Abstract
The first year of a calf’s life is a critical phase as its digestive system and immunity are underdeveloped. A high level of stress caused by separation from mothers, transportation, antibiotic treatments, dietary shifts, and weaning can have long-lasting health effects, which can reduce future production parameters, such as milk yield and reproduction, or even increase the mortality of calves. The early succession of microbes throughout the gastrointestinal tract of neonatal calves follows a sequential pattern of colonisation and is greatly influenced by their physiological state, age, diet, and environmental factors; this leads to the establishment of region- and site-specific microbial communities. This review summarises the current information on the various potential factors that may affect the early life microbial colonisation pattern in the gastrointestinal tract of calves. The possible role of host–microbe interactions in the development and maturation of host gut, immune system, and health are described. Additionally, the possibility of improving the health of calves through gut microbiome modulation and using antimicrobial alternatives is discussed. Finally, the trends, challenges, and limitations of the current research are summarised and prospective directions for future studies are highlighted.
Keywords: Calve, Microbiome, Gastrointestinal tract, Feeding, Host health, Immune system
1. Introduction
The development of the gastrointestinal tract (GIT) in neonatal humans and animals is a highly dynamic process that is influenced by genetic and environmental factors, nutrition, and the concomitant development of the intestinal microbial communities. This is also true for ruminants, where the first month of life is even more challenging as the rumen is less developed. The rumen is the largest forestomach in ruminants and is highly important for the conversion of ingested feed particles into metabolites that are absorbed and utilised by the host and the formation of microbial protein sources used by the animals [1]. Young ruminants are functionally monogastric at birth with an underdeveloped forestomach system, including the rumen, reticulum, and omasum. During these first months of life, the abomasum and intestines serve as their major digestion sites [2]. The establishment of a fully mature system requires the development of the reticulo-rumen and the associated microbiomes [3]. The microbial communities in the rumen follow a sequential pattern of colonisation with bacteria as the first colonisers, followed by the methanogenic archaea, anaerobic fungi, and protozoa [4], [5], [6]. However, studies using molecular-based techniques showed initial rumen colonisation with facultative anaerobic bacteria (Enterococcus and Streptococcus) in new-born calves as well as archaea within a few hours after birth [7], [8]. A recent study by Malmuthuge et al. (2019) reported on rumen colonisation in neonatal calves with an active bacterial community at birth. The rumen of one-week-old calves were already colonised by active complex-carbohydrate-fermenting bacterial species even in the absence of solid substrates in the diet [9]. These initial gut colonisers utilise the oxygen available in the gut, thus, creating an anaerobic environment favourable for the growth of strict anaerobic gut communities, including Bifidobacterium and Bacteroides [10], [11]. The strict anaerobic bacterial community, including cellulolytic and proteolytic bacteria, together with niche specialists, establish and dominate the gut microbiome within the first two weeks of life [7], [12], [13], [14].
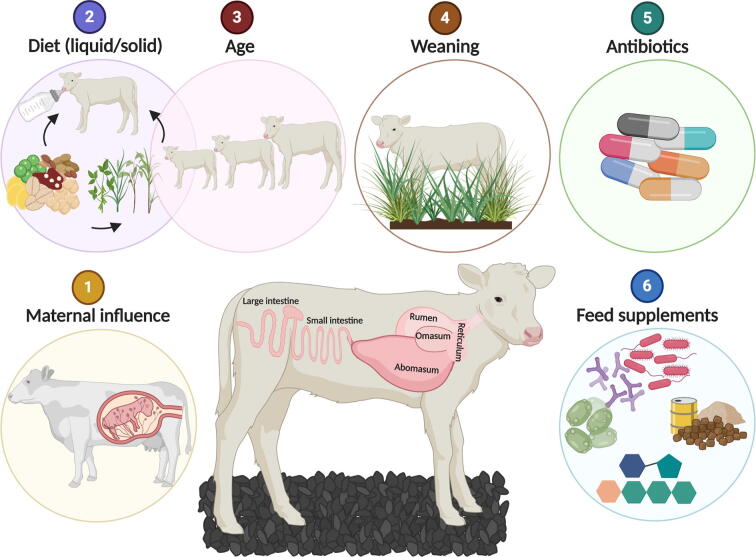
The establishment of a strict anaerobic bacterial community in the GIT of neonates plays an essential role in mucosal immune system development, and is therefore, a critical phase for the host [15], [16]. After the initial gut colonisation, constant exposure of the host GIT to specific microbes is necessary to maintain the host’s energy metabolism, health, and mucosal immune system maturation [17], [18]. Once the GIT is fully mature and the climax microbial community is established, the intestinal microbiome is considered stable thereafter, except for changes in the host’s health, physiological state, and diet [19], [20], [21]. However, considerable differences exist in microbial community profiles in different regions of the GIT in ruminants [14]. Similarly, the mucosa-associated microbial communities were found to differ from those occupying the lumen [14], [22], [23], [24], [25], suggesting a possible role of host–microbe interactions in defining such diverse microbial community structures. In this review, the development of microbial communities across the GIT of calves under the influence of maternal microbiota, age, diet, weaning, and environmental factors (antibiotics and pre/probiotics) (Fig. 1 and Table1), and the possible role of host–microbe interactions in the development of the host’s gut, immunity, and health is summarised.
Fig. 1.
Factors that influence the initial establishment and development of microbial communities throughout the GIT of neonatal calves. Figure created with BioRender.com.
Table 1.
Overview of major factors that affect the initial colonization of microbial communities throughout the GIT of neonatal calves, host gut and immune system development.
| Sample type | Calf age at the time of sampling1 | Diet1, 2 | Method3 | Year | Reference |
|---|---|---|---|---|---|
| MATERNAL INFLUENCE | |||||
| Faeces | 0, 6, 12, 24 and 48 h, 3, 7, 14, and 42 days | N.D. | DNA, PCR single strand conformation polymorphism (PCR-SSCP) of V4-V5 region | 2012 | [28] |
| 4 days–20 days | |||||
| Faeces | 24 h and 7 days | Colostrum: 4–6 h after birth, followed by pooled cow milk | DNA, qPCR, V3-V4 amplicon sequencing (Illumina) | 2018 | [29] |
| Overall GIT | 0, 1, 2, 3, 4, 5, 7, 14, and 21 days | Milk replacer (MR) throughout the study | DNA, V3-V4 amplicon sequencing (Illumina) | 2018 | [25] |
| Faeces and mouth | Faeces (0.5, 6,12, 24, and 48 h); mouth (0.5 h) | Colostrum: after 0.5 h till the end of trial | DNA, V3, V4, V5 amplicon sequencing (Illumina) | 2019 | [30] |
| WEANING | |||||
| Rumen and faeces | 36 and 54 days | Abrupt weaning: MR until day 48, reduction to 0 within 24 h; Gradual weaning: MR slowly reduced from day 36 to day 49; all calves had ad lib. access to water, starter and chopped straw from day 7 to day 54 | DNA, V4 amplicon sequencing (Illumina) | 2016 | [79] |
| Rumen | N.D., after weaning | Fresh milk: day 1 to day 7; half fresh milk and half MR until day 13; MR and dry feed starter till the end of trial; starter, grass hay and water were available ad lib. | DNA, qPCR | 2017 | [80] |
| Rumen and faeces | 5, 7, and 9 weeks | Ad libitum access to water, starter, chopped straw and oat straw from birth till the end of trial | DNA, V4 amplicon sequencing (Illumina) | 2017 | [84] |
| ANTIBIOTICS | |||||
| Rectal swabs and faeces | Newborn, 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10 weeks | Trial 1: Milk substitute without antibiotics or antibiotic containing fresh milk or fermented milk | Culture-based assays | 1990 | [143] |
| Trial 2: Standard milk substitute, containing growth promoter or antibiotic containing milk | |||||
| Rectal swabs | N.D. | Colostrum: within 24 h after birth; ad lib. milk with penicillin G and water: until day 37 | Culture-based assays | 2003 | [144] |
| Faeces | N.D. | Bulk milk (BM) and grain concentrates with or without oxytetracycline: 12 weeks trial | Culture-based assays and PCR for screening of drug resistance genes | 2004 | [133] |
| Rectal faecal swabs | 0, 2, 4, and 6 weeks | N.D. | Culture-based assays | 2005 | [145] |
| Faeces | 9 time points during first 6 months | Pasteurized or non-pasteurized waste milk before weaning | Culture-based assays | 2012 | [146] |
| Faeces | 6, 7, and 12 weeks | Colostrum: within 2–6 h after birth; MR without antibiotics or with neomycin sulfate and oxytetracycline hydrochloride antibiotics; all calves ad lib. access to starter grain from day 1; alfalfa hay offered post-weaning | DNA, qPCR, sequencing of target genes | 2012 | [150] |
| Faeces | 2, 14, 28, and 56 days | Colostrum: within 2–4 h after birth; ad lib. hay: from day 1; pasteurized or non-pasteurized (WM and BM): from day 3; pelleted calf starter: from day 8 until day 56 | Culture-based assays | 2013 | [132] |
| Faeces | 12 days | MR: from day 0 with or without bacitracin methylene disalicylate. all calves: ad lib. to concentrate from day 3 until day 56 | DNA, V4-V6 amplicon sequencing (454) | 2013 | [137] |
| Faeces | 3, 5, and 6 weeks | Pasteurized hospital milk throughout the study. Water and calf starter ad lib. | DNA, V1-V2 amplicon sequencing (454) | 2015 | [138] |
| Faeces | Newborn, 1, 2, 3, 4, 5, and 6 weeks | Colostrum: within 4 h after birth; raw milk without antibiotics or with low concentrations of ampicillin, ceftiofur, penicillin, and oxytetracycline: from day 1 till the end of trial; pelleted calf starter: offered from day 7 until day 42 | DNA, V4 amplicon sequencing (Illumina) | 2016 | [139] |
| Faecal and nasal swabs | 42 days and 1 year | Colostrum: after birth; MR or WM: for 6–12 weeks | Culture-based assays | 2017 | [147] |
| Faecal swabs | 3, 35, and 56 days | Colostrum: within the 24 h after birth; MR without antimicrobials or pasteurized WM with β-lactam residues: until day 49. all calves ad lib. water and textured calf starter: from day 1 to day 56 | Culture-based assays and PCR of antimicrobial resistance genes | 2017 | [148] |
| Faeces | 0, 1, 3, and 6 weeks | Milk without antimicrobials or with low concentrations of ceftiofur, penicillin, ampicillin and oxytetracycline: birth till 6 weeks of age | DNA, and whole genome sequencing (Illumina) | 2018 | [151] |
| Faeces, ileum, colon | 35 days | Colostrum: within 1 h after birth; MR without antibiotics or with low concentrations of antibiotics. all calves ad lib. water and starter feed from day 4 until end of trial | DNA, V3-V4 amplicon sequencing (Illumina) | 2018 | [140] |
| Faeces | N.D. | Colostrum: within hours after birth; Pasteurized non-saleable milk: until 56 days of age. Ad lib. water | DNA, whole genome sequencing (Illumina) | 2019 | [141] |
| Rumen fluid and tissues | 15, 25, and 35 days | Colostrum: within 1 h after birth; MR without antibiotics or with low concentrations of penicillin, streptomycin, tetracycline and ceftiofur. all calves ad lib. starter and water from day 2 until end of trial | DNA, V3-V4 amplicon sequencing (Illumina) | 2019 | [142] |
| Faeces | N.D. | Colostrum: within 1 h after birth; pasteurized non-saleable milk until 56 days of age. ad lib. water | Culture-based assays and PCR | 2020 | [149] |
| FEED SUPPLEMENTS | |||||
| Probiotics | |||||
| Faeces | 7–35 days | Trial 1: MR without or with B. pseudolongum / L. acidophilus: from day 7 to day 42 d; starter: from day 14 to day 56. ad lib. water and dried grass | Culture-based assays | 1995 | [154] |
| Trial 2: MR without or with B. thermophilum, E. faecium and L. acidophilus; ad lib. MR without antibiotics and water | |||||
| Rumen contents and faeces | 31–33 days | MR until 6 weeks of age, afterwards a mixture of alfalfa pellets and sweet feed with ad lib. water throughout the trial | Culture-based assays and genomic DNA fingerprinting | 1998 | [157] |
| Faeces and blood | 1, 3, 5, and 7 weeks | Non-pasteurized colostrum: after birth; acidified non-saleable milk: day 1 - day 56. Ad lib. water and calf starter | DNA, V4 amplicon sequencing (Illumina) | 2015 | [155] |
| Blood, and tissue and digesta of jejunum, ileum and colon | Blood (1 and 12 h, 1–7 days); Tissue and digesta (1 week) | Colostrum replacer: first 12 h; MR: from day 1 to day 7 with or without supplementation of Saccharomyces cerevisiae boulardii. Ad lib. water. | Radial immunodiffusion analysis, ELISA, immunohistochemistry, RNA and DNA, RT-qPCR | 2020 | [159] |
| Prebiotics | |||||
| Rumen fluid and blood | N.D. | Milk and concentrate feed (with or without cellooligosaccharides or kraft pulp supplements): from 4 weeks before weaning till 12- or 16-weeks post-weaning | DNA, qPCR | 2019 | [161] |
| Rumen fluid and blood | 6.5, 7, 7.5, and 8 months | Ad lib. starter concentrate, chopped oat hay and water: for 1 week; oat hay and concentrate (3:7) with or without astragalus root extract: afterwards | Manual assay for serum; DNA, V3-V4 amplicon sequencing (Illumina) | 2020 | [162] |
| Dietary supplements | |||||
| Rumen | 90 and 160 days | Whole milk: first 30 days; MR and starter concentrate (with or without calcium propionate supplement): day 30 to day 90; starter feed: day 91 till the end of trial; alfalfa hay was only provided at day 91. | DNA, V4 (bacteria) and V8 (archaea) amplicon sequencing (Illumina) | 2020 | [164] |
| Faeces and blood | Faeces (1, 3, 7, and 14 days); Blood (14 days) | Colostrum: within 1 h after birth; Raw milk: day 2 to day 4; starter concentrate (with or without zinc supplement): day 4 till the end of trial | ELISA, DNA, V3-V4 amplicon sequencing (Illumina) | 2020 | [163] |
| HOST IMMUNE SYSTEM DEVELOPMENT | |||||
| Ileum and colon tissues, plasma, adrenal glands | Plasma samples (72 h); other (75 h) | Colostrum: immediately after birth. three groups: a) colostrum, b) whole milk, c) mixture of 50% colostrum and 50% whole milk: for 72 h | RNA, qRT-PCR and qPCR | 2020 | [113] |
| Jejunal mucosa | 80 days | Colostrum: immediately after birth; acidified transition milk: first 3 days; MR: day 4 until 8 weeks of age with linear reduced amount during week 9 to 10. Ad lib. water, hay and concentrate from day 10 | RNA, Illumina HiSeq sequencing | 2018 | [115] |
| Blood, jejunum mucosa | Blood (1, 2, 7, 14, 21, 28, 35, 42, 49, 56, 63, 70, and 77 days) Jejunum (day 80) | Colostrum: within 2 h after birth; acidified transition milk until day 3; MR: day 4 until day 70. Ad lib. water, hay and concentrate from day 10 | RNA, whole transcriptome sequencing | 2018 | [117] |
| Rumen, jejunum, ileum, cecum, and colon | 3 weeks | Fresh whole milk and calf supplement throughout the trial | DNA, V1-V3 amplicon sequencing (454), qPCR | 2014 | [14] |
| Mucosa of rumen, jejunum, ileum, cecum and colon | 3 weeks and 6 months | Non-pasteurized whole milk and calf supplement: first 12 weeks; alfalfa hay and oats: for the next 4 months | DNA, fingerprinting, clone libraries, qPCR | 2012 | [119] |
| HOST GUT DEVELOPMENT | |||||
| Rumen, jejunum and ileum tissues | Newborn, 7, 21, and 42 days | Colostrum: after 30 min. of birth; whole milk: until day 7; ad lib. starter: from day 7 until day 42 | DNA and RNA; Illumina RNA-sequencing and qRT-PCR | 2014 | [122] |
| Rumen tissue and content | Newborn, 1, 3, and 6 weeks | Colostrum: within the first 3 days; whole milk: day 4 till the end of trial. Ad lib. starter from second week of life | DNA, whole genome sequencing (Illumina), qPCR, RNA, transcriptome (host) | 2019 | [9] |
N.D. = Not defined.
