Highlights
-
•
The bacterial strain isolated from traditional dairy products well satisfies probiotic criteria.
-
•
Cell-free supernatant of the strains has significant cytotoxicity against cancerous cells.
-
•
This bacterial compound increase BAX and decrease Bcl-2 protein expression.
-
•
This bacterial agent mostly induces early apoptosis in MCF-7 cells.
Keywords: Pediococcus, Probiotics, Apoptosis, Anti-cancer, Breast cancer, Bacterial derivatives
Abstract
Herein, 18 lactic acid bacteria isolated from 30 samples of traditional dairy products were identified, and their probiotic potential was evaluated. According to the results, almost all strains showed the probiotic properties sufficiently, though M1 had better characterise. 16S rRNA gene sequencing revealed that this strain belongs to the Pediococcus sp. (<95 % similarity). This strain had substantial antipathogenic activity and did not show any worrying antibiotic resistance. Also, the strain was resistant to high concentrations of bile salt (1 %), NaCl (6.5 %), and low pH (2). Furthermore, it was revealed that cell-free supernatant (CFS), heat-killed cells and live cells derived from M1 significantly decreased the viability of MCF-7 cells so that the CFS resulted in 85 % cell death. Flow cytometry and western blot analysis determined that this compound induced apoptosis in the cancerous cells through increasing the BAX protein expression and decreasing the Bcl-2 protein expression.
1. Introduction
Cancer is known as a disease that can occur as a result of unhealthy environmental conditions and dietary habits [1]. Up to now, many types of cancers have been described and the number of deaths (cancer mortality) corresponding to each one recorded. According to the World Health Organization (WHO), breast cancer is one of the most prevalent cancers in women worldwide. In 2018, over 2 million cases of cancer were reported which was responsible for more than 600,000 deaths [2]. Hence, during the past decades, the human being has been concentrating on finding effective means of preventing and curing cancer. Currently, there are several conventional anticancer treatments, including surgery, chemotherapy, and radiotherapy. But, these treatments are costly and have harmful side effects [1]. Chemotherapy and synthetic drugs are known as standard treatments, though most of them are usually toxic to normal human cells and tissues. Therefore, the exploration of sophisticated anticancer agents with low side effects is essential to remedy the situation [3,4].
Even though researchers have achieved promising results in cancer diagnosis and therapy in recent years, there are still severe challenges to attain definitive cancer treatment [5]. At the individual level, studies have discovered that lifestyle and diet can be effective in cancer prevention and treatment [1,4,6]. During past decades, the global probiotic consumption has been rising rapidly, and many companies have been supplying and supporting these food supplements. So, the United Nations and the WHO introduced a universal definition for probiotic. They described probiotics as “Live microorganisms which when administered in adequate amounts confer a health benefit on the host” [7,8].
Among all probiotics, Lactic acid bacteria (LAB) have a long history of utilization in fermented foods [9,10]. A few studies have already revealed that probiotics, especially LAB, have promising results in the treatment and prevention of cancer. Also, it has been confirmed that they raise health indicators of their host. Promotion of human intestinal health, amelioration of lactose intolerance symptoms, and decrease of the risk of various other diseases are among the well-known effects of probiotics [11,12]. Both in vitro and in vivo studies have demonstrated that bacterial constituents of LAB strains suppress the progression of breast cancer cell lines through affecting the immune system [[13], [14], [15]].
Probiotics induce their anti-carcinogenic activities through multiple mechanisms. They start their performance by releasing soluble metabolites that can stimulate the immune system and release natural killer cells (NK cells). Also, they may increase the reproduction and differentiation of T-cells, accelerate the growth of bone marrow stem cells (B cell), and stimulate macrophages by producing nitric oxide (NO). Furthermore, they can disturb the metabolism of tumour cells, inducing apoptosis in these cells [3,14,16]. Interestingly, it has been demonstrated that oral administration of probiotics can increase immune cells in Peyer’s patches, which can migrate to other lymphoid tissues and exocrine glands, including mammary glands. Therefore, oral administration of probiotics and fermented dairy products can be effective against non-gastrointestinal tumours, such as breast cancer [13].
Some studies have been done on the anticancer activity of LAB on colon cancer [[17], [18], [19]], though, there are few reports about their activity against breast cancer. Given the benefits of probiotics and the increase of the consumption of probiotic dairy products in the world, looking for original, native LAB strains is required. In this regard, the evaluation of the probiotic potential of different LAB strains, as well as their anticancer activities, is crucial to select a suitable strain. Thus, this study aimed to identify and characterize one of the best native LAB that presents probiotic potential alongside anti-cancer activities. After evaluation of the probiotic properties, cytotoxicity of the isolated strains and their derivatives were examined against MCF-7 tumour cell line. Then, to investigate their activity in detailed, the expression of apoptotic markers (Bax, and Bcl-2) was studied using flow cytometry and western blotting methods.
2. Materials and methods
2.1. Collection and identification of strains
To do this study, 30 samples were collected from three types of traditional dairy products (milk, yoghurt, and cheese) from Kerman province, Iran, and transported to the microbiology laboratory of the Shahid Bahonar University of Kerman, under sterile conditions at 4 °C. Then, 1 mL of each sample was diluted in 5 mL sterilized normal saline (0.9 %), and 0.5 mL of them were transferred to the 5 ml MRS broth medium (Sigma Aldrich) and incubated at 37 °C for 18−24 h in the anaerobic condition in an isolated Jar. The anaerobic atmosphere was provided by using Anaerocult A-strip (Merck, Germany). After that, the overnight bacterial suspensions were adjusted to 0.5 McFarland standard and 100 μL of each sample was separated on MRS agar plates and anaerobically incubated at 37 °C for 24−48 h. Morphological, biochemical, and physiological characteristics of the strains were examined as described in the reported literature [20,21]. These tests included the evaluation of the morphology of the bacterial colonies and their Gram staining. Also, the ability of the bacteria to the production of catalase, oxidase, and spore was investigated. Finally, the Gram-positive bacilli/coccobacilli, catalase-negative, and non-spore-forming strains were chosen to further study on their probiotic properties.
2.2. Evaluation of the tolerance of the strains to the low pH, bile salts and NaCl
The resistance of the isolated strains to the low pH, bile salts, and different concentrations of NaCl was examined as previously described by our group [22]. Briefly, the pH of the bacterial suspensions (0.05 McFarland) was adjusted to 2, 3, and 4, and after 4 h, 50 μL of them were transferred to an MRS agar plate and their growth was tested after incubation at 37 °C in anaerobic conditions. The bacterial cell viability was determined by using the colony count method. Also, the viability of the strains exposed to different concentrations of bile salts (Sigma-Aldrich; 0.3 %, 0.5 %, 1 % w/v), and their tolerance to NaCl (2.5 %, 4.5 %, 6.5 % w/v), was examined through the same method.
2.3. Evaluation of the strains’ antibiotic resistance
The susceptibility of the strains to several antibiotics was put to the test through the disk diffusion method, as described by the Clinical and Laboratory Standards Institute (CLSI) [23]. For this purpose, at first, the 0.5 McFarland standard of each overnight strain was provided and swabbed on MRS agar plates. Then, the antibiotic disks were embedded in the medium, and the plats were incubated for 24 h. The antibiotics were Penicillin (10 μg), Tetracycline (30 μg), Kanamycin (30 μg), Chloramphenicol (30 μg), Oxacillin (1 μg), Ceftriaxone (30 μg), and Trimethoprim/Sulfamethoxazole (1.25/23.75 μg) that were purchased from MAST Group, Merseyside, UK. Subsequently, the diameter of the inhibition zone of each disk was measured and interpreted based on the guidelines of the CLSI.
