonic dystrophy locus insulator DM-1, and Hyaluronidase | Phase I | NCT03799744 |
| Refractory retinoblastoma (RTB) | VCN-01 | E1A under control of E2F-1 promoter and myotonic dystrophy locus insulator DM-1, and Hyaluronidase | Phase I | NCT03284268 |
| Resectable colon cancer, non-small cell lung cancer, bladder cancer, renal cell carcinoma | ColoAd1 (Enadenotucirev) | NA | Phase I | NCT02053220 |
| Locally advanced rectal cancer | Chemoradiation + Enadenotucirev | NA | Phase I | NCT03916510 |
| Metastatic cancer, epithelial tumor | NG-641 | FAP-TAc antibody /CXCL9/CXCL10/IFN-α | Phase I | NCT04053283 |
| Metastatic cancer, epithelial tumor | NG-350A | anti-CD40 antibody | Phase I | NCT03852511 |
CD cytosine deaminase, CD40L CD40 ligand, CMV cytomegalovirus, CXCL chemokine ligand, FAP-Tac fibroblast-activating protein/CD3, GM-CSF granulocyte-macrophage colony-stimulating factor, hIL12 human interleukin-12, HSV-TK herpes simplex virus thymidine kinase, hTERT human telomerase reverse transcriptase, IFN interferon, NA not applicable, NTR Escherichia coli nfsB bacterial nitroreductase, PNP Escherichia coli Pruine nucleoside phosphorylase, PSA prostate-specific antigen.
i. Delivery of suicide genes. Based on the well-established fact that many tumor types display dysfunction in the p53 tumor repressor pathway,83 Ad vectors have been engineered to induce p53 expression to cause cell-cycle arrest and apoptosis in tumor cells.84,85 However, not all tumor types lack p53 function. Other applications for Ad vectors in anticancer therapy have been tested in the targeted expression of pro-drug-converting enzymes to achieve tumor cell killing. For example, the enzyme purine nucleoside phosphorylase (PNP) converts the pro-drug fludarabine monophosphate (F-ara-AMP) into fluoroadenine, which confers cytotoxicity to proliferating cells. A dose-escalation phase I trial to treat patients with advanced tumors was conducted to test the efficacy of an Ad vector encoding Escherichia coli PNP (Ad/PNP), followed by intravenous administration of F-araAMP (NCT01310179).86
The herpes simplex virus thymidine kinase (HSV-TK) can convert the pro-drug ganciclovir (GCV) to a cytotoxic nucleotide to selectively kill dividing cells. In 2004, the first randomized, controlled clinical trial with the non-replicable Ad HSV-TK/GCV (AdHSV-TK/GCV) gene therapy was carried out (NCT00005025).87 Since then, Ad-TK vectors have been applied in multiple clinical trials around the world for treating numerous types of solid tumor cancers. In 2006, a phase I/II clinical trial was conducted with an Ad-TK vector under the control of the Rous sarcoma virus long terminal repeat (LTR) promoter (Ad-RSV-TK) to treat prostate cancer (NCT01913106).88 Another phase I/II trial used an Ad vector to express TK with the bone-specific osteocalcin promoter to treat patients with hormone-refractory prostate cancer exhibiting metastasis to the bone.89
Cytosine deaminase (CD) can convert the pro-drug 5-fluorocytosine (5-FC) to toxic 5-fluorouridine (5-FU), which is further processed in cells to 5-FUTP and 5-FdUMP. These products cause a blockage of thymidylate synthase and subsequent damage to DNA. The HAd5-CD/TKrep vector, which contains the CD/HSV-TK chimeric enzyme transgene, showed long-term effectiveness when systemically delivered along with 5-FC and GCV, and was used in conjunction with intensity-modulated radiotherapy (IMRT) (NCT00583492).90 A modified version of this treatment (HAd5-yCD/mutTK(SR39)rep-ADP), which delivers a yeast CD fused to a mutated TK enzyme and the Ad death protein (ADP) showed promise in a phase I clinical trial for advanced pancreatic cancer (NCT02894944).91 A variant of this vector, HAd5-yCD/mutTK(SR39)rep-hNIS, also co-delivers a transgene encoding the human sodium iodide transporter (hNIS) to enable imaging of tumors to monitor viral spread and efficacy by single photon radionuclide computed tomography (SPRCT) and positron emission tomography.92 Another vector that was designed by the same research group also harbors the pro-inflammatory cytokine IL-12 to induce an antitumor immune response.93 Currently, two clinical trials using this strategy to treat prostate and pancreatic cancer are ongoing (NCT02555397 and NCT03281382, respectively).
ii. Delivery of immuno-regulatory genes. Ad vectors can also be armed with immune-regulating genes to stimulate antitumor immune responses in the patient. Intrapleural administration of Ad-delivered interferon (IFN)-β or IFN-α-2b to the lungs have been proven to be safe treatments for malignant pleural mesothelioma.94,95 Most recently, treatments using Ad-IFN-α-2b in combination with celecoxib and gemcitabine are being actively tested in a phase III trial (NCT03710876).
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is known to induce activation of immune cells to trigger an antitumor response. Replication-competent HAd5/3 chimeric viruses expressing GM-CSF (Oncos-102, HAd5/3-D24-GM-CSF) were tested in a phase I clinical trial to target solid tumors.96,97 Since then, Oncos-102 has been tested in a phase I clinical trial, and in combination with durvalumab and autologous dendritic cell immunotherapy (DCVAC/PCa) in phase I/II trials for treating solid tumors (NCT01598129, NCT02963831, and NCT03514836). CG0070 is also a replication-competent oncolytic Ad vector that expresses GM-CSF under control of the human E2F-1 promoter to target bladder cancer. The treatment underwent a phase II clinical trial (NCT02365818).98 The interim results demonstrate that CG0070 confers acceptable levels of toxicity and an overall 47% complete response rate after six months for all patients and 50% for patients with carcinoma in situ.99
CD40 is a cell surface receptor that has also been shown to prevent cell proliferation and promote apoptosis once it interacts with CD40 ligand (CD40L). This association stimulates T-helper 1 immunity via maturation of dendritic cells and promotion of M2 to M1 macrophage differentiation.100 In most breast cancer cells, CD40 is overexpressed, permitting the possibility of using Ad vectors to deliver CD40L to target breast cancers. A vector that expresses CD40L under the control of a promoter containing a hypoxia-response element and an estrogen response element (AdEHCD40L) showed cancer-specific killing ability.101 Another Ad vector expressing CD40L under the hTERT promoter (CGCT-401) was developed to inhibit tumor growth via oncolysis and apoptosis.102 LOAd703 is an immuno-stimulatory oncolytic virus developed by the same research group and uses an HAd5/35 vector to express trimerized CD40L and 4-1BBL, another peptide known to enhance immunologic memory and expand natural killer cells to stimulate innate and adaptive immune responses.103 LOAd703 is currently being tested in phase I/II trials for pancreatic cancer patients (NCT03225989, NCT02705196, and NCT04123470).104
iii. Chimeric and tropism-modified oncolytic Ad vectors. Low expression levels of CARs, the primary Ad receptor on tumor cells, often results in resistance to infection by CAR-dependent oncolytic Ad vectors. To further enhance the cancer-selectivity of Ad vectors, researchers have sought alternative Ad-receptor interactions. Delta-24-RGD (DNX-2401) is an oncolytic Ad vector containing a deletion of the conserved region 2 of E1A (E1AΔCR2) and an insertion of an ACDCRGDCFCG peptide sequence (RGD-4C) into the HI loop of the fiber knob protein. The RGD-4C sequence has been shown to bind strongly to αvβ3 and αvβ5 integrins and enhances virus tropism in a CAR-independent fashion.105 Phase I trials to test these vectors in recurrent malignant gliomas patients are ongoing (NCT00805376, NCT01956734, NCT02197169, NCT03178032, and NCT03896568).106 Another group of oncolytic Ad vectors that can infect host cells via RGD-4C sequences and independent of CAR binding includes ICOVIR-5 and ICOVIR-7. ICOVIR-5/7 clinical trials have been initiated in cancer patients with advanced metastatic tumors (NCT01864759).107,108
The gene therapy vector, VCN-01, is characterized by its putative heparin sulfate glycosaminoglycan-binding site (KKTK) within the fiber shaft replaced by an RGDK motif. The vector delivers the human glycosylphosphatidylinositol-anchored enzyme, PH20 hyaluronidase, to promote viral spread in solid tumor stroma. VCN-01 has been shown to confer antitumor efficacy in patients with pancreatic cancer, especially when combined with chemotherapy (NCT02045589, EudraCT: 2012-005556-42),109 head and neck squamous cell carcinoma (NCT03799744), and refractory retinoblastoma (NCT03284268).
An HAd5/3 chimeric vector was created by substituting the receptor binding fiber knob domain of the HAd5 CR2-deleted vector with the knob domain of serotype 3 (Ad3), which binds to a tumor cell-enriched Ad3 receptor, circumventing CAR interaction.110 A phase I clinical trial of HAd5/3-Δ24 was conducted in patients with recurrent ovarian cancer.111 Many additional modifications have been made based on this chimeric Ad vector. For example, HAd5/3-Cox2L-D24 utilizes the tumor-specific cyclooxygenase 2 gene promoter to control E1A expression, to further enforce tumor selectivity.112 The vector design demonstrated safety and extended virus circulation in patients with metastatic and refractory solid tumors. The Oncos-102 drug mentioned above is also an HAd5/3 chimeric virus that targets solid tumors.96,97
Another tumor-selective chimeric Ad vector ColoAd1 (enadenotucirev), which was directly evolved via facilitating recombination among an array of serotypes and selecting highly potent Ad variants under stringent conditions, showed potent oncolytic activity in colon cancer cell lines (HT29). This hybrid Ad vector harbors the Ad11p (B group Ad virus) backbone and has a near complete deletion of the E3 region, a smaller deletion in the E4 region, and a chimeric Ad3/Ad11p E2B region.113 ColoAd1 conferred potent and unique tumor cell killing properties in multiple solid tumors.114 A phase l clinical trial test the synergistic effect of combining ColoAd1 with standard chemoradiotherapy has been launched to treat advanced rectal cancer (NCT03916510). Most recently, two independent phase I trials using ColoAd1 to express FAP-TAc antibody, together with an immune enhancer module (CXCL9/CXCL10/IFNα) (NCT04053283) and anti-CD40 antibody (NCT03852511), have been initiated for safety validation.
Challenges for Ad vector-based gene therapy
Although different types of Ad vectors are currently available for different preclinical applications, extensive study in Ad vector development have highlighted serious challenges associated with the high worldwide prevalence of pre-existing immunity against common human Ad serotypes, including HAd5. The prevalence rates for NAbs against HAd5 range from 35% in the United States to over 90% in Cote d’Ivoire.115,116 Circulating anti-HAd5 antibodies have been shown to significantly impair the ability of HAd5 vectors to transduce target cells and dampen the resultant adaptive immune responses.117 Moreover, immunogenicity and cellular toxicity continue to be major obstacles for Ad gene therapy, despite the fact that Ad vector-based vaccines and oncolytic therapies benefit from these properties. Therefore, proper control of Ad vector-mediated host innate immune responses is key for the success of these approaches.
The future for Ad vectors
To avoid pre-existing immunity against Ad vectors, several strategies are being employed. First, several “rare” human serotypes with low seroprevalence, such as HAd2, HAd26, and HAd35, were identified and developed into vectors to minimize pre-existing immunity. However, the ability of these vectors to induce an immune response have been shown to be less potent compared with most commonly used HAd5.118 In addition, various non-human Ad vectors were developed from bovine (BAd), canine (CAd), chimpanzee (ChAd), ovine (OAd), porcine (PAd), and fowl (FAd) to limit cross-reactive immunity. Chimpanzee-derived Ad vectors are most widely used, as NAbs against these in human blood circulation are significantly lower.119 Thus far, more than ten clinical trials using ChAd3-derived vaccine have demonstrated the safety of such replication-deficient vectors.120 Moreover, high-capacity HCAd vectors can achieve long-term transgene expression in vivo, since they have dampened host immune responses against viral proteins that may be residually expressed.121 To combat contamination by HV, self-inactivating HVs such as AdTetCre have been developed, in which a chimeric MerCreMer recombinase is regulated by a TetOn system to ensure effective elimination of HV.122 In addition to this advantage, the lack of viral coding sequences in HCAd genomes expands the cloning capacity to 37 kb, allowing the accommodation of site-specific nucleases such as zinc-finger nucleases (ZFNs), transcription activator-like effector nucleases, and clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 systems for genome editing.
AAV vectors: a small vector with huge potential
Structure and genome
AAV was discovered in 1965 by Bob Atchison as a contaminant of Ad preparations.123 Due to its dependence on Ad, or any virus that can serve helper function to complete its life cycle, it is classified as a dependoparvovirus. Despite not causing any known human diseases, AAVs are remarkably well-studied in the short 40 years since their discovery. Much of our knowledge is owed to its relatively simple genome and its capacity to be experimentally manipulated in cloning plasmids.
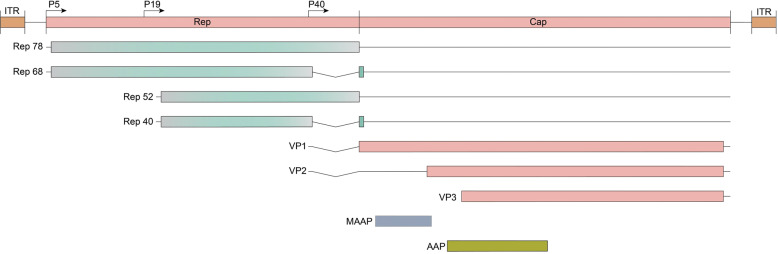
As a dependoparvovirus, AAV lacks the essential genes needed for replication and expression of its own genome. These functions are provided by the Ad E1, E2a, E4, and VA RNA genes.124,125 The AAV genome itself, is a single-stranded DNA that houses four known open reading frames (ORFs) (Fig. 4). The first ORF encodes the four replication genes (rep), which are named after their molecular weights: Rep40, Rep52, Rep 68, and Rep78.126 The second ORF is the cap gene that encodes for the three viral capsid proteins, VP1, VP2, and VP3, respectively.126 The third and fourth are nested sub-genomic mRNAs, named the assembly-activating protein (AAP),127 which is involved in the shuttling of capsid monomers to the nucleolus where capsid assembly takes place; and the recently discovered membrane-associated accessory protein (MAAP),128 whose function is not completely understood. The 4.7-kb genome is flanked by 145-nt ITRs on both ends of the genome (Fig. 4). The ITRs serve as self-priming structures for replication, and provides the signal for Rep-mediated packaging.129
Fig. 4. Schematic of the AAV genome and sites used for PCR screening.
The AAV genome comprised four known open reading frames, rep (green), cap (salmon), MAAP (orange), and AAP (yellow). The rep and cap ORFs encode four and three isoforms, respectively. Transcription is driven by the viral P5, P19, and P40 promoters (arrows). The genome is flanked by inverted terminal repeat (ITR, cyan) sequences
The AAV capsid is a T = 1, icosahedral, 60-mer capsid formed at a 1 : 1 : 10 ratio of VP1, VP2, and VP3 subunits, respectively. The assembled capsid is best characterized by the five-fold axis of symmetry that forms the face for rep binding and contains the five-fold pore, where DNA is inserted under rep-mediated ATPase activity; and the threefold axis of symmetry that is defined by the threefold protrusions comprising of variable loop regions V, VII, VIII, and IV.130 These variable loops establish the main surface epitope for antibody recognition and receptor binding. There are at least 12 natural serotypes that can be categorized into five main clades (Clades A–E) found in primates.131 Its sero-epidemiology is wide-spread and can be found in 50–80% of the human population, depending on the geographical region.132 On their own, AAVs are thought to be non-pathogenic and have yet to be concretely linked to any human diseases.
Infection pathway
There are more than a thousand AAV variants, falling within the five primate clades. Despite the known diversity, our knowledge of AAV’s infection pathway is based on only a few serotypes. The prevailing understanding is that serotypes bind to different, or have differential affinities to an array of primary cell surface glycoprotein receptors and secondary receptors.133 For example, AAV2 binds heparan sulfate proteoglycans.134 The identification of universal receptors has been sought after. Such efforts have discovered transmembrane proteins, such as AAVR that may act to help AAV traffic through the cell via intracellular vesicles,135 although not all serotypes turn out to be dependent on AAVR for transduction.136
Upon binding to the cell surface, clathrin-mediated endocytosis is triggered. The AAV particle is then trafficked in endosomal vesicles and are transported through late endosomal and lysosomal compartments. Due to the low pH environments of these vesicles, the capsid undergoes a conformational change to expose the VP1/2 domains.137 Through a mechanism that is not entirely understood, the VP1-unique region, which contains a phospholipase domain escapes the endosome or lysosome compartment, and is then shuttled into the nucleus via nuclear localization signals found on VP2. Once within the nucleus, the AAV genome undergoes second-strand synthesis to form the double-stranded genome configuration required for gene transcription. In addition, the ITRs confer intra- and inter-molecular recombination to form circular dsDNA species or concatemerized genomes.138 This establishes stability for the AAV genome in an epichromosomal state. Cell culture evidence and in vivo characterizations have suggested that the wild-type AAV can integrate into the genome of the host cell.139–141 The best known integration site in the human genome is a position in chromosome 19 called AAVS1.140 Although, genomic insertion is likely opportunistic with no general hotspots for wild-type AAV integration that is well-accepted.
AAV as a vector for gene therapy
The vector genome. Samulski et al.142 first cloned the AAV genome into expression plasmids and found that transfection of these plasmids into mammalian cell lines in the presence of Ad could produce infectious viruses. Further engineering of recombinant AAV (rAAV) demonstrated that packaging of transgenes could be achieved by gutting the genome, save for the ITR elements, and replacing it with any promoter and gene of interest. The first demonstration of an AAV vector used in humans was performed in 1995 and involved the delivery of the cystic fibrosis transmembrane regulator (CFTR) gene packaged with the AAV2 capsid (rAAV2-CFTR), into a patient with cystic fibrosis.143 Since this first demonstration, multiple vector designs have been reported. The design of vectors in a way is an art form, the reasons for which is manifold. The main consideration for AAV vector design is that the wild-type genome is ~4.7 kb in size. Thus, vectors based on them are irrevocably limited to a ~5 kb capacity. All components needed for proper expression therefore need to be abbreviated/truncated/minimized to fit into the small capsid. Alternatively, strategies that exploit ITR-mediated recombination have produced dual-vector systems that can express “oversized” transgenes, by way of transcript splicing across intermolecularly recombined ITRs from two complementary vector genomes.144–146 Other means of promoting vector-size expansion through vector recombination by homology,147 RNA trans-splicing,148 or protein “trans-splicing” via split intein designs149,150 have also been developed.
