Abstract
Cancer remains the second leading life-threatening disease worldwide. Increasing evidence indicates that long non-coding RNAs (lncRNAs) play an important role in multiple physiological and pathological processes, including gene amplification, mutation, rearrangement, and overexpression regulations. In this review, we comprehensively summarize the current knowledge of lncRNA AGAP2-AS1 from a cancer perspective. As a member of the lncRNA family, lncRNA AGAP2-AS1 is upregulated in solid tumor malignancies, functions as an oncogene, and plays a key role in tumorigenesis and tumor progression. AGAP2-AS1 expression is significantly increased in clinical cancer tissue samples, cell lines, and in vivo, and is closely related to an unfavorable prognosis in several cancers. Upregulated lncRNA AGAP2-AS1 binds with microRNAs (miRNAs) and promotes activation of downstream genes. This aberrant regulation induces carcinogenesis and tumorigenesis. Here we provide a comprehensive overview of AGAP2-AS1 in cancer progression that leads to an improved understanding of the effects of AGAP2-AS1 on early detection and therapeutic approaches. This information is essential for the future development of lncRNA AGAP2-AS1 as a potential therapy against these devastating cancers.
Keywords: AGAP2-AS1, lncRNAs, oncogene, cancers
Introduction
Cancer has become the second leading life-threatening disease in the world, and its incidence and mortality are increasing rapidly [1]. Globally, approximately 18.1 million people were diagnosed with cancers and 9.6 million deaths resulted from cancers in 2018 [2,3]. Lung, colorectum, stomach, and liver cancers remain the top four types of cancer-causing deaths worldwide [2-4]. Genomic and chromosomal instability are fundamental characteristics of human cancer [5,6]. Indeed, the hallmark of cancer is increased genomic instability, which is usually detected at an early stage and contributes to carcinogenesis and disease progression [7]. Thus, advanced diagnostics and therapies may increase the effectiveness of early detection and cancer therapy, respectively. Genome-wide cancer mutation analyses show that genomic mutations in cancers occur in non-coding regions [8,9]. RNAs located in non-coding regions are frequently transcribed into long non-coding RNAs (lncRNAs) that are 200 nt-100 kb in length [10]. Also, microRNAs (miRNAs) are a class of short non-coding RNAs [11]. The lncRNAs and miRNAs account for 90% of human genomes that regulate mRNA expression both directly and indirectly [9]. More and more studies have reported that lncRNAs play an important regulatory role in gene transcription, post-transcription modification, gene translation, gene expression, and protein degradation [12,13]. Overall, lncRNAs regulate the pathophysiological process and maintain hemostasis. Aberrant lncRNA expression may alter the expression of lncRNA target genes and thereby contribute to a variety of chronic diseases, including malignancies [14-16]. Additionally, miRNAs have been recognized as tumor suppressors, suggesting a potential for miRNA-based novel therapeutic targets for cancer patients [17]. Another RNA gene affiliated with the lncRNA class is Arf GAP [ADP-ribosylation factor (Arf), GTPase-activated protein (GAP)], which has a GTPase domain, ankyrin repeat, and PH domain 2 isoform 2 antisense RNA 1 (AGAP2-AS1) [18]. The lncRNA AGAP2-AS1 is located on a cytogenetic band in chromosome 12q14.1, which contains 1567 nucleotides (12q14.1 is the gene symbol of AGAP2-AS1 in HUGO Gene Nomenclature Committee 48633, entrez gene: 100130776, Ensemble: ENSG00000255737). AGAP2-AS1 is on the opposite strand of the AGAP2 gene and includes the intronic and untranslated (UTR) regions of the opposite protein-coding gene (https://www.genecards.org/) [19]. AGAP2 is a protein that belongs to the Arf GAP protein family and is involved in transportation of cellular signals. However, there is no significant evidence that lncRNA AGAP2-AS1 and protein-coding gene AGAP2 have common or shared functions. LncRNA AGAP2-AS1 is primarily located in the nucleus and weakly expressed in the plasma membrane, extracellular matrix, cytoskeleton, endosome, and cytosol. Clinical research studies and experiments have demonstrated that compared with normal tissue, AGAP2-AS1 is highly expressed in solid tumor tissue, such as lung cancer, colorectal cancer, breast cancer, and esophageal cancer [20-24]. Abnormal expression of AGAP2-AS1 functions as a key regulator in tumorigenesis and progression [25-30]. In this review, we comprehensively summarize the current research progress regarding the role of lncRNA AGAP2-AS1 in clinical human solid tumors and its in vivo regulatory mechanism, and discuss the potential value of lncRNA AGAP2-AS1 as a diagnostic biomarker and therapeutic target.
