Abstract
HBV infection plays a crucial role in primary liver cancer development. Also, HBV related liver cancer has higher invasiveness and earlier discovered distant metastasis. HBV-encoded X protein (HBx) exerts various biological functions on liver cancer progression, including proliferation, invasion, and venous metastasis. There is evidence that High-mobility group box 1 (HMGB1) promotes epithelial-mesenchymal transition (EMT) and angiogenesis of tumors, including liver cancer. Therefore, this study investigates whether HMGB1 mediates HBx-induced EMT and angiogenesis in HBV related liver cancer. We collected 76 tumor samples of primary liver cancer patients to analyze the relationship between HMGB1 and portal vein tumor thrombus (PVTT) in HBV related liver cancer. To test the influence of HMGB1 on EMT and angiogenesis, we constructed HBx lentivirus transfected HepG2/Huh7 cell lines and performed invasion assays, tube formation and in vivo metastatic experiments. We evaluated HMGB1 and STAT3/miR-34a/NF-κB pathway in vivo and in vitro by immunoblot, quantitative real-time polymerase chain reaction (qRT-PCR), immunofluorescence and immunohistochemistry analysis. Subsequent RNA interference (RNAi) and luciferase reporter assay were conducted to detect the functional correlation between HMGB1 and STAT3/miR-34a/NF-κB pathway. Our results showed enhanced expression of HMGB1 in HBV related liver cancer, especially with PVTT, while HMGB1 expression was associated with tumor invasion and metastasis. Further experiments indicated that the activation of STAT3 mediated HBx-induced HMGB1, which is involved in EMT and tumor angiogenesis. Besides, HMGB1 expression stimulated by HBx was dependent on the activation of the NF-κB signaling pathway, which was inhibited by miR-34a, while STAT3 suppressed the expression of miR-34a. Moreover, extracellular HMGB1 induced the IL-6/STAT3/miR-34a axis activation, which indicated a reciprocal relationship between HMGB1 and miR-34a. Collectively, our study provided evidence to reveal that HBx-mediated high expression of HMGB1 accounted for EMT and tumor angiogenesis in HBV related liver cancer, and HMGB1 may be a potential target for predicting venous metastasis.
Keywords: HBx, HMGB1, liver cancer, EMT, angiogenesis, miR-34a
Introduction
Primary liver cancer is one of the most common malignancies worldwide [1]. Since liver cancer is often diagnosed with intrahepatic metastasis and has a high rate of postsurgical recurrence, the prognosis of patients with liver cancer is reduced, and the 5-year survival rate is around only 30%-40% [2]. The development of liver cancer is likely to be related to hepatitis B virus (HBV) and hepatitis C virus (HCV) infection because more than 50% of liver cancer results from chronic HBV/HCV infection [3]. Metastasis is the leading cause of cancer-related death in HBV related liver cancer, in which hepatitis B virus X protein (HBx) might play a critical role [4-7]. Portal vein tumor thrombus (PVTT) is a significant risk factor of metastasis in liver cancer [8,9]. It has been reported that PVTT is strongly linked to HBV infection status [10]. However, the molecular basis of HBV infection induced metastasis remains unclear.
HBx is a ~17 kd protein that plays a vital role in cell proliferation, apoptosis and invasion [5,6,11]. HBx facilitates liver cancer metastasis by promoting the EMT process and angiogenesis [12,13]. Accumulated data have suggested that HBx acts as an oncogene in liver cancer’s metastasis by modulating ERK, MAPK and NF-κB signaling pathways [6]. A previous study revealed that HBV induced TGF-β-miR-34a-CCL22 signaling promoted venous metastasis of HBV related liver cancer, suggesting that HBx may affect the progress of PVTT [10].
Members of miR-34 family, including miR-34a, miR-34b, and miR-34c, have been demonstrated to be direct targets of p53 and MYN [14]. Since p53 is often mutated and inactivated in many tumors, miR-34a is poorly expressed and functions as a tumor suppressor [15]. Mounting evidence shows that miR-34a is involved in proliferation, apoptosis and senescence by regulating CDK4/6, E2F3, Bcl-2, and MYCN [14,16]. Additionally, it is reported that miR-34a inhibited liver cancer’s metastasis by targeting c-met and be repressed by HBx via TGF-beta [10,17].
High mobility group box 1 (HMGB1) is a highly conserved nuclear protein and acts as a DNA chaperone, which regulates DNA replication, repair and transcription [18,19]. HMGB1 plays a critical role in proliferation, angiogenesis and metastasis in many types of tumor, including primary liver cancer [18,20-23]. Accumulating studies indicate that HMGB1 promotes the EMT in liver cancer and colorectal carcinoma (CRC) [20,24,25]. Interestingly, many miRNAs, such as miR-181, miR-22, miR-325-3p and miR-34a, have been reported to modulate HMGB1 expression [26]. Recently, Chen demonstrates that HBx induces HMGB1 via calcium-dependent cascades [27]. However, the molecular mechanism of regulating HMGB1 expression in tumors is still not well understood.
Here, we provided evidence that HMGB1 induced by HBx was responsible for EMT and angiogenesis in HBV related liver cancer. Ablation of HMGB1 inhibited EMT process and VEGF expression of HBx transfected liver cancer cell lines, impaired tumor angiogenesis and metastasis in vivo. Moreover, we demonstrated that the HBx-induced STAT3 promoted the secretion of HMGB1 in liver cancer cells, eventually led to the formation of PVTT by promoting EMT and angiogenesis. Furthermore, HBx inhibited the expression of miR-34a through STAT3 signaling, then affected the expression and secretion of downstream HMGB1 by blocking the activation of NF-κB. We verified that extracellular HMGB1 up-regulated IL-6/STAT3 activation, enhanced the inhibitory effect of STAT3 on miR-34a, there by forming a positive feedback loop of IL-6/STAT3/miR-34a, ultimately promoting the secretion of HMGB1. In conclusion, our study clarified a mechanism of EMT and tumor angiogenesis progression relies on HMGB1 in HBV related liver cancer and provide a potential predictive target for venous metastasis.
Methods
Patients and specimens
We achieved tumor samples from 76 patients who had undergone liver resection between 2015 and 2017 and were pathologically confirmed primary liver cancer at Affiliated Drum Tower Hospital of Nanjing University Medical School. The experiments proceeded with the understanding and written consent of each subject, and the study methodologies conformed to the standards set by the Declaration of Helsinki. The Ethical Committee of Affiliated Drum Tower Hospital of Nanjing University Medical School approved this project. All patients signed informed consent for the present study before the commencement of the experiments. Table S1 summarized the clinical signatures of all patients.
Animals and chemical reagents
Male BALB/c nu/nu mice (6-8 weeks old, Shanghai Institute of Material Medicine, Chinese Academy of Science) were housed in specific pathogen-free conditions. All animals received humane care according to the criteria “Guide for the Care and Use of Laboratory Animals”, which was prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH publication 86-23 revised 1985).
Cell culture
The human liver cancer cell line HepG2, Huh7 were achieved from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Cells were all cultured in 4.5 g/L glucose DMEM containing 10% FBS (Gbico), penicillin (100 U/mL), and streptomycin (100 μg/mL), incubated at 37°C in humidified air with 5% CO2. All human cell lines have been authenticated using STR (or SNP) profiling within the last three years.
Construction of stably transfected cell lines with HBx lentivirus transfection
For the further experiment of HBx function in tumors, we constructed stably transfected liver cancer cell lines with HBx lentivirus transfection. Details of the cell line construction process are consistent with our previous research [28]. The verification results are shown in Figure S1.
