Introduction
Hidradenitis suppurativa (HS) is a chronic, inflammatory disorder characterized by recurrent, painful, suppurative or inflammatory lesions in intertriginous sites. Many pharmacologic treatments provide improvement of lesions but do not completely prevent the progression of the disease.1 Localized or wide surgical excision of the affected areas is often necessary for extensive or advanced disease; however, biologic agents provide a favorable non-surgical modality. We present a case of severe HS successfully treated with ixekizumab, an interleukin (IL)-17 inhibitor. Additionally, we report the unique clinical appearance of HS superinfected with herpes simplex virus (HSV)-1.
Case report
A woman in her 30's presented to the dermatology clinic for follow-up of Hurley stage 3 HS affecting her bilateral axillae, inframammary region, groin, thighs, and buttocks. Her condition had been refractory to many treatments including surgery, radiation, adalimumab, infliximab, acitretin, spironolactone, intralesional and oral corticosteroids, and oral and topical antibiotics. At the present visit, she reported multiple open sores in the buttock region resulting in significant pain with sitting; she described this as a different pain sensation than her routine HS lesions. She also stated that she had recently been hospitalized for hyponatremia in the setting of decreased oral intake.
Physical examination revealed large, hyperpigmented, indurated plaques with overlying large ulcerations in linear and serpiginous arrangements with undermined borders and with underlying sinus tract formation and tunneling (Fig 1). Given the appearance of punched-out ulcerations, bacterial and viral cultures were obtained from the buttock region. Bacterial culture grew methicillin-resistant Staphylococcus aureus, and immunofluorescent stain and culture were positive for HSV-1. Of note, the patient was prescribed oral trimethoprim-sulfamethoxazole twice daily several weeks prior to presentation; however, this was held during her recent hospitalization. She was advised to restart this medication following the positive bacterial culture results. Additionally, she was initiated on oral acyclovir 400 mg 3 times daily for 10 days. Mild improvement in the ulcerations was noted at close follow-up. She was treated with an additional week of oral acyclovir 400 mg 3 times daily and was continued on trimethoprim-sulfamethoxazole twice daily for treatment of her HS. Following the antiviral treatment, the ulcerations healed, and follow-up HSV polymerase chain reaction was negative. She continued to have severe baseline HS disease with the presence of subcutaneous nodules, sinus tracts, and drainage. Off-label use of ustekinumab was discussed with the patient and was initiated for 7 months without improvement. Off-label use of ixekizumab was discussed with the patient, and she was initiated on subcutaneous ixekizumab 160 mg once, followed by 80 mg at weeks 2, 4, 6, 8, 10, and 12, and then 80 mg every 4 weeks thereafter. Thirteen months after initiating ixekizumab, the patient demonstrated significant improvement in her condition. Physical examination demonstrated extensive scarring of the bilateral buttocks with improvement and even resolution of many of the hyperpigmented nodules, plaques, and sinus tracts, with significant improvement in drainage (Fig 2).
Fig 1.
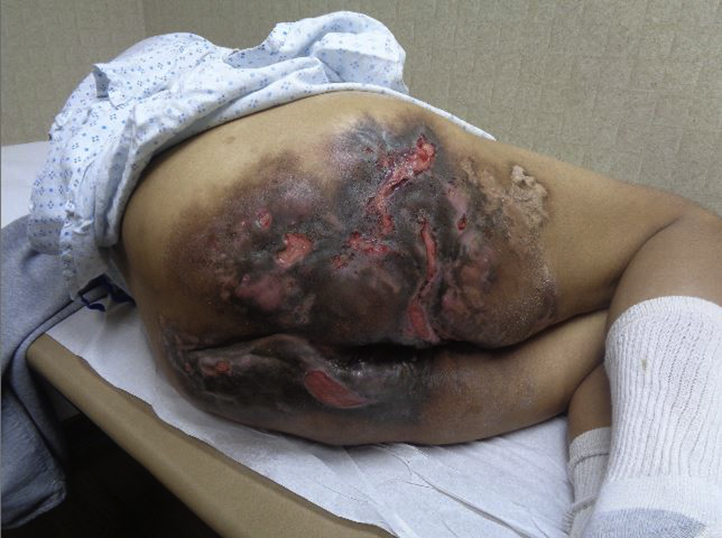
HS affecting the bilateral buttocks with concurrent HSV-1 and methicillin-resistant Staphylococcus aureus superinfection. HS, Hidradenitis suppurativa; HSV, herpes simplex virus.
