Abstract
Hepatocellular carcinoma (HCC) is the most frequent malignancy of the liver, which is considered the fourth leading cause of cancer-related death in the United States. Liver transplant and surgical resection are curative treatments for HCC, but only 10-15% of HCC patients are eligible candidates. The FDA-approved sorafenib is a multi-kinase inhibitor systemic therapy for advanced HCC that extends the overall survival by over 3 months when compared with placebo. Adoptive transfer of Natural Killer (NK) cells holds great promise for clinical cancer treatment. However, only limited clinical benefit has been achieved in cancer patients. Therefore, there is currently considerable interest in development of the combination of sorafenib and NK cells for the treatment of HCC patients. However, the mechanism of how sorafenib affects the function of NK cells remains to be comprehensively clarified. In this paper, we will discuss NK cell-based immunotherapies that are currently under preclinical and clinical investigation and its potential combination with sorafenib for improving the survival of HCC patients.
Keywords: Natural killer cells, sorafenib, hepatocellular carcinoma, combination therapies
Introduction
Hepatocellular carcinoma (HCC) is the most frequent primary malignancy in the liver with a high mortality rate worldwide [1]. Chronic infection in the liver with hepatitis B or hepatitis C viruses remains the major risk of HCC. Alcoholic cirrhosis and nonalcoholic steatohepatitis with metabolic syndrome are considered other well-recognized factors that escalate the risk for the development of HCC [2-4]. HCC is a high therapy-resistant tumor that is most frequently diagnosed at advanced stages and thus difficult to treat. Although liver transplant and surgical resection are considered curative treatment options for HCC, it is generally only offered to patients without extrahepatic metastases [5]. Patients who do not qualify for major surgical therapeutic procedures can be treated by other minimally invasive procedures, including radiofrequency ablation, transarterial chemoembolization (TACE), microwave ablation, and irreversible electroporation [6]. However, these therapeutic approaches most often do not provide a complete cure as a high rate of recurrences has been reported [7]. Recently, sorafenib, an oral multi-target kinase inhibitor that can impair tumor proliferation and angiogenesis, has gained recognition in the clinical treatment development for advanced or metastatic HCC patients who have no viable therapeutic strategies [8,9]. Despite the fact that sorafenib can bring clinical benefits, the median survival rate for patients who have progressed into the terminal stage is less than 10% [10]. Therefore, novel therapies for HCC remain an urgent medical need.
Immunotherapy, including vaccines, immune-modulatory reagents, and adoptive transfer of immune cells, has been explored in HCC for decades and is considered a promising avenue, in light of the recent progress in the management of other malignancies [11]. The adoptively transferring of Natural killer (NK) cells immunotherapy is a potent and well tolerated treatment for a broad range of malignancies [12]. NK cells have significant advantages for cancer therapy, since they do not depend on antigen presentation and spontaneously kill cancer cells, and they are key effectors in cancer immunosurveillance [13,14]. Recently, adoptive transfer of NK cells has been investigated for tumor immunotherapy in patients and was demonstrated effective anti-tumor effects without any significant adverse effects (AEs) [15]. Furthermore, one of the human NK cell lines, NK-92 cell, has been tested in clinical studies for cancer therapy and is considered safe [16]. However, only limited clinical benefits have been observed thus far using adoptive transfer NK cell immunotherapies for the treatment of cancers including HCC.
The focus of the present article is on the combination of sorafenib with NK cell-based immunotherapy to treat HCC, based on the hypothesis that the combination will augment the therapeutic efficacy. We summarize the preclinical studies and the results of clinical trials, discuss the underlying mechanisms involved in the antitumor effects of NK cells and sorafenib as well as their functional interaction, which will provide a theoretic basis for the development of a combined treatment strategy for HCC.
Sorafenib
Sorafenib (NEXAVAR®), the first FDA approved agent for the systemic treatment of HCC, is a multi-targeted tyrosine kinase inhibitor (TKI) that impairs angiogenesis, cancer apoptosis, and proliferation by blocking the activity of vascular endothelial growth factor receptor (VEGFR)-1, VEGFR-2, VEGFR-3, platelet-derived growth factor receptor β (PDGFRβ), tyrosine-protein kinase (KIT), fibrosarcoma (Raf) kinases, FLT3, Ret, and fibroblast growth factor receptors (FGFR) (Figure 1) [9,17]. According to two international randomized controlled trials, sorafenib brings an obvious survival benefit to patients with HCC [8,18]. However, sorafenib is related to multiple AEs, including hand-foot skin reaction, cardiovascular events, gastrointestinal disturbances, renal toxicity, and fatigue, which can potentially cause treatment discontinuation [8,18,19]. Recently, the development of resistance to sorafenib has also raised concerns due to the high heterogeneity of HCC, which can result in different sensitivity to the treatment among patients [20,21]. Therefore, in recent years it has been suggested that sorafenib combining with other molecular targeted drugs could overcome such limitations by expanding the HCC treatment efficacy.
Figure 1.
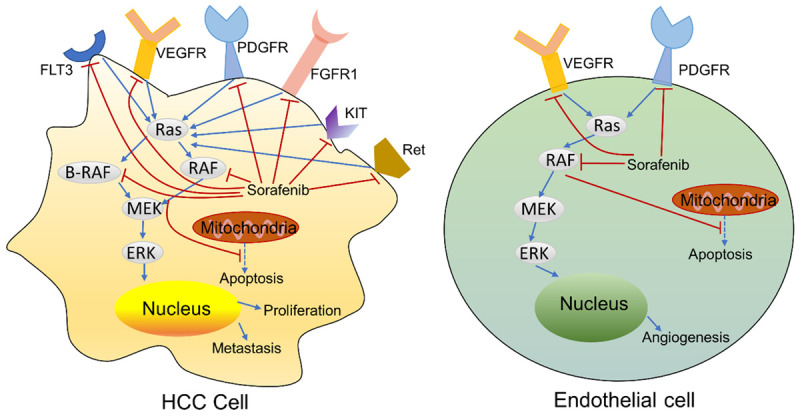
The mechanism of action of Sorafenib: tumor proliferation and angiogenesis.
Current status of sorafenib based combination therapy
The initiation and progression of HCC is a multi-step and multi-factor process, which suggests that a combination of agents that target multiple critical pathways or key molecules implicated in the hepatocarcinogenesis may achieve considerable improvements in the management of this resilient tumor. Sorafenib has been co-administrated with antiangiogenic drugs and inhibitors or agents targeting MEK/ERK pathway, PI3K/AKT/mTOR signal pathway, histone deacetylase, EGF/EGFR pathway, and HGF/c-Met pathway [22]. Combination of sorafenib with other agents, such as doxorubicin [23-25], selumetinib [26] interferon [27], capecitabine [28], tegafur-uracil [29], modified FOLFOX (5-fluorouracil (5-FU), leucovorin, and oxaliplatin) [30], gemcitabine and oxaliplatin [31,32], and gemcitabine lonely [33] have also been evaluated. However, until now, no combination treatments involving sorafenib did go through phase III trials.