Ad lib. = ad libitum.
Hypervariable regions (V1, V2, V3, V4, V5 V6 and V8) of prokaryotic 16S rRNA gene.
2. Early succession of microbes throughout the GIT of neonatal calves and maternal influence
Birth exposes neonates to the vaginal, skin, and colostrum microbiome of the mother [26], [27], which initiates the microbial colonisation of the neonatal GIT. The neonatal microbiome must undergo several modifications prior to weaning (6–12 weeks), and it may take a year for the establishment of a fully functional and stable GIT microbial community [7]. To date, only a few culture-independent studies have examined the effect of maternal sources on the early establishment of microbes in neonatal calves’ GIT [25], [28], [29], [30]. At the genus level, the rectal microbiota of the new-born calves was more similar to the dam’s oral microbiota (39%) as compared to the microbiota on the dam’s vagina (24%) or faeces (15%), indicating an in utero transfer route for the inoculation of neonatal gut microbiota [29]. However, the faecal microbiota during the first 48 h of calf life showed a close resemblance to the dam’s vaginal microbiota than other maternal sources (faeces or colostrum), indicating the possible transfer of microbes to the neonates via the birth canal [30]. In contrast, Yeoman et al. reported high similarity between the dam’s udder skin and calf’s GIT microbiota during the first three weeks of life [25]. The inconsistencies among these studies are probably due to differences in sampling sites (calf faeces vs. dam’s mouth, vagina, faeces, udder skin, or colostrum), and sampling time. In addition to the influence of maternal interaction/microbiome on the early succession of microbes throughout the neonatal calves’ GIT, the facility, farm or location where the calves are born and raised also reported to have a significant impact on the gut microbiota of Holstein dairy cows [31] as well as beef calves [32], [33]. Thus, the management practices must be carefully considered because of their unidentified role in shaping gut microbial community structures besides several other factors including genetics, breed, age, diet and study method etc.
3. Effect of early feeding regimen and age on the initial establishment and development of microbial communities in the GIT of neonatal calves
Young ruminants are pseudo-monogastric at birth with an underdeveloped reticulo-rumen, relying solely on a milk-based diet [2]. In pre-weaned calves, most of the liquid feed flows straight into the abomasum without entering the rumen; thus, the small and large intestines serve as their major digestion sites. The forestomach system in neonatal calves changes tremendously during the first year of life, with a shift in the activity of intestinal enzymes (lactase and maltase), which facilitates the development of the salivary apparatus, other digestive compartments, and rumination behaviour in calves [34], [35], [36]. In addition, rumen volume increases, and rumen papillary shape and size proliferate, providing better niche environments for the microbial colonisation of the rumen and its subsequent functioning [37]. Concomitant with these morphophysiological adaptations, the changes in microbial composition of pre-weaned calves’ GIT are driven by the rearing environment, age, and diet [17], [33], [36], [38], [39]. The diet of pre-weaned calves is changed gradually from milk or milk replacer (MR)-based diets to solid feed within the first few weeks of their lives [40]. These dietary shifts seem to have prominent effects on the neonatal calf microbiome. Many studies have explored the effect of liquid/solid diets, including fresh or heated colostrum [41], [42], whole milk, waste milk (WM), pasteurised waste milk (pWM) or MR [43], [44], [45], starter concentrate [23], [46], [47] and roughage [48], [49], [50], [51], on the initial establishment of bacterial communities in the GIT of neonatal calves.
3.1. Colostrum and other liquid feeds
New-born calves are immunodeficient and depend solely on colostrum-associated immunoglobulins [52]. Feeding high-quality colostrum is highly recommended as it can inhibit the growth of pathogens, stimulate the colonisation of the small intestines with beneficial microorganisms [41], increase body weight gain, improve the development and function of the GIT, reduce the risk of diarrhoea [53] and thereby, decrease the mortality rate in calves [54]. However, the lack of proper hygiene practises increases the risk of colostrum contamination with microbes [55]; therefore, adequate heating of colostrum is recommended. Feeding heat-treated colostrum within the first 12 h of life inhibited pathogenic Escherichia coli and Shigella, and increased the growth of Bifidobacterium [41], [42]. The increase in Bifidobacterium was also observed in 51-hour-old dairy calves using a similar treatment [56].
After colostrum feeding, the nutrient composition of the subsequent feeding again defines the microbiome composition. In general, the rumen bacterial community of one- to three-day-old colostrum-fed calves was dominated by Proteobacteria [7], [57], but as the calves aged and started to consume MR and starter concentrate-based diet, Proteobacteria was slowly replaced by Bacteroidetes in the rumen [7], [12], [57]. Similar to the rumen, Proteobacteria dominated the faecal microbiota of 24–48-hour-old calves, showing a depletion and a subsequent increase in Firmicutes within the first seven days of a calf’s life without any diet change [29], [30]. Similarly, Firmicutes was the dominant phylum in the faecal microbiota of one- to seven-week-old calves [13]. Yeoman et al. also reported higher abundance of Firmicutes in the colon and faeces, while Bacteroidetes was more abundant in the rumen, reticulum, omasum, and abomasum within the first three weeks of a calf’s life [25].
Shifting the diet of pre-weaned calves (7–28 days) from colostrum to whole milk increased the abundance of typical milk-utilising bacteria (Lactobacillus, Parabacteroides, and Bacteroides) in their rumen [47]. Feeding milk to two-week-old calves also increased the abundance of Ruminococcus flavefaciens, a fibrolytic bacterium in the rumen [46]. Similarly, a recent study by Malmuthuge et al. reported the colonisation of a whole milk-fed one-week-old calf’s rumen with active R. flavefaciens, whose density increased with increasing age, suggesting the possible use of milk as a substrate for R. flavefaciens [9]. Feeding a milk-based diet also had prominent effects on the lower gut microbiota of pre-weaned calves as indicated by the high levels of the Bacteroides–Prevotella group and Faecalibacterium in the faecal samples of MR-fed one-week-old calves [58]. Similar levels were also reported in the colon samples of three-week-old whole milk-fed calves [14], indicating that faecal samples represent the microbiome of the large intestine in an adequate manner [14]. Similarly, Alipour et al. also observed a high dominance of Faecalibacterium and Bacteroides in the faecal samples of seven-day-old milk-fed calves [29].
The cost benefits of WM over whole milk and MR [59], [60] and the increased use of on-farm pasteurisers have facilitated the use of waste milk in calf feeding programmes. Feeding WM modified the rumen bacterial community composition by decreasing Prevotella 7 and increasing Butyrivibrio 2, the Rikenellaceae RC9 gut group, and Prevotellaceae UCG-003 in two-month-old calves [45]. The opposite was true when WM feeding was prolonged during the first six months, and higher abundance of Prevotella 7 and Succinvibrionaceae UCG-001 and lower abundance of Prevotellaceae UCG-003, Rikenellaceae RC9 gut group, Selenomonas 1, and others were observed [45]. Pasteurisation inactivates the vegetative bacterial cells, reduces the risk of disease transmission and mortality and improves the growth rate of calves [61]. A relatively high abundance of Prevotella and low abundance of Streptococcus and Histophilus were observed in the nasal microbiota of pWM-fed 42-day-old calves [44]. In addition, feeding pWM increased faecal bacterial diversity from two weeks to six months of age; a higher prevalence of faecal Bacteroidetes and lower prevalence of Firmicutes, and no Salmonella were detected in young pWM-fed calves [43]. An opposite, but non-significant, ratio of Firmicutes and Bacteroidetes was also found in pWM-fed calves as compared to that in MR-fed calves [44]. The effects of MR-compositions on faecal microbial communities were studied recently, and it was found that the faecal microbiota of seven-day-old calves fed with MR enriched with conjugated milk oligosaccharides had higher relative abundance of Faecalibacterium prausnitzii and Bifidobacterium species than did those consuming MR with high free milk oligosaccharides [62]. F. prausnitzii is a beneficial bacterium for neonatal calves due to its positive correlation with body weight gain and reduced diarrhoea [13]. Furthermore, Yak calves reared in isolation on a standard MR-, starter concentrate-, and hay-based diet were found to have better organ development, growth rate, immune function, and higher abundance of non-fibrous carbohydrate-utilising bacterial genera [63] than the maternally nursed and grazed calves that had a higher abundance of fibrous carbohydrate-utilising bacterial genera [63], [64]. Thus, the early feeding regimen shapes the microbiome structure in pre-weaned calves by providing different substrates for growth and establishment of various ecological niches.
In addition, drinking water offered to the calves immediately after birth seems to have a prominent impact on gut microbial composition, as indicated by the increased abundances of Faecalibacterium and Bacteroides at two weeks and Faecalibacterium and Bifidobacterium in the six-week-old calves [65]. In addition, calves consuming drinking water from birth had higher body weight, digestibility of fibre, and feed efficiency than the calves that started to receive drinking water from 17 days of age [66].