The promoters used in AAV vectors, also known as regulatory cassettes, to drive transgene expression are also an important aspect of vector design. As contemporary AAV serotypes or engineered capsids have the capacity to transduce multiple tissue/cell types, cell-type-specific transgene expression is by and large controlled at the level of gene transcription. Designing tissue-specific promoters is the main approach for improving on-target tissue expression. In addition, not only do regulatory cassettes need to be tissue-specific, they must be powerful enough to confer therapeutic levels of transgene expression. For example, the use of muscle-specific regulatory cassettes based on the muscle creatine kinase gene has been employed for muscle gene therapy treatments,151 such as Duchenne muscular dystrophy (DMD) and limb-girdle muscular dystrophy (LGMD). To meet the challenges of the small packaging size, bidirectional vectors have also been employed for delivery of dual therapeutic gene cassettes. An example of this is the bidirectional chicken β-actin ubiquitous promoter that drives the simultaneous expression of the hexosaminidase α- and β-subunits of the HexA enzyme, the two respective genes involved in Tay-Sachs and Sandhoff diseases.152
Other non-promoter vector design strategies to control transgene expression exploits the host cell’s response to foreign pathogens. A standing challenge for AAV-mediated gene therapy is how innate immunity plays a role in muting transgene expression. There is evidence that Toll-like receptor 9 (TLR9) activation and MyD88 can be activated by the presence of unmethylated CpG dinucleotides.153 One straightforward way to circumvent this challenge is to deplete the vector genome of CpGs by codon optimization and mutation of promoter elements;153 although, overall expression can be affected.154,155 Other methods for reducing the activation of the immune system is to employ the cell’s own RNA interference machinery to inhibit expression in antigen-presenting cells (APCs) by inserting binding sites for microRNAs (miRNAs) that are highly expressed in these cell types,156 such as miR142, into the vector genome. This approach effectively eliminates MHC class I-mediated antigen presentation in APCs, hence suppressing CD8+ T-cell activation.157–159 Alternatively, designs to include DNA sequences that are derived from human telomeres have been reported to inhibit TLR9 activation and reduce rAAV-associated immune responses.160
One frontier that has yet to be fully explored is the rational engineering of ITR regions.161 Currently, the only widely used modification to this essential element is to make one ITR unresolvable during replication. By mutating one of the ITRs so that Rep can no longer nick the terminal resolution sequence, a double-stranded genome is packaged into capsids, yielding vectors termed “self-complementary” AAVs (scAAVs).162,163 This modification allows for the bypass of second-strand synthesis, the rate-limiting step for rAAV transduction. The scAAV platform significantly enhances transduction onset and efficiency. The drawback is that the maximum genome size is effectively halved, restricting the packaging limit of the vector. In addition, scAAV genomes can trigger a stronger innate immune response to the transgene compared to single-stranded vectors.164–166 Aside from manipulating how the AAV genome is packaged, is has long been evidenced that the ITR harbors promoter function.167 The extent by which ITRs can impact transgene expression is not well-studied. However, new evidence suggests that the ITRs of different serotypes can confer differential promoter activity.168 Furthermore, there is evidence that the ITRs can produce transcripts on the minus strand, independent of a classical promoter on the reverse strand.169,170 The result of such action is the formation of double-stranded RNAs, which can trigger the host innate immune system. Our lack of full understanding for the AAV vector transduction pathway, and ways in which the ITRs can serve to impact vector genetic fate, has limited our ability to modulate the very last viral elements still residing in current vectors used for gene therapy.
Capsids. The advancement of AAV to expand the capsid toolbox has been the focal point for improving AAV vectors in recent years. The capsid is not only critical for the recognition of cell surface proteins that impact tissue/cell tropism,133 but also serves as the epitope for antibody recognition,171 functions as the substrate for phosphorylation (either prior to or during viral egress from the late endosome for proteasomal degradation),172 and has been shown to remain bound to the genome to impact second-strand synthesis and transcription in a cell-type-specific manner.173,174 As AAVs cannot be easily engineered with capsid proteins from other non-related viruses to yield chimeric vectors with modified properties (pseudotyped), work in the past two decades has focused on developing new capsids with improved properties via alternative approaches. There are currently four main methods for capsid discovery: (1) vectorization of AAV capsids from natural isolates; (2) rational design of capsids using pre-existing capsid as scaffolds; (3) directed evolution of capsid libraries generated by error-prone PCR and/or shuffling of pre-existing capsids with desired properties; and (4) in silico approaches involving the use of computational tools to design novel capsids not observed in nature. We will highlight some of the milestones met in these four categories.
i. Natural isolates. Serotypes AAV1 and AAV2 were first discovered from tissue culture. Afterwards, AAV serotypes were isolated from a range of mammalian species and tissue types. Between 1965 to 2001, only a handful of AAVs, mostly from human clinical samples, were derived from viral isolates, vectorized, and tested. Following these efforts, which demonstrated that each serotype had unique tropism profiles, larger studies to isolate AAVs from human and non-human primate tissues were accomplished by using PCR- amplification of proviral cap sequences. At the end of 2009, more than a hundred variants across six different clades were discovered.131,175,176 Although only a few research groups continue to screen natural variants as potential gene therapy vectors, they are still the predominant capsids used in clinical studies today.
ii. Rational design. The engineering of pre-existing capsids by rational design is an efficient means of developing new capsids with specific properties.177 Rational design approaches have produced some capsids with beneficial characteristics that are improved over natural variants. Such strategies began with the grafting of peptide sequences known to bind surfaces of target tissues onto the surface of the capsid.178 The latest achievement using such a strategy was the development of bone-homing capsid named AAV9.DSS-Nter.179 However, the generation of functional capsids through these means are in general difficult. Part of the reason behind this difficulty is that the AAV capsid and it’s role during the viral life cycle is not completely understood, i.e., changes on the capsid may theoretically improve binding of AAV to specific cell receptors, but may negatively impact other aspects, such as vector production or cellular trafficking.
iii. Directed evolution. Iterative selection of randomly mutated capsids in cell culture or in animals, coupled with next-gen sequencing strategies have given researchers a manageable means of developing new capsids with defined properties.177 Randomized mutations can be achieved by error-prone PCR180 or by the generation of chimeric capsids (capsid shuffling) from the primary sequences of pre-existing serotypes.181–183 The most noteworthy examples of evolved capsids are derived from platforms based on the insertion of short, randomized peptide sequences onto the threefold protrusions, which are highly amenable for capsid manipulation.184 Among the first in this class of capsids are AAV2.7m8 and AAVPHP.B.185,186 The AAV2.7m8 capsid was derived by the insertion of a random seven-amino acid sequence into loop VIII of the AAV2 capsid and iterative rounds of random mutagenesis and selection of positively transduced photoreceptor cells. The development of high-throughput sequencing approaches in the early 2000s marked not only the capacity to profile the transcriptome and epigenetic marks on a whole-genome scale but also allowed for AAV investigators to expand screening depth to test millions of variants at once. Unfortunately, these screening methodologies are limited in that they can only evolve capsids that perform well in the systems they are screened in. For example, the discovery of AAV.PHP.B, which was screened for its ability to traverse the blood–brain barrier (BBB) in C57BL6 mice, produced a capsid that exhibited strain-specific differences.187 Nonetheless, this work was able to reveal the species- and strain-dependent LYS6A gene as the critical receptor that permits AAV.PHP.B to traverse the BBB.188 Additional capsids have thus far been evolved that do not rely on Lys6A host expression, such as AAV-F.189 Further improvements to these methodologies are ongoing and they offer a rapid means of developing new capsids for specific targets.
iv. In silico approaches. The use of computational approaches to develop novel AAV capsids for gene therapy is a relatively new concept.177 Within the past few years, this approach is best exemplified by the discovery of Anc80, a capsid whose sequence is predicted to be the “ancestral” capsid by phylogenetic connectivity between contemporary capsids. Importantly, Anc80 has shown strong tropism to the mouse liver, muscle, retina, and cochlea.190,191 The evaluation of germline endogenous viral elements (EVEs) also represent attempts to reconstruct novel ancestral capsids with the potential for vectorization.192 By using bioinformatics approaches to identify and analyze EVEs among several marsupial species, “fossilized” viral integration events within host DNA have been uncovered. Recently, the single-amino acid substitution across the entire AAV2 cap ORF in combination with machine learning approaches have been used to further increase the depth of variants that can be queried for improved vector performance. The use of machine learning approaches truly represents a new frontier in the gene therapy field. Computer-assisted structure–function analyses has great potential for producing capsids sequences not seen in nature. These tools are developed to consume and integrate large amounts of data on the performance of capsids with single or multiple amino acid changes (production and transduction efficacies), to ultimately derive novel capsids with desired properties. These means can potentially bypass the iterative steps required for directed evolution and can overcome the trial and error process attributed to rational design approaches. Machine-guided strategies have already demonstrated their utility by uncovering a previously unknown ORF encoding for the MAAP gene.128
The nuances of AAV vector biology
AAV vectors have been universally recognized as versatile vectors for gene therapy. Much of this is owed to their wide-ranging tropism profiles, even by the contemporary serotypes. As a result, most AAV vector platforms have been developed to target diseases of the central nervous system, the eyes, liver, heart, and muscle. Similar to their wild-type counterparts, AAV vector genomes undergo circularization via ITR recombination to form stable and persistent episomal configurations that can be detected well beyond 10 years following administration in non-dividing, terminally differentiated cell populations.193–195
Compared to other current viral vectors, rAAVs are accepted as the least immunogenic, with much less vector-related toxicity. In contrast to Ad vectors, AAVs are ideal for their lack of transgene immunity when expressing self-antigens and confer relatively low innate immunity and viral immunity within a broad dose range.196,197
One final nuance for AAV vector research is that assessing AAV vector performance is not ideal with in vitro models. Typically, transduction of rAAVs in vivo does not tend to reflect what is observed in vitro. Furthermore, the field is increasingly aware that results gleaned from small animal models may not necessarily be reproduced when tested in human trials. This failure has led to the necessity for researchers to carry out studies in non-human primate models such as cynomolgus or rhesus macaques before clinical trials are realized.
Production platforms
Although AAV-based vectors have shown great promise as gene therapy drugs, one of the limitations for rAAVs is achieving scalable and economical production of vectors that are free of impurities. Since the initial vectorization studies to produce single-strand rAAVs in the 1980s, clinically relevant advancements in AAV vectorology have been intermittent. The scAAV platform is sometimes referred to as the second generation of rAAVs, but they were developed nearly 20 years ago. In contrast, vector production platforms have undergone multiple advancements. Since both single-stranded AAV and scAAV vectors are absent of all viral components save for the ITRs, the universal strategy for different production schemes is to achieve optimal trans expression of obligatory factors required for packaging AAV. Knowing that impurities continue to be a burden for generating high-quality vectors, efforts to streamline and improve vector yields have been the focus for improving manufacturing practices in recent years. Currently, vector production schemes for clinical use have yet to adopt strategies that are completely cell free.
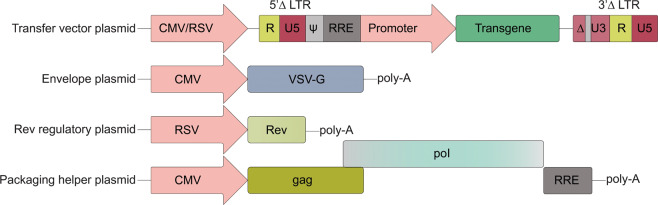
Triple transfection: HEK293. Preclinical/research-grade AAVs are still predominantly produced by the standard triple-transfection method in HEK293 cells. Typically, this employs the co-transfection of the cis plasmid containing the transgene cassette of interest, flanked by ITRs; the trans plasmid, which houses the rep and cap genes, and the Ad helper plasmid, which provides the E2a/b, E4, and VARNA genes. As HEK293 cells are transformed by Ad,198 they express the essential E1a and E1b genes. These cells are typically incubated as semi-adherent cultures and reach maximum productivity at 2–3 days post transfection, losing viability overtime.199
Mammalian stable cell lines. Stable cell lines that constitutively express vector components have been developed to streamline the production process. These platforms free the need to deliver, for example, capsid expression cassettes and/or helper genes on a plasmid. They only require the delivery of the vector genome to be packaged. Clark et al.200 first developed a HeLa-based producer cell line in the mid-1990s, by cloning rep/cap and the rAAV vector genome into an expression plasmid, and then using it to stably transfect HeLa cells. Such a cell line would only require infection by wtAd5 to trigger high-yield vector production. One obvious limitation of this strategy, is that a cell line must be produced for every vector design. Alternatively, hybrid rAAV/rAd vectors were developed to deliver both the transgene vector and adenoviral elements to provide robust rep-cap expression from stably integrated AAV genes in HeLa cell lines. These platforms yield high-titer vector for large-scale production needs.201,202 Producer cell lines derived from A549, a lung-epithelial carcinoma cell line, have also been developed.203 Other vector-based production methods such as HSV vectors have been engineered to deliver within them AAV vector components into baby hamster kidney fibroblast cells (BHK21).204 These platforms are great for production scale-up and enables the generation of clinical-grade vectors. In addition, the use of stable cell lines limits the auxiliary components necessary for production and removes sources of contamination, like adventitious viruses or plasmid contaminants originating from DNA preparation steps. Unfortunately, the concern with using immortalized or transformed cell lines is that the elements that confer their characteristics can be packaged into the vectors as impurities, raising the possibility of genotoxicity in the host, as well as potentially harmful carryover helper viral impurities. Furthermore, unlike the HEK293-based platforms, these stable cell lines lack E1a/E1b expression and therefore require replication-competent Ad helper and/or Ad-AAV (replication-defective) hybrid virus infection. Thus, many methods have been developed to determine whether potential oncogenes or transforming factors end up in manufactured AAVs. Most recently, SSV-seq, AAV-GPseq, and Fast-seq approaches have all been developed to address the relative abundance of mispackaged nuclear material that winds up in preparations.205–207
Baculovirus/Sf9. An alternative production scheme to mammalian cell lines is the use of baculovirus expression vector (BEV) system in Spodoptera frugiperda insect cells (Sf9). This involves the generation of recombinant baculoviruses: one BEV carrying the AAV vector genome, flanked by ITRs, and in another, the rep and cap genes. The BEVs are in turn used to infect Sf9 cells. Since the baculovirus provides helper function,208 a single BEV system in conjunction with Sf9 cell lines that stably express rep, can be employed for flexible and high-titer large-scale vector production.209 Interestingly, a report that vectors produced from Sf9 cells are differentially posttranslationally modified, as compared to those generated from human- or mammalian-derived cell lines raised question as to whether differences in production schemes may impact tropism and transduction efficacies,210 although further work in this area is necessary to cross-validate these findings. Nonetheless, BEV/Sf9 systems exhibit reduced encapsidation of contaminating DNAs205 and therefore remain an attractive production strategy for large-scale, clinical-grade vectors.
Therapeutic AAV vectors and commercial availability
To date, there are have been three AAV-based gene therapy drugs worldwide that have made it to the commercial market. Although they symbolize the things to come for these promising platforms, two major challenges remain: cost of treatment and safety. Manufacturing represents a third challenge. However, the demand for these drugs has yet to reach levels in which manufacturing has created significant bottlenecks. Although manufacturing limitations can still slow clinical trials, most of these drugs are currently developed towards monogenic diseases, which belong to the category of rare diseases and the patient population eligible for trials remains relatively low.
Glybera (alipogene tiparvovec) was the world’s first AAV-based gene therapy to gain regulatory approval for commercialization from the European Medicines Agency in 2012.211 Glybera is an AAV1-based gene therapy that delivered lipoprotein lipase (LPL) to patients with LPL deficiency.212 It was also infamous for gaining the moniker of “the world’s first million-dollar drug.” Due to low demand and its high cost, Glybera was withdrawn from European markets and has yet to gain approval in other countries. Altogether, only four patients outside of clinical trials ever received the drug, three of which payed only €1 to expend the remaining unused stocks.
Luxturna (voretigene neparvovec-rzyl) was the second AAV drug that gained commercial approval in 2017—first by the U.S. Food and Drug Administration (FDA). Luxturna is an AAV2-based vector that delivers the retinoid isomerohydrolase RPE65,213 the effected gene in Leber’s congenital amaurosis, which causes progressive blindness. On its launch, the drug held a price tag of $425,000 per eye.
Zolgensma (onasemnogene abeparvovec) is the third and the most recently approved AAV drug, developed for the treatment of spinal muscular atrophy (SMA).214 It gained approval in the United States and Japan in 2019, and received approval in the EU in 2020. Zolgensma is a one-time gene therapy that delivers the survival motor neuron (SMN1) transgene to replace the non-functional SMN1 gene mutated in patients with SMA. During its launch, it was marketed at $2.125 million per dose—currently, the most expensive medication in the world.
Clinical trials
With the approval of the first AAV-based drugs and continued promising clinical data across the field, several gene therapy platforms have sprung forth and are currently under investigation in the clinic. As a result, there have been over 200 clinical trials based on AAV worldwide. For many trials, enrollment is limited to only a few individuals and the selection criteria are many times very restricted. Combined with the fact that many current gene therapy strategies are presently only developed for rare diseases, the advancement of these new drugs are slow. The FDA and National Institutes of Health are now recognizing that the number of eligible patients is limited and many AAV treatments are for lethal diseases with no alternative options. Therefore, many combined phase I/II or II/III trials have been granted. Table 2 summarizes all current AAV-based clinical trials. The following clinical trials highlight some of the most noteworthy outcomes.
Table 2.
