Abstract
This study described the expression of beclin-1 and mTOR proteins in 86 ovarian tumor tissue samples (n=25 benign ovarian tumor tissues; n=16 borderline ovarian tumor tissues; n=45 malignant epithelial ovarian tumor tissues) and 20 normal ovarian tissue samples and determined the diagnostic value of serum beclin-1 and mTOR protein levels for ovarian tumors. Serum and tissue beclin-1 and mTOR protein levels were detected with enzyme-linked immunosorbent assay, western blot, and immunohistochemistry. Receiver operating characteristic (ROC) curves were constructed to determine the significance of serum beclin-1 and mTOR protein levels for diagnosing borderline and malignant epithelial ovarian tumor. Mean beclin-1 protein levels were highest in normal ovarian tissue and were progressively decreased in benign, borderline, and malignant epithelial ovarian tumor. Mean mTOR protein levels were lowest in normal ovarian tissue and were progressively increased in benign, borderline, and malignant epithelial ovarian tumor. The area under the ROC curve was 0.93 or 0.97 when a combination of serum beclin-1 and mTOR protein levels was used to diagnose borderline or malignant epithelial ovarian tumor. There was a significant negative correlation between beclin-1 and mTOR protein expression in malignant epithelial ovarian tumor (rs=-0.78, P<0.05), but no correlation between beclin-1 and mTOR protein expression in borderline ovarian tumor. Combined detection of serum beclin-1 and mTOR protein levels may have improved diagnostic accuracy for malignant epithelial ovarian tumor compared to each marker alone.
Keywords: Ovarian tumor, ovarian cancer, diagnostic value, beclin-1, mTOR
Introduction
Ovarian cancer is the most lethal gynecologic cancer. Globally, in 2018, there were 295,414 new cases of ovarian cancer and 184,799 deaths from the disease [1]. Ovarian cancer may go undetected until it is late stage and has spread within the pelvis and abdomen. Diagnosis of ovarian cancer at an early stage increases the potential for long-term survival after treatment.
Autophagy is a cellular self-digestion process that is essential for maintaining homeostasis in eukaryotes [2]. Autophagy has a complex role in cancer. During early stage tumorigenesis, autophagy contributes to tumor suppression. During late stage tumorigenesis, autophagy promotes malignant progression [3]. Detection of autophagy-related mechanisms may have clinical utility for the diagnosis of ovarian tumors.
Beclin-1 is a protein that regulates autophagy and cell death through interactions with B-cell lymphoma-2 (BCL-2) or phosphatidylinositol-3-hydroxykinase (PI3k) class III. mTOR is a kinase that inhibits autophagy following activation by protein kinase B (AKT) and mitogen-activated protein kinase (MAPK) signaling. Studies describing the clinical significance of combined detection of serum beclin-1 and mTOR protein levels for diagnosis of malignant epithelial ovarian tumor are scarce. The objective of this study was to describe the expression of beclin-1 and mTOR protein in ovarian tumors and determine the diagnostic value of serum beclin-1 and mTOR protein levels for ovarian tumors. Beclin-1 and mTOR may represent new biomarkers for the early detection and monitoring of disease progression in patients with ovarian cancer.
Materials and methods
In accordance with the journal’s guidelines, we will provide our data for the reproducibility of this study in other centers if such is requested.
Subjects
This study included patients with ovarian tumors and patients with healthy ovarian tissue who underwent ovariectomy at the First Affiliated Hospital of Dali University between Nov. 2017 and Oct. 2019. Subjects mean age was 52 years (range, 19-73 years). Samples were divided into 4 groups: normal (Group A, normal ovarian tissues; n=20); benign (Group B, benign ovarian tumor tissues; n=25); borderline (Group C, borderline ovarian tumor tissues; n=16); and malignant (Group D, malignant epithelial ovarian tumor tissues; n=45). Metastasis was detected by computed tomography (CT) scan, ultrasonography, or observation during surgery (Table 1). The study was approved by the Ethical Board Committee of the First Affiliated Hospital of Dali University (20200714) and was conducted according to the ethical guidelines of the 1975 Declaration of Helsinki. All included subjects provided written informed consent.
Table 1.