Fig 2.
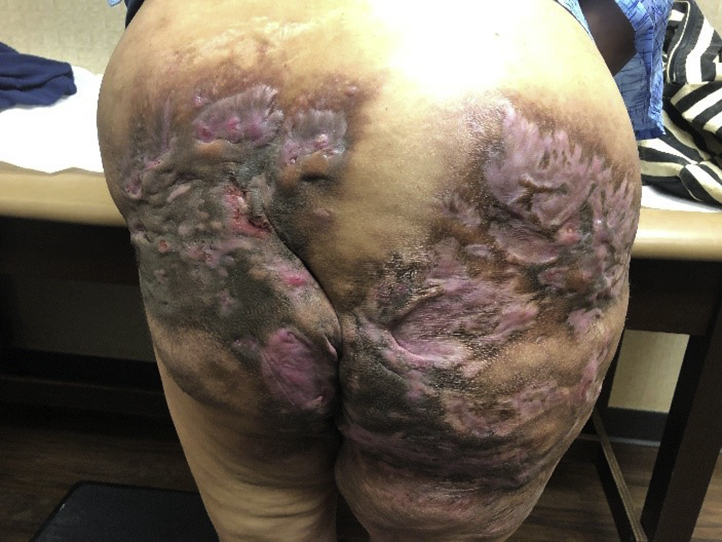
HS affecting the bilateral buttocks after 13 months of IL-17 inhibitor therapy. HS, Hidradenitis suppurativa; IL, interleukin.
Discussion
Our case serves to emphasize 2 important considerations in regard to severe HS: Lack of clinical response to traditional treatment regimens combined with the recognition of a punched-out morphology should prompt consideration of HSV. In our case, the patient's positive HSV culture, improvement on antiviral therapy, and negative follow-up HSV polymerase chain reaction suggest that the superinfection was clinically relevant. Furthermore, our case demonstrates that IL-17 inhibitors hold potential for the treatment of HS. After multiple treatment failures with antibiotics and various biologics, initiation of ixekizumab prompted a sustained improvement in her HS.
Matusiak et al found a statistically significant elevation of mean serum IL-17 levels in HS individuals compared with healthy matched controls.1 They also found statistically significant differences in IL-17 serum concentrations between groups according to the Hurley stage, with patients with the more severe disease having a markedly elevated serum level of IL-17. A literature review revealed isolated case reports of HS treated with ixekizumab with clinical improvement2,3 in addition to case reports with other IL-17 inhibitors.4 These findings lend support to the further evaluation of IL-17 inhibitors in patients with HS.
Treatment for HS is often unsatisfactory. Currently, the only Food and Drug Administration-approved medication to treat HS is adalimumab. Our case demonstrates that IL-17 inhibitors may represent another promising therapeutic modality. Additionally, in cases of HS with new ulcerations, concomitant HSV superinfection should be considered.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
References
- 1.Matusiak L., Szczech J., Bieniek A., Nowicka-Suszko D., Szepietowski J.C. Increased interleukin (IL)-17 serum levels in patients with hidradenitis suppurativa: implications for treatment with anti-IL-17 agents. J Am Acad Dermatol. 2017;76:670–675. doi: 10.1016/j.jaad.2016.10.042. [DOI] [PubMed] [Google Scholar]
- 2.Cotter C., Tobin A.M., O'Connor R., Gallagher C., Connolly M. Severe refractory hidradenitis suppurativa: treatment with ixekizumab, two case reports. Br J Dermatol. 2018;179:70. [Google Scholar]
- 3.Odorici G., Pellacani G., Conti A. Ixekizumab in hidradenitis suppurativa: a case report in a psoriatic patient. G Ital Dermatol Venereol. 2019 doi: 10.23736/S0392-0488.18.06135-7. [DOI] [PubMed] [Google Scholar]
- 4.Pandey K., Sekhri R., Sekhri V. Interleukin-17 inhibitor secukinumab in refractory hidradenitis suppurativa: a case report. J Am Acad Dermatol. 2018;79:AB156. [Google Scholar]