A selection of ongoing clinical trials employing AAV vectors
| Condition | Intervention | Sponsor | Trial stage | Identifier |
|---|---|---|---|---|
| AADC deficiency | AADC | Krystof Bankiewicz UCSF | Phase I | NCT02852213 |
| AADC | National Taiwan University Hospital | Phase II | NCT02926066 | |
| Batten disease (CLN2) | CLN2 | Weill Cornell | Phase I/II | NCT01414985 |
| Batten disease (CLN6) | CLN6 | Nationwide Children’s Hospital | Phase I/II | NCT02725580 |
| MPS-IIIB | NAGLU | UniQure | Phase I/II | NCT03300453 |
| Parkinson disease | AADC | Jichi Medical University | Phase I/II | NCT02418598 |
| GDNF | NINDS | Phase I | NCT01621581 | |
| Neurturin | Sangamo | Phase I/II | NCT00985517 | |
| AADC | Voyager | Phase I | NCT03065192 | |
| SMA | SMN | AveXis | Phase III | NCT03461289 |
| GAN | GAN | NINDS | Phase I | NCT02362438 |
| Achromatopsia | CNGB3 | AGTC | Phase I/II | NCT02599922 |
| CNGB3 | MeiraGTx | Phase I/II | NCT03001310 | |
| Choroideremia | REP1 | Nightstar | Phase III | NCT03496012 |
| REP1 | Spark | Phase I/II | NCT02341807 | |
| REP1 | STZ Eyetrial | Phase II | NCT02671539 | |
| REP1 | University of Oxford | Phase II | NCT02407678 | |
| LCA | RPE65 | Spark | Phase III | NCT00999609 |
| RPE65 | MeiraGTx | Phase I/II | NCT02781480 | |
| LHON | ND4 | GenSight | Phase III | NCT03293524 |
| ND4 | John Guy University of Miami | Phase I | NCT02161380 | |
| RP (RLBP1) | RLBP1 | Novartis | Phase I/II | NCT03374657 |
| Wet AMD | Anti-VEGF antibody | Regenxbio | Phase I | NCT03066258 |
| Anti-VEGF protein | Adverum Biotechnologies | Phase I | NCT03748784 | |
| X-Linked RP | RPGR | AGTC | Phase I/II | NCT03316560 |
| RPGR | MeiraGTx | Phase I/II | NCT03252847 | |
| RPGR | Nightstar | Phase I/II | NCT03116113 | |
| X-linked retinoschisis | RS1 | AGTC | Phase I/II | NCT02416622 |
| RS1 | NEI | Phase I/II | NCT02317887 | |
| Crigler–Najjar syndrome | UGT1A1 | Audentes | Phase I/II | NCT03223194 |
| UGT1A1 | Genethon | Phase I/II | NCT03466463 | |
| FH (homozygous) | LDLR | University of Pennsylvania | Phase I/II | NCT02651675 |
| GSD1a | G6PC | Ultragenyx | Phase I/II | NCT03517085 |
| Hemophilia A | FVIII | Shire | Phase I/II | NCT03370172 |
| FVIII | Bayer | Phase I/II | NCT03588299 | |
| FVIII | BioMarin | Phase III | NCT03392974 | |
| FVIII | Sangamo | Phase I/II | NCT03061201 | |
| FVIII | Spark | Phase I/II | NCT03003533 | |
| FVIII | UCL | Phase I | NCT03001830 | |
| Hemophilia B | FIX | Shire | Phase I/II | NCT01687608 |
| FIX | Pfizer | Phase II | NCT02484092 | |
| FIX | Pfizer | Phase III | NCT03587116 | |
| FIX | Sangamo | Phase I | NCT02695160 | |
| FIX | St. Jude Children’s Research Hospital | Phase I | NCT00979238 | |
| FIX | UniQure | Phase III | NCT03569891 | |
| FIX | UCL | Phase I | NCT03369444 | |
| FIX | Freeline Therapeutics | Phase II/III | NCT03641703 | |
| MPS-I | ZFN1, ZFN2, IDUA donor | Sangamo | Phase I | NCT02702115 |
| MPS-II | ZFN1, ZFN2, IDS donor | Sangamo | Phase I | NCT03041324 |
| MPS-IIIA | SGSH | LYSOGENE | Phase II/III | NCT03612869 |
| MPS-VI | ARSB | Fondazione Telethon | Phase I/II | NCT03173521 |
| OTC deficiency | OTC | Ultragenyx | Phase I/II | NCT02991144 |
| A1AT deficiency | A1AT | UMMS | Phase I | NCT00377416 |
| CMT1A | NTF3 | Nationwide Children’s Hospital | Phase I/II | NCT03520751 |
| DMD | Microdystrophin | Nationwide Children’s Hospital | Phase I/II | NCT03375164 |
| Mini-dystrophin | Pfizer | Phase I | NCT03362502 | |
| Microdystrophin | Solid Biosciences | Phase I/II | NCT03368742 | |
| Microdystrophin | Sarepta Therapeutics | Phase II | NCT03769116 | |
| LGMD, type 2E | LGMD2E | Sarepta Therapeutics | Phase I/II | NCT03652259 |
| Dysferlinopathy | DYSF | Nationwide Children’s Hospital | Phase I | NCT02710500 |
| HIV infections | PG9 antibody | International AIDS Vaccine Initiative | Phase I | NCT01937455 |
| VRC07 antibody | NIAID | Phase I | NCT03374202 | |
| Pompe disease | GAA | Actus Therapeutics | Phase I/II | NCT03533673 |
| GAA | University of Florida | Phase I | NCT02240407 | |
| X-linked MTM | MTM1 | Audentes | Phase I/II | NCT03199469 |
A1AT α1 antitrypsin, AADC aromatic l-amino acid decarboxylase, AGTC Applied Genetic Technologies Corporation, AMD age-related macular degeneration, ARSB arylsulfatase B, CLN2 neuronal ceroid lipofuscinosis type 2, CMT1A Charcot–Marie–Tooth disease type 1A, CNGB3 cyclic nucleotide-gated channel-β3, DMD Duchenne muscular dystrophy, DYSF dysferlin, FH familial hypercholesterolemia, FVIII factor VIII, G6PC glucose-6-phosphatase catalytic subunit, GAA α-glucosidase, GAN gigaxonin, GDNF glial cell line-derived neurotrophic factor, GSD1a glycogen storage disease type 1a, LCA Leber congenital amaurosis, LDLR low-density lipoprotein receptor, LHON Leber hereditary optic neuropathy, mAb monoclonal antibody, MPS mucopolysaccharidosis, MTM myotubular myopathy, NAGLU N-α-acetylglucosaminidase, ND not disclosed, ND4 NADH-ubiquinone oxidoreductase chain 4, NEI National Eye Institute, NIAID National Institute of Allergy and Infectious Diseases, NINDS National Institute of Neurological Disorders and Stroke, NTF3 neurotrophin 3, OTC ornithine transcarbamylase, REP1 RAB escort protein 1, RLBP1 retinaldehyde-binding protein 1, RP retinitis pigmentosa, RPE65 retinal pigment epithelium-specific 65 kDa protein, RPGR retinitis pigmentosa GTPase regulator, RS1 retinoschisin 1, SGSH N-sulfoglucosamine sulfohydrolase, SMA spinal muscular atrophy, SMN survival of motor neuron, UCL University College London, UCSF University of California San Francisco, UGT1A1 UDP glucuronosyltransferase family 1 member A1, UMMS University of Massachusetts Medical School, VEGF vascular endothelial growth factor, ZFN zinc-finger-containing protein.
Age-related and diabetic macular degeneration (AMD). ADVM-022 is an intravitreal injection of an AAV vector carrying aflibercept, an anti-VEGF fusion protein that blocks blood vessel growth and leakage related to AMD and diabetic retinopathy. What makes this gene therapy stand out is the use of AAV2.7m8, the evolved capsid that is strongly tropic to photoreceptors.185 Current phase I clinical trials aim to assess safety and tolerability of a single intravitreal injection in patients with AMD, correction of visual acuity, and the elimination of anti-VEGF rescue injections (NCT03748784). The most recent and positive outcomes from patients receiving treatments showed mean best corrected visual acuity improvements (+6.8 letters) and central subfield macular thickness reduction (−137.8 μM), indicators of disease reversal.215
Muscular dystrophies. The fight against DMD has a long history and, thanks to awareness efforts throughout the decades, groundbreaking research has resulted in successful treatments of the disease via AAV-based gene therapy.216 DMD is a fatal X-linked neuromuscular genetic disease that occurs in ~1 in every 3500–5000 males worldwide. DMD is caused by mutation(s) in dystrophin, a structural gene that provides tensile strength and integrity to striated muscles, and is one of the largest proteins in the human body. As a result of many years of work, several groups have developed platforms with clinical trials currently underway.
SRP-9001 is an AAVrh.74 capsid-based vector that harbors a shortened form of dystrophin. The discovery of microdystrophin, whose shortened design was inspired by the mutant form of the dystrophin gene observed in Becker muscular dystrophy, is the basis of the vector’s success.217 As AAV is limited in its packaging capacity, the relatively short transgene allows for packaging of the transgene along with the striated muscle-specific regulatory cassette called αMHCK7, a combined α-myosin heavy chain enhancer and version 7 of the creatine kinase promoter.151 The treatment confers systemic and robust therapeutic transgene delivery to skeletal and cardiac muscles. A similar gene therapy strategy to tackle LGMD (SRP-9003) has also implemented. SRP-9003 delivers β-sarcoglycan—a gene when missing, causes LGMD Type 2E. Trials to test these gene therapy platforms are under the respective clinical trial identifiers NCT03769116 and NCT03652259.
Despite the promise of some DMD gene therapy trials, others have been met with challenge. SGT-001 is also an AAV vector packaging microdystrophin. Although clinical outcomes for trials testing SGT-001 (NCT03368742) showed that 50–70% of muscle fibers express the therapeutic transgene and conferred a decline in muscle damage, some patients had adverse effects, where at least one patient had an immunological response to the gene therapy.218 Albeit the adversity was resolved, the FDA’s decision to halt trials are a reflection of its dedication to ensuring that trials are safe for the patients. Similarly, patients who were systemically administered with high doses of PF-06939926 (NCT02310763) experienced transient and/or acute renal impairment, accompanied by activation of the complement system.219
Hemophilia. The gene therapy field for hemophilia, a family of severe blood clotting diseases, has long had its ups and downs. The numerous human trials in the mid-1990s to the early 2000s reflected the diversity of strategies aimed at correcting this blood clotting disease. In fact, AAVs, retrovirus, and Ad vectors were all explored. The first AAV-based hemophilia strategies aimed to target muscle tissues to act as biofactories for expressing factor IX (F.IX) treat hemophilia B220 showed no toxicity and sustained transgene presence beyond multiple years post administration. However, these studies also demonstrated that the therapeutic dose via intramuscular injections could not be achieved by muscle-targeted transgene expression without larger vector doses or clinically impractical injection regimens.221,222 In a milestone trial, delivery of AAV-F.IX to the liver showed successful transduction to human hepatocytes. However, this result was also met by immunological responses to capsid protein and subsequent decline in circulating F.IX levels, showing that immunotoxicity was a critical barrier for therapeutic vectors.223 Nearing the end of 2017, AAV delivery of FVIII to the liver with AAV5224 and a high-activity F.IX variant (F.IX-Padua), which exhibits eight times more activity than the normal protein, also targeting the liver,225 were demonstrated in trials. There are currently at least 12 ongoing clinical trials that are being pursued by 5 pharmaceutical companies. Many of these are in phase II/III stages and thus show promise for commercialization, namely fidanacogene elaparvovec, developed to treat hemophilia B; SPK-8011, a gene therapy for hemophilia A; Valrox (valoctocogene roxaparvovec or BMN 270), a gene therapy to treat hemophilia A; and AMT-061, a gene therapy for hemophilia B, are currently being tested in phase III clinical trials (NCT03392974 and NCT03569891). FLT180a is also in a phase II/III study with hemophilia B patients in the U.K (NCT03641703).
X-Linked myotubular myopathy (XLMTM). Recently, a clinical trial involving high-dose administration of AT132 (NCT03199469), an investigational gene therapy product candidate for XLMTM, led to the deaths of three patients who had progressive liver dysfunction, characterized by hyperbilirubinemia.226–228 The patients were notably of older age, heavier weight, and had prior histories of hepatobiliary disease. Nevertheless, this unfortunate outcome signals how important it is to determine safe, therapeutic doses. It is a continuing challenge for researchers and clinicians to advance their therapeutic strategies to human patients, knowing that there are risks involved. Still, these trials are very much needed.
Challenges
The growing number of clinical trials using rAAVs is a positive sign that these vector-based gene therapies hold a lot of promise; yet, there are still quite a lot of challenges that the field has yet to address. Immunogenicity towards the vector remains the largest challenge for AAV-based gene therapies. In fact, the immune system will always be a major barrier for any gene therapy approach. Thus far, adaptive immunity to the capsid and the foreign transgene represents major factors for decreased efficacies. Although the single administration aspect and sustainability of AAV gene therapy mean that the need for redosing can be avoided, patients that have been exposed to AAV serotypes that a gene therapy is based on, will have a high-chance of presenting NAbs against the vector capsid.171 The goal of discovering new capsids seeks to partially address the presence of NAbs against serotypes commonly found in populations. For circumstances where tailoring the vector platform to the patient is not possible, plasmapheresis is a plausible means of removing anti-AAV antibodies from the bloodstream229 or pre-treatment with IgG-cleaving endopeptidases such as imlifidase (IdeS) can nonspecifically reduce IgG antibodies from the sera of patients.230 IdeZ, a homolog of IdeS, isolated from Streptococcus zooepidemicus, demonstrated efficacious removal of capsid NAbs in non-human primate (NHP) studies.231 Adaptive immunity towards the transgene may be more straightforward to address. As mentioned above, detargeting from professional APCs, such as dendritic cells with tissue-specific promoters and the design of miRNA binding sites for miR142 to silence transgene expression, can circumvent adaptive immunity.157–159
Mechanisms for innate immunity have been well-described in response to viruses, but exploration of innate immune response towards AAV vectors is understudied. Serotype-specific responses have been observed and can be directly addressed. Namely, differences in receptor binding between AAV2 and AAV8, whereby AAV2’s preference for heparin proteoglycans may confer higher T cell response, may lead to strategies to mitigate immunity via rational design.232 In addition, evidence is accumulating for the possibility that the AAV vector genome can elicit an innate immune response,233 necessitating an area of research that is critically needed. TLR9 detection of methylation-free CpG dinucleotides is one means by which cells can identify foreign DNA. Other foreign DNA- and RNA-sensing mechanisms, involving RIG-I (Ddx58), CGAS (Mb21d1), and STING (Tmem173), may also play roles.234
Finally, a challenge that must be confronted is managing the right treatment doses, which may be at the heart of the strong immunological responses and subsequent toxicities seen in recent trials. Although AAV-based gene therapy for SMA has been proven to be safe, interpatient differences may impact the response. In a preclinical study in piglets and non-human primates, where supraphysiological transgene expression was achieved, proprioceptive deficits and ataxia was observed, and was attributable to high transduction of the dorsal root ganglia (DRG) and subsequent toxicity.235 In fact, a recent meta-analysis of 33 non-clinical studies encompassing 256 NHPs showed that the majority of animals administered via cerebrospinal fluid (CSF) exhibited DRG pathologies.236 Using miRNA-detargeting can reduce toxicity.237 These studies and others indicate that further evaluation of the appropriate routes of administration, capsid choice, and vector genome designs are still needed, even for approved drugs.
The future for AAV vectors
Despite some setbacks, AAV vectors still hold huge promise for correcting disease. Now with the preclinical successes seen with CRISPR/Cas systems, which allow programmed editing nearly anywhere within the genome, the potential for AAVs seems limitless.238 However, every approach has their downsides. AAV vectors have always been considered relatively safe, yet recent demonstration that AAV vector genomes carrying CRISPR components can integrate into the host cell genome at the site of double-strand breaks239 has raised concerns over their efficacies and long-term safety profiles. In fact, in a 10-year follow-up study in six dogs receiving gene therapy vector for F.VIII, vector genomes were found to be stably integrated into the host genome and has re-raised concerns for oncogenic integration of AAV.240 Despite the overall positive safety track record for AAV treatments in humans, continued efforts in gauging the long-term and short-term safety for AAV vectors are certainly warranted.
Lentivirus vectors: a robust vector for genetically modified cell therapies
Structure and genome
Lentiviruses constitute a genus of the retroviridae family. Retroviruses are spherical, enveloped, single-stranded RNA viruses that are ~100 nm in diameter.241,242 The lentiviral particle encapsidates two sense-strand RNAs that are bound by nucleocapsid proteins. The particle also contains reverse transcriptase, integrase, and protease proteins. Retroviruses can be classified into simple or complex viruses, based on their genome organization. Gammaretroviruses are an example of simple retroviruses, whereas the HIV-1, a lentivirus, is an example of a complex retrovirus. The 9.7 kb HIV-1 genome is flanked by 5′- and 3′ LTRs, which are integral to viral genome replication. The LTR is composed of U5 and U3 sequences that are unique to the 5′ and the 3′ termini of the viral genome, respectively, and R, which is a common sequence at both ends. The cis-acting sequence, also known as psi, resides just outside of the LTR and is pivotal for signaling viral genome encapsidation.243,244 Retroviruses have common essential core protein genes, such as gag, pol, and env. Although the gag gene encodes for the protective capsid and matrix proteins, the env gene carries information for transmembrane and envelope glycoproteins that dictate the virus’ cellular tropism.245 Reverse transcriptase and integrase are transcribed from the pol gene. As part of an intricate regulatory machinery that facilitates viral replication, lentiviruses have additional genes called tat and rev. Tat supports transcriptional activation and RNA polymerase II elongation by binding adjacently to the LTR.246 Rev orchestrates nuclear export of spliced and un-spliced viral RNA by binding to a motif in the env gene region.247 Lentiviruses, such as HIV-1, have an additional set of auxiliary genes (vif, vpr, vpu, and nef), which elevate the viral titer and pathogenesis of the virus.248,249
Infection pathway
The entry of infectious lentiviral particles to the host cell is mediated by interactions between glycoproteins anchored on the outer envelope and a specific cell receptor. Successful binding to cell surface receptors prompts a series of events that lead to the fusion of the viral particle lipid bilayer and the cell, and subsequent unloading of the viral genetic cargo into the cytoplasm.250–252 Lentiviral DNA integrates into the host genome in a non-random manner with preference for transcriptionally active sites.253–255
Lentiviruses as vectors in gene therapy
Lentiviral vectors have several features that make them amenable to transgene delivery for therapeutic purposes. Lentiviral vectors are integrating vectors that permit long-term transgene expression. They have a packaging capacity of up to 9 kb.256 High-level expression of multiple genes may be a requisite for achieving a therapeutic outcomes for certain diseases. Employing two separate vectors carrying co-dependent transgenes may not be an optimal solution, as successful transduction of multiple viral vectors to the same cell is not efficient. Lentiviral vectors are demonstrated to have the ability to express multiple genes from a single vector.257–259 Lentiviral vectors can transduce postmitotic and quiescent cells, whereas other retrovirus-based platforms, such as gammaretroviral vectors, require active cell division for successful infection. Although quiescent cells are mostly recalcitrant to infection, partly as a result of the innate immune response,260 stimulation of mitotic entry can facilitate viral transduction.261–264 Depending on the viral vector design, lentiviral vectors elicit relatively weak immune responses.265,266
Lentiviral vector systems that are derived from the HIV-1 virus have evolved through the years. These advancements have been made in part to mitigate the potential risks associated with the virus that the platform is based on. One obvious safety consideration in designing a lentiviral vector system for gene therapy is the unintended generation of replication-competent provirus. The first generation of HIV-1-based vectors retained most of the viral genome within the trans packaging construct, including the viral core, regulatory protein coding sequences, and accessory regulatory genes.256 In the three-plasmid design to generate vector, the env gene is replaced by the glycoprotein of vesicular stomatitis virus (VSV-G) and is separately provided in trans by a second plasmid. In this way, pseudotyping the viral particle with VSV-G facilitates viral entry independent of its native host cell receptor. The therapeutic transgene is provided in a third plasmid construct along with the cis-acting elements that confer viral encapsidation. The second generation of safer lentiviral vectors are devoid of vif, vpr, vpu, and nef auxiliary genes, which promote viral proliferation and infection.267 To limit the formation of unintended replication-competent provirus, third-generation vectors that lack the tat and rev regulatory genes in the packaging construct were developed (Fig. 5).268 The rev gene, which is required for replication, is supplied in trans, creating a conditional packaging platform. The safety profile of the third-generation lentiviral system is further improved by deleting part of the 3′-LTR, which contains the TATA box and transcription factor-binding sites; thus, creating a vector packaging system that is self-inactivating.269
Fig. 5. Third-generation HIV-1-based lentiviral vector design.