Characteristics of the study population
| A | B | C | D | Total | |
|---|---|---|---|---|---|
| Case number | 20 | 25 | 16 | 45 | 106 |
| Average age | 43.5 ± 12.3 | 46.2 ± 13.1 | 42.8 ± 8.5 | 52.4 ± 10.4 | - |
| Nationality (n) | |||||
| Han nationality | 12 | 16 | 8 | 25 | 61 |
| Bai nationality | 5 | 6 | 4 | 13 | 28 |
| Others | 3 | 3 | 4 | 7 | 17 |
| Preoperative time (d) | 4.2 ± 1.8 | 3.9 ± 1.7 | 4.5 ± 2.3 | 3.7 ± 2.1 | - |
Group A, normal ovarian tissues; Group B, benign ovarian tumor tissues; Group C, borderline ovarian tumor tissues; Group D, malignant epithelial ovarian tumor tissues.
Sampling
Fasting venous blood samples (5 ml) were collected from all subjects in the morning 3 days before surgery. Sera were stored at -20°C until use. Ovarian tissue samples were collected during surgery. A portion of the tissue was stored at -80°C, and the remainder was formalin-fixed and paraffin-embedded following standard protocols.
Enzyme linked immunosorbent assay
Beclin-1 and mTOR protein were detected in serum using ELISA kits (Sun Red Biotechnology Company, Shanghai, China), according to the manufacturer’s instructions. Serum beclin-1 and mTOR protein levels were calculated by measuring absorbance at a wavelength of 450 nm.
Western blotting
Total protein was isolated from tissue samples. 30 g protein was separated by electrophoresis on a 10% sodium dodecyl sulfate (SDS) gel and transferred to a polyvinylidene difluoride (PVDF) membrane. The membrane was incubated in Tris-buffered saline containing 0.1% Tween-20 (TBST; 50 mM Tris-Cl, pH 7.5, and 150 mM NaCl) and 3% bovine serum albumin (BSA) for 3 h at room temperature to block non-specific binding. Subsequently, the membrane was incubated with primary antibody against beclin-1 (1:500) or mTOR (1:500) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) overnight at 4°C. The membrane was washed three times with TBST and incubated with a horseradish peroxidase-conjugated secondary antibody (anti-mouse IgG, 1:5000; Cell Signaling Technology, Danvers, MA, USA). Protein bands were visualized using an enhanced chemiluminescence (ECL) reagent (Thermo Fisher Scientific, Waltham, MA, USA). Band intensities were semi-quantified via densitometry analysis using Image-Pro Plus (IPP 6.0, Media Cybernetics, Maryland, U.S.). Relative protein expression was normalized to GAPDH.
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissues were stained with hematoxylin and eosin to preview the tissue and select sections suitable for immunohistochemistry. Sections were deparaffinized in xylene, rehydrated in a series of graded alcohols, and autoclaved for five minutes in ethylene diamine tetra-acetic acid (EDTA). Endogenous peroxidase was quenched with 3% H2O2. Tissue sections were incubated with a primary antibody against beclin-1 (rabbit anti-human polyclonal antibody, 1:100; Abcam, UK) or mTOR (rabbit anti-human polyclonal antibody 1:100; Abcam, UK) overnight at 4°C. Sections were incubated with horseradish peroxidase conjugated streptavidin for 30 min and visualized with diaminobenzidine (DAKO, Glostrup, Denmark).
Immunohistochemistry was visually scored, as previously described [4], independently by two researchers who were blinded to the patients’ information. Staining intensity was scored as 0, no staining; 1, faint staining; 2, moderate staining; or 3, strong staining. The distribution of labeled protein was defined as a percentage of the whole area and scored as: 0 (<5%), 1 (5%-25%), 2 (26%-50%), 3 (51%-75%) and 4 (>75%). Total scores were determined by combining scores for staining intensity and distribution.
Beclin-1 and mTOR protein have a predominantly cytoplasmic staining pattern. The proportion of cells with strong beclin-1 and mTOR cytoplasmic expression was recorded in all available optical fields (×200; whole section), and a mean value was used to score each case.
Statistical analysis
Statistical analyses were performed with IBM SPSS version 20.0 software and GraphPad Prism version 7 software. Between group comparisons of median serum beclin-1 and mTOR protein levels and the rate of beclin-1 positive cells and mTOR positive cells in normal ovarian tissue, benign ovarian tumor tissue, borderline ovarian tumor tissue and malignant epithelial ovarian tumor tissue were performed with the Mann-Whitney U test. Correlation between serum beclin-1 and mTOR protein levels in 86 ovarian tumor tissue samples was assessed using Spearman’s correlation test. The value of individual or combined detection of serum beclin-1 and mTOR protein levels in the diagnosis of malignant epithelial ovarian tumor was evaluated with receiver operating characteristic (ROC) curve and logistic regression analyses. Area under the curve (AUC), sensitivity, and specificity were calculated. Serum beclin 1 and mTOR protein levels were independent variables and the presence/absence of ovarian cancer was the dependent variable. A two-sided p value <0.05 was considered significant.
