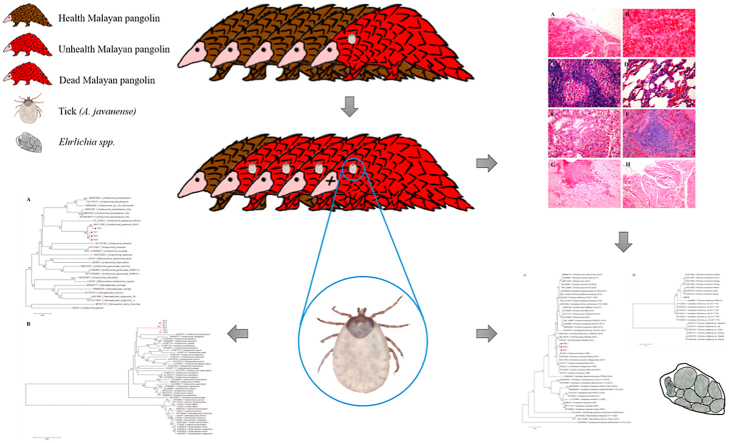
Abstract
Due to habitat destruction and illegal hunting and trade, the number of pangolins has been sharply reduced. To protect pangolins from extinction, relevant departments are combined and active action have been taken. A total of 21 confiscated Malayan pangolins were rescued in 2019, but died continuously for unknown reasons. This study aimed to investigate the reasons for the death of these pangolin and rescue them. 19 of the 21 confiscated pangolins had ticks on their body integument. A total of 303 ticks were collected and identified as Amblyomma javanense (A. javanense) according to their morphology and the sequences of 16S rRNA and internal transcribed spacer 2 (ITS2). There were multi-organ damages in the dead pangolins, especially congestion and hemorrhage in lung, heart and kidney and inflammation of which were observed using HE staining. Pathogens' nucleic acid detection showed ticks were only positive for Ehrlichia spp, with 56.7% positive rate of collected ticks (127/224), which was further confirmed in tissues from dead pangolins. Our findings confirm that ehrlichiosis caused by Ehrlichia spp. from A. javanense might accelerate the confiscated pangolin's death. More attention should be payed to tick-elimination work and the diagnoses and treatment of tick-borne diseases in the follow-up rescue operation.
Keywords: Malayan pangolin, Tick, Tick-borne pathogen, Amblyomma javanense, Ehrlichia spp., Ehrlichiosis
Abbreviations: PCR, polymerase chain reaction; 16S rRNA, 16 Svedberg ribosomal ribonucleic acid; BLAST, basic local alignment search tool
Graphical abstract
Highlights
-
•
Identification of Amblyomma javanense tick in confiscated Malayan Pangolins.
-
•
Internal transcribed spacer 2 (ITS2) regions of Amblyomma javanense were newly sequenced and uploaded in public database.
-
•
Ehrlichiosis caused by Ehrlichia spp. from A. javanense might accelerate the death of the confiscated Malayan pangolins.
-
•
This research provides an important reference for the diagnosis and prevention of pangolin-related diseases in the future.
1. Introduction
Ticks (Acari: Ixodidae) are parasites of obligate blood-sucking arthropod on the body surface of animals and are important vectors for many kinds of pathogens because of its extensive host range (Anderson, 2002; Khatri-Chhetri et al., 2016). When ticks bit into the nude skin, they can not only cause animal or human itch, anemia and emaciation, but also spread pathogens such as bacteria, viruses, nematodes and protozoa, which would seriously threaten human health, animal husbandry production and wildlife survival. Tick-borne diseases mostly occur in temperate, tropical and subtropical regions (Boucher et al., 2020; Chen et al., 2010; Jiang et al., 2018; Reye et al., 2012; Vesco et al., 2011).
The genus Ehrlichia belongs to the family Anaplasmataceae and consists of six recognized species, Ehrlichia minasensis (E. minasensis), E. canis, E. muris, E. chaffeensis, E. ruminantium and E. ewingii. All members of Ehrlichia are tick-borne pathogens for mammals like ruminants (E. ruminantium), dogs (E. canis, E. chaffeensis and E. ewingii), mice (E. muris) and humans (E. canis, E. ruminantium, E. chaffeensis and E. ewingii) worldwide (Cabezas-Cruz et al., 2016; Paddock and Childs, 2003; Zhang et al., 2017). E. ruminantium, initially named as Rickettsia ruminantium, was the first species of Ehrlichia to be discovered. E. ruminantium infected ruminants mainly in African areas such as Kenya and Uganda (Allsopp, 2010; Peter et al., 2019; Tumwebaze et al., 2020), and in other areas like Northwest China (Guo et al., 2018) and Oman (El-Neweshy et al., 2019). However, E. ruminantium were also reported in free-living jaguars in Brazil (Widmer et al., 2011). E. chaffeensis came into the view of mankind firstly from patients presumptive diagnosed of ehrlichiosis in middle state of America in 1986 (Fishbein, 1990), and formally named after isolation and molecular characterization analysis in 1991 (Anderson et al., 1991). Since then, increasing evidence of E. chaffeensis infection in human throughout the world were reported in Mali (Uhaa et al., 1992), Thailand (Heppner et al., 1997), Mexico (Gongora-Biachi et al., 1999), Italy (Nuti et al., 1998) and Israel (Keysary et al., 1999). White-tailed deer have been believed to be the most pivotal element in the emergence of E. chaffeensis in the United States during the 20th century (Paddock and Childs, 2003). Recently, E. chaffeensis were found in autochthonous cervidae from Argentina (Guillemi et al., 2019) and white-tailed deer from south Texas of America (Yu et al., 2020). Ehrlichia infection in mammal host usually induces a kind of zoonosis ehrlichiosis, which can parasitize in the cells of spleen, lymph node, bone marrow and peripheral blood and induce lesions and dysfunction of multiorgans. The clinical symptoms of ehrlichiosis were deemed to be caused by host inflammatory reaction instead of direct damage caused by these bacteria (Parola et al., 2003b). Pangolin ehrlichiosis has been reported since 2010 (Cabezas-Cruz et al., 2016; Chen et al., 2010), but hasn't caused much serious consequences.
Pangolins are a kind of toothless mammals that are famous for their full armor of scales.Pangolin armor was considered a valuable traditional Chinese medicinal material and its meat was also regarded as a nourishing treasure in the past. Coupled with the serious destruction of habitats and illegal smuggling, their survival is threatened, and the number of wild pangolins has continued to decline. In addition, the decrease of pangolin may also be related to the occurrence of disease. They are extremely susceptible to diseases such as blood parasites, bacteria and viruses in the wild field, which further exacerbates the decrease of pangolin population (Parola et al., 2003a). There are limited reports about the diseases of pangolins caused by ticks, bacteria, virus and other pathogens (Hua et al., 2015; Mohapatra et al., 2016), such as Anaplasma spp., Sendai virus and coronavirus in Malayan pangolin (Manis javanica) (Koh et al., 2016; Liu et al., 2019; Parola et al., 2003b), Rickettsia africae in African giant pangolin (Mediannikov et al., 2012), Trypanosoma brucei gambiense in long tailed pangolin (M. tetradactyla) and tree pangolin (M. tricuspis) (Njiokou et al., 2006), fatal canine parvovirus-2 (CPV-2) and canine distemper virus (CDV) in Taiwanese pangolin (M. pentadactyla pentadactyla) (Wang et al., 2020). All kinds of pangolin are included in Appendix I to the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES). Chinese authorities are also actively taking action to protect and rescue these species to prevent population extinction (Wang et al., 2020).