The third generation of lentiviral vectors are produced using four plasmids. The first plasmid has a construct carrying the gene of interest driven by a desired promoter flanked by long terminal repeats (LTRs). Both 5′ and 3′ LTRs in wild-type HIV-1 is composed of U3, R, and U5 sequences. The U3 sequence constitutes promoter/enhancer elements. Part of the U3 sequence in the 3′-LTR is deleted, and the entire U3 sequence within the 5′-LTR is replaced by a strong viral promoter, such as CMV. The psi (ψ) packaging signal is followed by the rev response element (RRE). The envelope glycoprotein is encoded by VSV-G (vesicular stomatitis virus) and is expressed under a strong promoter, such as CMV. The rev gene is split from the packaging plasmid and is provided on a separate plasmid construct. The packaging plasmid harbors the viral gag and pol genes, and notably lacks the tat regulatory gene
Additional modifications have been made to improve the expression and transduction efficiency of lentiviral vectors. Incorporating transcriptional regulatory elements, such as a central polypurine tract (cppt) and a matrix attachment region (MAR) in the cis expression vector augments viral transduction.270 Inclusion of scaffold attachment regions from the β-interferon gene into the vector design enhances the expression of vector transgenes in quiescent T cells.271 In addition, incorporating woodchuck hepatitis virus post-transcriptional regulatory element (WPRE) as a posttranscriptional regulatory element in the 3′-untranslated region of the ORF significantly enhances transgene expression.272
Similar to other viral vectors, achieving tissue or cell-type specificity is a key component for designing efficient and safe lentiviral vectors. Numerous tissues or cell types have been specifically targeted by employing tissue-specific promoters to drive transgene expression.273,274 For instance, temporal regulation of lentiviral vector expression can be achieved by using tetracycline-inducible promoters.275 In addition, cell-specific targeting can be improved by utilizing endogenous miRNAs to posttranscriptionally regulate transgene expression, thereby reducing immune response mounted against the transgene.156 Pseudotyping has paramount importance to the success of transducing the desired cell or tissue type. For example, lentivirus pseudotyped with glycoproteins of simian immunodeficiency virus and Ebola virus have improved transduction to the retina and hematopoietic stem cells (HSCs), respectively.276,277
Production platforms
Large-scale good manufacturing practice (GMP) of lentiviral vectors for clinical application involves intricate production, purification, and quality assessment steps. The process of lentiviral vector production can be roughly divided into upstream production and downstream purification procedures. For clinical applications, viral production of third-generation vectors can be achieved by quadruple plasmid transfection with the transfer vector, along with the three separate plasmids carrying packaging and helper genes.278–280 HEK293 or HEK293T producer cell lines are routinely used. Although the HEK293T cell line is preferred, as it confers higher viral titers,278,281 the HEK293T line carries the oncogenic simian vacuolating virus 40 large T antigen, which poses safety concerns. Although the four-plasmid transfection method is highly modular, batch-to-batch variability in viral titers remains a challenge.
Alternatively, lentivirus vectors can be manufactured by using a stable producer cell line that harbors functional lentiviral helper and packaging genes. In such stable producer cell lines, tetracycline-inducible systems are customarily used to control VSV-G and gag-pol expression, which would otherwise be toxic to cells.282,283 Pseudotyping with VSV-G also enhances the stability of the viral particle during vector manufacturing.284,285 Cumate- and tetracycline-inducible systems have been concomitantly used to limit leaky viral protein expression.286,287 In addition to inducible stable cell lines, constitutive packaging cell lines have been developed for a number of envelope proteins that are less toxic than VSV-G, such as cocal288 and RD114-TR.289 The use of producer cell lines has an edge over transient transfection methods in terms of cost, reproducibility, and scalability. However, it can be cumbersome to produce a stable cell line for each pseudotyping envelope protein and helper gene combination.
Suspension cell culture production is becoming a widely adopted method for scalable large-scale lentiviral manufacturing because of its superiority over adherent cell culture production schemes. Different cell clones amenable for suspension culture system have been developed.287,290 Serum-free media is commonly used for GMP-manufacturing of large-scale vector intended for clinical use, as this reduces biological contaminants inherent to sera.291,292 In downstream processes, initial centrifugation and filtration steps help to remove cellular debris. These procedures are followed by purification of viral particles with various chromatographic techniques. Buffer exchange and viral particle concentration can be carried out by ultracentrifugation and tangential flow filtration.293–295 The purification process needs to be optimized for each pseudotyping strategy. Large-scale production of lentiviral vector for clinical application needs to be in compliance with current GMPs (cGMPs), which include the use of cGMP-certified reagents and manipulation under aseptic conditions.
Therapeutic lentiviral vectors and commercial availability
The HIV virus has killed about 32 million people to date (www.who.int/gho/hiv/en/). In transforming this lethal pathogenic virus into a delivery vehicle for gene therapy, we have come a long way towards understanding lentiviruses. For instance, in recent years, ex vivo applications for lentiviruses in the form of generating chimeric antigen receptor (CAR) T cells for cancer immunotherapy have revolutionized how cancer is treated.
Engineered CAR-T cells have been developed to specifically target cancer-related antigens. The use of lentiviral vectors is critical for developing CAR-T cells to treat refractory hematologic malignancies. This approach has been demonstrated in a safety assessment clinical trial that involved 30 children and adult patients with relapsed and refractory acute lymphoblastic leukemia (ALL).296 In these patients, autologous T cells were transduced with lentiviral vectors carrying a transgene to express a CAR that binds CD19. The study showed that a remission rate as high as 90% could be achieved in patients who underwent the CAR-T cell infusion treatment.296 Going by the commercial name Kymriah, the treatment later became the first drug to be approved by the FDA to treat pediatric B-cell ALL. The second CAR-T cell-based drug approved by the FDA for the treatment of refractory large B cell lymphoma, named Yescarta, uses gammaretrovirus to stably deliver the CAR gene construct to cells.297
Lentiviral vectors in clinical trials
Lentiviral vectors have been tested in many successful clinical trials. The first endeavor employed a lentiviral vector to treat HIV-positive patients via ex vivo transduction of patients’ CD4 cells by antisense sequence against the wild-type HIV viral envelope gene.298 The study demonstrated that the vector lowered viral load and did not result in any major adverse effects. To date, there are more than a dozen completed clinical trials that have used lentiviral vectors for treating a range of diseases, including metabolic disorders, cancers, immune disorders, and rare congenital diseases (Table 3). Moreover, due to its safety profile compared to other retroviruses, the number of ongoing trials that use lentiviral vector is dramatically increasing.
Table 3.
A selection of ongoing and completed clinical trials employing lentiviral vector
| Condition | Intervention | Sponsor/collaborators | Trial stage | NCT number |
|---|---|---|---|---|
| Acute lymphoblastic leukemia | CART-19 T cells | Union Stem Cell & Gene Engineering Co. Ltd, Juventas Cell Therapy Ltd | Phase I | NCT02975687 |
| University of Pennsylvania | NCT01029366 | |||
| University of Pennsylvania | Phase II | NCT02030847 | ||
| ADA-SCID | Autologous EFS-ADA LV CD34+ cells | Orchard Therapeutics, NIAID, NHGRI, NHLBI, UCLA | Phase I/II | NCT01852071 |
| Orchard Therapeutics, UCLA | Phase III | NCT04140539 | ||
| NHGRI, UCLA, Duke University, NCI, CC | Phase I | NCT02022696 | ||
| Orchard Therapeutics, CIRM, UCLA | Phase I/II | NCT02999984 | ||
| NHS Foundation Trust, Orchard Therapeutics | Phase I/II | NCT01380990 | ||
| Age-related macular degeneration | Endostatin and Angiostatin LV | Oxford BioMedica | Phase I | NCT01301443 |
| Non-Hodgkin lymphoma | CART-19 T cells | Peking Union Medical College Hospital, Cellular Biomedicine Group Ltd | Phase I | NCT03483688 |
| Cerebral adrenoleukodystrophy (CALD) | Autologous ABCD1 LV CD34+ cells | Bluebird Bio | Phase II/III | NCT01896102 |
| β-Thalassemia | Autologous βA-T87Q-globin LV CD34+ cells | Bluebird Bio | Phase I/II | NCT01745120 |
| Bluebird Bio | Phase III | |||
| β-Thalassemia major, sickle cell disease | Autologous βA-T87Q-globin LV CD34+ cell | Bluebird Bio | Phase I/II | NCT02151526 |
| Sickle cell disease | Autologous βA-T87Q-globin LV CD34+ cells | Bluebird Bio | Phase III | NCT04293185 |
| Fanconi anemia | Autologous FANCA LV CD34+ cells | Hospital Universitari Vall d’Hebron Research Institute, CIEMAT, CIBERER | Phase II | NCT02931071 |
| HIV infection | Autologous C34-CXCR4 LV CD4 T cells | University of Pennsylvania | Early Phase I | NCT03020524 |
| Wt gag-TCR and α/6-gag-TCR-modified T cells | University of Pennsylvania, Adaptimmune | Phase I | NCT00991224 | |
| HIV antigen LV | Theravectys S.A. | Phase I/II | NCT02054286 | |
| Lymphoma | rHIV7-shI-TAR-CCR5RZ LV HPCs | City of Hope Medical Center, NCI | Phase I | NCT00569985 |
| Multiple myeloma | CART-19 T cells | University of Pennsylvania | Phase I | NCT02135406 |
| Refractory diffuse large B-cell lymphoma | CART-19 T cells | Cellular Biomedicine Group Ltd, Nanjing Medical University | Phase I | NCT02976857 |
| Covid-19 infection | SARS-CoV-2 antigen in aAPC | Shenzhen Geno-Immune Medical Institute | Phase I | NCT04299724 |
| Wiskott–Aldrich syndrome | Autologous WAS LV CD34+ cells | Genethon, Hôpital Necker-Enfants Malades | Phase I/II | NCT01347346 |
| Genethon, NHS Foundation Trust, Institute of Child Health | Phase I/II | NCT01347242 |
ADA-SCID adenosine deaminase severe combined immunodeficiency, CART-19 T chimeric antigen receptor 19 T cells, CC National Institutes of Health Clinical Center, EFS-ADA elongation factor 1α promoter driving adenosine deaminase, FANCA Fanconi anemia complementation group gene, HIV human immunodeficiency viruses, HPCs hematopoietic progenitor cells, LV lentiviral vector, NCI National Cancer Institute, NHGRI National Human Genome Research Institute, NHLBI National Heart, Lung, and Blood Institute, NIAID National Institute of Allergy and Infectious Diseases, SARS-CoV-2 severe acute respiratory syndrome coronavirus, TCR T-cell receptor, UCLA University of California Los Angeles, WAS Wiskott–Aldrich syndrome gene, wt wild type.
In particular, the use of lentiviruses in ex vivo gene therapy strategies for treating genetic diseases have enjoyed substantial advancements. For example, 18 β-thalassemia patients carrying mutations in the HBB globin gene received infusion of autologous CD34+ cells transduced with lentiviral vectors encoding the human βA-T87Q-globin gene in phase I/II studies.299 The treatment proved to be successful in replacing long-term allogeneic hematopoietic cell transplantations. In patient follow-ups, there were no significant toxicity effects related to the vector after three years post infusion. This effort has led to the development of LentiGlobinBB305, which has now advanced to a multinational phase III clinical trial for the treatment of β-thalassemia (NCT03207009 and NCT02906202) and sickle cell disease (NCT04293185). Similarly, a lentiviral based gene therapy approach has shown promising results for the treatment of cerebral adrenoleukodystrophy, which typically has a very poor prognosis without allogeneic hematopoietic stem cell transplantation (HSCT). Nearly 90% of cerebral adrenoleukodystrophy patients who received hematopoietic stem cells transduced with lentiviral vectors carrying the ABCD1 transgene have overcome major functional impairment.300 No genotoxicity or adverse effects related to the treatment have been reported for any of the test subjects.
Ex vivo gene therapy has also been a viable alternative to allogeneic HSCT treatment of inherited primary immune deficiency disorders. To this end, lentiviral gene therapy has been demonstrated to be safe in patients with ADA-SCID that is caused by a mutation in the ADA gene. In a non-randomized clinical trial conducted at two different sites in the USA, 30 pediatric ADA-SCID patients were infused with autologous CD34+ hematopoietic stem/progenitor cells transduced with lentiviral vectors containing the ADA transgene.301 With the exception of one patient exhibiting non-engraftment, all other patients were taken off of enzyme replacement therapy and showed event-free survival beyond two years post treatment. Following this success, the lentiviral vector drug named OTL-101, entered a phase III clinical trial for the treatment of ADA-SCID (NCT04140539). In another primary immune deficiency disorder, eight pediatric patients with Wiskott–Aldrich syndrome were infused with autologous CD34+ transduced with a lentiviral vector carrying the WAS transgene. Interim analysis at 3.6 years post treatment showed an overall 100% survival rate with improved immunity and removal from immunoglobulin supplementation for seven of the eight treated patients.302
In comparison to gammaretroviral vectors, lentiviral vectors are preferentially used in many advanced (phase III) clinical trials for CAR-T-cell therapies. Ongoing phase III clinical trials for CAR-T cells targeting refractory B-cell lymphoma (NCT03391726), ALL (NCT03027739 and NCT03937544), and refractory B-cell acute myeloid leukemia (NCT03631576) were developed by lentiviral vectors.
Engineering of T-cell receptors (TCRs) to specifically recognize cancer-specific proteins is another cancer immunotherapy approach being explored. In a preclinical study, lentiviral vectors were successfully used to transduce hematopoietic stem cells with NY-ESO-1 TCR, with no measurable genotoxicity.303 A recently reported clinical trial indicated that a TCR targeting the NY-ESO-1 antigen in multiple myeloma patients had good efficacy and minimal toxicity (NCT01352286).304
Lentiviral vectors are also being tested to tackle viral infection including the development of novel vaccines against COVID-19. In two recently launched clinicals, lentiviral vectors are being used to express synthetic viral minigenes and immune modulatory genes to engineer artificial APCs to activate the immune system against COVID-19 (NCT04299724).
Challenges
An early clinical trial that used a gammaretroviral vector to treat X-linked SCID led to serious adverse effects.305 Four out of the nine patients who received the treatment went on to develop T-cell leukemias, as a result of insertional mutagenesis. Thus, lentiviral vectors have become the preferred vehicle for transgene delivery, owing to their reduced genotoxicity profile when compared to gammaretroviral vectors.306–308 Although both vectors preferentially integrate into transcriptionally active regions, one possible factor contributing to differences in genotoxicity is that gammaretroviral vectors often integrate within the vicinity of transcriptional start sites, and has affinities toward oncogenes.307,309–311 The use of third-generation, self-inactivating lentiviral vectors have helped to mitigate the risk of insertional mutagenesis.269 Nonetheless, it has been reported that even self-inactivating lentiviral vectors with strong promoter and enhancer elements can activate neighboring genes. Incorporation of insulator sequences to neutralize the effect of enhancers acting in trans can reduce these effects.312–315 In addition, integration can potentially form chimeric gene fusions made up of proviral and host sequences.316–318 Finally, lentiviral vectors have been shown to cause aberrant splicing of cellular transcripts.318,319
The future for lentivirus vectors
The number of viral vectors being developed as delivery platforms for genetic vaccines is increasing. Due to their ability to transduce non-dividing cells, such as dendritic cells, recombinant lentiviral vectors have been shown to elicit B cell- and T cell-mediated immunity against infectious diseases and different tumors.320 Recent advances in the development of non-integrating lentiviral vectors have greatly reduced insertional mutagenesis.321 Another advantage of non-integrating lentiviral vectors is their transient expression in actively dividing cells, where sustained transgene expression is not necessary. Application of non-integrating lentiviral vectors to circumvent immune responses mounted against prolonged expression of genome editing tools, such as CRISPR/Cas, facilitates the use of such systems in therapeutic gene editing.322 In addition to non-integrating lentiviruses, self-restricted CRISPR systems with shortened duration of cas9 expression, could reduce off-target effects and enhance CRISPR-based therapeutics.323
Integrase-defective lentiviral vectors have been designed as a vaccine platform to deliver vaccination against influenza and malaria antigens.324,325 Such lentiviral vectors are being continuously developed and optimized, and could enjoy a broader application in future gene therapies. In addition, the development of safer vectors via photo-switchable non-canonical amino acids to regulate transgene expression in a spatial and temporal manner,326 also represent the next generation of lentiviral vectors.
Lentiviral vector has become one of the preferred tools for ex vivo transgene delivery for gene therapy, because it has many attractive features. These vectors have proven to be invaluable for ex vivo gene correction and gene transfer. Lentiviral vector systems have evolved a great deal and are increasingly being improved. This effort will result in wider adoption of lentiviral vectors to treat human diseases.
Closing remarks
In this review, we have discussed the current state of viral vectors, specifically those based on Ad, AAV, and lentivirus. It is abundantly clear that the future for viral-based vectors is bright and the ability to address many human genetic diseases is within reach. Unfortunately, the current tools at our disposal are still wrought with complications, and for many diseases, a long path awaits until viable treatments are available. Further exploration into viral biology, as well as advanced and interdisciplinary approaches are needed to overcome the current challenges that still curb the promise for the numerous vector platforms currently in preclinical and clinical testing phases. What the past 30 years has taught us is that every great idea has unexpected pitfalls. Nevertheless, every obstacle that is faced and overcome gifts us with a clearer sense of where our goals towards improving human health are set.