2.4. Antagonistic activity of the strains against pathogens
The antagonistic activity of the isolated strains was examined through the agar well diffusion method, as previously described by our group [22]. The overnight culture of each isolated strain was centrifuged, its supernatant was filtered and added to the wells made in the MRS agar medium. This medium had already been inoculated by Gram-negative (Escherichia coli PTCC 1330) and Gram-positive (Listeria monocytogenes PTCC 1298, Staphylococcus aureus PTCC 25923, Enterococcus faecalis PTCC 1237, and Bacillus cereus PTCC 1715) pathogens. In this test, the disks of Trimethoprim/Sulfamethoxazole (SXT) were applied as a control.
2.5. Biofilm formation
The capability of biofilm formation of the isolated strains was evaluated as previously described by our group [24]. Briefly, 100 μL (0.5 McFarland) of the fresh culture of each strain alongside 50 μL uncultured medium was added to the wells of a 96-well microtiter plate, and the plate was anaerobically incubated (24 h, 37 °C). Then, the contents of the wells were drained, washed, and dried in the lab temperature. Next, to stabilize the biofilm, 150 μL ethanol (96 % v/v) was added to each well and after 15 min it was drained, and the wells were stained by using crystal violet (2 %) for 45 min. The wells were then rewashed to eliminate the extra stain. After that, 150 μL glacial acetic acid 33 % was used to solubilize the dye combined with bacterial cells. Finally, the absorbance of each well was recorded at 492 nm via a microplate reader, and the thickness of biofilm was interpreted according to Table 1 [25]. Wells filled by 150 μL uncultured medium were considered as controls.
Table 1.
Interpretation of the biofilm production of the strains.
| Absorbance status | Biofilm production status |
|---|---|
| At ≤ Ac | non-biofilm producers |
| Ac < At ≤ 2Ac | weak biofilm producers |
| 2Ac < At ≤ 4Ac | moderate biofilm producers |
| At > 4Ac | strong biofilm producers |
| At = Absorbance of wells of tests; Ac = Absorbance of wells of the control | |
2.6. MCF-7 Cell culture
The anticancer activity of the isolated strains was evaluated against a breast cancer cell line (MCF-7) purchased from the Pasteur Institute, Tehran, Iran. Also, Roswell Park Memorial Institute 1640 (RPMI, Merck, Germany) supplemented with 10 % (v/v) fetal bovine serum (FBS) and 1% penicillin/streptomycin (10,000 IU ml−1 and 10,000 L g ml−1 Gibco) was used as cell culture medium during this study. The cells were cultured in 25*2 cm plastic flasks containing 5 mL RPMI medium and incubated at 37 °C in a humidified 5% CO2 atmosphere for 24 h. The cells were passaged weekly [26].
2.7. Preparation of the strains derivatives
2.7.1. Live bacterial cells (LC)
Overnight cultures of the strains were centrifuged (9000 g, 10 min, 4 °C) to harvest live bacterial cells mass. The deposited bacterial cells were washed twice with sterile PBS (pH 7.2), and 108 CFU/mL were suspended in the cell culture medium (RPMI without antibiotics).
2.7.2. Heat-killed cells (HK cell)
Firstly, the bacterial cells were prepared as explained in subsection 2.7.i. Next, the cells were washed twice with PBS buffer and resuspended in the buffer and heated at 95 °C for 1 h. After that, the mixtures were lyophilized, and the 50 μg of lyophilized cells mass were suspended in 1 mL cell culture medium [27]. The prepared stocks were stored at −20 °C until they were used in further experiments.
2.7.3. Cell-free supernatant (CFS)
The CFS samples were provided by centrifuging overnight bacterial cultures (9000 g, 10 min, 4 °C), lyophilizing the supernatant, and solving 50 μg of them in 1 mL RPMI media. The final suspension was sterilized using 0.22 μm Millipore filters. In a parallel path, to minimize the probable effects of organic acids, the pH of the CFS was adjusted to 7.4 by adding NaOH (1 M), which resulted in neutralized cell-free supernatant (NCFS).
2.8. Trypan blue exclusion assay
The dye exclusion test was done to determine the number of living MCF-7 cells exposed to bacterial derivatives. At first, 106 MCF-7 cells were added to each well of the 24-well plates containing 1 mL RPMI medium and incubated for 24 h. Then, 1 mL of each bacterial derivatives (LC, HK, CFS, and NCFS) was added into the wells, and the plates were incubated for 24 h. The cells treated with cell culture medium were considered as control. After that, the cells were washed by using PBS, and 1 mL Trypan Blue (0.4 %, Sigma-Aldrich) was added to each well, and the mixture was allowed to incubate ∼3 min at room temperature. Then, a drop of the trypan blue/cell mixture was dropped to a hemocytometer, and via a microscope, the unstained (viable) and stained (nonviable) cells were separately counted. Finally, the percentage of cell viability was evaluated as below [28].
2.9. MTT assay
The cytotoxic activity of LC and HK cells (108 CFU/mL), as well as that of CFS and NCFS (50 μg/mL), was assessed after 24 h via MTT assay (3-(4, 5-dimethylthiazol-z-yl)-2, 5-diphenyltetrazolium bromide, Sigma-Aldrich, Darmstadt, Germany) [29]. At first, MCF-7 cells were seeded into the 96-well plates and incubated for 24 h. Next, the previous medium was replaced with a new medium containing HK cells, CFS, and NCFS, and the plates were incubated for 24 h. The medium was then replaced with a fresh medium containing the MTT solution, followed by a 4- hour incubation. After that time, the wells were drained and filled using DMSO (150 μL), and the plates were incubated for 40 min in the dark. Finally, the percentage of cell death was calculated by measuring the absorbance of each well at 517 nm. The cells treated with fresh RPMI medium without any bacterial agents were considered as control.
2.10. Flow cytometry analysis
To understanding whether the isolated strain and its products induce apoptosis in the cancerous cells, the FITC Annexin V Apoptosis Detection Kit (BD Biosciences) was applied by following the instructions of the manufacturer. Flow cytometry detection was performed using a 488 nm argon-ion light source, a 515 nm bandpass filter for FITC fluorescence, and a 630 nm bandpass filter for PI fluorescence. The experimental data analysis was done by Cell Quest software [3].
2.11. Western blot analysis
To conduct Western blot analysis, firstly, the MCF-7 cells were treated with cell-free supernatant (CFS, 50 μg/mL) and incubated for 24 h. After incubation, the pleats were washed twice with ice-cold PBS, the cells were lysed by adding 250 μL lysis buffer, and protease inhibitors to each well. After that, the lysates were centrifuged at 13000 rpm for 15 min at 4 °C, and the total protein concentration was evaluated by the Bradford protein assay kit (Bio-Rad Laboratories, Munchen, Germany). In the next step, 50 μg of the proteins were separated by 12 % SDS-PAGE gel and moved to nitrocellulose membranes (Hybond ECL, GE Healthcare Bio-Sciences Corp., Piscataway, NJ, USA). Five per cent of non-fat dried milk in Tris-buffered saline with TBST buffer was used to blocking step for overnight at 4 °C. Membranes were then probed with Bax (D 21): sc-6236 and Bcl-2 (C-2): sc-7382 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), 1:1000 at room temperature for 3 h. Next, TBST was used to washing, and blots were incubated with a horseradish peroxidase-conjugated secondary antibody diluted in blocking buffer (1:150, 00, GE Healthcare Bio-Sciences Corp.). Then, using the ECL system, the antibody-antigen complexes were spotted and exposed to Lumi-Film chemiluminescent detection film (Roche Applied Science, Germany). Finally, the intensity of the protein expression was evaluated using LabWork analyzing software (UVP, Cambridge, UK). It is worth mentioning that GAPDH immunoblotting was used as a loading control [17].