Metastatic model in vivo
5×106 HepG2-con, HepG2-HBx or HepG2-HBx-shHMGB1 cells suspended in 200 ul PBS were injected via tail vein in Male BALB/c nu/nu mice (6-8 weeks old, Shanghai Institute of Material Medicine, Chinese Academy of Science). Three groups of mice were sacrificed 14 days after injection, the lungs and livers were obtained for measuring colonies.
Immunoblot analysis
Total protein was extracted by lysing cells in RIPA buffer containing protease inhibitor cocktail. Protein samples boiled with 1× loading buffer were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene fluoride (PVDF) membranes. After blocking with 5% BSA in TBS-T, membranes were incubated with the primary antibody at 4°C overnight. Goat-anti-rabbit or mouse IgG conjugated to horseradish peroxidase (HRP) was used as the secondary antibody. The results were imaged by Tanon.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNAs of tumor tissues and cells were isolated using Trizol reagent (Life Technology). 2 μg of total RNA were reverse-transcribed (Takara). The specific primers used to amplify relevant genes are shown in Table S2. Quantitative PCR was carried out in triplicate using SYBR Green real-time PCR master mix (Takara) in an ABI StepOne Plus system (Thermo Fisher Scientific). All results are normalized to 18S rRNA expression.
RNA interference (RNAi)
For stably knockdown of HMGB1, 2×105 cells were plated into 6-well plates. After 24 h, the mixture containing diluted shRNA-plasmid and lipofectin 2000 was added to the medium according to protocol. After 6 h of incubation, cells were cultured in complete DMEM with 10 μg/ml puromycin (Sigma-Aldrich) for 24-48 h to select stable transfectants. MiR-34a mimic and miR-34a inhibitor (Riobio) were transfected into cells using lipofectin 2000 according to the manufacturer’s instructions. At the end of RNAi, all cells were collected for immunoblot analysis and qRT-PCR.
Immunofluorescence
Immunofluorescence analysis proceeded according to protocols. Cells were implanted in 24-well dishes and fixed by 4% paraformaldehyde 24 h later. Fixed cells were incubated by the primary antibody of target protein (Cell Signaling Technology) and subsequently incubated by FITC-conjugated anti-mouse IgG and Cy3-conjugated anti-rabbit IgG (Abcam). Representative images were detected by fluorescent microscopy (Leica) and data was analyzed via ImagePro Plus.
Immunohistochemistry
Immunohistochemistry of primary liver cancer samples was conducted as previously described [29]. Samples were incubated with anti-HMGB1 (Abcam), anti-EMT related markers (Cell Signaling Technology), anti-CD31 (Cell Signaling Technology), p-STAT3/snail (Abcam) and anti-p65 (Cell Signaling Technology), followed by incubation with secondary antibody. IHC results were scored according to 0, <25%; 1, <50%; 2, <75%; 3, >75% by two experienced pathologists. Data are shown as means ± SEM.
Invasion assays
The liver cancer cells’ invasive ability was measured via 24-well transwell chambers separated by polycarbonate membranes with 8-µm pores and precoated with Matrigel (Colin). The lower chamber was filled with complete DMEM as a chemoattractant. Cells in serum-free medium were seeded at 5×104 in the upper chamber and incubated at 37°C in humidified air with 5% CO2. Cells that migrated to the membrane’s underside were fixed and stained with Giemsa (Sigma), then detected and calculated with a microscope (Leica). All experiments were carried out in triplicate.
In vitro tube formation
Human umbilical vein endothelial cells (HUVECs) were cultured in an essential medium containing 2% FBS and 1% penicillin/streptomycin or the conditioned media (CM) collected from HepG2 and Huh7 cells. HUVECs (1×104) were seeded into a 96-well culture plate precoated with Matrigel (BD Biosciences) and then cultured in the indicated condition. After 8 h of incubation, the tubes’ formation was observed, photographed with a phase-contrast microscope and quantified by counting the total length of vascular tubes in five randomly selected microscope fields per well. The experiments were performed three times.
Dual-luciferase reporter assay
Following the protocol indication, pmirGLO, pmirGLO-IKKβ, or pmirGLO-IKKβ-mut was cotransfected with miR-34a mimics/miR NC into HepG2 cells by Lipofectamine-mediated gene transfer. The relative luciferase activity was normalized to Renilla 48 h after transfection.
Statistical analysis
Fisher’s exact tests and χ2 tests were used to determine clinicopathological correlations. Spearman’s correlation analysis evaluated the association between HMGB1, EMT markers and MVD in liver cancer tissues. The Student’s t-test and one-way ANOVA were used for comparison between variables. GraphPad Prism 6 was used for all statistical analysis. P<0.05 was considered statistically significant.
Result
HMGB1 expression and HBV infection status are positively related to PVTT development in primary liver cancer
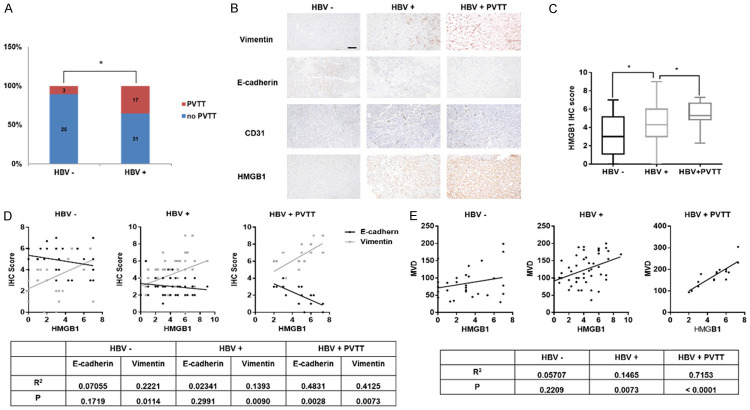
Primary liver cancer patients diagnosed with PVTT have been proven to have a poorer prognosis and a high rate of tumor metastasis [9]. However, the molecular basis of venous tumor metastasis remains unclear. Several studies revealed a tight relationship between HBV infection status and PVTT [8,10,30]. As expected, we found that the rate of PVTT occurrence in HBV related liver cancer patients is significantly higher than HBV negative patients (Figure 1A). Recently, HBx, the main effector of HBV infection, has been demonstrated to stimulate HMGB1 secretion and enhance tumor metastasis [27,31]. HMGB1 is considered as an important tumor-promoting protein [20,32]. To investigate the correlation of HMGB1 and HBV status in liver cancer, we performed immunohistochemistry staining of HMGB1 in 76 primary liver cancer patients. The results showed that HMGB1 expression was positively associated with HBV infection. Besides, compared to HBV positive patients with no PVTT, those with PVTT were characterized with elevated expression of HMGB1 (Figure 1B, 1C).
Figure 1.
HMGB1 expression and HBV infection status are positively related to PVTT development in primary liver cancer. A. Rate of PVTT occurrence in HBV positive or negative liver cancer patients, analyzed by χ2 test. B. IHC of HMGB1 in liver cancer samples. Representative images show different intensities of staining of Vimentin, E-cadherin, CD31 and HMGB1. Scale bars, 100 um. Lower panels represent magnified pictures of the boxed area in the corresponding upper panels. C. Box plot graph shows the quantitative evaluation of HMGB1 staining intensity. The statistical differences between the three groups were analyzed by one-way analysis of variance. D, E. Correlation between HMGB1 and EMT markers/MVD in three groups, analyzed by unpaired student T-test. Data are means ± SEM from 3 independent experiments. * means P<0.05.
