Abstract
Dengue is a rapidly spreading mosquito-borne flavivirus infection that is prevalent in tropical and sub-tropical regions. Humans are known to be the main reservoir host maintaining the epidemic cycles of dengue but it is unclear if dengue virus is also maintained in a similar enzootic cycle. The systematic review was conducted in accordance to Cochrane's PRISMA recommendations. A search was done on PubMed, EMBASE, Scopus and Cochrane Library. Key data on animal dengue positivity was extracted and classified according to animal type and diagnostic modes. Of the 3818 articles identified, 56 articles were used in this review. A total of 16,333 animals were tested, 1817 of which were positive for dengue virus by RT-PCR or serology. Dengue positivity was detected in bats (10.1%), non-human primates (27.3%), birds (11%), bovid (4.1%), dogs (1.6%), horses (5.1%), pigs (34.1%), rodents (3.5%), marsupials (13%) and other small animals (7.3%). While majority of dengue positivity via serology suggests potential enzootic transmission, but regular dengue virus spillback cannot be excluded. With the exception of bats, acute infection among animals is limited. Further investigation on animals is critically required to better understand their role as potential reservoir in dengue transmission.
Keywords: Dengue infection, Animal reservoir, Enzootic transmission, Systematic review
Highlights
-
•
Besides non-human primates, dengue infection occurred in pigs, marsupials, bats, birds, horses, bovid, rodents and dogs.
-
•
There is potential enzootic transmission, but regular dengue virus spillback cannot be excluded.
-
•
With the exception of bats, acute dengue infection among animals is still limited in evidence.
-
•
Though the role of animals as potential dengue reservoir cannot be concluded, neither can it be excluded yet.
1. Introduction
Dengue is the most rapidly spreading mosquito-borne viral disease in the last decade. Transmission is the most active in the tropical and subtropical regions of the world. At least 128 countries, including 36 previously dengue-free countries in temperate regions, with a total estimated population of 3.97 billion people are at risk of dengue outbreak [1]. Globally, the estimated number of dengue infections is 390 million per year. Among these infections, only 25% are symptomatic and at least 1% are severe cases, mainly in children [2]. The number of symptomatic dengue cases has increased more than twice in every new decade between 1990 and 2013. This has resulted in about 1.14 million disability-adjusted life-year and about 9221 dengue deaths per year [3]. The total global cost due to dengue illness is estimated to be US$8.9 billion per year [4].
Dengue infection is caused by single-stranded RNA virus of the genus Flavivirus and family Flaviviridae. There are four antigenically-related dengue serotypes (DENV 1, 2, 3 and 4) which are genetically diverse, with a number of genotypes and clades of each serotype that have been associated with dengue disease severity [5] as well as a number of severe dengue epidemics [6]. Dengue virus infection is usually self-limiting and mild, but can also result in severe diseases classified as dengue hemorrhagic fever/dengue shock syndrome [7] or severe dengue [8] based on World Health Organization guidelines. Prevention measures are usually focused on mosquitoes' population control as well as removal and reduction of mosquito's breeding grounds. These measures can be effective but only over a short period of time and it is expensive to sustain as a long-term national strategic intervention [9]. While recent trials of the release of wolbachia-infected mosquitoes had demonstrated the feasibility and efficacy to reduce the mosquito populations, more prospective investigation and surveillance are necessary to assess any long-term implications among the Wolbachia/mosquito/virus interactions [10]. The only approved dengue vaccine does offer some form of protection, but remains limited in efficacy [11] and in vaccine implementation among seronegatives [12]. Furthermore, treatment remains largely supportive with appropriate fluid management as there is still no effective antivirals against dengue despite decades of search.
Dengue virus transmission likely originated from sylvatic cycles maintained between susceptible non-human primates and Aedes mosquitoes in the forests of Asia [13]. Sylvatic transmission cycles have also been described in the forests of Africa, but no evidence indicates likewise in the Americas [14]. Spillover of sylvatic dengue virus can occur in both rural and urban areas as zones of emergence. Given the adaptability of sylvatic virus for the human host and documented past infections that resulted in severe clinical manifestations, this can potentially sustain the natural horizontal human to human transmission via Aedes aegypti and Aedes albopictus mosquitoes [15,16]. Humans are known to be the main reservoir host in maintaining urban epidemic cycles of dengue [17]. While animals are commonly thought to be the reservoir host for flaviviruses transmitted by Aedes mosquitoes such as Zika virus [18] and yellow fever virus (YFV), there is insufficient evidence suggesting that for urban dengue transmission. Notably, Zika virus was first discovered in a monkey in Uganda [19] while non-human primates in South America are well established as reservoirs of YFV given their high susceptibility to YFV infection [14,20]. As such, the aim of this study is to systematically evaluate the role of animals in dengue transmission.
2. Material and methods
2.1. Search strategy and selection criteria
This study was carried out following the recommendations of the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) [21]. There were two phases for our search strategy. The first phase involved using all the identified relevant index terms and keywords to do an extensive search in four different databases – EMBASE, PubMed, Scopus and the Cochrane Library. The second phase involved searching for additional articles based on the references found in the selected articles from the first phase but were not found in the database search. The following keywords were used in the first phase of search: ‘dengue’, ‘dengue virus’, ‘dengue infection’, ‘DENV’, ‘animals’, ‘dogs’, ‘cats’, ‘pigs’, ‘horses’, ‘bats’, ‘monkey’, ‘swine’, ‘chicken’, ‘poultry’, ‘rodents’, ‘rats’, ‘primates’, ‘ducks’, ‘insects’ and ‘birds’. The search was done in late October 2019 with no restriction on time. Importation of references and removal of duplicate references were done using the bibliographical software package, Endnote version X7 (Thomas Reuters, New York, NY, USA). The inclusion criteria was any of the following types of peer-reviewed articles involving dengue diagnosis in animals through direct testing of DENV1/2/3/4 or flaviviruses: observational studies, seroprevalence studies, cross-sectional studies, case-control studies or reviews. The exclusion criterion was any of the following types of peer-reviewed articles: case reports, case series, clinical trials, non-randomized/randomized controlled trials, animal model/in-vivo studies, in-vitro/cell-based studies, experimental studies or vaccine-related studies. All titles, abstracts and selected full reports were screened independently by two authors based on the inclusion and exclusion criteria. Discrepancies were resolved by consensus.
2.2. Data extraction
The following variables of interest were extracted from the selected studies: animal type, country, location of study, study period, species of animal involved, biological samples collected, dengue positivity, dengue serotypes and reported dengue outbreak. The data extracted were cross-checked by another author and discrepancies were resolved by consensus. Data was classified into three categories of animals – bats, non-human primates and other mammals, and two main diagnostic modes – serology or RT-PCR. Other animals include specified animals that were neither bats nor non-human primates, or unspecified animals in their respective studies. Serology includes diagnosis by enzyme-linked immunosorbent assay (ELISA), plaque reduction neutralization test (PRNT), hemagglutination inhibition (HI), complement fixation (CF), and antigen tests (NS1). Studies were also classified into either forest or urban/rural setting, which encompassed non-forest sites near human settlement. Studies that involved both settings due to sample collection at multiple sites had their results separated into each of the categories as much as the literature informs.
2.3. Data analysis
On several occasions, a single study used different number of animal samples in multiple diagnostic methods. For the most of this review including the meta-analysis, dengue prevalence for each animal type was pooled by including one platform of diagnostic method, either serology or RT-PCR, that involved the largest number of samples in each study. Since some NHP and bat studies utilized multiple methods, dengue prevalence by serology and RT-PCR was pooled respectively by including both the serologic method that used the biggest sample and RT-PCR if applicable. Meta-analysis was conducted using STATA 14.2, with the metaprop software command. Each animal type was analysed separately, stratified by diagnostic type whenever possible. Forest plots that display prevalence of dengue positivity with 95% confidence intervals (CI) were generated for graphical representations. I2 statistic values were calculated to quantify degree of heterogeneity among studies, where values of 25–50% represented moderate heterogeneity and values of >50% large heterogeneity among studies.
3. Results
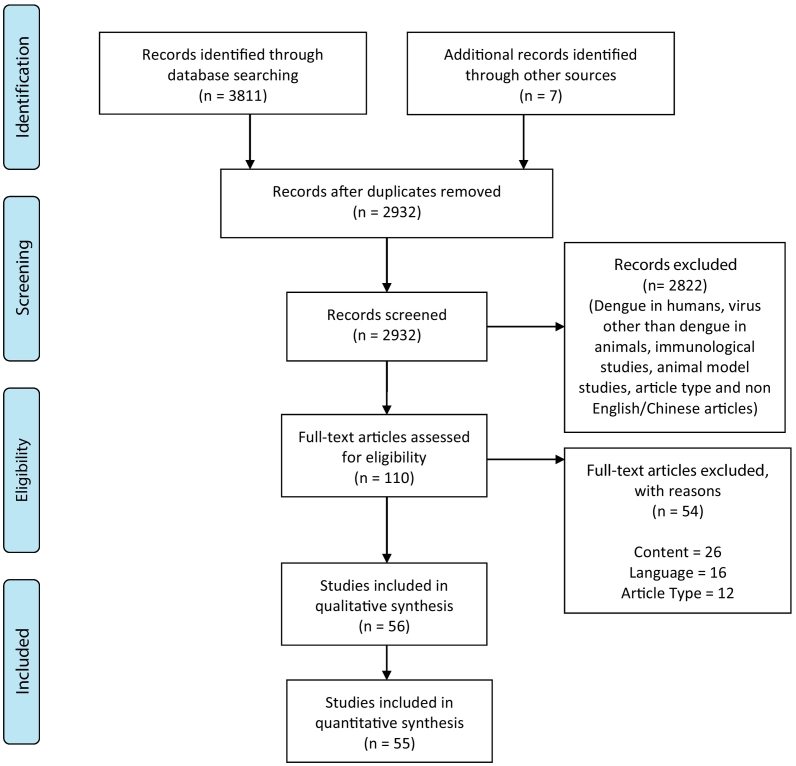
A total of 3811 articles were identified from the initial database search, of which 886 were duplicates and removed. The titles and abstracts of the remaining 2932 articles were screened for their relevance yielding the selection of 110 articles for full-text review. After the review of these 110 articles based upon our inclusion and exclusion criteria (Fig. 1), a final list of 56 articles were selected for comprehensive study. Data from 55 articles were utilized in this review – two [22,23] different papers were identified to have utilized an identical sample, resulting in our exclusion of one [23] in the tabulation of figures. The following animal types were involved the final selected studies – bats (n = 18 studies), non-human primates (n = 22), birds (n = 9), rodents (n = 5), dogs (n = 2), pigs (n = 3), bovid (n = 6), horses (n = 4) and marsupial (n = 3). A collective of other mammals and reptile – one study involved caimans – not included in the list above were also mentioned across 8 articles. Overall, 31 studies exclusively studied a single animal type – bats (n = 13 studies), non-human primates (n = 18), other animals (n = 10) including pigs (n = 1), rodents (n = 1), dogs (n = 1), horses (n = 1) and birds (n = 6).
