Abstract
The renin-angiotensin system (RAS) regulates physiological functions of the cardiovascular system, kidneys, and other tissues. Various in vivo and in vitro studies have shown that RAS plays a pivotal role in the development of malignant tumors, while several retrospective studies have confirmed that patients undergoing long-term RAS inhibitors (RASi) treatment have a lowered risk of cancer. Moreover, blocking RAS has been shown to inhibit tumor growth, metastasis, and angiogenesis in various experimental models of malignant tumors. Herein, we review the available RASi-related literature and provide an analysis using the scientific atlas software VOSviewer. We observed that recent studies have primarily focused on gene expression, tumor biology, and survival analysis. Through an in-depth data analysis from the Cancer Genome Atlas (TCGA) and Genotype Tissue Expression (GTEx), we identified the impact of AGTR1, an essential component of RAS, on tumors, and we discuss the underlying biological mechanism of RASi. Furthermore, we outline the research progress and potential use of RASi in tumor treatment. Overall, RASi may be a promising adjunct in cancer therapy.
Keywords: Renin-angiotensin system, RAS inhibitors, angiotensin receptor blockers, AGTR1, tumor
Introduction
The renin-angiotensin system (RAS) is a vital system for the regulation of human bodily fluids of not only in the circulatory system but also in several other tissues and organs (Figure 1) [1]. Renin-angiotensin system inhibitors (RASi) are widely used in the treatment of hypertension and related complications. In addition to this role, RAS is closely associated with the pathogenesis of malignant tumors [2]. Therefore, the potential use of RASi to treat tumors has attracted attention. A meta-analysis showed that the use of angiotensin receptor blockers (ARBs) may increase the risk of cancer [3], but the study had several limitations [4]. Subsequently, multi-institutional studies have reported that RASi do not increase the incidence of cancer [5]. Although the effect of RAS blockade on the incidence of cancer remains controversial, most experimental models have verified that RAS blockade could improve the patient prognosis by suppressing angiogenesis and inhibiting tumor cell proliferation and metastasis. Therefore, the role of RASi in tumor treatment has become a hotspot in research.
Figure 1.
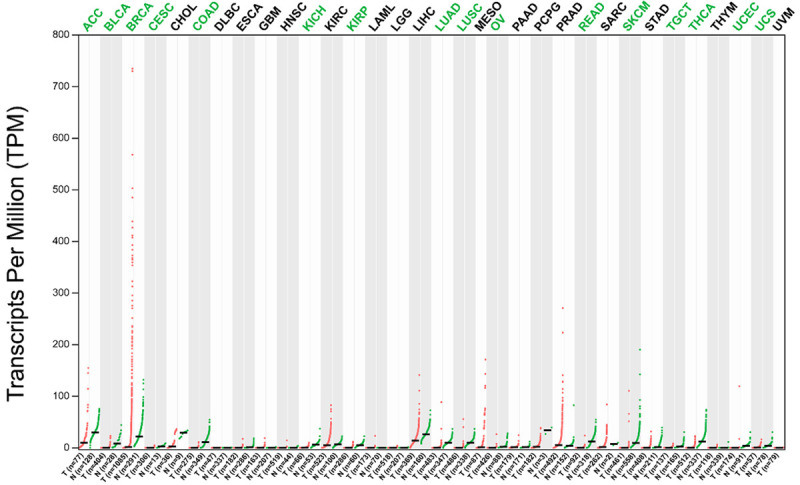
Gene expression profile of AGTR1 in 33 cancer types and matched non-tumor samples. Each point represents a different tumor or normal sample. Short black lines represent the median gene expression level. The data were obtained through Gene Expression Profiling Interactive Analysis (GEPIA). T: tumor tissue; N: normal tissue; n: number; ACC: adrenocortical carcinoma; BLCA: bladder urothelial carcinoma; BRCA: breast invasive carcinoma; CESC: cervical squamous cell carcinoma and endocervical adenocarcinoma; CHOL: cholangiocarcinoma; COAD: colon adenocarcinoma; DLBC: lymphoid neoplasm diffuse large B-cell lymphoma; ESCA: esophageal carcinoma; GBM: glioblastoma multiforme; HNSC: head and neck squamous cell carcinoma; KICH: kidney chromophobe; KIRC: kidney renal clear cell carcinoma; KIRP: kidney renal papillary cell carcinoma; LAML: acute myeloid leukemia; LGG: brain lower grade glioma; LIHC: liver hepatocellular carcinoma; LUAD: lung adenocarcinoma; LUSC: lung squamous cell carcinoma; MESO: mesothelioma; OV: ovarian serous cystadenocarcinoma; PAAD: pancreatic adenocarcinoma; PCPG: pheochromocytoma and paraganglioma; PRAD: prostate adenocarcinoma; READ: rectum adenocarcinoma; SARC: sarcoma; SKCM: skin cutaneous melanoma; STAD: stomach adenocarcinoma; TGCT: testicular germ cell tumors; THCA: thyroid carcinoma; THYM: thymoma; UCEC: uterine corpus endometrial carcinoma; UCS: uterine carcinosarcoma.
Cancer is one of the leading causes of suffering and death worldwide, apart from being a severe economic burden on patients and their families [6,7]. The research and development of novel anticancer drugs can be time-consuming and costly. Therefore, the repurposing and studies of existing therapies, such as RASi, may be useful to help expand their application and provide evidence for their use in tumor treatment, which would be more cost-effective than developing new drugs or treatment strategies. Exploration of the positive association between RASi and the prognosis of patients with specific cancer types, malignant features, or stages may help to optimize the recovery from treatment and promote the progress of individualized treatment plans.
Classification of RASi
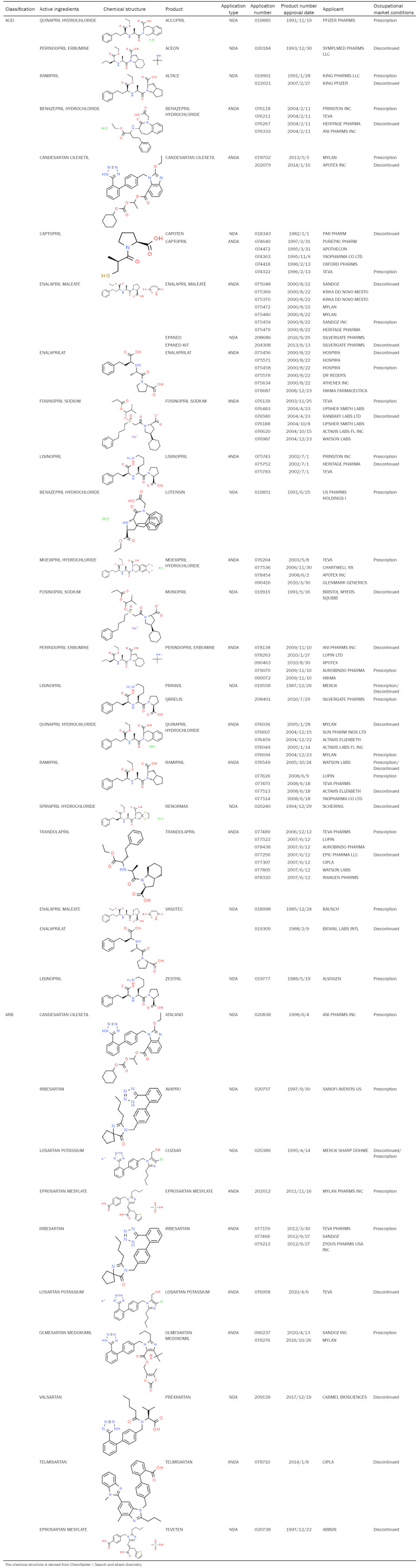
RASi, include angiotensin-converting enzyme inhibitors (ACEI) and hemotensin II receptor antagonists (ARBs), and those that are approved by the Food and Drug Administration (FDA) are listed in Table 1. ACEI reduce the production of angiotensin II by inhibiting angiotensin-converting enzyme (ACE), whereas ARB primarily blocks the effect of AngII by antagonizing AT1R.