TACE is currently considered as the standard of care for patients with intermediate-stage HCC according to international guidelines [34]. Over the past decade, numerous studies have tried to combination of TACE and sorafenib for patients with unresectable HCC, while the results of previous trials have been inconclusive [35-57]. Some clinical trials have shown that the combination of sorafenib and TACE brings encouraging efficacy and survival benefits for HCC patients [35,38,40-45,47-50,52-57]. Contrasting with these findings, other studies showed that sorafenib did not significantly extend overall survival in patients who have responses to TACE [37,46,51]. Although selective internal radiation therapy (SIRT) shows efficacy in unresectable HCC, the combination of SIRT and sorafenib did not achieve an improved response in overall survival when compared with sorafenib monotherapy [58].
In recent years, immunotherapy for the treatment of several types of cancer malignancies has advanced rapidly and has shown great promise especially when combined with traditional therapies. The combination of immunotherapy and sorafenib is a very promising therapeutic approach for the HCC. A recent trial reported that the cell-based immune primer ilixadencel in combination with sorafenib can induce antitumor specific immunological responses in patients with advanced HCC [59]. Moreover, in a clinical study, the combination of sorafenib with the treatment of dendritic cells and cytokine-induced killers improved the tumor response rate and prolonged overall survival of advanced HCC patients without increasing the incidence of AEs [60]. Furthermore, the combination of sorafenib with other immunotherapies, including TLR3 agonists [61], anti-programmed death-ligand 1 monoclonal antibodies [62], dendritic cell therapy [63], chimeric antigen receptor (CAR) T-cell therapy [64], have been shown to induce a considerable reduction in tumor growth in preclinical HCC models.
The NK cells in HCC
NK cells, characterized in humans as CD3-CD56+ lymphocytes and in mice as CD3-NKp46+ or CD3-NK1.1+ cells, are major effectors of innate immunity in defense against pathogens and malignancies. NK cells can recognize and lyse viral infected cells or tumor cells based on the delivery of cytotoxic granules, the secretion of effector cytokines, and their expression of ligands, including inhibitory, activating, adhesion, and cytokine receptors. Additionally, NK cells can effectively kill tumor cells through antibody-dependent cell-mediated cytotoxicity (ADCC).
It has been shown that NK cells are enriched in the liver and play key roles in immune surveillance and in controlling the initiation and progression of HCC [65,66]. Importantly, the ligands of several activating NK receptors, including the major histocompatibility complex class I chain-related protein A and B (MICA/B), CD133, and CD155, are frequently up-regulated on HCC cells [67,68]. Moreover, a preclinical study observed that the NK cell frequency and function are disrupted during HCC onset and progression in a mouse model [69]. Meanwhile, the liver tumor area showed a lower frequency of NK cells when compared to the nontumor area, and NK cell function with regard to cytotoxic ability, including cytoplasmic granules secretion, TNF-α and IFN-γ production, was impaired in HCC patients [70,71]. Available evidence showed that the low frequency of NK cells in peripheral blood (PB) and liver is associated with the initiation and progression of HCC in humans [70,72,73]. Additionally, these patients showed disturbed distributions of NK subtypes, with a dramatic reduction in the CD56dimCD16+ NK cells (more mature and cytotoxic).
Targeting NK cell function in the HCC microenvironment
Among the activating NK receptor pathways, Fc RIII (also known as CD16) is one of the most potent receptors that can induce a strong enough activating signal to trigger cytokine production and degranulation via ADCC [74]. It has been reported that an improved outcome after targeting CD16 monoclonal antibody (mAb) treatment can be achieved in HCC patients who show a high affinity to FcRIII [75], which suggests that the CD16 can serve as a promising treatment target in HCC. A humanized antibody, codrituzumab, targets the glypican-3 (GPC-3) which is up-regulated in HCC cells but generally not in normal hepatocytes, is proven to interact with CD16/FcγRIIIa and trigger ADCC [76]. Importantly, a recent clinical trial showed that elevated CD16 expression on peripheral NK cells and GPC-3 expression on the tumors were correlated with prolonged overall survival and progression-free survival in HCC patients [77]. Therefore, these studies suggest the potential for CD16 and/or GPC-3 as targets to enhance NK cell function in HCC.
As an activating receptor, NKG2D has received increased attention since it mediates the cytotoxicity of NK cells via binding to its ligands such as MICA/MICB which are up-regulated in malignant cells but are generally lacking in the normal cells [78]. It has been reported that the levels of soluble MICA were increased in advanced HCC patients, which was correlated with down-regulated expression of NKG2D and disrupted activation of NK cells [79]. Accordingly, in a recent report, Easom et al. observed that NK cells derived from malignant liver tissue show a down-regulated expression of NKG2D compared with adjacent non-invasive tissue in HCC patients [80]. In contrast, a recent preclinical study showed that activating the receptor NKG2D resulted in tumor growth in a model of HCC. These mixed pieces of evidence suggest that the roles of NKG2D in the progression of HCC are highly complicated.
Suppressing inhibitory receptors may provide an alternative method to boost the function of NK cells. Recently, the monoclonal antibodies that target inhibitory receptors (i.e., immunoglobulin-like receptors (KIR) and NKG2A)) have been tested on multiple myeloma patients [81,82]. However, strategies for blocking inhibitory receptors to activate NK cells’ antitumor function need to be further evaluated in patients and mouse models of HCC. To date, several cytokine genes, including IL-12, IL-15, IL-2, IFN-α, and stem cell factors, have been applied to modify NK cell lines to enhance NK cell activity against tumor cells [83,84]. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is constitutively expressed on hepatic NK cells and plays a key role in the surveillance of tumor initiation, progression, and metastasis [85]. It has also shown that combined gene-based virotherapy involving TRAIL and IL-12 genes have obvious anti-HCC effects through upregulating the production of IFN-γ and infiltration of NK cells in the tumor microenvironment [86]. Recently, IL-15 has also been assessed in clinical studies as a potential pharmacological candidate for cancer therapy because of its critical role in the regulation of NK cell proliferation, survival, and cytotoxicity [87]. However, IL-15 can induce severe toxicity which is correlated with consequent IFN-γ secreted by NK cells [88]. Additionally, despite advances in NK cell expansion, the administration of IL-15 showed no sustained antitumor responses [89]. Figure 2 describes various strategies to augment the function of NK cells in the HCC microenvironment.
Figure 2.
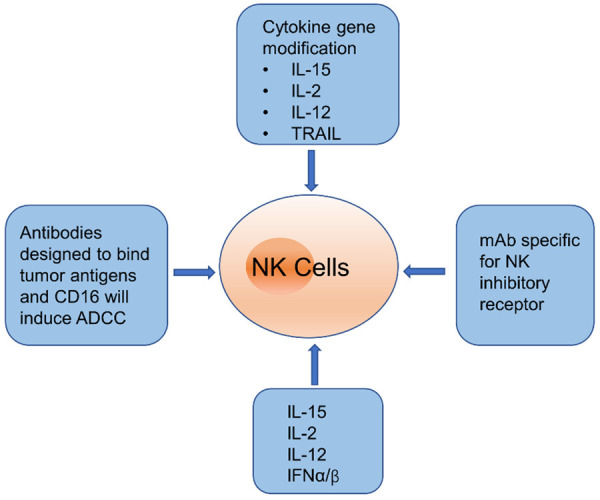
Strategies to enhance NK cell function in HCC.