Results
Serum beclin-1 and mTOR protein levels
Median serum beclin-1 protein levels in patients with normal ovarian tissue and benign ovarian tumor were not significantly different (P=0.945). Median serum beclin-1 protein levels were significantly lower in patients with borderline ovarian tumor compared to benign ovarian tumor (P=0.033), and in patients with malignant epithelial ovarian tumor compared to normal ovarian tissue (P<0.001), benign ovarian tumor (P<0.001), and borderline ovarian tumor (P<0.05).
Median serum mTOR protein levels in patients with normal ovarian tissue and benign ovarian tumor were not significantly different (P=0.918). Median serum mTOR protein levels were significantly higher in patients with borderline ovarian tumor compared to benign ovarian tumor (P<0.001), and in patients with malignant epithelial ovarian tumor compared to normal ovarian tissue (P<0.001), benign ovarian tumor (P<0.001), and borderline ovarian tumor (P<0.05) (Figure 1).
Figure 1.
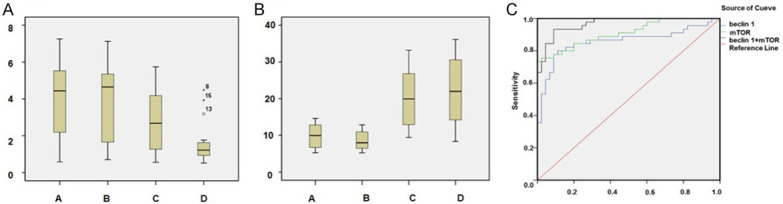
Serum beclin-1 and mTOR protein levels and ROC curve analysis. A. Serum beclin-1; B. Serum mTOR (Group A, normal ovarian tissues; Group B, benign ovarian tumor tissues; Group C, borderline ovarian tumor tissues; Group D, malignant epithelial ovarian tumor tissues); C. The diagnostic value of serum beclin-1 and/or mTOR protein levels (malignant epithelial ovarian tumor, AUCbeclin-1 + mTOR=0.97, AUCmTOR=0.91, AUCbeclin-1=0.86).
Diagnostic value of serum beclin-1 and mTOR protein levels in ovarian tumor
Assessment of the value of individual or combination detection of serum beclin-1 and mTOR protein levels in the diagnosis of malignant epithelial ovarian tumor showed AUCbeclin-1 + mTOR was 0.97, which was greater than AUCmTOR (0.91) and AUCbeclin-1 (0.86). For borderline ovarian tumor, AUCbeclin-1 + mTOR was 0.93, which was greater than AUCmTOR (0.84) and AUCbeclin-1 (0.71). Individual or combined detection of serum beclin-1 and mTOR protein levels had no value for differentiating benign ovarian tumor from normal ovarian tissue (Table 2; Figure 1).
Table 2.
Diagnostic value of serum beclin-1 and mTOR protein levels in ovarian tumors
| Epithelial ovarian tumor | Borderline ovarian tumor | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Beclin-1 | mTOR | Beclin-1 + mTOR | Beclin-1 | mTOR | Beclin-1 + mTOR | |
| AUC | 0.86 | 0.91 | 0.97 | 0.71 | 0.84 | 0.93 |
| 95% CI | 0.77-0.94 | 0.85-0.97 | 0.94-1.00 | 0.55-0.88 | 0.70-0.97 | 0.83-1.00 |
| P-value | <0.001 | <0.001 | <0.001 | 0.029 | 0.001 | <0.001 |
| Youden index | 0.689 | 0.734 | 0.689 | 0.4 | 0.58 | 0.79 |
| Sensitivity (%) | 80 | 75.6 | 77.8 | 75 | 62.5 | 93.75 |
| Specificity (%) | 88.9 | 97.8 | 91.1 | 65 | 95 | 85 |
| Critical values | 1.809 | 18.27 | - | 3.96 | 17.13 | - |
| Positive predictive value (%) | 81.6 | 97.1 | 97.3 | 63.16 | 90.91 | 83.33 |
| Negative predictive value (%) | 87.8 | 80 | 83 | 76.47 | 76 | 94.44 |
Beclin-1 and mTOR protein levels in ovarian tumor tissue
Mean beclin-1 protein levels were highest in normal ovarian tissue and were progressively decreased in benign ovarian tumor, borderline ovarian tumor, and malignant epithelial ovarian tumor.