Table 1.
U.S. Food and Drug Administration (FDA) listed renin-angiotensin system inhibitors, including angiotensin-converting enzyme inhibitors (ACEI) and angiotensin receptor inhibitors (ARB)
Basis for the application of RASi in cancer treatment
Bibliometric analysis
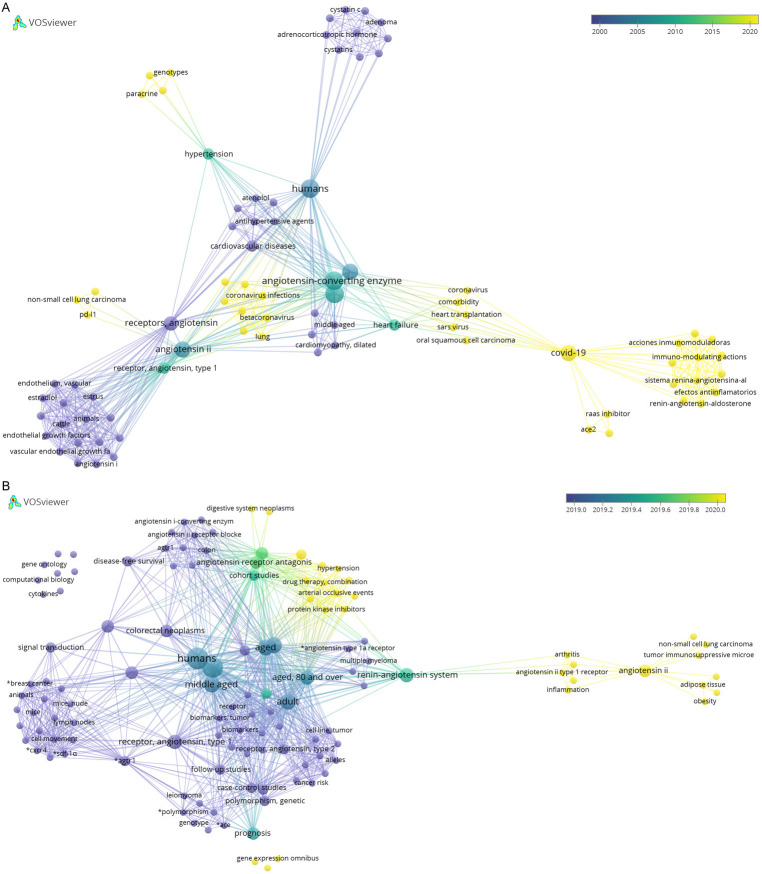
We searched the RAS, RASi, AGTR1, ACE, and tumor-related literature through PubMed database and used the scientific knowledge map software “VOSviewer” to construct and visualize the relationships between “network data” (document knowledge units). We observed that the relevant literature catalogs showed changes at several time points (Figure 2). Between 2000 and 2015, the literature primarily focused on cardiovascular diseases, such as hypertension and heart failure, as well as the impact of the use of RASi on patient survival analysis (Figure 2A). Over the past 5 years, researchers have begun to focus on cancer-related factors such as AngII and ATR1, vascular endothelial growth factor, tumor immunosuppressive microenvironment, and PD-L1. The literature search for the past two years showed that the keywords “AGTR1” and “RASi” appeared frequently in cancer research-related literature, indicating that AGTR1 and ACE are closely associated with the occurrence and development of tumors. Specifically, the articles based on AGTR1 mainly focused on the effects of gene expression and polymorphism on “cell apoptosis”, “tumor metastasis”, and “tumor immunosuppressive microenvironment”; whereas articles based on the ACE research primarily focused on genotype-phenotype and apoptosis related studies, as well as cancers such as cervical cancer and lymphoma. Research on RASi has focused on the treatment of digestive system tumors, chronic myeloid leukemia, and breast cancer, as well as the combined use of protein kinase inhibitors and other drugs (Figure 2B). Collectively, these studies demonstrated the potential of RASi in tumor treatment. Due to the high incidence of cancer and the acquisition of tolerance for traditional treatments, an increasing number of studies have focused on the search for new treatment strategy, such as targeted therapy, immunotherapy, and combination therapy. In recent years, researchers have also explored the role of RASi, besides their effects on the cardiovascular system, and have made significant advances.
Figure 2.
A. Bibliometric map of RASi application during year 2000 to 2020. B. Bibliometric map of the application of RASi and tumor from 2000 to 2020. Dot size is proportional to the frequencies of particular keywords in analyzed articles. Lines between two dots indicate that these two keywords appear in the same article. The thicker the line, the more frequently the two keywords appear in the same article. The color indicates the year when a keyword appears most often in an article.
The controversial role of RASi in different tumors
Although results reported from multiple studies have shown that the use of RASi can improve patient prognosis, no correlation has been reported between RASi and cancer prognosis, and in fact, some studies have even indicated that it may even increase the risk of cancer.
A cohort study on British individuals had reported that the use of RASi was not associated with a reduction in the risk of pancreatic cancer [8]. In contrast, in a retrospective study on lung cancer, the use of RASi could reduce tumor progression and improve patient prognosis [9]. A recent review showed that patients with anti-VEGF-responsive tumors, such as hepatocellular carcinoma (HCC), appear to be more sensitive to treatment with RASi, which can significantly improve their prognosis [10]. In addition, the use of RASi is an independent prognostic factor for longer cancer-specific and overall survival in patients with bladder cancer [11]. However, in a meta-review that included 13 breast cancer studies, only two studies reported beneficial effects of RSAi, whereas three studies reported poor outcomes [10]. In an acute myeloid leukemia cell model, combined treatment with losartan and doxorubicin could increase the sensitivity of certain cell lines to doxorubicin, whereas no changes in the therapeutic effect was observed in other cell lines [12]. A large epidemiological study showed that the benefits of RASi for cancer treatment reported in case-control studies and cohort studies were not observed in randomized controlled trials (RCTs) [13].
