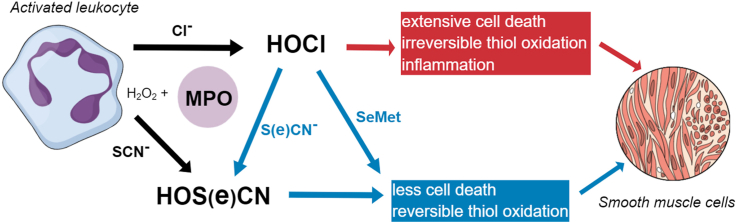
Abstract
The production of hypochlorous acid (HOCl) by myeloperoxidase (MPO) plays a key role in immune defense, but also induces host tissue damage, particularly in chronic inflammatory pathologies, including atherosclerosis. This has sparked interest in the development of therapeutic approaches that decrease HOCl formation during chronic inflammation, including the use of alternative MPO substrates. Thiocyanate (SCN−) supplementation decreases HOCl production by favouring formation of hypothiocyanous acid (HOSCN), which is more selectively toxic to bacterial cells. Selenium-containing compounds are also attractive therapeutic agents as they react rapidly with HOCl and can be catalytically recycled. In this study, we examined the ability of SCN−, selenocyanate (SeCN−) and selenomethionine (SeMet) to modulate HOCl-induced damage to human coronary artery smooth muscle cells (HCASMC), which are critical to both normal vessel function and lesion formation in atherosclerosis. Addition of SCN− prevented HOCl-induced cell death, altered the pattern and extent of intracellular thiol oxidation, and decreased perturbations to calcium homeostasis and pro-inflammatory signaling. Protection was also observed with SeCN− and SeMet, though SeMet was less effective than SeCN− and SCN−. Amelioration of damage was detected with sub-stoichiometric ratios of the added compound to HOCl. The effects of SCN− are consistent with conversion of HOCl to HOSCN. Whilst SeCN− prevented HOCl-induced damage to a similar extent to SCN−, the resulting product hyposelenocyanous acid (HOSeCN), was more toxic to HCASMC than HOSCN. These results provide support for the use of SCN− and/or selenium analogues as scavengers, to decrease HOCl-induced cellular damage and HOCl production at inflammatory sites in atherosclerosis and other pathologies.
Keywords: Myeloperoxidase, Thiocyanate, Selenocyanate, Selenomethionine, Inflammation, Atherosclerosis
Graphical abstract
Highlights
-
•
HOCl induces extensive smooth muscle cell death and irreversible thiol oxidation.
-
•
Addition of SCN− decreases the extent of HOCl-induced cell damage.
-
•
SeCN− has similar protective effects to SCN− towards HOCl-induced cell damage.
-
•
HOSeCN is less toxic than HOCl but more damaging than HOSCN.
-
•
SeMet modulates HOCl-induced damage but less effectively than SCN− or SeCN−.
Abbreviations
- Br−
bromide
- Ca2+
calcium
- Cl−
chloride
- GSH
glutathione
- GSSG
glutathione disulfide
- GPx
glutathione peroxidase
- HCASMC
human coronary artery smooth muscle cells
- H2O2
hydrogen peroxide
- HOBr
hypobromous acid
- HOCl
hypochlorous acid
- HOI
hypoiodous acid
- HOSCN
hypothiocyanous acid
- HOSeCN
hyposelenocyanous acid
- I−
iodide
- LPO
lactoperoxidase
- MPO
myeloperoxidase
- Nrf2
nuclear factor erythroid 2–related factor 2
- SeCN−
selenocyanate
- SeMet
selenomethionine
- SeMetO
selenomethionine selenoxide
- SCN−
thiocyanate
- SMC
smooth muscle cells
- TrxR
thioredoxin reductase
- VSMC
vascular smooth muscle cells
1. Introduction
Hypochlorous acid (HOCl) is a potent oxidant produced by the heme enzyme myeloperoxidase (MPO), which is released by activated neutrophils and related immune cells at sites of inflammation [1,2]. The production of HOCl is a key innate immune defense against bacteria and other invading pathogens [3,4]. However, the excessive production of HOCl during chronic inflammation promotes host tissue damage, which is strongly linked to the progression of many inflammatory diseases [2,4], including atherosclerosis [5,6]. Thus, MPO is elevated in human atherosclerotic plaques [7], and serves as both a risk factor for the development of coronary artery disease and a prognostic marker for severity and survival following a myocardial infarction [[8], [9], [10]]. Vascular smooth muscle cells are an important target for HOCl, highlighted by the ability of MPO to promote the weakening of the fibrous cap and plaque rupture (reviewed [6]).
Evidence for the role of MPO as a driver of chronic inflammatory disease, has raised interest in the development of therapies targeting MPO to modulate its activity, and reduce the formation of HOCl in vivo [2,11,12]. MPO inhibitors could be beneficial in reducing detrimental outcomes in acute situations, such as in high-risk coronary artery disease patients, on the basis of studies showing that the irreversible MPO inhibitor AZM198, increased fibrous cap thickness in unstable plaques [13]. However, the implications of long-term MPO inhibition on innate immunity are not fully understood. Therefore, alternative strategies to limit HOCl production without preventing bacterial cell killing and hence compromising the immune system, could be highly beneficial.
MPO forms HOCl by catalyzing the reaction of hydrogen peroxide (H2O2) with chloride (Cl−) ions, which are highly abundant in plasma (100–140 mM) [[1], [2], [3], [4]]. MPO can also utilize bromide (Br−), iodide (I−) and the pseudo-halide, thiocyanate (SCN−) to form hypobromous acid (HOBr), hypoiodous acid (HOI) and hypothiocyanous acid (HOSCN), respectively, in a manner dependent on the relative concentrations of each halide ion [14]. MPO has a higher specificity constant for SCN− compared to Cl− by a factor of 730 [15]. For this reason, increased concentrations of SCN− favour the formation of HOSCN at the expense of HOCl [[14], [15], [16]]. Additionally, SCN− can react directly with HOCl and related N-chloramines, which are reactive secondary products of this oxidant, to form HOSCN [17,18]. It has been proposed that supplementation with SCN− could be a potential therapeutic strategy, as HOSCN is both bactericidal, and appears to be less toxic to host cells than bacteria [16]. This may be due to the selectivity and reversibility of HOSCN-mediated damage, which occurs primarily with thiols [19,20], and a higher capacity for repair in mammalian cells [[21], [22], [23]]. Thus, supplementation with SCN− has protective effects in vitro [16,23,24] and in vivo, in models of cystic fibrosis [25] and atherosclerosis [26,27]. It is presumed that the protective effects are related to the ability of SCN− to modulate HOCl production by MPO, but the underlying mechanisms responsible for these effects have not been clearly defined.
Supplementation with selenium-containing compounds can also be beneficial in the context of cardiovascular disease [28] which is, at least in part, related to the upregulation of endogenous antioxidant selenoproteins, including glutathione peroxidase (GPx) and thioredoxin reductase (TrxR) [29,30]. Selenium can also modulate the inflammatory response, and reduce endothelial dysfunction in atherosclerosis [31,32]. Selenium-containing compounds, including selenomethionine (SeMet), react rapidly with HOCl [33,34] giving the corresponding selenoxide that can be catalytically recycled [35], and thereby diminishing HOCl-induced damage [32,36]. In addition, the selenium analogue of SCN−, selenocyanate (SeCN−), can be metabolized by lactoperoxidase (LPO) to give an antimicrobial agent, which is presumed to be hyposelenocyanous acid (HOSeCN) [37]. HOSeCN has been reported to have more potent antibacterial activity than HOSCN, and also showed selective toxicity to bacterial cells, which was attributed to its detoxification by mammalian, but not bacterial, TrxR [37]. The redox potentials of HOCl and SeCN− indicate that direct oxidation of SeCN− by HOCl is favourable [38], and likely to be more rapid than for SCN−, on the basis of other kinetic data for selenium-versus sulfur-containing compounds [33].
In this study, we examined the ability of SCN−, SeCN− and SeMet to influence HOCl-induced damage to vascular smooth muscle cells (VSMC), which have an important role in the development and progression of atherosclerosis and maintaining the integrity of the fibrous cap [39]. We focus on how SCN−, SeCN− and SeMet affect the extent of HOCl-induced cell death, oxidation of intracellular thiols and expression of genes relating to inflammation and VSMC phenotype. We also examined the reactivity of HOSeCN with SMC for the first time. Together, these data provide insights into the pathways responsible for SCN−-mediated cellular protection during chronic inflammation. We also demonstrate that SeCN− could be an alternative approach to combat HOCl-induced inflammatory damage, while preserving the antimicrobial activity of MPO in innate immunity.
2. Materials and methods
2.1. Reagents and chemicals
All buffers and solutions were prepared with nanopure water (npH2O), filtered through a four stage Milli-Q system. All chemicals were from Sigma-Aldrich/Merck (St. Louis, MI, USA) unless stated otherwise. Sodium hypochlorite (10–15% w/v) was purchased from Acros Organics (Acros Organics, Waltham, MA, USA) and was standardized by measuring the absorbance at 292 nm at pH 11, using an extinction coefficient of 350 M−1 cm−1 [40]. HOSeCN was generated enzymatically using LPO and H2O2 as described previously [37]. HOSeCN was kept on ice and used immediately after rapid quantification by measuring the conversion of 5-thio-2-nitrobenzoic acid (TNB) to 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) at 412 nm as previously [41], using an extinction coefficient of 14,150 M−1 cm−1 [42].
2.2. Cell culture
Human coronary artery smooth muscle cells (HCASMC) were purchased from Cell Applications (San Diego, CA, USA). Experiments were performed on different days with cells from at least 3 individual donors (Table S1), and used between passages 2 and 5 unless stated otherwise. The cells were cultured under sterile conditions in HCASMC growth medium (Cell Applications) at 37 °C in a 5% CO2 incubator. The cells were seeded in 96-, 12- or 6-well plates at a density of 1 × 105 cells mL−1 using volumes of 100 μL, 1 mL or 2 mL respectively, and were allowed to adhere overnight. Prior to the treatments, cells were washed with warm (37 °C) Hanks’ buffered salt solution (HBSS) to prevent reactions of the medium with the hypohalous acids. HCASMC were exposed to HOCl in HBSS for 15 min without or with the addition of increasing concentrations of SCN−, SeCN− or SeMet (Carbolution Chemicals GmbH, Germany), at a constant ratio of oxidant per cell, accounting for the different well sizes.
2.3. Viability
Metabolic activity was assessed as a measure of viability using a commercial reagent MTS (CellTiter 96® AQueous One Solution Assay, Promega, WI, USA). Cells in 96-well tissue culture plates (1 × 104 cells/well) were exposed to 100 μL of 100 μM HOCl or 100 μM HOCl containing SCN−, SeCN− or SeMet (10–400 μM) for 15 min. After incubation, the treatment solution was aspirated and the cells were washed with warm HBSS. The metabolic activity was measured directly after treatment or following the re-culture of the cells in growth medium for 24 h. Cells were incubated with cell growth medium (100 μL) containing MTS reagent (10 μL), for 4 h at 37 °C, before measuring the change in absorbance at 490 nm, using a microplate reader (Spectra Max M, Molecular Devices, San Jose, CA, USA). Experiments were also performed with SeCN− and SeMet (25–1000 μM), and HOSeCN (0–100 μM).