Fig. 1.
PRISMA flow diagram.
3.1. Non-human primates
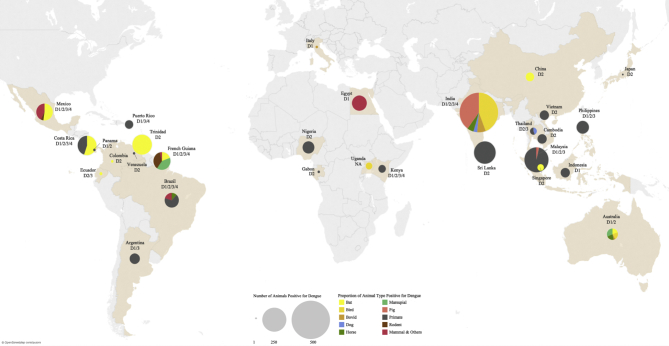
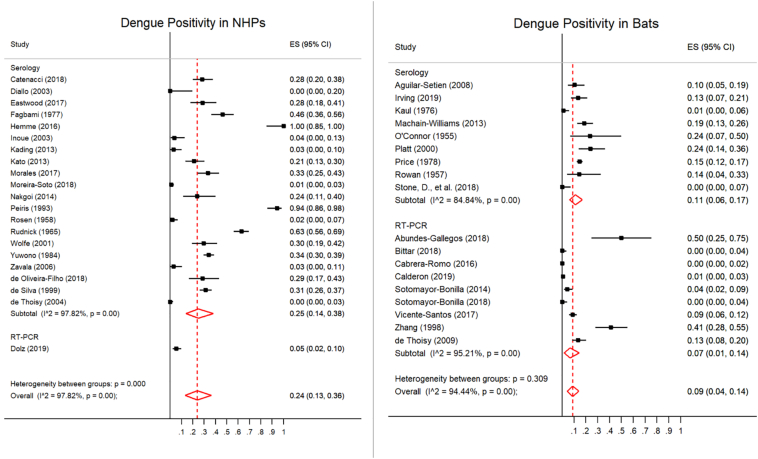
Between 1954 and 2017, there were 2509 non-human primates (NHP) originating from 20 different countries (Argentina, Brazil, Costa Rica, Democratic Republic of Congo, French Guiana, Gabon, India, Japan, Kenya, Malaysia, Nigeria, Panama, Philippines, Puerto Rico, Rwanda, Senegal, Sri Lanka, Thailand, Venezuela and Uganda) that were tested for evidence of dengue virus infection (Table 1, Fig. 2). The names of species involved in each study are detailed in Supplementary Table 2. Of all studies that tested for evidence of DENV infection in NHPs, fourteen study sites from which NHP samples were collected reported dengue endemicity and past outbreaks (Table 2). Among NHPs (mainly monkeys) tested, 684 (27.3%) were found to be dengue positive, albeit mostly through serological tests (Fig. 3). All but one study reported the identification of anti-dengue antibodies through serological means. The remaining study identified the presence of antibodies against flaviviruses in general. Overall, there is a dearth in studies employing RT-PCR to detect dengue infection in non-human primates. Despite 13 of 22 studies were conducted after or examined samples dated after 2000, only 4 studies employed RT-PCR diagnostic to some extent. Only a single study utilized RT-PCR to identify dengue RNA genetic material in all their samples, identifying 8 positives of 155 (5.2%) monkeys tested. Our meta-analysis of studies based on one platform of detection method found a dengue prevalence of 24% (CI: 13–36%) in NHPs (Fig. 4). There was significant intra-group heterogeneity in the serology group (I2: 97.82%), and pooling of results from both serology and RT-PCR methods was not supported as given significant inter-group heterogeneity was noted (p = 0.000). There were 15 studies involving a forest setting [22,[24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37]], 12 of which were based solely in a forest. Of 7 studies involving the urban/rural setting [26,31,[38], [39], [40], [41], [42]], only 5 were exclusively based at such. Only one study involved the coastal setting [29]. Four studies constituted samples obtained from a mix of settings or from an unidentified source [43]. While most of the animals tested were dengue positive for serotype 2, it was observed that 8 of 18 studies with clearly published results distinguishing serotypes tested specifically for serotype 2 only (Table 1).
Table 1.
Study characteristics and dengue positivity among non-human primates.
| Animal Types | Country | Location | Site Setting | Study Period | Dengue RT-PCR Positive Only (%); Serotype | Dengue Serology Positive Only (%); Serotype | Serological Test Type; Serotype Tested | Human Dengue Cases; Serotype | Dengue Serotype (Mosquitoes) | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Monkeys | Philippines | Animal Breeding Facility | Urban/rural | 2010 | 2/9 (22.2); D2.Asian Epidemic DENV (NS1 & E)⁎, D2.Asian Epidemic DENV (NS1)⁎ | 21/100 (21.0); D1,2,3 | PRNT; Tested for D1/2/3/4 | Remarkably high number of DENV cases was reported in 2010, more than triple the number reported in 2009 & Number of cases exceeded the Alert and Epidemic thresholds. |
[40] | |
| Monkeys | Asia | Imported Samples | Unknown | Before 1984 | 145/358 (40.5); D1,2,3,4 | PRNT; Tested for D1/2/3/4 | [43] | |||
| India | 0/33 | PRNT; Tested for D1 only | ||||||||
| Japan | 1/37 (2.7); D2 | PRNT; Unspecified | ||||||||
| Monkeys | Philippines | Luzon Island, Monkey Farm (Monkeys originated from Zamboanga in Mindanao island and Tanay) |
Forest | 1999 | 2/54 (3.7) | ELISA; Unspecified | 35.2% and 16.9% of confirmed DENV and probable DENV cases in 770 patients | [28] | ||
| Monkeys | Sri Lanka | Nature & Archaeological Reserve at Polonnaruwa | Forest | 1986 | 2/16 (12) | ELISA; Unspecified | 1986–1987 epizootic | [33] | ||
| 1987 | 41/44 (94) | ELISA; Unspecified | ||||||||
| 1995 | 52/244 (21); D2 | ELISA; Tested for D2 only | ||||||||
| Monkey | Sri Lanka | Nature & Archaeological Reserve at Polonnaruwa | Forest | 1987 | 64/68 (94); D2 | PRNT; Tested for D2 only | All 4 serotypes isolated from humans before | [32] | ||
| Monkeys | Puerto Rico | Southwestern Region | Urban/rural | 2010 & 2012 | 23/23 (100); D1, 3, 4 | MNT; Tested for D1/2/3/4 | Circulation of all 4 DENV types during 1992 to 2012. | [42] | ||
| Orang Utans | Malaysia | Sabah | Forest (Free Ranging) | 1996–1998 | 11/40 (28) | IFA/ELISA; Unspecified | [23] | |||
| Forest (Semi-captive) | 10/31 (32) | |||||||||
| Orang Utans | Malaysia | Sabah | Forest (Wild) | 1996–1997 | 11/40 (28); D2 | PRNT; Tested for D2 only | Denv-2 seroprevalence in native Bornean and migrants at 11/30 (37%) and 59/80 (74%) respectively. Humans sampled lived or worked on the boundaries of the Sepilok Forest Reserve. | [22] | ||
| Forest (Semi-captive) | 10/31 (32); D2 | |||||||||
| Monkeys | Nigeria | Nupeko Forest | Forest | 1969 (Lowland monkeys) 1971–1972 (Nupeko monkeys) | 45/92 (48.9); D2 | PRNT; Tested for D2 only | 486/1275 (38%) and 811/1816 (45%) seropositivity in humans by HI and PRNT respectively. | [25] | ||
| Galagos | 3/12 (25%); D2 | |||||||||
| NHP | Kenya | Kwale & Kakamega County (Western & Coastal) |
Forest | 2014 | 14/34 (41%) (Coastal); 5/33 (15%) (Western); D1,2,3,4 | ELISA; Tested for D1/2/3/4 | [29] | |||
| Monkeys | Brazil | Atlantic Forest, Bahia, Northeast Brazil, municipalities of Ilhéus and Una | Forest (Free Ranging) | 2006–2014 | 31/110 (28.2); D1,2,3,4 | HI; Tested for D1/2/3/4 | D1,2,3,4 are endemic to both municipalities with sporadic outbreaks | [36] | ||
| Monkeys | Brazil | Atlantic forest (Border of Pernambuco and Paraíba States) | Forest (Free Ranging) | 2015–2016 | 14/49 (28.6); D1,2,3,4 | PRNT; Tested for D1/2/3/4 | D1, 2, 3 and 4 are endemic to the human population | [34] | ||
| Zoo & Wildlife Screening and Recovery Center, Recife | Urban/rural (Captive) | |||||||||
| NHP, Monkeys | Brazil | Zoos in Salvador, Brasília, Itapetinga National primate centre in Cabedelo, Salvador, Vitória da Conquista & Barreiras. Urban & Peri Urban sites; Lucena, Sapé & Santa Rita, |
Urban/Rural | 2012–2017 | 0/207 (Tested against Flavivirus) | 2/6 (33.3); D1 | PRNT; Tested for D1 only | [39] | ||
| NHP, Monkeys | Venezuela | Parque Zoológico Bararida, Barquisimeto, Parque Zoológico El Pinar, Caracas & Guri dick (8 free living) | Urban (Captive) & Forest (Free-ranging) |
Before 2006 | 2/62 (3.2); D2 | HI; Tested for D2 only | Dengue highly endemic in Venezuela since 1989. | [31] | ||
| Argentina | Unknown | Unknown | 0/4 | |||||||
| NHP, Monkeys | Costa Rica | 31 Lowlands locations | Forest & Urban/rural (Private land) | 1993–1996, 2000–2012 | 8/155 (5.2); D2,3,4 (C/prM) | 53/209 (25.4) (Tested against flavivirus) | ELISA | Co-circulation of D1,2,3,4 since re-emergence in 1993. | [26] | |
| NHP, Monkeys | Malaysia | Unknown | Forest | 1962–1964 | 139/221 (62.9); D2 | PRNT; Tested for D2 only | [35] | |||
| NHP, Monkeys | French Guiana | Petit Saut Hydroelectric Dam | Forest | 1994–1995 | 0/145 | PRNT; Tested for D2 only | Dramatic increase in cases over the past 30 years, epidemic in 1991–1992 with up to 3000 cases. | [37] | ||
| Mandrill | Gabon | Lope National Park | Forest | 1998–2006 | 2/25 (8); D2 | PRNT; Tested for D2 only | [30] | |||
| NHP, Monkeys | Congo Basin; Uganda, Democratic Republic of Congo & Rwanda | Bwindi forest, Park National of the Volcanoes, Kinigi & Unknown locations | Forest | 2001–2009 | 0/44 | |||||
| NHP, Monkeys | Panama | Localities near the Canal zone (unknown setting) | Urban/rural | 1954–1955 | 2/105 (1.9); D1,2 | HI; Tested against D1/2 only (Only 2 positive samples further tested using PRNT) |
Dengue epidemic in 1904, 1912, late 1941 to early 1942. At least one local outbreak occurred during 1934–1936. Sporadic cases observed in 1946. 57/213 (27%) residents of Caledonia (non-forested area) with D1/2 HI antibodies 195/892 (22%) residents of forested areas of Panama with D1/2 HI antibodies. |
[41] | ||
| NHP, Monkeys | Senegal | Forest Gallery Near Ngari | Forest | 2000 | 0/17; D2 | ELISA; Tested for D2 only | D2 (Aedes furcifer, A. taylori, A. luteocephalus, A. aegypti) | [24] | ||
| NHP, Monkeys | Thailand | Chiang Mai (Captive Monkey) | Urban/rural | 2008–2009 | 7/21 (33) | PRNT; Tested for D1/2/3/4 but results unspecified | 6052 cases of DHF in 2008 & 2009. | [38] | ||
| Chiang Rai (Captive Monkey) | 1/12 (8.3) | 1974 cases of DHF in 2008 & 2009. | ||||||||
| Maehongsorn (Captive Monkey) | 1/5 (20) | 942 cases of DHF in 2008 & 2009. | ||||||||
| NHP, Monkeys | Argentina | San Cayetano, Corrientes | Forest | 2010 | 0/51 (Tested against flavivirus; NS5) | 36/108 (33.3); D1,3 | PRNT; Tested against D1/3 only | Indigenous circulation of DENV in Northern and Central Argentina since 1988. | [27] | |
| Isla del Cerrito | ||||||||||
| Isla Brasilera |
Phylogenetic analysis linked to human cases.