NK cell adoptive immunotherapy for HCC
Adoptive transfer NK cell immunotherapy requires the NK ex vivo expansion, maximal in vivo activity, in vivo long-term persistence, and cytotoxic cells with high specificity. Currently, the source of NK cells can be derived from stem cells, PB NK cells of a healthy donor (allogeneic setting) or from the patient (autologous setting), and the NK cell lines such as the NK-92 cells. Allogeneic NK cells adoptive transfer immunotherapy for HCC have been demonstrated in two recent clinical trials [90,91]. However, it might cause serious graft-versus-host disease (GVHD) and can limit the clinical benefits due to the inadequate depletion of T cells in grafts. In this regard, autologous NK cell immunotherapy is safer than allogeneic settings with minimal side effects. A recent study observed that co-administration with autologous cytokine-induced killer cells increased overall survival and progression-free survival for patients with HCC [92]. In ClinicalTrials.gov, 6 trials for HCC were found (search with disease and condition: “Hepatocellular Carcinoma”; other term: “NK cell”; recruitment status: “Recruiting” or “Active, not recruiting”; search date 17th Sep 2020). The results are listed in Table 1.
Table 1.
On-going clinical trials on NK cell therapy for HCC
| Identifier | Phase | Status | Start Year | Title | Condition | Country | NK Cell Source |
|---|---|---|---|---|---|---|---|
| NCT04162158 | II | Recruiting | 2019 | Safety and Efficacy of Allogeneic NK Cells Therapy in Patients with Advanced Hepatocellular Carcinoma | Hepatocellular Carcinoma | China | Allogeneic PB |
| NCT04011033 | II | Recruiting | 2019 | Study of Adoptive Transfer of iNKT Cells Combined with TACE to Treat Advanced HCC | Hepatocellular Carcinoma | China | Invariant Natural Killer T |
| NCT03319459 | I | Active, not recruiting | 2017 | FATE-NK100 as Monotherapy and in Combination with Monoclonal Antibody in Subjects with Advanced Solid Tumors | Advanced Solid Tumor, including Hepatocellular Carcinoma | United States | Donor-derived NK cell |
| NCT03841110 | I | Recruiting | 2019 | FT500 as Monotherapy and in Combination with Immune Checkpoint Inhibitors in Subjects with Advanced Solid Tumors | Advanced Solid Tumors, including Hepatocellular Carcinoma | United States | Allogeneic, iPSC-derived NK cell |
| NCT03592706 | II/III | Recruiting | 2018 | Autologous Immune Killer Cells to Treat Liver Cancer Patients as an Adjunct Therapy | Hepatocellular Carcinoma | Taiwan | Autologous immune killer cells |
| Liver Cancer | |||||||
| NCT04106167 | Not applicable | Recruiting | 2019 | Long-term, Non-interventional, Observational Study Following Treatment with Fate Therapeutics FT500 Cellular Immunotherapy | Advanced Solid Tumors, including Hepatocellular Carcinoma | United States | Allogeneic, iPSC-derived NK cell |
The alternative option is NK cell lines that show strong antitumor activities and can be reproducibly and easily expanded and purified. The NK-92 cell, an IL-2 dependent human NK cell line, is comprised of 100% activated NK cells [93]. It is easier to expand and manipulate genetically with transfection efficiency being superior to that of PB NK cells. The NK-92 cells adoptive immunotherapy has been tested in patients with advanced malignancies and showed some antitumor responses in treatment-resistant lung cancer patients [94]. Additionally, genetically engineered NK-92 cells, GPC3-specific CAR-modified NK-92 cells were demonstrated to have significant antitumor effects against HCC both in vitro and in xenografts [95-97]. These preclinical studies suggest that CAR-engineered NK cells have the potential for further development as an investigational novel therapeutic approach for HCC patients.
The potential of combination sorafenib and NK cell for HCC
Although NK cells play a key role in the immune surveillance of HCC, the overall efficacy of NK cell-based therapeutic strategies alone is low. Researchers have recently focused on studying the immunological mechanism of sorafenib on NK cells to better inhibit HCC progression (Figure 3). It has been reported that the enhanced cytotoxic sensitivity of tumor cells to NK cells is associated with the up-regulated expression of NKG2D ligands after incubation with sorafenib [98]. Meanwhile, a previous study reported that administration of sorafenib stimulates the activation of tumor-associated macrophages and consequently induces the activation of NK cells by a cytokine- and NF-κB-dependent manner in mice liver [99]. This study also showed that NK cells activated by sorafenib-treated macrophages have up-regulated degranulation and IFN-γ secretion. On the other hand, it has been reported that expanded NK cells significantly enhanced the antitumor effects of sorafenib and that the cytotoxicity of NK cells has not been affected in the presence of sorafenib [100]. A recent study also reported an immunomodulatory mechanism of sorafenib by unleashing NK cell cytotoxicity against HCC tumors [101]. They indicated that sorafenib can down-regulate MHC class I expression of HCC cells, which may then induce tumor resistance to immune checkpoint therapies and increase sensitivity to NK cell responses. Moreover, Shi et al. demonstrated that androgen receptor (AR) can directly bind to the IL12A promoter region and subsequently down-regulate IL12A expression at the transcriptional level, which resulted in inhibited NK cell cytotoxicity against HCC, whereas sorafenib treatment can boost IL12A signals through suppressing AR signals [102]. Furthermore, our recent study observed that sorafenib can also affect the sub-populations and functions of peripheral CD56brightCD16- and CD56dimCD16+ NK cells, which were correlated with the treatment outcomes such as the overall survival of HCC patients [103]. Additionally, Lohmeyer et al. reported that sorafenib enhanced the cytotoxicity of NK cells in a time- and dose-dependent fashion via the RAS/RAF/ERK pathway [104], whereas Li et al. suggested that sorafenib decreased the function of NK cells via suppressing ERK1/2 [105]. Therefore, there is a need to further investigate the comprehensive functional interactions between sorafenib and NK cells.
Figure 3.
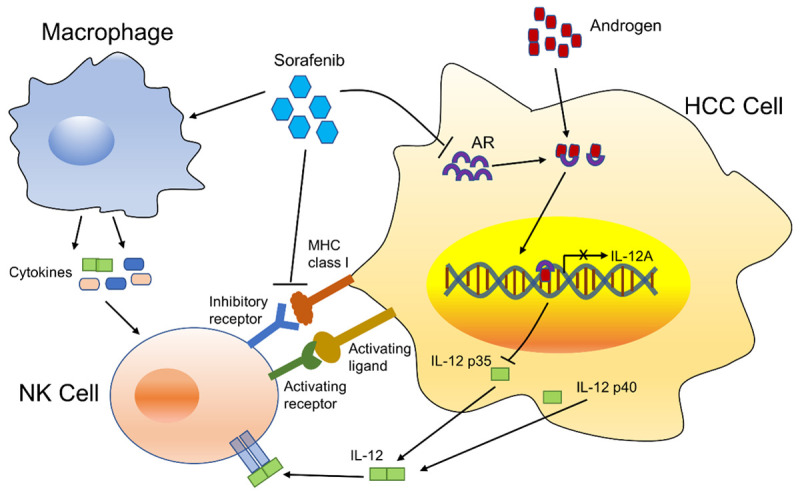
Hypothetic modes of the effects of sorafenib on NK cell activation against HCC cells.