2.12. Molecular identification of the isolated strain
Molecular identification of the selected strain that showed the best properties was carried out by the sequencing of 16S rRNA. The extraction of bacterial genomic DNA was done using the Genomic DNA extraction kit (Bioneer Co., Korea) based on the manufacturer’s instructions. Universal 16S rRNA PCR primers set were used to amplify the 16S rRNA gene. In this experiment, U8F (5′-AGAGTTTGATCCTGGCTCAG-3′) and U1390R (5′-GACGGGCGGTGTGTACAA-3′) were applied as the forward and reverse primers, respectively. It was done in a total volume of 50 μL comprising genomic DNA (50 ng), Taq DNA polymerase (1.25 units), primers (20 pmol), PCR Buffer, and dNTPs (200 μM). The PCR steps were as following; the early denaturation (35 cycles, 3 min, 94 °C), the recreation of denaturation (30 s, 94 °C), recombination of the double-stranded (30 s, 58 °C), and extension (2 min at 72 °C followed by 7 min at 72 °C to final extension). PCR products were electrophoresed on agarose gel (1%), and afterwards, the purification of amplified 16S rRNA bands was performed via DNA extraction kit (Cinaclone) and the Bioneer Company (South Korea) carried out the DNA sequencing on both strands. The BLAST analysis was done by applying the sequence data of 16S rRNA (http://www.ncbi.nlm.nih.gov/BLAST/), and the neighbour-joining phylogenetic tree was made using the Molecular Evolutionary Genetics Analysis (MEGA) Software Version 6.0.
2.13. Statistical analysis
All tests were done three times, and the results were expressed as mean and standard deviation (S.D). The values in the tolerance test were compared by One Way ANOVA by SPSS software vision 21. P-values less than 0.05 and 0.001 were considered as a significant difference.
3. Results
3.1. Sample collection and identification of LAB strains
Eighteen bacterial strains were primarily isolated from the 30 traditional dairy products collected in Kerman, Iran. These strains were Gram-positive, coccobacilli or bacilli in shape, catalase-negative, and oxidase negative (Table 2). These are the main characteristics of lactic acid bacteria [21,22].
Table 2.
Morphological, biochemical and physiological characteristics of the strains.
| strains | source | gram stain | spore | catalase | oxidase | colony morphology |
|---|---|---|---|---|---|---|
| M1 | milk | Gram-positive bacilli with bipolar staining | – | – | – | tiny, white |
| M2 | milk | Gram-positive bacilli with various size | – | – | – | smooth, round |
| M3 | milk | Gram-positive short bacilli | – | – | – | smooth, round |
| M4 | milk | Gram-positive bacilli | – | – | – | smooth, round |
| M5 | milk | Gram-positive bacilli | – | – | – | smooth, round, colorless |
| M6 | milk | Gram-positive long bacilli | – | – | – | smooth, round, big |
| M7 | milk | Gram-positive bacilli | – | – | – | tiny, white |
| M8 | milk | Gram-positive cocci | – | + | – | white |
| M9 | milk | Gram-positive bacilli | – | + | – | tiny, white |
| M10 | milk | Gram-positive bacilli | – | + | – | medium, white |
| Y1 | yogurt | Gram-positive bacilli | – | – | – | mucoid |
| Y2 | yogurt | Gram-positive cocci | – | – | – | medium, white |
| Y3 | yogurt | Gram-positive bacilli | – | – | – | big, mucoid |
| Y4 | yogurt | Gram-positive bacilli | – | – | – | medium, mucoid |
| Y5 | yogurt | Gram-positive bacilli | – | – | – | mucoid, colorless |
| Y6 | yogurt | Gram-positive bacilli | – | – | – | medium, white |
| Y7 | yogurt | Gram-positive coccobacilli | – | + | – | tiny, white |
| Y8 | yogurt | Gram-positive bacilli | – | + | – | white |
| Y9 | yogurt | Gram-positive cocci | – | + | – | white |
| Y10 | yogurt | Gram-positive bacilli | – | + | – | tiny, white |
| CH1 | cheese | Gram-positive bacilli | – | – | – | colorless |
| CH2 | cheese | Gram-positive bacilli | – | – | – | big, white |
| CH3 | cheese | Gram-positive bacilli | – | – | – | smooth, round |
| CH4 | cheese | Gram-positive bacilli | – | – | – | colorless |
| CH5 | cheese | Gram-positive cocci | – | – | – | mucoid, white |
| CH6 | cheese | Gram-positive bacilli | – | – | – | medium, white |
| CH7 | cheese | Gram-positive bacilli | – | – | – | tiny, white |
| CH8 | cheese | Gram-positive bacilli | – | + | – | white |
| CH9 | cheese | Gram-positive coccobacilli | – | + | – | colorless |
| CH10 | cheese | Gram-positive cocci | – | + | – | medium, white |
3.2. The tolerance of the strains to low pH, bile salt and NaCl
The results of the assessment of the strain's resistance to low pH, bile salt, and NaCl has been shown in Table 3. According to the results, all isolated strains were highly tolerant to low pH (2, 3, and 4). While, M1, CH1, and CH4 offered the highest level of resistance, their viability was more than 70 % even in pH 2. Furthermore, the isolated strains showed more than 50 % viability when they were exposed to 0.3 % bile salt. This behaviour was dose-depend and, interestingly, M1, and CH4 were two strains that preserved their durability at the highest concentration of bile salt (1 %).
Table 3.
The viability of the isolated LAB strains exposed to low pH, bile salt, and NaCl.
| strains | samples | Resistance to low pH (%) |
Resistance to bile salt (%) |
Resistance to NaCl |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| pH 2 | pH 3 | pH4 | 0.3 % | 0.5 % | 1 % | 2.5 % | 4.5 % | 6.5 % | ||
| M1 | Milk | 70 | 72.86 | 74.75 | 67.62 | 50.1 | 49.52 | + | + | + |
| M2 | Milk | 55.1 | 59 | 63 | 66 | 54.2 | 41.6 | – | + | + |
| M3 | Milk | 57.45 | 62.95 | 63.73 | 47.38 | 34.5 | 33.84 | + | – | – |
| M4 | Milk | 50.36 | 57.95 | 59.56 | 55.65 | 33.94 | 33.59 | + | – | – |
| M5 | Milk | 59.08 | 63.34 | 64.26 | 38.31 | 32.83 | 32.42 | + | + | – |
| M6 | Milk | 60 | 66.43 | 67.7 | 41.22 | 33.83 | 29.74 | + | – | – |
| M7 | Milk | 57.08 | 60.36 | 63.31 | 42.27 | 40.05 | 38.26 | + | – | – |
| Y1 | Yogurt | 61.1 | 75.26 | 76.54 | 58.64 | 49.1 | 41.52 | + | – | – |
| Y3 | Yogurt | 50.28 | 55 | 55.75 | 35.27 | 31.89 | 27.46 | + | – | – |
| Y4 | Yogurt | 59.22 | 67.9 | 73 | 40.39 | 35.87 | 33.19 | + | – | – |
| Y5 | Yogurt | 54.09 | 63.48 | 66.27 | 52.65 | 37.38 | 37.56 | + | – | – |
| Y6 | Yogurt | 55.53 | 66.86 | 68.86 | 50.88 | 36.38 | 36.12 | + | – | – |
| CH1 | Cheese | 70.51 | 85.26 | 85.88 | 48.79 | 33.06 | 22.22 | + | – | – |
| CH2 | Cheese | 63.34 | 67.65 | 73.01 | 42.34 | 36.94 | 29.57 | + | – | – |
| CH3 | Cheese | 58.91 | 61.72 | 62.19 | 52.27 | 36.96 | 35.62 | + | – | – |
| CH4 | Cheese | 79.56 | 83.56 | 84.78 | 68.98 | 67.99 | 49.19 | + | + | – |
| CH6 | Cheese | 58.86 | 70.85 | 78.72 | 69.2 | 45.74 | 40.51 | + | – | – |
| CH7 | Cheese | 54.25 | 63.62 | 64.68 | 59.1 | 36.27 | 34.53 | + | + | – |
Values expressed as a percentage of survival after 4 h. +: growth observed, -: No growth observed.