Fig. 2.
Geographical distribution and proportion of dengue positivity reported among different animal types. NHP: Non-human primates; Others: Shrew, Sloths, Acouchy, Agouti, Porcupine, Armadillo and Kangaroo.
Table 2.
Study characteristics and dengue positivity among bats.
| Animal Types | Country | Location | Site Setting | Study Period | Dengue RT-PCR Positive Only (%); Serotype | Dengue Serology Positive Only (%); Serotype | Serological Test Type; Serotype Tested | Human Dengue Cases; Serotype | Dengue Serotype (Mosquitoes) | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Bats | Mexico | Colima & Jialisco (Pacific Coast) | Urban/rural | 2005 | 5/40 (12.5) | ELISA; Unspecified | 2339 cases reported in 2005–2006 | [46] | ||
| Veracruz (Gulf Coast) | 2006 | 4/30 (13.3); D2 | 4/46 (8.7) | 7272 cases reported in 2005–2006; D2 outbreak in 2006 | ||||||
| Bats | Mexico | Calakmul Biosphere Reserve |
Forest | 2010–2011 | 4/85 (4.7); D2 | Reported among human populations (20 km away); D1,2,4 | [58] | |||
| Montes Azules Biosphere Reserve | 2/61 (3.3); D2 | |||||||||
| Bats | Mexico | Morelos, Central Mexico |
Urban/Rural | 2011–2012 | 0/149 | 0/149 | PRNT; Tested for D2/4 only | 196 cases reported in 2011–2012; Endemic with D1,2 | [52] | |
| Campeche, South-east Mexico | Forest | 2012 | 0/91 | 0/91 | 764 cases reported in 2012 in the city; Endemic with D1,2 | |||||
| Bats | Mexico | Progreso, Hidalgo (Cave Roost) | Urban/rural | 2014–2015 | 8/16 (50); D2 (NS5)* | [48] | ||||
| Bats | China | Shankouxiang, Mei Wen Village, Hainan Island | Urban/rural | 1995 | 23/56 (41.1%) | 16/20 (80); D2 | ELISA; Tested for D1/2/3/4 | Outbreaks recorded from 1980 to 1982, 1986–1991 (Coastal region of dengue region), with each outbreak affecting hundreds of thousand | 1/3 lots [1/30 (3.3) – 10/30 (33.3)] of female Aedes aegypti captured from endemic area positive by RT-PCR (NS1) | [47] |
| Bats | French Guiana | le Camp du Tigre | Forest | 2001, 2006, 2007 | 19/125 (15.2); D1,2,3 D1.G1 (C/prM)* |
Active outbreaks of D1/2/3 since 1991, sporadic detection of D4; Dengue endemic. | [44] | |||
| Site of Sain Georges de l'pyapock | Edge of forest & rural area | 2006, 2007 | 0/16 | Dengue practically absent in this rural site. | ||||||
| Bats | Costa Rica | La Virgen from Sarapiquı (rural households) | Urban/rural | 2013–2014 | 6/102 (5.9); D2.Asian/American (C/prM)⁎⁎, D3, D4.G2 (C/prM)⁎ | 23/75 (30.7); D1,2,3 | PRNT; Tested for D1/2/3/4 | 16.7% seroprevalence (4/24) | D3 (Culex sp) | [51] |
| Nicoya (semi-urban households) | 6/98 (6.1); D2.Asian/America (C/prM), D4.G2 (C/prM)⁎ | 14/82 (17.1); D1,2,3,4 | 82.6% seroprevalence (19/23) | |||||||
| Central Valley (urban households) | 16/118 (13.6); D1, D2.Asian/America (C/prM)⁎⁎, D4.G2 (C/prM)⁎ | 14/84 (16.7); D1,2,3 | 8.3% seroprevalence (1/12) | D1 (Ae aegypti), D2.Asian/American (C/prM; Culex sp)⁎* | ||||||
| Bats | Mexico | Parque Hundido | Urban/rural | 2010 | 0/59 (Flavivirus RT-PCR targeting NS5) | 14/59 (23.7); D1,2,3,4 | PRNT; Tested for D1/2/3/4 | Presence of all 4 serotypes | [55] | |
| Parque Metropolitano | 0/42 | 2/42 (4.8); D1,2,4 | ||||||||
| Acuaparque | 0/21 | 2/21 (9.5); D1,2,4 | ||||||||
| Merida zoo | 0/14 | 8/14 (57.1); D1,2,3,4 | ||||||||
| Santa Gertrudis Copo | 0/4 | 0/4 | ||||||||
| Bats | Trinidad | Unknown | Unknown | 1972–1974 | 126/857 (14.7); D2 | HI; Tested for D2 only | [61] | |||
| Bats | Grenada | All 6 parishes of Grenada | Urban/rural | 2015 | 0/50 | PRNT; Tested for D1/2/3/4 | All 4 serotypes documented between 2000 and 2003; Dengue endemic. | [57] | ||
| Bats | Costa Rica | Puntarenas & Liberia | Urban/rural | 1998 | 12/53 (22.6); D1,2 | PRNT; Tested for D1/2/3/4 | Active outbreaks of dengue, all 4 serotypes have be isolated in the past 6 years of the study. | [49] | ||
| Ecuador | Tena | 3/10 (30); D2,3 | Unknown | |||||||
| Bats | Mexico | Cuitzmala River Basin, Jalisco | Forest | 2014 | 0/83 (Tested against flavivirus; NS5) | DENV reported in humans | [59] | |||
| Bats | Brazil | São Jose ´ do Rio Preto, São Paulo State | Urban/rural | 2014–2017 | 0/64 (Tested against flavivirus; NS5) | 0/46 | HI; Tested for D1/2/3/4 | High incidence of arbovirus infection in humans | [50] | |
| Barreiras, Bahia State | 0/39 (Tested against flavivirus; NS5) | 0/27 | Known occurrences of DENV | |||||||
| Bats | Columbia | San Carlos & Ayapel, Córdoba & Sucre | Urban/rural | Before 2019 | 2/286 (0.7); D2 (NS5)* | [45] | ||||
| Bats | Singapore | Unknown | Urban/rural | Before 2019 | 14/106 (13.2); D2 (NS1) | Luciferase LIPS; Tested for D2 NS1 antigen only | [54] | |||
| Bats | Australia | Townsville, Northern Queensland | Urban/rural | 1954 | 4/17 (23.5); D1 | Mouse protection test; Tested for D1 only | Extensive epidemic from Dec 1953 – Mar 1954, sporadic cases from Dec 1954 – Nov 1955. | [53] | ||
| Bats | Australia | Townsville, Northern Queensland | Coastal | 1954 | 4/28 (14.3); D1,2 | Mouse protection test; Tested for D1/2 only | Dengue outbreak in the early months of 1954 | [60] | ||
| Bats | India | Bankura Distict, West Bengal | Urban/ Rural | 1973 | 1/91 (1.1); D3 | HI; Tested against D1/2/3 only | [56] |
Phylogenetic analysis linked to human cases.
Phylogenetic analysis linked to both human cases and mosquitoes.
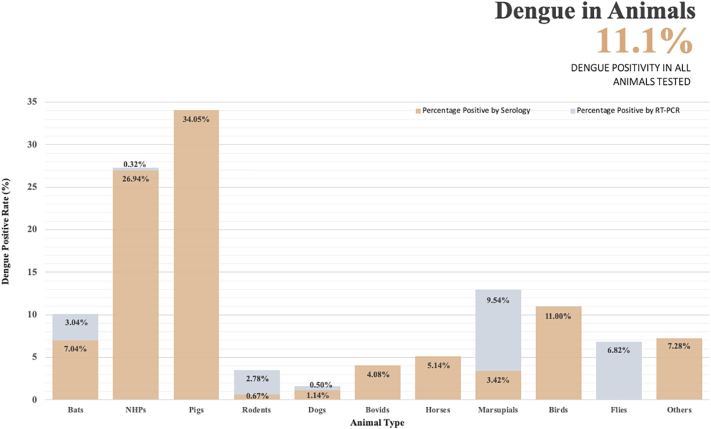
Fig. 3.