2.4. Flow cytometry analysis
HCASMC (2 × 105 cells/well in 6-well plates) were treated with HOCl (100 μM, 2 mL) in the absence or presence of SCN− (0–100 μM) for 15 min before assessing cell viability using an APC Annexin V Apoptosis Detection Kit with propidium iodide (PI) (BioLegend, San Diego, CA, USA). Briefly, after treatment the cells were washed with HBSS and incubated in fresh cell media for 24 h. After this re-incubation period, the cells were harvested using trypsin/EDTA solution (0.025% trypsin, 0.01% EDTA, in PBS), re-suspended in binding buffer containing APC Annexin V and PI, incubated in the dark at 22 °C for 30 min and analyzed with a FACSVerse™ flow cytometer (BD, Franklin Lakes, NJ, USA), with 15000 events counted per sample. Changes in cytosolic calcium were measured using the cell permeant Fluo-4AM probe (Thermo Fisher Scientific, Waltham, MA, USA). Directly after the treatment period, the cells were washed with HBSS (Ca2+-free) to remove any residual oxidant and Ca2+ from the buffer solution, and were incubated for 45 min with 5 μM Fluo-4AM in Ca2+-free HBSS (in the dark, at 37 °C). The cells were then carefully scraped from the tissue culture plates and were analyzed by flow cytometry.
2.5. Quantification of cell thiols
HCASMC (1 × 105 cells/well in 12-well plates) were exposed to HOCl (100 μM) in the absence or presence of SCN−, SeCN− or SeMet or HOSeCN (0–200 μM) for 15 min before measuring the concentration of intracellular thiols immediately after the treatment, or following re-incubation in growth medium for 24 h, using the ThioGlo 1 reagent (Berry & Associates Inc, Dexter, MI, USA) as described previously [41]. The concentration of thiols was determined using a GSH standard curve, and normalized to the protein concentration determined using the Pierce BCA Protein Assay (Thermo Fisher Scientific, Waltham, MA, USA).
2.6. Determination of GSH/GSSG
HCASMC (1 × 105 cells/well in 12-well plates) were treated with HOCl or HOCl/SCN− for 15 min, with and without re-incubation in cell media for 24 h. The ratio of reduced GSH and oxidized glutathione (GSSG) was quantified by using a GSH/GSSG-Glo™ Assay Kit (Promega, WI, USA). The samples were prepared according to the manufacturer's manual, and the change in luminescence recorded using a microplate reader (Spectra Max i3, Molecular Devices, Sunnyvale, CA, USA).
2.7. Quantitative real-time polymerase chain reaction (qPCR)
HCASMC (2 × 105 cells/well in 6-well plates) were exposed to HOCl (50 μM) in the absence or presence of SCN−, SeCN− or SeMet (100 μM) for 15 min before washing and re-incubating the cells in media for 24 h. RNA extraction was completed using a RNAeasy® Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions, including a DNase digestion step (RNase-Free DNase Set, Qiagen). After the quantification of RNA concentration, cDNA synthesis was achieved using the SensiFAST cDNA Synthesis Kit (Bioline, London, UK) following the manufacturer's manual. The qPCR analysis was performed using a SensiFAST SYBR Hi-ROX Kit (Bioline) and the following conditions: 95 °C for 30 s, 60 °C for 30 s, 72 °C for 30 s for 39 cycles, followed by dissociation curve analysis. The data were normalized to two housekeeping genes, 18S ribosomal RNA and beta-2-microglobulin (B2M), and expressed as a fold change compared to the control cells. Housekeeping and target gene primer sequences, are listed in Table S2.
2.8. Quantification of SeCN− and SeMet by HPLC-ICP-MS
HCASMC (2 × 105 cells/well in 6-well plates) were exposed to increasing concentrations of SeCN− and SeMet for 15 min, followed by washing and harvesting with trypsin/EDTA solution (0.025% trypsin, 0.01% EDTA, in PBS). The cell suspension was centrifuged for 5 min (5000 g at 4 °C), before collecting the media, washing the cells, and lysing with 20 mM Tris-HCl (pH 7.5) for 30 min at 4 °C. Cell lysates were collected after centrifugation for 10 min (10000 g at 4 °C) before quantification of SeCN− and SeMet by HPLC and inductively coupled plasma mass spectrometry (ICP-MS). Lysates and media samples were diluted with mobile phase containing 200 mM ammonium acetate in 5% (v/v) methanol (pH 7). Samples were analyzed with a Dionex Ultimate 3000 UPLC system (Thermo Scientific) with a Gemini C18 (5 μm, 250 × 2 mm) column (Phenomenex) and a flow rate of 200 μL min−1. The UPLC system was coupled to an Agilent 8800 ICP Triple Quadrupole MS equipped with a 1.5 mm ID quartz injector torch, with sampler and skimmer cones made of platinum (Agilent Technologies, Santa Clara, USA). The system was optimized using a Se-standard solution (50 μg L−1 Se in mobile phase), and the analyses were carried out in MS/MS mode with oxygen as the reaction gas (monitoring 77Se → 93SeO and 80Se → 96SeO).
2.9. Statistical analyses
Statistical analysis was performed using GraphPad Prism 8 (GraphPad Software, San Diego, USA) using an ANOVA test with p < 0.05 taken as significant. Details of the specific statistical tests are given in the figure legends. Data are shown as mean ± SEM from triplicate determinations from 3 individual cell donors, unless otherwise stated in the figure legends.
3. Results
3.1. SCN− prevents HOCl-induced loss in metabolic activity and viability
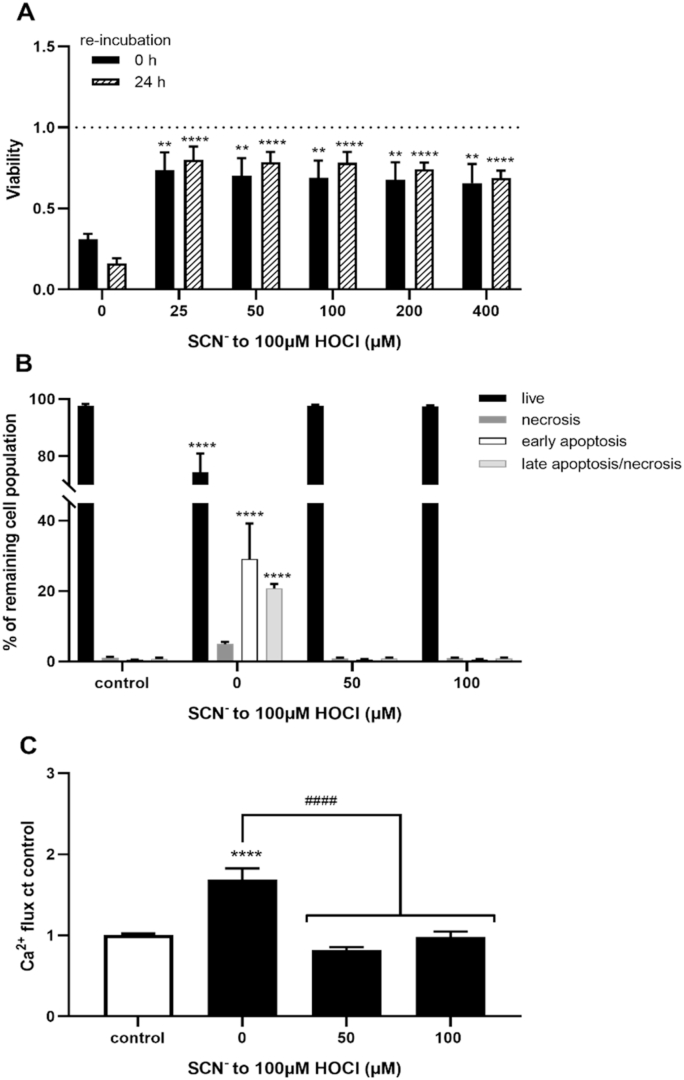
Initial studies were performed to assess whether SCN− could prevent HOCl-induced cell death using the MTS assay to assess changes in metabolic activity. HCASMC were treated with HOCl (100 μM), or HOCl and SCN− (25–400 μM), for 15 min, before measuring their metabolic activity, over a 4 h incubation period with the MTS reagent. Exposure of the cells to HOCl resulted in a significant loss in metabolic activity, which was prevented by the presence of SCN− on addition to HOCl (Fig. 1A, black bars). This protective effect of SCN− was apparent even with sub-stoichiometric amounts (25 and 50 μM) of SCN− (Fig. 1A). Similar results were observed in experiments where the cells were washed and re-incubated in growth media for 24 h (at 37 °C, 5% CO2) after the initial oxidant exposure (Fig. 1A, dashed bars), to assess possible effects on signaling and survival pathways. No protective effect of SCN− on the loss of metabolic activity induced by HOCl (100 μM) was observed when HCASMC were pre-incubated with SCN− (25–100 μM in HBSS; Fig. S1), before washing and exposure to the oxidant. Similar results were obtained in experiments with a lower concentration of HOCl (50 μM; Fig. S1).
Fig. 1.
Prevention of HOCl-induced cell death and intracellular Ca2+accumulation by SCN−. HCASMC were exposed to 100 μM HOCl with addition of increasing concentrations of SCN− for 15 min. (A) The changes in metabolic activity were assessed by MTS assay directly after treatment (black bars) and after treatment with re-incubation for 24 h in growth media (dashed bars) and expressed as a fold change compared to control cells. Data in (B) show the cell populations 24 h post-treatment, following a washing step which removes any non-adherent, dead cells resulting from the initial HOCl exposure, assessed using APC Annexin V/PI cell death kit and flow cytometry analysis. Percentage of live cell population (A-/PI-, black bars), of necrotic (A-/PI+, dark grey bars), early apoptotic (A+/PI-, white bar) and late apoptotic/necrotic (A+/PI+, light grey bar) cell populations. (C) Accumulation of intracellular Ca2+ in HCASMC immediately after the treatment with flow cytometry and staining with Fluo-4AM. Data represent the geometric means as a fold change compared to control. Statistical significance indicated by **p < 0.01, ****p < 0.0001, ####p < 0.0001 by two-way ANOVA with Dunnett's post hoc testing. In (A) the asterisks indicate significance compared to HOCl treatment alone, in (B–C) asterisks indicate significance compared to the non-treated control, and # indicates significance compared to the HOCl treatment.