Acknowledgements
We thank Dan Wang, Jun Xie, and the Gao Lab for the insightful discussions, which served as the inspiration for the content of this review. G.G. is supported by grants from the University of Massachusetts Medical School (an internal grant) and by the National Institutes of Health (R01NS076991-01, 1P01AI100263-01, 4P01HL131471-02, UG3 HL147367-01, 1U19AI149646, and R01HL097088).
Competing interests
G.G. is a scientific co-founder of Voyager Therapeutics, Adrenas Therapeutics, and Aspa Therapeutics, and holds equity in these companies. G.G. is an inventor on patents with potential royalties licensed to Voyager Therapeutics, Aspa Therapeutics, and other biopharmaceutical companies. The remaining authors declare no competing interests.
Footnotes
These authors contributed equally: Jote T. Bulcha, Yi Wang
Contributor Information
Phillip W. L. Tai, Email: phillip.tai2@umassmed.edu
Guangping Gao, Email: Guangping.Gao@umassmed.edu.
References
- 1.Terheggen HG, et al. Unsuccessful trial of gene replacement in arginase deficiency. Z. Kinderheilkd. 1975;119:1–3. doi: 10.1007/BF00443566. [DOI] [PubMed] [Google Scholar]
- 2.Cline MJ. Perspectives for gene therapy: inserting new genetic information into mammalian cells by physical techniques and viral vectors. Pharm. Ther. 1985;29:69–92. doi: 10.1016/0163-7258(85)90017-8. [DOI] [PubMed] [Google Scholar]
- 3.Anderson WF. Human gene therapy. Science. 1992;256:808–813. doi: 10.1126/science.1589762. [DOI] [PubMed] [Google Scholar]
- 4.Kohn DB, et al. Establishment and characterization of adenosine deaminase-deficient human T cell lines. J. Immunol. 1989;142:3971–3977. [PubMed] [Google Scholar]
- 5.Arrand JR, Roberts RJ. The nucleotide sequences at the termini of adenovirus-2 DNA. J. Mol. Biol. 1979;128:577–594. doi: 10.1016/0022-2836(79)90294-8. [DOI] [PubMed] [Google Scholar]
- 6.Shinagawa M, Padmanabhan RV, Padmanabhan R. The nucleotide sequence of the right-hand terminal SmaI-K fragment of adenovirus type 2 DNA. Gene. 1980;9:99–114. doi: 10.1016/0378-1119(80)90169-9. [DOI] [PubMed] [Google Scholar]
- 7.Gingeras TR, et al. Nucleotide sequences from the adenovirus-2 genome. J. Biol. Chem. 1982;257:13475–13491. doi: 10.1016/S0021-9258(18)33473-2. [DOI] [PubMed] [Google Scholar]
- 8.Yang ZR, et al. Recent developments in the use of adenoviruses and immunotoxins in cancer gene therapy. Cancer Gene Ther. 2007;14:599–615. doi: 10.1038/sj.cgt.7701054. [DOI] [PubMed] [Google Scholar]
- 9.Tooze, J., Acheson, N. H., Broker, T. R. & Flint, S. J. DNA Tumor Viruses (Cold Spring Harbor Laboratory, 1981).
- 10.Fields, B. N., Knipe, D. M. & Howley, P. M. Fields’ Virology (Wolters Kluwer, 2007).
- 11.Russell WC. Adenoviruses: update on structure and function. J. Gen. Virol. 2009;90:1–20. doi: 10.1099/vir.0.003087-0. [DOI] [PubMed] [Google Scholar]
- 12.Saban SD, Silvestry M, Nemerow GR, Stewart PL. Visualization of alpha-helices in a 6-angstrom resolution cryoelectron microscopy structure of adenovirus allows refinement of capsid protein assignments. J. Virol. 2006;80:12049–12059. doi: 10.1128/JVI.01652-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.San Martin C, et al. Localization of the N-terminus of minor coat protein IIIa in the adenovirus capsid. J. Mol. Biol. 2008;383:923–934. doi: 10.1016/j.jmb.2008.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wodrich H, et al. A capsid-encoded PPxY-motif facilitates adenovirus entry. PLoS Pathog. 2010;6:e1000808. doi: 10.1371/journal.ppat.1000808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fabry CM, et al. A quasi-atomic model of human adenovirus type 5 capsid. EMBO J. 2005;24:1645–1654. doi: 10.1038/sj.emboj.7600653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu, H., Naismith, J. H. & Hay, R. T. In Adenoviruses: Model and Vectors in Virus-Host Interactions: Virion-Structure, Viral Replication and Host-Cell Interactions (eds. Doerfler, W. & Böhm, P.) 131–164 (Springer, 2003).
- 17.Ahi YS, Mittal SK. Components of adenovirus genome packaging. Front Microbiol. 2016;7:1503. doi: 10.3389/fmicb.2016.01503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomko RP, Xu R, Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc. Natl Acad. Sci. Usa. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bergelson JM, et al. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 20.Gaggar A, Shayakhmetov DM, Lieber A. CD46 is a cellular receptor for group B adenoviruses. Nat. Med. 2003;9:1408–1412. doi: 10.1038/nm952. [DOI] [PubMed] [Google Scholar]
- 21.Wang H, et al. Desmoglein 2 is a receptor for adenovirus serotypes 3, 7, 11 and 14. Nat. Med. 2011;17:96–104. doi: 10.1038/nm.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nilsson EC, et al. The GD1a glycan is a cellular receptor for adenoviruses causing epidemic keratoconjunctivitis. Nat. Med. 2011;17:105–109. doi: 10.1038/nm.2267. [DOI] [PubMed] [Google Scholar]
- 23.Gaden F, et al. Gene transduction and cell entry pathway of fiber-modified adenovirus type 5 vectors carrying novel endocytic peptide ligands selected on human tracheal glandular cells. J. Virol. 2004;78:7227–7247. doi: 10.1128/JVI.78.13.7227-7247.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiethoff CM, Wodrich H, Gerace L, Nemerow GR. Adenovirus protein VI mediates membrane disruption following capsid disassembly. J. Virol. 2005;79:1992–2000. doi: 10.1128/JVI.79.4.1992-2000.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiethoff CM, Nemerow GR. Adenovirus membrane penetration: tickling the tail of a sleeping dragon. Virology. 2015;479-480:591–599. doi: 10.1016/j.virol.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bremner KH, et al. Adenovirus transport via direct interaction of cytoplasmic dynein with the viral capsid hexon subunit. Cell Host Microbe. 2009;6:523–535. doi: 10.1016/j.chom.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhong L, Granelli-Piperno A, Choi Y, Steinman RM. Recombinant adenovirus is an efficient and non-perturbing genetic vector for human dendritic cells. Eur. J. Immunol. 1999;29:964–972. doi: 10.1002/(SICI)1521-4141(199903)29:03<964::AID-IMMU964>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 28.Mast TC, et al. International epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type-36 neutralizing antibodies: correlates of high Ad5 titers and implications for potential HIV vaccine trials. Vaccine. 2010;28:950–957. doi: 10.1016/j.vaccine.2009.10.145. [DOI] [PubMed] [Google Scholar]
- 29.Barouch DH, et al. International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine. 2011;29:5203–5209. doi: 10.1016/j.vaccine.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munoz FM, Piedra PA, Demmler GJ. Disseminated adenovirus disease in immunocompromised and immunocompetent children. Clin. Infect. Dis. 1998;27:1194–1200. doi: 10.1086/514978. [DOI] [PubMed] [Google Scholar]
- 31.Wold, W. S. M. & Ison MG. In Fields Virology (eds. Knipe, D. M. & Howley, P. M.) 6th edn, 1732–1767 (Lippincott, 2013).
- 32.Ledgerwood JE, et al. A replication defective recombinant Ad5 vaccine expressing Ebola virus GP is safe and immunogenic in healthy adults. Vaccine. 2010;29:304–313. doi: 10.1016/j.vaccine.2010.10.037. [DOI] [PubMed] [Google Scholar]
- 33.Buchbinder SP, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee CS, et al. Adenovirus-mediated gene delivery: potential applications for gene and cell-based therapies in the new era of personalized medicine. Genes Dis. 2017;4:43–63. doi: 10.1016/j.gendis.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh, S., Kumar, R. & Agrawal, B. In: Adenoviral Vector-based Vaccines and Gene Therapies: Current Status and Future Prospects. Adenoviruses, (Desheva, Y. A. ed.) Vol. 1, 1−39 (Intech Open Publishers: London, United Kingdom, 2018).
- 36.McGrory WJ, Bautista DS, Graham FL. A simple technique for the rescue of early region I mutations into infectious human adenovirus type 5. Virology. 1988;163:614–617. doi: 10.1016/0042-6822(88)90302-9. [DOI] [PubMed] [Google Scholar]
- 37.Akusjärvi G. Proteins with transcription regulatory properties encoded by human adenoviruses. Trends Microbiol. 1993;1:163–170. doi: 10.1016/0966-842X(93)90085-6. [DOI] [PubMed] [Google Scholar]
- 38.Yang Y, et al. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc. Natl Acad. Sci. USA. 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gorziglia MI, et al. Elimination of both E1 and E2 from adenovirus vectors further improves prospects for in vivo human gene therapy. J. Virol. 1996;70:4173–4178. doi: 10.1128/JVI.70.6.4173-4178.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Engelhardt JF, Ye X, Doranz B, Wilson JM. Ablation of E2A in recombinant adenoviruses improves transgene persistence and decreases inflammatory response in mouse liver. Proc. Natl Acad. Sci. USA. 1994;91:6196–6200. doi: 10.1073/pnas.91.13.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amalfitano A, et al. Production and characterization of improved adenovirus vectors with the E1, E2b, and E3 genes deleted. J. Virol. 1998;72:926. doi: 10.1128/JVI.72.2.926-933.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Osada T, et al. Optimization of vaccine responses with an E1, E2b and E3-deleted Ad5 vector circumvents pre-existing anti-vector immunity. Cancer Gene Ther. 2009;16:673–682. doi: 10.1038/cgt.2009.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao GP, Yang Y, Wilson JM. Biology of adenovirus vectors with E1 and E4 deletions for liver-directed gene therapy. J. Virol. 1996;70:8934. doi: 10.1128/JVI.70.12.8934-8943.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lusky M, et al. In vitro and in vivo biology of recombinant adenovirus vectors with E1, E1/E2A, or E1/E4 deleted. J. Virol. 1998;72:2022–2032. doi: 10.1128/JVI.72.3.2022-2032.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Q, Finer MH. Second-generation adenovirus vectors. Nat. Med. 1996;2:714–716. doi: 10.1038/nm0696-714. [DOI] [PubMed] [Google Scholar]
- 46.Alba R, Bosch A, Chillon M. Gutless adenovirus: last-generation adenovirus for gene therapy. Gene Ther. 2005;12:S18–S27. doi: 10.1038/sj.gt.3302612. [DOI] [PubMed] [Google Scholar]
- 47.Hartigan-O’Connor D, Amalfitano A, Chamberlain JS. Improved production of gutted adenovirus in cells expressing adenovirus preterminal protein and DNA polymerase. J. Virol. 1999;73:7835. doi: 10.1128/JVI.73.9.7835-7841.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bischoff JR, et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 49.Fueyo J, et al. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene. 2000;19:2–12. doi: 10.1038/sj.onc.1203251. [DOI] [PubMed] [Google Scholar]
- 50.Heise C, et al. An adenovirus E1A mutant that demonstrates potent and selective systemic anti-tumoral efficacy. Nat. Med. 2000;6:1134–1139. doi: 10.1038/80474. [DOI] [PubMed] [Google Scholar]
- 51.Lin Y, et al. Identification and characterization of alphavirus M1 as a selective oncolytic virus targeting ZAP-defective human cancers. Proc. Natl Acad. Sci. USA. 2014;111:E4504–E4512. doi: 10.1073/pnas.1408759111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roberts MS, Lorence RM, Groene WS, Bamat MK. Naturally oncolytic viruses. Curr. Opin. Mol. Ther. 2006;8:314–321. [PubMed] [Google Scholar]
- 53.Rosenfeld M, et al. Adenovirus-mediated transfer of a recombinant alpha 1-antitrypsin gene to the lung epithelium in vivo. Science. 1991;252:431–434. doi: 10.1126/science.2017680. [DOI] [PubMed] [Google Scholar]
- 54.Jaffe HA, et al. Adenovirus-mediated in vivo gene transfer and expression in normal rat liver. Nat. Genet. 1992;1:372–378. doi: 10.1038/ng0892-372. [DOI] [PubMed] [Google Scholar]
- 55.Zabner J, et al. Adenovirus-mediated gene transfer transiently corrects the chloride transport defect in nasal epithelia of patients with cystic fibrosis. Cell. 1993;75:207–216. doi: 10.1016/0092-8674(93)80063-K. [DOI] [PubMed] [Google Scholar]
- 56.Rosengart Todd K, et al. Angiogenesis gene therapy. Circulation. 1999;100:468–474. doi: 10.1161/01.CIR.100.5.468. [DOI] [PubMed] [Google Scholar]
- 57.Crystal RG. Adenovirus: the first effective in vivo gene delivery vector. Hum. Gene Ther. 2014;25:3–11. doi: 10.1089/hum.2013.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raper SE, et al. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol. Genet. Metab. 2003;80:148–158. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 59.Schnell MA, et al. Activation of innate immunity in nonhuman primates following intraportal administration of adenoviral vectors. Mol. Ther. 2001;3:708–722. doi: 10.1006/mthe.2001.0330. [DOI] [PubMed] [Google Scholar]
- 60.Zhang Y, et al. Acute cytokine response to systemic adenoviral vectors in mice is mediated by dendritic cells and macrophages. Mol. Ther. 2001;3:697–707. doi: 10.1006/mthe.2001.0329. [DOI] [PubMed] [Google Scholar]
- 61.Muruve DA. The innate immune response to adenovirus vectors. Hum. Gene Ther. 2004;15:1157–1166. doi: 10.1089/hum.2004.15.1157. [DOI] [PubMed] [Google Scholar]
- 62.Peng Z. Current status of gendicine in China: recombinant human Ad-p53 agent for treatment of cancers. Hum. Gene Ther. 2005;16:1016–1027. doi: 10.1089/hum.2005.16.1016. [DOI] [PubMed] [Google Scholar]
- 63.Heise C, et al. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nat. Med. 1997;3:639–645. doi: 10.1038/nm0697-639. [DOI] [PubMed] [Google Scholar]
- 64.Ganly I, et al. A phase I study of Onyx-015, an E1B attenuated adenovirus, administered intratumorally to patients with recurrent head and neck cancer. Clin. Cancer Res. 2000;6:798. [PubMed] [Google Scholar]
- 65.Yu W, Fang H. Clinical trials with oncolytic adenovirus in China. Curr. Cancer Drug Targets. 2007;7:141–148. doi: 10.2174/156800907780058817. [DOI] [PubMed] [Google Scholar]
- 66.Lasaro MO, Ertl HCJ. New insights on adenovirus as vaccine vectors. Mol. Ther. 2009;17:1333–1339. doi: 10.1038/mt.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang C, Zhou D. Adenoviral vector-based strategies against infectious disease and cancer. Hum. Vaccines Immunother. 2016;12:2064–2074. doi: 10.1080/21645515.2016.1165908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Milligan ID, et al. Safety and immunogenicity of novel adenovirus type 26- and modified vaccinia Ankara-vectored Ebola vaccines: a randomized clinical trial. JAMA. 2016;315:1610–1623. doi: 10.1001/jama.2016.4218. [DOI] [PubMed] [Google Scholar]
- 69.Kennedy SB, et al. Phase 2 placebo-controlled trial of two vaccines to prevent Ebola in Liberia. N. Engl. J. Med. 2017;377:1438–1447. doi: 10.1056/NEJMoa1614067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.De Santis O, et al. Safety and immunogenicity of a chimpanzee adenovirus-vectored Ebola vaccine in healthy adults: a randomised, double-blind, placebo-controlled, dose-finding, phase 1/2a study. Lancet Infect. Dis. 2016;16:311–320. doi: 10.1016/S1473-3099(15)00486-7. [DOI] [PubMed] [Google Scholar]
- 71.Tapia MD, et al. Safety, reactogenicity, and immunogenicity of a chimpanzee adenovirus vectored Ebola vaccine in children in Africa: a randomised, observer-blind, placebo-controlled, phase 2 trial. Lancet Infect. Dis. 2020;20:719–730. doi: 10.1016/S1473-3099(20)30019-0. [DOI] [PubMed] [Google Scholar]
- 72.Tapia MD, et al. Safety, reactogenicity, and immunogenicity of a chimpanzee adenovirus vectored Ebola vaccine in adults in Africa: a randomised, observer-blind, placebo-controlled, phase 2 trial. Lancet Infect. Dis. 2020;20:707–718. doi: 10.1016/S1473-3099(20)30016-5. [DOI] [PubMed] [Google Scholar]
- 73.Sebastian S, Lambe T. Clinical advances in viral-vectored influenza vaccines. Vaccines (Basel). 2018;6:29. doi: 10.3390/vaccines6020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Antrobus RD, et al. Clinical assessment of a novel recombinant simian adenovirus ChAdOx1 as a vectored vaccine expressing conserved Influenza A antigens. Mol. Ther. 2014;22:668–674. doi: 10.1038/mt.2013.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Coughlan L, et al. Heterologous two-dose vaccination with Simian adenovirus and poxvirus vectors elicits long-lasting cellular immunity to influenza virus A in healthy adults. EBioMedicine. 2018;29:146–154. doi: 10.1016/j.ebiom.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sekaly R-P. The failed HIV Merck vaccine study: a step back or a launching point for future vaccine development? J. Exp. Med. 2008;205:7–12. doi: 10.1084/jem.20072681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Churchyard GJ, et al. A phase IIA randomized clinical trial of a multiclade HIV-1 DNA prime followed by a multiclade rAd5 HIV-1 vaccine boost in healthy adults (HVTN204) PLoS ONE. 2011;6:e21225–e21225. doi: 10.1371/journal.pone.0021225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baden LR, et al. Induction of HIV-1-specific mucosal immune responses following intramuscular recombinant adenovirus serotype 26 HIV-1 vaccination of humans. J. Infect. Dis. 2015;211:518–528. doi: 10.1093/infdis/jiu485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fuchs JD, et al. Safety and immunogenicity of a recombinant adenovirus serotype 35-vectored HIV-1 vaccine in adenovirus serotype 5 seronegative and seropositive individuals. J. AIDS Clin. Res. 2015;6:461. doi: 10.4172/2155-6113.1000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhu F-C, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395:1845–1854. doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Folegatti PM, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shaw AR, Suzuki M. Immunology of adenoviral vectors in cancer therapy. Mol. Ther. Methods Clin. Dev. 2019;15:418–429. doi: 10.1016/j.omtm.2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bressy C, Hastie E, Grdzelishvili VZ. Combining oncolytic virotherapy with p53 tumor suppressor gene therapy. Mol. Ther. Oncolytics. 2017;5:20–40. doi: 10.1016/j.omto.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tazawa H, Kagawa S, Fujiwara T. Advances in adenovirus-mediated p53 cancer gene therapy. Expert Opin. Biol. Ther. 2013;13:1569–1583. doi: 10.1517/14712598.2013.845662. [DOI] [PubMed] [Google Scholar]