Dengue positivity rate of different animal types detected by PCR and serology-based assays. NHP: Non-human primates; Others: Shrew, Sloths, Acouchy, Agouti, Porcupine, Armadillo and Kangaroo.
Fig. 4.
Forest plot of dengue prevalence in NHPs. Forest plot of dengue prevalence in bats.
3.2. Bats
Between 1954 and 2018, a total of 2827 bats captured from twelve different countries were tested for evidence of dengue virus infection (Table 2, Fig. 2). The species names are detailed in Supplementary Table 1. Bats originated from Australia, Brazil, China, Columbia, Costa Rica, Ecuador, French Guiana, Grenada, India, Mexico, Singapore and Trinidad. All except five studies involved sites associated with high dengue endemicity and occurrence of outbreaks (Table 2). All except seven studies used reverse transcription polymerase chain reaction (RT-PCR) as one of the or only detection method/s to identify the presence of dengue RNA genetic material. Of these studies, only one was conducted before the 21st century, indicating a paradigm shift in bat studies towards using RT-PCR only in recent years. Among the 1559 bats tested with RT-PCR, 90 (5.8%) bats were found to be dengue virus RNA positive. All, except four studies, used serological assay as one of the or only detection method/s to identify the presence of dengue antibodies. Among 1974 bats tested with serological assay, 266 (13.5%) bats were found to have antibodies against dengue virus. Based on including only one platform of detection method in each study, 10.08% of bats were positive, with 3.04% and 7.04% of dengue positivity observed from RT-PCR and serology assays respectively (Fig. 3). Overall, our meta-analysis of studies based on one platform of detection method found a 9% dengue prevalence (CI: 4–14%) in bats (Fig. 4). There was significant intra-group heterogeneity as I2 exceeded 85% for both studies, but pooling of results from both serology and RT-PCR methods was supported as no inter-group heterogeneity was noted (p = 0.309). Among the urban/rural setting where bats were captured, there were 5.9% (65/1098) of bats positive for dengue virus RNA as compared to 5.6% (25/445) of bats captured in the forest setting (p > 0.05). Among the urban/rural setting where bats were captured, there were 13.1% (136/1036) of bats that were dengue positive by serology while no bats (0/91) captured in the forest setting was positive by serology (p < 0.05). There were 14 studies involving the urban/rural setting [[44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57]], 4 involving the forest setting [44,52,58,59]. Of which, 12 and 2 studies respectively were based in those settings exclusively. Only 1 study was of the coastal setting [60] and 1 was of unidentified origin [61]. The sole study conducted in the coastal setting reported 14.3% positivity for dengue by serology, comparable to the rate observed in the urban/rural setting. Most of the bats tested in Mexico and Costa Rica were reported to be infected with dengue serotype 2, even though dengue serotype 1, 3 and 4 were also detected. This observation may be associated to the predominant dengue serotype 2 circulation in the human community during the same period of time (Table 2).
3.3. Other animals
Between 1954 and 2016, there were 1406 dogs, 2083 bovid (e.g. sheep, cattle, goat), 1244 horses, 2218 birds (including hens, ducks & geese), 608 pigs, 1187 rodents, 31 elephants and a collective of small animals reported from 17 different countries (Australia, Brazil, Central African Republic, Chad, Czechoslovakia, Democratic Republic of Congo, Egypt, French Guiana, Gabon, India, Italy, Malaysia, Mexico, South Pacific Islands, Thailand, Uganda and Zambia) that were tested against dengue (Table 3, Fig. 2). Eleven of 24 studies involved sites associated with dengue endemicity and outbreaks. Names of the species are detailed in Supplementary Tables 3–11. Among the animals tested, 11.0% of birds, 4.1% of bovid, 1.6% of dogs, 5.1% of horses, 34.1% of pigs, 3.5% of rodent, 13.0% of marsupials and 7.3% of other small animals were found to be dengue positive (Fig. 3). All except three studies employed serological testing to identify the presence of antibodies against dengue - hemagglutination inhibition (HI; 12 studies), plague reduction neutralization test (PRNT; 5 studies), enzyme-linked immunosorbent assay (ELISA) screening followed by PRNT (2 studies) and mouse protection test (1 study). Majority of the studies on pigs and birds had utilized HI instead of PRNT, the gold standard for serological testing. Among the dogs tested with RT-PCR test, only 0.5% were found to be positive. Of 1187 rodents tested, 33 were positive by RT-PCR while 8 were positive by serology. Most of the positive rats (36/41) were from the forest. Meta-analysis of all animal types displayed high heterogeneity (Supplementary Table 14). Overall, most of the dengue positive animals were reportedly from the urban and rural areas [35,36,44,48,56,[62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74]]. Otherwise, six studies involved the forest setting [30,36,37,44,59,75], one was in the coastal setting [60], one was unknown [76]. Notably, most of the animals tested were dengue positive for serotype 2.
Table 3.
Study characteristics and dengue positivity among other animals.
| Animal Types | Country | Location | Site Setting | Study Period | Dengue RT-PCR Positive Only (%); Serotype | Dengue Serology Positive Only (%); Serotype | Serological Test Type; Serotype Tested | Human Dengue Cases; Serotype | Dengue Serotype (Mosquitoes) | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Bat Flies | Mexico | Progreso, Hidalgo (Cave Roost) | Urban/rural | 2014–2015 | 38/557 (6.8%) to 342/557 (61.4%); D2 (NS5) | [48] | ||||
| Rodents | French Guiana | le Camp du Tigre | Forest | 2001, 2006, 2007 | 28/109 (25.7); D1,2,3,4 | Active outbreaks of D1/2/3 since 1991, sporadic detection of D4; Dengue endemic. | [44] | |||
| Site of Sain Georges de l'pyapock | Edge of forest & rural area | 2006, 2007 | 5/21 (23.8); D3,4 D3.G5 (C/prM)⁎, D4.G1 (C/prM)⁎ |
Dengue practically absent in this rural site. | ||||||
| Marsupials | French Guiana | le Camp du Tigre | Forest | 2001, 2006, 2007 | 39/273 (14.3); D1,2,3,4 D2.American-Asian (C/prM)⁎, D2.Native American (C/prM)⁎, D3.G5 (C/prM)⁎, D4.G1 (C/prM)⁎ |
Active outbreaks of D1/2/3 since 1991, sporadic detection of D4; Dengue endemic. | ||||
| Site of Sain Georges de l'pyapock | Edge of forest & rural area | 2006, 2007 | 0/15 | Dengue practically absent in this rural site. | ||||||
| Birds (seashore) | Australia | Townsville, Northern Queensland | Coastal | 1954 | 8/38 (21.1); D1 | Mouse protection test; Tested for D1 only | Dengue outbreak in the early months of 1954 | [60] | ||
| Birds | India | West Bengal, Bankura Distict | Urban/ Rural | 1973 | 3/104 (2.9); D1 | HI; Tested for D1/2/3 only | [56] | |||
| Rodent | 0/32 | |||||||||
| Shrew | 0/4 | |||||||||
| Sloths | Brazil | Atlantic Forest, Bahia, Northeast Brazil, municipalities of Ilheus and Una | Forest & Urban/rural (enclosure) | 2006–2014 | 14/29 (48.3); D1,2,3,4 | HI; Tested for D1/2/3/4 | D1,2,3,4 are endemic to both municipalities with sporadic outbreaks | [36] | ||
| Pigs | Malaysia | Penang | Urban | 1962–1964 | 7/34 (20.6); D2 | PRNT; Tested for D2 only | [35] | |||
| House Shrew | 1/18 (5.6); D2 | |||||||||
| Acouchy | French Guiana | Petit Saut Hydroelectric Dam | Forest | 1994–1995 | 0/29 | PRNT; Tested for D2 only | Dramatic increase in cases over the past 30 years, epidemic in 1991–1992 with up to 3000 cases. | [37] | ||
| Agouti | 1/29 (3.4); D2 | |||||||||
| Porcupine | 2/42 (4.8); D2 | |||||||||
| Paca | 0/17 | |||||||||
| Rat | 0/39 | |||||||||
| Opposum | 1/99 (1); D2 | |||||||||
| Sloth | 0/55 | |||||||||
| Armadillo | 3/60 (5); D2 | |||||||||
| Anteater | 0/26 | |||||||||
| Kinkajou | 0/9 | |||||||||
| Coati | 0/4 | |||||||||
| Tayra | 0/3 | |||||||||
| Collared Peccary | 0/3 | |||||||||
| Brocket Deer | 1/10 (10); D2 | |||||||||
| Buffalo+ | Democratic Republic of Congo & Gabon | Garamaba National Park & Lope National Park | Forest | 1994–2002 | 1/24 (4.2); D2 | PRNT; Tested for D2 only | [30] | |||
| Duiker+ | Democratic Republic of Congo | Ituri Rain Forest | Forest | 1991–1992 | 0/33 | |||||
| Elephant | Zambia, Democratic Republic of Congo, Chad & Central African Republic | Lungunya, Garamba National Park, Odzala National Park, Zakouma National Park, Gobounga Bai, Dzanga-Sangha National Park | Forest | 1991–2002 | 0/31 | |||||
| Pigs | India | Darbhanga | Urban/rural | 1978–1979 | 5/16 (31.3); D2 | HI; Tested for D2 only | [69] | |||
| Patna | 26/40 (65); D2 | |||||||||
| Begusarai | 8/16 (50); D2 | |||||||||
| Nawada | 13/35 (37.1); D2 | |||||||||
| Dhanbad | 11/32 (34.4); D2 | |||||||||
| Singhbhum | 3/14 (21.4); D2 | |||||||||
| Hazaribagh | 50/111 (45); D2 | |||||||||
| Ranchi | 64/140 (45.7); D2 | |||||||||
| Dog | India | Uttar Pradesh, Bareilly | Urban/rural | Before 1995 | 16/104 (15.4); D2 | HI; Tested for D2 only | [72] | |||
| Pig | 20/170 (11.8); D2 | |||||||||
| Horse | 27/170 (15.9); D2 | |||||||||
| Buffalo+ | 26/333 (7.8); D2 | |||||||||
| Goat+ | 10/252 (4); D2 | |||||||||
| Cattle+ | 0/252 | |||||||||
| Sheep+ | 0/168 | |||||||||
| Rodents | Mexico | Cuitzmala River Basin, Jalisco | Forest | 2014 | 0/713 (Tested against flavivirus; NS5) | 0/708 | ELISA; Tested for D2 only | DENV reported in humans | [59] | |
| Rodents | Mexico | Merida, Residential Housings | Urban/ rural | 2011–2012 | 5/161 (3.1); D2 | PRNT; Tested for D2/4 only | Dengue endemic with outbreaks | [64] | ||
| Dogs (Domestic) | Thailand | Nakhon Sawan Province (Urban City) | Urban/rural | 2008–2009 | 6/1057 (0.6); D2,3 (C/prM)^⁎ | Dengue endemic | [63] | |||