HCASMC viability 24 h post-treatment was examined further by flow cytometry and staining with APC Annexin V and PI to evaluate whether SCN− could influence the extent and/or pathway involved in cell death following prolonged incubation. The cells were exposed to HOCl (100 μM) in the absence and presence of SCN− (50 and 100 μM) for 15 min, before washing and re-incubation in growth medium for 24 h (37 °C, 5% CO2). After the treatment, the cell media and any non-adherent cells were removed, and the remaining cell population was analyzed 24 h post-treatment, to evaluate effects from possible activation of signaling/survival pathways (Fig. 1B and S2). A significant decrease in the live cell population that remained after the initial HOCl treatment, together with a corresponding increase in the cell population stained with either/or Annexin V and PI (Fig. 1B), was observed in the absence of SCN−. These data are consistent with the induction of apoptotic cell death, with a low percentage of the cells found to be stained only with PI consistent with necrosis (Fig. 1B). It is important to note that these data do not reflect the total extent of cell death induced by HOCl, as the washing step before the re-incubation period removes non-adherent cells. Our previous studies show that the non-adherent cells resulting from exposure of HCASMC to HOCl are predominantly necrotic, staining positively with PI (Flouda et al., submitted). In contrast, there was no evidence for cell death by apoptosis or necrosis on co-treatment of the cells with HOCl and SCN−, or on treatment with SCN− alone (Fig. 1B and S2). This suggests that SCN− prevents the induction of apoptosis seen on re-incubation of the population of HCASMC that survive the initial exposure to HOCl. These studies were extended to examine the effect of SCN− on HOCl-induced enhancement of intracellular Ca2+ concentrations, which is often associated with the induction of programmed cell death. Exposure of HCASMC to HOCl resulted in an increase in intracellular Ca2+, which was not observed on co-treatment of the cells with HOCl and SCN− (Fig. 1D and S3).
3.2. Influence of SCN− on the extent and nature of HOCl-induced thiol oxidation
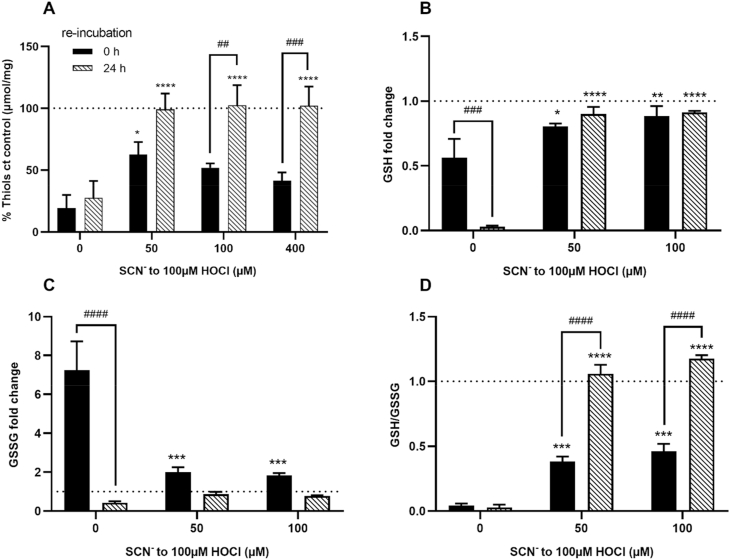
It has been established previously that HOCl reacts with protein thiols, resulting in thiol oxidation and a decrease of intracellular GSH in multiple different cell types, including neutrophils, macrophages and endothelial cells [[43], [44], [45], [46]]. To examine the effects of SCN− on HOCl-induced thiol oxidation in HCASMC, cells were exposed to HOCl (100 μM) without and with increasing concentrations of SCN− (50–400 μM) for 15 min. The total cell thiol concentration was quantified by the ThioGlo 1 reagent and was normalized to the cell protein concentration, directly after oxidant exposure, and after re-incubation of cells in growth medium for 24 h, to assess possible reversible modifications. HOCl treatment induced a significant decrease in cellular thiols compared to the non-treated, control cells, both when analyzed immediately after treatment, and also following 24 h re-incubation in growth media (Fig. 2A). There was no significant difference in the extent of thiol loss seen in cells exposed to HOCl for 15 min compared to cells re-incubated for 24 h, consistent with non-reversible thiol oxidation. The extent of thiol loss observed immediately following co-treatment with HOCl and SCN− was dependent on the concentration of SCN−, with a significant prevention of thiol oxidation seen at concentrations ≤50 μM SCN− (Fig. 2A, black bars). This is consistent with the formation of HOSCN at higher concentrations of SCN−, which also reacts with thiols [21]. However, the presence of SCN− (50–400 μM) resulted in a more marked and significant restoration of thiol concentration when analyzed 24 h after the initial treatment of the cells with HOCl, which was comparable to that seen in the non-treated, control cells (Fig. 2A, dashed bars). This indicates that the thiol oxidation seen on co-treatment of cells with HOCl and SCN− is reversible, which is again consistent with formation of HOSCN (Flouda et al., submitted).
Fig. 2.
Modulation of HOCl-induced thiol oxidation and GSH/GSSG by SCN−in HCASMC. HCASMC were exposed to 100 μM HOCl with and without addition of SCN− for 15 min. Data in (A) show the total intracellular thiol concentration as determined by the ThioGlo1 assay, immediately following treatment (black bars) or following washing and re-incubation for 24 h (dashed bars) in growth media. Data are normalized to cell protein concentration and expressed as a percentage compared to (ct) the controls. Data in (B), (C) and (D) show the GSH, GSSG and GSH/GSSG ratio, respectively as measured using the GSH/GSSG-Glo™ Assay Kit. GSH and GSSG were measured immediately following the treatments (black bars) and after 24 h re-incubation in cell media (dashed bars) and are expressed as a fold change compared to the non-treated control cells. Statistical significance indicated by *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001, ##p < 0.01, ###p < 0.0001 and ####p < 0.0001 by two-way ANOVA with Dunnett's post hoc testing. Asterisks indicate significance compared to HOCl treatment alone, and # indicates significance between the immediate analysis data and the 24 h re-incubation data.
These studies were extended to examine the effect of SCN− addition on the concentration of intracellular GSH. Exposure of HCASMC to HOCl (100 μM for 15 min) resulted in a decrease in GSH (Fig. 2B), an increase in GSSG (Fig. 2C), and a corresponding change in the ratio of GSH:GSH when compared to control cells (Fig. 2D). These changes were less extensive in the presence of SCN−. A greater extent of GSH loss was apparent in the cells 24 h following the initial treatment with HOCl in the absence of SCN− (Fig. 2B). However, the amount of GSSG decreased (Fig. 2C), resulting in a comparable GSH:GSSG ratio between the 2 incubation conditions (Fig. 2D). This could reflect the formation of other oxidation products, such as glutathione sulfonamide or cell death, as the assay was not normalized to total protein concentration. With SCN−, there was no further change in GSH concentration, though the ratio of GSH:GSSG increased, owing to a decrease in GSSG to levels comparable to the non-treated control cells (Fig. 2B–D).
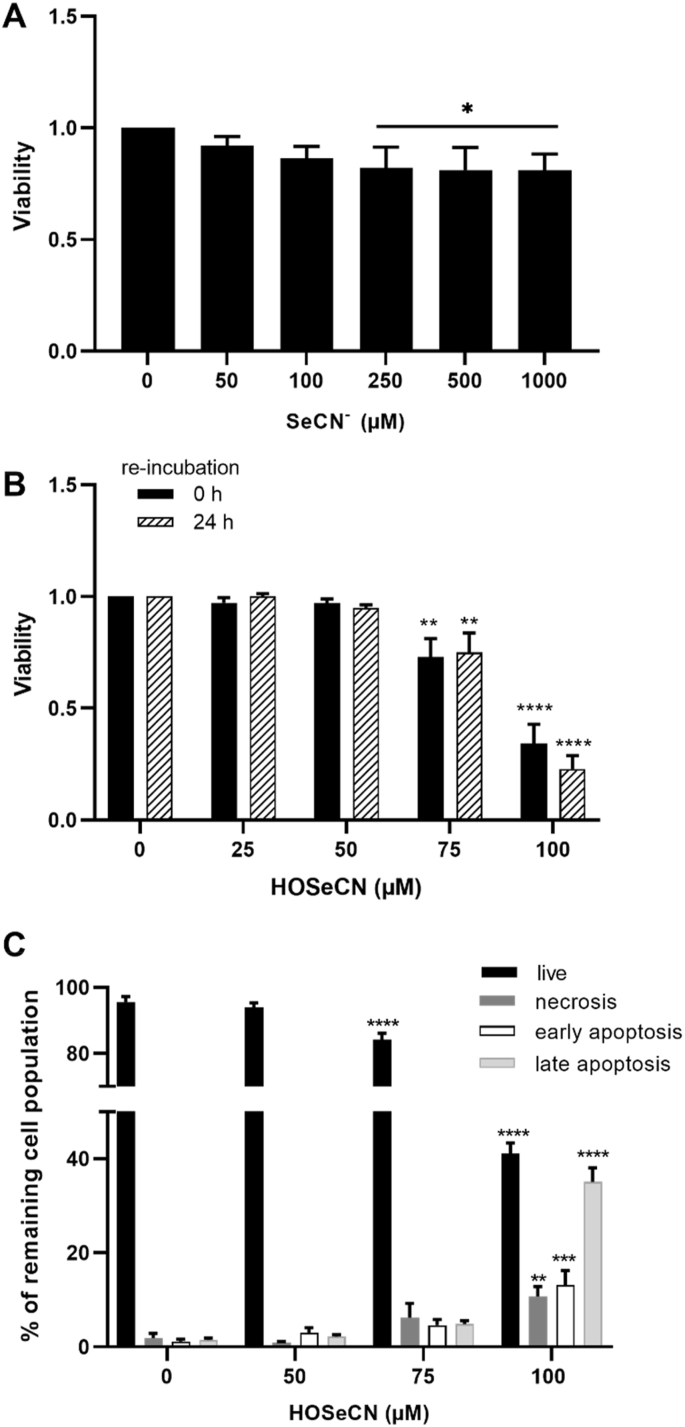
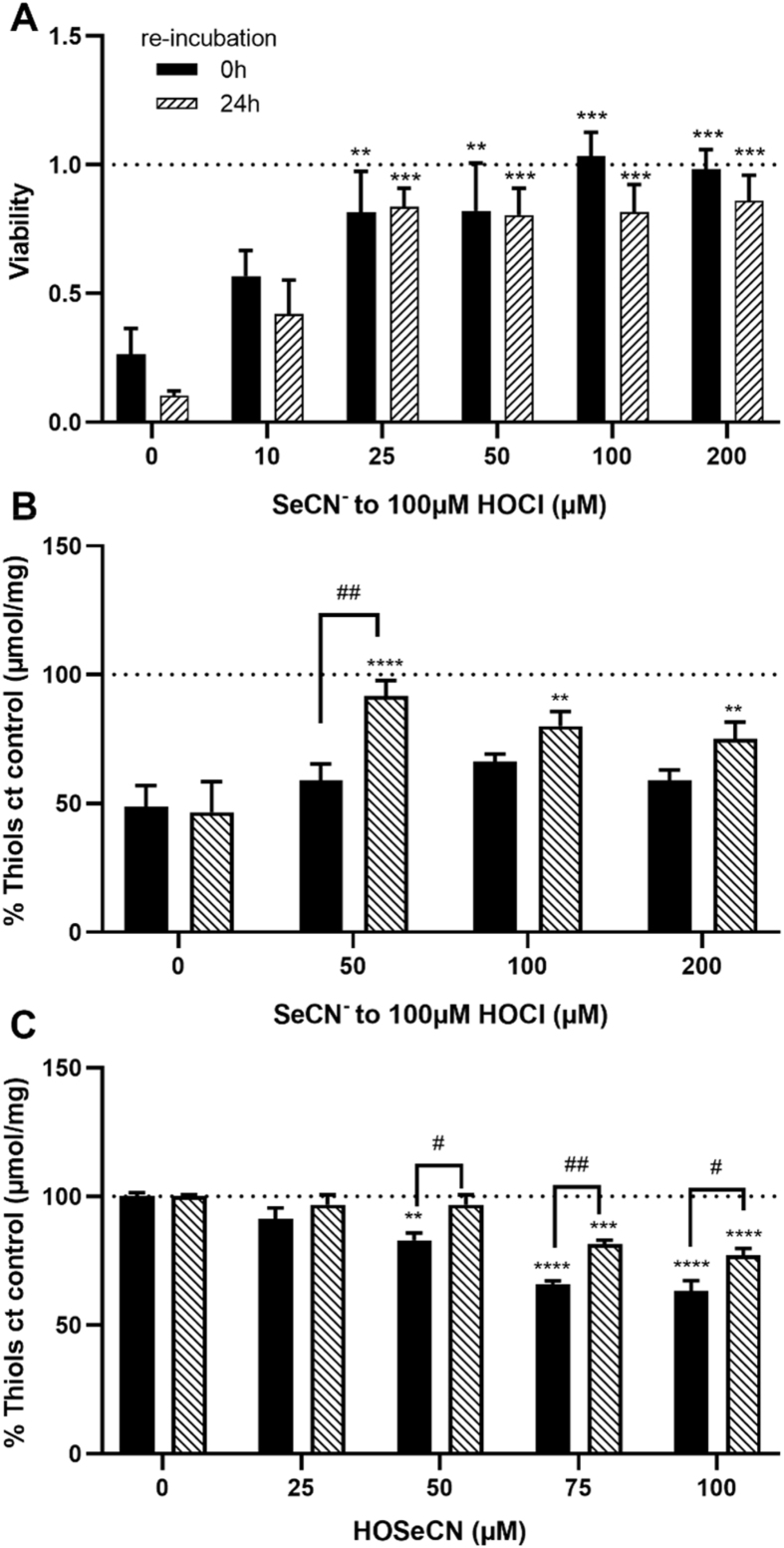
3.3. Effect of SeCN− and HOSeCN on cell viability