Effect of high AGTR1 expression in malignant tissue on patient survival
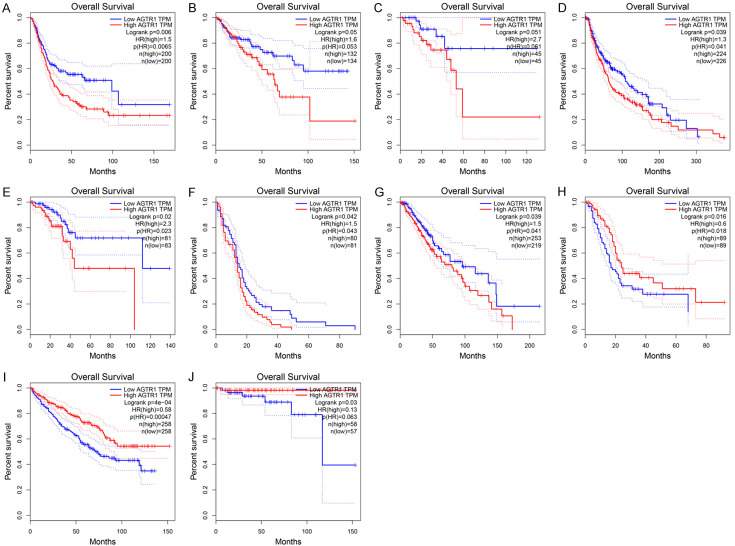
AGTR1 is one of the most studied genes in the RAS and plays a crucial role in tumors. Based on an analysis of the large TCGA and GTEx data found in the GEPIA database, we found that AGTR1 is expressed at lower levels in tumor tissue than normal tissue (Figure 1). We analyzed the relationship between the expression level of AGTR1 and survival in 31 types of tumor tissues (excluding mesothelioma and uveal melanoma lacking the control group), and the results are listed in Table 2. AGTR1 expression is associated with survival in most tumors and is differentially expressed in tumor and normal tissues. Examples of tumors showing differential expression of AGTR1 include uterine corpus endometrial carcinoma, colon adenocarcinoma, cutaneous skin melanoma, urothelial bladder carcinoma, and rectum adenocarcinoma (Table 2). In addition, in tumors with differential AGTR1 expression between tumor and normal tissue, higher expression of AGTR1 in tumor tissue was negatively correlated with patient survival (Figure 3A-E). In tumor tissues where there was no difference in the expression of AGTR1 between normal and tumor tissues, higher expression of AGTR1 was negatively correlated (Figure 3F, 3G) or positively correlated (Figure 3J) with patient survival.
Table 2.
Expression levels of AGTR1 in 31 cancer types and matched non-tumor samples and the relationship between high AGTR1 expression in tumor tissues and patient survival
| Tumor | T Median | N Median | HR (high) | P (HR) | logrankP |
|---|---|---|---|---|---|
| ACC | 10.04 | 29.98 | 1.2 | 0.63 | 0.62 |
| BLCA | 0.21 | 8.32 | 1.5 | 0.0065 | 0.006 |
| BRCA | 1.89 | 21.87 | 1.1 | 0.47 | 0.47 |
| CESC | 0.05 | 3.01 | 0.97 | 0.89 | 0.89 |
| CHOL | 2.83 | 29.62 | 2.1 | 0.14 | 0.13 |
| COAD | 0.09 | 11.07 | 1.6 | 0.053 | 0.05 |
| DLBC | 0.05 | 0.02 | 1.8 | 0.42 | 0.41 |
| ESCA | 0.17 | 1.01 | 0.75 | 0.22 | 0.22 |
| GBM | 0.35 | 0.11 | 1.5 | 0.043 | 0.042 |
| HNSC | 0.08 | 0.21 | 1.2 | 0.24 | 0.24 |
| KICH | 0.7 | 6.33 | 0.76 | 0.66 | 0.66 |
| KIRC | 4.94 | 6.92 | 0.58 | 0.00047 | 4.00E-04 |
| KIRP | 0.29 | 5.4 | 0.68 | 0.21 | 0.21 |
| LAML | 0.1 | 0.02 | 1.2 | 0.44 | 0.44 |
| LGG | 0.09 | 0.11 | 1.5 | 0.041 | 0.039 |
| LIHC | 14.21 | 26.29 | 0.96 | 0.83 | 0.83 |
| LUAD | 0.61 | 10.09 | 0.84 | 0.26 | 0.26 |
| LUSC | 0.28 | 10.2 | 1.3 | 0.09 | 0.09 |
| OV | 0.16 | 2.06 | 0.96 | 0.77 | 0.78 |
| PAAD | 1.17 | 1.36 | 0.6 | 0.018 | 0.016 |
| PCPG | 2.14 | 34.36 | 0.25 | 0.22 | 0.19 |
| PRAD | 5.54 | 4.19 | 1.8 | 0.37 | 0.37 |
| READ | 0.26 | 12.72 | 2.7 | 0.061 | 0.051 |
| SARC | 1.92 | 7.69 | 0.76 | 0.17 | 0.17 |
| SKCM | 0.11 | 9.43 | 1.3 | 0.041 | 0.039 |
| STAD | 0.27 | 1.38 | 1.4 | 0.063 | 0.062 |
| TGCT | 0.39 | 2.3 | 3.1 | 0.33 | 0.31 |
| THCA | 0.45 | 12.09 | 0.91 | 0.85 | 0.85 |
| THYM | 0.14 | 0.02 | 0.13 | 0.063 | 0.03 |
| UCEC | 0.05 | 4.11 | 2.3 | 0.023 | 0.02 |
| UCS | 0.83 | 4.22 | 1.2 | 0.65 | 0.67 |
Blue indicates that the expression of AGTR1 in tumor groups and normal tissues is significantly different. The data were obtained through Gene Expression Profiling Interactive Analysis (GEPIA). T median: Median expression of tumor tissue; N median: median expression of normal tissue.
Figure 3.
The effect of high expression of AGTR1 in tumor tissue on the survival of patients. The median expression level of AGTR1 was divided into one-to-one groups. A. Bladder urothelial carcinoma (BLCA); B. Colon adenocarcinoma (COAD); C. Rectal adenocarcinoma (READ); D. Skin cutaneous melanoma (SKCM); E. Uterine corpus endometrial carcinoma (UCEC); F. Glioblastoma multiforme (GBM); G. Brain lower-grade glioma (LGG); H. Pancreatic adenocarcinoma (PAAD); I. Kidney renal clear cell carcinoma (KIRC); J. Thymoma (THYM). The data was obtained through Gene Expression Profiling Interactive Analysis (GEPIA).
The above mentioned studies indicated that the response to treatment with RASi may vary depending on various factors including tumor type, characteristics or stage, and study design. In some tumors, detailed analysis revealed that elevated expression of AGTR1 is closely related to survival, which may also explain why RASi do not show significant benefits for patients in some studies. Therefore, it is necessary to carry out treatment with RASi according to the types and characteristics of the tumor, and the maximum therapeutic benefit is expected to be realized using personalized treatment plans.