Malayan pangolin mainly distributing in Asia and Africa is one of the eight existing pangolin species (Hassan et al., 2013). According to the study of Heinrich in 2016, there were 1485 pangolin trade events between 1977 and 2014, of which approximately 809,723 pangolins were traded internationally (Heinrich et al., 2016), which increased the risk of pangolin infectious pathogens. During the illegal transfer process, pangolins probably carrying parasite ticks and other infectious pathogens were crowed in hostile environments, therefore increasing the risk of tick-borne diseases transmission among individuals. Recently, pangolin has become a research hotspot, but little research has been done on the disease of pangolins and its etiology.
In this study, to investigate the reasons for the death of this batch of confiscated Malayan pangolin, ticks and tissues from the dead pangolins were detected for reported viral, bacterial and protozoa pathogens. The results showed more than 90% pangolins were parasitized by ticks, which were further identified as Amblyomma javanense (A. javanense) by their morphology and molecular marksGross and microscopic lesions of the dead pangolins showed marked presence of inflammation in tissues which might related to ehrlichiosis of confiscated pangolins. Further studies revealed Ehrlichia spp, were positive in tick and pangolin tissues. Our findings confirmed that ehrlichiosis caused by Ehrlichia spp. from A. javanense might accelerate the rescued pangolins' death. This research fills the gaps in pangolin rescue operation at home and abroad, and provides an important reference for the diagnosis and prevention of pangolin-related diseases in the future.
2. Materials and methods
2.1. Pangolin
Twenty-one live Malayan pangolins in this study were ferreted out and confiscated by Customs and Department of Forestry of Guangdong Province on March 2019, and then were kept in a quiet and dark room for health assessment and rehabilitation carried out by Guangdong Provincial Wildlife Rescue Center Guangzhou Zoo, and Guangdong Institute of Applied Biological Resources.
2.2. Tick collection and morphological identification
The skin of infested pangolin was carefully examined for the presence of ticks. Ticks were gently removed from pangolins with tweezers and observed for species, life stage and sex according to standard protocols (Kwak et al., 2018; Voltzit and Keirans, 2003) using a stereomicroscope (Olympus®, Tokyo, Japan) by two of the co-authors separately. All tick specimens from each animal were separately kept in clean tubes plugged with cotton and stored at −80 °C after identification until further detection.
2.3. Pangolin blood and tissue collection
Whole blood from the jugular vein along the ventral midline of the tail of live pangolin or from the heart of pangolin immediately after its' death were put into clean blood collection tubes and kept at −80 °C before needed.
Diagnostic necropsy of pangolin was carried out within 6 h after pangolin's death as reported (Parkinson et al., 2011). Briefly, marked gross changes in body integument, external orifices and major organs, including brain, thyroid, heart, lung, liver, kidney, spleen, pancreas, bladder and gastrointestinal tract, were observed and recorded. Tissues with typical lesions were removed from pangolin carcass, collected and stored in 10% neutral buffered formalin (NBF) in clean containers or in sterile tubes, which were kept at room temperature and at −80 °C until further investigation, respectively.
2.4. Hematoxylin and eosin (HE) staining
Pangolin tissue from dead Malayan pangolins were observed for histopathological lesions after HE staining as described before (Xiao et al., 2020). Briefly, tissues stored in 10% NBF at room temperature for at least 48 h were embedded with molten paraffin wax and sectioned in 7 μm with a microtome. Then the sections were moved onto grease-free glass slides, deparaffinized and rehydrated in ethanol solution in with descending grades. Then sections on slides were stained with HE staining kit (Beyotime Biotechnology, Nantong, China) according to the guidance, mounted with coverslips and observed using a suit of Olympus equipment (BX53 with PM-C 35 digital camera).
2.5. Nucleic acid extraction of samples
The samples of tick and tissue stored at −80 °C were thawed and gently washed twice with ddH2O in individual Eppendorf tubes, then were added with PBS at a weight/volume (g/ml) ratio of 1/10 and thoroughly homogenized for 60 s twice with 30 s break on ice using handheld homogenizer (Jingxin F6/10, China). Homogenate of ticks and tissues were prepared for next investigation.
2.6. Molecular detection of ticks and pathogens using PCR
For molecular identification of tick species, total RNA was extracted from tick homogenate using Trizol reagent (TIANGEN, China) and reverse-transcribed using PrimeScript™ 1st Strand cDNA Synthesis Kit (Takara, China) according to manufacturer's instructions. 16S rRNA and internal transcribed spacer 2 (ITS2) of ticks were targeted using specific primer pairs shown in Table 1. cDNA pool was used as template and the reaction was performed in total volume of 25 μl using the Phusion Hot Start II High-Fidelity PCR Master Mix (Thermo Scientific, USA) according to the product information.
Table 1.
Primers used for detection of pathogens in this study.
| Primer name | Primer sequence | Product length | Reference* |
|---|---|---|---|
| Tick-16S–F | CTGCTCAATGATTTTTTAAATTGCTGTGG | 450 bp | Norris et al. (1996) |
| Tick-16S-R | CCGGTCTGAACTCAGATCAAGT | ||
| Tick-ITS2-F | CGAGACTTGGTGTGAATTGCA | 1231 bp | Zou et al. (2018) |
| Tick-ITS2-R | TCCCATACACCACATTTCCCG | ||
| Hepatozoon-F | GGTAATTCTAGAGCTAATACATGAGC | 574 bp | Almeida et al. (2012) |
| Hepatozoon-R | ACAATAAAGTAAAAAACAYTTCAAAG | ||
| Ehrlichia-16S–F | CTAGAGGTCGAAAGAGGATAG | 555 bp | This study |
| Ehrlichia-16S-R | GTGCTGATTTGACATCATCC | ||
| Ehrlichia-gltA-F Ehrlichia-gltA-R |
GAGTATTAACTTATGATCCAGG | 1066 bp | This study |
| TTCATACCAYTGAGCAGACC | |||
| Babesia-18S–F | CCGTGCTAATTGTAGGGCTAATACA | 551 bp | Almeida et al. (2012) |
| Babesia 18S-R | GCTTGAAACACTCTARTTTTCTCAAAG | ||
| Theileria-SSU-F | CTTCAGCACCTTGAGAGAAAT | 477 bp | Sloboda et al. (2011) |
| Theileria-SSU-R | TCDATCCCCRWCACGATGCRBAC | ||
| AIV-F | ATGAGYCTTCTAACCGAGG | 299 bp | This study |
| AIV-R | CGTCTACGCTGCAGTCCT | ||
| PIV5–F | GATCATTCCGCTTAATCCC | 449 bp | This study |
| PIV5-R | CTTTCCAACATCCCCTACC | ||
| CPV-F | GTAAGCTTCCAGGAGACTTT | 600 bp | This study |
| CPV-R | GTAAGCTTCGTCGTGTTCTT | ||
| CDV-F | ATAGATGTCTTGACACCGCTCTT | 587 bp | This study |
| CDV-R | GTACATACCTTGGCTTTGGAACT | ||
| EMCV-F | TCTGTTGAATGTCGTGAAGGA | 286 bp | This study |
| EMCV-R | AGGCCCCAGATCAGATCC | ||
| CHV–F | GGTAGACCCTCCTCGTAGGTAT | 357 bp | This study |
| CHV-R | GGGGCAGCTAAAACTAATCCCA |
(NOTE: *, primers without indicated references were designed in this study.).