Initial studies were performed to assess the cytotoxicity of SeCN− alone, in the absence of HOCl. Exposure of HCASMC to increasing concentrations of SeCN− (50 μM–1 mM) for 24 h in growth medium resulted in a small decrease (10–15%) in metabolic activity with >250 μM SeCN− compared to the control cells (Fig. 3A). Subsequent studies examined the reactivity of the SeCN−-derived oxidant, HOSeCN, with HCASMC. HCASMC were exposed to increasing concentrations of HOSeCN (25–100 μM) for 1 h, with and without re-incubation for 24 h in growth media, with metabolic activity assessed using the MTS assay. A significant decrease in metabolic activity was observed at concentrations of HOSeCN ≥75 μM, both after 1 h, and with 24 h re-incubation (Fig. 3B). Flow cytometry experiments using Annexin V and PI on cells treated in a similar manner, showed a dose-dependent loss in the live cells in the remaining population (Fig. 3C, black bars), and a corresponding increase in cell populations stained positively with PI and Annexin V (Fig. 3C).
Fig. 3.
Cell viability of HCASMC after exposure to SeCN−and HOSeCN. The changes in metabolic activity were measured by MTS after treatment of HCASMC with (A) SeCN− for 24 h in growth media and (B) HOSeCN for 1 h in HBSS, directly after the treatment (black bars) and after following 24 h re-incubation in growth media (dashed bars). In (C) cells were exposed to HOSeCN for 1 h and were analyzed after washing and 24 h re-incubation in growth media. The percentages of live (A-/PI-, black bars), necrotic (A-/PI+, dark grey bars), early apoptotic (A+/PI-, white bars) and late apoptotic/necrotic (A+/PI+, light grey bars) cells in the remaining population were assessed using the APC Annexin V/Pi cell death detection kit and flow cytometry analysis. Statistical significance compared to the non-treated control indicated by *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001 by one- or two-way ANOVA with Dunnett's post hoc testing.
3.4. SeCN− prevents HOCl-induced cell death and cellular thiol oxidation
The effect of SeCN− on HOCl-induced cell damage was examined by treating HCASMC with HOCl (100 μM) in the absence and presence of SeCN− (10–200 μM) for 15 min, with and without 24 h re-incubation. Co-treatment with HOCl and SeCN− resulted in a less extensive loss in metabolic activity compared to that seen with HOCl alone, with this being dependent on the SeCN− concentration, and significant above ≥25 μM (Fig. 4A). In contrast, no protection by SeCN− was observed on pre-incubation of the HCASMC with SeCN− (25–100 μM) for 15 min prior to washing and addition of HOCl (100 μM) (Fig. S4). ICP-MS experiments performed to examine the cellular uptake of SeCN− showed that only very low intracellular concentrations (<0.2 μM) of SeCN− were achieved under these conditions (Fig. S5). Therefore, HCASMC were pre-treated with higher amounts of SeCN− (200 μM) for 24 h in cell media rather than 15 min, before washing and addition of HOCl (10–100 μM). However, this treatment had no significant effect on the loss of metabolic activity observed on addition of HOCl (Fig. S4). These data suggest that SeCN− reacts directly with HOCl, rather than by cellular accumulation and upregulation of antioxidant defense systems.
Fig. 4.
Prevention of HOCl-induced cell death and modulation of thiol oxidation with SeCN−in HCASMC. HCASMC were co-treated with 100 μM HOCl and increasing concentrations of SeCN− for 15 min. (A) The changes in metabolic activity by MTS were assessed immediately after exposure (black bars) and after following 24 h re-incubation in growth media (dashed bars) and are expressed as a fold change compared to control cells. (B) Total cellular thiols were measured after HOCl – SeCN− co-treatment (15 min) 0 h (black bars) and 24 h post-treatment (dashed bars), by ThioGlo1 assay. In (C) data show the thiol concentration of HCASMC exposed to HOSeCN (1 h) immediately after the treatment (black bars) and after following re-incubation for 24 h (dashed bars). Thiol data are normalized to the protein concentration and expressed as a percentage compared to (ct) the controls. Statistical significance indicated by **p < 0.01, ***p < 0.001 and ****p < 0.0001, #p < 0.05 and ##p < 0.01, by two-way ANOVA with Dunnett's post hoc testing. * indicate significance compared to HOCl treatment alone, with # showing significance comparing no re-incubation with 24 h re-incubation.
The effect of SeCN− on HOCl-induced thiol oxidation was also examined in experiments where the HCASMC were treated with HOCl (100 μM), or co-treated with HOCl and SeCN− (50–200 μM) for 15 min with and without a subsequent 24 h re-incubation in growth medium. Addition of SeCN− had no significant effect on the extent of thiol loss induced by HOCl when the cells were analyzed immediately after oxidant treatment (Fig. 4B). However, a significant restoration of thiols was observed in the co-treatment experiments, compared to HOCl alone, when analyzed after 24 h re-incubation in growth media (Fig. 4B). The reactivity of HOSeCN with thiols was also assessed, as this may influence the extent of thiol loss in the co-treatment experiments with HOCl and SeCN−. Exposure of HCASMC to HOSeCN (25–100 μM) for 1 h, resulted in a dose-dependent decrease in cellular thiols, with a significant recovery detected after 24 h re-incubation (Fig. 4C). However, the concentration of intracellular thiols did not return to the levels seen in the non-treated control cells in experiments with >50 μM HOSeCN (Fig. 4C). This suggests that the thiol oxidation induced by HOSeCN is partially reversible in HCASMC, in contrast to that seen with HOCl (Fig. 2A).
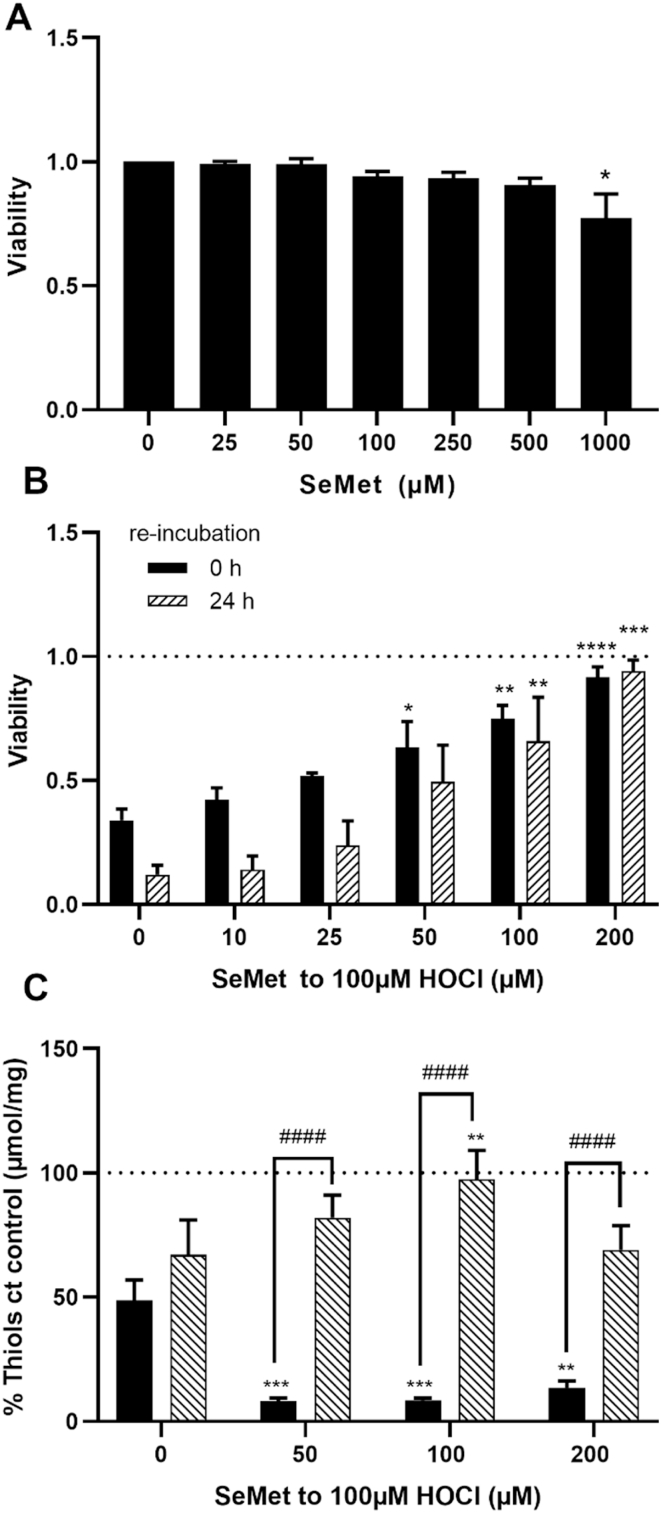
3.5. Role of SeMet in the prevention of HOCl-induced cellular damage
Recent studies have highlighted a potential role for SeMet in modulating damage induced by MPO-derived oxidants, owing to its ability to catalytically scavenge HOCl [35]. The toxicity of SeMet was examined by treating HCASMC with increasing concentrations of SeMet (25 μM–1 mM) in growth medium for 24 h and assessing the metabolic activity using the MTS assay. A significant decrease in metabolic activity was apparent only with 1 mM SeMet (Fig. 5A). The HCASMC were treated with HOCl (100 μM) in the absence and presence of increasing concentrations of SeMet (10–200 μM) for 15 min, or 15 min followed by washing and re-incubation for 24 h in growth media. SeMet co-treatment resulted in a dose-dependent decrease in the extent of HOCl-induced cell death determined by the MTS assay, which was significant in experiments with ≥50 μM SeMet on analysis immediately after treatment. Similar results were observed on re-incubation of the cells for 24 h after the initial treatment, although in this case, a significant protective effect against HOCl-induced cell death was apparent with ≥75 μM SeMet (Fig. 5B).