| Rayong Province (Rubber Plantation) | 2009–2010 | 1/174 (0.6); D3 (C/prM)^⁎ | ||||||||
| Koh Chang (Tourist Island) | 2012 | 0/71 | ||||||||
| Equines | Brazil | Nhecolândia Sub-region of Pantanal | Urban/rural | 2009–2010 | 8/760 (1.1); Screened against flavivirus; D1,2,4 |
Screened with ELISA, 396 positive ELISA samples tested using PRNT; Tested for D1/2/3/4 for PRNT | Dengue endemic | [71] | ||
| Sheep+ | 0/238; Tested against flavivirus | ELISA | ||||||||
| Caimans | 0/61; Tested against flavivirus | ELISA | ||||||||
| Cattle+ | Sicily, Italy | Madonie, Mazara del Vallo, Casteltermini, Castelvetrano, Acamo, Canicattì & Palermo | Rural | 1969 | 1/410 (0.2); D1 | HI; Tested for D1 only | [74] | |||
| Sheep+ | 1/130 (0.8); D1 | |||||||||
| Goat+ | 0/27 | |||||||||
| Horses | Australia | Junee | Unknown | 1954 | 2/4 (50); D1,2 | PRNT; Tested for D1/2 only | 7/14 (50) Seropositivity in Neutralization Test. 110/142 (77.5) and 103/141 (73) seropositivity by to D1 & D2 respectively Individuals had been screened for Group B virus using MVE HI. | [76] | ||
| Rockhampton | 1955 | 4/14 (28.6); D1 | ||||||||
| Mt Surprise | 1955 | 2/2 (100); D1 | ||||||||
| Gympie | 1960 | 1/1 (100); D1/2 | ||||||||
| Cattle+ | Innisfail | 1954 | 2/5 (0.4); D1 | |||||||
| Tambo | 1958 | 0/1 | ||||||||
| Kangeroo | Western Australia | 1958 | 5/12 (41.7); D1 | |||||||
| Muckadilla | 1/1 (100); D1 | |||||||||
| Tambo | 1/1 (100); D1 | |||||||||
| South-east Queensland | 1960 | 6/8 (75); D1/2 | ||||||||
| Cockatoo | Unknown | 1959 | 1/2 (50); D1 | |||||||
| Horses | South Pacific Islands | New Caledonia (Includes imported horses) |
Urban/Rural | 2015 | 10/163 (6.1); D1 | Screened with ELISA, Positive samples tested with MNT; Tested for D1 only in MNT | Outbreaks in 2008–2009, 2012–2013 (>10, 000 cases); D1. Circulation of all 4 serotypes in 2013. | [68] | ||
| Marquesas Islands, French Polynesia (Includes imported horses) | 2016 | 10/130 (7.7); D1 | D1 epidemic in 2006–2007 (circulation in 2013–2017). D3 circulation in 2013 | |||||||
| Domestic Animals (Mammals & Birds) | Egypt | Cairo Slaughter Abattoir | Urban/Rural | 1969 | 74/964 (7.7); D1 | HI; Tested for D1 only | 159/1133 (14%); 4/1133 (0.3) (accounted for WNV) seropositivity against D1 in 1969 survey. Dengue epidemics in 1927, 1928 and 1937 | [73] | ||
| Ducks | India | Ranchi viz. Kanke, Bihar | Urban/Rural | 1981 | 34/64 (53.1); D2 | HI; Tested for D2 only | [70] | |||
| Fowls | Ranchi viz. Kanke, Bihar | 30/81 (37); D2 | ||||||||
| Sparrows | Poultry Farm, Kanke, Bihar | 1/16 (6.25) – 16/16 (100); D2 | ||||||||
| Fowls | Hotwar, Bihar | 26/63 (41.3); D2 | ||||||||
| Fowls | Daily Market, Bihar | 33/68 (48.5); D2 | ||||||||
| Geese | Czechoslovakia | South Morovia | Urban/rural | 1972 | 0/196 | HI; Unspecified | [65] | |||
| Ducks | 0/141 | |||||||||
| Hens | 0/100 | |||||||||
| Birds | Czechoslovakia | South Morovia | Urban/rural | 1973 | 0/280 | HI; Unspecified | [66] | |||
| Birds | India | Shimoga district, Karnataka | Urban/rural | 1959–1962 | 93/759 (12.1); D1,2,3,4 | HI (17 sera positive for HI tested for PRNT); Tested for D1/2/3/4 | [67] | |||
| Birds | Uganda | Bunyoro, Karmoja, Acholi, Ankole & West Mengo | Forest (Including savannah) | 1970 | 15/221 (6.8); Unknown | HI; Unspecified | [75] | |||
| Birds | Brazil | Mangal das Garças Natural Park in Belém, state of Pará | Urban | 2015 | 0/85 | HI; Tested for D1/2/3/4 | [62] |
Bovids.
Phylogenetic analysis linked to human cases.
4. Discussion
Dengue transmission is well-known to involve sylvatic (enzootic) cycle and urban endemic cycle which involve non-human primates in sylvatic habitat and humans in urban setting as reservoir hosts [17], respectively. As demonstrated in our study, a number of reports suggest other animals may play a role in both the sylvatic and urban endemic cycles as potential secondary hosts. Moreover, with increasing deforestation due to globalization and urbanization, animal and human populations are increasingly staying closer together with higher frequency of potential contacts [16,77]. It is not clear if dengue transmission can evolve to involve an enzootic cycle in an urban setting like the West Nile virus transmission among birds and/or a rural epizootic cycle that involves amplification of dengue virus within domestic animals. As a result, these may increase the risk of spillover and outbreaks affecting the human population in the rural and urban settings.
This systematic review summarized evidence that dengue virus can infect at least 84 species of 23 animal types, including 28 species of non-human primates (Supplementary Tables 1–11). On the contrary, West Nile Virus (WNV), another flavivirus, has also been shown to infect at least 225 species of birds and at least 29 animals (including horses, cattle, llamas, alligators, cats, dogs, wolves and sheep [78,79]. Animal species in both the Old and the New World have antibodies to WNV, but virus is rarely isolated from animals in the Old World compared with those in the New World. Unfortunately, this phenomenon is not clear for dengue virus as most studies were performed only with serological tests but not RT-PCR. Japanese Encephalitis virus (JEV) similarly infects an unusually wide range of animals, including equines, birds, dogs, bats and snakes [80], but they are reported to be dead-end hosts that are unable to infect mosquitoes. Pigs and birds are the major amplifying hosts of JEV, although infection usually does not produce clinical disease.
This systematic review showed that NHPs (27.3%), pigs (34.1%; by serology only) and bats (13.5%; by serology only) are likely more vulnerable to dengue virus infection than the other animals reported in the literature. Dengue virus has been known to infect NHPs such as monkey, which serves as an efficient amplification host for enzootic dengue transmission [13,14,16]. However, based on this review and existing studies [14], not all species of primates may be susceptible to dengue virus infection. As the only natural vertebrate host of dengue apart from humans, their susceptibility to dengue virus has been extensively leveraged as models for studies on dengue pathogenesis and therapeutic interventions [81,82]. Rhesus macaque (Macaca mulata), cynomolgus macaque (Macaca fascicularis), green monkeys (Cercopithecus aethiops) and yellow baboons (Papio cynocephalus) to a less extent, are among species commonly used in experimental studies [13,82]. This study corroborates the infectability of these monkey species through serological identification of dengue exposure. Interestingly, these same species – baboons (Papio) and Macaque (Macaca) – were found to be more behaviorally and ecologically resilient, enabling their survival even in urban areas in the face of anthropogenic stressors [83]. African green monkeys also reportedly sustained longer viremia when infected with human-endemic strains of DENV-2 as compared to sylvatic DENV-2 [84]. This represents a longer viremic duration that could allow infection of more vectors in the event the monkeys are infected, serving as an amplification host. However, findings cannot be generalized to species of monkeys that were not tested.
While the long-tailed macaque (Macaca fascicularis) and banded surili (Presbytis femoralis) are both native to Singapore [85,86], only the former – known to be more susceptible to dengue and adaptable to human environments – is commonly seen in Singapore, numbering some 1500 individuals in the population. Globally, Brazil, Madagascar, Indonesia and the Democratic Republic of Congo are home to two-thirds of 504 primate species in existence. Yet, all four countries are carrying out massive destructions to their primate's habitats with human activities [87], which could force NHPs and humans to interface at increasing levels.
Kato et al. demonstrated a spillback event in cynomolgus macaques in the Philippines, proving that NHPs can be involved in the human/urban DENV cycle [40]. However, their potential to act as viable reservoirs depends on whether they can sustain sufficient viremia to enable mosquitoes to become infected, causing subsequent transmission to NHPs. Further investigation of their potential for zoonotic transmission of urban strains should be conducted on NHPs living near human settlements and vector studies. The spillover of sylvatic DENV strains, albeit uncommon, have been documented on several occasions in individuals who entered the forests. With persistent encroachment of human settlements into undeveloped forests, the increasing occurrence of spillover from the sylvatic cycle can endanger local populations active near the zones of emergence as well.
Bats are evolutionary successful creatures that are widely distributed globally. At least one species in each of the 19 families constituting the order Chiroptera is known to roost in buildings [88]. Myotis yumanensis, M. lucifugus, Tadarida brasiliensis [89,90] (one of the most abundant species in North America), Eptesicus fuscus [91] and Pteropus lylei [92] are just some examples of bats staying in close proximity with human population in both rural and urban settings [88]. Of more than a thousand species of bats in existence, there are at least 25 native species that roost in both natural and man-made habitats in Singapore [85,93]. They include Old world fruit bats (Pteropodidae), horseshoe bats (Rhinolophidae), false vampires (Megadermatidae), hollow-faced bats (Nycteridae), free-tailed bats (Molossidae) and evening bats (Vespertilionidae). Specifically, the lesser dog-faced fruit bat (Cynopterus brachyotis) and whiskered myotis (Myotis muricola) are reported to commonly roost in residential estates [94]. In a study involving Cynopterus brachyotis captured in Singapore, dengue exposure by D2 anti-NS1 antibodies was noted in 13.2% of bats [54].