For molecular identification of procaryotic and viral pathogens in ticks and tissues, the homogenate was centrifuged at 2000 rpm for 3 min at 4 °C and the supernatant was collected for extraction of both RNA and DNA using AxyPrep Body Fluid Viral DNA/RNA Miniprep Kit (Axygen biosciences, Cat. AP-MN-BF-VNA-250) according to users' guide. For the detection of RNA viral pathogens including encephalomyocarditis virus (ECMV), parainfluenza virus type 5 (PIV5), avian influenza virus (AIV), canine distemper virus (CDV), cDNA was reverse-transcribed from extraction nucleic acid pool and specifically amplified using primers as described above. For DNA viral pathogens detection (canine herpes virus (CHV) and canine parvovirus (CPV)) and procaryotic pathogens detection, including Ehrlichia (16S rRNA and citrate synthase (gltA)), Babesia, Theileria and Hepatozoon, specific primers were used to amplified indicated sequences from extraction nucleic acid pool. Primers used in this study were listed in Table 1.
2.7. Gel electrophoresis, sequencing and high similarity analysis
To separate DNA fragments, PCR products prepared above were added into 5% agarose gel with Golden View™ (Takara) and electrophoresed at 120 V for 25 min, together with DL2000 DNA marker (Takara). Bands of nucleic acid product were visualized using a ChemiDoc™ MP imaging system (Bio-Rad). Specific positive products were sequenced by Ruibiotech company in China. The nucleotide sequences were analyzed against sequences deposited in GenBank using BLASTn (https://blast.ncbi.nlm.nih.gov/Blast.cgi) for high similarity analysis of sequences.
2.8. Phylogenetic tree analysis
Phylogenetic tree analyses of obtained sequences of PCR product from ticks or pangolin tissues were conducted using MEGA 6.0 with the Maximum Likelihood algorithm. Bootstrap values were calculated with 1000 replicates.
3. Results
3.1. Observation and clinical symptoms of confiscated Malayan pangolins
A total of 21 confiscated Malayan pangolins were rescued in 2019. When confiscated, they were in bad situation, including poor spirit, drowsiness, anorexia, edema of extremities, snotty and coughing. Furthermore, there were inexplicable wounds and bleeding on pangolins' body and lasted for 10–26 days. Dozens of adult and subadult ticks on pangolins' integument were gently removed (Fig. 1A), and the wounds were cleaned and applied with antibiotics. However, they died continuously for unknown reasons. Before died, most of pangolins were found to excrete tarry stool and hematuria and exhibit nervous symptoms such as convulsions.
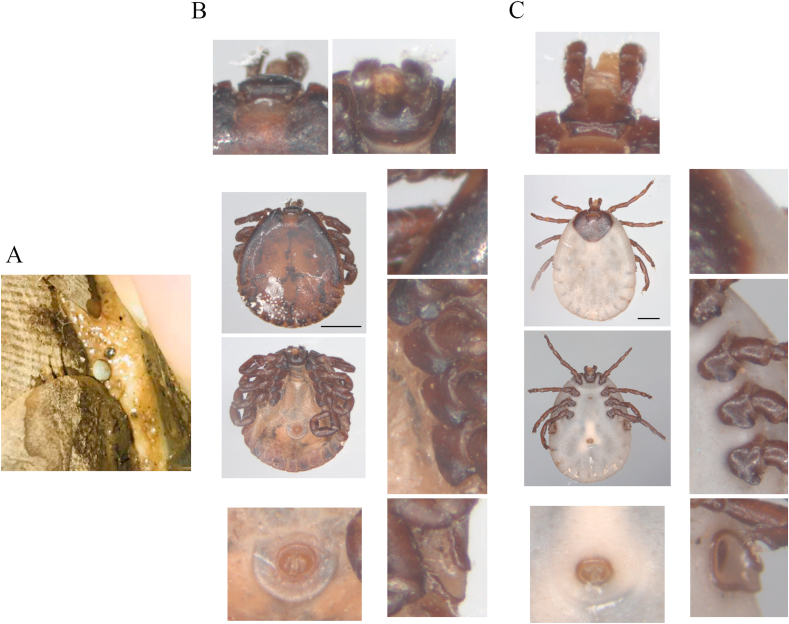
Fig. 1.
Morphologic identification of ticks on the surface of confiscated Malayan pangolins. Tick on Malayan pangolin surface (A). Dorsa view, ventral view and typical feature details of male (B) and female (C) Amblyomma javanense ticks collected from confiscated Malayan pangolins. Black bar, 2 mm.
3.2. Identification and phylogenetic tree analysis of Amblyomma javanense from Malayan pangolins
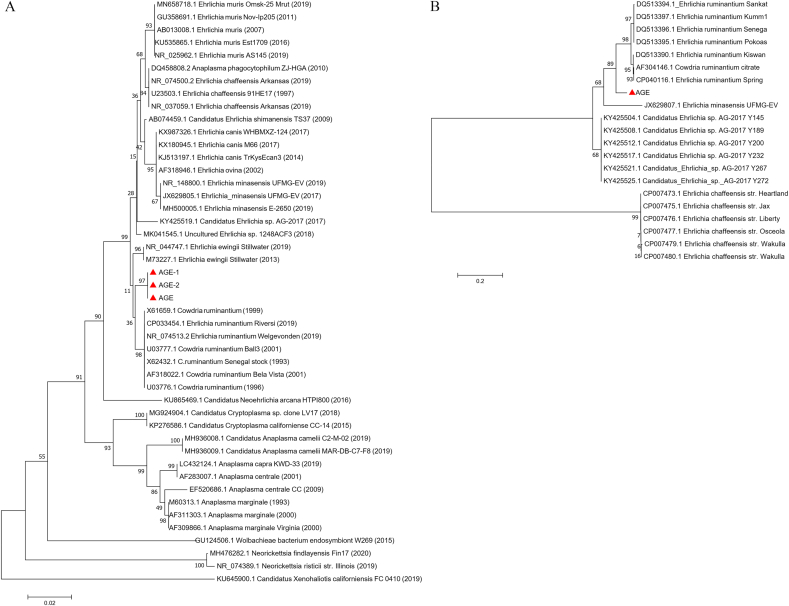
Ticks collected from the surface of pangolins were identified using morphological method and molecular biotechnology (Fig. 1). Morphologic analysis implied that these ticks collected from the confiscated Malayan pangolins were Amblyomma javanense (A. javanense) (Fig. 1B and C). Long slender palps and festoons on the adult tick idiosoma were typical features of members of the genus Amblyomma. Other observed characteristics for A. javanense distinguished from other Amblyomma members including (Fig. 1B and C): rectangular basic capitula; small and deep porose area; the wide anterior and narrow posterior of palp, with the longer second segment than the third; dark auburn and heart-shaped scutum with no enamel; small and flat eyes with only one trace; the anal groove behind the anus; comma-shaped peritreme. Moreover, coxa I with two extremely wide, blunt, well separated spurs, and coxa II, III and IV each with an extremely wide, blunt, rounded spur (Fig. 1B and C). Subsequently, specific DNA sequences were amplified from cDNA of 6 individual ticks and sequenced for 16S rRNA (4/6) and ITS2 (6/6) (Supplementary Figs. 1A and B) for tick identification. BLAST results showed that 16S rRNA gene of ticks was close to Amblyomma javanense (A. javanense) with a similarity of more than 99%, and ITS2 region was close to Amblyomma paulopunctatum with a similarity of nearly 92% (data now shown). Genetic evolution showed that the selected sequences of 16S rRNA gene belonged to the same branch as A. javanense (Fig. 2A) and ITS2 region sequences formed an individual branch (Fig. 2B) using phylogenetic tree analysis. Based the results above, the ticks were finally defined as A. javanense. and ITS2 sequences identified in this study were uploaded as A. javanense (Accession number: MK928428.1 and MK928429.1).