Conclusion
There is increasing evidence that sorafenib can regulate the function of immune cells, particularly NK cells. In addition, it has been shown that the combination of NK immunotherapy with sorafenib can be developed as a promising and effective therapeutic approach for the treatment of HCC. However, the key pathways involved in the regulation of NK cell distribution and function in HCC by sorafenib have not been fully illustrated. Therefore, a comprehensive understanding of the mechanism is necessary in order to optimize the combination of sorafenib and NK cell-based immunotherapy for the treatment of HCC.
Acknowledgements
This study was supported by the National Cancer Institute (grants R01CA209886, R01CA241532); 2019 Harold E. Eisenberg Foundation Scholar Award; and SIR Foundation Pilot Grant (PR-0000000012).
Disclosure of conflict of interest
None.
References
- 1.Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, Gores G. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. doi: 10.1038/nrdp.2016.18. [DOI] [PubMed] [Google Scholar]
- 2.Liu J, Yang HI, Lee MH, Lu SN, Jen CL, Batrla-Utermann R, Wang LY, You SL, Hsiao CK, Chen PJ, Chen CJ. Spontaneous seroclearance of hepatitis B seromarkers and subsequent risk of hepatocellular carcinoma. Gut. 2014;63:1648–1657. doi: 10.1136/gutjnl-2013-305785. [DOI] [PubMed] [Google Scholar]
- 3.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 4.Morgan TR, Mandayam S, Jamal MM. Alcohol and hepatocellular carcinoma. Gastroenterology. 2004;127:S87–96. doi: 10.1053/j.gastro.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 5.Song TJ, Wai Kit Ip E, Fong Y. Hepatocellular carcinoma: current surgical management. Gastroenterology. 2004;127:S248–S260. doi: 10.1053/j.gastro.2004.09.039. [DOI] [PubMed] [Google Scholar]
- 6.Llovet JM. Updated treatment approach to hepatocellular carcinoma. J Gastroenterol. 2005;40:225–235. doi: 10.1007/s00535-005-1566-3. [DOI] [PubMed] [Google Scholar]
- 7.Lai EC, Tang CN, Yang GP, Li MK. Minimally invasive surgical treatment of hepatocellular carcinoma: long-term outcome. World J Surg Oncol. 2009;33:2150–2154. doi: 10.1007/s00268-009-0155-7. [DOI] [PubMed] [Google Scholar]
- 8.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 9.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, Cao Y, Shujath J, Gawlak S, Eveleigh D, Rowley B, Liu L, Adnane L, Lynch M, Auclair D, Taylor I, Gedrich R, Voznesensky A, Riedl B, Post LE, Bollag G, Trail PA. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 10.El-Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008;134:1752–1763. doi: 10.1053/j.gastro.2008.02.090. [DOI] [PubMed] [Google Scholar]
- 11.Greten TF, Manns MP, Korangy F. Immunotherapy of HCC. Rev Recent Clin Trials. 2008;3:31–39. doi: 10.2174/157488708783330549. [DOI] [PubMed] [Google Scholar]
- 12.Hu W, Wang G, Huang D, Sui M, Xu Y. Cancer immunotherapy based on natural killer cells: current progress and new opportunities. Front Immunol. 2019;10:1205. doi: 10.3389/fimmu.2019.01205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiessling R, Klein E, Wigzell H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975;5:112–117. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- 14.Herberman RB, Nunn ME, Holden HT, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int J Cancer. 1975;16:230–239. doi: 10.1002/ijc.2910160205. [DOI] [PubMed] [Google Scholar]
- 15.Cheng M, Chen Y, Xiao W, Sun R, Tian Z. NK cell-based immunotherapy for malignant diseases. Cell Mol Immunol. 2013;10:230–252. doi: 10.1038/cmi.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tonn T, Becker S, Esser R, Schwabe D, Seifried E. Cellular immunotherapy of malignancies using the clonal natural killer cell line NK-92. J Hematother Stem Cell Res. 2001;10:535–544. doi: 10.1089/15258160152509145. [DOI] [PubMed] [Google Scholar]
- 17.Ibrahim N, Yu Y, Walsh WR, Yang JL. Molecular targeted therapies for cancer: sorafenib monotherapy and its combination with other therapies (Review) Oncol Rep. 2012;27:1303–1311. doi: 10.3892/or.2012.1675. [DOI] [PubMed] [Google Scholar]
- 18.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 19.Tovoli F, Ielasi L, Casadei-Gardini A, Granito A, Foschi FG, Rovesti G, Negrini G, Orsi G, Renzulli M, Piscaglia F. Management of adverse events with tailored sorafenib dosing prolongs survival of hepatocellular carcinoma patients. J Hepatol. 2019;71:1175–1183. doi: 10.1016/j.jhep.2019.08.015. [DOI] [PubMed] [Google Scholar]
- 20.Chen J, Jin R, Zhao J, Liu J, Ying H, Yan H, Zhou S, Liang Y, Huang D, Liang X, Yu H, Lin H, Cai X. Potential molecular, cellular and microenvironmental mechanism of sorafenib resistance in hepatocellular carcinoma. Cancer Lett. 2015;367:1–11. doi: 10.1016/j.canlet.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 21.Chen W, Yang J, Zhang Y, Cai H, Chen X, Sun D. Regorafenib reverses HGF-induced sorafenib resistance by inhibiting epithelial-mesenchymal transition in hepatocellular carcinoma. FEBS Open Bio. 2019;9:335–347. doi: 10.1002/2211-5463.12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao JJ, Shi ZY, Xia JF, Inagaki Y, Tang W. Sorafenib-based combined molecule targeting in treatment of hepatocellular carcinoma. World J Gastroenterol. 2015;21:12059–12070. doi: 10.3748/wjg.v21.i42.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richly H, Henning BF, Kupsch P, Passarge K, Grubert M, Hilger RA, Christensen O, Brendel E, Schwartz B, Ludwig M, Flashar C, Voigtmann R, Scheulen ME, Seeber S, Strumberg D. Results of a Phase I trial of sorafenib (BAY 43-9006) in combination with doxorubicin in patients with refractory solid tumors. Ann Oncol. 2006;17:866–873. doi: 10.1093/annonc/mdl017. [DOI] [PubMed] [Google Scholar]
- 24.Abou-Alfa GK, Johnson P, Knox JJ, Capanu M, Davidenko I, Lacava J, Leung T, Gansukh B, Saltz LB. Doxorubicin plus sorafenib vs. doxorubicin alone in patients with advanced hepatocellular carcinoma: a randomized trial. JAMA. 2010;304:2154–2160. doi: 10.1001/jama.2010.1672. [DOI] [PubMed] [Google Scholar]