Vicente-Santos et al. made an interesting conjecture on the infection of bats via oral ingestion of mosquitoes when they could not find evidence supporting bat infection through infected mosquito bite [51]. While evidence is still very limited [51], this could explain the conflicting observation of DENV presence in bats, which has been proven on several occasions [51,58], and the general lack of substantial findings from experimental inoculation studies [[95], [96], [97]], which cast doubts on their role as competent hosts/reservoirs. Apart from non-human primates, bats are the most extensively studied for presence of dengue infection, with the most studies conducted involving RT-PCR. Although the proportion of bats positive by RT-PCR was higher than that in non-human primates, it could be attributed to the greater number of bat studies than employed RT-PCR as compared to NHP studies. This study noted a lower proportion of bats positive for dengue by RNA detection as compared to serology based on both the inclusion of studies utilising more than one diagnostic platform (serology vs RT-PCR: 13.5% vs 5.8%), and the meta-analysis of studies based on one platform of detection (serology vs RT-PCR: 11% vs 7%). This indicate prior dengue infections in bats sampled instead of active ones. Of the six studies that conducted both serological testing and RT-PCR, three studies [46,47,51] yielded comparative results while the remaining three [50,52,55] did not detect any dengue positivity for comparison. Overall, the infection and amplification mechanism of DENV in bats warrants deeper investigation. The detection of DENV in bat flies by RT-PCR in a single study also highlights the importance of studying hematophagous bats and the interactions between bats and ectoparasitic organisms in infection. In view of the diverse range of bat species in existence compared to the few tested, species staying near/in the urban and rural areas may still play a role as reservoirs conserving dengue transmission.
While the study notes a high seroprevalence of DENV in pigs, the available literature were dated some time back and utilized HI predominately. Domestic pigs common in rural setting may act as one of the potential reservoir for dengue epizootic transmission resulting in increased spillover and epidemic in rural human population, similar to Japanese encephalitis virus (JEV) [98]. Pigs are also susceptible to Zika – a flavivirus similar to dengue – infection and can generate viremia upon experimental inoculation [99]. A relatively high DENV seroprevalence was also observed in marsupials, well-known to be primary reservoirs of Ross River virus, a mosquito-borne alphavirus [100]. No in-depth studies has been conducted to explore the potential of pigs and marsupials as DENV reservoirs hitherto. Dengue positivity was also observed in birds, dogs and rodents, which are animals commonly found in the urban setting. The high seroprevalence observed in birds ought to also be interpreted with caution given that detection was mainly done using HI and birds are known to be reservoirs of JEV [101]. Given the hypothesized feeding plasticity of Aedes albopictus, plausible DENV presence in the blood of these animals means that mosquitoes can be infected when biting them [102]. By extension, abundance of these animals in urban environments translates to heightened risk of dengue exposure in humans who become infected through mosquito bites as well. Furthermore, studies have also observed a wide feeding host range of the mosquitoes vectors that transmit dengue efficiently, namely Aedes albopictus [103] and Aedes aegypti [104].
5. Limitations
This systematic review is limited by a lack of homogeneity across assays employed in the respective studies. Inconsistency in assays used, differential laboratory setting and sample handling may have affected the results consolidated in our study as seen with the high heterogeneity in dengue prevalence results from our meta-analysis. In addition, usage of different RT-PCR and serological assays from different time periods, different testing strategies whereby some studies only tested specific DENV serotypes, or tested for viral genera were also contributing factors.
The capture of sufficient sample size and a viable specimen volume for each animal are implicated by time, environmental constraints, and size of the animal caught. This is not an infrequent observation in the available literature, as seen from one selected study [70] having to pool serum from 16 sparrows to constitute one bird sample. Insufficient sample volumes collected or a small overall sample size in certain studies hence, limit their analysis given that a larger sample size increases the significance level of the findings and produce more generalizable results. This subsequently affects the accuracy of our review in generalizing the trends across specific animal types.
Third, many of the reports lacked evidence of live DENV, which is best confirmed by virus isolation, the gold standard in dengue diagnosis. Only two studies [48,63] in the available literature attempted and successfully isolated virus from its RT-PCR positive samples. While positivity by serology or RT-PCR test constitute indirect evidence of dengue presence in animals, they do not confirm an active infection and/or circulation of the virus among animal population. The dearth of studies that have successfully isolated virus from the animal samples, or even attempted to do so demonstrates a lack in substantial evidence supporting the roles of animals as potential reservoirs in the urban dengue transmission cycle.
Furthermore, there have been limited studies exploring DENV infection and replication in bats and animals that are commonly found in the urban setting. Without strong evidence of sustained dengue virus replication in animals, it is uncertain if animals currently can or as they evolve over time may play a role as potential reservoirs or are simply incidental hosts in the dengue transmission cycle. While the inability to generate sufficient viremia, hence, adequate antibody titers could underlie any lack of genetic material or antibody detected, experimental inoculation/immunological studies are still required to investigate the mechanism of DENV infection and the viability of replication in different animal types to consider their true plausibility as reservoirs.
Lastly, cross-reaction with other flaviviruses should not be ruled out in the serological assays. Individuals infected with a flavivirus are well known to produce broadly reactive antibodies that are highly cross-reactive with other JEV serocomplex viruses [40,71]. The ELISA and HI assays do not differentiate between broadly reactive or serotype-specific antibodies, nor differentiate neutralizing antibodies like PRNT. Yet, most of the studies compiled by this systematic review had ascertained dengue positivity through serological tests and did not follow-up with diagnostic tests to exclude other viruses of the same genera. Taken together, the dengue seropositivity observed by our study may represent an inflation of the actual dengue presence in animals. Results should be interpreted with caution.
6. Conclusion
Dengue virus is capable of infecting a number of animal species, however, their role as amplifying reservoirs is uncertain due to various limitations in the evidence. It seems plausible that some animals in an urban settings may play an indirect role in dengue ecology, possibly even with low titers of viral load. However, this requires more investigation.
Author contributions
GSXW carried out the search, extraction of data and data analysis. PJ checked and analyzed the data and wrote the first manuscript draft. Both GSXW and PJ edited subsequent versions of the draft. GCG conceived of the study. SJAL and GCG reviewed drafts of the manuscript and suggested changes.
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by Ministry of Defence, Singapore [grant number N-608-000-065-001, 2017]. Pang J. was recipient of the Fulbright United States-ASEAN Visiting Scholar Award 2018 (IIE ID: PS00266705). The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.onehlt.2021.100216.
Appendix A. Supplementary data
Tables of dengue positivity among animals with their species information.
References
- 1.Brady O.J. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl. Trop. Dis. 2012;6 doi: 10.1371/journal.pntd.0001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatt S. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stanaway J.D. The global burden of dengue: an analysis from the global burden of disease study 2013. Lancet Infect. Dis. 2016;16:712–723. doi: 10.1016/S1473-3099(16)00026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shepard D.S., Undurraga E.A., Halasa Y.A., Stanaway J.D. The global economic burden of dengue: a systematic analysis. Lancet Infect. Dis. 2016;16:935–941. doi: 10.1016/S1473-3099(16)00146-8. [DOI] [PubMed] [Google Scholar]
- 5.OhAinle M. Dynamics of dengue disease severity determined by the interplay between viral genetics and serotype-specific immunity. Sci. Transl. Med. 2011;3:114ra128. doi: 10.1126/scitranslmed.3003084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rico-Hesse R. Dengue virus virulence and transmission determinants. Curr. Top. Microbiol. Immunol. 2010;338:45–55. doi: 10.1007/978-3-642-02215-9_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO . Second edition. World Health Organization; Geneva: 1997. Dengue Hemorrhagic Fever: Diagnosis, Treatment, Prevention and Control. [Google Scholar]
- 8.WHO/TDR . New edition. World Health Organization; Geneva: 2009. Dengue Guidelines for Diagnosis, Treatment, Prevention and Control. [PubMed] [Google Scholar]
- 9.Morrison A.C., Zielinski-Gutierrez E., Scott T.W., Rosenberg R. Defining challenges and proposing solutions for control of the virus vector Aedes aegypti. PLoS Med. 2008;5 doi: 10.1371/journal.pmed.0050068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lambrechts L. Assessing the epidemiological effect of wolbachia for dengue control. Lancet Infect. Dis. 2015;15:862–866. doi: 10.1016/S1473-3099(15)00091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Capeding M.R. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial. Lancet. 2014;384:1358–1365. doi: 10.1016/S0140-6736(14)61060-6. [DOI] [PubMed] [Google Scholar]