Clinicopathologic characteristics of AGAP2-AS1 in cancer diseases
AGAP2-AS1 mRNA expression and prognosis in pan-cancer analyses
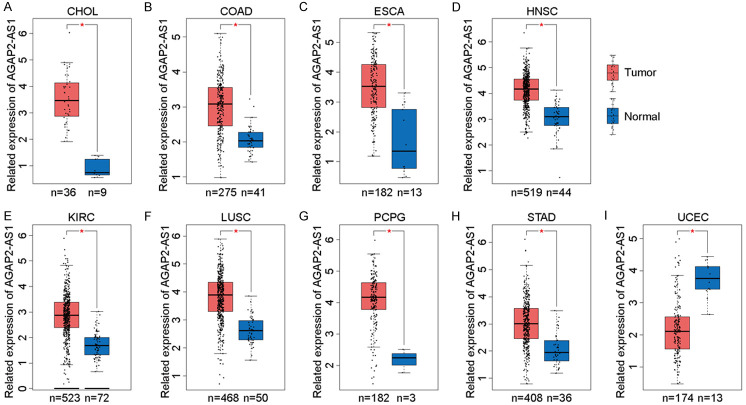
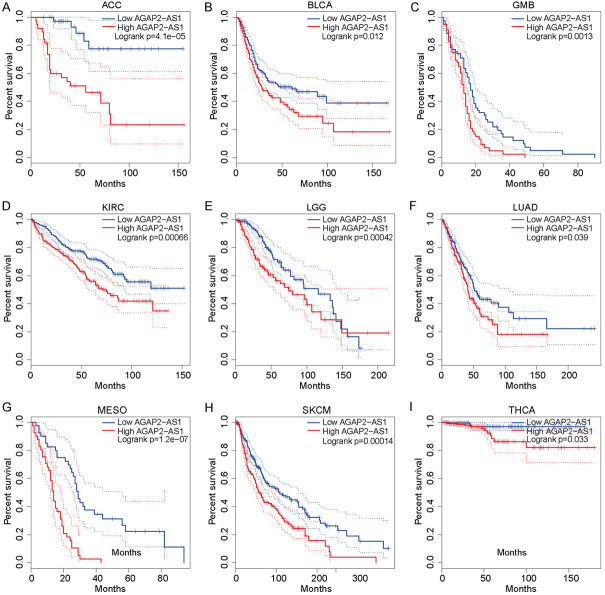
Recognized as novel biomarkers in clinical settings, lncRNAs have an important impact on the development of solid tumors [31,32]. RNA sequence profiling has provided novel insights into the potential regulatory mechanisms and clinical features of lncRNAs [33,34]. Bioinformatics applications, such as differential gene expression, and survival analysis have been utilized to describe the characteristics of lncRNAs in the clinic [31,34,35]. Increasing evidence demonstrates that lncRNAs have a close relationship with the prognosis of solid tumors. In this study, we utilized the Gene Expression Profiling Interactive Analysis (GEPIA) online analysis tools based on The Cancer Genome Atlas (TCGA) databases to analyze AGAP2-AS1 expression data and potential prognosis value. We found that lncRNA AGAP2-AS1 is overexpressed in many solid tumors. We used the GEPIA online tool based on the TCGA database to explore the expression of AGAP2-AS1 in 22 common tumors and matched normal tissues. The results indicate that AGAP2-AS1 is significantly (P<0.05) upregulated in eight solid tumors, including cholangiocarcinoma, colon adenocarcinoma, esophageal cancer (EC), head and neck squamous cell carcinoma, kidney renal clear cell carcinoma (KIRC), lung squamous cell carcinoma, pheochromocytoma and paraganglioma, stomach adenocarcinoma, and downregulated in uterine corpus endometrial carcinoma (UCEC) (Figure 1). We further explored the prognostic value of AGAP2-AS1 among some solid tumors and found that the increased AGAP2-AS1 expression in nine tumors [i.e., adrenocortical carcinoma, glioblastoma multiforme (GBM), KIRC, lung adenocarcinoma, mesothelioma (MESO), skin cutaneous melanoma, thyroid carcinoma] show an unfavorable prognosis (Figure 2). In addition, current advanced literatures have reported that AGAP2-AS1 play important role in many carcinogenesis and tumor progression processes, such as e [36,37], Non-small cell lung cancer (NSCLC) [38], breast cancer [39], colorectal cancer (CRC) [20,23] and some other cancers [40,41]. The specific regulation mechanisms have been validated that lncRNA AGAP2-AS1 function as sponge and competing endogenous RNAs, targeting microRNAs and therefore facilitating the downstream oncogenes expression and promoting tumor progression.