- 25.Abou-Alfa GK, Shi Q, Knox JJ, Kaubisch A, Niedzwiecki D, Posey J, Tan BR Jr, Kavan P, Goel R, Lammers PE, Bekaii-Saab TS, Tam VC, Rajdev L, Kelley RK, El Dika I, Zemla T, Potaracke RI, Balletti J, El-Khoueiry AB, Harding JJ, Suga JM, Schwartz LH, Goldberg RM, Bertagnolli MM, Meyerhardt J, O’Reilly EM, Venook AP. Assessment of treatment with sorafenib plus doxorubicin vs. sorafenib alone in patients with advanced hepatocellular carcinoma: phase 3 calgb 80802 randomized clinical trial. JAMA Oncol. 2019;5:1582–1588. doi: 10.1001/jamaoncol.2019.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tai WM, Yong WP, Lim C, Low LS, Tham CK, Koh TS, Ng QS, Wang WW, Wang LZ, Hartano S, Thng CH, Huynh H, Lim KT, Toh HC, Goh BC, Choo SP. A phase Ib study of selumetinib (AZD6244, ARRY-142886) in combination with sorafenib in advanced hepatocellular carcinoma (HCC) Ann Oncol. 2016;27:2210–2215. doi: 10.1093/annonc/mdw415. [DOI] [PubMed] [Google Scholar]
- 27.Itokawa N, Atsukawa M, Tsubota A, Okubo T, Arai T, Nakagawa A, Kondo C, Iwakiri K. Effects of sorafenib combined with low-dose interferon therapy for advanced hepatocellular carcinoma: a pilot study. Int J Clin Oncol. 2016;21:676–683. doi: 10.1007/s10147-015-0942-0. [DOI] [PubMed] [Google Scholar]
- 28.Patt Y, Rojas-Hernandez C, Fekrazad HM, Bansal P, Lee FC. Phase II trial of sorafenib in combination with capecitabine in patients with hepatocellular carcinoma: INST 08-20. Oncologist. 2017;22:1158–e1116. doi: 10.1634/theoncologist.2017-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Azim HA, Omar A, Atef H, Zawahry H, Shaker MK, Abdelmaksoud AK, EzzElarab M, Abdel-Rahman O, Ismail M, Kassem L, Waked I. Sorafenib plus tegafur-uracil (UFT) versus sorafenib as first line systemic treatment for patients with advanced stage HCC: a phase II trial (ESLC01 study) J Hepatocell Carcinoma. 2018;5:109–119. doi: 10.2147/JHC.S169285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goyal L, Zheng H, Abrams TA, Miksad R, Bullock AJ, Allen JN, Yurgelun MB, Clark JW, Kambadakone A, Muzikansky A, Knowles M, Galway A, Afflitto AJ, Dinicola CF, Regan E, Hato T, Mamessier E, Shigeta K, Jain RK, Duda DG, Zhu AX. A phase II and biomarker study of sorafenib combined with modified FOLFOX in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2019;25:80–89. doi: 10.1158/1078-0432.CCR-18-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Assenat E, Pageaux GP, Thézenas S, Peron JM, Bécouarn Y, Seitz JF, Merle P, Blanc JF, Bouché O, Ramdani M, Poujol S, de Forges H, Ychou M, Boige V. Sorafenib alone vs. sorafenib plus GEMOX as 1(st)-line treatment for advanced HCC: the phase II randomised PRODIGE 10 trial. Br J Cancer. 2019;120:896–902. doi: 10.1038/s41416-019-0443-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y, Yue H, Xu S, Wang F, Ma N, Li K, Qiao L, Wang J. First-line gemcitabine and oxaliplatin (GEMOX) plus sorafenib, followed by sorafenib as maintenance therapy, for patients with advanced hepatocellular carcinoma: a preliminary study. Int J Clin Oncol. 2015;20:952–959. doi: 10.1007/s10147-015-0796-5. [DOI] [PubMed] [Google Scholar]
- 33.Srimuninnimit V, Sriuranpong V, Suwanvecho S. Efficacy and safety of sorafenib in combination with gemcitabine in patients with advanced hepatocellular carcinoma: a multicenter, open-label, single-arm phase II study. Asia Pac J Clin Oncol. 2014;10:255–260. doi: 10.1111/ajco.12191. [DOI] [PubMed] [Google Scholar]
- 34.European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Hoffmann K, Glimm H, Radeleff B, Richter G, Heining C, Schenkel I, Zahlten-Hinguranage A, Schirrmacher P, Schmidt J, Büchler MW, Jaeger D, von Kalle C, Schemmer P. Prospective, randomized, double-blind, multi-center, phase III clinical study on transarterial chemoembolization (TACE) combined with Sorafenib®versus TACE plus placebo in patients with hepatocellular cancer before liver transplantation-HeiLivCa [ISRCTN24081794] . BMC Cancer. 2008;8:349. doi: 10.1186/1471-2407-8-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dufour JF, Hoppe H, Heim MH, Helbling B, Maurhofer O, Szucs-Farkas Z, Kickuth R, Borner M, Candinas D, Saar B. Continuous administration of sorafenib in combination with transarterial chemoembolization in patients with hepatocellular carcinoma: results of a phase I study. Oncologist. 2010;15:1198–1204. doi: 10.1634/theoncologist.2010-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kudo M, Imanaka K, Chida N, Nakachi K, Tak WY, Takayama T, Yoon JH, Hori T, Kumada H, Hayashi N, Kaneko S, Tsubouchi H, Suh DJ, Furuse J, Okusaka T, Tanaka K, Matsui O, Wada M, Yamaguchi I, Ohya T, Meinhardt G, Okita K. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer. 2011;47:2117–2127. doi: 10.1016/j.ejca.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 38.Park JW, Koh YH, Kim HB, Kim HY, An S, Choi JI, Woo SM, Nam BH. Phase II study of concurrent transarterial chemoembolization and sorafenib in patients with unresectable hepatocellular carcinoma. J Hepatol. 2012;56:1336–1342. doi: 10.1016/j.jhep.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 39.Qu XD, Chen CS, Wang JH, Yan ZP, Chen JM, Gong GQ, Liu QX, Luo JJ, Liu LX, Liu R, Qian S. The efficacy of TACE combined sorafenib in advanced stages hepatocellullar carcinoma. BMC Cancer. 2012;12:263. doi: 10.1186/1471-2407-12-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sansonno D, Lauletta G, Russi S, Conteduca V, Sansonno L, Dammacco F. Transarterial chemoembolization plus sorafenib: a sequential therapeutic scheme for HCV-related intermediate-stage hepatocellular carcinoma: a randomized clinical trial. Oncologist. 2012;17:359–366. doi: 10.1634/theoncologist.2011-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bai W, Wang YJ, Zhao Y, Qi XS, Yin ZX, He CY, Li RJ, Wu KC, Xia JL, Fan DM, Han GH. Sorafenib in combination with transarterial chemoembolization improves the survival of patients with unresectable hepatocellular carcinoma: a propensity score matching study. J Dig Dis. 2013;14:181–190. doi: 10.1111/1751-2980.12038. [DOI] [PubMed] [Google Scholar]
- 42.Chung YH, Han G, Yoon JH, Yang J, Wang J, Shao GL, Kim BI, Lee TY, Chao Y. Interim analysis of START: study in asia of the combination of TACE (transcatheter arterial chemoembolization) with sorafenib in patients with hepatocellular carcinoma trial. Int J Cancer. 2013;132:2448–2458. doi: 10.1002/ijc.27925. [DOI] [PubMed] [Google Scholar]
- 43.Huang YH, Chen W, Li JP, Chen B, Yang JY. Clinical value of continuous administration of sorafenib in combination with modified transarterial chemoembolization in patients with unresectable hepatocellular carcinoma. Chin Med J (Engl) 2013;126:385–386. [PubMed] [Google Scholar]