- 85.Wold WSM, Toth K. Adenovirus vectors for gene therapy, vaccination and cancer gene therapy. Curr. Gene Ther. 2013;13:421–433. doi: 10.2174/1566523213666131125095046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rosenthal EL, et al. Phase I dose-escalating trial of Escherichia coli purine nucleoside phosphorylase and fludarabine gene therapy for advanced solid tumors. Ann. Oncol. 2015;26:1481–1487. doi: 10.1093/annonc/mdv196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Colombo F, et al. Combined HSV-TK/IL-2 gene therapy in patients with recurrent glioblastoma multiforme: biological and clinical results. Cancer Gene Ther. 2005;12:835–848. doi: 10.1038/sj.cgt.7700851. [DOI] [PubMed] [Google Scholar]
- 88.Ayala G, et al. Biological response determinants in HSV-tk + Ganciclovir gene therapy for prostate cancer. Mol. Ther. 2006;13:716–728. doi: 10.1016/j.ymthe.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 89.Shirakawa T, et al. Long-term outcome of phase I/II clinical trial of Ad-OC-TK/VAL gene therapy for hormone-refractory metastatic prostate cancer. Hum. Gene Ther. 2007;18:1225–1232. doi: 10.1089/hum.2007.074. [DOI] [PubMed] [Google Scholar]
- 90.Freytag SO, et al. Prospective randomized phase 2 trial of intensity modulated radiation therapy with or without oncolytic adenovirus-mediated cytotoxic gene therapy in intermediate-risk prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2014;89:268–276. doi: 10.1016/j.ijrobp.2014.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee J-C, et al. Tolerability and safety of a replication-competent adenovirus-mediated double suicide gene therapy (Ad5-yCD/mutTK(SR39)rep-ADP) with chemotherapy in locally advanced pancreatic cancer: Phase 1 trial. J. Clin. Oncol. 2019;37:e15761–e15761. doi: 10.1200/JCO.2019.37.15_suppl.e15761. [DOI] [Google Scholar]
- 92.Barton KN, et al. Feasibility of adenovirus-mediated hNIS gene transfer and 131I radioiodine therapy as a definitive treatment for localized prostate cancer. Mol. Ther. 2011;19:1353–1359. doi: 10.1038/mt.2011.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Freytag SO, Barton KN, Zhang Y. Efficacy of oncolytic adenovirus expressing suicide genes and interleukin-12 in preclinical model of prostate cancer. Gene Ther. 2013;20:1131–1139. doi: 10.1038/gt.2013.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sterman DH, et al. A phase I clinical trial of single-dose intrapleural IFN-beta gene transfer for malignant pleural mesothelioma and metastatic pleural effusions: high rate of antitumor immune responses. Clin. Cancer Res. 2007;13:4456–4466. doi: 10.1158/1078-0432.CCR-07-0403. [DOI] [PubMed] [Google Scholar]
- 95.Sterman DH, et al. A trial of intrapleural adenoviral-mediated Interferon-alpha2b gene transfer for malignant pleural mesothelioma. Am. J. Respir. Crit. Care Med. 2011;184:1395–1399. doi: 10.1164/rccm.201103-0554CR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ranki T, et al. Phase I study with ONCOS-102 for the treatment of solid tumors - an evaluation of clinical response and exploratory analyses of immune markers. J. Immunother. Cancer. 2016;4:17. doi: 10.1186/s40425-016-0121-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Koski A, et al. Treatment of cancer patients with a serotype 5/3 chimeric oncolytic adenovirus expressing GMCSF. Mol. Ther. 2010;18:1874–1884. doi: 10.1038/mt.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Packiam VT, et al. An open label, single-arm, phase II multicenter study of the safety and efficacy of CG0070 oncolytic vector regimen in patients with BCG-unresponsive non-muscle-invasive bladder cancer: Interim results. Urol. Oncol. 2018;36:440–447. doi: 10.1016/j.urolonc.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 99.Packiam VT, et al. An open label, single-arm, phase II multicenter study of the safety and efficacy of CG0070 oncolytic vector regimen in patients with BCG-unresponsive non–muscle-invasive bladder cancer: Interim results. Urol. Oncol. 2018;36:440–447. doi: 10.1016/j.urolonc.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 100.Eriksson E, et al. Activation of myeloid and endothelial cells by CD40L gene therapy supports T-cell expansion and migration into the tumor microenvironment. Gene Ther. 2017;24:92–103. doi: 10.1038/gt.2016.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gomes EM, et al. Antitumor activity of an oncolytic adenoviral-CD40 ligand (CD154) transgene construct in human breast cancer cells. Clin. Cancer Res. 2009;15:1317. doi: 10.1158/1078-0432.CCR-08-1360. [DOI] [PubMed] [Google Scholar]
- 102.Diaconu I, et al. Immune response is an important aspect of the antitumor effect produced by a CD40L-encoding oncolytic adenovirus. Cancer Res. 2012;72:2327. doi: 10.1158/0008-5472.CAN-11-2975. [DOI] [PubMed] [Google Scholar]
- 103.Wang C, Lin GH, McPherson AJ, Watts TH. Immune regulation by 4-1BB and 4-1BBL: complexities and challenges. Immunol. Rev. 2009;229:192–215. doi: 10.1111/j.1600-065X.2009.00765.x. [DOI] [PubMed] [Google Scholar]
- 104.Eriksson E, et al. Shaping the tumor stroma and sparking immune activation by CD40 and 4-1BB signaling induced by an armed oncolytic virus. Clin. Cancer Res. 2017;23:5846. doi: 10.1158/1078-0432.CCR-17-0285. [DOI] [PubMed] [Google Scholar]
- 105.Wang L, Arnold K. Press release: modified adenovirus offers new approach to treating aggressive brain tumors. J. Natl Cancer Inst. 2003;95:633–633. [Google Scholar]
- 106.Lang FF, et al. Phase I study of DNX-2401 (delta-24-RGD) oncolytic adenovirus: replication and immunotherapeutic effects in recurrent malignant glioma. J. Clin. Oncol. 2018;36:1419–1427. doi: 10.1200/JCO.2017.75.8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nokisalmi P, et al. Oncolytic adenovirus ICOVIR-7 in patients with advanced and refractory solid tumors. Clin. Cancer Res. 2010;16:3035–3043. doi: 10.1158/1078-0432.CCR-09-3167. [DOI] [PubMed] [Google Scholar]
- 108.Cascallo M, et al. Systemic toxicity-efficacy profile of ICOVIR-5, a potent and selective oncolytic adenovirus based on the pRB pathway. Mol. Ther. 2007;15:1607–1615. doi: 10.1038/sj.mt.6300239. [DOI] [PubMed] [Google Scholar]
- 109.Hidalgo M, et al. Proof of concept clinical study by US-guided intratumor injection of VCN-01, an oncolytic adenovirus expressing hyaluronidase in patients with pancreatic cancer. Ann. Oncol. 2019;30:v171–v172. doi: 10.1093/annonc/mdz244.021. [DOI] [Google Scholar]
- 110.Kanerva A, et al. Targeting adenovirus to the serotype 3 receptor increases gene transfer efficiency to ovarian cancer cells. Clin. Cancer Res. 2002;8:275–280. [PubMed] [Google Scholar]
- 111.Kim KH, et al. A phase I clinical trial of Ad5/3-Δ24, a novel serotype-chimeric, infectivity-enhanced, conditionally-replicative adenovirus (CRAd), in patients with recurrent ovarian cancer. Gynecol. Oncol. 2013;130:518–524. doi: 10.1016/j.ygyno.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pesonen S, et al. Prolonged systemic circulation of chimeric oncolytic adenovirus Ad5/3-Cox2L-D24 in patients with metastatic and refractory solid tumors. Gene Ther. 2010;17:892–904. doi: 10.1038/gt.2010.17. [DOI] [PubMed] [Google Scholar]
- 113.Kuhn I, et al. Directed evolution generates a novel oncolytic virus for the treatment of colon cancer. PLoS ONE. 2008;3:e2409–e2409. doi: 10.1371/journal.pone.0002409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Garcia-Carbonero R, et al. Phase 1 study of intravenous administration of the chimeric adenovirus enadenotucirev in patients undergoing primary tumor resection. J. Immunother. Cancer. 2017;5:71. doi: 10.1186/s40425-017-0277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xiang Z, et al. Chimpanzee adenovirus antibodies in humans, sub-Saharan Africa. Emerg. Infect. Dis. 2006;12:1596–1599. doi: 10.3201/eid1210.060078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ludwig SL, et al. Prevalence of antibodies to adenovirus serotypes 4 and 7 among unimmunized US Army trainees: results of a retrospective nationwide seroprevalence survey. J. Infect. Dis. 1998;178:1776–1778. doi: 10.1086/314498. [DOI] [PubMed] [Google Scholar]
- 117.Zak DE, et al. Merck Ad5/HIV induces broad innate immune activation that predicts CD8+ T-cell responses but is attenuated by preexisting Ad5 immunity. Proc. Natl Acad. Sci. USA. 2012;109:E3503–E3512. doi: 10.1073/pnas.1208972109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen H, et al. Adenovirus-based vaccines: comparison of vectors from three species of adenoviridae. J. Virol. 2010;84:10522–10532. doi: 10.1128/JVI.00450-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Quinn KM, et al. Comparative analysis of the magnitude, quality, phenotype, and protective capacity of simian immunodeficiency virus gag-specific CD8+ T cells following human-, simian-, and chimpanzee-derived recombinant adenoviral vector immunization. J. Immunol. 2013;190:2720–2735. doi: 10.4049/jimmunol.1202861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bots STF, Hoeben RC. Non-Human Primate-Derived Adenoviruses for Future Use as Oncolytic Agents? Int J Mol Sci. 2020;21:4821. doi: 10.3390/ijms21144821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Brunetti-Pierri N, et al. Transgene expression up to 7 years in nonhuman primates following hepatic transduction with helper-dependent adenoviral vectors. Hum. Gene Ther. 2013;24:761–765. doi: 10.1089/hum.2013.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gonzalez-Aparicio M, et al. Self-inactivating helper virus for the production of high-capacity adenoviral vectors. Gene Ther. 2011;18:1025–1033. doi: 10.1038/gt.2011.58. [DOI] [PubMed] [Google Scholar]
- 123.Atchison RW, Casto BC, Hammon WM. Adenovirus-associated defective virus particles. Science. 1965;149:754–756. doi: 10.1126/science.149.3685.754. [DOI] [PubMed] [Google Scholar]
- 124.Matsushita T, et al. Adeno-associated virus vectors can be efficiently produced without helper virus. Gene Ther. 1998;5:938–945. doi: 10.1038/sj.gt.3300680. [DOI] [PubMed] [Google Scholar]
- 125.Xiao X, Li J, Samulski RJ. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J. Virol. 1998;72:2224–2232. doi: 10.1128/JVI.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Linden RM, Berns KI. Molecular biology of adeno-associated viruses. Contrib. Microbiol. 2000;4:68–84. doi: 10.1159/000060327. [DOI] [PubMed] [Google Scholar]
- 127.Sonntag F, Schmidt K, Kleinschmidt JA. A viral assembly factor promotes AAV2 capsid formation in the nucleolus. Proc. Natl Acad. Sci. USA. 2010;107:10220–10225. doi: 10.1073/pnas.1001673107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ogden PJ, Kelsic ED, Sinai S, Church GM. Comprehensive AAV capsid fitness landscape reveals a viral gene and enables machine-guided design. Science. 2019;366:1139–1143. doi: 10.1126/science.aaw2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lusby E, Fife KH, Berns KI. Nucleotide sequence of the inverted terminal repetition in adeno-associated virus DNA. J. Virol. 1980;34:402–409. doi: 10.1128/JVI.34.2.402-409.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tseng YS, Agbandje-McKenna M. Mapping the AAV capsid host antibody response toward the development of second generation gene delivery vectors. Front Immunol. 2014;5:9. doi: 10.3389/fimmu.2014.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gao G, et al. Clades of adeno-associated viruses are widely disseminated in human tissues. J. Virol. 2004;78:6381–6388. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Calcedo R, et al. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J. Infect. Dis. 2009;199:381–390. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mietzsch M, et al. Differential adeno-associated virus serotype-specific interaction patterns with synthetic heparins and other glycans. J. Virol. 2014;88:2991–3003. doi: 10.1128/JVI.03371-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Summerford C, Samulski RJ. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J. Virol. 1998;72:1438–1445. doi: 10.1128/JVI.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Summerford C, Johnson JS, Samulski RJ. AAVR: a multi-serotype receptor for AAV. Mol. Ther. 2016;24:663–666. doi: 10.1038/mt.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dudek AM, et al. An alternate route for adeno-associated virus (AAV) entry independent of AAV receptor. J. Virol. 2018;92:e02213–e02217. doi: 10.1128/JVI.02213-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sonntag F, et al. Adeno-associated virus type 2 capsids with externalized VP1/VP2 trafficking domains are generated prior to passage through the cytoplasm and are maintained until uncoating occurs in the nucleus. J. Virol. 2006;80:11040–11054. doi: 10.1128/JVI.01056-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Duan D, et al. Circular intermediates of recombinant adeno-associated virus have defined structural characteristics responsible for long-term episomal persistence in muscle tissue. J. Virol. 1998;72:8568–8577. doi: 10.1128/JVI.72.11.8568-8577.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nault JC, et al. Recurrent AAV2-related insertional mutagenesis in human hepatocellular carcinomas. Nat. Genet. 2015;47:1187–1193. doi: 10.1038/ng.3389. [DOI] [PubMed] [Google Scholar]
- 140.Kotin RM, Menninger JC, Ward DC, Berns KI. Mapping and direct visualization of a region-specific viral DNA integration site on chromosome 19q13-qter. Genomics. 1991;10:831–834. doi: 10.1016/0888-7543(91)90470-Y. [DOI] [PubMed] [Google Scholar]
- 141.Kotin RM, et al. Site-specific integration by adeno-associated virus. Proc. Natl Acad. Sci. USA. 1990;87:2211–2215. doi: 10.1073/pnas.87.6.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Samulski RJ, Berns KI, Tan M, Muzyczka N. Cloning of adeno-associated virus into pBR322: rescue of intact virus from the recombinant plasmid in human cells. Proc. Natl Acad. Sci. USA. 1982;79:2077–2081. doi: 10.1073/pnas.79.6.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Flotte T, et al. A phase I study of an adeno-associated virus-CFTR gene vector in adult CF patients with mild lung disease. Hum. Gene Ther. 1996;7:1145–1159. doi: 10.1089/hum.1996.7.9-1145. [DOI] [PubMed] [Google Scholar]
- 144.Duan D, Yue Y, Yan Z, Engelhardt JF. A new dual-vector approach to enhance recombinant adeno-associated virus-mediated gene expression through intermolecular cis activation. Nat. Med. 2000;6:595–598. doi: 10.1038/75080. [DOI] [PubMed] [Google Scholar]
- 145.Nakai H, Storm TA, Kay MA. Increasing the size of rAAV-mediated expression cassettes in vivo by intermolecular joining of two complementary vectors. Nat. Biotechnol. 2000;18:527–532. doi: 10.1038/75390. [DOI] [PubMed] [Google Scholar]
- 146.Sun L, Li J, Xiao X. Overcoming adeno-associated virus vector size limitation through viral DNA heterodimerization. Nat. Med. 2000;6:599–602. doi: 10.1038/75087. [DOI] [PubMed] [Google Scholar]
- 147.Ghosh A, Yue Y, Lai Y, Duan D. A hybrid vector system expands adeno-associated viral vector packaging capacity in a transgene-independent manner. Mol. Ther. 2008;16:124–130. doi: 10.1038/sj.mt.6300322. [DOI] [PubMed] [Google Scholar]
- 148.Lai Y, et al. Efficient in vivo gene expression by trans-splicing adeno-associated viral vectors. Nat. Biotechnol. 2005;23:1435–1439. doi: 10.1038/nbt1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chew WL, et al. A multifunctional AAV-CRISPR-Cas9 and its host response. Nat. Methods. 2016;13:868–874. doi: 10.1038/nmeth.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Li J, et al. Protein trans-splicing as a means for viral vector-mediated in vivo gene therapy. Hum. Gene Ther. 2008;19:958–964. doi: 10.1089/hum.2008.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Salva MZ, et al. Design of tissue-specific regulatory cassettes for high-level rAAV-mediated expression in skeletal and cardiac muscle. Mol. Ther. 2007;15:320–329. doi: 10.1038/sj.mt.6300027. [DOI] [PubMed] [Google Scholar]
- 152.Lahey HG, et al. Pronounced therapeutic benefit of a single bidirectional AAV vector administered systemically in Sandhoff mice. Mol. Ther. 2020;28:2150–2160. doi: 10.1016/j.ymthe.2020.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Faust SM, et al. CpG-depleted adeno-associated virus vectors evade immune detection. J. Clin. Invest. 2013;123:2994–3001. doi: 10.1172/JCI68205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chanda D, et al. Effects of cellular methylation on transgene expression and site-specific integration of adeno-associated virus. Genes. 2017;8:232. doi: 10.3390/genes8090232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wright JF. Codon modification and PAMPs in clinical AAV vectors: the tortoise or the hare? Mol. Ther. 2020;28:701–703. doi: 10.1016/j.ymthe.2020.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Brown BD, et al. Endogenous microRNA regulation suppresses transgene expression in hematopoietic lineages and enables stable gene transfer. Nat. Med. 2006;12:585–591. doi: 10.1038/nm1398. [DOI] [PubMed] [Google Scholar]