3.2. Consumption of solid feed reshapes the gut microbiota in pre-weaned calves
The solid feed intake begins around two to three weeks of life, which initiates the critical transition process leading to the establishment of a fully functional rumen. It is usually characterised by a constant or gradual supply of concentrate and ad-libitum hay in addition to milk feeding. Thus, the effects of solid feed intake should be considered as complex responses to enhanced starch-rich and moderate fibrous feed ingredients together. Generally, an increased abundance of amylolytic and fibrolytic bacteria, such as Succinovibrionaceae, Fibrobacteraceae, and Prevotellaceae, in the rumen microbiome has been described in almost all studies of this feeding period [7], [46], [48], [49], [50], [57], [67], [68], [69], [70]. Prevotellaceae is the predominant family in the rumen fluid and has a broad genetic capacity to use a variety of soluble sugars, starch, protein, and peptides [71], [72], [73]. The enzymes involved include carbohydrate-degrading enzymes (CAZYmes), such as glycoside hydrolases (GH2, GH3, GH42, and GH92), which are detectable in pre-ruminant rumen samples [12]. The activity of amylase and xylanase has already been shown in two-day-old calves, even in the absence of complex dietary carbohydrates [74]. Thus, the presence of glycoside hydrolase activity together with the production of short-chain fatty acids (SCFA) reveals that the metabolically active rumen microbiome is established soon after birth in neonatal calves, even in the absence of solid feed. SCFA are important for rumen tissue metabolism, rumen papillae, and epithelium development [9], [40] and they are absorbed into the bloodstream through the papillae and provide energy for calf metabolism and growth [40]. Depending on the solid feed source, changes in the pH and SCFA amount and composition are observed. Forage feeding improves the ruminal environment by increasing rumen liquid pH [40], [48], reducing the chances of subacute ruminal acidosis, and modifying the structure of the rumen microbiome, leading to the establishment of a fully functional rumen during weaning [49], [75]. Furthermore, the particle size as well as the physical form of diet seems to influence the morphophysiological and microbial development of the rumen [76], [77]. Feeding a ground diet to calves reduces the growth of their rumen papillae, lowers the pH of their rumen liquid, reduces the number of cellulose-degrading bacteria, and increases the number of amylose degraders [76]. This finding strongly indicates the potential role of effective fibre feeding for the modification of the rumen environment as well as the associated microbial community composition.
The establishment of an archaeal community in the GIT of calf is important for the required hydrogen balance during bacterial fermentation. The dietary modifications also seemed to have obvious effects, and a higher abundance of Methanosphaera and lower abundance of Methanobrevibacter were observed in the rumen of pre-weaned calves fed a milk plus starter concentrate-based diet as compared to the milk-fed calves [47]. Starter concentrate feeding also increased the dominance of Methanomicrobiales mobile in the abomasum, caecum, and faeces and Methanobrevibacter in the caecum and faeces of 20-day-old calves, as well as decreasing the abundance of Methanococcales votae [46]. Additionally, a decrease in the rumen bacterial diversity, and an increase in the rumen archaeal diversity as well as fungal richness were observed with silage supplementation [51].
4. Effect of weaning age and management on microbial colonisation of the GIT in calves
Among the most important factors influencing further animal development in general, and the forestomach system in particular, are the date (age) and strategy of weaning. The abrupt weaning of calves from a milk-based diet to the consumption of solid feed decreases their solid feed intake and average daily gain [40], [78]. However, no effect of the weaning strategy (abrupt vs. gradual) was observed on the establishment of rumen and faecal microbial community composition [79], suggesting that the progressive development of the microbial community into a mature state occurs with age [12]. The date of weaning is an important factor in the development of the rumen. Weaning calves, at eight weeks of age, increased their average daily gain [80], and improved carcass quality, feedlot growth, and performance [81], [82]. Rumen enzyme activity was also improved [80], probably due to a greater concentrate intake [83], indicating that the consumption of solid feed triggers the development of the adult-like rumen bacterial community. However, calves weaned six weeks after birth abruptly shifted the β-diversity of their rumen and faecal microbiomes compared to the calves weaned eight weeks after birth [84].This sudden change in the microbial community structure of early weaned calves reflects pre-mature rumen development, paralleled by their reduced growth rate [85], whereas gradual rumen development, [84] improved feed intake, and growth rates were observed when calves were weaned at eight weeks of age [85]. Thus, a balanced weaning management and an appropriate weaning age are important to minimise the side effects.
Rumen fermentation activity begins with the addition of solid feed in the diet and concomitantly alters the microbial composition of a calf’s GIT. An increase in the abundance of Firmicutes and Proteobacteria and a decrease in the abundance of Bacteroidetes were observed in the rumen microbial community from pre- to post-weaned state [79]. Bacteroidetes dominated the rumen microbiota of 42-day-old [12] and two-month-old pre-weaned calves [7]. A similar weaning-related decrease in the abundance of Bacteroidetes and a subsequent increase in Firmicutes were observed, regardless of the calf’s age at weaning [84]. This suggests that the rumen of pre-weaned calves contains the same dominant phyla, including Bacteroidetes, Firmicutes, and Proteobacteria, as found in the rumen of mature post-weaned calves, although the abundance of these phyla varies depending on the developmental stage [86]. At the genus level, Prevotella dominated the rumen microbial community of both pre- and post-weaned calves and showed no changes in the abundance regardless of weaning age or strategy [79], [84]. Similarly, high dominance of Prevotella in the mature rumen of two-month- to two-year-old cattle has previously been reported [7], [12] Nevertheless, the genus level composition of MR-fed pre-weaned calves’ rumen showed a higher relative abundance of Bacteroides and Succinivibrio than did that of post-weaned calves fed a high-starch diet [79]. In contrast to this depletion, the abundance of Sharpea increased by weaning, making it the second dominant genus in the rumen of post-weaned calves [79]. The increase in starter and forage intake from pre- to post-weaned period [79], [87] was positively correlated with the calf’s body weight and the abundance of Sharpea [79]. However, the abundances of Shuttleworthia and Dialister increased drastically in early weaned calves across weaning, while no differences were observed in late-weaned calves before and after weaning [84]. Dialister spp. are capable of degrading starch [88], and the increased abundance of Dialister in early weaned calves was probably due to increased consumption of starter concentrate across weaning [84]. In addition, early weaned calves had higher number of Fibrobacter succinogenes and Ruminococcus albus, with a lower number of Butyrivibrio fibrisolvens, than did late-weaned calves [80]. Ruminococcus abundance was positively correlated with solid feed intake and body weight gain in calves [79], likely reflecting the cellulolytic capabilities of Ruminococcus species, which are found in the mature rumen [7], [89]. Therefore, it can be speculated that as soon as the calf started to consume the solid feed, the bacterial community resembling the mature rumen is established.
Contrary to the bacterial community of the rumen, the faecal bacterial community of pre-weaned calves showed a high dominance of Firmicutes being replaced by Bacteroidetes in post-weaned calves [79]. At the genus level, the abundance of faecal Bacteroides decreased due to weaning, but it remained the predominant genus in both the pre- and post-weaned state [79]. Furthermore, an increase in the abundance of Prevotella was observed due to weaning [79]. However, the abundances of the major faecal bacteria remained unaffected by weaning [84]. Nevertheless, an increased abundance of Ruminococcus and a decreased abundance of Blautia were observed in post-weaned calf faeces [79], [84], likely reflecting a shift from intestinal to ruminal fermentation in post-weaned calves.
Rumen carbohydrate metabolism showed an age-dependent increase between 5 and 9 weeks, regardless of weaning. Conversely, a decline in faecal carbohydrate metabolism was observed from the pre- to post-weaned state [84]. Additionally, a decrease in rumen bacterial diversity and evenness and an increase in faecal bacterial diversity, richness, and evenness were observed in post-weaned calves [79]. This was probably due to the higher solid feed intake in the post-weaning period, resulting in a greater amount of substrates reaching the lower intestine [79]. Thus, the higher substrate availability and lower pH variability of the hindgut favoured higher bacterial diversity in the lower digestive tract of ruminants.
5. Distinct bacterial communities are associated with the mucosal epithelium and luminal digesta of the GIT of calves
The bacterial composition in the GIT of animals and humans varies among the gut regions, with considerable differences between the microbes associated with the epithelial mucosa and those occupying the luminal digesta. This is also true for calves [14], [22], [23], [24], [25] and adult ruminants [90]. The mucosa-associated microbial community in calves is found to have higher individual variation, diversity, richness, and a lower microbial load than the microbiota in digesta samples [17], [22], [23], [24], [90]. These differences are caused by variations in host physiological state and immunity, interactions between the symbiotic bacteria and host epithelium, pH, oxygen gradient, nutrient profile, and dietary transition rates [91], [92]. Each of these factors defines the microbial colonisation potential of each site, thus resulting in site- and region-specific microbial community establishment.
Digesta-associated gut communities within the first 21 days of a calf’s life, except for the colon, showed a high dominance of Firmicutes [14], [25], whereas a higher abundance of Bacteroidetes was observed in the mucosa-associated communities, except jejunal tissues, suggesting that the early life mucosal environment favours the colonisation by Bacteroidetes than Firmicutes [14]. Proteobacteria were also more abundant in the mucosa than the digesta samples [14], [25], suggesting that the mucosa-associated Proteobacteria spp. might play an essential role in scavenging blood oxygen and ruminal ammonia oxidation [14]. This would promote an anaerobic environment for the colonisation and fermentative activities of rumen microorganisms [14]. Such compositional changes in the mucosa or digesta-associated communities were more prominent at the genus level, where Bacteroides dominated the digesta-associated communities in the reticulum, rumen, omasum, abomasum, caecum, and colon [14], [25]. In contrast, the mucosa-associated bacterial communities of the rumen, ileum, caecum, and colon were dominated by Prevotella [14]. Moreover, the abundance of Escherichia exceeded Bacteroides in the mucosal samples of the omasum, abomasum, ileum, colon, and faeces [25]. Similar to this study, the hindgut microbiota of one-week-old calves showed a high dominance of mucosa-associated Escherichia-Shigella groups, indicating greater disease susceptibility in young calves [24]. The digesta-associated community of the duodenum was dominated by Lactobacillus, while Pseudomonas dominated in the mucosa [25]. Furthermore, the mucosa-associated communities of the jejunum showed high abundances of Prevotella, Pseudomonas, Acinetobacter, Rikenellaceae RC9 group, and Delftia [25]. The high dominance of aerobic/facultative anaerobic bacteria (Pseudomonas, Acinetobacter, Delftia, and Escherichia) in several mucosal samples suggests that these bacteria prefer gastrointestinal epithelium for growth due to higher availability of oxygen concentration [93]. In contrast, the jejunal digesta-associated communities were dominated by Sharpea, Butyrivibrio, Ruminococcus, Lactobacillus [14], Streptococcus, and Escherichia [25]. Sharpea spp. are capable of fermenting a vast variety of sugars [94]. Their high dominance in jejunal digesta of three-week-old calves is indicative of their important role in the fermentation of milk during early calf life [14].
The mucosa-associated bacterial community composition was also affected by calves’ age and was significantly correlated with SCFA concentrations, indicating that the host physiology as well as diet play a role in shaping mucosal microbial communities [24]. The abundance of mucosa-associated Escherichia-Shigella was negatively correlated with acetate concentration and the inhibition of E. coli growth was observed due to high concentration of acetate [95]. SCFA can also influence the turnover of intestinal epithelial cells [96], indicating a possible interaction between mucosa-associated microbial communities and digesta-associated microbial metabolites [24].
6. Influence of host genetics on gut microbial colonisation and systemic immunity in neonatal calves
In recent years, many studies have evaluated the influence of host genetics on gut microbiota in cattle [97], [98], [99], [100], [101] and the possible association of heritable gut microbes with nutrition and gut health in calves [102], methane emissions and feed efficiency in beef and dairy cattle [98], [101]. Majority of these studies used animals belonging to different populations with variable genetic distance, age and diet, thus, masking the real influence of host genetics on gut microbiota. However, a recent study by Fan and colleagues reported genetic influences on gut microbiota based on 228 calves with linearly varying breed (Angus to Brahman), raised under controlled diet and environmental conditions [102]. The three-month-old pre-weaned calves with higher Brahman proportion harboured more butyrate-producing and fibre-digesting bacteria, carbohydrate metabolism genes, less opportunistic pathogenic bacteria and mucin-degraders, lower level of primary antibody (plasma IgG1) and less weight gain than higher Angus proportion calves that harboured bacterial taxa rapidly involved in amino acids and lipids metabolism [102]. This indicates that the host genetics not only shapes the early life gut microbiota composition but can also have strong impact on systemic immunity, which is further associated with health and growth of an animal. However, the studies addressing the role of host genetic influence on neonatal calves’ microbiota are still very scare and needed to be explore further.