Likewise, the tolerance of the strains towards NaCl was substantial. As mentioned in Table 3, the isolated strains all survived and grew in the concentration of 2.5 % NaCl. Four strains endured 4.5 % NaCl, and M1 was the only strain that resisted to the concentration of 6.5 % NaCl.
3.3. Antibiotic resistance of the isolated strains
The antibiotic resistance of the strains was assessed toward seven antibiotics via the disk diffusion method. According to the results (Table 4), strains all were sensitive to tetracycline, chloramphenicol, and trimethoprim/sulfamethoxazole, while they were resistant to kanamycin. Also, they showed variable reactions to penicillin, oxacillin, and ceftriaxone. Among these strains, M1 showed no warring resistance to the common antibiotics. This strain was only resistant toward kanamycin.
Table 4.
Antibiotic susceptibility of the isolated LAB strains.
| strains | Antibiotics |
||||||
|---|---|---|---|---|---|---|---|
| Pen | Tet | Kan | Chl | Oxa | Cro | SXT | |
| M1 | S | S | R | S | I | S | S |
| M2 | S | S | R | S | R | I | I |
| M3 | S | S | R | S | R | I | I |
| M4 | S | S | R | S | S | S | S |
| M5 | I | S | R | S | I | S | S |
| M6 | I | S | R | S | R | I | S |
| M7 | I | S | R | S | S | S | S |
| Y1 | I | S | R | S | R | S | S |
| Y3 | S | S | R | S | R | S | S |
| Y4 | S | S | R | S | I | S | S |
| Y5 | I | S | R | S | R | S | S |
| Y6 | S | S | R | S | R | S | S |
| CH1 | I | S | R | S | R | S | S |
| CH2 | I | S | R | S | I | S | S |
| CH3 | S | S | R | S | R | S | S |
| CH4 | S | S | R | S | I | S | S |
| CH6 | I | S | R | S | I | S | S |
| CH7 | I | S | R | S | R | S | S |
S: susceptible, I: intermediate susceptible, R: resistant.
Pen: Penicillin, Tet: Tetracycline, Kan: Kanamycin, Chl: Chloramphenicol, OXA: Oxacillin, Cro: Ceftriaxone, SXT: Trimethoprim/Sulfamethoxazole.
3.4. Antagonistic activity of the isolated strains
The antagonistic activity of the strains was evaluated against five pathogens. This evaluation revealed that all strains have significant antimicrobial activity. Notably, M1 and seven other strains led to the inhibition zone diameters ≥ 20 mm (Table 5), indicating the substantial antibacterial activity of the isolated LAB.
Table 5.
The antagonistic activity of the isolated LAB strains against pathogens.
| Strains |
E. coli PTCC 1330 |
E. faecalis PTCC 1237 |
S. aureus PTCC 25923 |
L. monocytogenes PTCC 1298 |
B. cereus PTCC 1715 |
|---|---|---|---|---|---|
| M1 | ++ | ++ | ++ | ++ | ++ |
| M2 | ++ | + | ++ | + | + |
| M3 | ++ | ++ | ++ | ++ | ++ |
| M4 | ++ | ++ | + | + | + |
| M5 | + | ++ | ++ | + | ++ |
| M6 | ++ | ++ | + | ++ | ++ |
| M7 | + | ++ | ++ | ++ | ++ |
| Y1 | ++ | ++ | ++ | ++ | ++ |
| Y3 | + | ++ | ++ | ++ | + |
| Y4 | ++ | ++ | ++ | ++ | ++ |
| Y5 | ++ | ++ | ++ | ++ | ++ |
| Y6 | + | ++ | + | ++ | + |
| CH1 | + | + | ± | + | + |
| CH2 | +++ | ++ | ++ | ++ | ++ |
| CH3 | + | ++ | ++ | + | + |
| CH4 | +++ | ++ | ++ | ++ | ++ |
| CH6 | + | ++ | + | + | + |
| CH7 | ++ | ++ | ++ | ++ | ++ |
-: ≤ 0 mm; ±: 1−9 mm; +: 10−19 mm; ++: 20−29 mm; +++ : >30mm.
3.5. Biofilm formation of the isolated strains
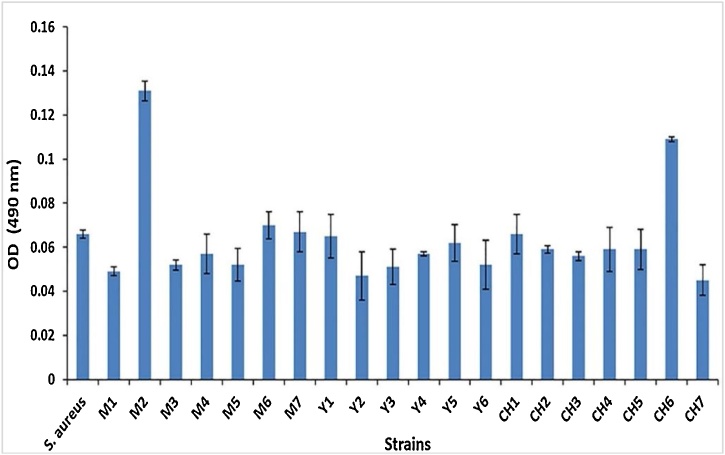
Based on the results, surprisingly, only two strains, M2 and CH6, formed a weak biofilm (Fig. 1). Others were not capable of forming any biofilm in this study.
Fig. 1.
The biofilm formation status of the strains. M2 and CH6 are two strains weakly produce biofilm. S. aureus was used as a well-known biofilm producer strain. The data presented are the mean numbers ± standard deviations.