Basic mechanisms of RASi in tumor treatment
The above mentioned data analysis and a variety of experimental evidence indicated that components of the RAS exist in a variety of solid tumors, such as in the breast, liver, and gastrointestinal tract tumors (Figure 1), and they are involved in the pathological and physiological processes of cancer, including proliferation, migration, apoptosis, and angiogenesis, suggesting that dysfunction of RAS contributes to tumor progression [4,14-17]. At the same time, abnormal expression levels of RAS have been observed in the tumor microenvironment, including tumor-associated macrophages (TAM), regulatory T cells (Tregs), fibroblasts, and the surrounding matrix. These are related to the regulation of immune function, vascular endothelial growth factor (VEGF), hypoxia, and acidosis in the matrix [4,18-20]. Therefore, whether the antagonistic effects of RAS can produce the expected antitumor effects remains a controversial subject. Many experimental studies have shown that RASi play a beneficial role in certain aspects of cancer, although the molecular mechanisms underlying its benefits are not fully understood.
The role of RASi in cell proliferation, apoptosis, and survival
Mounting experimental evidence indicates that RASi have potential anti-proliferation and pro-apoptotic properties. For example, in a mouse liver cancer model induced by diethyl nitrosamine, the inhibition of ACE or blocking of AT1R inhibited tumor development by inactivating the NF-κB pathway and increasing the survival rate of mice [21]. Captopril can inhibit the growth of colorectal cancer liver metastases in the regenerating liver by anti-angiogenesis and promoting tumor cell apoptosis, without affecting the regeneration of normal liver tissue following partial hepatectomy [22]. In HepG2 cell line, the angiotensin receptor blocker azilsartan increased the rate of apoptosis induced by Bay 11-7082 (an NF-κB inhibitor) by inducing oxidative stress to inhibit the growth of tumor cells [23]. In an experimental model, treatment of a breast cancer (MCF-7) cell line with the angiotensin II receptor antagonist olmesartan and an NF-κB inhibitor, Bay11-7082, could inhibit tumor growth individually or in combination by enhancing cytotoxicity and inducing cell apoptosis [24]. A mouse model of breast cancer treated with losartan showed a significant decrease in the number of invasive cancer cells, which indicated inhibition of tumor cell proliferation and reduction of inflammatory cytokines [25]. Similarly, in experimental studies, telmisartan reduced the viability of melanoma cells by inducing mitochondrial dysfunction, changing cell bioenergy, and inducing apoptosis [26]. In addition, in a lung adenocarcinoma model, mice treated with captopril had significantly reduced tumor volume compared with those of the control group. This effect was closely related to the blockade of the energy uptake pathway in tumor cells, which resulted in a decrease in the number of proliferating cells [27]. Another meta-analysis showed that the survival rate improved for patients with renal cancer who received treatment with RASi compared to those who did not [28].
Role of RASi in tumor invasion and metastasis
The N87 and MKN45 cell lines derived from gastric cancer became less aggressive following olmesartan treatment [29]. The AT1R antagonist TCV-116 is equivalent to the ACE inhibitor lisinopril and exerts inhibitory effects on tumor angiogenesis, growth, and metastasis [30]. An in vitro experimental model confirmed that candesartan could inhibit the invasion, angiogenesis, and peritoneal dissemination of ovarian cancer [31]. A retrospective study reported that the expression of AGTR1 in colorectal cancer was significantly upregulated and that the treatment with ACEI and ARBs reduced the tumor recurrence rate of colorectal cancer, thereby improving its prognosis [32].
Role of RASi in tumor angiogenesis
The blockade of angiogenesis has long been considered an effective mechanism for inhibiting tumor growth. Increasing evidence has shown that targeting the Ang II/AT1R axis can inhibit tumor growth and metastasis by reducing the expression of VEGF to inhibit angiogenesis and reduce vascular permeability [33-35]. In an in vitro model of hepatocellular carcinoma, the ACE inhibitor perindopril and the AT1R blocker losartan prevented hepatocellular carcinoma by inhibiting growth factor-mediated angiogenesis and enhancing endostatin-mediated anti-angiogenesis [21,36]. The new angiotensin II antagonist, olmesartan, can target the VEGF-A gene by upregulating miR-205 in cervical cancer cell lines, thereby inhibiting tumor proliferation [37]. In prostate cancer cell lines, AT1R blockade can inhibit the expression of hypoxia-inducible factor alpha (HIF-1α) and Ets-1, thereby inhibiting tumor cell angiogenesis [38]. In pancreatic ductal adenocarcinoma, the expression levels of ACE and AT1R are positively correlated with the expression of VEGF, and captopril and losartan significantly inhibited cell proliferation [39].
Role of RASi in the tumor microenvironment
Angiotensin II can reduce tumor perfusion, leading to acidosis and hypoxia in the tumor stroma [40]. Tumor acidosis and hypoxia can trigger the expression of a series of inflammatory cytokines, such as HIF, VEGF, and transforming growth factor-β (TGF-β). At the same time, modulation of some inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), monocyte chemoattractant protein-1 (MCP-1), osteopontin (OPN), and inducible nitric oxide synthase (iNOS) influence the regulation of immune suppression and immune escape [41,42]. Acidosis and hypoxia contribute to the establishment of an immunosuppressive environment and promote tumor growth and metastasis [43,44]. Antagonizing VEGF receptors can normalize tumor blood vessels, thereby effectively alleviating hypoxia and acidosis, reprogramming the tumor immunosuppressive microenvironment, and improving the efficacy of immunotherapy [44,45].
Ang II targets AT1R to release a variety of tumor-supporting cytokines, such as MCP-1, cyclooxygenase 2 (COX-2) and C-reactive protein (CRP) can upregulate the immunosuppressive pathway through COX-dependent pathways [46,47]. In addition, after AT1R is activated, Ang II can promote the production of reactive oxygen species (ROS) and related proteins in tumor and stromal cells [48]. ROS can impair the function of T cells in the tumor microenvironment (TME), while enhancing the functions of Tregs and TAM [47]. The ARB inhibitor candesartan can reduce the production of ROS and inhibit the oxidative stress response [49].
Local RAS in the tumor microenvironment can inhibit the induction of tumor antigen-specific treatment. Antitumor efficacy was augmented by enhancing the induction and infiltration of tumor antigen-specific T cells [19]. In addition, RASi can enhance antitumor effects by inducing neutrophil polarization to an antitumor phenotype [50]. In mouse tumor models, the blockade of local RAS reverses the immunosuppressive microenvironment of tumor and triggers the immune activation cytokine profile of cancer cells [51].
In addition, RAS can affect the immune response by establishing a proliferative environment. For example, cancer-associated fibroblasts (CAFs) inhibit the function of T cells and NK cells, promote the accumulation of immunosuppressive cells, and maintain an inflammatory environment that affects the immune system and hinders its normal physiological functions [52]. Dense tumor fibrosis can also compress blood vessels by increasing solid stress [53,54], reducing tumor perfusion, and leading to hypoxia and acidosis in the tumor microenvironment. This, in turn, can promote immune cell reprogramming to an immunosuppressive phenotype, inhibiting the normal killing of tumor cells by immune cells and enhancing the expression of various immunosuppressive checkpoint molecules [43,53-57]. Several experimental studies have shown that RASi can improve tumor-stromal fibrosis. In various malignant tumor models, losartan and telmisartan reduced the expression of transforming growth TGF-β and collagen I, thereby reducing tumor-stromal proliferation and improving vascular perfusion, and improving the distribution and efficacy of anticancer drugs and nanotherapy in tumors [58-61].