- 44.Muhammad A, Dhamija M, Vidyarthi G, Amodeo D, Boyd W, Miladinovic B, Kumar A. Comparative effectiveness of traditional chemoembolization with or without sorafenib for hepatocellular carcinoma. World J Hepatol. 2013;5:364–371. doi: 10.4254/wjh.v5.i7.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao Y, Wang WJ, Guan S, Li HL, Xu RC, Wu JB, Liu JS, Li HP, Bai W, Yin ZX, Fan DM, Zhang ZL, Han GH. Sorafenib combined with transarterial chemoembolization for the treatment of advanced hepatocellular carcinoma: a large-scale multicenter study of 222 patients. Ann Oncol. 2013;24:1786–1792. doi: 10.1093/annonc/mdt072. [DOI] [PubMed] [Google Scholar]
- 46.Liu L, Chen H, Wang M, Zhao Y, Cai G, Qi X, Han G. Combination therapy of sorafenib and TACE for unresectable HCC: a systematic review and meta-analysis. PLoS One. 2014;9:e91124. doi: 10.1371/journal.pone.0091124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chao Y, Chung YH, Han G, Yoon JH, Yang J, Wang J, Shao GL, Kim BI, Lee TY. The combination of transcatheter arterial chemoembolization and sorafenib is well tolerated and effective in Asian patients with hepatocellular carcinoma: final results of the START trial. Int J Cancer. 2015;136:1458–1467. doi: 10.1002/ijc.29126. [DOI] [PubMed] [Google Scholar]
- 48.Wan X, Zhai X, Yan Z, Yang P, Li J, Wu D, Wang K, Xia Y, Shen F. Retrospective analysis of transarterial chemoembolization and sorafenib in Chinese patients with unresectable and recurrent hepatocellular carcinoma. Oncotarget. 2016;7:83806–83816. doi: 10.18632/oncotarget.11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yao X, Yan D, Zeng H, Liu D, Li H. Concurrent sorafenib therapy extends the interval to subsequent TACE for patients with unresectable hepatocellular carcinoma. J Surg Oncol. 2016;113:672–677. doi: 10.1002/jso.24215. [DOI] [PubMed] [Google Scholar]
- 50.Aktas G, Kus T, Emin Kalender M, Kervancioglu S, Sevinc A, Kul S, Camci C. Sorafenib with TACE improves the survival of hepatocellular carcinoma patients with more than 10 cm tumor: a single-center retrospective study. J BUON. 2017;22:150–156. [PubMed] [Google Scholar]
- 51.Meyer T, Fox R, Ma YT, Ross PJ, James MW, Sturgess R, Stubbs C, Stocken DD, Wall L, Watkinson A, Hacking N, Evans TRJ, Collins P, Hubner RA, Cunningham D, Primrose JN, Johnson PJ, Palmer DH. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol. 2017;2:565–575. doi: 10.1016/S2468-1253(17)30156-5. [DOI] [PubMed] [Google Scholar]
- 52.Varghese J, Kedarisetty C, Venkataraman J, Srinivasan V, Deepashree T, Uthappa M, Ilankumaran K, Govil S, Reddy M, Rela M. Combination of TACE and sorafenib improves outcomes in BCLC stages B/C of hepatocellular carcinoma: a single centre experience. Ann Hepatol. 2017;16:247–254. doi: 10.5604/16652681.1231583. [DOI] [PubMed] [Google Scholar]
- 53.Yao Q, Zhang H, Xiong B, Zheng C. Combination of sorafenib and TACE inhibits portal vein invasion for intermediate stage HCC: a single center retrospective controlled study. Oncotarget. 2017;8:79012–79022. doi: 10.18632/oncotarget.20745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lei XF, Ke Y, Bao TH, Tang HR, Wu XS, Shi ZT, Lin J, Zhang ZX, Gu H, Wang L. Effect and safety of sorafenib in patients with intermediate hepatocellular carcinoma who received transarterial chemoembolization: a retrospective comparative study. World J Clin Cases. 2018;6:74–83. doi: 10.12998/wjcc.v6.i5.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ren B, Wang W, Shen J, Li W, Ni C, Zhu X. Transarterial chemoembolization (TACE) combined with sorafenib versus TACE alone for unresectable hepatocellular carcinoma: a propensity score matching study. J Cancer. 2019;10:1189–1196. doi: 10.7150/jca.28994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yuan J, Yin X, Tang B, Ma H, Zhang L, Li L, Chen R, Xie X, Ren Z. Transarterial chemoembolization (TACE) combined with sorafenib in treatment of HBV background hepatocellular carcinoma with portal vein tumor thrombus: a propensity score matching study. Biomed Res Int. 2019;2019:2141859. doi: 10.1155/2019/2141859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cabrera R, Pannu DS, Caridi J, Firpi RJ, Soldevila-Pico C, Morelli G, Clark V, Suman A, George TJ Jr, Nelson DR. The combination of sorafenib with transarterial chemoembolisation for hepatocellular carcinoma. Aliment Pharmacol Ther. 2011;34:205–213. doi: 10.1111/j.1365-2036.2011.04697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ricke J, Klümpen HJ, Amthauer H, Bargellini I, Bartenstein P, de Toni EN, Gasbarrini A, Pech M, Peck-Radosavljevic M, Popovič P, Rosmorduc O, Schott E, Seidensticker M, Verslype C, Sangro B, Malfertheiner P. Impact of combined selective internal radiation therapy and sorafenib on survival in advanced hepatocellular carcinoma. J Hepatol. 2019;71:1164–1174. doi: 10.1016/j.jhep.2019.08.006. [DOI] [PubMed] [Google Scholar]
- 59.Rizell M, Sternby Eilard M, Andersson M, Andersson B, Karlsson-Parra A, Suenaert P. Phase 1 trial with the cell-based immune primer ilixadencel, alone, and combined with sorafenib, in advanced hepatocellular carcinoma. Front Oncol. 2019;9:19. doi: 10.3389/fonc.2019.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou Z, Qin H, Weng L, Ni Y. Clinical efficacy of DC-CIK combined with sorafenib in the treatment of advanced hepatocellular carcinoma. J BUON. 2019;24:615–621. [PubMed] [Google Scholar]
- 61.Ho V, Lim TS, Lee J, Steinberg J, Szmyd R, Tham M, Yaligar J, Kaldis P, Abastado JP, Chew V. TLR3 agonist and Sorafenib combinatorial therapy promotes immune activation and controls hepatocellular carcinoma progression. Oncotarget. 2015;6:27252–27266. doi: 10.18632/oncotarget.4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Y, Li H, Liang Q, Liu B, Mei X, Ma Y. Combinatorial immunotherapy of sorafenib and blockade of programmed death-ligand 1 induces effective natural killer cell responses against hepatocellular carcinoma. Tumour Biol. 2015;36:1561–1566. doi: 10.1007/s13277-014-2722-2. [DOI] [PubMed] [Google Scholar]