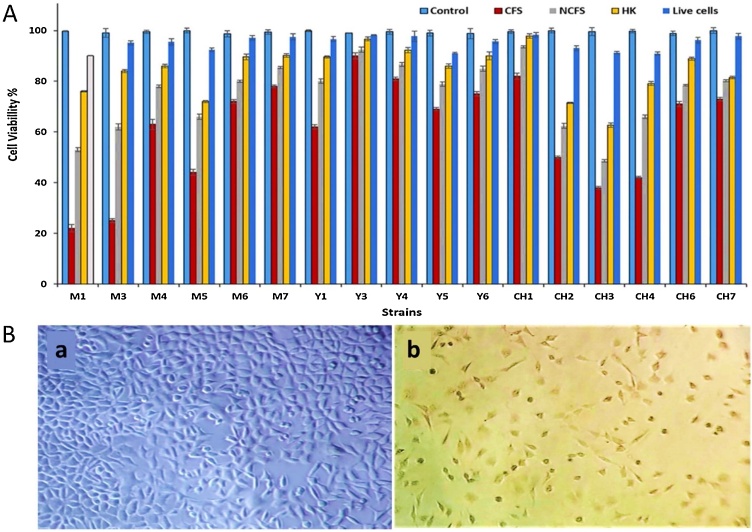
3.6. Trypan blue staining
The results of the elementary evaluation of the cytotoxicity of the bacterial derivatives (CFS, NCFS, HK, and Live cells) through Trypan blue staining revealed that except live cells, other three compounds considerably decreased the viability of the cells. Details of these results have been provided in Fig. 2A. Fig. 2B depicts the effect of CFS derived from M1 on the cancerous cells as an example. Noteworthy, this agent had by far the most cytotoxicity compared to others. Based on the results of this experiment, strains M1, M3, M5, CH2, CH3, and CH4 were chosen to further assessments because they had significant toxicity against MCF-7 cells.
Fig. 2.
(A) shows the effects of bacterial derivatives on MCF-7 cells evaluated via Trypan blue assay, and (B) shows the control MCF-7 cells treated with the cell culture medium (Ba) in comparison with the cells treated with the medium containing CFS (Bb).
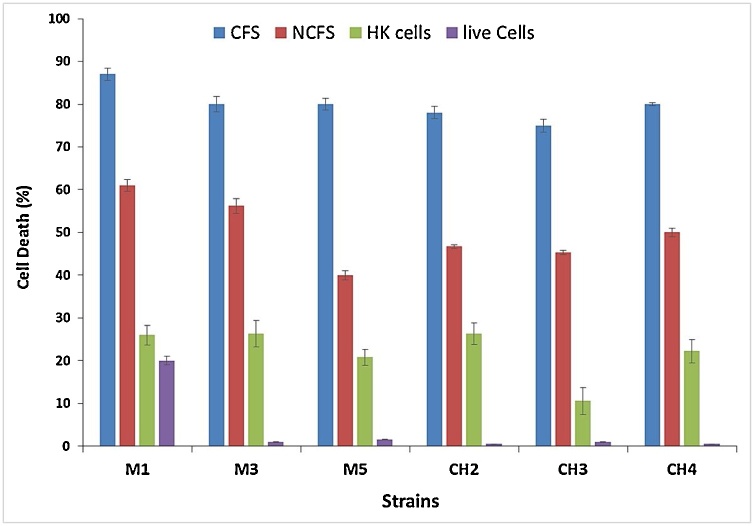
3.7. MTT assay results
As Fig. 3 shows, except for living cells, agents all were able to decrease the viability of the cancerous cells, and among them, CFS had by far the highest cytotoxicity against MCF-7 cells after 24 h. NCFS, also, appeared as the second most toxic agent. The CFS originated from M1 and CH4 strains, for example, destroyed more than 85 per cent of the cancerous cells. In contrast, except for M1, other live-cells did not show any considerable activity against the MCF-7 cells. Overall, due to its highest cytotoxicity and its desirable performance in other previous tests, M1 was selected as a great strain and considered for further investigations.
Fig. 3.
Cytotoxicity effects of isolated strains on MCF-7 after 24 h of treatment with bacterial derivatives. The data presented are the mean numbers ± standard deviations. All treatments are significantly different from control (p < 0.05).
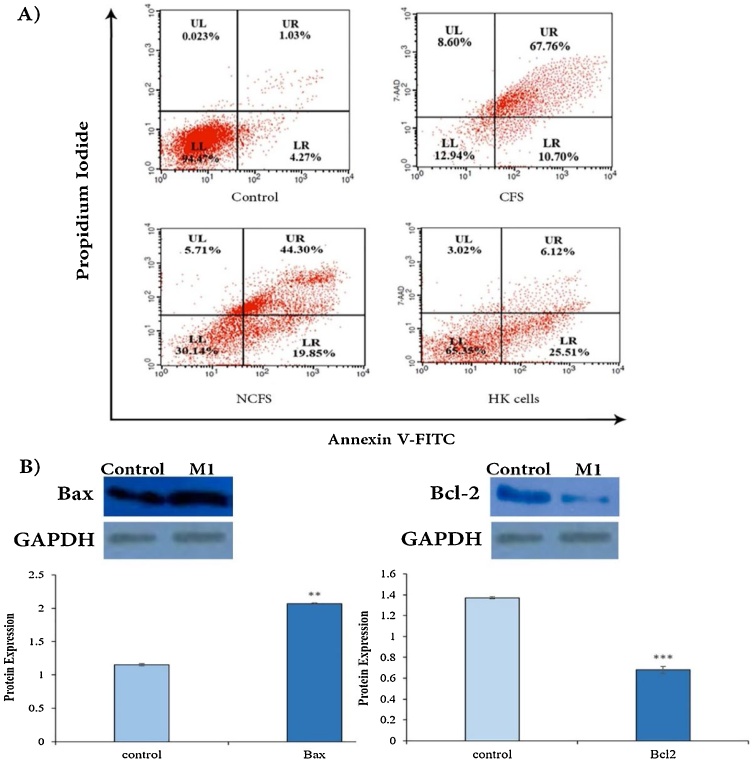
3.8. Flow cytometry analysis
To a thorough study of the M1 cytotoxicity, the percentage of the apoptosis induced in MCF-7 cells by CFS, NCFS, and HK Cells, originated from M1, was analyzed by flow cytometry. Fig. 4A illustrates that total apoptosis triggered by CFS, NCFS, and HK cells was 78.46 %, 64.15 %, and 31.63 %, respectively. Also, CFS and NCFS induced early apoptosis more than late apoptosis, but HK cells mostly induced late apoptosis. Given the results, CFS was nominated, and its effect on protein expression in the cell line was studied.
Fig. 4.
(A) Flow cytometry analysis of apoptosis in MCF-7 cells treated with the CFS, NCFS, and HK cells of M1 strain. The control was untreated MCF-7 cells. (B) Protein expression of Bax and Bcl-2 genes of MCF-7 cells in the control group and the group treated with CFS of M1 strain. **P < 0.05 and ***p < 0.001 significantly different from control.
3.9. Western blotting analysis
To understand how bacterial CFS kills cancerous cells, the expression of two essential proteins Bax and Bcl-2 that are known as pro-apoptotic and anti-apoptosis agents, respectively, was investigated in the MCF-7 cells exposed to CFS. The results revealed that the CFS not only up-regulates Bax expression but also down-regulates Bcl-2 expression compared to the control (Fig. 4B).
3.10. Molecular identification of the M1 strain
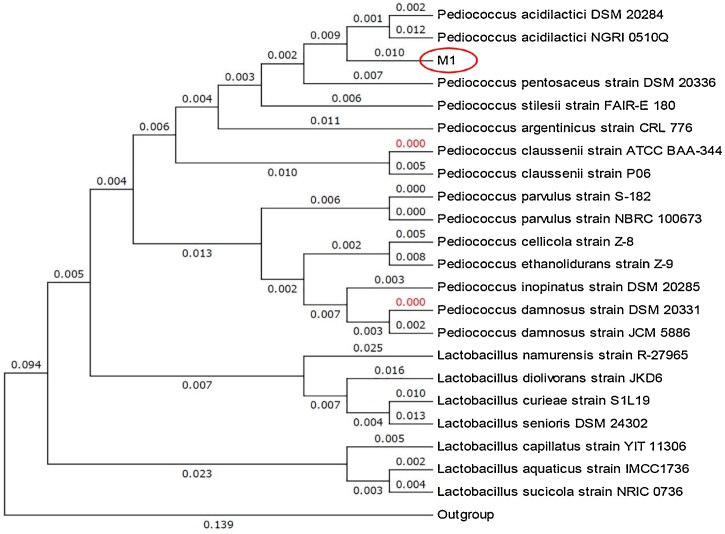
As the last stage of the present study, due to its unique properties, the M1 strain was selected and genetically identified by using information obtained from the sequencing of the 16S rRNA gene, and its phylogenetic tree provided by MEGA6 software. As Fig. 5 shows, M1 is determined as Pediococcus SP. This strain showed 95.9 % and 94.54 % similarities with Pediococcus acidlactici DSM 20284 and Pediococcus acidilactici NGRI 0510Q, respectively.