- 12.Wilder-Smith A. Deliberations of the Strategic Advisory Group of Experts on Immunization on the use of CYD-TDV dengue vaccine. Lancet Infect. Dis. 2018 doi: 10.1016/S1473-3099(18)30494-8. [DOI] [PubMed] [Google Scholar]
- 13.Hanley K.A. Fever versus fever: the role of host and vector susceptibility and interspecific competition in shaping the current and future distributions of the sylvatic cycles of dengue virus and yellow fever virus. Infect. Genet. Evol. 2013;19:292–311. doi: 10.1016/j.meegid.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valentine M.J., Murdock C.C., Kelly P.J. Sylvatic cycles of arboviruses in non-human primates. Parasit. Vectors. 2019;12:463. doi: 10.1186/s13071-019-3732-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rudnick A. Studies of the ecology of dengue in Malaysia. Bull. World Health Organ. 1966;35:78–79. [PMC free article] [PubMed] [Google Scholar]
- 16.Vasilakis N., Cardosa J., Hanley K.A., Holmes E.C., Weaver S.C. Fever from the forest: prospects for the continued emergence of sylvatic dengue virus and its impact on public health. Nat. Rev. Microbiol. 2011;9:532–541. doi: 10.1038/nrmicro2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weaver S.C., Barrett A.D. Transmission cycles, host range, evolution and emergence of arboviral disease. Nat. Rev. Microbiol. 2004;2:789–801. doi: 10.1038/nrmicro1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson S.J., Pearce J.M., Ramey A.M. Vectors, hosts, and control measures for Zika virus in the Americas. Ecohealth. 2017;14:821–839. doi: 10.1007/s10393-017-1277-2. [DOI] [PubMed] [Google Scholar]
- 19.Weaver S.C. Zika virus: history, emergence, biology, and prospects for control. Antivir. Res. 2016;130:69–80. doi: 10.1016/j.antiviral.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silva N.I.O. Recent sylvatic yellow fever virus transmission in Brazil: the news from an old disease. Virol. J. 2020;17:9. doi: 10.1186/s12985-019-1277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group, P Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolfe N.D. Sylvatic transmission of arboviruses among Bornean orangutans. Am. J. Trop. Med. Hyg. 2001;64:310–316. doi: 10.4269/ajtmh.2001.64.310. [DOI] [PubMed] [Google Scholar]
- 23.Kilbourn A.M. Health evaluation of free-ranging and semi-captive orangutans (Pongo pygmaeus pygmaeus) in Sabah, Malaysia. J. Wildl. Dis. 2003;39:73–83. doi: 10.7589/0090-3558-39.1.73. [DOI] [PubMed] [Google Scholar]
- 24.Diallo M. Amplification of the sylvatic cycle of dengue virus type 2, Senegal, 1999-2000: entomologic findings and epidemiologic considerations. Emerg. Infect. Dis. 2003;9:362–367. doi: 10.3201/eid0903.020219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fagbami A.H., Monath T.P., Fabiyi A. Dengue virus infections in Nigeria: a survey for antibodies in monkeys and humans. Trans. R. Soc. Trop. Med. Hyg. 1977;71:60–65. doi: 10.1016/0035-9203(77)90210-3. [DOI] [PubMed] [Google Scholar]
- 26.Dolz G. Detection of antibodies against flavivirus over time in wild non-human primates from the lowlands of Costa Rica. PLoS One. 2019;14 doi: 10.1371/journal.pone.0219271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morales M.A. Detection of the mosquito-borne flaviviruses, West Nile, dengue, Saint Louis encephalitis, Ilheus, Bussuquara, and yellow fever in free-ranging black howlers (Alouatta caraya) of northeastern Argentina. PLoS Negl. Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inoue S. Distribution of three arbovirus antibodies among monkeys (Macaca fascicularis) in the Philippines. J. Med. Primatol. 2003;32:89–94. doi: 10.1034/j.1600-0684.2003.00015.x. [DOI] [PubMed] [Google Scholar]
- 29.Eastwood G., Sang R.C., Guerbois M., Taracha E.L.N., Weaver S.C. Enzootic circulation of chikungunya virus in East Africa: serological evidence in non-human Kenyan Primates. Am. J. Trop. Med. Hyg. 2017;97:1399–1404. doi: 10.4269/ajtmh.17-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kading R.C., Borland E.M., Cranfield M., Powers A.M. Prevalence of antibodies to alphaviruses and flaviviruses in free-ranging game animals and nonhuman primates in the greater Congo basin. J. Wildl. Dis. 2013;49:587–599. doi: 10.7589/2012-08-212. [DOI] [PubMed] [Google Scholar]
- 31.Zavala R. Prevalence of antibodies to dengue virus, hepadnavirus and rotavirus in non-human primates. Acta Cient. Venez. 2006;57:22–27. [Google Scholar]
- 32.Peiris J.S., Dittus W.P., Ratnayake C.B. Seroepidemiology of dengue and other arboviruses in a natural population of toque macaques (Macaca sinica) at Polonnaruwa, Sri Lanka. J. Med. Primatol. 1993;22:240–245. [PubMed] [Google Scholar]
- 33.de Silva A.M., Dittus W.P., Amerasinghe P.H., Amerasinghe F.P. Serologic evidence for an epizootic dengue virus infecting toque macaques (Macaca sinica) at Polonnaruwa, Sri Lanka. Am. J. Trop. Med. Hyg. 1999;60:300–306. doi: 10.4269/ajtmh.1999.60.300. [DOI] [PubMed] [Google Scholar]
- 34.de Oliveira-Filho E.F. Seroprevalence of selected flaviviruses in free-living and captive capuchin monkeys in the state of Pernambuco, Brazil. Transbound. Emerg. Dis. 2018;65:1094–1097. doi: 10.1111/tbed.12829. [DOI] [PubMed] [Google Scholar]
- 35.Rudnick A. Studies of the ecology of dengue in Malaysia: a preliminary report. J. Med. Entomol. 1965;2:203–208. doi: 10.1093/jmedent/2.2.203. [DOI] [PubMed] [Google Scholar]
- 36.Catenacci L.S. Surveillance of arboviruses in primates and sloths in the Atlantic Forest, Bahia, Brazil. Ecohealth. 2018;15:777–791. doi: 10.1007/s10393-018-1361-2. [DOI] [PubMed] [Google Scholar]
- 37.de Thoisy B., Dussart P., Kazanji M. Wild terrestrial rainforest mammals as potential reservoirs for flaviviruses (yellow fever, dengue 2 and St Louis encephalitis viruses) in French Guiana. Trans. R. Soc. Trop. Med. Hyg. 2004;98:409–412. doi: 10.1016/j.trstmh.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 38.Nakgoi K. Dengue, Japanese encephalitis and chikungunya virus antibody prevalence among captive monkey (Macaca nemestrina) colonies of northern Thailand. Am. J. Primatol. 2014;76:97–102. doi: 10.1002/ajp.22213. [DOI] [PubMed] [Google Scholar]
- 39.Moreira-Soto A. Limited evidence for infection of urban and peri-urban nonhuman primates with zika and chikungunya viruses in Brazil. mSphere. 2018;3 doi: 10.1128/mSphere.00523-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kato F. Natural infection of cynomolgus monkeys with dengue virus occurs in epidemic cycles in the Philippines. J. Gen. Virol. 2013;94:2202–2207. doi: 10.1099/vir.0.055343-0. [DOI] [PubMed] [Google Scholar]
- 41.Rosen L. Observations on the epidemilogy of dengue in Panama. Am. J. Hyg. 1958;68:45–58. doi: 10.1093/oxfordjournals.aje.a119948. [DOI] [PubMed] [Google Scholar]
- 42.Hemme R.R. Serological evidence of infection with endemic human pathogens among free-ranging old world monkeys in Puerto Rico. Am. J. Trop. Med. Hyg. 2016;94:1095–1099. doi: 10.4269/ajtmh.15-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuwono J., Suharyono W., Koiman I., Tsuchiya Y., Tagaya I. Seroepidemiological survey on dengue and Japanese encephalitis virus infections in Asian monkeys. Southeast Asian J. Trop. Med. Public Health. 1984;15:194–200. [PubMed] [Google Scholar]
- 44.de Thoisy B. Dengue infection in neotropical forest mammals. Vector Borne Zoonotic Dis. 2009;9:157–170. doi: 10.1089/vbz.2007.0280. [DOI] [PubMed] [Google Scholar]
- 45.Calderon A. Dengue virus in bats from cordoba and sucre, Colombia. Vector Borne Zoonotic Dis. 2019;19:747–751. doi: 10.1089/vbz.2018.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aguilar-Setien A. Dengue virus in Mexican bats. Epidemiol. Infect. 2008;136:1678–1683. doi: 10.1017/S0950268808000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang H., Yang X., Li G. Detection of dengue virus genome RNA in some kinds of animals caught from dengue fever endemic areas in Hainan Island with reverse transcription-polymerase chain reaction. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 1998;12:226–228. [PubMed] [Google Scholar]
- 48.Abundes-Gallegos J. Detection of dengue virus in bat flies (Diptera: Streblidae) of common vampire bats, desmodus rotundus, in progreso, hidalgo, Mexico. Vector Borne Zoonotic Dis. 2018;18:70–73. doi: 10.1089/vbz.2017.2163. [DOI] [PubMed] [Google Scholar]
- 49.Platt K.B. Detection of dengue virus neutralizing antibodies in bats from Costa Rica and Ecuador. J. Med. Entomol. 2000;37:965–967. doi: 10.1603/0022-2585-37.6.965. [DOI] [PubMed] [Google Scholar]
- 50.Bittar C. Lack of serological and molecular evidence of arbovirus infections in bats from Brazil. PLoS One. 2018;13 doi: 10.1371/journal.pone.0207010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vicente-Santos A. Neotropical bats that co-habit with humans function as dead-end hosts for dengue virus. PLoS Negl. Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cabrera-Romo S. No evidence of dengue virus infections in several species of bats captured in central and southern Mexico. Zoonoses Public Health. 2016;63:579–583. doi: 10.1111/zph.12276. [DOI] [PubMed] [Google Scholar]
- 53.O’Connor J.L., Rowan L.C., Lawrence J.J. Relationships between the flying fox (genus Pteropus) and arthropod-borne fevers of North Queensland. Nature. 1955;176:472. doi: 10.1038/176472a0. [DOI] [PubMed] [Google Scholar]
- 54.Irving A.T. Robust dengue virus infection in bat cells and limited innate immune responses coupled with positive serology from bats in IndoMalaya and Australasia. Cell. Mol. Life Sci. 2019 doi: 10.1007/s00018-019-03242-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Machain-Williams C. Serologic evidence of flavivirus infection in bats in the Yucatan peninsula of Mexico. J. Wildl. Dis. 2013;49:684–689. doi: 10.7589/2012-12-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaul H.N., Venkateshan C.N., Mishra A.C., Modi G.B., Ghosh S.N. Serological evidence of arbovirus activity in birds and small mammals in Japanese encephalitis affected areas of Bankura district, West Bengal. Indian J. Med. Res. 1976;64:1735–1739. [PubMed] [Google Scholar]