Fig. 5.
Effects of SeMet on HOCl-induced cell death and thiol oxidation in HCASMC. The changes in metabolic activity were measured by MTS after treatment of HCASMC with (A) SeMet for 24 h in growth media and (B) 100 μM HOCl with addition of SeMet immediately after treatment (black bars) and after 24 h re-incubation (dashed bars) with data expressed as a fold change compared to control cells. (C) Total cellular thiols were measured directly after the treatment (black bars) and 24 h post-treatment (dashed bars), by ThioGlo1 assay, and are normalized to protein concentration and expressed as a change compared to (ct) the controls. In (A) statistical significance compared to non-treated cells indicated by *p < 0.05 by one-way ANOVA with Dunnett's post hoc testing. In (B) and (C) statistical significance indicated by *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ####p < 0.0001 by two-way ANOVA with Dunnett's post hoc testing. * indicate significance compared to HOCl treatment alone, with # showing significance comparing no re-incubation with 24 h re-incubation.
Experiments were also performed to examine the effect of pre-treatment of the HCASMC with SeMet (25–100 μM) for 15 min before washing, addition of HOCl (100 μM) and further incubation for 15 min. This pre-treatment had no significant effect on the extent of HOCl-induced loss in metabolic activity (Fig. S6). This may reflect the low concentration (<0.1 μM) of SeMet detected in the cells by ICP-MS experiments (Fig. S5). No protection was detected with a higher concentration of SeMet (200 μM) and pre-incubation for 24 h in cell media, before washing, addition of HOCl (10–100 μM) and further incubation for 15 min (Fig. S6, grey bars). In this case pre-incubation with SeMet resulted in a greater loss of metabolic activity on addition of HOCl, which was significant with 50 μM HOCl when compared to that observed with HOCl alone (Fig. S6, black bars).
The effect of SeMet on the intracellular thiols in HCASMC was examined directly after treatment with HOCl, and following 24 h re-incubation in growth medium. Co-treatment with HOCl (100 μM) and SeMet (50–200 μM) resulted in a greater loss of intracellular thiols compared to that seen in experiments with HOCl alone, when assayed immediately after oxidant treatment (Fig. 5C, black bars). This may reflect reaction of SeMet with HOCl to give SeMet selenoxide (SeMetO), which then reacts with intracellular thiols (therefore resulting in depletion) during regeneration of SeMet [35]. However, following re-incubation of the cells for 24 h in growth media, co-treatment with SeMet prevented the HOCl-induced loss in intracellular thiols (Fig. 5C, dashed bars).
3.6. Alterations in gene expression with SCN−, SeCN− and SeMet co-treatment with HOCl
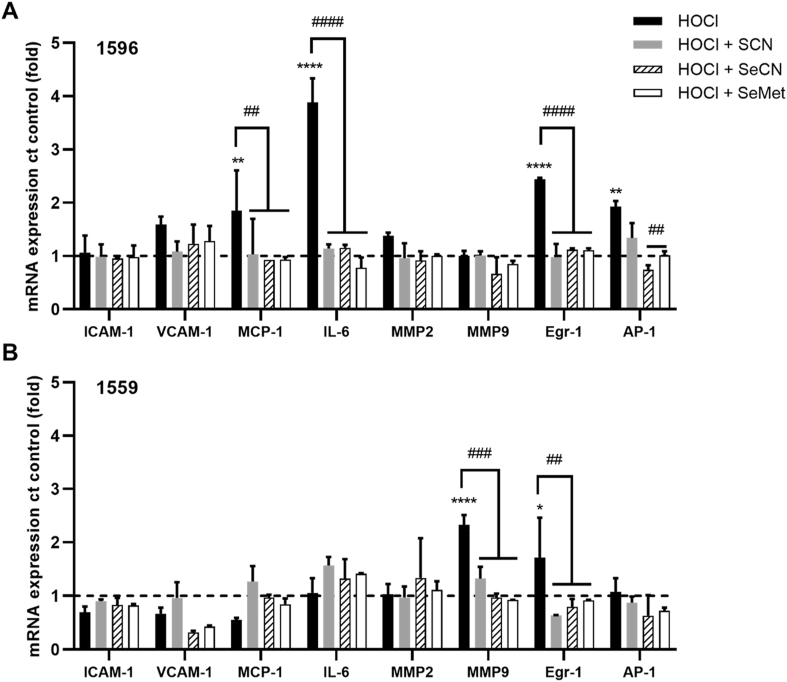
Exposure of cells to HOCl can activate pro-inflammatory signaling cascades, which could play a role in accelerating the development of chronic inflammatory pathologies [2,47]. Therefore, studies were carried out to examine the ability of SCN−, SeCN− and SeMet to modulate the alterations in pro-inflammatory and phenotypic gene expression induced by HOCl. In these experiments, HCASMC were exposed to a low concentration of HOCl (50 μM), so that the majority of the cells remained viable (Fig. 1), in both the absence and presence of SCN−, SeCN− or SeMet (100 μM) for 15 min. The mRNA expression of genes related to inflammation and SMC phenotype (Table S2), was examined by qPCR, 24 h post-treatment.
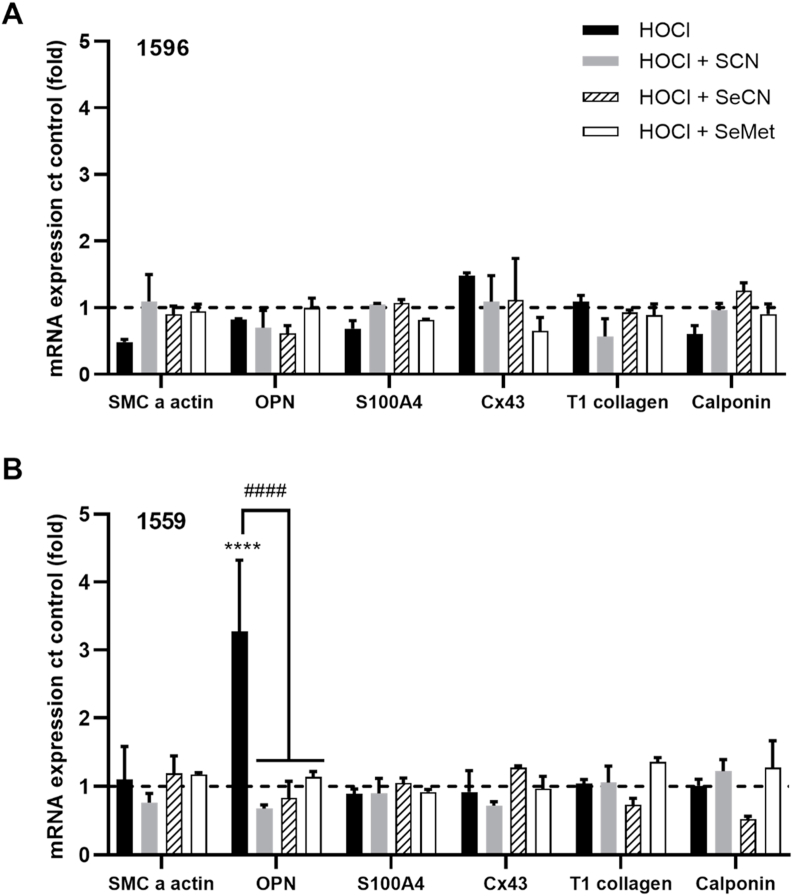
There was considerable variation between individual donors in the extent and pattern of mRNA expression of pro-inflammatory genes following HOCl exposure (Fig. 6). However, a significant increase in the expression of monocyte chemotactic protein 1 (MCP-1), interleukin 6 (IL-6), early growth response protein 1 (Egr-1), and activator protein 1 (AP-1) was seen in experiments with cells from donor 1596 following treatment with HOCl (Fig. 6A). A significant increase in the mRNA expression of Egr-1 was also observed with cells from donor 1559, together with an increase in matrix metalloproteinase 9 (MMP-9) (Fig. 6B). In contrast, there were no changes in gene expression on exposure of cells from donor 1522 to HOCl (Fig. S7). The increase in mRNA expression of MCP-1, IL-6, Egr-1 MMP-9 and AP-1 seen in HCASMC from donors 1596 and/or 1559 were not observed on co-treatment of the cells with HOCl and SCN−, SeCN− or SeMet (Fig. 6). Exposure of HCASMC to HOCl (50 μM) had no significant effect on the mRNA expression of genes associated with SMC phenotype in any of the cell donors, with the exception of donor 1559, where there was an increase in osteopontin (OPN) mRNA expression (Fig. 7). This alteration was not observed on co-treatment of the cells with HOCl and SCN−, SeCN− or SeMet (Fig. 7). There was no change in the expression of any of the genes examined on exposure of the cells to SCN−, SeCN− and SeMet alone (100 μM) compared to the non-treated, control cells (Fig. S8).
Fig. 6.