Several studies have shown that EMT, a crucial process involved in tumor invasion and angiogenesis, is a fundamental molecular biology basis for venous metastasis [33-38]. Thus, we performed immunohistochemistry staining E-cadherin, Vimentin and CD31 to investigate the relationship between HBV status and EMT/angiogenesis in liver cancer [39,40]. Data revealed that the EMT process and angiogenesis were significantly stimulated by HBV infection (Figures 1B, S2). Based on existing experiments, we further observed that HMGB1 expression was positively related to EMT phenotype and microvessel density (MVD), an index representing the degree of neoangiogenesis, in HBV related liver cancer with PVTT (Figure 1D, 1E). These clinical data indicated that HMGB1 might promote the formation of PVTT caused by HBV infection by affecting EMT and angiogenesis.
HBx promotes EMT progress and angiogenesis of liver cancer cells by up-regulating the expression of HMGB1
It has been reported that HBx, the most important pathogenic factor of HBV, promotes tumorigenesis by affecting EMT or angiogenesis [41,42]. Mounting data indicated that HBx and HMGB1 both promote liver cancer invasion and metastasis [27]. To investigate the relationship between HBx and HMGB1 in liver cancer progression, two liver cancer cell lines, Huh7 and HepG2, were stably established with lentivirus-HBx transfection for further experiments (Figure S1). Consistent with observations in clinical data, HBx increased HMGB1 expression in translation and secretion level (Figure S3), suggesting that the presence of HBx in liver cancer cells directly affects HMGB1 expression.
To further explore the role of HMGB1 in HBx induced EMT and angiogenesis, shHMGB1 was used to knockdown HMGB1 in HBx transfected cells for the following experiments. As shown in Figure 2A, the protein level of N-cadherin, Vimentin and VEGF, which are involved in EMT or angiogenesis, were induced after HBx transfection in liver cancer cells. However, knockdown of HMGB1 mRNA could alleviate such promotion. We observed that the invasive property caused by HBx was inhibited in the two cell lines with shHMGB1 transfection (Figure 2B, 2C). These observations indicated that HMGB1 was essential for HBx-mediated invasion. To test whether HMGB1 directly influences tumor angiogenesis, especially with HBV infection, we performed vascular formation experiments. HUVECs were cultured with various conditioned medium collected from Huh7/HepG2, HepG2-HBx/Huh7-HBx, or HepG2-HBx-sh/Huh7-HBx-sh for 8 hours. Results showed that knockdown of HMGB1 inhibited VEGF production and reduced the angiogenesis capacity promoted by HBx, which indicated that HBx mediated angiogenesis was dependent on HMGB1 expression (Figure 2D, 2E). In summary, we concluded that HBx promotes the EMT process and angiogenesis of liver cancer cells by up-regulating the expression of HMGB1.
Figure 2.

HBx promotes EMT progress and angiogenesis of liver cancer cells by up-regulating the expression of HMGB1. A. Immunoblot analysis shows that HBx promotes the protein production of HMGB1, EMT markers and VEGF in HepG2/Huh7 cells, which can be reversed by knockdown of HMGB1. B, C. Downregulation of HMGB1 in HepG2-HBx and Huh7-HBx cells markedly inhibits the invasive ability, measured by transwell experiments. D, E. HUVEC tube formation reveals that the downregulation of HMGB1 in HepG2-HBx and Huh7-HBx cells markedly inhibits tumor angiogenesis. The total length of tube formation was analyzed and calculated by Image J. Data are shown as means ± SEM from 3 independent experiments, * means P<0.05, ** means P<0.01, by one-way ANOVA.
Knockdown of HMGB1 inhibits tumor metastasis of liver cancer promoted by HBx in vivo
To examine the role of HMGB1 in vivo, we carried out experiments of subcutaneously implanted tumors in nude mice. We found that compared to mice bearing HepG2-HBx cells, growth inhibition of implanted tumors in mice bearing HMGB1 ablation of HepG2 cells was significantly observed (Figure 3A). Besides, knockdown of HMGB1 reversed the increased MVD level of implanted tumors after HepG2-HBx cells injection (Figure 3B). We then conducted a tumor metastasis experiment to clarify the role of HMGB1 in HBx promoted liver cancer metastasis. As shown in Figure 3C, injection of HepG2-HBx-sh cells reduced distant metastasis colonies in the lungs collected from mice. Besides, such results were observed in liver surface metastasis (Figure 3D). Consistent with experiments in vitro, the inhibition of HMGB1 might alleviate HBx induced tumor metastasis and angiogenesis.
Figure 3.

Knockdown of HMGB1 inhibits the tumor metastasis of liver cancer promoted by HBx in vivo. (A) 5×106 HepG2-con, HepG2-HBx and HepG2-HBx-shHMGB1 cells were subcutaneously injected into nude mice. Four weeks later, tumors derived from indicated cells were removed and shown. N=3. The tumor growth curves for the three groups described were measured. (B) The MVD level of the implanted tumor decreased with HMGB1 knockdown. (C, D) Metastasis experiments in vivo reveal that the downregulation of HMGB1 reduces the tumor metastasis. Colonies on the (C) lung and (D) liver surface (indicated by arrows). The numbers of tumor colonies were quantified. Data are means ± SEM from 3 independent experiments, * means P<0.05, ** means P<0.01, by one-way ANOVA.
HBx up-regulates EMT/angiogenesis in liver cancer cells via activation of STAT3, which affect the secretion of HMGB1
Previous evidence showed that STAT3, one kind of plasmosin, plays a vital role in facilitating tumor growth and metastasis of liver cancer [43-45]. STAT3 also induces tumor angiogenesis via the up-regulation of VEGF [46,47]. Teng, J discovered that HBx regulates EMT via the mediation of STAT3 in liver cells [48]. We performed immunohistochemistry staining p-STAT3/snail in liver cancer tissues, showed the highest expression level of p-STAT3/snail in HBV related liver cancer with PVTT samples (Figure 4A, 4B).
Figure 4.
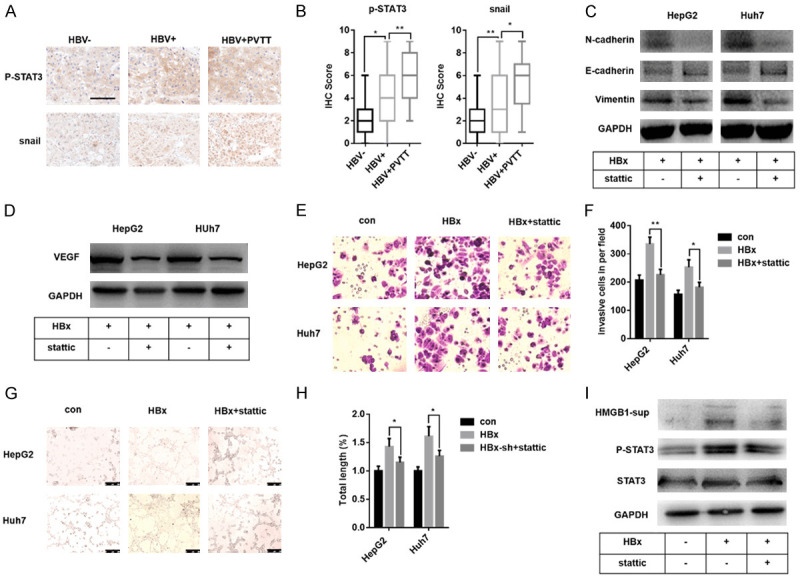
HBx up-regulates EMT/angiogenesis in liver cancer cells via activation of STAT3, which affect the secretion of HMGB1. A, B. Immunohistochemical results of p-STAT3/snail in liver cancer samples. C, D. Immunoblot analysis shows the change of protein level of EMT markers and VEGF in HepG2-HBx/Huh7-HBx cells with STAT3 inhibition. Stattic, a specific small-molecule inhibitor for STAT3. E, F. A transwell assay shows the down-regulation of the invasion ability of HepG2-HBx/Huh7-HBx cells with STAT3 inhibition. G, H. HUVEC tube formation reveals that inhibition of STAT3 in HepG2-HBx and Huh7-HBx cells markedly suppresses tumor angiogenesis. The total length of tube formation was analyzed by Image J. I. Immunoblot analysis of proteins in HepG2-HBx cells and their supernatants revealed that STAT3 might affect the secretion of HMGB1. Data are means ± SEM from 3 independent experiments, * means P<0.05, ** means P<0.01, by one-way ANOVA.