7. Gut microbiota and the host immune system development
Gut microbial communities are essential for the development of the mucosal epithelium and immune system of the host [18]. The mucosal epithelial cells line the upper respiratory tract, GIT, and uterus and are the primary responders to the microorganisms [103]. The mucosal immune system contains various physical and chemical barriers as well as pattern recognition receptors (PRRs), which enable the mucosal epithelium to coexist with its resident symbiotic microorganisms and provides protection against invading pathogens [104], [105], [106]. Notably, these signalling cascades are essential for maintaining the intestinal homoeostasis, integrity, antimicrobial peptide expression, and modulation of the mucosal barrier functions and immune responses [91], [107], [108]. The immune response at the mucosal surface is generally initiated by mucosa-associated lymphoid tissues (MALTs) [38], [103]. In ruminants, the initiation of MALT development occurs in utero when the microbial communities are not yet established [109]. These in utero MALTs are capable of initiating specific immune responses through secretory IgA production [110]. However, IgA+ and IgG+ cells appear in Peyer's patches (PPs) only after birth due to the absence of in utero infections [109]. The complete development of germinal centres of PPs requires exposure to the gut microbiome [18]. In the absence of gut microbial exposure, the ileal PPs of new-born lambs showed pre-mature lymphoid follicle involution; however, when the gut microbiome was restored at four weeks, the involution was reversed [111]. This finding demonstrates that the gut microbiome provides signals for the production of a vast variety of pre-immune B cells (Fig. 2A). In addition to the gut microbiome, diet (colostrum, intensive feeding of milk or MR), and environment (toxins) were also found to have a strong influence on the mucosal immune system development in neonatal calves [112]. Extended colostrum feeding during early life resulted in higher abundances of active mucosa-associated Lactobacillus and E. coli and upregulated the expressions of serotonin and adrenergic receptors genes in the calf’s intestines (Fig. 2B) [113]. These receptors are involved in the regulation of glucagon-like peptide-2 secretion by enteroendocrine L cells, which decreases the apoptosis of epithelial cells, reduces the motility and permeability of the gut, and increases mesenteric blood flow, intestinal growth, and nutrient absorption [114]. A positive correlation was observed between the abundances of Lactobacillus and E. coli and serotonin receptor gene expression in the colon, suggesting that the early feeding regimen may affect the host–microbe interactions, and thus play a critical role in host immune system development in new-born calves [113]. Likewise, the intensive feeding of milk or MR during the pre-weaning period stimulated the expression of long noncoding RNAs with a potential role in the synthesis of tight junction proteins in the jejunal mucosa of calves [115]. The tight junctions are protective mucosal barriers whose breakdown results in leaky gut syndrome (Fig. 2C) [103], [116]. In addition, it was shown that an ample supply of nutrients is essential for maturation of the intestinal immune system [117], suggesting that the pre-weaning period is critical for the development and maturation of the mucosal immune system in calves [39].
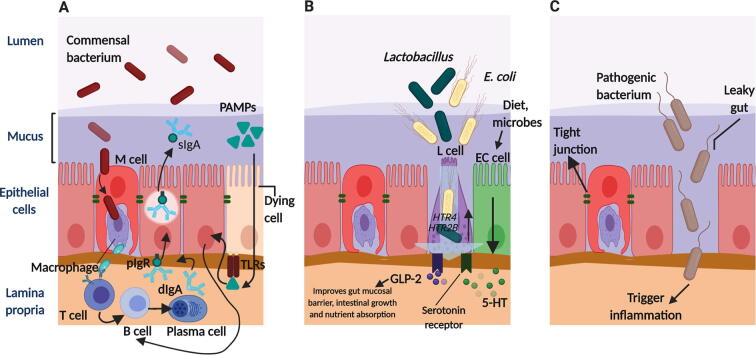
Fig. 2.
Mucosa-associated lymphoid tissues (MALTs) dependent activation of immune responses in mucosal surface of calves. A) Microfold (M) cell transport microbial antigens from the luminal surface to the underlying MALT cells, where they stimulate specific T- and B- lymphocytes, resulting in the production of dIgA by B-cells, which are translocated as sIgA to the apical epithelial surface. PAMPs can alter the expression of TLRs and activate host immunity. B) Upregulation of HTR4 and HTR2B genes expression by mucosa-associated bacteria. These gene codes for the serotonin receptors that regulate GLP-2 secretion by enteroendocrine L cells via interaction of 5-HT with serotonin receptors. C) Breakdown of tight junctions, transport of pathogens and activation of inflammatory responses. Abbreviations: PAMPs, pathogen-associated molecular patterns; dIgA, dimeric immunoglobulin A; sIgA, secretory immunoglobulin A; pIgR, polymeric Ig receptor; TLRs, toll-like receptors; EC cell, enterochromaffin cell; 5-HT, 5-hydroxytryptamine/serotonin; HTR4, 5-hydroxytryptamine receptor 4; HTR2B, 5-hydroxytryptamine receptor 2B; GLP-2, glucagon-like peptide-2. Figure created with BioRender.com.
The host identifies commensal microorganisms using PRRs such as toll-like receptors (TLRs) [107]. Mucosa-attached bacteria can also alter the expression of TLRs [118] and cause PRR-dependent activation of the host immunity [14]. In contrast, pathogen-dependent activation of TLR signalling generally activates inflammatory responses [107]. Furthermore, an age-dependent decrease in mucosal TLR gene expression [119] and an increase in T lymphocytes such as CD3+, CD4+, and CD8+ cells in the mucosa of the jejunum and ileum of calves were observed [120]. Such changes may cause a decrease in the innate immunity and an increase in the adaptive immunity with age. This age-dependent downregulation of the innate immunity protects the host from harmful inflammatory responses [121]. It has been suggested that TLRs act as a primary mechanism of innate immunity in neonatal calves. They are substituted by antimicrobial-peptide-dependent innate immune mechanisms over time and protect the animal from unnecessary inflammatory responses [119]. Additionally, a potential link between age-dependent alteration in mucosal immune mechanisms and the gut microbial communities was shown by the negative correlation between TLRs (TLR2, TLR6, and TLR9) in the mucosa of the rumen, jejunum, and caecum and the mucosa-attached bacterial population [119]. Moreover, the host–microbe interactions play a crucial role in the regulation of GIT development, as demonstrated by bovine transcriptome analyses [122].
A positive correlation was observed between the gene copy numbers of Lactobacillus or Bifidobacterium spp. and microRNAs (miRNA) expression levels. These miRNAs act as promoters of GIT development and include miR-15/16 (immune cells development), miR-29 (maturation of dendritic cells), and miR-196 (lymphoid tissue development) [122]. Likewise, the microbial-driven transcriptional regulation of developing rumen in calves via miRNAs was suggested recently [9]. They identified three miRNA-mRNA pairs involved in the development of rumen “miR-25 and fatty acid-binding protein 7, miR-29a and platelet-derived growth factor α polypeptide, and miR-30 and integrin-linked kinase” [9].
8. Role of the microbiota in gut health and treatment strategies
The previous sections have summarised the current knowledge about the essential co-evolution of GIT in ruminants and the colonising microbiome. Disturbances result in an imbalanced symbiosis, leading to gut microbial dysbiosis which can induce several enteric disorders [123]. The pre-weaning period is critical due to the high susceptibility of neonatal calves to a vast variety of bacterial and viral infections, which cause diarrhoea (the major cause of death in neonatal calves) [124]. A decreased incidence of diarrhoea was correlated with a higher abundance of Faecalibacterium in faecal samples of one-week-old calves and in the large intestine of three-week-old calves [13], [14], [58]. F. prausnitzii promotes anti-inflammatory responses, maintains intestinal homoeostasis [125] and produces butyrate in the large intestine [13]. A high abundance of this species during the pre-weaning period may provide health benefits to the neonates by decreasing their susceptibility to enteric infections. More recently, the idea of a microbiota transplantation to stabilise the gut microbiome was applied in ruminants by transferring the rumen microbiome of adult animals orally to young calves. Although the overall microbiome structure was not affected, the incidence of calf diarrhoea decreased [126].
8.1. Early life antimicrobial treatments and emergence of resistant bacterial strains in the calf gut
The dairy industry relies on the use of antimicrobials to cure various diseases, resulting in the production of milk with residual concentrations of antimicrobials [127], [128]. In addition to the presence of antimicrobial residues in the milk, it may contain a high number of pathogens and somatic cells [129]. Thus, the milk from antimicrobial-treated cows is generally used by the dairy industry as a feed for dairy calves [59], [60]. Antimicrobials are also fed directly to the calves as medicated MR to increase their growth rate and prevent diseases [123], [130]. Nevertheless, this direct or indirect exposure of neonatal calves to antimicrobials modifies their intestinal microbial community structure, resulting in the emergence of resistant bacterial strains as well as the transfer of resistance genes to other bacteria [131], [132]. There is increasing evidence of the presence of highly resistant enteric microbes in young animals compared to adults [133], [134], [135], probably due to high faecal–oral transmissions and higher antimicrobial usage in young animals [136]. Several studies have reported the effects of antimicrobial usage on the gut microbial composition [137], [138], [139], [140], [141], [142], the development of antimicrobial-resistant bacterial strains [132], [143], [144], [145], [146], [147], [148], [149], genes involved in antimicrobial resistance [133], [148], [150], and antimicrobial-dependent changes in the functional profile of gut microbiota [151].
Feeding calves with WM containing residual antibiotics (oxytetracycline, ceftiofur, ampicillin, and penicillin) resulted in lower abundances of faecal Clostridium and Streptococcus in pre-weaned calves [139]. Similarly, when calves were fed with medicated MR containing tetracycline, ceftiofur, penicillin, and streptomycin, reduced abundance of E. coli in the ileum [140] and Prevotella in the rumen [142] was observed. However, feeding calves with MR supplemented with only ceftiofur reduced the abundance of Comamonas in the ileum [140]. Decreased abundance of beneficial bacteria (Faecalibacterium, Roseburia, Prevotella, and Eubacterium) and increased abundance of pathogenic bacteria (Shigella, Escherichia, and Enterococcus) in calf faeces were observed using the antibiotic bacitracin methylene disalicylate antibiotics [137]. Enrofloxacin treatment decreased the abundance of Bacteroides and increased the abundance of Blautia, Desulfovibrio, and Coprococcus in calf faeces [141]. As the concentration of residual antibiotics in the WM increases, a higher number of antibiotic-resistant bacterial strains emerge in the gut [144]. A higher prevalence of antimicrobial-resistant faecal E. coli phenotypes and the increased detection of β-lactamase resistance genes in these populations was observed in WM-fed calves than in bulk milk or MR-fed calves [132], [147], [148]. Feeding drug residues containing milk to the pre-weaned calves also resulted in lower abundance of genes involved in regulation and cell signalling, stress response and nitrogen metabolism [151]. In addition, the direct treatment of calves with antibiotics may also result in the emergence of antibiotic-resistant bacterial strains [149]. However, other studies have reported that the occurrence of multi-drug resistant bacterial strains is not dependent on recent antimicrobial usage but rather on other environmental variables, age, and diet [145], [146], [147]. A decreased prevalence of multi-drug resistant faecal E. coli with increasing age of calves indicated that the underdeveloped digestive system of neonatal calves serves as an excellent niche for the growth of resistant microbes due to limited competition for resources [146]. However, Thames et al. reported an age-dependent increase in tetracycline resistance genes in calf faeces [150]. These studies suggest that the direct and indirect exposure of the gut of neonatal calves to the antimicrobials modifies the composition and functional profile of the microbiome and the development of antibiotic resistance is mainly influenced by host-specific factors.