- 157.Xiao Y, et al. Circumventing cellular immunity by miR142-mediated regulation sufficiently supports rAAV-delivered OVA expression without activating humoral immunity. JCI Insight. 2019;4:e99052. doi: 10.1172/jci.insight.99052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Majowicz A, et al. Mir-142-3p target sequences reduce transgene-directed immunogenicity following intramuscular adeno-associated virus 1 vector-mediated gene delivery. J. Gene Med. 2013;15:219–232. doi: 10.1002/jgm.2712. [DOI] [PubMed] [Google Scholar]
- 159.Boisgerault F, et al. Prolonged gene expression in muscle is achieved without active immune tolerance using microrRNA 142.3p-regulated rAAV gene transfer. Hum. Gene Ther. 2013;24:393–405. doi: 10.1089/hum.2012.208. [DOI] [PubMed] [Google Scholar]
- 160.Chan YK, et al. Reducing AAV-mediated immune responses and pathology in a subretinal pig model by engineering the vector genome. ASGCT 23rd Annu. Meet. 2020;27:298–298. [Google Scholar]
- 161.Berns KI. The unusual properties of the AAV inverted terminal repeat. Hum. Gene Ther. 2020;31:518–523. doi: 10.1089/hum.2020.017. [DOI] [PubMed] [Google Scholar]
- 162.McCarty DM, et al. Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Ther. 2003;10:2112–2118. doi: 10.1038/sj.gt.3302134. [DOI] [PubMed] [Google Scholar]
- 163.Wang Z, et al. Rapid and highly efficient transduction by double-stranded adeno-associated virus vectors in vitro and in vivo. Gene Ther. 2003;10:2105–2111. doi: 10.1038/sj.gt.3302133. [DOI] [PubMed] [Google Scholar]
- 164.Rogers GL, et al. Unique roles of TLR9- and MyD88-dependent and -independent pathways in adaptive immune responses to AAV-Mediated Gene Transfer. J. Innate Immun. 2015;7:302–314. doi: 10.1159/000369273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Martino AT, et al. The genome of self-complementary adeno-associated viral vectors increases Toll-like receptor 9-dependent innate immune responses in the liver. Blood. 2011;117:6459–6468. doi: 10.1182/blood-2010-10-314518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wu T, et al. Self-complementary AAVs induce more potent transgene product-specific immune responses compared to a single-stranded genome. Mol. Ther. 2012;20:572–579. doi: 10.1038/mt.2011.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Flotte TR, et al. Expression of the cystic fibrosis transmembrane conductance regulator from a novel adeno-associated virus promoter. J. Biol. Chem. 1993;268:3781–3790. doi: 10.1016/S0021-9258(18)53762-5. [DOI] [PubMed] [Google Scholar]
- 168.Earley LF, et al. Adeno-associated virus serotype-specific inverted terminal repeat sequence role in vector transgene expression. Hum. Gene Ther. 2020;31:151–162. doi: 10.1089/hum.2019.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Haberman RP, McCown TJ, Samulski RJ. Novel transcriptional regulatory signals in the adeno-associated virus terminal repeat A/D junction element. J. Virol. 2000;74:8732–8739. doi: 10.1128/JVI.74.18.8732-8739.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Shao W, et al. Double-stranded RNA innate immune response activation from long-term adeno-associated virus vector transduction. JCI Insight. 2018;3:e120474. doi: 10.1172/jci.insight.120474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mingozzi F, High KA. Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood. 2013;122:23–36. doi: 10.1182/blood-2013-01-306647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhong L, et al. Tyrosine-phosphorylation of AAV2 vectors and its consequences on viral intracellular trafficking and transgene expression. Virology. 2008;381:194–202. doi: 10.1016/j.virol.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Salganik M, et al. Adeno-associated virus capsid proteins may play a role in transcription and second-strand synthesis of recombinant genomes. J. Virol. 2014;88:1071–1079. doi: 10.1128/JVI.02093-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Powell SK, Samulski RJ, McCown TJ. AAV capsid-promoter interactions determine CNS cell-selective gene expression in vivo. Mol. Ther. 2020;28:1373–1380. doi: 10.1016/j.ymthe.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gao GP, et al. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl Acad. Sci. USA. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gao G, et al. Adeno-associated viruses undergo substantial evolution in primates during natural infections. Proc. Natl Acad. Sci. USA. 2003;100:6081–6086. doi: 10.1073/pnas.0937739100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wang D, Tai PWL, Gao G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019;18:358–378. doi: 10.1038/s41573-019-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Chen YH, Chang M, Davidson BL. Molecular signatures of disease brain endothelia provide new sites for CNS-directed enzyme therapy. Nat. Med. 2009;15:1215–1218. doi: 10.1038/nm.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yang YS, et al. Bone-targeting AAV-mediated silencing of Schnurri-3 prevents bone loss in osteoporosis. Nat. Commun. 2019;10:2958. doi: 10.1038/s41467-019-10809-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Maheshri N, Koerber JT, Kaspar BK, Schaffer DV. Directed evolution of adeno-associated virus yields enhanced gene delivery vectors. Nat. Biotechnol. 2006;24:198–204. doi: 10.1038/nbt1182. [DOI] [PubMed] [Google Scholar]
- 181.Gray SJ, et al. Directed evolution of a novel adeno-associated virus (AAV) vector that crosses the seizure-compromised blood-brain barrier (BBB) Mol. Ther. 2010;18:570–578. doi: 10.1038/mt.2009.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Grimm D, et al. In vitro and in vivo gene therapy vector evolution via multispecies interbreeding and retargeting of adeno-associated viruses. J. Virol. 2008;82:5887–5911. doi: 10.1128/JVI.00254-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lisowski L, et al. Selection and evaluation of clinically relevant AAV variants in a xenograft liver model. Nature. 2014;506:382–386. doi: 10.1038/nature12875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Muller OJ, et al. Random peptide libraries displayed on adeno-associated virus to select for targeted gene therapy vectors. Nat. Biotechnol. 2003;21:1040–1046. doi: 10.1038/nbt856. [DOI] [PubMed] [Google Scholar]
- 185.Dalkara D, et al. In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci. Transl. Med. 2013;5:189ra176. doi: 10.1126/scitranslmed.3005708. [DOI] [PubMed] [Google Scholar]
- 186.Deverman BE, et al. Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat. Biotechnol. 2016;34:204–209. doi: 10.1038/nbt.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hordeaux J, et al. The neurotropic properties of AAV-PHP.B are limited to C57BL/6J mice. Mol. Ther. 2018;26:664–668. doi: 10.1016/j.ymthe.2018.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Batista AR, et al. Ly6a differential expression in blood-brain barrier is responsible for strain specific central nervous system transduction profile of AAV-PHP.B. Hum. Gene Ther. 2020;31:90–102. doi: 10.1089/hum.2019.186. [DOI] [PubMed] [Google Scholar]
- 189.Hanlon KS, et al. Selection of an efficient AAV vector for robust CNS transgene expression. Mol. Ther. Methods Clin. Dev. 2019;15:320–332. doi: 10.1016/j.omtm.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Landegger LD, et al. A synthetic AAV vector enables safe and efficient gene transfer to the mammalian inner ear. Nat. Biotechnol. 2017;35:280–284. doi: 10.1038/nbt.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zinn E, et al. In silico reconstruction of the viral evolutionary lineage yields a potent gene therapy vector. Cell Rep. 2015;12:1056–1068. doi: 10.1016/j.celrep.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Smith RH, et al. Germline viral “fossils” guide in silico reconstruction of a mid-Cenozoic era marsupial adeno-associated virus. Sci. Rep. 2016;6:28965. doi: 10.1038/srep28965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Rivera VM, et al. Long-term pharmacologically regulated expression of erythropoietin in primates following AAV-mediated gene transfer. Blood. 2005;105:1424–1430. doi: 10.1182/blood-2004-06-2501. [DOI] [PubMed] [Google Scholar]
- 194.Nathwani AC, et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N. Engl. J. Med. 2014;371:1994–2004. doi: 10.1056/NEJMoa1407309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Nathwani AC, et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N. Engl. J. Med. 2011;365:2357–2365. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zaiss AK, et al. Differential activation of innate immune responses by adenovirus and adeno-associated virus vectors. J. Virol. 2002;76:4580–4590. doi: 10.1128/JVI.76.9.4580-4590.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Somanathan S, Breous E, Bell P, Wilson JM. AAV vectors avoid inflammatory signals necessary to render transduced hepatocyte targets for destructive T cells. Mol. Ther. 2010;18:977–982. doi: 10.1038/mt.2010.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Graham FL, Smiley J, Russell WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 199.Gao G, Sena-Esteves M. Introducing genes into mammalian cells: viral vectors. Mol. Cloning. Lab. Man. 2012;2:1209–1313. doi: 10.1101/pdb.top095513. [DOI] [PubMed] [Google Scholar]
- 200.Clark KR, Voulgaropoulou F, Fraley DM, Johnson PR. Cell lines for the production of recombinant adeno-associated virus. Hum. Gene Ther. 1995;6:1329–1341. doi: 10.1089/hum.1995.6.10-1329. [DOI] [PubMed] [Google Scholar]
- 201.Liu XL, Clark KR, Johnson PR. Production of recombinant adeno-associated virus vectors using a packaging cell line and a hybrid recombinant adenovirus. Gene Ther. 1999;6:293–299. doi: 10.1038/sj.gt.3300807. [DOI] [PubMed] [Google Scholar]
- 202.Gao GP, et al. High-titer adeno-associated viral vectors from a Rep/Cap cell line and hybrid shuttle virus. Hum. Gene Ther. 1998;9:2353–2362. doi: 10.1089/hum.1998.9.16-2353. [DOI] [PubMed] [Google Scholar]
- 203.Gao GP, et al. Rep/Cap gene amplification and high-yield production of AAV in an A549 cell line expressing Rep/Cap. Mol. Ther. 2002;5:644–649. doi: 10.1006/mthe.2001.0591. [DOI] [PubMed] [Google Scholar]
- 204.Thomas DL, et al. Scalable recombinant adeno-associated virus production using recombinant herpes simplex virus type 1 coinfection of suspension-adapted mammalian cells. Hum. Gene Ther. 2009;20:861–870. doi: 10.1089/hum.2009.004. [DOI] [PubMed] [Google Scholar]
- 205.Penaud-Budloo M, et al. Accurate identification and quantification of DNA species by next-generation sequencing in adeno-associated viral vectors produced in insect cells. Hum. Gene Ther. Methods. 2017;28:148–162. doi: 10.1089/hgtb.2016.185. [DOI] [PubMed] [Google Scholar]
- 206.Tai PWL, et al. Adeno-associated virus genome population sequencing achieves full vector genome resolution and reveals human-vector chimeras. Mol. Ther. Methods Clin. Dev. 2018;9:130–141. doi: 10.1016/j.omtm.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Maynard LH, et al. Fast-Seq: a simple method for rapid and inexpensive validation of packaged single-stranded adeno-associated viral genomes in academic settings. Hum. Gene Ther. Methods. 2019;30:195–205. doi: 10.1089/hgtb.2019.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kotin RM. Large-scale recombinant adeno-associated virus production. Hum. Mol. Genet. 2011;20:R2–R6. doi: 10.1093/hmg/ddr141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wu Y, et al. Development of versatile and flexible Sf9 packaging cell line-dependent OneBac system for large-scale recombinant adeno-associated virus production. Hum. Gene Ther. Methods. 2019;30:172–183. doi: 10.1089/hgtb.2019.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Rumachik NG, et al. Methods matter: standard production platforms for recombinant AAV produce chemically and functionally distinct vectors. Mol. Ther. Methods Clin. Dev. 2020;18:98–118. doi: 10.1016/j.omtm.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Yla-Herttuala S. Endgame: glybera finally recommended for approval as the first gene therapy drug in the European union. Mol. Ther. 2012;20:1831–1832. doi: 10.1038/mt.2012.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Burnett JR, Hooper AJ. Alipogene tiparvovec, an adeno-associated virus encoding the Ser(447)X variant of the human lipoprotein lipase gene for the treatment of patients with lipoprotein lipase deficiency. Curr. Opin. Mol. Ther. 2009;11:681–691. [PubMed] [Google Scholar]
- 213.Russell S, et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet. 2017;390:849–860. doi: 10.1016/S0140-6736(17)31868-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Mendell JR, et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N. Engl. J. Med. 2017;377:1713–1722. doi: 10.1056/NEJMoa1706198. [DOI] [PubMed] [Google Scholar]
- 215.Kiss, S., Osborne, A., Hoang, C. & Turpcu, A. In American Society of Gene and Cell Therapy (ASGCT) Virtual Annual Meeting 2020 Vol. 28, 220–221 (Molecular Therapy, 2020).
- 216.Crudele JM, Chamberlain JS. AAV-based gene therapies for the muscular dystrophies. Hum. Mol. Genet. 2019;28:R102–R107. doi: 10.1093/hmg/ddz128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Harper SQ, et al. Modular flexibility of dystrophin: implications for gene therapy of Duchenne muscular dystrophy. Nat. Med. 2002;8:253–261. doi: 10.1038/nm0302-253. [DOI] [PubMed] [Google Scholar]
- 218.Solid Biosciences, Inc. Solid Biosciences Reports Full Year 2017 Financial Results and Provides Corporate Update (GLOBE NEWSWIRE, 2018).
- 219.Pfizer, Inc. Pfizer Presents Initial Clinical Data on Phase 1b Gene Therapy Study for Duchenne Muscular Dystrophy (DMD), https://www.pfizer.com/news/press-release/press-release-detail/pfizer_presents_initial_clinical_data_on_phase_1b_gene_therapy_study_for_duchenne_muscular_dystrophy_dmd (2019).
- 220.Kay MA, et al. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat. Genet. 2000;24:257–261. doi: 10.1038/73464. [DOI] [PubMed] [Google Scholar]
- 221.Arruda VR, et al. Posttranslational modifications of recombinant myotube-synthesized human factor IX. Blood. 2001;97:130–138. doi: 10.1182/blood.V97.1.130. [DOI] [PubMed] [Google Scholar]
- 222.Herzog RW, et al. Influence of vector dose on factor IX-specific T and B cell responses in muscle-directed gene therapy. Hum. Gene Ther. 2002;13:1281–1291. doi: 10.1089/104303402760128513. [DOI] [PubMed] [Google Scholar]
- 223.Manno CS, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 224.Rangarajan S, et al. AAV5-factor VIII gene transfer in severe hemophilia A. N. Engl. J. Med. 2017;377:2519–2530. doi: 10.1056/NEJMoa1708483. [DOI] [PubMed] [Google Scholar]
- 225.George LA, et al. Hemophilia B gene therapy with a high-specific-activity factor IX variant. N. Engl. J. Med. 2017;377:2215–2227. doi: 10.1056/NEJMoa1708538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wilson JM, Flotte TR. Moving forward after two deaths in a gene therapy trial of myotubular myopathy. Hum. Gene Ther. 2020;31:695–696. doi: 10.1089/hum.2020.182. [DOI] [PubMed] [Google Scholar]
- 227.Srivastava A. AAV vectors: are they safe? Hum. Gene Ther. 2020;31:697–699. doi: 10.1089/hum.2020.187. [DOI] [PubMed] [Google Scholar]
- 228.Paulk NK. Gene therapy: it’s time to talk about high-dose AAV. Genet. Eng. Biotechnol. News. 2020;40:14–16. doi: 10.1089/gen.40.09.04. [DOI] [Google Scholar]
- 229.Bertin B, et al. Capsid-specific removal of circulating antibodies to adeno-associated virus vectors. Sci. Rep. 2020;10:864. doi: 10.1038/s41598-020-57893-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Leborgne C, et al. IgG-cleaving endopeptidase enables in vivo gene therapy in the presence of anti-AAV neutralizing antibodies. Nat. Med. 2020;26:1096–1101. doi: 10.1038/s41591-020-0911-7. [DOI] [PubMed] [Google Scholar]
- 231.Elmore ZC, et al. Rescuing AAV gene transfer from antibody neutralization with an IgG-degrading enzyme. JCI Insight. 2020;5:e139881. doi: 10.1172/jci.insight.139881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Vandenberghe LH, et al. Heparin binding directs activation of T cells against adeno-associated virus serotype 2 capsid. Nat. Med. 2006;12:967–971. doi: 10.1038/nm1445. [DOI] [PubMed] [Google Scholar]
- 233.Huang X, Yang Y. Innate immune recognition of viruses and viral vectors. Hum. Gene Ther. 2009;20:293–301. doi: 10.1089/hum.2008.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Chandler LC, et al. Enhancement of adeno-associated virus-mediated gene therapy using hydroxychloroquine in murine and human tissues. Mol. Ther. Methods Clin. Dev. 2019;14:77–89. doi: 10.1016/j.omtm.2019.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Hinderer C, et al. Severe toxicity in nonhuman primates and piglets following high-dose intravenous administration of an adeno-associated virus vector expressing human SMN. Hum. Gene Ther. 2018;29:285–298. doi: 10.1089/hum.2018.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Hordeaux J, et al. Adeno-associated virus-induced dorsal root ganglion pathology. Hum. Gene Ther. 2020;31:808–818. doi: 10.1089/hum.2020.167. [DOI] [PubMed] [Google Scholar]
- 237.Hordeaux J, et al. Method to prevent AAV-induced dorsal root ganglia toxicity & axonopathy in nonhuman primates, and insights into the pathophysiology. ASGCT 23rd Annu. Meet. 2020;28:214–215. [Google Scholar]
- 238.Wang D, Zhang F, Gao G. CRISPR-based therapeutic genome editing: strategies and in vivo delivery by AAV vectors. Cell. 2020;181:136–150. doi: 10.1016/j.cell.2020.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Hanlon KS, et al. High levels of AAV vector integration into CRISPR-induced DNA breaks. Nat. Commun. 2019;10:4439. doi: 10.1038/s41467-019-12449-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Nguyen, G. N. et al. 2019 American Society of Hematology Annual Meeting & Exposition 134 (American Society of Hematology, 2019).