- 57.Stone D. Serological evidence of widespread exposure of Grenada fruit bats to chikungunya virus. Zoonoses Public Health. 2018;65:505–511. doi: 10.1111/zph.12460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sotomayor-Bonilla J. Dengue virus in bats from southeastern Mexico. Am. J. Trop. Med. Hyg. 2014;91:129–131. doi: 10.4269/ajtmh.13-0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sotomayor-Bonilla J. Survey of mosquito-borne flaviviruses in the Cuitzmala River basin, Mexico: do they circulate in rodents and bats? Trop. Med. Health. 2018;46:35. doi: 10.1186/s41182-018-0117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rowan L.C., O’Connor J.L. Relationship between some coastal fauna and arthropod-borne fevers of North Queensland. Nature. 1957;179:786–787. doi: 10.1038/179786b0. [DOI] [PubMed] [Google Scholar]
- 61.Price J.L. Serological evidence of infection of Tacaribe virus and arboviruses in Trinidadian bats. Am. J. Trop. Med. Hyg. 1978;27:162–167. doi: 10.4269/ajtmh.1978.27.162. [DOI] [PubMed] [Google Scholar]
- 62.Ramos B.A. Clinical and serological tests for arboviruses in free-living domestic pigeons (Columba livia) Mem. Inst. Oswaldo Cruz. 2017;112:532–536. doi: 10.1590/0074-02760170014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thongyuan S., Kittayapong P. First evidence of dengue infection in domestic dogs living in different ecological settings in Thailand. PLoS One. 2017;12 doi: 10.1371/journal.pone.0180013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cigarroa-Toledo N. Serologic evidence of flavivirus infections in peridomestic rodents in Merida, Mexico. J. Wildl. Dis. 2016;52:168–172. doi: 10.7589/2015-05-116. [DOI] [PubMed] [Google Scholar]
- 65.Kolman J.M., Minár J., Horák Serologic examination of birds from the area of southern Moravia for the presence of antibodies against arboviruses of the groups alfa, flavo, Bunyamwera supergroup, and the virus Yaba 1-Lednice 110. I. Domestic fowls. Zentralblatt fur Bakteriologie, Parasitenkunde, Infektionskrankheiten und Hygiene. Erste Abteilung Originale. Reihe A: Medizinische Mikrobiologie und Parasitologie. 1975;233:279–287. [PubMed] [Google Scholar]
- 66.Kolman J.M., Folk C., Hudec K., Reddy G.N. Serologic examination of birds from the area of southern Moravia for the presence of antibodies against arboviruses of the groups Alfa, Flavo, Uukuniemi, Turlock and Bunyamwera supergroup. II. Wild living birds. Folia Parasitol. 1976;23:251–255. [PubMed] [Google Scholar]
- 67.Ghosh S.N., Rajagopalan P.K., Singh G.K., Bhat H.R. Serological evidence of arbovirus activity in birds of KFD epizootic--epidemic area, Shimoga District, Karnataka, India. Indian J. Med. Res. 1975;63:1327–1334. [PubMed] [Google Scholar]
- 68.Beck C. Serological evidence of infection with dengue and Zika viruses in horses on French Pacific Islands. PLoS Negl. Trop. Dis. 2019;13 doi: 10.1371/journal.pntd.0007162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kalimuddin M.D., Narayan K.G., Choudhary S.P. Serological evidence of Japanese encephalitis virus activity in Bihar. Int. J. Zoonoses. 1982;9:39–44. [PubMed] [Google Scholar]
- 70.Loach T.R., Narayan K.G., Choudhary S.P. Serological evidence of persistence of Japanese encephalitis virus activity in Bihar, India. Int. J. Zoonoses. 1983;10:7–14. [PubMed] [Google Scholar]
- 71.Pauvolid-Correa A. Serological evidence of widespread circulation of West Nile virus and other flaviviruses in equines of the Pantanal, Brazil. PLoS Negl. Trop. Dis. 2014;8 doi: 10.1371/journal.pntd.0002706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mall M.P., Kumar A., Malik S.V. Sero-positivity of domestic animals against Japanese encephalitis in Bareilly area, U.P. J. Commun. Disord. 1995;27:242–246. [PubMed] [Google Scholar]
- 73.Darwish M.A., Ibrahim A.H. Survey for antibodies to arboviruses in Egyptian sera. I. West Nile virus antihemagglutinins in human and animal sera. J. Egypt Public Health Assoc. 1971;46:61–70. [PubMed] [Google Scholar]
- 74.Albanese M., Di Cuonzo G., Randazzo G., Srihongse S., Tringali G. Survey for arbovirus antibodies in domestic animals of western Sicily. Ann. Sclavo. 1971;13:641–647. [PubMed] [Google Scholar]
- 75.Okia N.O. Arbovirus survey in wild birds in Uganda. East Afr. Med. J. 1971;48:725–731. [PubMed] [Google Scholar]
- 76.Doherty R.L., Carley J.G., Gorman B.M. Studies of arthropod-borne virus infections in Queensland. Iv. Further serological investigations of antibodies to group B arboviruses in man and animals. Aust. J. Exp. Biol. Med. Sci. 1964;42:149–164. doi: 10.1038/icb.1964.16. [DOI] [PubMed] [Google Scholar]
- 77.Young K.I. Abundance and distribution of sylvatic dengue virus vectors in three different land cover types in Sarawak, Malaysian Borneo. Parasit. Vectors. 2017;10:406. doi: 10.1186/s13071-017-2341-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McLean R.G. West Nile virus: emerging threat to public health and animal health. J. Vet. Med. Educ. 2003;30:143–144. doi: 10.3138/jvme.30.2.143. [DOI] [PubMed] [Google Scholar]
- 79.Marra P.P., Griffing S.M., McLean R.G. West Nile virus and wildlife health. Emerg. Infect. Dis. 2003;9:898–899. doi: 10.3201/eid0907.030277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mansfield K.L., Hernandez-Triana L.M., Banyard A.C., Fooks A.R., Johnson N. Japanese encephalitis virus infection, diagnosis and control in domestic animals. Vet. Microbiol. 2017;201:85–92. doi: 10.1016/j.vetmic.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 81.Sariol C.A., White L.J. Utility, limitations, and future of non-human primates for dengue research and vaccine development. Front. Immunol. 2014;5:452. doi: 10.3389/fimmu.2014.00452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Althouse B.M. Viral kinetics of primary dengue virus infection in non-human primates: a systematic review and individual pooled analysis. Virology. 2014;452-453:237–246. doi: 10.1016/j.virol.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Estrada A. Impending extinction crisis of the world’s primates: why primates matter. Sci. Adv. 2017;3 doi: 10.1126/sciadv.1600946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hanley K.A. Infection dynamics of sylvatic dengue virus in a natural primate host, the African green monkey. Am. J. Trop. Med. Hyg. 2014;91:672–676. doi: 10.4269/ajtmh.13-0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Parks N. List of Mammal Species Present in Singapore. 2020. https://www.nparks.gov.sg/biodiversity/wildlife-in-singapore/species-list/mammal>
- 86.Parks N. My Green Space. Monkey Musings: Respecting Singapore's Long-tailed Macaque. 2011. https://www.nparks.gov.sg/mygreenspace/issue-08-vol-1-2011/conservation/monkey-musings-respecting-singapore-s-long-tailed-macaque>
- 87.Bittel J. 2018. Four Countries Are Home to Two-Thirds of the Planet’s Primates—And Most of those Are Endangered. [Google Scholar]
- 88.Voigt C.C., Kingston T. Springer International Publishing; 2015. Bats in the Anthropocene: Conservation of Bats in a Changing World. [Google Scholar]
- 89.Krauel J.J., LeBuhn G. Patterns of bat distribution and foraging activity in a highly urbanized temperate environment. PLoS One. 2016;11 doi: 10.1371/journal.pone.0168927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hammerson G.A., Kling M., Harkness M., Ormes M., YOung B.E. Strong geographic and temporal patterns in conservation status of north American bats. Biol. Conserv. 2017;212:144–152. [Google Scholar]
- 91.Duchamp J.E., Sparks D.W., Whitaker J.O.J. Foraging-habitat selection by bats at an urban–rural interface: comparison between a successful and a less successful species. Can. J. Zool. 2004;82:1157–1164. [Google Scholar]
- 92.Choden K. Pteropus lylei primarily forages in residential areas in Kandal, Cambodia. Ecol. Evol. 2019;9:4181–4191. doi: 10.1002/ece3.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Parks N. Bats. 2019. https://www.nparks.gov.sg/gardens-parks-and-nature/dos-and-donts/animal-advisories/bats>
- 94.Tian Ang Tian. Bats Flying into Homes a Common Phenomenon in Singapore. 2017. https://www.tnp.sg/news/singapore/bats-flying-homes-common-phenomenon-singapore>
- 95.Cabrera-Romo S. Experimental inoculation of Artibeus jamaicensis bats with dengue virus serotypes 1 or 4 showed no evidence of sustained replication. Am. J. Trop. Med. Hyg. 2014;91:1227–1234. doi: 10.4269/ajtmh.14-0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Reagan R., Brueckner A.L. Studies of dengue fever virus in the cave bat (Myotus lucifugus) J. Infect. Dis. 1952;91:145–146. doi: 10.1093/infdis/91.2.145. [DOI] [PubMed] [Google Scholar]
- 97.Perea-Martinez L. Experimental infection of Artibeus intermedius bats with serotype-2 dengue virus. Comp. Immunol. Microbiol. Infect. Dis. 2013;36:193–198. doi: 10.1016/j.cimid.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 98.Scherer W.F., Buescher E.L., Flemings M.B., Noguchi A., Scanlon J. Ecologic studies of Japanese encephalitis virus in Japan. III. Mosquito factors. Zootropism and vertical flight of Culex tritaeniorhynchus with observations on variations in collections from animal-baited traps in different habitats. Am. J. Trop. Med. Hyg. 1959;8:665–677. [PubMed] [Google Scholar]
- 99.Darbellay J. Neonatal pigs are susceptible to experimental Zika virus infection. Emerg. Microbes. Infect. 2017;6 doi: 10.1038/emi.2016.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Flies E.J., Lau C.L., Carver S., Weinstein P. Another emerging mosquito-borne disease? endemic ross river virus transmission in the absence of marsupial reservoirs. BioScience. 2018;68:288–293. doi: 10.1093/biosci/biy011. [DOI] [Google Scholar]
- 101.Filgueira L., Lannes N. Review of emerging Japanese encephalitis virus: new aspects and concepts about entry into the brain and inter-cellular spreading. Pathogens. 2019;8:111. doi: 10.3390/pathogens8030111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Goodman H., Egizi A., Fonseca D.M., Leisnham P.T., LaDeau S.L. Primary blood-hosts of mosquitoes are influenced by social and ecological conditions in a complex urban landscape. Parasit. Vectors. 2018;11:218. doi: 10.1186/s13071-018-2779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kek R. Feeding host range of Aedes albopictus (Diptera: Culicidae) demonstrates its opportunistic host-seeking behavior in rural Singapore. J. Med. Entomol. 2014;51:880–884. doi: 10.1603/me13213. [DOI] [PubMed] [Google Scholar]
- 104.Barrera R. Vertebrate hosts of Aedes aegypti and Aedes mediovittatus (Diptera: Culicidae) in rural Puerto Rico. J. Med. Entomol. 2012;49:917–921. doi: 10.1603/me12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables of dengue positivity among animals with their species information.
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).