8.2. Improvement of calf gut health by feed supplements
The use of antimicrobials to support calves’ health and to prevent or treat certain diseases can be avoided by using direct-fed microbes, prebiotics, and probiotics. This has been widely practised in order to improve gut health and productivity of livestock [152], [153]. Supplementation of new-born calves with Lactobacillus and Bifidobacterium within the first seven days of life decreased diarrhoea and increased feed conversion ratio and weight gain [154]. Similarly, supplementation with F. prausnitzii in the first week of calf life decreased the calf death rate and diarrhoea [155]. Administration of Lactobacillus spp. to young calves also increased their serum IgG levels, suggesting a potential role of the host–microbe interactions in modulating calf health [156]. Apart from influencing host health, microbial manipulations also affect the gut microbial community structure. Feeding pre-weaned calves with probiotic strains decreased their intestinal colonisation with pathogenic E. coli [157]. Similarly, a decrease in faecal E. coli load was observed using direct-fed microbes [158]. Supplementation of the diet of neonatal calves with Saccharomyces cerevisiae boulardii immediately after birth increased the abundance of beneficial bacteria (F. prausnitzii and Lactobacillus) in the intestinal microbiota, as well as increasing the concentrations of endogenous secretory IgA, thus enhancing immunity and intestinal homoeostasis of calf GIT [159]. Feeding heated colostrum soon after birth benefited young calves with increased colonisation with Bifidobacterium and decreased colonisation with E. coli in the small intestine, suggesting the potential role of colostrum as a natural prebiotic associated with reduced risk of diarrhoea [41], [53]. Prebiotics supplementation immediately after birth was found to have more prominent effects than supplementation at a later stage. Higher abundances of Bifidobacterium and Lactobacillus were detected in the colon of two-week-old than in four-week-old calves fed with galactooligosaccharides [160]. Supplementation of grazing calf diet with cellooligosaccharides decreased the proportions of archaea at weaning and Fibrobacter within the first four weeks post-weaning. In contrast, an increase in Fibrobacter was detected using kraft pulp as prebiotics at four weeks post-weaning [161]. Addition of astragalus root extract in the diet of early weaned calves at a dose of 2% dry matter intake, increased the body weight, average daily gain, serum concentrations of interleukin-2 (IL-2), IgG, superoxide dismutase, and the abundance of fibrolytic bacteria [162]. Increasing the dose of astragalus root extract to 5% and 8% dry matter intake fortified these effects [162]. Supplementation of calf diet with zinc oxide (104 mg/d) effectively reduced the incidence of diarrhoea from days 1–3, increased the abundance of beneficial Faecalibacterium and Lactobacillus within the first seven days of life and improved the immunity by increasing the concentrations of serum immunoglobulins (IgM and IgG) [163]. However, when zinc methionine (457 mg/d) was supplemented, a prolonged reduction in diarrhoea was observed from days 1–14, and increased abundances of Faecalibacterium and Collinsella (day 7), and Ruminococcus (2 weeks) were detected [163]. These results suggest the essential role of zinc in the treatment of neonatal calf diarrhoea. In addition, calcium propionate supplementation increased the body weight and decreased the relative abundance of Bacteroidetes in both pre- and post-weaning groups, but increased Proteobacteria (Succinivibrionaceae) and Methanobrevibacter only the post-weaning group [164]. These studies suggest that microbial manipulations are easier to perform during early life, and these effects may persist longer when manipulations are performed in early life of animal.
9. Summary and outlook
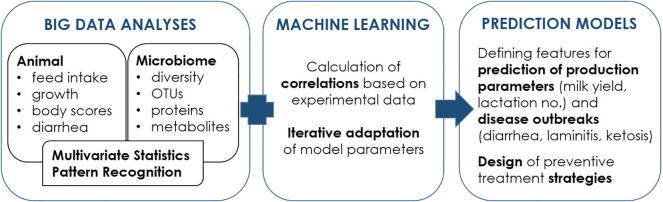
Understanding the pattern of microbial succession throughout the GIT of pre-weaned calves is essential as it influences the development and maturation of the host gut, immune system, and health. The microbial colonisation of the GIT of neonatal calves begins during the birthing process or even in utero, but the microbial community structure changes rapidly within the first few weeks of life and is strongly affected by the genetic background, rearing environment, early life antibiotic treatments, age and feeding conditions. The majority of the studies reported the early life microbial succession patterns using DNA-based methods without any information about the viability, genetic potential (metagenomics), or even gene or protein expression (metatranscriptomics and metaproteomics) of the detected microbial communities. Thus, there is still a lot to understand the underlying mechanisms of the possible interactions between the gut microbial communities and their mammalian host. In addition, the results obtained by various DNA-based studies are limited by different sample types and locations, extraction methods, gene regions being sequenced, sequencing methods, sequence depth, and the pipeline used for the analysis. In addition to the region-specific establishment of microbial communities along the GIT of calves, the microbiota associated with the epithelial mucosa was clearly different from those occupying the luminal digesta and had a potential role in host immune system development [113]. Thus, to better understand the host–microbe interactions, a thorough knowledge of microbial segregation between mucosal epithelium and luminal digesta throughout the pre-weaning period is of utmost importance. In the future, genome-wide association studies should be conducted to track the possible associations between host single nucleotide polymorphisms and the abundances of commensal bacterial taxa. Furthermore, more emphasis should be placed on the microbial dysbiosis caused by in-feed antimicrobials and the possibility of using the gut microbiome, prebiotics, and probiotics as antimicrobial substitutes. In addition to the control of neonatal calf diseases using antimicrobial alternatives, one can also predict the onset of diseases based on early life gut microbiota composition, and the predictive modelling approach was recently suggested by Ma et al. [165]. The combination of collecting big data with machine learning algorithms can support the establishment of prediction tools for output targets or disease outbreaks, and helps to design preventive treatment strategies (Fig. 3). We conclude by mentioning that future studies must focus on the ecologic as well as metabolic activity of the detected microbiome based on advanced machine learning and prediction modelling approaches.
Fig. 3.
Combination of big data repositories with machine learning algorithms to create prediction tools for sustainable animal productions strategies.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We would like to thank the Deutsche Forschungsgemeinschaft (DFG) for funding (SE2059/2-2).
References
- 1.Mackie R.I. Mutualistic fermentative digestion in the gastrointestinal tract: diversity and evolution. Integr Comp Biol. 2002;42:319–326. doi: 10.1093/icb/42.2.319. [DOI] [PubMed] [Google Scholar]
- 2.Davis C.L., Drackley J.K. Iowa State University Press; Ames (IA): 1998. The development, nutrition, and management of the young calf. [Google Scholar]
- 3.Heinrichs A. Rumen development in the dairy calf. Adv Dairy Technol. 2005;17:179–187. [Google Scholar]
- 4.Anderson KL, Nagaraja TG, Morrill JL, Avery TB, Galitzer SJ, et al. Ruminal microbial development in conventionally or early-weaned calves. J Anim Sci 1987;64:1215-26. [DOI] [PubMed]
- 5.Fonty G., Gouet P., Jouany J.-P., Senaud J. Establishment of the microflora and anaerobic fungi in the rumen of lambs. J Gen Microbiol. 1987;133:1835–1843. [Google Scholar]
- 6.Minato H., Otsuka M., Shirasaka S., Itabashi H., Mitsumori M. Colonization of microorganisms in the rumen of young calves. J Gen App Microbiol. 1992;38:447–456. [Google Scholar]
- 7.Jami E., Israel A., Kotser A., Mizrahi I. Exploring the bovine rumen bacterial community from birth to adulthood. ISME J. 2013;7:1069–1079. doi: 10.1038/ismej.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guzman C.E., Bereza-Malcolm L.T., De Groef B., Franks A.E., Suen G. Presence of selected methanogens, fibrolytic bacteria, and proteobacteria in the gastrointestinal tract of neonatal dairy calves from birth to 72 hours. PLoS One. 2015;10:e0133048. doi: 10.1371/journal.pone.0133048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malmuthuge N., Liang G., Guan L.L. Regulation of rumen development in neonatal ruminants through microbial metagenomes and host transcriptomes. Genome Biol. 2019;20:1–16. doi: 10.1186/s13059-019-1786-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fanaro S., Chierici R., Guerrini P., Vigi V. Intestinal microflora in early infancy: composition and development. Acta Paediatr. 2003;91:48–55. doi: 10.1111/j.1651-2227.2003.tb00646.x. [DOI] [PubMed] [Google Scholar]
- 11.Jost T., Lacroix C., Braegger C.P., Chassard C., Ravel J. New insights in gut microbiota establishment in healthy breast fed neonates. PLoS One. 2012;7:e44595. doi: 10.1371/journal.pone.0044595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li R.W., Connor E.E., Li C., Baldwin Vi R.L., Sparks M.E. Characterization of the rumen microbiota of pre-ruminant calves using metagenomic tools. Environ Microbiol. 2012;14:129–139. doi: 10.1111/j.1462-2920.2011.02543.x. [DOI] [PubMed] [Google Scholar]
- 13.Oikonomou G, Teixeira AGV, Foditsch C, Bicalho ML, Machado VS, et al. Fecal microbial diversity in pre-weaned dairy calves as described by pyrosequencing of metagenomic 16S rDNA. Associations of Faecalibacterium species with health and growth. PLoS ONE 2013;8:e63157. [DOI] [PMC free article] [PubMed]
- 14.Malmuthuge N., Griebel P.J., Guan L.L. Taxonomic identification of commensal bacteria associated with the mucosa and digesta throughout the gastrointestinal tracts of preweaned calves. Appl Environ Microbiol. 2014;80:2021–2028. doi: 10.1128/AEM.03864-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansen C.H.F., Nielsen D.S., Kverka M., Zakostelska Z., Klimesova K., Hudcovic T. Patterns of early gut colonization shape future immune responses of the host. PLoS One. 2012;7:e34043. doi: 10.1371/journal.pone.0034043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lei Y.M.K., Nair L., Alegre M.-L. The interplay between the intestinal microbiota and the immune system. Clin Res Hepatol Gastroenterol. 2015;39:9–19. doi: 10.1016/j.clinre.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malmuthuge N., Griebel P.J., Guan L.L. The gut microbiome and its potential role in the development and function of newborn calf gastrointestinal tract. Front Vet Sci. 2015;2:36. doi: 10.3389/fvets.2015.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sommer F., Bäckhed F. The gut microbiota-masters of host development and physiology. Nat Rev Microbiol. 2013;11:227–238. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- 19.Russell J.B., Rychlik J.L. Factors that alter rumen microbial ecology. Science. 2001;292:1119–1122. doi: 10.1126/science.1058830. [DOI] [PubMed] [Google Scholar]
- 20.Weimer P.J., Stevenson D.M., Mantovani H.C., Man S.L.C. Host specificity of the ruminal bacterial community in the dairy cow following near-total exchange of ruminal contents. J Dairy Sci. 2010;93:5902–5912. doi: 10.3168/jds.2010-3500. [DOI] [PubMed] [Google Scholar]
- 21.Henderson G., Cox F., Ganesh S., Jonker A., Young W., Janssen P.H. Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Sci Rep. 2015;5:14567. doi: 10.1038/srep14567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malmuthuge N., Li M., Chen Y., Fries P., Griebel P.J., Baurhoo B. Distinct commensal bacteria associated with ingesta and mucosal epithelium in the gastrointestinal tracts of calves and chickens. FEMS Microbiol Ecol. 2012;79:337–347. doi: 10.1111/j.1574-6941.2011.01220.x. [DOI] [PubMed] [Google Scholar]
- 23.Malmuthuge N., Li M., Goonewardene L.A., Oba M., Guan L.L. Effect of calf starter feeding on gut microbial diversity and expression of genes involved in host immune responses and tight junctions in dairy calves during weaning transition. J Dairy Sci. 2013;96:3189–3200. doi: 10.3168/jds.2012-6200. [DOI] [PubMed] [Google Scholar]