Fig. 5.
Phylogenetic analysis of the 16S rRNA sequence obtained from the amplified product of M1 strain. The analysis was constructed using the neighbor-joining method in MEGA6.
4. Discussion
Cancer is considered as a complex disease and incurable in some cases [30]. Breast cancer, for instance, is one of the most common cancers in women and causes many deaths worldwide. Currently, conventional treatments such as surgery, chemotherapy, and radiotherapy are being operated as breast cancer treatments, notwithstanding their dangerous side effects. Hence, searching for a safer and effective alternative approach is essential [6,31]. Food sources have been nominated as a group that has the potential for cancer prevention and treatment. In this regard, probiotics have become striking in recent years due to their promising activities against cancerous cells and their positive effects on consumers’ health [4,6]. The nature and probiotic efficacy of the lactobacilli species largely depend on their environment; therefore the source from which they are isolated is crucial [32]. This study aimed to look for a novel, potent probiotic strain that is qualified to combat cancer without the need for aggressive methods.
Being tolerant of gastrointestinal conditions, low pH, high concentrations of bile salts, and high concentrations of NaCl for probiotic strains are as crucial as being susceptible to widespread antibiotics [33,34]. Fortunately, the results of the present study demonstrated that all isolated strains are highly tolerant toward low pH, high concentrations of bile salts, and NaCl. Furthermore, CH4, M1, and M5 strains presented unparalleled durability in extreme conditions. These results are in agreement with previous reports [35,36]. It has been found that lactic acid bacteria can resist these extreme environments due to their efficient H+ ATPase pump, cytoplasmic membrane constituents that balance the osmotic potential of cytoplasm, and the production of enzymes hydrolysis bile salts [[36], [37], [38]].
The susceptibility of probiotic strains to antibiotics is also crucial because the transformation of the resistance genes to pathogens is feasible in the human gastrointestinal tract and might result in a catastrophe [39]. In the present study, the strains showed high sensitivity to antibiotics, whereas they were resistant to kanamycin and partially to oxacillin. Other researchers have already reported the resistance of lactobacilli to kanamycin [35,40]. According to them, the resistance of some lactobacilli species against kanamycin, Oxacillin, and vancomycin is an intrinsic feature [41,42].
Concerning the formation of biofilm by LAB strains, recent studies have reported that this depends on some factors such as strains, medium composites, and growth conditions [43]. In the study previously conducted by our group, it was observed that different types of LAB could form a biofilm with different thicknesses [22]. However, in the present study, except for two strains (M2 and CH6), others could not form a considerable biofilm. These results are in agreement with some of the previous studies [43,44].
In the present study, all strains showed antibacterial activity against the pathogenic bacteria. Since the LAB are considered as microflora of the human gastrointestinal tract, the most desirable ones are those that have antagonistic activity against pathogens. The effectiveness of LAB in the treatment and prevention of gastrointestinal infectious diseases has already been proven [[45], [46], [47]]. Recently, Aarti1 et al. have reported the antibacterial activity of Lactobacillus brevis 35 strain LAP2, isolated from a local fermented product. They believe that antibacterial activity refers to compounds other than acids because this activity was not affected by low pH or neutral pH [48]. The precise mechanism through which probiotics induce their effect on pathogens has not been known yet. However, it may attribute to the production of bacteriocins (like nisin), or decreasing the environment pH by secreting acidic compounds such as lactic acid. They also compete with pathogens for nutrients and surface and interfere with their colonization. Additionally, the production of active enzymes that inhibit pathogenic bacteria has been observed in a few strains [49].
Both Trypan blue staining and MTT assay confirmed the cytotoxicity of the derivatives of the isolated strains, making them more advantageous compared to other strains. Researchers have suggested that the anti-proliferative activity of LAB can be contributed to producing short-chain fatty acids such as butyric acid and propionic acid, secretion of a verity of active compounds like polysaccharide segments of the bacterial cell wall (exopolysaccharides, etc.), and the compounds existing in the cytoplasmic extract [3,13,19]. In this study, a significant difference between the cell-free supernatant’s (CFS) activity and that of the neutralized CFS appeared, indicating their activity against cancerous cells much depends on the availability of their acidic compounds. Incidentally, some recent studies have revealed that exopolysaccharides produced by LAB show antiproliferative activity [50]. Whereby the relatively moderate activity of neutralized CFS can be justified. It could be concluded that the CFS has the highest toxicity against cancerous cells because it consists of a group of agents such as organic acids and exopolysaccharides.
Considering that the CFS originated from the M1 strain showed the most effect on MCF-7 cells, it was selected to investigate the way by which it induces cell death. Flow cytometry analysis shows a high percentage of apoptosis (78.46 %) in the cells exposed to CFS. On the other hand, it was revealed that this agent increases the expression of Bax protein and decreases that of Bcl-2, two critical regulators of apoptosis in cells. Consequently, the activation of the apoptotic pathway in the cancerous cells is inevitable [19]. Similarly, previous studies performed on different cancer cells have determined LAB strains induce apoptosis in the cells, and they not only increased Bax but also decreased Bcl-2 expression in cancer cells [17,19,51].
5. Conclusion
The Pediococcus spp. isolated from local dairy products had satisfactory probiotic properties. It was adaptable to gastrointestinal conditions, susceptible to antibiotics, and showed antimicrobial activity. Besides, derivatives of the strain, particularly its cell-free supernatant, significantly induced apoptosis in the MCF-7 cell line by increasing the Bax protein expression and decreasing that of the Bcl-2. Overall, according to the current findings, Pediococcus sp. isolated and characterized in this study can be considered as one of the most promising probiotic strains to apply as both alternative cancer treatment and functional food supplements.
Ethical approval
This article contains no studies with human participants or animals performed by any of the authors.
Funding
Not applicable
CRediT authorship contribution statement
Tayebeh Jafari-Nasab: Investigation, Writing - original draft, Methodology. Moj Khaleghi: Resources, Conceptualization, Supervision. Alireza Farsinejad: Supervision. Sadegh Khorrami: Writing - original draft, Writing - review & editing, Investigation.
Declaration of Competing Interest
The authors declare that they have no conflicts of interest.
Acknowledgements
The authors would like to express their very great appreciation to Professor Saeed Esmaeili Mahani for his valuable and constructive suggestions, as well as providing facilities while the "western blot" technique was being done in this research work.
Contributor Information
Moj Khaleghi, Email: m.khaleghi@uk.ac.ir.
Sadegh Khorrami, Email: s.khorrami.992@gmail.com.