Effects of HOCl and co-treatments with SCN−, SeCN−and SeMet on inflammatory gene expression. HCASMC were exposed to 50 μM HOCl (black bars) with addition of 100 μM SCN− (grey bars) or SeCN− (dashed bars) or SeMet (white bars) for 15 min, before re-incubation in cell media for 24 h. Graphs show the mRNA expression of a range of inflammatory genes analyzed by qPCR and normalized to the housekeeping genes 18S and B2M. The data for 2 individual donors 1596 (A) and 1559 (B) are shown separately. Results represent the mean ± SD of 3 replicates and are expressed as an increase compared to (ct) the non-treated control. *p < 0.05, **p < 0.01 and ****p < 0.0001 indicate a significant increase compared to non-treated control, with ##p < 0.01, ###p < 0.001 and ####p < 0.0001 indicating a significance difference compared to HOCl treatment using two-way ANOVA with Dunnett's multiple comparisons post hoc test.
Fig. 7.
Effect of HOCl and HOCl co-treatments with SCN−, SeCN−and SeMet on phenotypic gene expression. HCASMC were exposed to 50 μM HOCl (black bars) with addition of 100 μM SCN- (grey bars) or SeCN− (dashed bars) or SeMet (white bars) for 15 min, before re-incubation in cell media for 24 h. Graphs show the mRNA expression of a range of phenotypic genes, analyzed by qPCR and normalized to the housekeeping genes 18S and B2M. The data for 2 individual donors 1596 (A) and 1559 (B) are shown separately. Results represent the mean ± SD of 3 replicates and are expressed as an increase compared to (ct) the non-treated control. ****p < 0.0001 indicates a significant increase compared to non-treated control, with ####p < 0.0001 indicating a significance difference compared to HOCl treatment using two-way ANOVA with Dunnett's multiple comparisons post hoc test.
4. Discussion
It is well established that the MPO-derived oxidant HOCl, and its downstream reaction products, modify the function of numerous cell types [47], including VSMCs, with implications for the progression of inflammatory diseases, particularly atherosclerosis [2,4,47,48]. The alternative MPO substrate SCN− and selenium-containing compounds may therefore have potential therapeutic value by either promoting the formation of less damaging oxidants by MPO (e.g. HOSCN, HOSeCN) or by acting as direct scavengers of HOCl [35,37,49]. In this study, we show that SCN−, SeCN− or SeMet, can inhibit HOCl-induced HCASMC damage. These compounds also influenced the extent and nature of intracellular thiol oxidation, and expression of inflammatory genes. In addition, we evaluated the effects of the SeCN− product, HOSeCN, with HCASMC, and have shown that this is less toxic than HOCl, but has greater toxicity compared to HOSCN.
Exposure of HCASMC to HOCl resulted in a dose-dependent decrease of cell viability, which was attenuated by SCN− and SeCN−, at a sub-stoichiometric ratio of anion: HOCl (1 : 4). This protective effect was only apparent on co-treatment with HOCl, and was not seen when cells were pre-incubated with these anions prior to oxidant treatment. These results are consistent with a direct reaction of HOCl with SCN− or SeCN−, yielding HOSCN or HOSeCN, respectively. The sub-stoichiometric protection afforded by SCN− is attributed to the initial conversion of HOCl to HOSCN, and subsequent further oxidation by HOCl to form products such as HO2SCN [18]. It is presumed that similar reactions occur with SeCN−, forming HOSeCN, and potentially HO2SeCN with residual HOCl. These reactions have not been studied previously, and no data are available on the biological reactivity of these products, although decomposition of HO2SCN is reported to form SO42− and OCN− [18]. These data agree well with other studies indicating that an excess of SCN− is required to give “clean”, conversion of HOCl to HOSCN [18], and studies performed with macrophages exposed to HOCl in the presence of SCN− [23].
Whether SCN− or SeCN− will modulate HOCl concentration in vivo by direct scavenging or by competing with Cl− as a substrate for MPO is not certain. It has been postulated that the presence of SCN− in physiological fluids, such as plasma, will limit the lifetime of HOCl, owing to the high rate constant for this reaction (k = 2.3 × 107 M−1 s−1) compared to other low molecular mass plasma components [17]. However, these kinetic modelling studies [17] neglect the contribution of plasma proteins, which are a key target for HOCl [50]. In addition, the opportunity for the secondary reaction of HOCl with HOSCN or HOSeCN may be more limited in vivo in the presence of other competing substrates. While SCN− is the favoured substrate for MPO compared to Cl− [15], the specificity of SeCN− for MPO is not known, though this anion is a substrate for LPO [37].
Co-treatment with HOCl and SCN− prevented the alterations in cytosolic Ca2+ and the induction of apoptotic cell death seen in HCASMC exposed to HOCl. Evidence was also obtained for a switch from non-reversible to reversible thiol loss in the presence of SCN−, which supports the conversion of HOCl to HOSCN, particularly at high ratios of SCN- to HOCl, in the co-treatment experiments. Thus, it is well established that thiols are key targets for HOSCN, although the reactivity of this species is lower than that of HOCl [33,[51], [52], [53], [54]], and that reversible oxidation products, including sulfenic acids, are formed [21,22]. Addition of SCN− also influenced the extent and nature of HOCl-induced GSH oxidation, with SCN− supplementation resulting in a decreased loss of GSH, and a smaller change in the GSH:GSSG ratio. A more pronounced increase in GSH to levels above that seen in the non-treated control cells, was observed in re-incubation experiments over 24 h with HOCl and SCN− (both 100 μM). The reason for this is not certain, but could reflect the activation of pro-survival signaling cascades, such as nuclear factor erythroid 2–related factor 2 (Nrf2). For example, exposure of macrophages to HOCl results in an initial decrease in cellular GSH, with a rebound effect seen 24 h after treatment, with GSH concentrations increasing above basal levels [55]. The ability of HOSCN to activate Nrf2 has not been widely examined but appears to be cell-type dependent, with evidence for increased Nrf2-related gene expression detected in cardiac myoblasts [56], but not macrophages [23], on exposure to HOSCN.
Sulfur and selenium have somewhat similar physical and chemical properties but selenium is more easily oxidized [38]. Thus, selenium-containing compounds are usually more reactive than their sulfur analogues, as demonstrated by comparing their reactivity with different MPO-derived oxidants [33,34,53,54]. Co-treatment of HCASMC with HOCl and SeCN−, had similar protective effects to those seen with SCN−, with concentrations of SeCN− ≥ 25 μM preventing HOCl-induced cell death. Analysis of the metabolic activity of cells directly after oxidant treatment, indicated that the protective effect of SeCN− was greater than SCN−, particularly at concentrations ≥100 μM. Addition of SeCN− also attenuated thiol oxidation in HCASMC exposed to HOCl, but only when examined 24 h post-treatment. This suggests that HOSeCN is also reactive with thiols, and like HOSCN, can form reversible oxidation products. This conclusion is supported by the results from experiments where HCASMC were exposed directly to HOSeCN. Interestingly, the extent of thiol oxidation and the formation of reversible products was not as great with HOSeCN when compared to HOSCN (Flouda et al. submitted). This suggests that HOSeCN is a stronger oxidant than HOSCN, with the ability to form non-reversible thiol oxidation products and potentially react with other cellular targets. This is supported by the HCASMC viability data (Fig. S9), where the ability of the oxidants to decrease metabolic activity diminished in the order HOCl > HOSeCN > HOSCN.
These data are supported by studies comparing the ability of HOSCN and HOSeCN to kill bacterial cells. Thus, supplementation with SeCN− has been shown to be more effective in killing bacteria and fungi when compared to SCN− [37,57,58]. Similarly, SeCN−-containing compounds have therapeutic applications for cancer treatment and prevention [59]. SeCN− is a bioavailable inorganic form of selenium with low toxicity in cell and rodent studies, that can also be incorporated into selenoproteins [60,61]. For HCASMC, SeCN− was more cytotoxic than SeMet, but a significant loss of cell viability was only seen at high concentrations (≥250 μM) and at prolonged incubation times (24 h). No protective effect was observed when the cells were pre-incubated with SeCN− prior to HOCl addition, although there is evidence that SeCN− can be metabolized by mammalian cells, and contribute to the intrinsic selenium pool to increase the synthesis of selenoproteins [60,62]. The intracellular concentration of SeCN− was relatively low (<0.05 μM with 200 μM SeCN−) in HCASMC when analyzed 15 min after exposure to SeCN−, which may reflect slow uptake by the cells. However, there was also no evidence for protection following a 24 h pre-treatment, in experiments with lower concentrations of HOCl (<100 μM).
Similar negative results were obtained on pre-treatment of the cells with SeMet. However, protection from HOCl-induced HCASMC death was observed on co-treatment with SeMet (≥50 μM). This suggests that like SeCN−, the main pathway of cellular protection is via oxidant scavenging rather than upregulation of cellular defense systems, in accord with previous studies with cardiac myoblasts [36]. Reaction of SeMet with HOCl generates SeMet selenoxide (SeMetO) [33], which can react with GSH and be recycled by intracellular reducing systems such as the glutathione reductase and TrxR systems [35,63]. Such reactions would explain the initial thiol depletion seen on exposure of HCASMC to HOCl in the presence of SeMet, with these returning to basal levels after 24 h.
Of the three compounds examined, SeMet afforded the lowest level of protection against loss of viability, with higher concentrations required to attenuate cell death to the same extent as SCN− and SeCN−. Which compound is most effective in vivo remains to be established, as each of the product species (SeMetO, HOSCN and HOSeCN), can potentially cause cellular toxicity (e.g. via GSH depletion). While the cellular reactivity of HOSCN has been studied in multiple cells [22,36,45,47,51,52], data for HOSeCN are limited. No significant toxicity was observed on exposure of human epithelial cells to a bactericidal flux of HOSeCN (from an enzymatic LPO/H2O2/SeCN− system [37]), however the concentration of HOSeCN generated in these experiments was very low (~5 μM) compared to those examined in the current study, where apoptotic HCASMC death was observed with >50 μM HOSeCN for 1 h.
In addition to preventing cell death, co-treatment with SCN−, SeCN− or SeMet attenuated the HOCl-induced upregulation of inflammatory and phenotypic genes, including MCP-1, IL-6. Egr-1 and AP-1, MMP9 and OPN. Although the changes in gene expression were not consistent between the different cell donors, a protective effect was apparent on co-treatment in all cases. SCN−, SeCN− or SeMet alone had no significant effect on gene expression (Fig. S8). Interestingly, cells isolated from a diseased artery (donor 1596) were more sensitive to altered inflammatory gene expression compared to those from non-diseased arteries (donors 1522 and 1559). This might be relevant in vivo, as it may potentially enhance immune cell recruitment to lesions. IL-6 has potent pro-inflammatory effects on a variety of cells including VSMC, and plays a key role in the inflammatory response responsible for atherosclerosis [64]. MMPs can enhance the migration, proliferation and survival of VSMC, and OPN has an active role in the progression of vascular remodeling and proliferation of VSMC [65,66]. Further experiments with cells from a wider range of donors are needed to confirm these observations. As these genes are of relevance to the development of atherosclerosis, targeting their expression with SCN−, SeCN− and/or SeMet may be beneficial. The pathways involved have not been examined in detail, but are likely to reflect HOCl scavenging, as a 2-fold molar excess of SCN−, SeCN− and SeMet was present in these experiments. These data also suggest that HOSCN and HOSeCN do not have marked activating effects on inflammatory gene expression under these conditions.