- 24.Song Y., Malmuthuge N., Steele M.A., Guan L.L. Shift of hindgut microbiota and microbial short chain fatty acids profiles in dairy calves from birth to pre-weaning. FEMS Microbiol Ecol. 2018;94:179. doi: 10.1093/femsec/fix179. [DOI] [PubMed] [Google Scholar]
- 25.Yeoman C.J., Ishaq S.L., Bichi E., Olivo S.K., Lowe J., Aldridge B.M. Biogeographical differences in the influence of maternal microbial sources on the early successional development of the bovine neonatal gastrointestinal tract. Sci Rep. 2018;8:1–14. doi: 10.1038/s41598-018-21440-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dominguez-Bello M.G., Costello E.K., Contreras M., Magris M., Hidalgo G., Fierer N. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107:11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taschuk R., Griebel P.J. Commensal microbiome effects on mucosal immune system development in the ruminant gastrointestinal tract. Anim Health Res Rev. 2012;13:129–141. doi: 10.1017/S1466252312000096. [DOI] [PubMed] [Google Scholar]
- 28.Mayer M., Abenthum A., Matthes J.M., Kleeberger D., Ege M.J., Hölzel C. Development and genetic influence of the rectal bacterial flora of newborn calves. Vet Microbiol. 2012;161:179–185. doi: 10.1016/j.vetmic.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 29.Alipour M.J., Jalanka J., Pessa-Morikawa T., Kokkonen T., Satokari R., Hynönen U. The composition of the perinatal intestinal microbiota in cattle. Sci Rep. 2018;8:1–14. doi: 10.1038/s41598-018-28733-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein-Jöbstl D., Quijada N.M., Dzieciol M., Feldbacher B., Wagner M., Drillich M. Microbiota of newborn calves and their mothers reveals possible transfer routes for newborn calves’ gastrointestinal microbiota. PLoS ONE. 2019;14:e0220554. doi: 10.1371/journal.pone.0220554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Indugu N., Vecchiarelli B., Baker L.D., Ferguson J.D., Vanamala J.K.P., Pitta D.W. Comparison of rumen bacterial communities in dairy herds of different production. BMC Microbiol. 2017;17:190. doi: 10.1186/s12866-017-1098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weese J.S., Jelinski M. Assessment of the fecal microbiota in beef calves. J Vet Intern Med. 2017;31:176–185. doi: 10.1111/jvim.14611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Hara E, Kenny DA, McGovern E, Byrne CJ, McCabe MS, et al. Investigating temporal microbial dynamics in the rumen of beef calves raised on two farms during early life. FEMS Microbiol Ecol 2020;96:203. [DOI] [PubMed]
- 34.Baldwin R.L., McLeod K.R., Klotz J.L., Heitmann R.N. Rumen development, intestinal growth and hepatic metabolism in the pre- and postweaning ruminant. J Dairy Sci. 2004;87:E55–E65. [Google Scholar]
- 35.Guilloteau P., Zabielski R., Blum J. Gastrointestinal tract and digestion in the young ruminant: ontogenesis, adaptations, consequences and manipulations. J Physiol Pharmacol. 2009:37–46. [PubMed] [Google Scholar]
- 36.Khan M.A., Bach A., Weary D.M., von Keyserlingk M.A.G. Invited review: transitioning from milk to solid feed in dairy heifers. J Dairy Sci. 2016;99:885–902. doi: 10.3168/jds.2015-9975. [DOI] [PubMed] [Google Scholar]
- 37.Li R.W., Sparks M., Connor E.E. Dynamics of the rumen microbiota. In: Li R., editor. Metagenomics and its applications in agriculture. Nova Science Publishers; New York, NY: 2011. pp. 135–164. [Google Scholar]
- 38.Yáñez-Ruiz D.R., Abecia L., Newbold C.J., Wrighton K., Ishaq S.L. Manipulating rumen microbiome and fermentation through interventions during early life: a review. Front Microbiol. 2015;6:1133. doi: 10.3389/fmicb.2015.01133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malmuthuge N., Guan L.L. Understanding the gut microbiome of dairy calves: opportunities to improve early-life gut health. J Dairy Sci. 2017;100:5996–6005. doi: 10.3168/jds.2016-12239. [DOI] [PubMed] [Google Scholar]
- 40.Khan M.A., Weary D.M., von Keyserlingk M.A.G. Hay intake improves performance and rumen development of calves fed higher quantities of milk. J Dairy Sci. 2011;94:3547–3553. doi: 10.3168/jds.2010-3871. [DOI] [PubMed] [Google Scholar]
- 41.Malmuthuge N., Chen Y., Liang G., Goonewardene L.A., Guan L.L. Heat-treated colostrum feeding promotes beneficial bacteria colonization in the small intestine of neonatal calves. J Dairy Sci. 2015;98:8044–8053. doi: 10.3168/jds.2015-9607. [DOI] [PubMed] [Google Scholar]
- 42.Song Y., Malmuthuge N., Li F., Guan L.L. Colostrum feeding shapes the hindgut microbiota of dairy calves during the first 12 h of life. FEMS Microbiol Ecol. 2018;95:203. doi: 10.1093/femsec/fiy203. [DOI] [PubMed] [Google Scholar]
- 43.Edrington T.S., Dowd S.E., Farrow R.F., Hagevoort G.R., Callaway T.R., Anderson R.C. Development of colonic microflora as assessed by pyrosequencing in dairy calves fed waste milk. J Dairy Sci. 2012;95:4519–4525. doi: 10.3168/jds.2011-5119. [DOI] [PubMed] [Google Scholar]
- 44.Maynou G., Chester-Jones H., Bach A., Terré M. Feeding pasteurized waste milk to preweaned dairy calves changes fecal and upper respiratory tract microbiota. Front Vet Sci. 2019;6:159. doi: 10.3389/fvets.2019.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang R., Zhang W.-B., Bi Y.-L., Tu Y., Beckers Y., Du H.-C. Early feeding regime of waste milk, milk, and milk replacer for calves has different effects on rumen fermentation and the bacterial community. Animals. 2019;9:443. doi: 10.3390/ani9070443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guzman C.E., Bereza-Malcolm L.T., De Groef B., Franks A.E. Uptake of milk with and without solid feed during the monogastric phase: effect on fibrolytic and methanogenic microorganisms in the gastrointestinal tract of calves. Anim Sci J. 2016;87:378–388. doi: 10.1111/asj.12429. [DOI] [PubMed] [Google Scholar]
- 47.Dias J., Marcondes M.I., Noronha M.F., Resende R.T., Machado F.S., Mantovani H.C. Effect of pre-weaning diet on the ruminal archaeal, bacterial, and fungal communities of dairy calves. Front Microbiol. 2017;8:1553. doi: 10.3389/fmicb.2017.01553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Castells L., Bach A., Aris A., Terré M. Effects of forage provision to young calves on rumen fermentation and development of the gastrointestinal tract. J Dairy Sci. 2013;96:5226–5236. doi: 10.3168/jds.2012-6419. [DOI] [PubMed] [Google Scholar]
- 49.Kim Y.-H.-H., Nagata R., Ohtani N., Ichijo T., Ikuta K. Effects of dietary forage and calf starter diet on ruminal pH and bacteria in holstein calves during weaning transition. Front Microbiol. 2016;7:1575. doi: 10.3389/fmicb.2016.01575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin X., Wang J., Hou Q., Wang Y., Hu Z., Shi K. Effect of hay supplementation timing on rumen microbiota in suckling calves. MicrobiologyOpen. 2018;7:e00430. doi: 10.1002/mbo3.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dill-McFarland K.A., Weimer P.J., Breaker J.D., Suen G., Dudley E.G. Diet influences early microbiota development in dairy calves without long-term impacts on milk production. Appl Environ Microbiol. 2019;85 doi: 10.1128/AEM.02141-18. e02141-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Godden S. Colostrum management for dairy calves. Vet Clin North Am Food Anim Pract. 2008;24:19–39. doi: 10.1016/j.cvfa.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Godden S.M., Smolenski D.J., Donahue M., Oakes J.M., Bey R., Wells S. Heat-treated colostrum and reduced morbidity in preweaned dairy calves: results of a randomized trial and examination of mechanisms of effectiveness. J Dairy Sci. 2012;95:4029–4040. doi: 10.3168/jds.2011-5275. [DOI] [PubMed] [Google Scholar]
- 54.Priestley D., Bittar J.H., Ibarbia L., Risco C.A., Galvão K.N. Effect of feeding maternal colostrum or plasma-derived or colostrum-derived colostrum replacer on passive transfer of immunity, health, and performance of preweaning heifer calves. J Dairy Sci. 2013;96:3247–3256. doi: 10.3168/jds.2012-6339. [DOI] [PubMed] [Google Scholar]
- 55.Morrill K.M., Conrad E., Lago A., Campbell J., Quigley J., Tyler H. Nationwide evaluation of quality and composition of colostrum on dairy farms in the United States. J Dairy Sci. 2012;95:3997–4005. doi: 10.3168/jds.2011-5174. [DOI] [PubMed] [Google Scholar]
- 56.Fischer A.J., Song Y., He Z., Haines D.M., Guan L.L., Steele M.A. Effect of delaying colostrum feeding on passive transfer and intestinal bacterial colonization in neonatal male Holstein calves. J Dairy Sci. 2018;101:3099–3109. doi: 10.3168/jds.2017-13397. [DOI] [PubMed] [Google Scholar]
- 57.Rey M., Enjalbert F., Combes S., Cauquil L., Bouchez O., Monteils V. Establishment of ruminal bacterial community in dairy calves from birth to weaning is sequential. J Appl Microbiol. 2014;116:245–257. doi: 10.1111/jam.12405. [DOI] [PubMed] [Google Scholar]
- 58.Uyeno Y, Sekiguchi Y, Kamagata Y. rRNA-based analysis to monitor succession of faecal bacterial communities in Holstein calves. Lett Appl Microbiol 2010;51:570-7. [DOI] [PubMed]
- 59.Brunton L.A., Duncan D., Coldham N.G., Snow L.C., Jones J.R. A survey of antimicrobial usage on dairy farms and waste milk feeding practices in England and Wales. Vet Rec. 2012;171:296. doi: 10.1136/vr.100924. [DOI] [PubMed] [Google Scholar]
- 60.Duse A., Waller K.P., Emanuelson U., Unnerstad H.E., Persson Y., Bengtsson B. Farming practices in Sweden related to feeding milk and colostrum from cows treated with antimicrobials to dairy calves. Acta Vet Scand. 2013;55:49. doi: 10.1186/1751-0147-55-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Godden S.M., Fetrow J.P., Feirtag J.M., Green L.R., Wells S.J. Economic analysis of feeding pasteurized nonsaleable milk versus conventional milk replacer to dairy calves. J Am Vet Med Assoc. 2005;226:1547–1554. doi: 10.2460/javma.2005.226.1547. [DOI] [PubMed] [Google Scholar]
- 62.Badman J., Daly K., Kelly J., Moran A.W., Cameron J., Watson I. The effect of milk replacer composition on the intestinal microbiota of pre-ruminant dairy calves. Front Vet Sci. 2019;6:371. doi: 10.3389/fvets.2019.00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cui Z., Wu S., Liu S., Sun L.u., Feng Y., Cao Y. From maternal grazing to barn feeding during pre-weaning period: altered gastrointestinal microbiota contributes to change the development and function of the rumen and intestine of Yak calves. Front Microbiol. 2020;11:485. doi: 10.3389/fmicb.2020.00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Klevenhusen F., Petri R.M., Kleefisch M.T., Khiaosa-ard R., Metzler-Zebeli B.U. Changes in fibre-adherent and fluid-associated microbial communities and fermentation profiles in the rumen of cattle fed diets differing in hay quality and concentrate amount. FEMS Microbiol Ecol. 2017;93 doi: 10.1093/femsec/fix100. [DOI] [PubMed] [Google Scholar]
- 65.Wickramasinghe H.K.J.P., Anast J.M., Schmitz-Esser S., Serão N.V.L., Appuhamy J.A.D.R.N. Beginning to offer drinking water at birth increases the species richness and the abundance of Faecalibacterium and Bifidobacterium in the gut of preweaned dairy calves. J Dairy Sci. 2020;103:4262–4274. doi: 10.3168/jds.2019-17258. [DOI] [PubMed] [Google Scholar]
- 66.Wickramasinghe H.K.J.P., Kramer A.J., Appuhamy J.A.D.R.N. Drinking water intake of newborn dairy calves and its effects on feed intake, growth performance, health status, and nutrient digestibility. J Dairy Sci. 2019;102:377–387. doi: 10.3168/jds.2018-15579. [DOI] [PubMed] [Google Scholar]
- 67.Klein-Jöbstl D., Schornsteiner E., Mann E., Wagner M., Drillich M. Pyrosequencing reveals diverse fecal microbiota in Simmental calves during early development. Front Microbiol. 2014;5:622. doi: 10.3389/fmicb.2014.00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dill-Mcfarland K.A., Breaker J.D., Suen G. Microbial succession in the gastrointestinal tract of dairy cows from 2 weeks to first lactation. Sci Rep. 2017;7:40864. doi: 10.1038/srep40864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dias J., Marcondes M.I., Motta de Souza S., Cardoso da Mata e Silva B., Fontes Noronha M., Tassinari Resende R. Bacterial community dynamics across the gastrointestinal tracts of dairy calves during preweaning development. Appl Environ Microbiol. 2018;84 doi: 10.1128/AEM.02675-17. e02675-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lourenco JM, Kieran TJ, Seidel DS, Glenn TC, Silveira MFd, et al. Comparison of the ruminal and fecal microbiotas in beef calves supplemented or not with concentrate. PLOS ONE 2020;15:e0231533. [DOI] [PMC free article] [PubMed]