References
- 1.Elfahri K.R., Vasiljevic T., Yeager T., Donkor O.N. Anti-colon cancer and antioxidant activities of bovine skim milk fermented by selected Lactobacillus helveticus strains. J. Dairy Sci. 2016;99:31–40. doi: 10.3168/jds.2015-10160. [DOI] [PubMed] [Google Scholar]
- 2.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Wang S.-M., Zhang L.-W., Fan R.-B., Han X., Yi H.-X., Zhang L.-L., Xue C.-H., Li H.-B., Zhang Y.-H., Shigwedha N. Induction of HT-29 cells apoptosis by lactobacilli isolated from fermented products. Res. Microbiol. 2014;165:202–214. doi: 10.1016/j.resmic.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Kahouli I., Malhotra M., Alaoui-Jamali M., Prakash S. In-vitro characterization of the anti-cancer activity of the probiotic bacterium Lactobacillus fermentum NCIMB 5221 and potential against colorectal cancer. J. Cancer Sci. Ther. 2015;7:224–235. [Google Scholar]
- 5.Patel S. Breast cancer: lesser-known facets and hypotheses. Biomed. Pharmacother. 2018;98:499–506. doi: 10.1016/j.biopha.2017.12.087. [DOI] [PubMed] [Google Scholar]
- 6.Dasari S., Kathera C., Janardhan A., Kumar A.P., Viswanath B. Surfacing role of probiotics in cancer prophylaxis and therapy: a systematic review. Clin. Nutr. 2017;36:1465–1472. doi: 10.1016/j.clnu.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 7.FAO/WHO . Rep. from FAO/WHO Expert Consult. 2001. Evaluation of health and nutritional properties of powder milk and live lactic acid bacteria; pp. 1–4. [Google Scholar]
- 8.Reid G. The growth potential for dairy probiotics. Int. Dairy J. 2015;49:16–22. doi: 10.1016/j.idairyj.2015.04.004. [DOI] [Google Scholar]
- 9.Zhong L., Zhang X., Covasa M. Emerging roles of lactic acid bacteria in protection against colorectal cancer. World J. Gastroenterol. WJG. 2014;20:7878. doi: 10.3748/wjg.v20.i24.7878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haghshenas B., Abdullah N., Nami Y., Radiah D., Rosli R., Khosroushahi A.Y. Different effects of two newly-isolated probiotic Lactobacillus plantarum 15HN and Lactococcus lactis subsp. Lactis 44Lac strains from traditional dairy products on cancer cell lines. Anaerobe. 2014;30:51–59. doi: 10.1016/j.anaerobe.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Kechagia M., Basoulis D., Konstantopoulou S., Dimitriadi D., Gyftopoulou K., Skarmoutsou N., Fakiri E.M. Health benefits of probiotics: a review. Int. Sch. Res. Not. 2013;2013 doi: 10.5402/2013/481651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zangeneh M., Khorrami S., Khaleghi M. Bacteriostatic activity and partial characterization of the bacteriocin produced by L. Plantarum sp. Isolated from traditional sourdough. Food Sci. Nutr. 2020 doi: 10.1002/fsn3.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aragón F., Carino S., Perdigón G., de M. de LeBlanc A. The administration of milk fermented by the probiotic Lactobacillus casei CRL 431 exerts an immunomodulatory effect against a breast tumour in a mouse model. Immunobiology. 2014;219:457–464. doi: 10.1016/j.imbio.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Maroof H., Hassan Z.M., Mobarez A.M., Mohamadabadi M.A. Lactobacillus acidophilus could modulate the immune response against breast cancer in murine model. J. Clin. Immunol. 2012;32:1353–1359. doi: 10.1007/s10875-012-9708-x. [DOI] [PubMed] [Google Scholar]
- 15.Rachid M., Matar C., Duarte J., Perdigon G. Effect of milk fermented with a Lactobacillus helveticus R389 (+) proteolytic strain on the immune system and on the growth of 4T1 breast cancer cells in mice, FEMS Immunol. Med. Microbiol. 2006;47:242–253. doi: 10.1111/j.1574-695X.2006.00088.x. [DOI] [PubMed] [Google Scholar]
- 16.Raman M., Ambalam P., Doble M. Springer; 2016. Probiotics and Bioactive Carbohydrates in Colon Cancer Management. [DOI] [Google Scholar]
- 17.Chondrou P., Karapetsas A., Kiousi D.E., Tsela D., Tiptiri-Kourpeti A., Anestopoulos I., Kotsianidis I., Bezirtzoglou E., Pappa A., Galanis A. Lactobacillus paracasei K5 displays adhesion, anti-proliferative activity and apoptotic effects in human colon cancer cells. Benef. Microbes. 2018;9:975–983. doi: 10.3920/BM2017.0183. [DOI] [PubMed] [Google Scholar]
- 18.Dubey V., Ghosh A.R., Bishayee K., Khuda-Bukhsh A.R. Appraisal of the anti-cancer potential of probiotic Pediococcus pentosaceus GS4 against colon cancer: in vitro and in vivo approaches. J. Funct. Foods. 2016;23:66–79. [Google Scholar]
- 19.Tukenmez U., Aktas B., Aslim B., Yavuz S. The relationship between the structural characteristics of lactobacilli-EPS and its ability to induce apoptosis in colon cancer cells in vitro. Sci. Rep. 2019;9:1–14. doi: 10.1038/s41598-019-44753-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arasu M.V., Al-Dhabi N.A., Ilavenil S., Choi K.C., Srigopalram S. In vitro importance of probiotic Lactobacillus plantarum related to medical field. Saudi J. Biol. Sci. 2016;23:S6–S10. doi: 10.1016/j.sjbs.2015.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Axelsson L. Lactic acid bacteria: classification and physiology. FOOD Sci. Technol. YORK-MARCEL DEKKER. 2004;139:1–66. [Google Scholar]
- 22.Zangeneh M., Khaleghi M., Khorrami S. Isolation of Lactobacillus plantarum strains with robust antagonistic activity, qualified probiotic properties, and without antibiotic-resistance from traditional sourdough. Avicenna J. Clin. Microbiol. Infect. 2019;6:66–74. [Google Scholar]
- 23.Cockerill F.R. Clinical and Laboratory Standards Institute (CLSI); 2011. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-first Informational Supplement. [Google Scholar]
- 24.Khaleghi M., Khorrami S., Ravan H. Identification of Bacillus thuringiensis bacterial strain isolated from the mine soil as a robust agent in the biosynthesis of silver nanoparticles with strong antibacterial and anti-biofilm activities. Biocatal. Agric. Biotechnol. 2019;18 doi: 10.1016/j.bcab.2019.101047. [DOI] [Google Scholar]
- 25.Stepanović S., Vuković D., Dakić I., Savić B., Švabić-Vlahović M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods. 2000;40:175–179. doi: 10.1016/S0167-7012(00)00122-6. [DOI] [PubMed] [Google Scholar]
- 26.Nami Y., Abdullah N., Haghshenas B., Radiah D., Rosli R., Khosroushahi A.Y. Probiotic potential and biotherapeutic effects of newly isolated vaginal Lactobacillus acidophilus 36YL strain on cancer cells. Anaerobe. 2014;28:29–36. doi: 10.1016/j.anaerobe.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 27.Choi S.S., Kim Y., Han K.S., You S., Oh S., Kim S.H. Effects of Lactobacillus strains on cancer cell proliferation and oxidative stress in vitro. Lett. Appl. Microbiol. 2006;42:452–458. doi: 10.1111/j.1472-765X.2006.01913.x. [DOI] [PubMed] [Google Scholar]