Figure 1.
The gene expression level of lncRNA AGAP2-AS1 in various cancers. A-H. LncRNA AGAP2-AS1 is significantly upregulated in tumor tissue compared with adjacent tumor tissue (CHOL, COAD, ESCA, HNSC, KIRC, LUSC, PCPG, and STAD). I. LncRNA AGAP2-AS1 is downregulated in UCEC tumor tissue compared with para-tumor tissue.
Figure 2.
The prognostic value of lncRNA AGAP2-AS1 in various cancers. A-I. The highly expressed lncRNA AGAP2-AS indicated unfavorable prognosis in various cancers such as ACC, BLCA, GMB, KIRC, LGG, LUAD, MESO, SKCM, and THCA.
Glioma and glioblastoma multiforme (GBM)
GBM has become the most frequent primary malignant brain tumor with a poor prognosis [42,43]. The increased expression of lncRNA AGAP2-AS1 in gliomas has been validated in many studies, which is consistent with the involvement of lncRNAs in glioma initiation, proliferation, and other malignant activities [44,45]. LncRNA AGAP2-AS1 has been reported to be significantly increased in glioma, GBM tissue samples, and human glioma cell lines [37,46,47]. In addition, AGAP2-AS1 expression was reported as positively correlated with tumor size; that is, tumor sizes larger than 5 cm have significantly higher AGAP2-AS1 expression [46]. Studies also investigated whether the AGAP2-AS1 expression level is higher in advanced stages and grades and found that increased AGAP2-AS1 expression is positively related with poor prognosis. These results suggest that AGAP2-AS1 may function as an oncogene and may have potential as a biomarker and prognosis prediction factor in glioma and GBM.
Non-small cell lung cancer (NSCLC)
Lung cancer is ranked first in cancer-related mortality worldwide, and NSCLC accounts for 80% of all lung cancers [48]. An increasing number of studies have demonstrated that lncRNAs may act as a promising biomarker in diagnosis and prognosis [48]. Also, lncRNA AGAP2-AS1 was validated as being overexpressed in blood samples and tumor tissues [26,38]. Studies found that NSCLC patients had increased levels of exosomal AGAP2-AS1 in the bloodstream compared with cancer-free subjects. Exosomal lncRNA AGAP2-AS1 is stable in the bloodstream, which contributes to its potential to be used to distinguish malignancies from healthy controls [38].
In addition, researchers have demonstrated that AGAP2-AS1 expression is upregulated in NSCLC tumor tissues compared with adjacent tumor tissues [49]. They also confirmed that increased AGAP2-AS1 expression is strongly correlated with greater tumor size, an advanced tumor stage, and lymph node metastasis status [49,50]. In another study, receiver operating characteristic (ROC) curve analysis was used to confirm that the ROC of lncRNA AGAP2-AS1 is 0.845 [50]. These studies indicate that lncRNA AGAP2-AS1 may be an efficient biomarker for early detection and prognosis prediction.
Breast cancer
Breast cancer is the most common cancer in women and has a high mortality rate and poor prognosis [31,51]. As in other cancers, lncRNAs are involved in tumorigenesis and chemoresistance of breast cancer [52,53]. The expression level of lncRNA AGAP2-AS1 has been shown by reverse-transcription quantitative polymerase chain reaction (RT-qPCR) to be upregulated in breast cancer tissues compared with adjacent non-cancer tissues [22,39]. Studies of exosomes have provided novel insights into the discovery of new therapeutic strategies for cancer patients. Exosomal lncRNA AGAP2-AS1 is upregulated in breast cancer patients compared with healthy controls [22,39]. The increased AGAP2-AS1 expression level enhances chemotherapy resistance [39]. Also, lncRNA AGAP2-AS1 is significantly increased in exosomes from breast cancer cells and tumor tissues and facilitates trastuzumab resistance and unfavorable prognosis. Overall, these studies indicate that AGAP2-AS1 may function as an oncogenic biomarker for detection and chemotherapeutic response.