- 71.Avgustin G., Wright F., Flint H.J. Genetic diversity and phylogenetic relationships among strains of Prevotella (Bacteroides) ruminicola from the rumen. Int J Syst Bacteriol. 1994;44:246–255. doi: 10.1099/00207713-44-2-246. [DOI] [PubMed] [Google Scholar]
- 72.Matsui H., Ogata K., Tajima K., Nakamura M., Nagamine T., Aminov R.I. Phenotypic characterization of polysaccharidases produced by four Prevotella type strains. Curr Microbiol. 2000;41:45–49. doi: 10.1007/s002840010089. [DOI] [PubMed] [Google Scholar]
- 73.Purushe J., Fouts D.E., Morrison M., White B.A., Mackie R.I., Coutinho P.M. Comparative genome analysis of Prevotella ruminicola and Prevotella bryantii: insights into their environmental niche. Microb Ecol. 2010;60:721–729. doi: 10.1007/s00248-010-9692-8. [DOI] [PubMed] [Google Scholar]
- 74.Rey M., Enjalbert F., Monteils V. Establishment of ruminal enzyme activities and fermentation capacity in dairy calves from birth through weaning. J Dairy Sci. 2012;95:1500–1512. doi: 10.3168/jds.2011-4902. [DOI] [PubMed] [Google Scholar]
- 75.Cui Z., Wu S., Li J., Yang Q.-E., Chai S., Wang L. Effect of alfalfa hay and starter feeding intervention on gastrointestinal microbial community, growth and immune performance of yak calves. Front Microbiol. 2020;11:994. doi: 10.3389/fmicb.2020.00994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Beharka A.A., Nagaraja T.G., Morrill J.L., Kennedy G.A., Klemm R.D. Effects of form of the diet on anatomical, microbial, and fermentative development of the rumen of neonatal calves. J Dairy Sci. 1998;81:1946–1955. doi: 10.3168/jds.S0022-0302(98)75768-6. [DOI] [PubMed] [Google Scholar]
- 77.Pazoki A., Ghorbani G.R., Kargar S., Sadeghi-Sefidmazgi A., Drackley J.K., Ghaffari M.H. Growth performance, nutrient digestibility, ruminal fermentation, and rumen development of calves during transition from liquid to solid feed: effects of physical form of starter feed and forage provision. Anim Feed Sci Technol. 2017;234:173–185. [Google Scholar]
- 78.Sweeney B.C., Rushen J., Weary D.M., de Passillé A.M. Duration of weaning, starter intake, and weight gain of dairy calves fed large amounts of milk. J Dairy Sci. 2010;93:148–152. doi: 10.3168/jds.2009-2427. [DOI] [PubMed] [Google Scholar]
- 79.Meale S.J., Li S., Azevedo P., Derakhshani H., Plaizier J.C., Khafipour E. Development of ruminal and fecal microbiomes are affected by weaning but not weaning strategy in dairy calves. Front Microbiol. 2016;7:582. doi: 10.3389/fmicb.2016.00582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mao H., Xia Y., Tu Y., Wang C., Diao Q. Effects of various weaning times on growth performance, rumen fermentation and microbial population of yellow cattle calves. Asian-Australas J Anim Sci. 2017;30:1557–1562. doi: 10.5713/ajas.16.0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Myers S.E., Faulkner D.B., Ireland F.A., Berger L.L., Parrett D.F. Production systems comparing early weaning to normal weaning with or without creep feeding for beef steers. J Anim Sci. 1999;77:300–310. doi: 10.2527/1999.772300x. [DOI] [PubMed] [Google Scholar]
- 82.Myers S.E., Faulkner D.B., Ireland F.A., Parrett D.F. Comparison of three weaning ages on cow-calf performance and steer carcass traits. J Anim Sci. 1999;77:323–329. doi: 10.2527/1999.772323x. [DOI] [PubMed] [Google Scholar]
- 83.Roth B.A., Keil N.M., Gygax L., Hillmann E. Influence of weaning method on health status and rumen development in dairy calves. J Dairy Sci. 2009;92:645–656. doi: 10.3168/jds.2008-1153. [DOI] [PubMed] [Google Scholar]
- 84.Meale S.J., Li S.C., Azevedo P., Derakhshani H., DeVries T.J., Plaizier J.C. Weaning age influences the severity of gastrointestinal microbiome shifts in dairy calves. Sci Rep. 2017;7:198. doi: 10.1038/s41598-017-00223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Eckert E., Brown H.E., Leslie K.E., DeVries T.J., Steele M.A. Weaning age affects growth, feed intake, gastrointestinal development, and behavior in Holstein calves fed an elevated plane of nutrition during the preweaning stage. J Dairy Sci. 2015;98:6315–6326. doi: 10.3168/jds.2014-9062. [DOI] [PubMed] [Google Scholar]
- 86.Jami E., Mizrahi I., López-García P. Composition and similarity of bovine rumen microbiota across individual animals. PLoS One. 2012;7:e33306. doi: 10.1371/journal.pone.0033306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Steele M.A., Leal L.E., Soberon F., Doelman J.H., Carson M. Gradual weaning affects pre- and post-weaning feed intake, growth, and gastrointestinal development in Holstein calves fed an elevated plane of nutrition during the pre-weaning period. J Anim Sci. 2015;98:242. [Google Scholar]
- 88.Wang W., Li C., Li F., Wang X., Zhang X., Liu T. Effects of early feeding on the host rumen transcriptome and bacterial diversity in lambs. Sci Rep. 2016;6:32479. doi: 10.1038/srep32479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Flint H.J., Bayer E.A., Rincon M.T., Lamed R., White B.A. Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis. Nat Rev Microbiol. 2008;6:121–131. doi: 10.1038/nrmicro1817. [DOI] [PubMed] [Google Scholar]
- 90.Mao S., Zhang M., Liu J., Zhu W. Characterising the bacterial microbiota across the gastrointestinal tracts of dairy cattle: membership and potential function. Sci Rep. 2015;5:16116. doi: 10.1038/srep16116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Abreu M.T. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. 2010;10:131–144. doi: 10.1038/nri2707. [DOI] [PubMed] [Google Scholar]
- 92.Laarman A.H., Ruiz-Sanchez A.L., Sugino T., Guan L.L., Oba M. Effects of feeding a calf starter on molecular adaptations in the ruminal epithelium and liver of Holstein dairy calves. J Dairy Sci. 2012;95:2585–2594. doi: 10.3168/jds.2011-4788. [DOI] [PubMed] [Google Scholar]
- 93.Glover LE, Lee JS, Colgan SP. Oxygen metabolism and barrier regulation in the intestinal mucosa. J Clin Invest 2016;126:3680-8. [DOI] [PMC free article] [PubMed]
- 94.Morita H., Shiratori C., Murakami M., Takami H., Toh H., Kato Y. Sharpea azabuensis gen. nov., sp. nov., a grampositive, strictly anaerobic bacterium isolated from the faeces of thoroughbred horses. Int J Syst Evol Microbiol. 2008;58:2682–2686. doi: 10.1099/ijs.0.65543-0. [DOI] [PubMed] [Google Scholar]
- 95.Fukuda S., Toh H., Hase K., Oshima K., Nakanishi Y., Yoshimura K. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469:543–547. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- 96.Park J.-h., Kotani T., Konno T., Setiawan J., Kitamura Y., Imada S. Promotion of intestinal epithelial cell turnover by commensal bacteria: role of short-chain fatty acids. PLoS One. 2016;11:e0156334. doi: 10.1371/journal.pone.0156334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Roehe R., Dewhurst R.J., Duthie C.-A., Rooke J.A., McKain N., Ross D.W. Bovine host genetic variation influences rumen microbial methane production with best selection criterion for low methane emitting and efficiently feed converting hosts based on metagenomic gene abundance. PLoS Genet. 2016;12:e1005846. doi: 10.1371/journal.pgen.1005846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Difford G.F., Plichta D.R., Løvendahl P., Lassen J., Noel S.J., Højberg O. Host genetics and the rumen microbiome jointly associate with methane emissions in dairy cows. PLoS Genet. 2018;14:e1007580. doi: 10.1371/journal.pgen.1007580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gonzalez-Recio O., Zubiria I., García-Rodríguez A., Hurtado A., Atxaerandio R. Short communication: signs of host genetic regulation in the microbiome composition in 2 dairy breeds: Holstein and Brown Swiss. J Dairy Sci. 2018;101:2285–2292. doi: 10.3168/jds.2017-13179. [DOI] [PubMed] [Google Scholar]
- 100.Clemmons B.A., Voy B.H., Myer P.R. Altering the gut microbiome of cattle: considerations of host-microbiome interactions for persistent microbiome manipulation. Microb Ecol. 2019;77:523–536. doi: 10.1007/s00248-018-1234-9. [DOI] [PubMed] [Google Scholar]
- 101.Li F., Li C., Chen Y., Liu J., Zhang C., Irving B. Host genetics influence the rumen microbiota and heritable rumen microbial features associate with feed efficiency in cattle. Microbiome. 2019;7:92. doi: 10.1186/s40168-019-0699-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fan P., Bian B., Teng L., Nelson C.D., Driver J., Elzo M.A. Host genetic effects upon the early gut microbiota in a bovine model with graduated spectrum of genetic variation. ISME J. 2020;14:302–317. doi: 10.1038/s41396-019-0529-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chase C., Kaushik R.S. Mucosal immune system of cattle: all immune responses begin here. Vet Clin North Am Food Anim Pract. 2019;35:431–451. doi: 10.1016/j.cvfa.2019.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hooper L.V., Littman D.R., Macpherson A.J. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McGhee J.R., Fujihashi K. Inside the mucosal immune system. PLoS Biol. 2012;10:e1001397. doi: 10.1371/journal.pbio.1001397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kuhn K.A., Stappenbeck T.S. Peripheral education of the immune system by the colonic microbiota. Semin Immunol. 2013;25:364–369. doi: 10.1016/j.smim.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rakoff-Nahoum S., Paglino J., Eslami-Varzaneh F., Edberg S., Medzhitov R. Recognition of commensal microflora by Toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 108.Ulluwishewa D, Anderson RC, McNabb WC, Moughan PJ, Wells JM, et al. Regulation of tight junction permeability by intestinal bacteria and dietary components. J Nutr 2011;141:769-76. [DOI] [PubMed]
- 109.Yasuda M., Fujino M., Nasu T., Murakami T. Histological studies on the ontogeny of bovine gut-associated lymphoid tissue: appearance of T cells and development of IgG+ and IgA + cells in lymphoid follicles. Dev Comp Immunol. 2004;28:357–369. doi: 10.1016/j.dci.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 110.Gerdts V., Babiuk L.A., van Drunen Littel-van den Hurk S., Griebel P.J. Fetal immunization by a DNA vaccine delivered into the oral cavity. Nat Med. 2000;6:929–932. doi: 10.1038/78699. [DOI] [PubMed] [Google Scholar]
- 111.Reynolds J.D., Morris B. The effect of antigen on the development of Peyer's patches in sheep. Eur J Immunol. 1984;14:1–6. doi: 10.1002/eji.1830140102. [DOI] [PubMed] [Google Scholar]
- 112.Tourneur E., Chassin C. Neonatal immune adaptation of the gut and its role during infections. Clin Dev Immunol. 2013;2013:1–17. doi: 10.1155/2013/270301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hromádková J., Suzuki Y., Pletts S., Pyo J., Ma T., Chen Y. Effect of colostrum feeding strategies on the expression of neuroendocrine genes and active gut mucosa-attached bacterial populations in neonatal calves. J Dairy Sci. 2020;103:8629–8642. doi: 10.3168/jds.2019-17710. [DOI] [PubMed] [Google Scholar]
- 114.Connor E.E., Evock-Clover C.M., Walker M.P., Elsasser T.H., Kahl S. Comparative gut physiology symposium: comparative physiology of glucagon-like peptide-2: implications and applications for production and health of ruminants. J Anim Sci. 2015;93:492–501. doi: 10.2527/jas.2014-8577. [DOI] [PubMed] [Google Scholar]
- 115.Weikard R., Hadlich F., Hammon H.M., Frieten D., Gerbert C., Koch C. Long noncoding RNAs are associated with metabolic and cellular processes in the jejunum mucosa of pre-weaning calves in response to different diets. Oncotarget. 2018;9:21052–21069. doi: 10.18632/oncotarget.24898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Marchiando A.M., Graham W.V., Turner J.R. Epithelial barriers in homeostasis and disease. Annu Rev Pathol. 2010;5:119–144. doi: 10.1146/annurev.pathol.4.110807.092135. [DOI] [PubMed] [Google Scholar]
- 117.Hammon H.M., Frieten D., Gerbert C., Koch C., Dusel G., Weikard R. Different milk diets have substantial effects on the jejunal mucosal immune system of pre-weaning calves, as demonstrated by whole transcriptome sequencing. Sci Rep. 2018;8:1693. doi: 10.1038/s41598-018-19954-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang Y, Devkota S, Musch MW, Jabri B, Nagler C, et al. Regional mucosa-associated microbiota determine physiological expression of TLR2 and TLR4 in murine colon. PLoS ONE 2010;5:e13607. [DOI] [PMC free article] [PubMed]