Mean mTOR protein levels were lowest in normal ovarian tissue and were progressively increased in benign ovarian tumor, borderline ovarian tumor, and malignant epithelial ovarian tumor (Figure 2).
Figure 2.
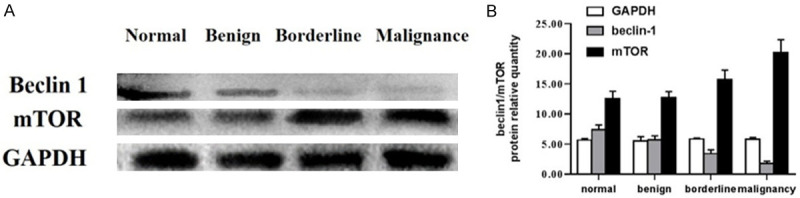
Beclin-1 and mTOR protein levels in ovarian tumor tissue. Tissue lysates were analyzed for beclin-1, mTOR, and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) protein levels. Data represent normalized protein expression from triplicate experiments.
Immunohistochemistry
Beclin-1 and mTOR protein appeared as brown, yellow or tan cytoplasmic staining. The rate of beclin-1 positive cells was significantly lower in borderline ovarian tumor and malignant epithelial ovarian tumor compared to normal ovarian tissue or benign ovarian tumor (P≤0.005).
The rate of mTOR positive cells was significantly higher in borderline ovarian tumor and malignant epithelial ovarian tumor compared to normal ovarian tissue or benign ovarian tumor (P≤0.007) (Table 3; Figure 3).
Table 3.
Beclin-1 and mTOR protein expression in ovarian tissue
| Group (n) | Beclin-1 | mTOR | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Positive | Negative | Positive rate (%) | Positive | Negative | Positive rate (%) | |
| Group A (20) | 17 | 3 | 85.0 | 6 | 14 | 30.0 |
| Group B (25) | 19 | 6 | 76.0 | 9 | 16 | 36.0 |
| Group C (16) | 5 | 11 | 31.3 | 12 | 4 | 75.0 |
| Group D (45) | 13 | 32 | 28.9 | 37 | 8 | 82.2 |
| P | <0.001 (24.23) | <0.001 (24.33) | ||||
| P A vs B | 0.453 (0.56) | 0.672 (0.18) | ||||
| P A vs C | 0.001 (10.81) | 0.007 (7.20) | ||||
| P A vs D | <0.001 (17.54) | <0.001 (16.86) | ||||
| P B vs C | 0.005 (8.05) | 0.015 (5.94) | ||||
| P B vs D | <0.001 (14.37) | <0.001 (15.24) | ||||
| P C vs D | 0.859 (0.03) | 0.533 (0.39) | ||||
Figure 3.
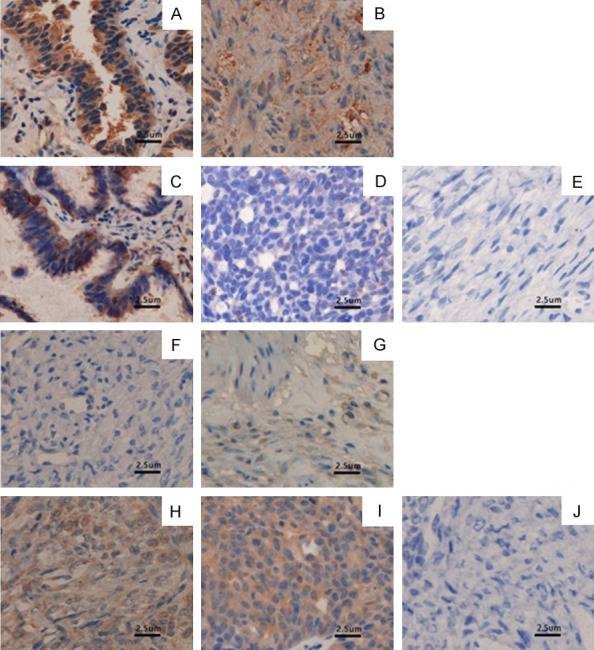
Proteins beclin-1 and mTOR expression in ovarian tumor. A. Beclin-1 in normal ovarian tissue; B. Beclin-1 in benign ovarian tumor tissue; C. Beclin-1 in borderline ovarian tumor tissue; D. Beclin-1 in malignant epithelium; E. Negative control; F. mTOR in normal ovarian tissue; G. mTOR in benign ovarian tumor tissue; H. mTOR in borderline ovarian tumor tissue; I. mTOR in malignant epithelial ovarian tumor tissue; J. Negative control (400×).