Colorectal cancer (CRC)
CRC is the third ranking cancer in the world and has become the second leading cause of cancer-related deaths [54,55]. Evidence implies that lncRNAs have a close relationship with CRC tumor initiation, progression, and metastatic activities [56,57]. Previous studies demonstrate that lncRNA AGAP2-AS1 is significantly upregulated in CRC tissue samples and cell lines and increased AGAP2-AS1 expression is associated with an adverse prognosis [20,23]. Multivariate analysis further validated that lncRNA AGAP2-AS1 functions as an independent prognostic factor for CRC [23]. These studies imply that upregulated AGAP2-AS1 plays an important role in carcinogenesis and has a potential function as a biomarker for CRC [20,23].
Other cancers
Research studies have indicated that AGAP2-AS1 is overexpressed in CRC, pancreatic cancer (PC), gastric cancer (GC), EC, hepatocellular carcinoma (HCC), prostate cancer [41], papillary thyroid cancer [40], and clear cell renal cell carcinoma (ccRCC) [18,19,29,58]. Evidence implies that enhanced AGAP2-AS1 expression is positively correlated with poor prognosis, greater tumor sizes, distance metastasis, lymph node metastasis, and advanced grades and tumor stages in ccRCC and PC [29,58]. Further, overexpressed AGAP2-AS1 can serve as an oncogene and correlates with poor prognosis of GC and HCC [18,19]. Recent study results also indicate that high expression of AGAP2-AS1 shows excellent clinical diagnostic value and may potentially become a biomarker for diagnosis and prognosis [58].
Regulatory role of AGAP2-AS1 in cell lines experiments
Tumor growth, progression, invasion, migration, and epithelial mesenchymal transformation (EMT)
To uncover the molecular mechanisms underlying AGAP2-AS1 function in cancers, many researchers have demonstrated that AGAP2-AS1 has an important role in malignant processes. The enhanced expression of AGAP2-AS1 has been detected in various cancer cell lines, such as GBM, lung cancer, breast cancer, etc. Also, AGAP2-AS1 is a classic lncRNA and functions as an oncogene in tumorigenesis. The lncRNA-miRNA regulatory network is a major mechanism of lncRNA regulation in cancer development [59]. Indeed, lncRNA AGAP2-AS1 could band with miRNA and function as a sponge, thus upregulating the downstream target gene and triggering malignant activities. The regulatory mechanism landscape in solid tumor cells is illustrated in Figure 3 and Table 1. Studies have implied that in GBM and glioma cell lines, AGAP2-AS1 acts as a sponge by banding with miR-15a/b-5p and then enhancing the expression of downstream target gene HDGF, activating the Wnt/β-catenin signaling pathway, and thereby inducing the progression of cancer cells. In prostate cancer cell lines, the lncRNA AGAP2-AS1 is upregulated, the increased AGAP2-AS1 function as a sponge and banding with miR-195-5p, which targeted PDZ and LIM domain 5 (PDLIM5) and subsequently upregulated its expression, resulting in promoting tumor growth [41]. In addition, lncRNA AGAP2-AS1 bands with miR-4668-3p and enhances SRSF1 expression. The AGAP2-AS1/miR-4668-3p/SRSF1 axis is involved in promoting cell proliferation, migration, and the EMT process in CRC cell lines [20]. Similarly, overexpressed AGAP2-AS1 targets miRNA miR-195-5p and downregulates target gene FOSL1 expression [24]. These results suggest that the AGAP2-AS1/miR-195-5p/FOSL1 axis acts as a prognostic biomarker leading to proliferation, invasion, migration, tumor growth, and inhibition of apoptosis in EC cells. The sponge effect was also validated in HCC cell lines. Upregulated AGAP2-AS1 directly targeted miR-16-5P and functioned as a sponge, inducing ANX1 and AKT expression. Therefore, the AGAP2-AS1/miR-16-5p/ANXA11/AKT axis is an important potential therapeutic target for HCC treatments [19]. Enhancement of zeste homolog 2 (EZH2) functions as a key epigenetic regulator and plays an important role in tumor progression and metastasis. Lysine-specific demethylase 1 (LSD1), a histone demethylase, had critical functions in carcinogenesis and tumorigenesis [60]. Tissue factor pathway inhibitor 2 (TFPI2) was recognized as the most frequently hypermethylated gene and may contribute to tumor progression [61]. Together these findings reveal that EZH2 and LSD1 may be potential therapeutic targets for the treatment of cancer. Recent studies have shown that AGAP2-AS1 could recruit EZH2 and LSD1 to the TFPI2 promoter region and suppress TFPI2 transcription, resulting in trimethylation of H3K27 or demethylation of H3K4 at this region in GBM cells [47]. Another study demonstrated that the EZH2 and LSD1 could directly bind to large tumor suppressor 2 (LATS2) and Kruppel-like factor 2 (KLF2) promoter regions and mediate downstream trimethylation and demethylation modification in lung cancer cells. Researchers focusing on breast cancer found that AGAP2-AS1 levels were increased in exosomes and upregulated by heterogeneous nuclear ribonucleoprotein A2/B1 (hnRNPA2B1) overexpression. HnRNPA2B1 plays an important role in oncogenic regulation and is associated with tumor progression [49,62,63].