- 241.Campbell S, Vogt VM. In vitro assembly of virus-like particles with Rous sarcoma virus Gag deletion mutants: identification of the p10 domain as a morphological determinant in the formation of spherical particles. J. Virol. 1997;71:4425–4435. doi: 10.1128/JVI.71.6.4425-4435.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Vogt, V. M. Retroviruses (eds. Coffin, J. M., Hughes, S. H. & Varmus H. E.) (Cold Spring Harbor, 1997). [PubMed]
- 243.Beasley BE, Hu WS. cis-Acting elements important for retroviral RNA packaging specificity. J. Virol. 2002;76:4950–4960. doi: 10.1128/JVI.76.10.4950-4960.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Watanabe S, Temin HM. Encapsidation sequences for spleen necrosis virus, an avian retrovirus, are between the 5′ long terminal repeat and the start of the gag gene. Proc. Natl Acad. Sci. USA. 1982;79:5986–5990. doi: 10.1073/pnas.79.19.5986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Hanawa H, et al. Comparison of various envelope proteins for their ability to pseudotype lentiviral vectors and transduce primitive hematopoietic cells from human blood. Mol. Ther. 2002;5:242–251. doi: 10.1006/mthe.2002.0549. [DOI] [PubMed] [Google Scholar]
- 246.Bieniasz PD, Grdina TA, Bogerd HP, Cullen BR. Recruitment of cyclin T1/P-TEFb to an HIV type 1 long terminal repeat promoter proximal RNA target is both necessary and sufficient for full activation of transcription. Proc. Natl Acad. Sci. USA. 1999;96:7791–7796. doi: 10.1073/pnas.96.14.7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Neville M, et al. The importin-beta family member Crm1p bridges the interaction between Rev and the nuclear pore complex during nuclear export. Curr. Biol. 1997;7:767–775. doi: 10.1016/S0960-9822(06)00335-6. [DOI] [PubMed] [Google Scholar]
- 248.Basmaciogullari S, Pizzato M. The activity of Nef on HIV-1 infectivity. Front. Microbiol. 2014;5:232. doi: 10.3389/fmicb.2014.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Seissler T, Marquet R, Paillart JC. Hijacking of the ubiquitin/proteasome pathway by the HIV auxiliary proteins. Viruses. 2017;9:322. doi: 10.3390/v9110322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.McClure MO, Marsh M, Weiss RA. Human immunodeficiency virus infection of CD4-bearing cells occurs by a pH-independent mechanism. EMBO J. 1988;7:513–518. doi: 10.1002/j.1460-2075.1988.tb02839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Sinangil F, Loyter A, Volsky DJ. Quantitative measurement of fusion between human immunodeficiency virus and cultured cells using membrane fluorescence dequenching. FEBS Lett. 1988;239:88–92. doi: 10.1016/0014-5793(88)80551-9. [DOI] [PubMed] [Google Scholar]
- 252.Stein BS, et al. pH-independent HIV entry into CD4-positive T cells via virus envelope fusion to the plasma membrane. Cell. 1987;49:659–668. doi: 10.1016/0092-8674(87)90542-3. [DOI] [PubMed] [Google Scholar]
- 253.Ciuffi A. Mechanisms governing lentivirus integration site selection. Curr. Gene Ther. 2008;8:419–429. doi: 10.2174/156652308786848021. [DOI] [PubMed] [Google Scholar]
- 254.Marshall HM, et al. Role of PSIP1/LEDGF/p75 in lentiviral infectivity and integration targeting. PLoS ONE. 2007;2:e1340. doi: 10.1371/journal.pone.0001340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Qian W, et al. Prolonged integration site selection of a lentiviral vector in the genome of human keratinocytes. Med. Sci. Monit. 2017;23:1116–1122. doi: 10.12659/MSM.903094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Naldini L, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 257.Zhu Y, et al. Multigene lentiviral vectors based on differential splicing and translational control. Mol. Ther. 2001;4:375–382. doi: 10.1006/mthe.2001.0469. [DOI] [PubMed] [Google Scholar]
- 258.Yu X, et al. Lentiviral vectors with two independent internal promoters transfer high-level expression of multiple transgenes to human hematopoietic stem-progenitor cells. Mol. Ther. 2003;7:827–838. doi: 10.1016/S1525-0016(03)00104-7. [DOI] [PubMed] [Google Scholar]
- 259.Tian J, Andreadis ST. Independent and high-level dual-gene expression in adult stem-progenitor cells from a single lentiviral vector. Gene Ther. 2009;16:874–884. doi: 10.1038/gt.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Rausell A, et al. Innate immune defects in HIV permissive cell lines. Retrovirology. 2016;13:43. doi: 10.1186/s12977-016-0275-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Bukrinsky MI, Stanwick TL, Dempsey MP, Stevenson M. Quiescent T lymphocytes as an inducible virus reservoir in HIV-1 infection. Science. 1991;254:423–427. doi: 10.1126/science.1925601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Ohashi K, Park F, Kay MA. Role of hepatocyte direct hyperplasia in lentivirus-mediated liver transduction in vivo. Hum. Gene Ther. 2002;13:653–663. doi: 10.1089/10430340252837242. [DOI] [PubMed] [Google Scholar]
- 263.Park F, et al. Efficient lentiviral transduction of liver requires cell cycling in vivo. Nat. Genet. 2000;24:49–52. doi: 10.1038/71673. [DOI] [PubMed] [Google Scholar]
- 264.Stevenson M, Stanwick TL, Dempsey MP, Lamonica CA. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J. 1990;9:1551–1560. doi: 10.1002/j.1460-2075.1990.tb08274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Abordo-Adesida E, et al. Stability of lentiviral vector-mediated transgene expression in the brain in the presence of systemic antivector immune responses. Hum. Gene Ther. 2005;16:741–751. doi: 10.1089/hum.2005.16.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Baekelandt V, et al. Optimized lentiviral vector production and purification procedure prevents immune response after transduction of mouse brain. Gene Ther. 2003;10:1933–1940. doi: 10.1038/sj.gt.3302094. [DOI] [PubMed] [Google Scholar]
- 267.Zufferey R, et al. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat. Biotechnol. 1997;15:871–875. doi: 10.1038/nbt0997-871. [DOI] [PubMed] [Google Scholar]
- 268.Dull T, et al. A third-generation lentivirus vector with a conditional packaging system. J. Virol. 1998;72:8463–8471. doi: 10.1128/JVI.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Zufferey R, et al. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J. Virol. 1998;72:9873–9880. doi: 10.1128/JVI.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Park F, Kay MA. Modified HIV-1 based lentiviral vectors have an effect on viral transduction efficiency and gene expression in vitro and in vivo. Mol. Ther. 2001;4:164–173. doi: 10.1006/mthe.2001.0450. [DOI] [PubMed] [Google Scholar]
- 271.Agarwal M, et al. Scaffold attachment region-mediated enhancement of retroviral vector expression in primary T cells. J. Virol. 1998;72:3720–3728. doi: 10.1128/JVI.72.5.3720-3728.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Zufferey R, Donello JE, Trono D, Hope TJ. Woodchuck hepatitis virus posttranscriptional regulatory element enhances expression of transgenes delivered by retroviral vectors. J. Virol. 1999;73:2886–2892. doi: 10.1128/JVI.73.4.2886-2892.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Fernandez-Rubio P, Torres-Rusillo S, Molina IJ. Regulated expression of murine CD40L by a lentiviral vector transcriptionally targeted through its endogenous promoter. J. Gene Med. 2015;17:219–228. doi: 10.1002/jgm.2837. [DOI] [PubMed] [Google Scholar]
- 274.Marodon G, et al. Specific transgene expression in human and mouse CD4+ cells using lentiviral vectors with regulatory sequences from the CD4 gene. Blood. 2003;101:3416–3423. doi: 10.1182/blood-2002-02-0578. [DOI] [PubMed] [Google Scholar]
- 275.Vigna E, et al. Robust and efficient regulation of transgene expression in vivo by improved tetracycline-dependent lentiviral vectors. Mol. Ther. 2002;5:252–261. doi: 10.1006/mthe.2002.0542. [DOI] [PubMed] [Google Scholar]
- 276.Duisit G, et al. Five recombinant simian immunodeficiency virus pseudotypes lead to exclusive transduction of retinal pigmented epithelium in rat. Mol. Ther. 2002;6:446–454. doi: 10.1006/mthe.2002.0690. [DOI] [PubMed] [Google Scholar]
- 277.MacKenzie TC, et al. Efficient transduction of liver and muscle after in utero injection of lentiviral vectors with different pseudotypes. Mol. Ther. 2002;6:349–358. doi: 10.1006/mthe.2002.0681. [DOI] [PubMed] [Google Scholar]
- 278.Ausubel LJ, et al. Production of CGMP-grade lentiviral vectors. Bioprocess Int. 2012;10:32–43. [PMC free article] [PubMed] [Google Scholar]
- 279.Gandara C, Affleck V, Stoll EA. Manufacture of third-generation lentivirus for preclinical use, with process development considerations for translation to good manufacturing practice. Hum. Gene Ther. Methods. 2018;29:1–15. doi: 10.1089/hgtb.2017.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Merten OW, et al. Large-scale manufacture and characterization of a lentiviral vector produced for clinical ex vivo gene therapy application. Hum. Gene Ther. 2011;22:343–356. doi: 10.1089/hum.2010.060. [DOI] [PubMed] [Google Scholar]
- 281.Gama-Norton L, et al. Lentivirus production is influenced by SV40 large T-antigen and chromosomal integration of the vector in HEK293 cells. Hum. Gene Ther. 2011;22:1269–1279. doi: 10.1089/hum.2010.143. [DOI] [PubMed] [Google Scholar]
- 282.Stewart HJ, et al. A stable producer cell line for the manufacture of a lentiviral vector for gene therapy of Parkinson’s disease. Hum. Gene Ther. 2011;22:357–369. doi: 10.1089/hum.2010.142. [DOI] [PubMed] [Google Scholar]
- 283.Wielgosz MM, et al. Generation of a lentiviral vector producer cell clone for human Wiskott-Aldrich syndrome gene therapy. Mol. Ther. Methods Clin. Dev. 2015;2:14063. doi: 10.1038/mtm.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Burns JC, et al. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc. Natl Acad. Sci. USA. 1993;90:8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Hopkins N. High titers of retrovirus (vesicular stomatitis virus) pseudotypes, at last. Proc. Natl Acad. Sci. USA. 1993;90:8759–8760. doi: 10.1073/pnas.90.19.8759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Broussau S, et al. Inducible packaging cells for large-scale production of lentiviral vectors in serum-free suspension culture. Mol. Ther. 2008;16:500–507. doi: 10.1038/sj.mt.6300383. [DOI] [PubMed] [Google Scholar]
- 287.Manceur AP, et al. Scalable lentiviral vector production using stable HEK293SF producer cell lines. Hum. Gene Ther. Methods. 2017;28:330–339. doi: 10.1089/hgtb.2017.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Humbert O, et al. Development of third-generation cocal envelope producer cell lines for robust lentiviral gene transfer into hematopoietic stem cells and T-cells. Mol. Ther. 2016;24:1237–1246. doi: 10.1038/mt.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Stornaiuolo A, et al. RD2-MolPack-Chim3, a packaging cell line for stable production of lentiviral vectors for anti-HIV gene therapy. Hum. Gene Ther. Methods. 2013;24:228–240. doi: 10.1089/hgtb.2012.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Witting SR, et al. Efficient large volume lentiviral vector production using flow electroporation. Hum. Gene Ther. 2012;23:243–249. doi: 10.1089/hum.2011.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Gelinas JF, Davies LA, Gill DR, Hyde SC. Assessment of selected media supplements to improve F/HN lentiviral vector production yields. Sci. Rep. 2017;7:10198. doi: 10.1038/s41598-017-07893-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Kuroda H, Kutner RH, Bazan NG, Reiser J. Simplified lentivirus vector production in protein-free media using polyethylenimine-mediated transfection. J. Virol. Methods. 2009;157:113–121. doi: 10.1016/j.jviromet.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 293.Boudeffa D, et al. Toward a scalable purification protocol of GaLV-TR-pseudotyped lentiviral vectors. Hum. Gene Ther. Methods. 2019;30:153–171. doi: 10.1089/hgtb.2019.076. [DOI] [PubMed] [Google Scholar]
- 294.Merten OW, Hebben M, Bovolenta C. Production of lentiviral vectors. Mol. Ther. Methods Clin. Dev. 2016;3:16017. doi: 10.1038/mtm.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Olgun HB, Tasyurek HM, Sanlioglu AD, Sanlioglu S. High-grade purification of third-generation HIV-based lentiviral vectors by anion exchange chromatography for experimental gene and stem cell therapy applications. Methods Mol. Biol. 2019;1879:347–365. doi: 10.1007/7651_2018_154. [DOI] [PubMed] [Google Scholar]
- 296.Maude SL, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Kochenderfer JN, et al. Construction and preclinical evaluation of an anti-CD19 chimeric antigen receptor. J. Immunother. 2009;32:689–702. doi: 10.1097/CJI.0b013e3181ac6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Levine BL, et al. Gene transfer in humans using a conditionally replicating lentiviral vector. Proc. Natl Acad. Sci. USA. 2006;103:17372–17377. doi: 10.1073/pnas.0608138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Thompson AA, et al. Gene therapy in patients with transfusion-dependent beta-thalassemia. N. Engl. J. Med. 2018;378:1479–1493. doi: 10.1056/NEJMoa1705342. [DOI] [PubMed] [Google Scholar]
- 300.Eichler F, et al. Hematopoietic stem-cell gene therapy for cerebral adrenoleukodystrophy. N. Engl. J. Med. 2017;377:1630–1638. doi: 10.1056/NEJMoa1700554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Kohn DB, et al. Lentiviral gene therapy with autologous hematopoietic stem and progenitor cells (HSPCs) for the treatment of severe combined immune deficiency due to adenosine deaminase deficiency (ADA-SCID): results in an expanded cohort. Blood. 2019;134:3345–3345. doi: 10.1182/blood-2019-123432. [DOI] [Google Scholar]
- 302.Ferrua F, et al. Lentiviral haemopoietic stem/progenitor cell gene therapy for treatment of Wiskott-Aldrich syndrome: interim results of a non-randomised, open-label, phase 1/2 clinical study. Lancet Haematol. 2019;6:e239–e253. doi: 10.1016/S2352-3026(19)30021-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Puig-Saus C, et al. IND-enabling studies for a clinical trial to genetically program a persistent cancer-targeted immune system. Clin. Cancer Res. 2019;25:1000–1011. doi: 10.1158/1078-0432.CCR-18-0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Stadtmauer EA, et al. Long-term safety and activity of NY-ESO-1 SPEAR T cells after autologous stem cell transplant for myeloma. Blood Adv. 2019;3:2022–2034. doi: 10.1182/bloodadvances.2019000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Hacein-Bey-Abina S, et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J. Clin. Invest. 2008;118:3132–3142. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Beard BC, et al. Comparison of HIV-derived lentiviral and MLV-based gammaretroviral vector integration sites in primate repopulating cells. Mol. Ther. 2007;15:1356–1365. doi: 10.1038/sj.mt.6300159. [DOI] [PubMed] [Google Scholar]
- 307.Cattoglio C, et al. Hot spots of retroviral integration in human CD34+ hematopoietic cells. Blood. 2007;110:1770–1778. doi: 10.1182/blood-2007-01-068759. [DOI] [PubMed] [Google Scholar]
- 308.Montini E, et al. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat. Biotechnol. 2006;24:687–696. doi: 10.1038/nbt1216. [DOI] [PubMed] [Google Scholar]
- 309.Nowrouzi A, Glimm H, von Kalle C, Schmidt M. Retroviral vectors: post entry events and genomic alterations. Viruses. 2011;3:429–455. doi: 10.3390/v3050429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Schroder AR, et al. HIV-1 integration in the human genome favors active genes and local hotspots. Cell. 2002;110:521–529. doi: 10.1016/S0092-8674(02)00864-4. [DOI] [PubMed] [Google Scholar]
- 311.Wu X, Li Y, Crise B, Burgess SM. Transcription start regions in the human genome are favored targets for MLV integration. Science. 2003;300:1749–1751. doi: 10.1126/science.1083413. [DOI] [PubMed] [Google Scholar]
- 312.Cesana D, et al. Whole transcriptome characterization of aberrant splicing events induced by lentiviral vector integrations. J. Clin. Invest. 2012;122:1667–1676. doi: 10.1172/JCI62189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Emery DW. The use of chromatin insulators to improve the expression and safety of integrating gene transfer vectors. Hum. Gene Ther. 2011;22:761–774. doi: 10.1089/hum.2010.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Ramezani A, Hawley TS, Hawley RG. Combinatorial incorporation of enhancer-blocking components of the chicken beta-globin 5′HS4 and human T-cell receptor alpha/delta BEAD-1 insulators in self-inactivating retroviral vectors reduces their genotoxic potential. Stem Cells. 2008;26:3257–3266. doi: 10.1634/stemcells.2008-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Liu M, et al. Genomic discovery of potent chromatin insulators for human gene therapy. Nat. Biotechnol. 2015;33:198–203. doi: 10.1038/nbt.3062. [DOI] [PubMed] [Google Scholar]
- 316.Almarza D, et al. Risk assessment in skin gene therapy: viral-cellular fusion transcripts generated by proviral transcriptional read-through in keratinocytes transduced with self-inactivating lentiviral vectors. Gene Ther. 2011;18:674–681. doi: 10.1038/gt.2011.12. [DOI] [PubMed] [Google Scholar]
- 317.Modlich U, et al. Insertional transformation of hematopoietic cells by self-inactivating lentiviral and gammaretroviral vectors. Mol. Ther. 2009;17:1919–1928. doi: 10.1038/mt.2009.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Moiani A, et al. Lentiviral vector integration in the human genome induces alternative splicing and generates aberrant transcripts. J. Clin. Invest. 2012;122:1653–1666. doi: 10.1172/JCI61852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Cesana D, et al. Uncovering and dissecting the genotoxicity of self-inactivating lentiviral vectors in vivo. Mol. Ther. 2014;22:774–785. doi: 10.1038/mt.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Rowe HM, et al. Immunization with a lentiviral vector stimulates both CD4 and CD8 T cell responses to an ovalbumin transgene. Mol. Ther. 2006;13:310–319. doi: 10.1016/j.ymthe.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 321.Chen F, et al. Episomal lentiviral vectors confer erythropoietin expression in dividing cells. Plasmid. 2017;90:15–19. doi: 10.1016/j.plasmid.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 322.Hu J, et al. A non-integrating lentiviral approach overcomes Cas9-induced immune rejection to establish an immunocompetent metastatic renal cancer model. Mol. Ther. Methods Clin. Dev. 2018;9:203–210. doi: 10.1016/j.omtm.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Chen Y, et al. A self-restricted CRISPR system to reduce off-target effects. Mol. Ther. 2016;24:1508–1510. doi: 10.1038/mt.2016.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Gallinaro A, et al. Integrase defective lentiviral vector as a vaccine platform for delivering influenza antigens. Front. Immunol. 2018;9:171. doi: 10.3389/fimmu.2018.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Coutant F, et al. A nonintegrative lentiviral vector-based vaccine provides long-term sterile protection against malaria. PLoS ONE. 2012;7:e48644. doi: 10.1371/journal.pone.0048644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Wang Y, et al. Generation of a caged lentiviral vector through an unnatural amino acid for photo-switchable transduction. Nucleic Acids Res. 2019;47:e114. doi: 10.1093/nar/gkz659. [DOI] [PMC free article] [PubMed] [Google Scholar]