Clinical relevance of beclin1 and mTOR protein expression in malignant epithelial ovarian tumor
Spearman correlation analysis revealed a significant negative correlation between beclin-1 and mTOR protein expression (percentage of positive cells) in malignant epithelial ovarian tumor (rs=-0.78, P<0.05). There was no correlation between beclin-1 and mTOR protein expression in borderline ovarian tumor (rs=-0.45, P>0.05).
Discussion
An unmet clinical need exists for an effective screening strategy for ovarian cancer that has a high sensitivity for early-stage disease, which is usually asymptomatic. An estimated 60% of women with ovarian cancer are diagnosed at an advanced stage, which generally translates to poor prognosis [5].
Basal autophagy has a housekeeping role in most cell types where it maintains the integrity of intracellular organelles and proteins. Autophagy is upregulated as an adaptive response to intra- or extracellular stress, including starvation, infection, or oxidative stress, and as a result of oncogene activation during the progression of cancer [6]. Dynamic alterations in autophagy activation can be detected by monitoring changes in autophagy-related markers, such as beclin-1 and mTOR. Targeting these autophagy-related markers may yield significant clinical benefits for patients with ovarian cancer.
Beclin-1, located on chromosome 17q21, is a mammalian ortholog of the yeast autophagy-related gene 6 (Atg6) [7]. Targeted mutant mouse models demonstrated that beclin-1 is a haplo-insufficient tumor-suppressor gene. Specifically, beclin-1-/- mutant mice died early in embryogenesis and beclin-1+/- mutant mice suffered from a high incidence of spontaneous tumors [8], and heterozygous disruption of beclin-1 increased the frequency of spontaneous malignancies and accelerated the development of hepatitis B virus-induced premalignant lesions [9].
Abnormal beclin-1 expression in tumor tissues is associated with the pathogenesis of many cancers. High beclin-1 expression may represent a favorable prognostic factor in colorectal cancer [10] and oral squamous cell carcinoma [11], but an adverse prognostic factor in endometrioid adenocarcinoma [12], nasopharyngeal carcinoma [13], and pancreatic ductal adenocarcinoma [14]. The beclin-1 gene is monoallelically deleted in up to 75% of ovarian cancers, where low beclin-1 expression predicts an unfavorable response to platinum-based chemotherapy [9].
Serum beclin-1 protein levels are altered during the development of various disease conditions. In patients with diabetic kidney disease (DKD), serum beclin-1 protein levels were reduced, related to the stage of DKD, and correlated with the degree of albuminuria [15]. In patients with ulcerative colitis, serum beclin-1 protein levels were increased [16]. To the authors’ knowledge, the present study is the first to describe serum beclin-1 protein levels in patients with ovarian tumors. Median serum beclin-1 protein levels were lowest in patients with malignant epithelial ovarian tumor and progressively increased in patients with borderline ovarian tumor, benign ovarian tumor, and normal ovarian tissue.
Numerous studies have reported on beclin-1 protein expression in ovarian tumors. Using immunohistochemistry, Lin et al. showed the rate of beclin-1 positive cells was 97.1% in normal ovarian tissue, 84.6% in benign cystadenoma, 80% in borderline ovarian tumor, and 44.4% in epithelial ovarian tumor [17]. In the present study, immunohistochemistry revealed the rate of beclin-1 positive cells was 85.0% in normal ovarian tissue, 76.0% in benign ovarian tumor, 31.3% in borderline ovarian tumor, and 28.9% in epithelial ovarian tumor.
mTOR is a serine/threonine protein kinase that is a core component of the PI3K/AKT/mTOR signaling pathway that represses autophagy [18]. Accumulating evidence suggests that mTOR dysfunction contributes to cancer [19]. Genetic alterations in the PI3K/AKT/mTOR pathway have been detected in 36% of small cell lung tumors from Japanese patients [20], 42% of primary prostatic tumors and all metastatic tumors [21], and high-grade serous ovarian cancer [22]. High expression of mTOR in ovarian cancer may promote cell cycle progression and tumor cell proliferation [23]. In a previous study, 87% of clear cell ovarian carcinomas had elevated mTOR activity, while inhibition of the mTOR pathway sensitized these cells to cisplatin [24]. In another report, the expression of mTOR was obviously increased in advanced ovarian cancer [25]. In the present study, median serum mTOR protein levels were highest in patients with malignant epithelial ovarian tumor and progressively decreased in patients with borderline ovarian tumor, benign ovarian tumor, and normal ovarian tissue.