Supplementation with SeMet, inhibits atherosclerotic plaque formation in mice [32] and can decrease the inflammatory response and endothelial dysfunction during atherosclerosis development [31]. Similar results have been reported for SCN− [26,27]. Whether these effects are related solely to a reduction in HOCl production remain to be established. SeMet supplementation was associated with increased GPx activity, a decreased inflammatory response and altered macrophage phenotype to favour the wound-healing, M2 population, which is likely to be independent of HOCl production [32]. Supplementation with SeCN− has not been examined in the context of atherosclerosis, though studies show it is well tolerated in rodents and elevates selenoprotein synthesis, including GPx [60,61].
In summary, these data provide insights as to how supplementation with SCN− and the selenium-containing compounds SeCN− and SeMet, may influence HOCl-induced cell damage during the development of chronic inflammatory pathologies. The data for SCN− supplementation are consistent with a switch from HOCl to HOSCN-mediated effects, and the generation of reversible modifications in HCASMC. SeCN− is also protective, which is attributed to the conversion of HOCl to HOSeCN, which is less damaging than HOCl, but more reactive than HOSCN, with this cell type. Under the conditions employed in this study, SCN− and SeCN− appear to be more effective in preventing cell death than SeMet, despite the potential for catalytic recycling of the selenoxide generated from this compound. Whether SeCN− supplementation is of value in vivo in mitigating chronic inflammation has yet to be established, though promotion of HOSeCN formation may be beneficial in inflammatory situations where there is pathogen involvement (e.g. sepsis), in light of its superior bactericidal properties compared to HOSCN [37,57,58,67].
Declaration of competing interest
None.
Acknowledgements
The authors are grateful to the Novo Nordisk Foundation (Laureate Research Grant NNF13OC0004294 to MJD) and the Lundbeck Foundation (R317-2019-634) for financial support. We also thank Prof. Brian Day for helpful discussions, and Freja Grønbæk-Thorsen for technical assistance with the ICP-MS experiments.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2021.101873.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Van der Veen B.S., de Winther M.P., Heeringa P. Myeloperoxidase: molecular mechanisms of action and their relevance to human health and disease. Antioxidants Redox Signal. 2009;11(11):2899–2937. doi: 10.1089/ars.2009.2538. [DOI] [PubMed] [Google Scholar]
- 2.Davies M.J., Hawkins C.L. The role of myeloperoxidase in biomolecule modification, chronic inflammation, and disease. Antioxidants Redox Signal. 2020;32(13):957–981. doi: 10.1089/ars.2020.8030. [DOI] [PubMed] [Google Scholar]
- 3.Hampton M.B., Kettle A.J., Winterbourn C.C. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood. 1998;92(9):3007–3017. [PubMed] [Google Scholar]
- 4.Klebanoff S.J. Myeloperoxidase: friend and foe. J. Leukoc. Biol. 2005;77(5):598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 5.Nussbaum C., Klinke A., Adam M., Baldus S., Sperandio M. Myeloperoxidase: a leukocyte-derived protagonist of inflammation and cardiovascular disease. Antioxidants Redox Signal. 2013;18(6):692–713. doi: 10.1089/ars.2012.4783. [DOI] [PubMed] [Google Scholar]
- 6.Teng N., Maghzal G.J., Talib J., Rashid I., Lau A.K., Stocker R. The roles of myeloperoxidase in coronary artery disease and its potential implication in plaque rupture. Redox Rep. 2017;22(2):51–73. doi: 10.1080/13510002.2016.1256119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daugherty A., Dunn J.L., Rateri D.L., Heinecke J.W. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J. Clin. Invest. 1994;94(1):437–444. doi: 10.1172/JCI117342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schindhelm R.K., van der Zwan L.P., Teerlink T., Scheffer P.G. Myeloperoxidase: a useful biomarker for cardiovascular disease risk stratification? Clin. Chem. 2009;55(8):1462–1470. doi: 10.1373/clinchem.2009.126029. [DOI] [PubMed] [Google Scholar]
- 9.Zhang R., Brennan M.L., Fu X., Aviles R.J., Pearce G.L., Penn M.S., Topol E.J., Sprecher D.L., Hazen S.L. Association between myeloperoxidase levels and risk of coronary artery disease. J. Am. Med. Assoc. 2001;286(17):2136–2142. doi: 10.1001/jama.286.17.2136. [DOI] [PubMed] [Google Scholar]
- 10.Nicholls S.J., Tang W.H., Brennan D., Brennan M.L., Mann S., Nissen S.E., Hazen S.L. Risk prediction with serial myeloperoxidase monitoring in patients with acute chest pain. Clin. Chem. 2011;57(12):1762–1770. doi: 10.1373/clinchem.2011.166827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lazarevic-Pasti T., Leskovac A., Vasic V. Myeloperoxidase inhibitors as potential drugs. Curr. Drug Metabol. 2015;16(3):168–190. doi: 10.2174/138920021603150812120640. [DOI] [PubMed] [Google Scholar]
- 12.Davies M.J. Myeloperoxidase: mechanisms, reactions and inhibition as a therapeutic strategy in inflammatory diseases. Pharmacol. Therapeut. 2020 doi: 10.1016/j.pharmthera.2020.107685. [DOI] [PubMed] [Google Scholar]
- 13.Rashid I., Maghzal G.J., Chen Y.C., Cheng D., Talib J., Newington D., Ren M., Vajandar S.K., Searle A., Maluenda A., Lindstedt E.L., Jabbour A., Kettle A.J., Bongers A., Power C., Michaelsson E., Peter K., Stocker R. Myeloperoxidase is a potential molecular imaging and therapeutic target for the identification and stabilization of high-risk atherosclerotic plaque. Eur. Heart J. 2018;39(35):3301–3310. doi: 10.1093/eurheartj/ehy419. [DOI] [PubMed] [Google Scholar]
- 14.Morgan P.E., Pattison D.I., Talib J., Summers F.A., Harmer J.A., Celermajer D.S., Hawkins C.L., Davies M.J. High plasma thiocyanate levels in smokers are a key determinant of thiol oxidation induced by myeloperoxidase. Free Radic. Biol. Med. 2011;51(9):1815–1822. doi: 10.1016/j.freeradbiomed.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 15.van Dalen C.J., Whitehouse W.M., Winterbourn C.C., Kettle J.A. Thiocyanate and chloride as competing substrates for myeloperoxidase. Biochem. J. 1997;327(2):487–492. doi: 10.1042/bj3270487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandler J.D., Day B.J. Biochemical mechanisms and therapeutic potential of pseudohalide thiocyanate in human health. Free Radic. Res. 2015;49(6):695–710. doi: 10.3109/10715762.2014.1003372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashby M.T., Carlson A.C., Scott M.J. Redox buffering of hypochlorous acid by thiocyanate in physiologic fluids. J. Am. Chem. Soc. 2004;126(49):15976–15977. doi: 10.1021/ja0438361. [DOI] [PubMed] [Google Scholar]
- 18.Xulu B.A., Ashby M.T. Small molecular, macromolecular, and cellular chloramines react with thiocyanate to give the human defense factor hypothiocyanite. Biochemistry. 2010;49(9):2068–2074. doi: 10.1021/bi902089w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barrett T.J., Hawkins C.L. Hypothiocyanous acid: benign or deadly? Chem. Res. Toxicol. 2012;25(2):263–273. doi: 10.1021/tx200219s. [DOI] [PubMed] [Google Scholar]
- 20.Ashby M.T. Hypothiocyanite, Adv. Inorg. Chem. 2012;64:263–303. [Google Scholar]
- 21.Barrett T.J., Pattison D.I., Leonard S.E., Carroll K.S., Davies M.J., Hawkins C.L. Inactivation of thiol-dependent enzymes by hypothiocyanous acid: role of sulfenyl thiocyanate and sulfenic acid intermediates. Free Radic. Biol. Med. 2012;52(6):1075–1085. doi: 10.1016/j.freeradbiomed.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Love D.T., Barrett T.J., White M.Y., Cordwell S.J., Davies M.J., Hawkins C.L. Cellular targets of the myeloperoxidase-derived oxidant hypothiocyanous acid (HOSCN) and its role in the inhibition of glycolysis in macrophages. Free Radic. Biol. Med. 2016;94:88–98. doi: 10.1016/j.freeradbiomed.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 23.Guo C., Davies M.J., Hawkins C.L. Role of thiocyanate in the modulation of myeloperoxidase-derived oxidant induced damage to macrophages. Redox Biol. 2020;36 doi: 10.1016/j.redox.2020.101666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Y., Szép S., Lu Z. The antioxidant role of thiocyanate in the pathogenesis of cystic fibrosis and other inflammation-related diseases. Proc. Natl. Acad. Sci. U.S.A. 2009;106(48):20515–20519. doi: 10.1073/pnas.0911412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chandler J.D., Min E., Huang J., McElroy C.S., Dickerhof N., Mocatta T., Fletcher A.A., Evans C.M., Liang L., Patel M. Antiinflammatory and antimicrobial effects of thiocyanate in a cystic fibrosis mouse model. Am. J. Respir. Cell Mol. Biol. 2015;53(2):193–205. doi: 10.1165/rcmb.2014-0208OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgan P.E., Laura R.P., Maki R.A., Reynolds W.F., Davies M.J. Thiocyanate supplementation decreases atherosclerotic plaque in mice expressing human myeloperoxidase. Free Radic. Res. 2015;49(6):743–749. doi: 10.3109/10715762.2015.1019347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zietzer A., Niepmann S.T., Camara B., Lenart M.A., Jansen F., Becher M.U., Andrie R., Nickenig G., Tiyerili V. Sodium thiocyanate treatment attenuates atherosclerotic plaque formation and improves endothelial regeneration in mice. PloS One. 2019;14(4) doi: 10.1371/journal.pone.0214476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu H.M., Xu H.B., Huang K.X. Selenium in the prevention of atherosclerosis and its underlying mechanisms. Metallomics. 2017;9(1):21–37. doi: 10.1039/c6mt00195e. [DOI] [PubMed] [Google Scholar]
- 29.Wojcicki J., Rozewicka L., Barcew-Wiszniewska B., Samochowiec L., Juzwiak S., Kadlubowska D., Tustanowski S., Juzyszyn Z. Effect of selenium and vitamin E on the development of experimental atherosclerosis in rabbits. Atherosclerosis. 1991;87(1):9–16. doi: 10.1016/0021-9150(91)90227-t. [DOI] [PubMed] [Google Scholar]
- 30.Schwenke D.C., Behr S.R. Vitamin E combined with selenium inhibits atherosclerosis in hypercholesterolemic rabbits independently of effects on plasma cholesterol concentrations. Circ. Res. 1998;83(4):366–377. doi: 10.1161/01.res.83.4.366. [DOI] [PubMed] [Google Scholar]