- 119.Malmuthuge N., Li M., Fries P., Griebel P.J., Guan L.L. Regional and age dependent changes in gene expression of Toll-like receptors and key antimicrobial defence molecules throughout the gastrointestinal tract of dairy calves. Vet Immunol Immunopathol. 2012;146:18–26. doi: 10.1016/j.vetimm.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 120.Fries P.N., Popowych Y.I., Guan L.L., Griebel P.J. Age-related changes in the distribution and frequency of myeloid and T cell populations in the small intestine of calves. Cell Immunol. 2011;271:428–437. doi: 10.1016/j.cellimm.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 121.Teran R., Mitre E., Vaca M., Erazo S., Oviedo G., Hübner M.P. Immune system development during early childhood in tropical Latin America: evidence for the age-dependent down regulation of the innate immune response. Clin Immunol. 2011;138:299–310. doi: 10.1016/j.clim.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liang G, Malmuthuge N, McFadden TB, Bao H, Griebel PJ, et al. Potential regulatory role of microRNAs in the development of bovine gastrointestinal tract during early life. PLoS ONE 2014;9:e92592. [DOI] [PMC free article] [PubMed]
- 123.Constable P. Antimicrobial use in the treatment of calf diarrhea. J Vet Intern Med 2004;18:8-17. [DOI] [PMC free article] [PubMed]
- 124.Uetake K. Newborn calf welfare: a review focusing on mortality rates. Anim Sci J 2013;84:101-5. [DOI] [PMC free article] [PubMed]
- 125.Sokol H., Seksik P., Furet J.P., Firmesse O., Nion-Larmurier I., Beaugerie L. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm Bowel Dis. 2009;15:1183–1189. doi: 10.1002/ibd.20903. [DOI] [PubMed] [Google Scholar]
- 126.Bu D, Zhang X, Ma L, Park T, Wang L, et al. Repeated inoculation of young calves with rumen microbiota does not significantly modulate the rumen prokaryotic microbiota consistently but decreases diarrhea. Front Microbiol 2020;11:1403. [DOI] [PMC free article] [PubMed]
- 127.De Briyne N, Atkinson J, Borriello SP, Pokludová L. Antibiotics used most commonly to treat animals in Europe. Vet Rec 2014;175:325. [DOI] [PMC free article] [PubMed]
- 128.Pereira R.V., Siler J.D., Bicalho R.C., Warnick L.D. Multiresidue screening of milk withheld for sale at dairy farms in central New York State. J Dairy Sci. 2014;97:1513–1519. doi: 10.3168/jds.2013-7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ricci A., Allende A., Bolton D., Chemaly M., Davies R., Fernández Escámez P.S. Risk for the development of antimicrobial resistance (AMR) due to feeding of calves with milk containing residues of antibiotics. EFSA J. 2017;15 doi: 10.2903/j.efsa.2017.4665. e04665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Berge A.C.B., Moore D.A., Besser T.E., Sischo W.M. Targeting therapy to minimize antimicrobial use in preweaned calves: effects on health, growth, and treatment costs. J Dairy Sci. 2009;92:4707–4714. doi: 10.3168/jds.2009-2199. [DOI] [PubMed] [Google Scholar]
- 131.Thomas C.M., Nielsen K.M. Mechanisms of, and barriers to, horizontal gene transfer between bacteria. Nat Rev Microbiol. 2005;3:711–721. doi: 10.1038/nrmicro1234. [DOI] [PubMed] [Google Scholar]
- 132.Aust V, Knappstein K, Kunz HJ, Kaspar H, Wallmann J, et al. Feeding untreated and pasteurized waste milk and bulk milk to calves: effects on calf performance, health status and antibiotic resistance of faecal bacteria. J Anim Physiol Anim Nutr (Berl) 2013;97:1091-103. [DOI] [PubMed]
- 133.Khachatryan A.R., Hancock D.D., Besser T.E., Call D.R. Role of calf-adapted Escherichia coli in maintenance of antimicrobial drug resistance in dairy calves. Appl Environ Microbiol. 2004;70:752–757. doi: 10.1128/AEM.70.2.752-757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Donaldson S.C., Straley B.A., Hegde N.V., Sawant A.A., DebRoy C., Jayarao B.M. Molecular epidemiology of ceftiofur-resistant Escherichia coli isolates from dairy calves. Appl Environ Microbiol. 2006;72:3940–3948. doi: 10.1128/AEM.02770-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Edrington T.S., Callaway T.R., Anderson R.C., Nisbet D.J. Prevalence of multidrug-resistant Salmonella on commercial dairies utilizing a single heifer raising facility. J Food Prot. 2008;71:27–34. doi: 10.4315/0362-028x-71.1.27. [DOI] [PubMed] [Google Scholar]
- 136.Hinton M, Linton AM, Hedges AJ. The ecology of Escherichia coli in calves reared as dairy-cow replacements. J Appl Bacteriol 1985;58:131-8. [DOI] [PubMed]
- 137.Xie G., Duff G.C., Hall L.W., Allen J.D., Burrows C.D. Alteration of digestive tract microbiome in neonatal Holstein bull calves by bacitracin methylene disalicylate treatment and scours. J Anim Sci. 2013;91:4984–4990. doi: 10.2527/jas.2013-6304. [DOI] [PubMed] [Google Scholar]
- 138.Oultram J, Phipps E, Teixeira AGV, Foditsch C, Bicalho ML, et al. Effects of antibiotics (oxytetracycline, florfenicol or tulathromycin) on neonatal calves' faecal microbial diversity. Vet Rec 2015;117:598. [DOI] [PubMed]
- 139.Pereira RVV, Lima S, Siler JD, Foditsch C, Warnick LD, et al. Ingestion of milk containing very low concentration of antimicrobials: longitudinal effect on fecal microbiota composition in preweaned calves. PLOS ONE 2016;11:e0147525. [DOI] [PMC free article] [PubMed]
- 140.Yousif M., Li J.-H., Li Z.-Q., Maswayi Alugongo G., Ji S.-K., Li Y.-X. Low concentration of antibiotics modulates gut microbiota at different levels in pre-weaning dairy calves. Microorganisms. 2018;6:118. doi: 10.3390/microorganisms6040118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Foditsch C, Pereira RVV, Siler JD, Altier C, Warnick LD. Effects of treatment with enrofloxacin or tulathromycin on fecal microbiota composition and genetic function of dairy calves. PLOS ONE 2019;14:e0219635. [DOI] [PMC free article] [PubMed]
- 142.Li J.H., Yousif M.H., Li Z.Q., Wu Z.H., Li S.L., Yang H.J. Effects of antibiotic residues in milk on growth, ruminal fermentation, and microbial community of preweaning dairy calves. J Dairy Sci. 2019;102:2298–2307. doi: 10.3168/jds.2018-15506. [DOI] [PubMed] [Google Scholar]
- 143.Wray C., Furniss S., Benham C.L. Feeding antibiotic-contaminated waste milk to calves-effects on physical performance and antibiotic sensitivity of gut flora. Br Vet J. 1990;146:80–87. doi: 10.1016/0007-1935(90)90080-M. [DOI] [PubMed] [Google Scholar]
- 144.Langford F.M., Weary D.M., Fisher L. Antibiotic resistance in gut bacteria from dairy calves: a dose response to the level of antibiotics fed in milk. J Dairy Sci. 2003;86:3963–3966. doi: 10.3168/jds.S0022-0302(03)74006-5. [DOI] [PubMed] [Google Scholar]
- 145.Berge A.C.B., Atwill E.R., Sischo W.M. Animal and farm influences on the dynamics of antibiotic resistance in faecal Escherichia coli in young dairy calves. Prev Vet Med. 2005;69:25–38. doi: 10.1016/j.prevetmed.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 146.Edrington T.S., Farrow R.L., Carter B.H., Islas A., Hagevoort G.R. Age and diet effects on fecal populations and antibiotic resistance of a multi-drug resistant Escherichia coli in dairy calves. Agric Food Anal Bacteriol. 2012;2:162. [Google Scholar]
- 147.Maynou G., Bach A., Terré M. Feeding of waste milk to Holstein calves affects antimicrobial resistance of Escherichia coli and Pasteurella multocida isolated from fecal and nasal swabs. J Dairy Sci. 2017;100:2682–2694. doi: 10.3168/jds.2016-11891. [DOI] [PubMed] [Google Scholar]
- 148.Maynou G., Migura-Garcia L., Chester-Jones H., Ziegler D., Bach A., Terré M. Effects of feeding pasteurized waste milk to dairy calves on phenotypes and genotypes of antimicrobial resistance in fecal Escherichia coli isolates before and after weaning. J Dairy Sci. 2017;100:7967–7979. doi: 10.3168/jds.2017-13040. [DOI] [PubMed] [Google Scholar]
- 149.Pereira R.V., Altier C., Siler J.D., Mann S., Jordan D. Longitudinal effects of enrofloxacin or tulathromycin use in preweaned calves at high risk of bovine respiratory disease on the shedding of antimicrobial-resistant fecal Escherichia coli. J Dairy Sci. 2020;S0022–0302:30636–30646. doi: 10.3168/jds.2019-17989. [DOI] [PubMed] [Google Scholar]
- 150.Thames C.H., Pruden A., James R.E., Ray P.P., Knowlton K.F. Excretion of antibiotic resistance genes by dairy calves fed milk replacers with varying doses of antibiotics. Front Microbiol. 2012;3:139. doi: 10.3389/fmicb.2012.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pereira R.V.V., Carroll L.M., Lima S., Foditsch C., Siler J.D., Bicalho R.C. Impacts of feeding preweaned calves milk containing drug residues on the functional profile of the fecal microbiota. Sci Rep. 2018;8:554. doi: 10.1038/s41598-017-19021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Uyeno Y., Shigemori S., Shimosato T. Effect of probiotics/prebiotics on cattle health and productivity. Microbes Environ. 2015;30:126–132. doi: 10.1264/jsme2.ME14176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Weimer P.J. Redundancy, resilience, and host specificity of the ruminal microbiota: implications for engineering improved ruminal fermentations. Front Microbiol. 2015;6:296. doi: 10.3389/fmicb.2015.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Abe F., Ishibashi N., Shimamura S. Effect of administration of Bifidobacteria and lactic acid bacteria to newborn calves and piglets. J Dairy Sci. 1995;78:2838–2846. doi: 10.3168/jds.S0022-0302(95)76914-4. [DOI] [PubMed] [Google Scholar]
- 155.Foditsch C, Pereira RVV, Ganda EK, Gomez MS, Marques EC, et al. Oral administration of Faecalibacterium prausnitzii decreased the incidence of severe diarrhea and related mortality rate and increased weight gain in preweaned dairy heifers. PLOS ONE 2015;10:e0145485. [DOI] [PMC free article] [PubMed]
- 156.Al-Saiady M.Y. Effect of probiotic bacteria on iinmunoglobulin G concentration and other blood components of newborn calves. J Anim Vet Adv. 2010;9:604–609. [Google Scholar]
- 157.Zhao T, Doyle MP, Harmon BG, Brown CA, Mueller POE, et al. Reduction of carriage of enterohemorrhagic Escherichia coli O157:H7 in cattle by inoculation with probiotic bacteria. J Clin Microbiol 1998;36:641-7. [DOI] [PMC free article] [PubMed]
- 158.Krehbiel C., Rust S., Zhang G., Gilliland S. Bacterial direct-fed microbials in ruminant diets: performance response and mode of action. J Anim Sci. 2003;81:E120–E132. [Google Scholar]
- 159.Villot C., Chen Y., Pedgerachny K., Chaucheyras-Durand F., Chevaux E., Skidmore A. Early supplementation of Saccharomyces cerevisiae boulardii CNCM I-1079 in newborn dairy calves increases IgA production in the intestine at 1 week of age. J Dairy Sci. 2020;103:8615–8628. doi: 10.3168/jds.2020-18274. [DOI] [PubMed] [Google Scholar]
- 160.Marquez JC. Calf intestinal health: assessment and dietary interventions for its improvement. Dissertation. University of Illinois at Urbana-Champaign, Animal Sciences 2014. hdl.handle.net/2142/50681.
- 161.Kido K., Tejima S., Haramiishi M., Uyeno Y., Ide Y., Kurosu K. Provision of beta-glucan prebiotics (cellooligosaccharides and kraft pulp) to calves from pre- to post-weaning period on pasture. Anim Sci J. 2019;90:1537–1543. doi: 10.1111/asj.13299. [DOI] [PubMed] [Google Scholar]
- 162.Wei H., Ding L., Wang X., Yan Q.i., Jiang C., Hu C. Astragalus root extract improved average daily gain, immunity, antioxidant status and ruminal microbiota of early weaned yak calves. J Sci Food Agric. 2021;101:82–90. doi: 10.1002/jsfa.10617. [DOI] [PubMed] [Google Scholar]
- 163.Chang M.N., Wei J.Y., Hao L.Y., Ma F.T., Li H.Y., Zhao S.G. Effects of different types of zinc supplement on the growth, incidence of diarrhea, immune function, and rectal microbiota of newborn dairy calves. J Dairy Sci. 2020;103:6100–6113. doi: 10.3168/jds.2019-17610. [DOI] [PubMed] [Google Scholar]
- 164.Cao N., Wu H., Zhang X.Z., Meng Q.X., Zhou Z.M. Calcium propionate supplementation alters the ruminal bacterial and archaeal communities in pre- and postweaning calves. J Dairy Sci. 2020;103:3204–3218. doi: 10.3168/jds.2019-16964. [DOI] [PubMed] [Google Scholar]
- 165.Ma T., Villot C., Renaud D., Skidmore A., Chevaux E., Steele M. Linking perturbations to temporal changes in diversity, stability, and compositions of neonatal calf gut microbiota: prediction of diarrhea. ISME J. 2020;14:2223–2235. doi: 10.1038/s41396-020-0678-3. [DOI] [PMC free article] [PubMed] [Google Scholar]