RASi can therefore improve the activity of pathophysiological processes in the tumor microenvironment. In addition to modulating tumor angiogenesis, it can also increase tumor perfusion, reduce hypoxia and acidosis, improve the inflammatory environment, and enhance immune cell function to promote antitumor effects.
Practical application of RASi in cancer treatment
Preclinical and clinical studies have shown that RASi exhibit good anticancer properties in many types of cancer. RASi can not only be used in combination with radiotherapy and chemotherapy to improve the prognosis of patients, but can also be used to prevent the occurrence of certain cancers associated with high-risk factors. Currently, they are also used in immunotherapy and targeted therapy, In addition, several patients may require treatment termination because of the side effects of chemotherapy and radiotherapy. In such patients, RASi have also shown beneficial effects in reducing the side effects of cancer treatment. In short, RASi have great potential in the management of cancer.
A recent meta-analysis showed that a combination of RASi (including ACEI and ARBs) and chemotherapy significantly delayed the disease process, and the overall mortality was significantly reduced compared with treatment with chemotherapeutic drugs alone, suggesting that RASi can improve the prognosis of different types of cancer as an adjuvant therapy [62]. In a phase II trial of advanced renal cell carcinoma, a combined treatment with interferon-α, cimetidine, cyclooxygenase-2 inhibitor, and RAS inhibitor (I-CCA therapy) led to the majority of patients showing good tolerance with a low incidence of toxicity [63]. In an experimental study, the combined use of olmesartan and sorafenib significantly reduced the levels of angiogenic markers such as VEGF and IGF-I and their intracellular receptors and inhibited tumor angiogenesis, thereby enhancing the overall antitumor effect [64]. In a study on patients with rectal cancer, the pathological complete response rate (pCR) of patients using RASi to neoadjuvant radiotherapy was significantly increased compared to other drugs (such as statins) [65].
RASi can not only be used as a chemotherapy adjuvant to improve the prognosis of several tumor types, but can also be used to prevent the occurrence of cancer. Earlier studies have shown that ACEI/ARBs can effectively prevent liver cancer induced by diethylnitrosamine (DENA) and promoted by carbon tetrachloride (CCl(4)) [36]. An experimental study has shown that blocking RAS expression can effectively prevent the disorder of adenosine monophosphate activated protein kinase signal transduction pathway caused by unilateral nephrectomy, which leads to the carcinogenesis of renal tubular epithelial cells [66]. In addition, a meta-analysis of multiple observational studies found that the use of RASi can reduce the risk of keratinocyte carcinoma (basal and squamous cell carcinoma) [67].
As mentioned above, local RAS expression is associated with tumor immunosuppressive microenvironment, which provides a theoretical basis for the combination of local RAS blockade and immune checkpoints. Consistent with this, the combination of local RAS blockade and immune checkpoint blockade can change the immunosuppressive properties in the tumor microenvironment and significantly enhance the antitumor effect in a CD8+ T-cell-dependent manner [19,51]. In addition, the co-delivery system of gold nanoparticles modified by captopril-polyethyleneimine conjugated and gene drugs has shown strong tumor homing ability and antitumor effect in the treatment of breast cancer [68].
In addition to having a synergistic effect in the treatment of cancer, RASi can also reduce the side effects of molecular targeted therapy in cancer patients, such as left ventricular dysfunction. A clinical trial conducted by Gulati et al. showed that patients with early breast cancer treated with both desartan and anthracyclines had a decreased risk of reduced left ventricular function [69]. In addition, concurrent use of ACEI reduced the incidence of chest pneumonia in patients receiving radiotherapy for non-small cell lung cancer [70].
Conclusion
The benefits of treatment with RASi remains controversial, and the underlying mechanism of RASi in the treatment of different types of tumors warrants further investigations. However, existing research and data analysis have shown that the high expression of RAS has various effects on the survival of patients with different types of tumors. In addition, whether as an adjuvant in cancer treatment to improve the efficacy of chemoradiotherapy, immune and targeted therapy, or as a protective agent for normal tissues and organs in chemoradiotherapy to reduce toxic and side effects, RASi have demonstrated their infinite potential in cancer management.
Future research should continue to explore the role of RASi in the treatment of different types of tumors and the underlying mechanisms involved. Specifically, studies should focus on colorectal cancer and anti-VEGF-responsive tumors (such as hepatocellular carcinoma). The development of more effective and novel RASi analogs or complexes is needed to advance the applications of RASi in cancer therapy. Additionally, RASi are widely used in clinical practice and are tolerated well by the patients, Therefore, RASi can be used as potential therapeutic option for cancer patients, in the near future.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81472394 and 81803078), the Natural Science Foundation of Shanghai (19ZR1456500), and the Key Discipline Construction Project and Doctoral Supervisor Candidate of Shanghai Skin Disease Hospital (2019zdxk03, 17HBDS02, and 17HBDS03).
Disclosure of conflict of interest
None.
References
- 1.Bader M. Tissue renin-angiotensin-aldosterone systems: targets for pharmacological therapy. Annu Rev Pharmacol Toxicol. 2010;50:439–465. doi: 10.1146/annurev.pharmtox.010909.105610. [DOI] [PubMed] [Google Scholar]
- 2.George AJ, Thomas WG, Hannan RD. The renin-angiotensin system and cancer: old dog, new tricks. Nat Rev Cancer. 2010;10:745–759. doi: 10.1038/nrc2945. [DOI] [PubMed] [Google Scholar]
- 3.Sipahi I, Debanne SM, Rowland DY, Simon DI, Fang JC. Angiotensin-receptor blockade and risk of cancer: meta-analysis of randomised controlled trials. Lancet Oncol. 2010;11:627–636. doi: 10.1016/S1470-2045(10)70106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldstein MR, Mascitelli L, Pezzetta F. Angiotensin-receptor blockade, cancer, and concerns. Lancet Oncol. 2010;11:817–818. doi: 10.1016/S1470-2045(10)70160-1. author reply 821-812. [DOI] [PubMed] [Google Scholar]