Figure 3.
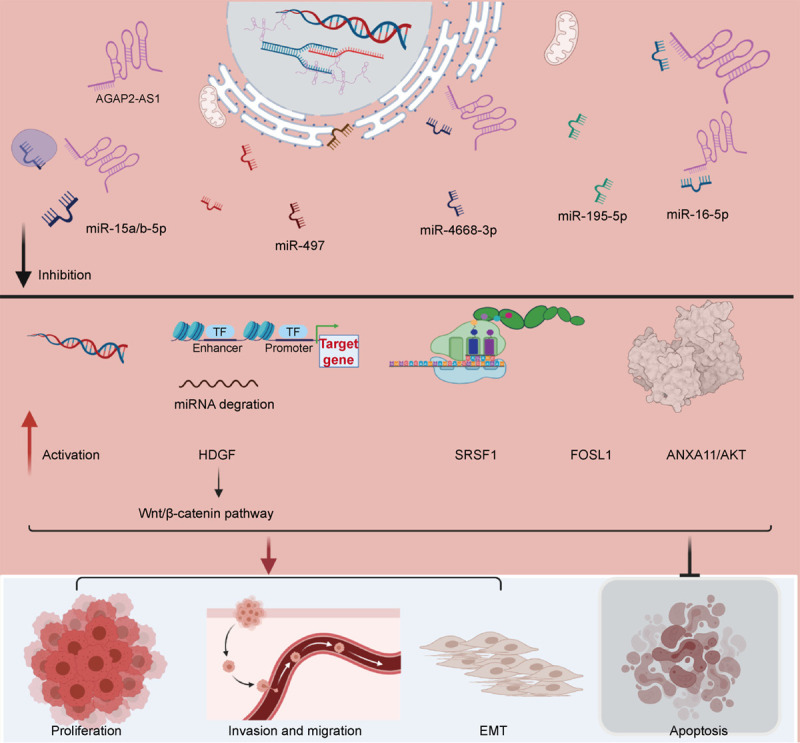
The regulatory mechanism of LncRNA AGAP2-AS1 in cancer cell. AGAP2-AS1 act as a sponge targeting downstream micro-RNAs, such as miR-628-5p, miR-15a/b-5p, miR-497, miR-4668-3p, and miR-16-5p. The downstream target genes, such as PTN, HDGF, FGFR1, SRSF1, FOSL1, and ANXA11/AKT, are dysregulated. The carcinogenesis and tumorigenesis related pathways are activated. LncRNA AGAP2-AS1 promotes cancer cell proliferation, invasion, migration, EMT, and inhibit tumor apoptosis.
Table 1.