- 63.Shi S, Rao Q, Zhang C, Zhang X, Qin Y, Niu Z. Dendritic cells pulsed with exosomes in combination with PD-1 antibody increase the efficacy of sorafenib in hepatocellular carcinoma model. Transl Oncol. 2018;11:250–258. doi: 10.1016/j.tranon.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu X, Luo H, Shi B, Di S, Sun R, Su J, Liu Y, Li H, Jiang H, Li Z. Combined antitumor effects of sorafenib and GPC3-CAR T cells in mouse models of hepatocellular carcinoma. Mol Ther. 2019;27:1483–1494. doi: 10.1016/j.ymthe.2019.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jenne CN, Kubes P. Immune surveillance by the liver. Nat Immunol. 2013;14:996–1006. doi: 10.1038/ni.2691. [DOI] [PubMed] [Google Scholar]
- 66.Guerra N, Tan YX, Joncker NT, Choy A, Gallardo F, Xiong N, Knoblaugh S, Cado D, Greenberg NM, Raulet DH. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28:571–580. doi: 10.1016/j.immuni.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gong J, Fang L, Liu R, Wang Y, Xing J, Chen Y, Zhuang R, Zhang Y, Zhang C, Yang A, Zhang X, Jin B, Chen L. UPR decreases CD226 ligand CD155 expression and sensitivity to NK cell-mediated cytotoxicity in hepatoma cells. Eur J Immunol. 2014;44:3758–3767. doi: 10.1002/eji.201444574. [DOI] [PubMed] [Google Scholar]
- 68.Mantovani S, Oliviero B, Lombardi A, Varchetta S, Mele D, Sangiovanni A, Rossi G, Donadon M, Torzilli G, Soldani C, Porta C, Pedrazzoli P, Chiellino S, Santambrogio R, Opocher E, Maestri M, Bernuzzi S, Rossello A, Clément S, De Vito C, Rubbia-Brandt L, Negro F, Mondelli MU. Deficient natural killer cell NKp30-mediated function and altered NCR3 splice variants in hepatocellular carcinoma. Hepatology. 2019;69:1165–1179. doi: 10.1002/hep.30235. [DOI] [PubMed] [Google Scholar]
- 69.Coulouarn C, Factor VM, Conner EA, Thorgeirsson SS. Genomic modeling of tumor onset and progression in a mouse model of aggressive human liver cancer. Carcinogenesis. 2011;32:1434–1440. doi: 10.1093/carcin/bgr133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cai L, Zhang Z, Zhou L, Wang H, Fu J, Zhang S, Shi M, Zhang H, Yang Y, Wu H, Tien P, Wang FS. Functional impairment in circulating and intrahepatic NK cells and relative mechanism in hepatocellular carcinoma patients. Clin Immunol. 2008;129:428–437. doi: 10.1016/j.clim.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 71.Fathy A, Eldin MM, Metwally L, Eida M, Abdel-Rehim M. Diminished absolute counts of CD56dim and CD56bright natural killer cells in peripheral blood from Egyptian patients with hepatocellular carcinoma. Egypt J Immunol. 2009;16:17–25. [PubMed] [Google Scholar]
- 72.Chew V, Chen J, Lee D, Loh E, Lee J, Lim KH, Weber A, Slankamenac K, Poon RT, Yang H, Ooi LL, Toh HC, Heikenwalder M, Ng IO, Nardin A, Abastado JP. Chemokine-driven lymphocyte infiltration: an early intratumoural event determining long-term survival in resectable hepatocellular carcinoma. Gut. 2012;61:427–438. doi: 10.1136/gutjnl-2011-300509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gehring AJ, Ho ZZ, Tan AT, Aung MO, Lee KH, Tan KC, Lim SG, Bertoletti A. Profile of tumor antigen-specific CD8 T cells in patients with hepatitis B virus-related hepatocellular carcinoma. Gastroenterology. 2009;137:682–690. doi: 10.1053/j.gastro.2009.04.045. [DOI] [PubMed] [Google Scholar]
- 74.Bryceson YT, March ME, Ljunggren HG, Long EO. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood. 2006;107:159–166. doi: 10.1182/blood-2005-04-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang W, Erbe AK, Hank JA, Morris ZS, Sondel PM. NK cell-mediated antibody-dependent cellular cytotoxicity in cancer immunotherapy. Front Immunol. 2015;6:368. doi: 10.3389/fimmu.2015.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ho M. Advances in liver cancer antibody therapies: a focus on glypican-3 and mesothelin. BioDrugs. 2011;25:275–284. doi: 10.2165/11595360-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Abou-Alfa GK, Puig O, Daniele B, Kudo M, Merle P, Park JW, Ross P, Peron JM, Ebert O, Chan S, Poon TP, Colombo M, Okusaka T, Ryoo BY, Minguez B, Tanaka T, Ohtomo T, Ukrainskyj S, Boisserie F, Rutman O, Chen YC, Xu C, Shochat E, Jukofsky L, Reis B, Chen G, Di Laurenzio L, Lee R, Yen CJ. Randomized phase II placebo controlled study of codrituzumab in previously treated patients with advanced hepatocellular carcinoma. J Hepatol. 2016;65:289–295. doi: 10.1016/j.jhep.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 78.Spear P, Wu MR, Sentman ML, Sentman CL. NKG2D ligands as therapeutic targets. Cancer Immun. 2013;13:8. [PMC free article] [PubMed] [Google Scholar]
- 79.Jinushi M, Takehara T, Tatsumi T, Hiramatsu N, Sakamori R, Yamaguchi S, Hayashi N. Impairment of natural killer cell and dendritic cell functions by the soluble form of MHC class I-related chain A in advanced human hepatocellular carcinomas. J Hepatol. 2005;43:1013–1020. doi: 10.1016/j.jhep.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 80.Easom NJW, Stegmann KA, Swadling L, Pallett LJ, Burton AR, Odera D, Schmidt N, Huang WC, Fusai G, Davidson B, Maini MK. IL-15 overcomes hepatocellular carcinoma-induced NK cell dysfunction. Front Immunol. 2018;9:1009–1009. doi: 10.3389/fimmu.2018.01009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Muntasell A, Ochoa MC, Cordeiro L, Berraondo P, López-Díaz de Cerio A, Cabo M, López-Botet M, Melero I. Targeting NK-cell checkpoints for cancer immunotherapy. Curr Opin Immunol. 2017;45:73–81. doi: 10.1016/j.coi.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 82.Carlsten M, Korde N, Kotecha R, Reger R, Bor S, Kazandjian D, Landgren O, Childs RW. Checkpoint inhibition of KIR2D with the monoclonal antibody IPH2101 induces contraction and hyporesponsiveness of NK cells in patients with myeloma. Clin Cancer Res. 2016;22:5211–5222. doi: 10.1158/1078-0432.CCR-16-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tatsumi T, Takehara T. Impact of natural killer cells on chronic hepatitis C and hepatocellular carcinoma. Hepatol Res. 2016;46:416–422. doi: 10.1111/hepr.12619. [DOI] [PubMed] [Google Scholar]
- 84.Jiang W, Zhang C, Tian Z, Zhang J. hIFN-α gene modification augments human natural killer cell line anti-human hepatocellular carcinoma function. Gene Ther. 2013;20:1062–1069. doi: 10.1038/gt.2013.31. [DOI] [PubMed] [Google Scholar]
- 85.Takeda K, Hayakawa Y, Smyth MJ, Kayagaki N, Yamaguchi N, Kakuta S, Iwakura Y, Yagita H, Okumura K. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat Med. 2001;7:94–100. doi: 10.1038/83416. [DOI] [PubMed] [Google Scholar]