- 5.Effects of telmisartan, irbesartan, valsartan, candesartan, and losartan on cancers in 15 trials enrolling 138,769 individuals. J Hypertens. 2011;29:623–635. doi: 10.1097/HJH.0b013e328344a7de. [DOI] [PubMed] [Google Scholar]
- 6.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 7.Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mandilaras V, Bouganim N, Yin H, Asselah J, Azoulay L. The use of drugs acting on the renin-angiotensin system and the incidence of pancreatic cancer. Br J Cancer. 2017;116:103–108. doi: 10.1038/bjc.2016.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei J, Zhou Z, Xu Z, Zeng S, Chen X, Wang X, Liu W, Liu M, Gong Z, Yan Y. Retrospective clinical study of renin-angiotensin system blockers in lung cancer patients with hypertension. PeerJ. 2019;7:e8188. doi: 10.7717/peerj.8188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinter M, Jain RK. Targeting the renin-angiotensin system to improve cancer treatment: implications for immunotherapy. Sci Transl Med. 2017;9:eaan5616. doi: 10.1126/scitranslmed.aan5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshida T, Kinoshita H, Fukui K, Matsuzaki T, Yoshida K, Mishima T, Yanishi M, Komai Y, Sugi M, Inoue T, Murota T, Matsuda T. Prognostic impact of renin-angiotensin inhibitors in patients with bladder cancer undergoing radical cystectomy. Ann Surg Oncol. 2017;24:823–831. doi: 10.1245/s10434-016-5534-3. [DOI] [PubMed] [Google Scholar]
- 12.Ghasemi M, Okay M, Turk S, Naeemaee R, Guver E, Malkan UY, Aksu S, Sayinalp N, Haznedaroglu IC. The impact of At1r inhibition via losartan on the anti-leukaemic effects of doxorubicin in acute myeloid leukaemia. J Renin Angiotensin Aldosterone Syst. 2019;20:1470320319851310. doi: 10.1177/1470320319851310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen J, Huang YM, Wang M, Hong XZ, Song XN, Zou X, Pan YH, Ling W, Zhu MH, Zhang XX, Sui Y, Zhao HL. Renin-angiotensin system blockade for the risk of cancer and death. J Renin Angiotensin Aldosterone Syst. 2016;17:1470320316656679. doi: 10.1177/1470320316656679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ager EI, Neo J, Christophi C. The renin-angiotensin system and malignancy. Carcinogenesis. 2008;29:1675–1684. doi: 10.1093/carcin/bgn171. [DOI] [PubMed] [Google Scholar]
- 15.Perdomo-Pantoja A, Mejía-Pérez SI, Gómez-Flores-Ramos L, Lara-Velazquez M, Orillac C, Gómez-Amador JL, Wegman-Ostrosky T. Renin angiotensin system and its role in biomarkers and treatment in gliomas. J Neurooncol. 2018;138:1–15. doi: 10.1007/s11060-018-2789-5. [DOI] [PubMed] [Google Scholar]
- 16.Arrieta O, Villarreal-Garza C, Vizcaíno G, Pineda B, Hernández-Pedro N, Guevara-Salazar P, Wegman-Ostrosky T, Villanueva-Rodríguez G, Gamboa-Domínguez A. Association between AT1 and AT2 angiotensin II receptor expression with cell proliferation and angiogenesis in operable breast cancer. Tumour Biol. 2015;36:5627–5634. doi: 10.1007/s13277-015-3235-3. [DOI] [PubMed] [Google Scholar]
- 17.Kinoshita J, Fushida S, Harada S, Yagi Y, Fujita H, Kinami S, Ninomiya I, Fujimura T, Kayahara M, Yashiro M, Hirakawa K, Ohta T. Local angiotensin II-generation in human gastric cancer: correlation with tumor progression through the activation of ERK1/2, NF-kappaB and survivin. Int J Oncol. 2009;34:1573–1582. doi: 10.3892/ijo_00000287. [DOI] [PubMed] [Google Scholar]
- 18.Stocks T, Van Hemelrijck M, Manjer J, Bjørge T, Ulmer H, Hallmans G, Lindkvist B, Selmer R, Nagel G, Tretli S, Concin H, Engeland A, Jonsson H, Stattin P. Blood pressure and risk of cancer incidence and mortality in the Metabolic Syndrome and Cancer Project. Hypertension. 2012;59:802–810. doi: 10.1161/HYPERTENSIONAHA.111.189258. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura K, Yaguchi T, Ohmura G, Kobayashi A, Kawamura N, Iwata T, Kiniwa Y, Okuyama R, Kawakami Y. Involvement of local renin-angiotensin system in immunosuppression of tumor microenvironment. Cancer Sci. 2018;109:54–64. doi: 10.1111/cas.13423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cortez-Retamozo V, Etzrodt M, Newton A, Ryan R, Pucci F, Sio SW, Kuswanto W, Rauch PJ, Chudnovskiy A, Iwamoto Y, Kohler R, Marinelli B, Gorbatov R, Wojtkiewicz G, Panizzi P, Mino-Kenudson M, Forghani R, Figueiredo JL, Chen JW, Xavier R, Swirski FK, Nahrendorf M, Weissleder R, Pittet MJ. Angiotensin II drives the production of tumor-promoting macrophages. Immunity. 2013;38:296–308. doi: 10.1016/j.immuni.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saber S, Mahmoud AAA, Goda R, Helal NS, El-Ahwany E, Abdelghany RH. Perindopril, fosinopril and losartan inhibited the progression of diethylnitrosamine-induced hepatocellular carcinoma in mice via the inactivation of nuclear transcription factor kappa-B. Toxicol Lett. 2018;295:32–40. doi: 10.1016/j.toxlet.2018.05.036. [DOI] [PubMed] [Google Scholar]
- 22.Koh SL, Ager EI, Costa PL, Malcontenti-Wilson C, Muralidharan V, Christophi C. Blockade of the renin-angiotensin system inhibits growth of colorectal cancer liver metastases in the regenerating liver. Clin Exp Metastasis. 2014;31:395–405. doi: 10.1007/s10585-014-9635-8. [DOI] [PubMed] [Google Scholar]
- 23.Ahmadian E, Khosroushahi AY, Eftekhari A, Farajnia S, Babaei H, Eghbal MA. Novel angiotensin receptor blocker, azilsartan induces oxidative stress and NFkB-mediated apoptosis in hepatocellular carcinoma cell line HepG2. Biomed Pharmacother. 2018;99:939–946. doi: 10.1016/j.biopha.2018.01.117. [DOI] [PubMed] [Google Scholar]
- 24.Bakhtiari E, Hosseini A, Boroushaki MT, Mousavi SH. Angiotensin II receptor antagonist olmesartan and NF-kappaB inhibitor as cytotoxic and apoptotic agents in MCF-7 human cell line. J Chemother. 2016;28:314–320. doi: 10.1179/1973947815Y.0000000055. [DOI] [PubMed] [Google Scholar]
- 25.Coulson R, Liew SH, Connelly AA, Yee NS, Deb S, Kumar B, Vargas AC, O’Toole SA, Parslow AC, Poh A, Putoczki T, Morrow RJ, Alorro M, Lazarus KA, Yeap EFW, Walton KL, Harrison CA, Hannan NJ, George AJ, Clyne CD, Ernst M, Allen AM, Chand AL. The angiotensin receptor blocker, Losartan, inhibits mammary tumor development and progression to invasive carcinoma. Oncotarget. 2017;8:18640–18656. doi: 10.18632/oncotarget.15553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grahovac J, Srdić-Rajić T, Francisco Santibañez J, Pavlović M, Čavić M, Radulović S. Telmisartan induces melanoma cell apoptosis and synergizes with vemurafenib in vitro by altering cell bioenergetics. Cancer Biol Med. 2019;16:247–263. doi: 10.20892/j.issn.2095-3941.2018.0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakaya K, Otsuka H, Kondo K, Otani T, Nagata M. Tumor growth-inhibitory effect of an angiotensin-converting enzyme inhibitor (captopril) in a lung cancer xenograft model analyzed using 18F-FDG-PET/CT. Nucl Med Commun. 2016;37:139–146. doi: 10.1097/MNM.0000000000000404. [DOI] [PubMed] [Google Scholar]