Based on this, we used stattic, a specific small-molecule inhibitor for STAT3, to demonstrate whether STAT3 is involved in HBx induced EMT progress and angiogenesis in liver cancer cells. As shown in Figure 4C and 4D, the protein level of E-cadherin, Vimentin and VEGF reduced after stattic addition in both two HBx-transfected liver cancer cells, while the changes of N-cadherin reversed. Further transwell assay found that STAT3 inhibition weakened the invasive ability of HBx-transfected liver cancer cells significantly (Figure 4E, 4F). Tube formation experiments observed that specific inhibition of STAT3 reduced the total length of vascular tubes (Figure 4G, 4H). We also verified that HBx induced phosphorylation of STAT3 (Figure 4I), as reported before [49]. These findings suggested that STAT3 is critical for HBx induced EMT/angiogenesis in liver cancer. Besides, inhibition of STAT3 suppressed the expression of HMGB1 (Figure S4) and interestingly reversed the induction of secreted HMGB1 by HBx in HepG2 cells (Figure 4I). Combined with the previous results, these data indicated that HBx activated STAT3 might mediate HMGB1 secretion.
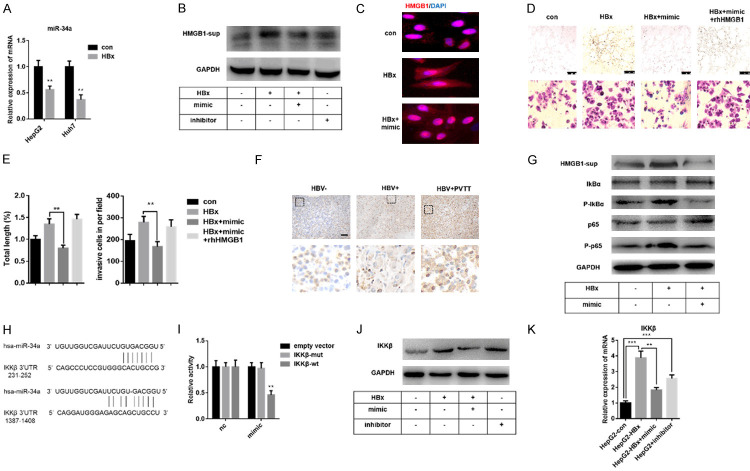
HBx promotes IKKβ-induced NF-κB activation by inhibiting miR-34a, thereby increasing the secretion of HMGB1
Pengyuan Yang has demonstrated that TGF-β-miR-34a-CCL22 is one of the essential mechanisms of PVTT development of HBV related liver cancer [10]. Besides, miR-34a has been demonstrated to target both VEGF and HMGB1 in many tumor types [17,26,50,51]. Our previous data have shown that HBx could increase the secretion level of HMGB1 (Figure 4I); however, the intracellular protein level of HMGB1 did not change obviously (Figure S2). Recently, HMGB1 secretion is demonstrated to be enhanced by HBx via regulating calcium-dependent cascades [27]. Thus we expected to investigate the role of miR-34a in HBx induced EMT/angiogenesis and whether miR-34a affects the promotion of HMGB1 secretion enhanced by HBx. HepG2-HBx/Huh7-HBx cells were transfected with miR-34a mimic or treated by inhibitor, respectively. As speculated, the content of miR-34a decreased with lentivirus-HBx transfection, which confirmed the previous results (Figure 5A) [10]. Transfection of miR-34a mimic significantly inhibited HBx induced HMGB1 content in cell supernatant (Figures 5B, S5), which might result from reversed HMGB1 nucleocytoplasmic translocation (Figure 5C). The vascular formation and transwell experiments result suggested that miR-34a regulates both invasion and angiogenesis induced by HBx (Figure 5D, 5E).
Figure 5.
HBx promotes IKKβ-induced NF-κB activation by inhibiting miR-34a, thereby increasing the secretion of HMGB1. (A) Q-PCR analysis indicates HBx inhibits miR-34a expression. (B) Immunoblot analysis reveals that miR-34a regulates the secretion of HMGB1 in HepG2 cells. (C) Immunofluorescence analysis shows that miR-34a mimic enhances HMGB1 nuclear translocation. (D, E) MiR-34a modulates angiogenesis and invasion in HepG2 cells, which is alleviated by exogenous HMGB1. (F) Immunohistochemical staining of p65, one of the most critical NF-κB isoforms, in liver cancer samples. (G) In HepG2 cells, HBx induces depolymerization of NF-κB/IκBα complex and activation of p65, which in turn is inhibited by miR-34a. (H) Putative miR-34a binding sites in the 3’UTR of IKKβ, which modulates the activation of NF-κB. (I) Dual-luciferase reporter gene assay shows a linear combination between the 3’UTR of IKKβ and miR-34a in HepG2 cells. (J, K) Immunoblot and q-PCR analysis reveal the suppression of miR-34a on the expression of IKKβ in HepG2 cells. Data are means ± SEM from 3 independent experiments, ** means P<0.01, by (A) unpaired student T-test and (E, I, K) one-way ANOVA.
It has been reported that the activation of NF-κB regulates HMGB1 expression/nucleocytoplasmic translocation [52,53]. Liang Duan revealed that HBx enhanced the transcriptional activity of NF-κB in liver cancer cells [29]. To clarify how HBx stimulation regulates the secretion of HMGB1, we detected the expression of p65 - the most critical NF-κB isoforms - in three groups of primary liver cancer tissues by immunohistochemistry staining and found a positive correlation between HBV infection and PVTT (Figure 5F). Further studies showed that miR-34a inhibited HBx induced HMGB1 secretion and NF-κB activation (Figure 5G). Since the depolymerization of NF-κB/IκBα and the activation of NF-κB are dependent on the activation of IKK, we performed miRNA target prediction for IKK and detected two binding sites at the 3’UTR region of IKKβ for miR-34a (Figure 5H). Dual-luciferase reporter gene assay confirmed that miR-34a could directly inhibit the expression of IKKβ by binding to its 3’UTR region (Figure 5I). As shown in Figure 5J, 5K, HBx induced the expression of IKKβ, while miR-34a negatively regulated such induction. Besides, the IKKβ protein level of HBV related liver cancer samples with PVTT is the highest among the three groups (Figure S6). In summary, these data suggested that HBx may affect EMT and angiogenesis of primary liver cancer via miR-34a/NF-κB/HMGB1 axis.
Extracellular HMGB1 facilitates the IL-6/STAT3/miR-34a pathway to form a feedback loop
As a member of damage-associated molecular patterns (DAMPs), extracellular HMGB1 exerts significant tumor progression effects [20]. We had confirmed that miR-34a reduced the level of extracellular HMGB1, while whether extracellular HMGB1 has a feedback effect on miR-34a, even tumor EMT progress or angiogenesis was still not clear. We treated HepG2 and Huh7 cells with various recombinant human HMGB1 (rhHMGB1) concentrations for 24 hours for follow-up experiments to clarify this speculation. Interestingly, we observed a gradual decrease of miR-34a expression with an increasing concentration of rhHMGB1 (Figure 6A). Besides, rhHMGB1 stimulated EMT phenotype in a dose-dependent manner, and VEGF expression also increased (Figure 6B). These data indicated that there is an interaction between HMGB1 and miR-34a.