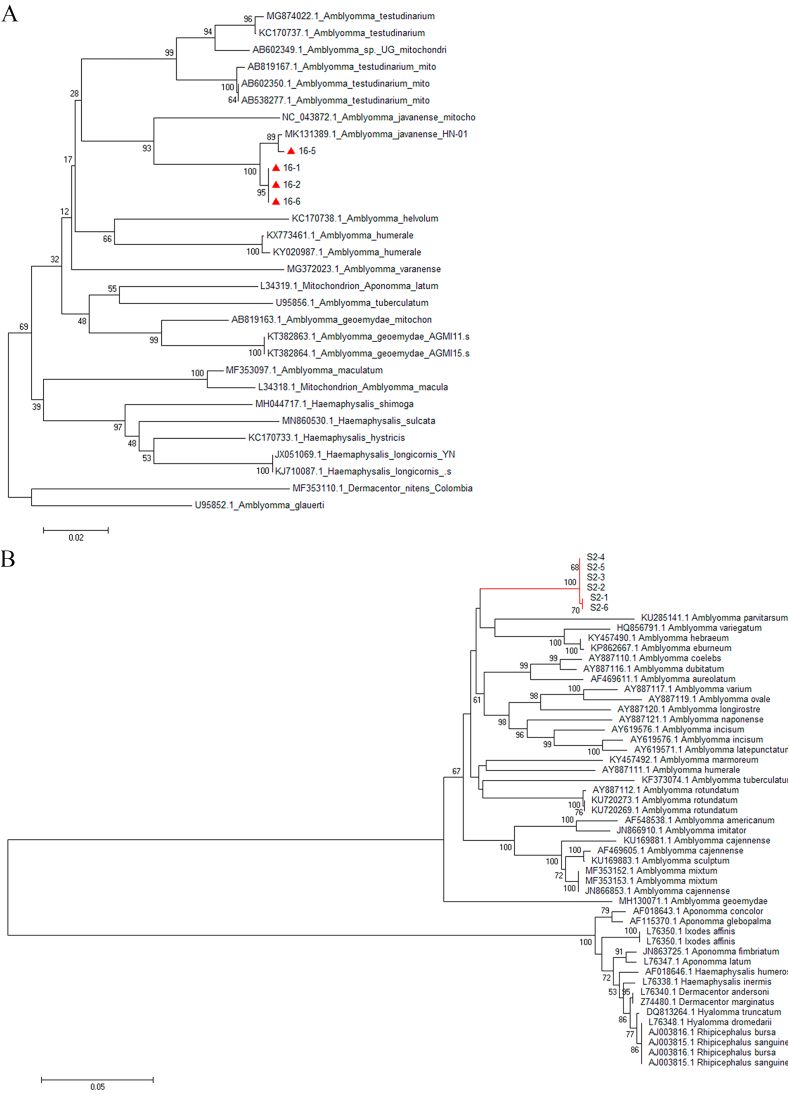
Fig. 2.
Phylogenetic tree based on the 16S rRNA (A) and ITS2 (B) of ticks from confiscated Malayan pangolins. Analyses were conducted using MEGA 6.0 with the Maximum Likelihood algorithm. Bootstrap values were calculated with 1000 replicates. The number on each branch indicates bootstrap value. Red triangles and red lines: sequences of ticks obtained in this study. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.3. Gross and microscopic lesion of dead Malayan pangolins
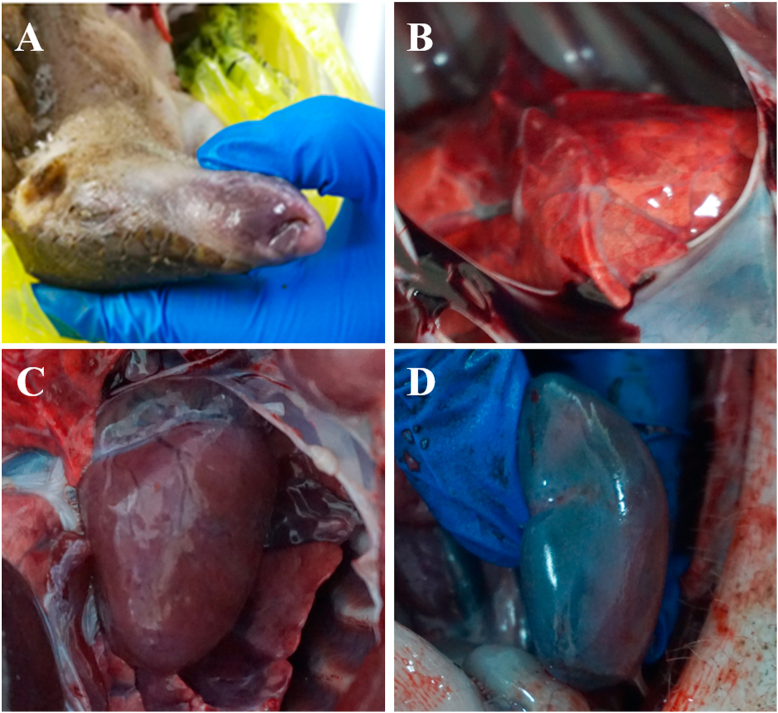
In the autopsy of pangolins, ulcerations and blood on the skin integument, edematous body and pale mucous membranes were observed (Fig. 3A). Congestion and edema were shown in most internal organs (Fig. 3B–D), including lung, pancreas, spleen, kidney and bladder mucosa. Bleeding points could be seen on the surface of lung, trachea, bronchus and kidney, and the cut surface of the lungs was infiltrated with foamy fluid (Fig. 3B). In the heart, there were signs of myocardial edema, pericardium effusion and ventricular congestion (Fig. 3C). Part of the interstina parva mucosa was congested, and the mesenteric lymph nodes were highly swollen, congested, edematous and hemorrhagic as well. Infiltrating hemorrhage was seen in the node section. In the kidney, corticomedullary differentiation was not clear and blood-like fluid accumulated in the renal calyes (Fig. 3D).
Fig. 3.