Several studies used immunohistochemistry to investigate the expression status of mTOR in ovarian tissues [26]. Yang et al. showed minimal mTOR expression in benign ovarian tumor and normal ovarian tissue. Xie et al. reported the rate of mTOR positive cells was 23.2% in normal ovarian tissue, 50.0% in benign ovarian tumor, and 68.5% in epithelial ovarian tumor [27]. Shang [28], and Ghoneum [29] showed that the PI3K/AKT/mTOR pathway was associated with aggressive disease, resistance to therapy, recurrence, and a poor prognosis in patients with ovarian cancer. In the present study, immunohistochemistry revealed the rate of mTOR positive cells was 30.0% in normal ovarian tissue, 36.0% in benign ovarian tumor, 75.0% in borderline ovarian tumor, and 82.2% in epithelial ovarian tumor.
These data imply that disruptions of autophagy may be a source of pathogenesis in ovarian cancer, and that beclin-1 and mTOR activity may be related to the pathological characteristics of ovarian tumors. Consistent with this, combined beclin-1 and mTOR expression may represent a biomarker for the evaluation of ovarian tumors. Previous studies show beclin-1 and mTOR protein expression are negatively correlated in breast and non-small cell lung cancer [30,31], and findings from the present study showed a negative correlation between serum beclin-1 and mTOR protein levels in patients with malignant epithelial ovarian tumor.
The sensitivity and specific of autophagy markers in the diagnosis of various diseases differs. Previous reports show mTOR could diagnose IgA nephropathy at a cut-off value of 0.930 (relative gene expression level) with a sensitivity of 90.2% and a specificity of 73.8% and renal fibrosis at a cut-off value of 0.301 with a sensitivity of 71.7% and a specificity of 64.8% [32], mTORC1 could diagnose preeclampsia at a cut-off value of 2113.35 ng/mL with a sensitivity of 93.5% and a specificity of 70.1% [33], and mTOR in cervical lesions could predict malignancy with a sensitivity of 94.0% and a specificity of 60.0% [34]. In the present study, serum beclin-1 protein levels alone could diagnose malignant epithelial ovarian tumor with a sensitivity of 81.6% and a specificity of 88.9%, serum mTOR protein levels alone could diagnose malignant epithelial ovarian tumor with a sensitivity of 75.6% and a specificity of 97.8%, and a combination of serum beclin-1 and mTOR protein levels could diagnose malignant epithelial ovarian tumor with a sensitivity of 77.8% and a specificity of 91.1%, a positive predictive value of 97.3% and a negative predictive value of 83.0%. These data suggest that combined detection of serum beclin-1 and mTOR protein levels may have improved diagnostic accuracy for malignant epithelial ovarian tumor compared to each marker alone.
In conclusion, this preliminary analysis described the expression of beclin-1 and mTOR protein in serum and tissue from patients with ovarian tumors. Findings suggest that autophagy may be a source of pathogenesis in ovarian cancer. Further studies are required to elucidate the molecular mechanisms underlying these data, and their clinical significance.
Acknowledgements
This work was supported by Natural Science Foundation of Yunnan Province (No. 2018FH001-073).
Disclosure of conflict of interest
None.