Figure 6.
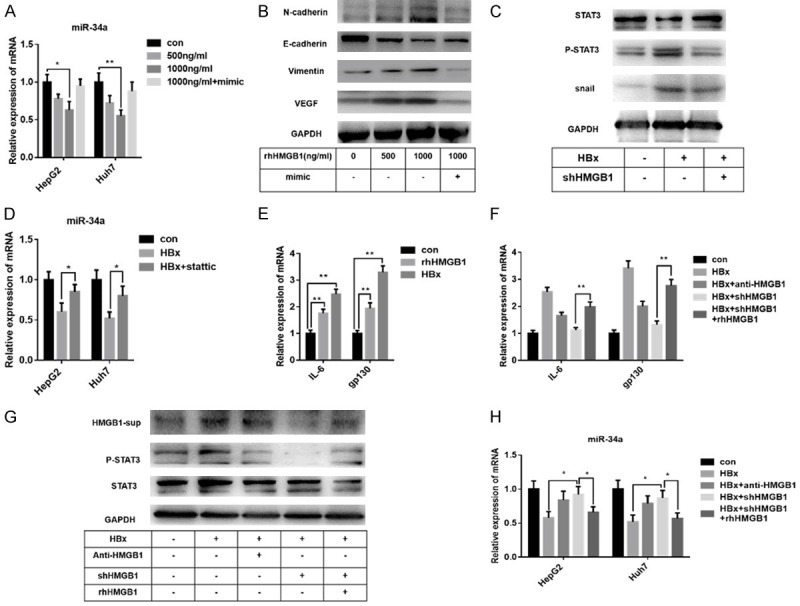
Extracellular HMGB1 facilitates the IL-6/STAT3/miR-34a pathway to form a feedback loop. (A) Q-PCR analysis shows a dose-related inhibition of miR-34a in HepG2/Huh7 cells by rhHMGB1. (B) With the increase of dose, the up-regulation of EMT-related factors and VEGF protein by rhHMGB1 is significant in HepG2 cells. (C) Immunoblot analysis reveals that knockdown of HMGB1 may inhibit the activation of STAT3 in HepG2-HBx cells. (D) Q-PCR analysis indicates that the inhibition of STAT3 could alleviate the suppression of miR-34a expression by HBx in HepG2/Huh7 cells. (E) RhHMGB1 up-regulates the expression of IL-6 and gp130 mRNA, which function as the upstream factor of STAT3 activation. (F-H) Immunoblot and q-PCR analysis reveal the promotion of rhHMGB1 on the IL-6/STAT3/miR-34a axis in HepG2-HBx/Huh7-HBx cells, while knockdown of HMGB1 reverses such promotion. The concentration of rhHMGB1 used in (E-H) is 1000 ng/ml. Data are means ± SEM from 3 independent experiments, * means P<0.05, ** means P<0.01, by one-way ANOVA.
As described above, HBx promoted the EMT progress and angiogenesis of liver cancer cells through up-regulating HMGB1 expression or activation of STAT3 (Figures 2 and 4C-H). However, the relationship between HMGB1 and STAT3 was not clear enough. As shown in Figure 6C, knockdown of HMGB1 affects the activation of STAT3 in HepG2 cells. It has been demonstrated that with IL-6 stimulation, STAT3 represses miR-34a expression by targeting its promoter region, thereby promotes EMT-mediated cancer invasion and metastasis [54,55]. In this study, we found that the inhibition of STAT3 could alleviate the suppression of miR-34a expression by HBx (Figure 6D). Furthermore, rhHMGB1 significantly increased the mRNA level of IL-6 and gp130, a receptor subunit of IL-6 and a critical factor in activating STAT3 [56] (Figure 6E). Thus, we speculated that there is a feedback effect of extracellular HMGB1 on the IL-6/STAT3/miR-34 axis in HBV related liver cancer. As shown in Figure 6F-H, HBx mediated IL-6/STAT3/miR-34a axis, while the treatment of rhHMGB1 reversed the inhibition of shHMGB1 on such signal pathway. With the above evidence, we concluded that extracellular HMGB1 promoted EMT/angiogenesis of HBx-transfected liver cancer cells through a feedback function on the IL-6/STAT3/miR-34a axis.
Conclusion
In summary, our study provided evidence that high expression of HMGB1 accounted for EMT and angiogenesis in HBV related liver cancer. HBx mediated HMGB1 expression was dependent on miR-34a reduction, activated by IL-6/STAT3. Moreover, extracellular HMGB1 enhanced EMT and angiogenesis in a miR-34a/NF-κB dependent way. These findings revealed a STAT3/miR-34a/NF-κB dependent HMGB1 up-regulation in metastasis of HBV related liver cancer (Figure 7).
Figure 7.
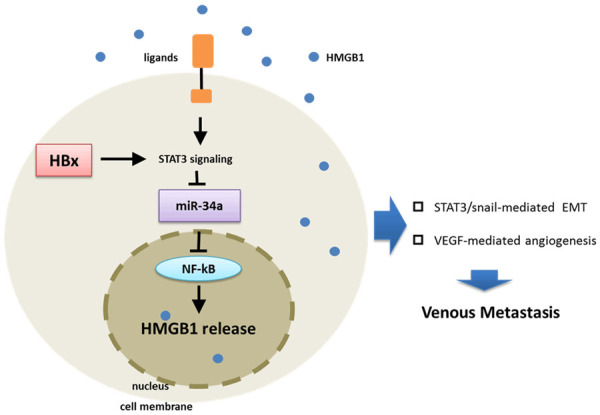
A model of HBx promoting venous metastasis of primary liver cancer. HBx promotes the activation of STAT3, which inhibits miR-34a expression. The inhibited miR-34a released its down-regulation of NF-κB, which promotes the expression of HMGB1. On the other hand, HMGB1 secreted extracellularly activates the IL-6/STAT3/miR-34a axis, and positive feedback promotes the secretion of HMGB1. Finally, induced HMGB1 leads to the formation of liver cancer PVTT by promoting EMT and angiogenesis.
Discussion
Primary liver cancer is one of the most common malignancies worldwide and the leading cause of cancer-related death [1]. Chronic inflammation resulted from hepatic B or C virus infection, alcoholic liver disease, and nonalcoholic steatohepatitis is the crucial feature of liver cancer progression [4]. Mounting evidence has shown that HBV infection promotes liver cancer initiation and development [3,4,7]. The previous study indicated that HBV status was tightly associated with PVTT occurrence in patients with liver cancer [10]. PVTT has been demonstrated as a significant risk factor for tumor metastasis and predicted poor prognosis [8-10]. However, the underlying mechanisms of liver cancer development caused by HBV infection need further elucidation.
HBx, a crucial effector of HBV infection, is involved in various aspects of liver cancer development [3]. HBx binds directly to DNA and inhibits transcriptional activation via interacting with tumor suppressors, such as p53 and Rb [3,11]. Many signal transduction pathways, including ERK, MAPK and NF-κB, are demonstrated to be affected by HBx [4]. Previous studies revealed that HBx promoted the EMT process via decreasing E-cadherin [57,58]. Moreover, VEGF production was enhanced by HBx, which contributed to tumor angiogenesis [59].