- 28.Strober W. Trypan blue exclusion test of cell viability. Curr. Protoc. Immunol. 2015;111 doi: 10.1002/0471142735.ima03bs111. A3-B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khorrami S., Zarrabi A., Khaleghi M., Danaei M., Mozafari M. Selective cytotoxicity of green synthesized silver nanoparticles against the MCF-7 tumor cell line and their enhanced antioxidant and antimicrobial properties. Int. J. Nanomed. 2018;13:8013–8024. doi: 10.2147/IJN.S189295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dalkic E., Wang X., Wright N., Chan C. Cancer-drug associations: a complex system. PLoS One. 2010;5 doi: 10.1371/journal.pone.0010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khorrami S., Abdollahi Z., Eshaghi G., Khosravi A., Bidram E., Zarrabi A. An improved method for fabrication of Ag-GO nanocomposite with controlled anti-cancer and anti-bacterial behavior; a comparative study. Sci. Rep. 2019;9:9167. doi: 10.1038/s41598-019-45332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aarti C., Khusro A., Varghese R., Arasu M.V., Agastian P., Al-Dhabi N.A., Ilavenil S., Choi K.C. In vitro investigation on probiotic, anti-Candida, and antibiofilm properties of Lactobacillus pentosus strain LAP1. Arch. Oral Biol. 2018;89:99–106. doi: 10.1016/j.archoralbio.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 33.Jena P.K., Trivedi D., Thakore K., Chaudhary H., Giri S.S., Seshadri S. Isolation and characterization of probiotic properties of lactobacilli isolated from rat fecal microbiota. Microbiol. Immunol. 2013;57:407–416. doi: 10.1111/1348-0421.12054. [DOI] [PubMed] [Google Scholar]
- 34.Dunne C., O’Mahony L., Murphy L., Thornton G., Morrissey D., O’Halloran S., Feeney M., Flynn S., Fitzgerald G., Daly C. In vitro selection criteria for probiotic bacteria of human origin: correlation with in vivo findings. Am. J. Clin. Nutr. 2001;73:386s–392s. doi: 10.1093/ajcn/73.2.386s. [DOI] [PubMed] [Google Scholar]
- 35.Hoque M.Z., Akter F., Hossain K.M., Rahman M.S.M., Billah M.M., Islam K.M.D. Isolation, identification and analysis of probiotic properties of Lactobacillus spp. From selective regional yoghurts. World J. Dairy Food Sci. 2010;5:39–46. [Google Scholar]
- 36.De Almeida Júnior W.L.G., Da Silva Ferrari Í., De Souza J.V., Da Silva C.D.A., Da Costa M.M., Dias F.S. Characterization and evaluation of lactic acid bacteria isolated from goat milk. Food Control. 2015;53:96–103. [Google Scholar]
- 37.Rani R.P., Anandharaj M., Ravindran A.D. Characterization of bile salt hydrolase from Lactobacillus gasseri FR4 and demonstration of its substrate specificity and inhibitory mechanism using molecular docking analysis. Front. Microbiol. 2017;8:1004. doi: 10.3389/fmicb.2017.01004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rushdy A.A., Gomaa E.Z. Antimicrobial compounds produced by probiotic Lactobacillus brevis isolated from dairy products. Ann. Microbiol. 2013;63:81–90. [Google Scholar]
- 39.Lerner A., Matthias T., Aminov R. Potential effects of horizontal gene exchange in the human gut. Front. Immunol. 2017;8:1630. doi: 10.3389/fimmu.2017.01630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo H., Pan L., Li L., Lu J., Kwok L., Menghe B., Zhang H., Zhang W. Characterization of antibiotic resistance genes from Lactobacillus isolated from traditional dairy products. J. Food Sci. 2017;82:724–730. doi: 10.1111/1750-3841.13645. [DOI] [PubMed] [Google Scholar]
- 41.Campedelli I., Mathur H., Salvetti E., Clarke S., Rea M.C., Torriani S., Ross R.P., Hill C., O’Toole P.W. Genus-wide assessment of antibiotic resistance in Lactobacillus spp. Appl. Environ. Microbiol. 2019;85:e01738–18. doi: 10.1128/AEM.01738-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Danielsen M., Wind A. Susceptibility of Lactobacillus spp. to antimicrobial agents. Int. J. Food Microbiol. 2003;82:1–11. doi: 10.1016/s0168-1605(02)00254-4. [DOI] [PubMed] [Google Scholar]
- 43.Ramírez M.D.F., Smid E.J., Abee T., Groot M.N.N. Characterisation of biofilms formed by Lactobacillus plantarum WCFS1 and food spoilage isolates. Int. J. Food Microbiol. 2015;207:23–29. doi: 10.1016/j.ijfoodmicro.2015.04.030. [DOI] [PubMed] [Google Scholar]
- 44.Aoudia N., Rieu A., Briandet R., Deschamps J., Chluba J., Jego G., Garrido C., Guzzo J. Biofilms of Lactobacillus plantarum and Lactobacillus fermentum: effect on stress responses, antagonistic effects on pathogen growth and immunomodulatory properties. Food Microbiol. 2016;53:51–59. doi: 10.1016/j.fm.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 45.Wang C.-Y., Lin P.-R., Ng C.-C., Shyu Y.-T. Probiotic properties of Lactobacillus strains isolated from the feces of breast-fed infants and Taiwanese pickled cabbage. Anaerobe. 2010;16:578–585. doi: 10.1016/j.anaerobe.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 46.Bao Y., Zhang Y., Zhang Y., Liu Y., Wang S., Dong X., Wang Y., Zhang H. Screening of potential probiotic properties of Lactobacillus fermentum isolated from traditional dairy products. Food Control. 2010;21:695–701. [Google Scholar]
- 47.Wang L., Zhang H., Rehman M.U., Mehmood K., Jiang X., Iqbal M., Tong X., Gao X., Li J. Antibacterial activity of Lactobacillus plantarum isolated from Tibetan yaks. Microb. Pathog. 2018;115:293–298. doi: 10.1016/j.micpath.2017.12.077. [DOI] [PubMed] [Google Scholar]
- 48.Aarti C., Khusro A., Varghese R., Arasu M.V., Al-dhabi N.A., Ilavenil S., Choi K.C. In vitro studies on probiotic and antioxidant properties of Lactobacillus brevis strain LAP2 isolated from Hentak, a fermented fish product of north-east India. LWT. 2017 doi: 10.1016/j.lwt.2017.07.055. [DOI] [Google Scholar]
- 49.Nigam A., Kumar A., Madhusuda H.V., Bhola N. In-vitro Screening of antibacterial activity of lactic acid bacteria against common enteric pathogens. J. Biomed. Sci. 2012;1 doi: 10.3823/1010. [DOI] [Google Scholar]
- 50.Rahbar Saadat Y., Yari Khosroushahi A., Pourghassem Gargari B. A comprehensive review of anticancer, immunomodulatory and health beneficial effects of the lactic acid bacteria exopolysaccharides. Carbohydr. Polym. 2019;217:79–89. doi: 10.1016/j.carbpol.2019.04.025. [DOI] [PubMed] [Google Scholar]
- 51.Rajoka M.S.R., Zhao H., Lu Y., Lian Z., Li N., Hussain N., Shao D., Jin M., Li Q., Shi J. Anticancer potential against cervix cancer (HeLa) cell line of probiotic Lactobacillus casei and Lactobacillus paracasei strains isolated from human breast milk. Food Funct. 2018;9:2705–2715. doi: 10.1039/c8fo00547h. [DOI] [PubMed] [Google Scholar]