Gross pathological lesions of dead Malayan pangolins after autopsy. A: Pale, even purple, superficial mucosa around the nose and mouth. B: Congestion and hemorrhage observed in the lung. C: Myocardial edema, pericardium effusion and ventricular congestion in the heart. D: Kidney congestion. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
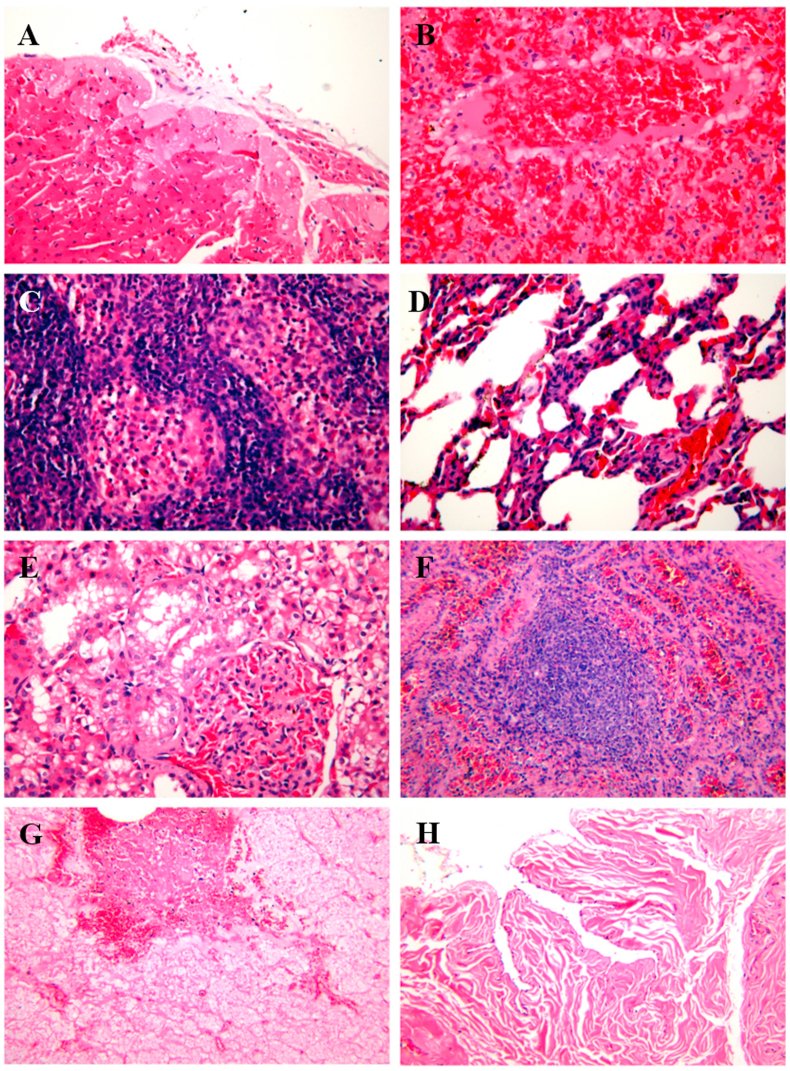
Histopathological analysis revealed marked presence of inflammation in tissues (Fig. 4). Microscopic lesions included myocardial failure (Fig. 4A); sinus hepaticus(Fig. 4B); lymphoid nodule dilatation with blood stasis, widened splenic cord (Fig. 4C); multiply lymphocytes, collapsed alveoli, glomerular capillary hyperemia and increased volume (Fig. 4D); the renal tubules were transparent, with capillaries congested and cystic spaces dilated in the glomeruli (Fig. 4E); medullary blood vessels were dilated and congested, and there were many macrophages in the medullary cord (Fig. 4F); epithelial cells of mucosa necrosis and submucosa congestion in the sialaden (Fig. 4G), and bladder mucosa folds inward (Fig. 4H). The results above indicated pangolins might die of multisystem failure.
Fig. 4.
The histopathological examination of Malayan pangolins tissue slide using hematoxylin-eosin (HE) staining. A: Heart, myocardial cells were necrotic, and the myoplasm at the necrosis was dissolved into vacuoles, some of which were lipid droplet vacuoles. B: Liver, sinus hepaticus was dilatate with blood stasis. C: Spleen, splenic cord widened and lymphocytes multiplied. D: Lung, alveoli collapse, inflammatory cell infiltration and capillaries congestion. E: Kidney, the renal tubules were transparent, with capillaries congested and cystic spaces dilated in the glomeruli. F: Lymph nodes, medullary blood vessels were dilated and congested, and there were many macrophages in the medullary cord. G: Salivary glands, epithelial cells of mucosa necrosis and submucosa congestion. H: Bladder, mucosa folds inward when empty. A-E, 400 X; F–H, 200X.
3.4. Identification and phylogenetic tree analysis of Ehrlichia spp. From Malayan pangolins and ticks
Ticks and the organs from dead Malayan pangolins were collected for the detection of viral and procaryotic pathogens using PCR or reverse transcription-PCR. Firstly, pangolins were tested for viral pathogens including EMCV, PIV5, AIV, CDV, CHV and CPV, and the results showed the specimens were negative (data not shown). On the other hand, protozoa and bacterial pathogens like Babesia, Hepatozoon, Theileria and Ehrlichia spp. were detected as described above, and the results showed specimens were only positive for 16S rRNA of Ehrlichia spp. in this study (data not shown). In the meantime, pangolin tissues and 224 individuals of ticks (male 140, female 84) were detected for Ehrlichia spp. 16S rRNA using PCR (Supplemental Fig. 1A). The results showed that most lung, spleen and blood of dead pangolins and 56.7% of the ticks were positive for Ehrlichia spp. (Table 2).
Table 2.
Detection of Ehrlichia spp. infection in ticks and tissues collected from Malayan pangolin.
| Pangolin ID (Gender) | NO. of ticks of each pangolin | NO. of ticks for detection | NO. of ticks positive for Ehrlichia spp. | Ehrlichia spp. in pangolin lung | Ehrlichia spp. in pangolin spleen | Ehrlichia spp. in pangolin blood |
|---|---|---|---|---|---|---|
| 1 (♂) | 12 | 9 (7♂, 2♀) | 5 | – | – | / |
| 2 (♀) | 1 | 1 (1♀) | 0 | + | / | / |
| 3 (♀) | 13 | 7 (3♂, 4♀) | 3 | / | / | / |
| 4 (♀) | 2 | 2 (2♂) | 1 | – | + | / |
| 5 (♂) | 16 | 7 (5♂, 2♀) | 4 | / | / | / |
| 6 (♀) | 1 | 1 (1♀) | 0 | / | / | / |
| 7 (♀) | 9 | 2 (1♂, 1♀) | 0 | / | / | / |
| 8 (♀) | 18 | 15 (11♂, 4♀) | 8 | + | / | / |
| 9 (♀) | 8 | 6 (4♂, 2♀) | 5 | + | / | / |
| 10 (♀) | 0 | 0 | 0 | / | / | / |
| 11 (♀) | 21 | 17 (14♂, 3♀) | 13 | + | + | + |
| 12 (♀) | 6 | 5 (5♂) | 4 | + | / | / |
| 13 (♂) | 0 | 0 | 0 | / | + | + |
| 14 (♀) | 38 | 34 (12♂, 22♀) | 30 | / | / | / |
| 15 (♂) | 5 | 2 (1♂, 1♀) | 0 | / | / | / |
| 16 (♂) | 31 | 28 (15♂, 13♀) | 13 | / | + | / |
| 17 (♂) | 5 | 4 (4♀) | 0 | / | / | – |
| 18 (♀) | 26 | 21 (17♂, 4♀) | 11 | / | + | / |
| 19 (♀) | 10 | 9 (6♂, 3♀) | 2 | / | / | – |
| 20 (♀) | 16 | 14 (8♂, 6♀) | 5 | / | / | / |
| 21 (♂) | 65 | 40 (29♂, 11♀) | 23 | / | + | + |
(NOTE: NO., numbers; ♂, male; ♀, female; +, positive; -, negative;/, not tested.).
Moreover, according to BLAST result, 3 randomly selected positive sequences of 16S rRNA of Ehrlichia spp. were found to be closest to E. chaffeensis and E. ruminantium, with a similarity of above 98%. This observation was consistent to a phylogenetic tree analysis (Fig. 5A). The obtained sequences of 16S rRNA of Ehrlichia (about 550 bp) were not long enough to identify to a particular species level. Then a 1066 bp length of gltA of Ehrlichia was amplified and sequenced. The phylogenetic analysis of gltA sequence (Fig. 5B) showed that Ehrlichia in this study was close to E. ruminantium, but in a relative individual clade still, which suggested this Ehrlichia from confiscated Malayan pangolin might be a new spice. In a word, this study Ehrlichia spp. infection might accelerate the death of confiscated Malayan pangolins.
Fig. 5.