HMGB1 is considered a conserved chromatin-binding protein and is involved in tumor progression [60]. Accumulating data indicated that extracellular HMGB1 also promoted tumor proliferation, metastasis and angiogenesis [32]. HMGB1 could be released passively or actively [60]. Recent studies showed that hypoxia and HBx lead to HMGB1 secretion from tumor cells [27]. After binding to its receptors, such as Rage and TLR2/4/9, HMGB1 promoted tumor development through activating relevant signaling pathways [32,60]. In our study, we found there was a strong relationship between HMGB1 and PVTT, especially in HBV related liver cancer. Moreover, the HMGB1 expression was higher in HBV related liver cancer tissues, and HBx up-regulated HMGB1 expression in liver cancer cells. Additionally, targeting HMGB1 in HBx transfected cells abolished HBx induced EMT and angiogenesis, which have been proven to be mediated by STAT3 signaling [46,56]. In vivo experiments confirmed the importance of HMGB1 for tumor angiogenesis and metastasis [8].
Previous studies showed that HBx reduced miR-34a expression [10]. Mounting evidence indicated that miR-34a was repressed in various types of tumors, associated with tumor metastasis and angiogenesis [17,50,61]. Both HMGB1 and VEGF have been confirmed to be direct targets for miR-34a [26,51]. In our study, we observed that miR-34a mimic treatment abrogated HBx induced HMGB1, while exogenous HMGB1 reversed the inhibitory effect of miR-34a on cell invasion and angiogenesis. Extracellular HMGB1 has been demonstrated to participate in liver cancer progression [32]. Moreover, SIRT-1 was considered to regulate the nucleocytoplasmic translocation of HMGB1 and promote HMGB1 release, which activated nuclear factor NF-κB then directly regulated HMGB1 expression, thus forming positive feedback HMGB1 secretion-NF-κB activation [52,62]. Our observations showed that HBx induced NF-κB activation and suggested up-regulation of HMGB1 secretion, indicated crosstalk between HMGB1 and NF-κB. The prediction of miR-34a-IKKβ binding sites and dual-luciferase assay confirmed that miR-34a directly inhibited IKKβ expression and blocked the activation of the NF-κB signaling pathway, thus clarified the interaction of miR-34a and NF-κB.
It was reported that IL-6R/STAT3/miR-34a feedback loop promotes EMT mediated invasion and metastasis in colorectal cancer [54]. Moreover, HBx promoted HMGB1 secretion via calcium-dependent cascades, which resulting in tumor metastasis. Our study showed that HMGB1 treatment induced the EMT process and VEGF expression of liver cancer cells in a dose-dependent way. Interestingly, similar to HBx, exogenous HMGB1 facilitated IL-6/STAT3/miR-34a activation, suggesting positive feedback between HMGB1 and STAT3/miR-34a.
Acknowledgements
We thank Dr. Ning Tang for the clinical assistance with the article. This work was supported by grants from the National Natural Science Foundation of China (Grant No. 81670566), Jiangsu Province’s Key Provincial Talents Program (Grant No. ZDRCA2016066). The present study was approved by the Ethics Committee of Nanjing Drum Tower Hospital (Nanjing, China). All patients provided written informed consent. In vivo experiments were approved by the Institutional Animal Care and Use Committee of Nanjing University, China, based on the NIH Guide for the Care and Use of Laboratory Animals.
Disclosure of conflict of interest
None.
Abbreviations
- HBx
HBV-encoded X protein
- HMGB1
high-mobility group box 1
- EMT
epithelial-mesenchymal transition
- PVTT
portal vein tumor thrombosis
- qRT-PCR
quantitative real-time polymerase chain reaction
- RNAi
RNA interference
- MVD
microvascular density
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- PVDF
polyvinylidene fluoride
- HRP
horseradish peroxidase
- HUVECs
human umbilical vein endothelial cells
Supporting Information
References
- 1.Han ZG. Functional genomic studies: insights into the pathogenesis of liver cancer. Annu Rev Genomics Hum Genet. 2012;13:171–205. doi: 10.1146/annurev-genom-090711-163752. [DOI] [PubMed] [Google Scholar]
- 2.Portolani N, Coniglio A, Ghidoni S, Giovanelli M, Benetti A, Tiberio GA, Giulini SM. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg. 2006;243:229–235. doi: 10.1097/01.sla.0000197706.21803.a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dienstag JL. Hepatitis B virus infection. N Engl J Med. 2008;359:1486–1500. doi: 10.1056/NEJMra0801644. [DOI] [PubMed] [Google Scholar]
- 4.Neuveut C, Wei Y, Buendia MA. Mechanisms of HBV-related hepatocarcinogenesis. J Hepatol. 2010;52:594–604. doi: 10.1016/j.jhep.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 5.Benhenda S, Cougot D, Buendia MA, Neuveut C. Hepatitis B virus X protein molecular functions and its role in virus life cycle and pathogenesis. Adv Cancer Res. 2009;103:75–109. doi: 10.1016/S0065-230X(09)03004-8. [DOI] [PubMed] [Google Scholar]
- 6.Diao J, Garces R, Richardson CD. X protein of hepatitis B virus modulates cytokine and growth factor related signal transduction pathways during the course of viral infections and hepatocarcinogenesis. Cytokine Growth Factor Rev. 2001;12:189–205. doi: 10.1016/s1359-6101(00)00034-4. [DOI] [PubMed] [Google Scholar]
- 7.Kew MC. Hepatitis B virus x protein in the pathogenesis of hepatitis B virus-induced hepatocellular carcinoma. J Gastroenterol Hepatol. 2011;26(Suppl 1):144–152. doi: 10.1111/j.1440-1746.2010.06546.x. [DOI] [PubMed] [Google Scholar]
- 8.Ye LY, Chen W, Bai XL, Xu XY, Zhang Q, Xia XF, Sun X, Li GG, Hu QD, Fu QH, Liang TB. Hypoxia-induced epithelial-to-mesenchymal transition in hepatocellular carcinoma induces an immunosuppressive tumor microenvironment to promote metastasis. Cancer Res. 2016;76:818–830. doi: 10.1158/0008-5472.CAN-15-0977. [DOI] [PubMed] [Google Scholar]
- 9.Takizawa D, Kakizaki S, Sohara N, Sato K, Takagi H, Arai H, Katakai K, Kojima A, Matsuzaki Y, Mori M. Hepatocellular carcinoma with portal vein tumor thrombosis: clinical characteristics, prognosis, and patient survival analysis. Dig Dis Sci. 2007;52:3290–3295. doi: 10.1007/s10620-007-9808-2. [DOI] [PubMed] [Google Scholar]
- 10.Yang P, Li QJ, Feng Y, Zhang Y, Markowitz GJ, Ning S, Deng Y, Zhao J, Jiang S, Yuan Y, Wang HY, Cheng SQ, Xie D, Wang XF. TGF-beta-miR-34a-CCL22 signaling-induced Treg cell recruitment promotes venous metastases of HBV-positive hepatocellular carcinoma. Cancer Cell. 2012;22:291–303. doi: 10.1016/j.ccr.2012.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouchard MJ, Schneider RJ. The enigmatic X gene of hepatitis B virus. J Virol. 2004;78:12725–12734. doi: 10.1128/JVI.78.23.12725-12734.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu LP, Liang HF, Chen XP, Zhang WG, Yang SL, Xu T, Ren L. The role of NF-kappaB in Hepatitis b virus X protein-mediated upregulation of VEGF and MMPs. Cancer Invest. 2010;28:443–451. doi: 10.3109/07357900903405959. [DOI] [PubMed] [Google Scholar]