References
- 1.Kossaï M, Leary A, Scoazec JY, Genestie C. Ovarian cancer: a heterogeneous disease. Pathobiology. 2018;85:41–49. doi: 10.1159/000479006. [DOI] [PubMed] [Google Scholar]
- 2.Gatica D, Lahiri V, Klionsky DJ. Cargo recognition and degradation by selective autophagy. Nat Cell Biol. 2018;20:233–242. doi: 10.1038/s41556-018-0037-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen C, Lu L, Yan S, Yi H, Yao H, Wu D, He G, Tao X, Deng X. Autophagy and doxorubicin resistance in cancer. Anticancer Drugs. 2018;29:1–9. doi: 10.1097/CAD.0000000000000572. [DOI] [PubMed] [Google Scholar]
- 4.Zhou WH, Tang F, Xu J, Wu X, Yang SB, Feng ZY, Ding YG, Wan XB, Guan Z, Li HG, Lin DJ, Shao CK, Liu Q. Low expression of beclin 1, associated with high Bcl-xL, predicts a malignant phenotype and poor prognosis of gastric cancer. Autophagy. 2012;8:389–400. doi: 10.4161/auto.18641. [DOI] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 6.Ding Z, Liu S, Wang X, Dai Y, Khaidakov M, Romeo F, Mehta JL. LOX-1, oxidant stress, mtDNA damage, autophagy, and immune response in atherosclerosis. Can J Physiol Pharmacol. 2014;92:524–530. doi: 10.1139/cjpp-2013-0420. [DOI] [PubMed] [Google Scholar]
- 7.Cai M, Hu Z, Liu J, Gao J, Liu C, Liu D, Tan M, Zhang D, Lin B. Beclin 1 expression in ovarian tissues and its effects on ovarian cancer prognosis. Int J Mol Sci. 2014;15:5292–5303. doi: 10.3390/ijms15045292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yue Z, Jin S, Yang C, Levine AJ, Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci U S A. 2003;100:15077–15082. doi: 10.1073/pnas.2436255100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh H, Troxel A, Rosen J, Eskelinen EL, Mizushima N, Ohsumi Y, Cattoretti G, Levine B. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest. 2003;112:1809–1820. doi: 10.1172/JCI20039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Z, Ghoorun RA, Fan X, Wu P, Bai Y, Li J, Chen H, Wang L, Wang J. High expression of Beclin-1 predicts favorable prognosis for patients with colorectal cancer. Clin Res Hepatol Gastroenterol. 2015;39:98–106. doi: 10.1016/j.clinre.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 11.Hu Z, Zhong Z, Huang S, Wen H, Chen X, Chu H, Li Q, Sun C. Decreased expression of beclin-1 is significantly associated with a poor prognosis in oral tongue squamous cell carcinoma. Mol Med Rep. 2016;14:1567–1573. doi: 10.3892/mmr.2016.5437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giatromanolaki A, Koukourakis MI, Koutsopoulos A, Chloropoulou P, Liberis V, Sivridis E. High beclin 1 expression defines a poor prognosis in endometrial adenocarcinomas. Gynecol Oncol. 2011;123:147–151. doi: 10.1016/j.ygyno.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 13.Wan XB, Fan XJ, Chen MY, Xiang J, Huang PY, Guo L, Wu XY, Xu J, Long ZJ, Zhao Y, Zhou WH, Mai HQ, Liu Q, Hong MH. Elevated Beclin 1 expression is correlated with HIF-1alpha in predicting poor prognosis of nasopharyngeal carcinoma. Autophagy. 2010;6:395–404. doi: 10.4161/auto.6.3.11303. [DOI] [PubMed] [Google Scholar]
- 14.Ko YH, Cho YS, Won HS, Jeon EK, An HJ, Hong SU, Park JH, Lee MA. Prognostic significance of autophagy-related protein expression in resected pancreatic ductal adenocarcinoma. Pancreas. 2013;42:829–835. doi: 10.1097/MPA.0b013e318279d0dc. [DOI] [PubMed] [Google Scholar]
- 15.Naguib M, Rashed LA. Serum level of the autophagy biomarker Beclin-1 in patients with diabetic kidney disease. Diabetes Res Clin Pract. 2018;143:56–61. doi: 10.1016/j.diabres.2018.06.022. [DOI] [PubMed] [Google Scholar]
- 16.Hao XQ. The pathogenesis of ulcerative colitis and the expression and significance of Beclin1 protein in intestinal mucosa and serum [D] Qingdao: Qingdao University; 2016. [Google Scholar]