Phylogenetic tree based on the 16S rRNA (A) and gltA (B) of pathogens found in ticks from confiscated pangolins. Analyses were conducted by using MEGA software version 6.0 with the Maximum Likelihood algorithm. Bootstrap values were calculated with 1000 replicates. The number on each branch indicates bootstrap values. Red triangles: sequences of Ehrlichia spp. obtained in this study. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
In the rescue operation of 21 Malayan pangolins confiscated by the Customs, 14 of them were female and 7 were male. More than 90% of pangolins (19/21) were carrying ticks on their integument, which was much higher than the parasitology survey of Formosan pangolins (Khatri-Chhetri et al., 2016) and our previous Malayan pangolin. In a parasitological survey of 52 Taiwan pangolins (25 males, 27 females), 25% of them were found to be infested with ticks (Khatri-Chhetri et al., 2016). A survey of 16 Malayan pangolins carried by Hassan et al., in 2013 showed a rate of 68.8% of the tick infection (Hassan et al., 2013). In the study by Hassan et al. a higher rate of tick was found in male pangolins rather than female pangolins, which might as a result of the difference behavior of male and female pangolin, especially during the spawning season (Hassan et al., 2013). In this study, 19 of the 21 Malayan pangolins were found to bearing ticks, 13 of the 14 female pangolins and 6 of the 7 male pangolins. Due to the small number and special situation of confiscated pangolins, the obtained data were not representative for tick infection of pangolins in wild circumstance. The high prevalence of tick in confiscated pangolins might as a result of the lack of suitable cages during the smuggling process, when individual pangolins were squeezed into restricted space, and got too close to each other, which may help ticks moving from one individual to another and causing cross-infection of ticks among this batch pangolins.
Amblyomma javanense (A. javanense) is commonly associated with reptiles and mammals including pangolins (Kwak et al., 2018). Previous researches reported that a total of 12 species of mammals and 4 species of reptiles were known to be infected with A. javanense, including Sunda pangolin, Chinese pangolin, Indian pangolin, Palawan pangolin, wild boar, bat, hyena, "bear", sambar deer, Indian crested porcupine, mouse deer, human, water monitor, "python", long-tailed skink and hill turtle (Hassan et al., 2013; Kollars and Sithiprasasna, 2000; Kwak et al., 2018). According to the research by Mihalca and Hassan., A. javanense was the most common tick parasitic on pangolins and may also be a common endangered species on Asian pangolins (Hassan et al., 2013; Mihalca et al., 2011; Nandi, 1981). In 1981, Nandi reported that the ticks captured from Indian pangolins were A. javanense in 1981, and Kwak also reported the same tick caught on Sunda Pangolin from Singapore in 2018 (Kwak et al., 2018; Nandi, 1981). A. javanense was found in Malayan pangolins and wild boars from Thailand in 2000 (Kollars and Sithiprasasna, 2000), and A. javanense was successfully identified on Chinese pangolin through species DNA barcode in 2019 (Jabin et al., 2019). In this study, the ticks collected from confiscated Malayan pangolin were finally determined as A. javanense through morphological and molecular biology analysis.
In this study a total of 224 ticks of 303 ticks collected were firstly tested for viral, bacterial and protozoa pathogens, 56.5% of which were found to be only positive for Ehrlichia spp., which was also found in the tissues of ticks' host pangolins. Sequencing and alignment analysis of partial sequence of 16S rRNA an ITS2 further confirmed that the bacteria detected in ticks and pangolin tissues in this study belong to Ehrlichia spp. Phylogenetic analysis using 16S rRNA of Ehrlichia spp. as the standard, these Ehrlichia spp. from ticks and pangolins in this study were most closely related to E. ruminantium (99.50%, 98.84% and 98.66%, respectively), and they formed a clade together with E. chaffeensis, E. muris, E. ewingii, E. minasensis and E. canis (Fig. 5).
Ehrlichia spp. are obligate intracellular parasitic bacteria and tends to favor hematopoietic cells, which cause ehrlichiosis in infected mammals. Common clinical symptoms of ehrlichiosis are anorexia, lymphadenopathy, cardiopulmonary dysfunction, leucopenia, neural and ocular lesions, and pathological lesions include pale mucous membranes, hepatomegaly, splenomegaly, edema, glomerulonephritis, interstitial mononuclear infiltration (de Castro et al., 2004). The symptoms and lesions of the pangolins were highly consistent with the above description of ehrlichiosis. In addition, lung, spleen and blood of the dead Malayan pangolins had also been confirmed to be infected with Ehrlichia spp.
In dogs, the infection lasts a life time even after injecting doxycycline (Hua et al., 2015). Although rickettsiosis is a fatal disease and the estimated death rate is 1%–10% in human (Blevins et al., 2008), prompt treatment for rickettsiosis can dramatically reduce mortality. However, the death rate of confiscated pangolins is staggering in this study. According to the retrospect of this case, confiscated pangolins might be extremely susceptible to pathogen infection and induce general damages because of poor physical state, low immunity and lack of immediate treatment. Further undergo stress and disturbance in the process of smuggling and trafficking would aggravate pangolin's pathogenetic process. According to this study, pangolins might die because of ehrlichiosis caused by Ehrlichia spp. infection from Amblyomma javanense. In addition, Ehrlichia spp. was resistant to most drugs, and the treatment effect was very poor, which might also be the reason for the high mortality after pangolin rescued. After medical treatment of removement of ticks on the surface and intramuscular injection with specific antibiotic doxycycline, sick pangolins reduced clinical symptoms and some of them kept alive before transfer.
5. Conclusions
Our findings confirm that ehrlichiosis caused by Ehrlichia spp. from A. javanense might accelerate the rescued pangolins' death. Through the study of this batch of confiscated Malayan pangolins, more attention should be payed to tick-elimination work and the diagnoses and treatment of tick-borne diseases in the follow-up rescue operation.
Authors' contributions
W.C. and N.Z. conceived the study and wrote the manuscript. J.Q.Z. and Y.J.W. performed the main molecular experiment. J.P.C and J.J.Z. performed the morphology analysis of ticks and autopsy of pangolins. F.S. and W.P.L. contributed to the analysis.
Funding
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests.
Acknowledgements
We thank Chen Wang, Xueqing Du and Jiaqi Sa in the Guangzhou Zoo for their kindly help in this work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijppaw.2021.01.008.
Contributor Information
Wu Chen, Email: guangzhouchenwu@sina.com.