- 28.Asgharzadeh F, Hashemzehi M, Moradi-Marjaneh R, Hassanian SM, Ferns GA, Khazaei M, Avan A. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers as therapeutic options in the treatment of renal cancer: a meta-analysis. Life Sci. 2020;242:117181. doi: 10.1016/j.lfs.2019.117181. [DOI] [PubMed] [Google Scholar]
- 29.Carl-McGrath S, Ebert MP, Lendeckel U, Röcken C. Expression of the local angiotensin II system in gastric cancer may facilitate lymphatic invasion and nodal spread. Cancer Biol Ther. 2007;6:1218–1226. doi: 10.4161/cbt.6.8.4412. [DOI] [PubMed] [Google Scholar]
- 30.Fujita M, Hayashi I, Yamashina S, Itoman M, Majima M. Blockade of angiotensin AT1a receptor signaling reduces tumor growth, angiogenesis, and metastasis. Biochem Biophys Res Commun. 2002;294:441–447. doi: 10.1016/S0006-291X(02)00496-5. [DOI] [PubMed] [Google Scholar]
- 31.Suganuma T, Ino K, Shibata K, Kajiyama H, Nagasaka T, Mizutani S, Kikkawa F. Functional expression of the angiotensin II type 1 receptor in human ovarian carcinoma cells and its blockade therapy resulting in suppression of tumor invasion, angiogenesis, and peritoneal dissemination. Clin Cancer Res. 2005;11:2686–2694. doi: 10.1158/1078-0432.CCR-04-1946. [DOI] [PubMed] [Google Scholar]
- 32.Ozawa T, Hashiguchi Y, Yagi T, Fukushima Y, Shimada R, Hayama T, Tsuchiya T, Nozawa K, Iinuma H, Ishihara S, Matsuda K. Angiotensin I-converting enzyme inhibitors/angiotensin II receptor blockers may reduce tumor recurrence in left-sided and early colorectal cancers. Int J Colorectal Dis. 2019;34:1731–1739. doi: 10.1007/s00384-019-03379-y. [DOI] [PubMed] [Google Scholar]
- 33.Ino K, Shibata K, Kajiyama H, Yamamoto E, Nagasaka T, Nawa A, Nomura S, Kikkawa F. Angiotensin II type 1 receptor expression in ovarian cancer and its correlation with tumour angiogenesis and patient survival. Br J Cancer. 2006;94:552–560. doi: 10.1038/sj.bjc.6602961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carbajo-Lozoya J, Lutz S, Feng Y, Kroll J, Hammes HP, Wieland T. Angiotensin II modulates VEGF-driven angiogenesis by opposing effects of type 1 and type 2 receptor stimulation in the microvascular endothelium. Cell Signal. 2012;24:1261–1269. doi: 10.1016/j.cellsig.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 35.Usui T, Sugisaki K, Iriyama A, Yokoo S, Yamagami S, Nagai N, Ishida S, Amano S. Inhibition of corneal neovascularization by blocking the angiotensin II type 1 receptor. Invest Ophthalmol Vis Sci. 2008;49:4370–4376. doi: 10.1167/iovs.07-0964. [DOI] [PubMed] [Google Scholar]
- 36.Mansour MA, Al-Ismaeel H, Al-Rikabi AC, Al-Shabanah OA. Comparison of angiotensin converting enzyme inhibitors and angiotensin II type 1 receptor blockade for the prevention of premalignant changes in the liver. Life Sci. 2011;89:188–194. doi: 10.1016/j.lfs.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 37.Yue Z, Yun-Shan Z, Feng-Xia X. miR-205 mediates the inhibition of cervical cancer cell proliferation using olmesartan. J Renin Angiotensin Aldosterone Syst. 2016;17:1470320316663327. doi: 10.1177/1470320316663327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kosaka T, Miyajima A, Shirotake S, Kikuchi E, Hasegawa M, Mikami S, Oya M. Ets-1 and hypoxia inducible factor-1alpha inhibition by angiotensin II type-1 receptor blockade in hormone-refractory prostate cancer. Prostate. 2010;70:162–169. doi: 10.1002/pros.21049. [DOI] [PubMed] [Google Scholar]
- 39.Arafat HA, Gong Q, Chipitsyna G, Rizvi A, Saa CT, Yeo CJ. Antihypertensives as novel antineoplastics: angiotensin-I-converting enzyme inhibitors and angiotensin II type 1 receptor blockers in pancreatic ductal adenocarcinoma. J Am Coll Surg. 2007;204:996–1005. doi: 10.1016/j.jamcollsurg.2007.01.067. discussion 1005-1006. [DOI] [PubMed] [Google Scholar]
- 40.Thews O, Kelleher DK, Vaupel P. Disparate responses of tumour vessels to angiotensin II: tumour volume-dependent effects on perfusion and oxygenation. Br J Cancer. 2000;83:225–231. doi: 10.1054/bjoc.2000.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riemann A, Reime S, Thews O. Tumor acidosis and hypoxia differently modulate the inflammatory program: measurements in vitro and in vivo. Neoplasia. 2017;19:1033–1042. doi: 10.1016/j.neo.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dayan F, Mazure NM, Brahimi-Horn MC, Pouysségur J. A dialogue between the hypoxia-inducible factor and the tumor microenvironment. Cancer Microenviron. 2008;1:53–68. doi: 10.1007/s12307-008-0006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell. 2014;26:605–622. doi: 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang Y, Goel S, Duda DG, Fukumura D, Jain RK. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res. 2013;73:2943–2948. doi: 10.1158/0008-5472.CAN-12-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang Y, Yuan J, Righi E, Kamoun WS, Ancukiewicz M, Nezivar J, Santosuosso M, Martin JD, Martin MR, Vianello F, Leblanc P, Munn LL, Huang P, Duda DG, Fukumura D, Jain RK, Poznansky MC. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci U S A. 2012;109:17561–17566. doi: 10.1073/pnas.1215397109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vinay DS, Ryan EP, Pawelec G, Talib WH, Stagg J, Elkord E, Lichtor T, Decker WK, Whelan RL, Kumara H, Signori E, Honoki K, Georgakilas AG, Amin A, Helferich WG, Boosani CS, Guha G, Ciriolo MR, Chen S, Mohammed SI, Azmi AS, Keith WN, Bilsland A, Bhakta D, Halicka D, Fujii H, Aquilano K, Ashraf SS, Nowsheen S, Yang X, Choi BK, Kwon BS. Immune evasion in cancer: mechanistic basis and therapeutic strategies. Semin Cancer Biol. 2015;35(Suppl):S185–S198. doi: 10.1016/j.semcancer.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 47.Zelenay S, van der Veen AG, Böttcher JP, Snelgrove KJ, Rogers N, Acton SE, Chakravarty P, Girotti MR, Marais R, Quezada SA, Sahai E, Reis e Sousa C. Cyclooxygenase-dependent tumor growth through evasion of immunity. Cell. 2015;162:1257–1270. doi: 10.1016/j.cell.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jackson SH, Devadas S, Kwon J, Pinto LA, Williams MS. T cells express a phagocyte-type NADPH oxidase that is activated after T cell receptor stimulation. Nat Immunol. 2004;5:818–827. doi: 10.1038/ni1096. [DOI] [PubMed] [Google Scholar]