- 86.El-Shemi AG, Ashshi AM, Na Y, Li Y, Basalamah M, Al-Allaf FA, Oh E, Jung BK, Yun CO. Combined therapy with oncolytic adenoviruses encoding TRAIL and IL-12 genes markedly suppressed human hepatocellular carcinoma both in vitro and in an orthotopic transplanted mouse model. J Exp Clin Cancer Res. 2016;35:74. doi: 10.1186/s13046-016-0353-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fehniger TA, Caligiuri MA. Interleukin 15: biology and relevance to human disease. Blood. 2001;97:14–32. doi: 10.1182/blood.v97.1.14. [DOI] [PubMed] [Google Scholar]
- 88.Conlon KC, Lugli E, Welles HC, Rosenberg SA, Fojo AT, Morris JC, Fleisher TA, Dubois SP, Perera LP, Stewart DM, Goldman CK, Bryant BR, Decker JM, Chen J, Worthy TA, Figg WD Sr, Peer CJ, Sneller MC, Lane HC, Yovandich JL, Creekmore SP, Roederer M, Waldmann TA. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J. Clin. Oncol. 2015;33:74–82. doi: 10.1200/JCO.2014.57.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Waldmann TA. The shared and contrasting roles of IL2 and IL15 in the life and death of normal and neoplastic lymphocytes: implications for cancer therapy. Cancer Immunol Res. 2015;3:219–227. doi: 10.1158/2326-6066.CIR-15-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lin M, Liang S, Wang X, Liang Y, Zhang M, Chen J, Niu L, Xu K. Cryoablation combined with allogenic natural killer cell immunotherapy improves the curative effect in patients with advanced hepatocellular cancer. Oncotarget. 2017;8:81967–81977. doi: 10.18632/oncotarget.17804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Alnaggar M, Lin M, Mesmar A, Liang S, Qaid A, Xu K, Chen J, Niu L, Yin Z. Allogenic natural killer cell immunotherapy combined with irreversible electroporation for stage IV hepatocellular carcinoma: survival outcome. Cell Physiol Biochem. 2018;48:1882–1893. doi: 10.1159/000492509. [DOI] [PubMed] [Google Scholar]
- 92.Lee JH, Lee JH, Lim YS, Yeon JE, Song TJ, Yu SJ, Gwak GY, Kim KM, Kim YJ, Lee JW, Yoon JH. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology. 2015;148:1383–1391. e1386. doi: 10.1053/j.gastro.2015.02.055. [DOI] [PubMed] [Google Scholar]
- 93.Gong JH, Maki G, Klingemann HG. Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia. 1994;8:652–658. [PubMed] [Google Scholar]
- 94.Tonn T, Schwabe D, Klingemann HG, Becker S, Esser R, Koehl U, Suttorp M, Seifried E, Ottmann OG, Bug G. Treatment of patients with advanced cancer with the natural killer cell line NK-92. Cytotherapy. 2013;15:1563–1570. doi: 10.1016/j.jcyt.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 95.Yu M, Luo H, Fan M, Wu X, Shi B, Di S, Liu Y, Pan Z, Jiang H, Li Z. Development of GPC3-specific chimeric antigen receptor-engineered natural killer cells for the treatment of hepatocellular carcinoma. Mol Ther. 2018;26:366–378. doi: 10.1016/j.ymthe.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huang Y, Zeng J, Liu T, Xu Q, Song X, Zeng J. DNAM1 and 2B4 costimulatory domains enhance the cytotoxicity of anti-GPC3 chimeric antigen receptor-modified natural killer cells against hepatocellular cancer cells in vitro. Cancer Manag Res. 2020;12:3247–3255. doi: 10.2147/CMAR.S253565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Luo H, Wu X, Sun R, Su J, Wang Y, Dong Y, Shi B, Sun Y, Jiang H, Li Z. Target-dependent expression of IL12 by synNotch receptor-engineered NK92 cells increases the antitumor activities of CAR-T cells. Front Oncol. 2019;9:1448. doi: 10.3389/fonc.2019.01448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Huang Y, Wang Y, Li Y, Guo K, He Y. Role of sorafenib and sunitinib in the induction of expressions of NKG2D ligands in nasopharyngeal carcinoma with high expression of ABCG2. J Cancer Res Clin Oncol. 2011;137:829–837. doi: 10.1007/s00432-010-0944-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sprinzl MF, Reisinger F, Puschnik A, Ringelhan M, Ackermann K, Hartmann D, Schiemann M, Weinmann A, Galle PR, Schuchmann M, Friess H, Otto G, Heikenwalder M, Protzer U. Sorafenib perpetuates cellular anticancer effector functions by modulating the crosstalk between macrophages and natural killer cells. Hepatology. 2013;57:2358–2368. doi: 10.1002/hep.26328. [DOI] [PubMed] [Google Scholar]
- 100.Kamiya T, Chang YH, Campana D. Expanded and activated natural killer cells for immunotherapy of hepatocellular carcinoma. Cancer Immunol Res. 2016;4:574–581. doi: 10.1158/2326-6066.CIR-15-0229. [DOI] [PubMed] [Google Scholar]
- 101.Hage C, Hoves S, Strauss L, Bissinger S, Prinz Y, Poschinger T, Kiessling F, Ries CH. Sorafenib induces pyroptosis in macrophages and triggers natural killer cell-mediated cytotoxicity against hepatocellular carcinoma. Hepatology. 2019;70:1280–1297. doi: 10.1002/hep.30666. [DOI] [PubMed] [Google Scholar]
- 102.Shi L, Lin H, Li G, Jin RA, Xu J, Sun Y, Ma WL, Yeh S, Cai X, Chang C. Targeting androgen receptor (AR)→IL12A signal enhances efficacy of sorafenib plus NK cells immunotherapy to better suppress HCC progression. Mol Cancer Ther. 2016;15:731–742. doi: 10.1158/1535-7163.MCT-15-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hu J, Wang E, Liu L, Wang Q, Xia D, Bai W, Tie J, Li X, Yuan J, Yang S, Jiang D, Shi J, Sun Y, Wang J, Zhang C, Niu J, Li K, He C, Guo W, Lv Y, Chen H, Yuan X, Yu T, Wang Z, Luo B, Han N, Zhu Y, Yin Z, Fan D, Zhang Z, Yang K, Han G. Sorafenib may enhance antitumour efficacy in hepatocellular carcinoma patients by modulating the proportions and functions of natural killer cells. Invest New Drugs. 2019;38:1247–1256. doi: 10.1007/s10637-019-00885-2. [DOI] [PubMed] [Google Scholar]
- 104.Lohmeyer J, Nerreter T, Dotterweich J, Einsele H, Seggewiss-Bernhardt R. Sorafenib paradoxically activates the RAS/RAF/ERK pathway in polyclonal human NK cells during expansion and thereby enhances effector functions in a dose- and time-dependent manner. Clin Exp Immunol. 2018;193:64–72. doi: 10.1111/cei.13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li C, Wei S, Xu X, Jiang Y, Xue L, Jiang P, Wang J. Sorafenib attenuated the function of natural killer cells infiltrated in HCC through inhibiting ERK1/2. Int Immunopharmacol. 2019;76:105855. doi: 10.1016/j.intimp.2019.105855. [DOI] [PubMed] [Google Scholar]