- 13.Lee SW, Lee YM, Bae SK, Murakami S, Yun Y, Kim KW. Human hepatitis B virus X protein is a possible mediator of hypoxia-induced angiogenesis in hepatocarcinogenesis. Biochem Biophys Res Commun. 2000;268:456–461. doi: 10.1006/bbrc.2000.2093. [DOI] [PubMed] [Google Scholar]
- 14.Wei JS, Song YK, Durinck S, Chen QR, Cheuk AT, Tsang P, Zhang Q, Thiele CJ, Slack A, Shohet J, Khan J. The MYCN oncogene is a direct target of miR-34a. Oncogene. 2008;27:5204–5213. doi: 10.1038/onc.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bommer GT, Gerin I, Feng Y, Kaczorowski AJ, Kuick R, Love RE, Zhai Y, Giordano TJ, Qin ZS, Moore BB, MacDougald OA, Cho KR, Fearon ER. p53-mediated activation of miRNA34 candidate tumor-suppressor genes. Curr Biol. 2007;17:1298–1307. doi: 10.1016/j.cub.2007.06.068. [DOI] [PubMed] [Google Scholar]
- 16.Sun F, Fu H, Liu Q, Tie Y, Zhu J, Xing R, Sun Z, Zheng X. Downregulation of CCND1 and CDK6 by miR-34a induces cell cycle arrest. FEBS Lett. 2008;582:1564–1568. doi: 10.1016/j.febslet.2008.03.057. [DOI] [PubMed] [Google Scholar]
- 17.Li N, Fu H, Tie Y, Hu Z, Kong W, Wu Y, Zheng X. miR-34a inhibits migration and invasion by down-regulation of c-Met expression in human hepatocellular carcinoma cells. Cancer Lett. 2009;275:44–53. doi: 10.1016/j.canlet.2008.09.035. [DOI] [PubMed] [Google Scholar]
- 18.Ge H, Roeder RG. The high mobility group protein HMG1 can reversibly inhibit class II gene transcription by interaction with the TATA-binding protein. J Biol Chem. 1994;269:17136–17140. [PubMed] [Google Scholar]
- 19.Mukherjee RM, Shravanti GV, Jakkampudi A, Kota R, Jangala AL, Reddy PB, Rao PN, Gupta R, Reddy DN. Reduced expression of DNA damage repair genes high mobility group Box1 and poly (ADP-ribose) polymerase1 in inactive carriers of hepatitis B virus infection-A possible stage of viral integration. J Clin Exp Hepatol. 2013;3:89–95. doi: 10.1016/j.jceh.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao J, Ding Y, Huang J, Li Q, Liu Y, Ni W, Zhang Y, Zhu Y, Chen L, Chen B. The association of HMGB1 gene with the prognosis of HCC. PLoS One. 2014;9:e89097. doi: 10.1371/journal.pone.0089097. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Ko YB, Kim BR, Nam SL, Yang JB, Park SY, Rho SB. High-mobility group box 1 (HMGB1) protein regulates tumor-associated cell migration through the interaction with BTB domain. Cell Signal. 2014;26:777–783. doi: 10.1016/j.cellsig.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 22.Chen M, Liu Y, Varley P, Chang Y, He XX, Huang H, Tang D, Lotze MT, Lin J, Tsung A. High-mobility group box 1 promotes hepatocellular carcinoma progression through miR-21-mediated matrix metalloproteinase activity. Cancer Res. 2015;75:1645–1656. doi: 10.1158/0008-5472.CAN-14-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan W, Chang Y, Liang X, Cardinal JS, Huang H, Thorne SH, Monga SP, Geller DA, Lotze MT, Tsung A. High-mobility group box 1 activates caspase-1 and promotes hepatocellular carcinoma invasiveness and metastases. Hepatology. 2012;55:1863–1875. doi: 10.1002/hep.25572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu L, Li X, Chen Y, Fang J, Ge Z. High-mobility group box 1: a novel inducer of the epithelial-mesenchymal transition in colorectal carcinoma. Cancer Lett. 2015;357:527–534. doi: 10.1016/j.canlet.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 25.Liu Z, Dou C, Wang Y, Jia Y, Li Q, Zheng X, Yao Y, Liu Q, Song T. Highmobility group box 1 has a prognostic role and contributes to epithelial mesenchymal transition in human hepatocellular carcinoma. Mol Med Rep. 2015;12:5997–6004. doi: 10.3892/mmr.2015.4182. [DOI] [PubMed] [Google Scholar]
- 26.Chandrasekaran KS, Sathyanarayanan A, Karunagaran D. Downregulation of HMGB1 by miR-34a is sufficient to suppress proliferation, migration and invasion of human cervical and colorectal cancer cells. Tumour Biol. 2016;37:13155–13166. doi: 10.1007/s13277-016-5261-1. [DOI] [PubMed] [Google Scholar]
- 27.Chen S, Dong Z, Yang P, Wang X, Jin G, Yu H, Chen L, Li L, Tang L, Bai S, Yan H, Shen F, Cong W, Wen W, Wang H. Hepatitis B virus X protein stimulates high mobility group box 1 secretion and enhances hepatocellular carcinoma metastasis. Cancer Lett. 2017;394:22–32. doi: 10.1016/j.canlet.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Li J, Wang S, Yang F, Zhou Y, Liu Y, Zhu W, Shi X. HBxassociated long noncoding RNA activated by TGFbeta promotes cell invasion and migration by inducing autophagy in primary liver cancer. Int J Oncol. 2020;56:337–347. doi: 10.3892/ijo.2019.4908. [DOI] [PubMed] [Google Scholar]
- 29.Duan L, Wu R, Zhang X, Wang D, You Y, Zhang Y, Zhou L, Chen W. HBx-induced S100A9 in NF-kappaB dependent manner promotes growth and metastasis of hepatocellular carcinoma cells. Cell Death Dis. 2018;9:629. doi: 10.1038/s41419-018-0512-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei X, Li N, Li S, Shi J, Guo W, Zheng Y, Cheng S. Hepatitis B virus infection and active replication promote the formation of vascular invasion in hepatocellular carcinoma. BMC Cancer. 2017;17:304. doi: 10.1186/s12885-017-3293-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu D, Liang H, Wang H, Duan C, Yazdani H, Zhou J, Pan Y, Shan B, Su Z, Wei J, Cui T, Tai S. Hepatitis B virus-X protein regulates high mobility group box 1 to promote the formation of hepatocellular carcinoma. Oncol Lett. 2018;16:4418–4426. doi: 10.3892/ol.2018.9178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X, Xiang L, Li H, Chen P, Feng Y, Zhang J, Yang N, Li F, Wang Y, Zhang Q, Li F, Cao F. The role of HMGB1 signaling pathway in the development and progression of hepatocellular carcinoma: a review. Int J Mol Sci. 2015;16:22527–22540. doi: 10.3390/ijms160922527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK, Vonderheide RH, Leach SD, Stanger BZ. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 35.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 37.Ding ZB, Shi YH, Zhou J, Shi GM, Ke AW, Qiu SJ, Wang XY, Dai Z, Xu Y, Fan J. Liver-intestine cadherin predicts microvascular invasion and poor prognosis of hepatitis B virus-positive hepatocellular carcinoma. Cancer. 2009;115:4753–4765. doi: 10.1002/cncr.24513. [DOI] [PubMed] [Google Scholar]
- 38.Lv G, Tan Y, Lv H, Fang T, Wang C, Li T, Yu Y, Hu C, Wen W, Wang H, Yang W. MXR7 facilitates liver cancer metastasis via epithelial-mesenchymal transition. Sci China Life Sci. 2017;60:1203–1213. doi: 10.1007/s11427-016-9042-y. [DOI] [PubMed] [Google Scholar]