Niu Zhou, Email: zhouniu@zju.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Allsopp B.A. Natural history of Ehrlichia ruminantium. Vet. Parasitol. 2010;167:123–135. doi: 10.1016/j.vetpar.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Almeida A.P., Marcili A., Leite R.C., Nieri-Bastos F.A., Domingues L.N., Martins J.R., Labruna M.B. Coxiella symbiont in the tick Ornithodoros rostratus (Acari: argasidae) Ticks Tick Borne Dis. 2012;3:203–206. doi: 10.1016/j.ttbdis.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Anderson B.E., Dawson J.E., Jones D.C., Wilson K.H. Ehrlichia chaffeensis, a new species associated with human ehrlichiosis. J. Clin. Microbiol. 1991;29:2838–2842. doi: 10.1128/jcm.29.12.2838-2842.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J.F. The natural history of ticks. Med. Clin. 2002;86:205–218. doi: 10.1016/s0025-7125(03)00083-x. [DOI] [PubMed] [Google Scholar]
- Blevins S.M., Greenfield R.A., Bronze M.S. Blood smear analysis in babesiosis, ehrlichiosis, relapsing fever, malaria, and Chagas disease. Cleve. Clin. J. Med. 2008;75:521–530. doi: 10.3949/ccjm.75.7.521. [DOI] [PubMed] [Google Scholar]
- Boucher F., Moutroifi Y., Peba B., Ali M., Moindjie Y., Ruget A.S., Abdouroihamane S., Madi Kassim A., Soule M., Charafouddine O., Cetre-Sossah C., Cardinale E. Tick-borne diseases in the Union of the Comoros are a hindrance to livestock development: circulation and associated risk factors. Ticks Tick Borne Dis. 2020;11:101283. doi: 10.1016/j.ttbdis.2019.101283. [DOI] [PubMed] [Google Scholar]
- Cabezas-Cruz A., Zweygarth E., Vancova M., Broniszewska M., Grubhoffer L., Passos L.M.F., Ribeiro M.F.B., Alberdi P., de la Fuente J. Ehrlichia minasensis sp nov., isolated from the tick Rhipicephalus microplus. Int. J. Syst. Evol. Microbiol. 2016;66:1426–1430. doi: 10.1099/ijsem.0.000895. [DOI] [PubMed] [Google Scholar]
- Chen Z., Yang X., Bu F., Yang X., Yang X., Liu J. Ticks (acari: ixodoidea: argasidae, ixodidae) of China. Exp. Appl. Acarol. 2010;51:393–404. doi: 10.1007/s10493-010-9335-2. [DOI] [PubMed] [Google Scholar]
- de Castro M.B., Machado R.Z., de Aquino L.P., Alessi A.C., Costa M.T. Experimental acute canine monocytic ehrlichiosis: clinicopathological and immunopathological findings. Vet. Parasitol. 2004;119:73–86. doi: 10.1016/j.vetpar.2003.10.012. [DOI] [PubMed] [Google Scholar]
- El-Neweshy M.S., Al Mawly J.H., Aboollo S.H., El-Manakhly E.M. Natural Ehrlichia ruminantium infection in two captive Arabian tahrs (Arabitragus jayakari) in Oman. Trop. Anim. Health Prod. 2019;51:2539–2545. doi: 10.1007/s11250-019-01970-7. [DOI] [PubMed] [Google Scholar]
- Fishbein D.B. Springer; Netherlands: 1990. Human Ehrlichiosis in the United States. [Google Scholar]
- Gongora-Biachi R.A., Zavala-Velazquez J., Castro-Sansores C.J., Gonzalez-Martinez P. First case of human ehrlichiosis in Mexico. Emerg. Infect. Dis. 1999;5:481. doi: 10.3201/eid0503.990327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemi E.C., Orozco M.M., Argibay H.D., Farber M.D. Evidence of Ehrlichia chaffeensis in Argentina through molecular detection in marsh deer (Blastocerus dichotomus) Int J Parasitol-Par. 2019;8:45–49. doi: 10.1016/j.ijppaw.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H., Yin C., Galon E.M., Du J., Gao Y., Adjou Moumouni P.F., Liu M., Efstratiou A., Lee S.H., Li J., Ringo A.E., Wang G., Li Y., Tumwebaze M.A., Xuan X. Molecular survey and characterization of Theileria annulata and Ehrlichia ruminantium in cattle from Northwest China. Parasitol. Int. 2018;67:679–683. doi: 10.1016/j.parint.2018.06.011. [DOI] [PubMed] [Google Scholar]
- Hassan M., Sulaiman M.H., Lian C.J. The prevalence and intensity of Amblyomma javanense infestation on Malayan Pangolins (Manis javanica Desmarest) from Peninsular Malaysia. Acta Trop. 2013;126:142–145. doi: 10.1016/j.actatropica.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Heinrich S., Wittmann T.A., Prowse T.A.A., Ross J.V., Delean S., Shepherd C.R., Cassey P. Where did all the pangolins go? International CITES trade in pangolin species. Glob Ecol Conserv. 2016;8:241–253. [Google Scholar]
- Heppner D.G., Wongsrichanalai C., Walsh D.S., McDaniel P., Eamsila C., Hanson B., Paxton H. Human ehrlichiosis in Thailand. Lancet. 1997;350:785–786. doi: 10.1016/S0140-6736(05)62571-8. [DOI] [PubMed] [Google Scholar]
- Hua L.S., Gong S.P., Wang F.M., Li W.Y., Ge Y., Li X.N., Hou F.H. Captive breeding of pangolins: current status, problems and future prospects. ZooKeys. 2015:99–114. doi: 10.3897/zookeys.507.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabin G., Dewan Y., Khatri H., Singh S.K., Chandra K., Thakur M. Identifying the tick Amblyomma javanense (Acari: ixodidae) from Chinese pangolin: generating species barcode, phylogenetic status and its implication in wildlife forensics. Exp. Appl. Acarol. 2019;78:461–467. doi: 10.1007/s10493-019-00393-1. [DOI] [PubMed] [Google Scholar]
- Jiang B.G., Jia N., Jiang J.F., Zheng Y.C., Chu Y.L., Jiang R.R., Wang Y.W., Liu H.B., Wei R., Zhang W.H., Li Y., Xu X.W., Ye J.L., Yao N.N., Liu X.J., Huo Q.B., Sun Y., Song J.L., Liu W., Cao W.C. Borrelia miyamotoi infections in humans and ticks, northeastern China. Emerg. Infect. Dis. 2018;24:236–241. doi: 10.3201/eid2402.160378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keysary A., Amram L., Keren G., Sthoeger Z., Potasman I., Jacob A., Strenger C., Dawson J.E., Waner T. Serologic evidence of human monocytic and granulocytic ehrlichiosis in Israel. Emerg. Infect. Dis. 1999;5:775–778. doi: 10.3201/eid0506.990605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri-Chhetri R., Wang H.C., Chen C.C., Shih H.C., Liao H.C., Sun C.M., Khatri-Chhetri N., Wu H.Y., Pei K.J. Surveillance of ticks and associated pathogens in free-ranging Formosan pangolins (Manis pentadactyla pentadactyla) Ticks Tick Borne Dis. 2016;7:1238–1244. doi: 10.1016/j.ttbdis.2016.07.007. [DOI] [PubMed] [Google Scholar]
- Koh F.X., Kho K.L., Panchadcharam C., Sitam F.T., Tay S.T. Molecular detection of Anaplasma spp. in pangolins (Manis javanica) and wild boars (Sus scrofa) in Peninsular Malaysia. Vet. Parasitol. 2016;227:73–76. doi: 10.1016/j.vetpar.2016.05.025. [DOI] [PubMed] [Google Scholar]
- Kollars T.M., Jr., Sithiprasasna R. New host and distribution record of Amblyomma javanense (Acari: ixodidae) in Thailand. J. Med. Entomol. 2000;37:640. doi: 10.1603/0022-2585-37.4.640. [DOI] [PubMed] [Google Scholar]
- Kwak M.L., Hsu C.D., Douay G., Ahmad A.A. The first authenticated record of the pangolin tick Amblyomma javanense (Acari: ixodidae) in Singapore, with notes on its biology and conservation. Exp. Appl. Acarol. 2018;76:551–557. doi: 10.1007/s10493-018-0310-7. [DOI] [PubMed] [Google Scholar]