- 31.Zhang F., Yu W., Hargrove J.L., Greenspan P., Dean R.G., Taylor E.W., Hartle D.K. Inhibition of TNF-alpha induced ICAM-1, VCAM-1 and E-selectin expression by selenium. Atherosclerosis. 2002;161(2):381–386. doi: 10.1016/s0021-9150(01)00672-4. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y., Cartland S.P., Henriquez R., Patel S., Gammelgaard B., Flouda K., Hawkins C.L., Rayner B.S. Selenomethionine supplementation reduces lesion burden, improves vessel function and modulates the inflammatory response within the setting of atherosclerosis. Redox Biol. 2020;29 doi: 10.1016/j.redox.2019.101409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carroll L., Pattison D.I., Fu S., Schiesser C.H., Davies M.J., Hawkins C.L. Reactivity of selenium-containing compounds with myeloperoxidase-derived chlorinating oxidants: second-order rate constants and implications for biological damage. Free Radic. Biol. Med. 2015;84:279–288. doi: 10.1016/j.freeradbiomed.2015.03.029. [DOI] [PubMed] [Google Scholar]
- 34.Carroll L., Gardiner K., Ignasiak M., Holmehave J., Shimodaira S., Breitenbach T., Iwaoka M., Ogilby P.R., Pattison D.I., Davies M.J. Interaction kinetics of selenium-containing compounds with oxidants. Free Radic. Biol. Med. 2020;155:58–68. doi: 10.1016/j.freeradbiomed.2020.05.007. [DOI] [PubMed] [Google Scholar]
- 35.Carroll L., Pattison D.I., Fu S., Schiesser C.H., Davies M.J., Hawkins C.L. Catalytic oxidant scavenging by selenium-containing compounds: Reduction of selenoxides and N-chloramines by thiols and redox enzymes. Redox Biol. 2017;12:872–882. doi: 10.1016/j.redox.2017.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reyes L., Bishop D.P., Hawkins C.L., Rayner B.S. Assessing the efficacy of dietary selenomethionine supplementation in the setting of cardiac ischemia/reperfusion injury. Antioxidants. 2019;8(11):546. doi: 10.3390/antiox8110546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Day B.J., Bratcher P.E., Chandler J.D., Kilgore M.B., Min E., LiPuma J.J., Hondal R.J., Nichols D.P. The thiocyanate analog selenocyanate is a more potent antimicrobial pro-drug that also is selectively detoxified by the host. Free Radic. Biol. Med. 2020;146:324–332. doi: 10.1016/j.freeradbiomed.2019.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cupp-Sutton K.A., Ashby M.T. Biological chemistry of hydrogen selenide. Antioxidants. 2016;5(4):42. doi: 10.3390/antiox5040042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bennett M.R., Sinha S., Owens G.K. Vascular smooth muscle cells in atherosclerosis. Circ. Res. 2016;118(4):692–702. doi: 10.1161/CIRCRESAHA.115.306361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morris J.C. The acid ionization constant of HOCl from 5 to 35 °C. J. Phys. Chem. 1966;70(12):3798–3805. [Google Scholar]
- 41.Hawkins C.L., Morgan P.E., Davies M.J. Quantification of protein modification by oxidants. Free Radic. Biol. Med. 2009;46(8):965–988. doi: 10.1016/j.freeradbiomed.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 42.Eyer P., Worek F., Kiderlen D., Sinko G., Stuglin A., Simeon-Rudolf V., Reiner E. Molar absorption coefficients for the reduced Ellman reagent: reassessment. Anal. Biochem. 2003;312(2):224–227. doi: 10.1016/s0003-2697(02)00506-7. [DOI] [PubMed] [Google Scholar]
- 43.Carr A.C., Winterbourn C.C. Oxidation of neutrophil glutathione and protein thiols by myeloperoxidase-derived hypochlorous acid. Biochem. J. 1997;327(1):275–281. doi: 10.1042/bj3270275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Degrossoli A., Müller A., Xie K., Schneider J.F., Bader V., Winklhofer K.F., Meyer A.J., Leichert L.I. Neutrophil-generated HOCl leads to non-specific thiol oxidation in phagocytized bacteria. Elife. 2018;7 doi: 10.7554/eLife.32288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lloyd M.M., van Reyk D.M., Davies M.J., Hawkins C.L. Hypothiocyanous acid is a more potent inducer of apoptosis and protein thiol depletion in murine macrophage cells than hypochlorous acid or hypobromous acid. Biochem. J. 2008;414(2):271–280. doi: 10.1042/BJ20080468. [DOI] [PubMed] [Google Scholar]
- 46.Vissers M.C.M., Winterbourn C.C. Oxidation of intracellular glutathione after exposure of human red blood cells to hypochlorous acid. Biochem. J. 1995;307(1):57–62. doi: 10.1042/bj3070057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rayner B.S., Love D.T., Hawkins C.L. Comparative reactivity of myeloperoxidase-derived oxidants with mammalian cells. Free Radic. Biol. Med. 2014;71:240–255. doi: 10.1016/j.freeradbiomed.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 48.Cai H., Chuang C.Y., Vanichkitrungruang S., Hawkins C.L., Davies M.J. Hypochlorous acid-modified extracellular matrix contributes to the behavioral switching of human coronary artery smooth muscle cells. Free Radic. Biol. Med. 2019;134:516–526. doi: 10.1016/j.freeradbiomed.2019.01.044. [DOI] [PubMed] [Google Scholar]
- 49.Chandler J.D., Day B.J. Thiocyanate: a potentially useful therapeutic agent with host defense and antioxidant properties. Biochem. Pharmacol. 2012;84(11):1381–1387. doi: 10.1016/j.bcp.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pattison D.I., Hawkins C.L., Davies M.J. What are the plasma targets of the oxidant hypochlorous acid? A kinetic modeling approach. Chem. Res. Toxicol. 2009;22(5):807–817. doi: 10.1021/tx800372d. [DOI] [PubMed] [Google Scholar]
- 51.Bozonet S.M., Scott-Thomas A.P., Nagy P., Vissers M.C. Hypothiocyanous acid is a potent inhibitor of apoptosis and caspase 3 activation in endothelial cells. Free Radic. Biol. Med. 2010;49(6):1054–1063. doi: 10.1016/j.freeradbiomed.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 52.Lloyd M.M., Grima M.A., Rayner B.S., Hadfield K.A., Davies M.J., Hawkins C.L. Comparative reactivity of the myeloperoxidase-derived oxidants hypochlorous acid and hypothiocyanous acid with human coronary artery endothelial cells, Free Radic. Biol. Med. 2013;65:1352–1362. doi: 10.1016/j.freeradbiomed.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 53.Skaff O., Pattison D.I., Davies M.J. Hypothiocyanous acid reactivity with low-molecular-mass and protein thiols: absolute rate constants and assessment of biological relevance. Biochem. J. 2009;422(1):111–117. doi: 10.1042/BJ20090276. [DOI] [PubMed] [Google Scholar]
- 54.Skaff O., Pattison D.I., Morgan P.E., Bachana R., Jain V.K., Priyadarsini K.I., Davies M.J. Selenium-containing amino acids are targets for myeloperoxidase-derived hypothiocyanous acid: determination of absolute rate constants and implications for biological damage. Biochem. J. 2012;441(1):305–316. doi: 10.1042/BJ20101762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pi J., Zhang Q., Woods C.G., Wong V., Collins S., Andersen M.E. Activation of Nrf2-mediated oxidative stress response in macrophages by hypochlorous acid. Toxicol. Appl. Pharmacol. 2008;226(3):236–243. doi: 10.1016/j.taap.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 56.Reyes L., Hawkins C.L., Rayner B.S. Characterization of the cellular effects of myeloperoxidase-derived oxidants on H9c2 cardiac myoblasts. Arch. Biochem. Biophys. 2019;665:132–142. doi: 10.1016/j.abb.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 57.Huang L., Xuan W., Sarna T., Hamblin M.R. Comparison of thiocyanate and selenocyanate for potentiation of antimicrobial photodynamic therapy. J. Biophot. 2019;12(1) doi: 10.1002/jbio.201800092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Quatrin P.M., Dalla Lana D.F., Bazana L.C.G., de Oliveira L.F.S., Teixeira M.L., Silva E.E., Lopes W., Canto R.F.S., Silveira G.P., Fuentefria A.M. 3-Selenocyanate-indoles as new agents for the treatment of superficial and mucocutaneous infections. New J. Chem. 2019;43(2):926–933. [Google Scholar]
- 59.Ali W., Álvarez-Pérez M., Marć M.A., Salardón-Jiménez N., Handzlik J., Domínguez-Álvarez E. The anticancer and chemopreventive activity of selenocyanate-containing compounds. Curr. Pharmacol. Rep. 2018;4(6):468–481. [Google Scholar]
- 60.Takahashi K., Ogra Y. Identification of the biliary selenium metabolite and the biological significance of selenium enterohepatic circulation. Metallomics. 2020;12(2):241–248. doi: 10.1039/c9mt00274j. [DOI] [PubMed] [Google Scholar]
- 61.Takahashi K., Suzuki N., Ogra Y. Effect of administration route and dose on metabolism of nine bioselenocompounds. J. Trace Elem. Med. Biol. 2018;49:113–118. doi: 10.1016/j.jtemb.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 62.Anan Y., Kimura M., Hayashi M., Koike R., Ogra Y. Detoxification of selenite to form selenocyanate in mammalian cells. Chem. Res. Toxicol. 2015;28(9):1803–1814. doi: 10.1021/acs.chemrestox.5b00254. [DOI] [PubMed] [Google Scholar]
- 63.Krause R.J., Elfarra A.A. Reduction of L-methionine selenoxide to seleno-L-methionine by endogenous thiols, ascorbic acid, or methimazole. Biochem. Pharmacol. 2009;77(1):134–140. doi: 10.1016/j.bcp.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hartman J., Frishman W.H. Inflammation and atherosclerosis: a review of the role of interleukin-6 in the development of atherosclerosis and the potential for targeted drug therapy. Cardiol. Review. 2014;22(3):147–151. doi: 10.1097/CRD.0000000000000021. [DOI] [PubMed] [Google Scholar]
- 65.Newby A.C. Matrix metalloproteinases regulate migration, proliferation, and death of vascular smooth muscle cells by degrading matrix and non-matrix substrates. Cardiovasc. Res. 2006;69(3):614–624. doi: 10.1016/j.cardiores.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 66.Lee S.J., Baek S.E., Jang M.A., Kim C.D. Osteopontin plays a key role in vascular smooth muscle cell proliferation via EGFR-mediated activation of AP-1 and C/EBPβ pathways. Pharmacol. Res. 2016;108:1–8. doi: 10.1016/j.phrs.2016.03.041. [DOI] [PubMed] [Google Scholar]
- 67.Huang L., Xuan W., Zadlo A., Kozinska A., Sarna T., Hamblin M.R. Antimicrobial photodynamic inactivation is potentiated by the addition of selenocyanate: Possible involvement of selenocyanogen? J. Biophot. 2018;11(8) doi: 10.1002/jbio.201800029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.