- 39.Giatromanolaki A, Koukourakis MI, Theodossiou D, Barbatis K, O’Byrne K, Harris AL, Gatter KC. Comparative evaluation of angiogenesis assessment with anti-factor-VIII and anti-CD31 immunostaining in non-small cell lung cancer. Clin Cancer Res. 1997;3:2485–2492. [PubMed] [Google Scholar]
- 40.Ansieau S, Caron de Fromentel C, Bastid J, Morel AP, Puisieux A. Role of the epithelial-mesenchymal transition during tumor progression. Bull Cancer. 2010;97:7–15. doi: 10.1684/bdc.2009.1025. [DOI] [PubMed] [Google Scholar]
- 41.Motavaf M, Safari S, Saffari Jourshari M, Alavian SM. Hepatitis B virus-induced hepatocellular carcinoma: the role of the virus x protein. Acta Virol. 2013;57:389–396. doi: 10.4149/av_2013_04_389. [DOI] [PubMed] [Google Scholar]
- 42.Yang SZ, Zhang LD, Zhang Y, Xiong Y, Zhang YJ, Li HL, Li XW, Dong JH. HBx protein induces EMT through c-Src activation in SMMC-7721 hepatoma cell line. Biochem Biophys Res Commun. 2009;382:555–560. doi: 10.1016/j.bbrc.2009.03.079. [DOI] [PubMed] [Google Scholar]
- 43.Yang X, Liang L, Zhang XF, Jia HL, Qin Y, Zhu XC, Gao XM, Qiao P, Zheng Y, Sheng YY, Wei JW, Zhou HJ, Ren N, Ye QH, Dong QZ, Qin LX. MicroRNA-26a suppresses tumor growth and metastasis of human hepatocellular carcinoma by targeting interleukin-6-Stat3 pathway. Hepatology. 2013;58:158–170. doi: 10.1002/hep.26305. [DOI] [PubMed] [Google Scholar]
- 44.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bromberg J, Darnell JE Jr. The role of STATs in transcriptional control and their impact on cellular function. Oncogene. 2000;19:2468–2473. doi: 10.1038/sj.onc.1203476. [DOI] [PubMed] [Google Scholar]
- 46.Chen X, Zheng Q, Li W, Lu Y, Ni Y, Ma L, Fu Y. SOX5 induces lung adenocarcinoma angiogenesis by inducing the expression of VEGF through STAT3 signaling. Onco Targets Ther. 2018;11:5733–5741. doi: 10.2147/OTT.S176533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao M, Gao FH, Wang JY, Liu F, Yuan HH, Zhang WY, Jiang B. JAK2/STAT3 signaling pathway activation mediates tumor angiogenesis by upregulation of VEGF and bFGF in non-small-cell lung cancer. Lung Cancer. 2011;73:366–374. doi: 10.1016/j.lungcan.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 48.Teng J, Wang X, Xu Z, Tang N. HBx-dependent activation of Twist mediates STAT3 control of epithelium-mesenchymal transition of liver cells. J Cell Biochem. 2013;114:1097–1104. doi: 10.1002/jcb.24450. [DOI] [PubMed] [Google Scholar]
- 49.Zheng Y, Ming P, Zhu C, Si Y, Xu S, Chen A, Wang J, Zhang B. Hepatitis B virus X protein-induced SH2 domain-containing 5 (SH2D5) expression promotes hepatoma cell growth via an SH2D5-transketolase interaction. J Biol Chem. 2019;294:4815–4827. doi: 10.1074/jbc.RA118.005739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Siemens H, Jackstadt R, Hunten S, Kaller M, Menssen A, Gotz U, Hermeking H. miR-34 and SNAIL form a double-negative feedback loop to regulate epithelial-mesenchymal transitions. Cell Cycle. 2011;10:4256–4271. doi: 10.4161/cc.10.24.18552. [DOI] [PubMed] [Google Scholar]
- 51.Kumar B, Yadav A, Lang J, Teknos TN, Kumar P. Dysregulation of microRNA-34a expression in head and neck squamous cell carcinoma promotes tumor growth and tumor angiogenesis. PLoS One. 2012;7:e37601. doi: 10.1371/journal.pone.0037601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lan KC, Chao SC, Wu HY, Chiang CL, Wang CC, Liu SH, Weng TI. Salidroside ameliorates sepsis-induced acute lung injury and mortality via downregulating NF-kappaB and HMGB1 pathways through the upregulation of SIRT1. Sci Rep. 2017;7:12026. doi: 10.1038/s41598-017-12285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang J, Xiong T, Zhang Z, Tan Y, Guo L. Inhibition of the receptor for advanced glycation inhibits lipopolysaccharide-mediated High mobility group protein B1 and Interleukin-6 synthesis in human gingival fibroblasts through the NF-kappaB signaling pathway. Arch Oral Biol. 2019;105:81–87. doi: 10.1016/j.archoralbio.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 54.Rokavec M, Oner MG, Li H, Jackstadt R, Jiang L, Lodygin D, Kaller M, Horst D, Ziegler PK, Schwitalla S, Slotta-Huspenina J, Bader FG, Greten FR, Hermeking H. IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated colorectal cancer invasion and metastasis. J Clin Invest. 2014;124:1853–1867. doi: 10.1172/JCI73531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin X, Lin BW, Chen XL, Zhang BL, Xiao XJ, Shi JS, Lin JD, Chen X. PAI-1/PIAS3/Stat3/miR-34a forms a positive feedback loop to promote EMT-mediated metastasis through Stat3 signaling in non-small cell lung cancer. Biochem Biophys Res Commun. 2017;493:1464–1470. doi: 10.1016/j.bbrc.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 56.Kuang Y, Guo W, Ling J, Xu D, Liao Y, Zhao H, Du X, Wang H, Xu M, Song H, Wang T, Jing B, Li K, Hu M, Wu W, Deng J, Wang Q. Iron-dependent CDK1 activity promotes lung carcinogenesis via activation of the GP130/STAT3 signaling pathway. Cell Death Dis. 2019;10:297. doi: 10.1038/s41419-019-1528-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu J, Lian Z, Han S, Waye MM, Wang H, Wu MC, Wu K, Ding J, Arbuthnot P, Kew M, Fan D, Feitelson MA. Downregulation of E-cadherin by hepatitis B virus X antigen in hepatocellullar carcinoma. Oncogene. 2006;25:1008–1017. doi: 10.1038/sj.onc.1209138. [DOI] [PubMed] [Google Scholar]
- 58.Ha HL, Kwon T, Bak IS, Erikson RL, Kim BY, Yu DY. IGF-II induced by hepatitis B virus X protein regulates EMT via SUMO mediated loss of E-cadherin in mice. Oncotarget. 2016;7:56944–56957. doi: 10.18632/oncotarget.10922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moon EJ, Jeong CH, Jeong JW, Kim KR, Yu DY, Murakami S, Kim CW, Kim KW. Hepatitis B virus X protein induces angiogenesis by stabilizing hypoxia-inducible factor-1alpha. FASEB J. 2004;18:382–384. doi: 10.1096/fj.03-0153fje. [DOI] [PubMed] [Google Scholar]
- 60.Naglova H, Bucova M. HMGB1 and its physiological and pathological roles. Bratisl Lek Listy. 2012;113:163–171. doi: 10.4149/bll_2012_039. [DOI] [PubMed] [Google Scholar]
- 61.Budhu A, Jia HL, Forgues M, Liu CG, Goldstein D, Lam A, Zanetti KA, Ye QH, Qin LX, Croce CM, Tang ZY, Wang XW. Identification of metastasis-related microRNAs in hepatocellular carcinoma. Hepatology. 2008;47:897–907. doi: 10.1002/hep.22160. [DOI] [PubMed] [Google Scholar]
- 62.Le K, Chibaatar Daliv E, Wu S, Qian F, Ali AI, Yu D, Guo Y. SIRT1-regulated HMGB1 release is partially involved in TLR4 signal transduction: a possible anti-neuroinflammatory mechanism of resveratrol in neonatal hypoxic-ischemic brain injury. Int Immunopharmacol. 2019;75:105779. doi: 10.1016/j.intimp.2019.105779. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.