- 49.Uemura H, Ishiguro H, Ishiguro Y, Hoshino K, Takahashi S, Kubota Y. Angiotensin II induces oxidative stress in prostate cancer. Mol Cancer Res. 2008;6:250–258. doi: 10.1158/1541-7786.MCR-07-0289. [DOI] [PubMed] [Google Scholar]
- 50.Shrestha S, Noh JM, Kim SY, Ham HY, Kim YJ, Yun YJ, Kim MJ, Kwon MS, Song DK, Hong CW. Angiotensin converting enzyme inhibitors and angiotensin II receptor antagonist attenuate tumor growth via polarization of neutrophils toward an antitumor phenotype. Oncoimmunology. 2016;5:e1067744. doi: 10.1080/2162402X.2015.1067744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xie G, Cheng T, Lin J, Zhang L, Zheng J, Liu Y, Xie G, Wang B, Yuan Y. Local angiotensin II contributes to tumor resistance to checkpoint immunotherapy. J Immunother Cancer. 2018;6:88. doi: 10.1186/s40425-018-0401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Öhlund D, Elyada E, Tuveson D. Fibroblast heterogeneity in the cancer wound. J Exp Med. 2014;211:1503–1523. doi: 10.1084/jem.20140692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jain RK. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J. Clin. Oncol. 2013;31:2205–2218. doi: 10.1200/JCO.2012.46.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jain RK, Martin JD, Stylianopoulos T. The role of mechanical forces in tumor growth and therapy. Annu Rev Biomed Eng. 2014;16:321–346. doi: 10.1146/annurev-bioeng-071813-105259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Noman MZ, Hasmim M, Messai Y, Terry S, Kieda C, Janji B, Chouaib S. Hypoxia: a key player in antitumor immune response. A review in the theme: cellular responses to hypoxia. Am J Physiol Cell Physiol. 2015;309:C569–79. doi: 10.1152/ajpcell.00207.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Palazón A, Aragonés J, Morales-Kastresana A, de Landázuri MO, Melero I. Molecular pathways: hypoxia response in immune cells fighting or promoting cancer. Clin Cancer Res. 2012;18:1207–1213. doi: 10.1158/1078-0432.CCR-11-1591. [DOI] [PubMed] [Google Scholar]
- 57.Voron T, Colussi O, Marcheteau E, Pernot S, Nizard M, Pointet AL, Latreche S, Bergaya S, Benhamouda N, Tanchot C, Stockmann C, Combe P, Berger A, Zinzindohoue F, Yagita H, Tartour E, Taieb J, Terme M. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med. 2015;212:139–148. doi: 10.1084/jem.20140559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chauhan VP, Martin JD, Liu H, Lacorre DA, Jain SR, Kozin SV, Stylianopoulos T, Mousa AS, Han X, Adstamongkonkul P, Popović Z, Huang P, Bawendi MG, Boucher Y, Jain RK. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat Commun. 2013;4:2516. doi: 10.1038/ncomms3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Diop-Frimpong B, Chauhan VP, Krane S, Boucher Y, Jain RK. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc Natl Acad Sci U S A. 2011;108:2909–2914. doi: 10.1073/pnas.1018892108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Godugu C, Patel AR, Doddapaneni R, Marepally S, Jackson T, Singh M. Inhalation delivery of Telmisartan enhances intratumoral distribution of nanoparticles in lung cancer models. J Control Release. 2013;172:86–95. doi: 10.1016/j.jconrel.2013.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Patel K, Doddapaneni R, Chowdhury N, Boakye CH, Behl G, Singh M. Tumor stromal disrupting agent enhances the anticancer efficacy of docetaxel loaded PEGylated liposomes in lung cancer. Nanomedicine (Lond) 2016;11:1377–1392. doi: 10.2217/nnm.16.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li XY, Sun JF, Hu SQ. The renin-angiotensin system blockers as adjunctive therapy for cancer: a meta-analysis of survival outcome. Eur Rev Med Pharmacol Sci. 2017;21:1375–1383. [PubMed] [Google Scholar]
- 63.Tatokoro M, Fujii Y, Kawakami S, Saito K, Koga F, Matsuoka Y, Iimura Y, Masuda H, Kihara K. Phase-II trial of combination treatment of interferon-α, cimetidine, cyclooxygenase-2 inhibitor and renin-angiotensin-system inhibitor (I-CCA therapy) for advanced renal cell carcinoma. Cancer Sci. 2011;102:137–143. doi: 10.1111/j.1349-7006.2010.01756.x. [DOI] [PubMed] [Google Scholar]
- 64.Abd-Alhaseeb MM, Zaitone SA, Abou-El-Ela SH, Moustafa YM. Olmesartan potentiates the anti-angiogenic effect of sorafenib in mice bearing Ehrlich’s ascites carcinoma: role of angiotensin (1-7) PLoS One. 2014;9:e85891. doi: 10.1371/journal.pone.0085891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morris ZS, Saha S, Magnuson WJ, Morris BA, Borkenhagen JF, Ching A, Hirose G, McMurry V, Francis DM, Harari PM, Chappell R, Tsuji S, Ritter MA. Increased tumor response to neoadjuvant therapy among rectal cancer patients taking angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. Cancer. 2016;122:2487–2495. doi: 10.1002/cncr.30079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang KK, Sui Y, Zhou HR, Zhao HL. Interaction of renin-angiotensin system and adenosine monophosphate-activated protein kinase signaling pathway in renal carcinogenesis of uninephrectomized rats. Tumour Biol. 2017;39:1010428317699116. doi: 10.1177/1010428317699116. [DOI] [PubMed] [Google Scholar]
- 67.Tang H, Fu S, Zhai S, Song Y, Asgari MM, Han J. Use of antihypertensive drugs and risk of keratinocyte carcinoma: a meta-analysis of observational studies. Pharmacoepidemiol Drug Saf. 2018;27:279–288. doi: 10.1002/pds.4384. [DOI] [PubMed] [Google Scholar]
- 68.Li M, Li Y, Huang X, Lu X. Captopril-polyethyleneimine conjugate modified gold nanoparticles for co-delivery of drug and gene in anti-angiogenesis breast cancer therapy. J Biomater Sci Polym Ed. 2015;26:813–827. doi: 10.1080/09205063.2015.1057991. [DOI] [PubMed] [Google Scholar]
- 69.Gulati G, Heck SL, Ree AH, Hoffmann P, Schulz-Menger J, Fagerland MW, Gravdehaug B, von Knobelsdorff-Brenkenhoff F, Bratland Å, Storås TH, Hagve TA, Røsjø H, Steine K, Geisler J, Omland T. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 × 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur Heart J. 2016;37:1671–1680. doi: 10.1093/eurheartj/ehw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kharofa J, Cohen EP, Tomic R, Xiang Q, Gore E. Decreased risk of radiation pneumonitis with incidental concurrent use of angiotensin-converting enzyme inhibitors and thoracic radiation therapy. Int J Radiat Oncol Biol Phys. 2012;84:238–243. doi: 10.1016/j.ijrobp.2011.11.013. [DOI] [PubMed] [Google Scholar]