- 17.Lin HX, Qiu HJ, Zeng F, Rao HL, Yang GF, Kung HF, Zhu XF, Zeng YX, Cai MY, Xie D. Decreased expression of Beclin 1 correlates closely with Bcl-xL expression and poor prognosis of ovarian carcinoma. PLoS One. 2013;8:e60516. doi: 10.1371/journal.pone.0060516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conciatori F, Ciuffreda L, Bazzichetto C, Falcone I, Pilotto S, Bria E, Cognetti F, Milella M. mTOR Cross-talk in cancer and potential for combination therapy. Cancers (Basel) 2018;10:23–52. doi: 10.3390/cancers10010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ciuffreda L, Di Sanza C, Incani UC, Milella M. The mTOR pathway: a new target in cancer therapy. Curr Cancer Drug Targets. 2010;10:484–495. doi: 10.2174/156800910791517172. [DOI] [PubMed] [Google Scholar]
- 20.Umemura S, Mimaki S, Makinoshima H, Tada S, Ishii G, Ohmatsu H, Niho S, Yoh K, Matsumoto S, Takahashi A, Morise M, Nakamura Y, Ochiai A, Nagai K, Iwakawa R, Kohno T, Yokota J, Ohe Y, Esumi H, Tsuchihara K, Goto K. Therapeutic priority of the PI3K/AKT/mTOR pathway in small cell lung cancers as revealed by a comprehensive genomic analysis. J Thorac Oncol. 2014;9:1324–1331. doi: 10.1097/JTO.0000000000000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva B, Antipin Y, Mitsiades N, Landers T, Dolgalev I, Major JE, Wilson M, Socci ND, Lash AE, Heguy A, Eastham JA, Scher HI, Reuter VE, Scardino PT, Sander C, Sawyers CL, Gerald WL. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18:11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mabuchi S, Kuroda H, Takahashi R, Sasano T. The PI3K/AKT/mTOR pathway as a therapeutic target in ovarian cancer. Gynecol Oncol. 2015;137:173–179. doi: 10.1016/j.ygyno.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Tang N, Zhang H. Expression of mTOR/p70S6K signaling pathways in epithelial ovarian carcinoma tissue and their significance. Medical Innovation of China. 2016;13:1–5. [Google Scholar]
- 24.Andorfer P, Heuwieser A, Heinzel A, Lukas A, Mayer B, Perco P. Vascular endothelial growth factor A as predictive marker for mTOR inhibition in relapsing high-grade serous ovarian cancer. BMC Syst Biol. 2016;10:33. doi: 10.1186/s12918-016-0278-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang CY, Li YQ, Zhang YQ. The clinical significance of mTOR and P-4EBP1 expression in ovarian cancer. J Changzhi Medical College. 2019;33:326–329. [Google Scholar]
- 26.Yang ZH. Expression and clinical significance of p-mTOR in epithelial ovarian cancer. J Liaoning Medical University. 2011;32:13–18. [Google Scholar]
- 27.Xie GJ. The study on the expression of Rheb, mTOR and TSC2 in ovarian cancer [D] Guangdong: Guangdong Medical College; 2012. [Google Scholar]
- 28.Shao WY, Yang YL, Yan H, Huang Q, Liu KJ, Zhang S. Phenethyl isothiocyanate suppresses the metastasis of ovarian cancer associated with the inhibition of CRM1-mediated nuclear export and mTOR-STAT3 pathway. Cancer Biol Ther. 2017;18:26–35. doi: 10.1080/15384047.2016.1264540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghoneum A, Said N. PI3K-AKT-mTOR and NFκB pathways in ovarian cancer: implications for targeted therapeutics. Cancers (Basel) 2019;11:949–1024. doi: 10.3390/cancers11070949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jing WJ, Zhao XH, Ma W, Wang JY, Zhang SL, Ma J, Li S. The expression and clinical significance of mTOR protein and Beclin 1 protein in triple-negative breast cancer. Modern Oncol. 2019;27:1745–1749. [Google Scholar]
- 31.Zhang XY, Xu HR, Jing WG, Sun YD, Sun XJ, He SJ. Expression and significance of P-mTOR Beclin 1 and LC3 proteins in non-small cell lung cancer. Guangdong Med. 2019;40:895–901. [Google Scholar]
- 32.Cao Y, Wang Y, Liu Y, Zhu X, Zhang G, Wang S, Chen X, Liu D, Fu C. Decreased expression of urinary mammalian target of rapamycin 2. mRNA is related to chronic renal fibrosis in IgAN. Dis Markers. 2019;2019:2424751. doi: 10.1155/2019/2424751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Zhang B, Wei ZL, Lv WJ, Yang YY, Chen Y. Diagnostic significance of phosphoinositide 3-Kinase and mammalian target of rapamycin complex 1 in preeclampsia. Reprod Sci. 2017;24:268–275. doi: 10.1177/1933719116653675. [DOI] [PubMed] [Google Scholar]
- 34.Guo X, Jin H, Kamilijiang M, Liu KJ, Hasimu H, Reyimu H, Wu GZ, Abudula A. Correlation of the dynamic changes of plasma apoA l content and mTOR activity with cervical carcinogenesis development in Uyghur women. Carcinogenesis, Teratogenesis & Mutagenesis. 2014;26:185–192. [Google Scholar]