- Liu P., Chen W., Chen J.P. 2019. Viral Metagenomics Revealed Sendai Virus and Coronavirus Infection of Malayan Pangolins (Manis Javanica). Viruses 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mediannikov O., Davoust B., Socolovschi C., Tshilolo L., Raoult D., Parola P. Spotted fever group rickettsiae in ticks and fleas from the Democratic Republic of the Congo. Ticks Tick-Borne Dis. 2012;3:370–372. doi: 10.1016/j.ttbdis.2012.10.015. [DOI] [PubMed] [Google Scholar]
- Mihalca A.D., Gherman C.M., Cozma V. Coendangered hard-ticks: threatened or threatening? Parasites Vectors. 2011;4 [Google Scholar]
- Mohapatra R.K., Panda S., Nair M.V., Acharjyo L.N. Check list of parasites and bacteria recorded from pangolins (Manis sp.) J. Parasit. Dis. 2016;40:1109–1115. doi: 10.1007/s12639-015-0653-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi N.C. vol. 4. 1981. (Amblyomma Javanense (Supino, 1897) from a New Host, Manis Crassicaudata Gray Together with a Description of its Nymph from Goa, India). [Google Scholar]
- Njiokou F., Laveissere C., Simo G., Nkinin S., Grebaut P., Cuny G., Herder S. Wild fauna as a probable animal reservoir for Trypanosoma brucei gambiense in Cameroon. Infect. Genet. Evol. 2006;6:147–153. doi: 10.1016/j.meegid.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Norris D.E., Klompen J.S., Keirans J.E., Black W.C.t. Population genetics of Ixodes scapularis (Acari: ixodidae) based on mitochondrial 16S and 12S genes. J. Med. Entomol. 1996;33:78–89. doi: 10.1093/jmedent/33.1.78. [DOI] [PubMed] [Google Scholar]
- Nuti M., Serafini D.A., Bassetti D., Ghionni A., Russino F., Rombola P., Macri G., Lillini E. Ehrlichia infection in Italy. Emerg. Infect. Dis. 1998;4:663–665. doi: 10.3201/eid0404.980420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddock C.D., Childs J.E. Ehrlichia chaffeensis: a prototypical emerging pathogen. Clin. Microbiol. Rev. 2003;16:37–64. doi: 10.1128/CMR.16.1.37-64.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson C.M., O'Brien A., Albers T.M., Simon M.A., Clifford C.B., Pritchett-Corning K.R. Diagnostic necropsy and selected tissue and sample collection in rats and mice. Jove-J Vis Exp. 2011 doi: 10.3791/2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parola P., Cornet J.P., Sanogo Y.O., Miller R.S., Thien H.V., Gonzalez J.P., Raoult D., Telford I.S., Wongsrichanalai C. Detection of Ehrlichia spp., Anaplasma spp., Rickettsia spp., and other eubacteria in ticks from the Thai-Myanmar border and Vietnam. J. Clin. Microbiol. 2003;41:1600–1608. doi: 10.1128/JCM.41.4.1600-1608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parola P., Cornet J.P., Sanogo Y.O., Miller R.S., Thien H.V., Gonzalez J.P., Raoult D., Telford S.R., Wongsrichanalai C. Detection of Ehrlichia spp., Anaplasma spp., Rickettsia spp., and other eubacteria in ticks from the Thai-Myanmar border and Vietnam. J. Clin. Microbiol. 2003;41:1600–1608. doi: 10.1128/JCM.41.4.1600-1608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter S.G., Gakuya D.W., Maingi N., Mulei C.M. Prevalence and risk factors associated with Ehrlichia infections in smallholder dairy cattle in Nairobi City County, Kenya. Vet. World. 2019;12:1599–1607. doi: 10.14202/vetworld.2019.1599-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reye A.L., Arinola O.G., Hubschen J.M., Muller C.P. Pathogen prevalence in ticks collected from the vegetation and livestock in Nigeria. Appl. Environ. Microbiol. 2012;78:2562–2568. doi: 10.1128/AEM.06686-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloboda M., Jirku M., Lukesova D., Qablan M., Batsukh Z., Fiala I., Horin P., Modry D., Lukes J. A survey for piroplasmids in horses and Bactrian camels in North-Eastern Mongolia. Vet. Parasitol. 2011;179:246–249. doi: 10.1016/j.vetpar.2011.01.064. [DOI] [PubMed] [Google Scholar]
- Tumwebaze M.A., Byamukama B., Tayebwa D.S., Byaruhanga J., Angwe M.K., Galon E.M., Liu M.M., Lee S.H., Ringo A.E., Moumouni P.F.A., Li J.X., Li Y.C., Ji S.W., Vudriko P., Xuan X.N. First molecular detection of Babesia ovis, Theileria spp., Anaplasma spp., and Ehrlichia ruminantium in goats from western Uganda. Pathogens. 2020;9 doi: 10.3390/pathogens9110895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhaa I.J., MacLean J.D., Greene C.R., Fishbein D.B. A case of human ehrlichiosis acquired in Mali: clinical and laboratory findings. Am. J. Trop. Med. Hyg. 1992;46:161–164. doi: 10.4269/ajtmh.1992.46.161. [DOI] [PubMed] [Google Scholar]
- Vesco U., Knap N., Labruna M.B., Avsic-Zupanc T., Estrada-Pena A., Guglielmone A.A., Bechara G.H., Gueye A., Lakos A., Grindatto A., Conte V., De Meneghi D. An integrated database on ticks and tick-borne zoonoses in the tropics and subtropics with special reference to developing and emerging countries. Exp. Appl. Acarol. 2011;54:65–83. doi: 10.1007/s10493-010-9414-4. [DOI] [PubMed] [Google Scholar]
- Voltzit O.V., Keirans J.E.J.A. vol. 11. 2003. pp. 135–214. (A Review of Asian Amblyomma Species (Acari, Ixodida, Ixodidae)). [Google Scholar]
- Wang S.L., Tu Y.C., Lee M.S., Wu L.H., Chen T.Y., Wu C.H., Tsao E.H.S., Chin S.C., Li W.T. Fatal canine parvovirus-2 (CPV-2) infection in a rescued free-ranging Taiwanese pangolin (Manis pentadactyla pentadactyla) Transboundary and Emerging Diseases. 2020 doi: 10.1111/tbed.13469. [DOI] [PubMed] [Google Scholar]
- Widmer C.E., Azevedo F.C., Almeida A.P., Ferreira F., Labruna M.B. Tick-borne bacteria in free-living jaguars (Panthera onca) in Pantanal, Brazil. Vector Borne Zoonotic Dis. 2011;11:1001–1005. doi: 10.1089/vbz.2011.0619. [DOI] [PubMed] [Google Scholar]
- Xiao K.P., Zhai J.Q., Feng Y.Y., Zhou N., Zhang X., Zou J.J., Li N., Guo Y.Q., Li X.B., Shen X.J., Zhang Z.P., Shu F.F., Huang W.Y., Li Y., Zhang Z.D., Chen R.A., Wu Y.J., Peng S.M., Huang M., Xie W.J., Cai Q.H., Hou F.H., Chen W., Xiao L.H., Shen Y.Y. Isolation of SARS-CoV-2-related coronavirus from Malayan pangolins. Nature. 2020;583:286. doi: 10.1038/s41586-020-2313-x. [DOI] [PubMed] [Google Scholar]
- Yu S.R.N., Modarelli J., Tomecek J.M., French J.T., Hilton C., Esteve-Gasent M.D. Prevalence of common tick-borne pathogens in white-tailed deer and coyotes in south Texas. Int J Parasitol-Par. 2020;11:129–135. doi: 10.1016/j.ijppaw.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Chang Z.Y., Mehmood K., Wang Y.J., Rehman M.U., Nabi F., Sabir A.J., Liu X.T., Wu X.X., Tian X.X., Zhou D.H. First report of Ehrlichia infection in goats, China. Microb. Pathog. 2017;110:275–278. doi: 10.1016/j.micpath.2017.07.012. [DOI] [PubMed] [Google Scholar]
- Zou Y., Tu Y., Feng M.X., Wei D.X., Lei C.Z. Molecular diagnosis and insect repellent test for ticker disease of a cattle farm in Guanling autonomous county. Journal of Animal Science and Veterinary Medicine. 2018;37:89–91. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.