AGAP2-AS1 expression and regulation mechanism in vivo
| Cancer types | Animal model | Cell lines transfection | Interventions and groups | Regulation Mechanism | References |
|---|---|---|---|---|---|
| GBM | BALB/C male nude mice | U87/MG | sh-NC | Knockdown of AGAP2-AS1 triggers a reduction of AGAP2-AS1 expression, an increase of TFPI2 protein level in excised tumor masses Silencing of AGAP2-AS1 obviously slows down the tumor growth | [47] |
| sh-AGAP2-AS1 | |||||
| CRC | 24 male BALB/c nude mice | RKO, HT29 | RKO/pWPXL, RKO/AGAP2-AS1, HT29/pWPXL, and HT29/AGAP2-AS1 | AGAP2-AS1 promotes the growth of RKO and HT29 cells in nude mice | [23] |
| PC | BALB/C male nude mice | BxPC-3 | sh-NC | sh-AGAP2-AS1 downregulates tumor growth as well as reduces tumor volume and weight | [29] |
| sh-AGAP2-AS1 | |||||
| GC | BALB/c nude mice | BGC823 | sh-AGAP2-AS1 | sh-AGAP2-AS1 inhibits GC cell tumor growth in vivo | [18] |
| sh-NC | |||||
| EC | 24 male BALB/c nude mice | KYSE70 | sh-NC & oe-NC | si-AGAP2-AS1 inhibits tumor volume and weight | [24] |
| sh-AGAP2-AS1 & oe-NC, | Suppressed in vivo tumorigenesis through downregulating FOSL1 | ||||
| sh-AGAP2-AS1 & oe-FOSL1 | |||||
| HCC | 4-6 week-old female BALB/c nude mice | Hep3B, HCCLM3 | sh-AGAP2-AS1 | AGAP2-AS1 knockdown inhibits the tumor growth of HCC cells in mice | [67] |
| sh-NC | AGAP2-AS1 overexpression increases the Ki67 positive staining cells and reduces the number of apoptotic cells | ||||
| AGAP2-AS1 knockdown inhibits proliferation and induces apoptosis cells |
Drug resistance
Drug resistance occurs at a high frequency and remains a major clinical challenge; therefore, the molecular mechanisms of chemoresistance in cancers still need further study [64]. It is essential to explore the mechanisms underlying this resistance to develop strategies to overcome drug resistance. Trastuzumab-resistant SKBR-3 and BT474 cells were used to investigate the regulatory mechanism of AGAP2-AS1 and the results indicate that AGAP2-AS1 is increased in breast cancer cells [57]. This increase enhances tumor growth and trastuzumab resistance and inhibits apoptosis [57]. Further, researchers found that MyD88 is induced by upregulation of AGAP2-AS1. Specificity protein 1 (SP1) plays a vital role in numerous cellular processes [65]. SP1 is a classical transcription factor that upregulates AGAP2-AS1 transcription in rastuzumab-resistant SKBR-3 and BT474 cells [66]. Overexpressed AGAP2-AS1 promotes MyD88 activation and facilitates the NF-κB signaling pathway, thus inducing tumor progression and rastuzumab-resistant abilities [39].
Overall, these findings indicate that lncRNA AGAP2-AS1 is involved in the SP1/AGAP2-AS1/Myd88/NF-κB signaling pathway and promotes tumorigenesis and chemotherapy drug resistance [57]. These results uncovered the regulatory mechanism by which the SP1/AGAP2-AS1/Myd88/NF-κB axis induces tumor growth and therapeutic resistance and may be a potential therapeutic target for breast cancer.
Regulatory role of AGAP2-AS1 in xenograft model
Xenograft models are used to validate the oncogenic role of AGAP2-AS1 and its tumorigenic abilities. In the majority of xenograft models, various cancer cell lines (e.g., glioma, GBM, CRC, PC, GC, EC, and HCC) were transferred into mice using the nude mouse model (Table 2). The results show that lncRNA AGAP2-AS1 is downregulated in the sh-AGAP2-AS1 group compared with the normal control group [18,23,29], and the tumor weight and mass are lower in the AGAP2-AS1-knockdown group compared with the control group (Figure 4) [24,29]. In the EC xenograft model, researchers determined that si-AGAP2-AS1 inhibits tumor volume and weight, and the knockdown of AGAP2-AS1 inhibits tumorigenesis via downregulating FOSL1 [24]. In addition, research has shown that AGAP2-AS1 overexpression increases proliferation and decreases apoptosis in vivo, and also results in decreased E-cadherin expression and increased vimentin in vivo [24,67]. These studies demonstrate that increased lncRNA-AGAP2-AS1 expression promotes tumor proliferation and metastasis in vivo and can be a useful prognostic biomarker and therapeutic target.
Table 2.
Expression and regulation mechanism of AGAP2-AS1 levels in cancers cell lines
| Cancer Type | Cell lines | Target miRNA | Target gene | Activity | Pathways | PMID |
|---|---|---|---|---|---|---|
| GBM | U87, U251 | Downregulation of AGAP2-AS1 inhibits proliferation, migration, and invasion, and promotes apoptosis | [46] | |||
| Glioma | U87, U251 LN229, normal human astrocyte | miR-15a/b-5p | HDGF | Know-down of AGAP2-AS1 decreases cell viability and proliferation, cell apoptosis, and cell growth | Wnt/β-catenin signaling pathway | [37] |
| GBM | U87/MG, U251/MG, A172 | EZH2, LSD1, TFPI2 | Knockdown of AGAP2-AS1 suppresses proliferation and invasion, and facilitated apoptosis | AGAP2-AS1/EZH2 and LSD1/TFPI2 patrhway | [47] | |
| Lung cancer | H1299, H1975 | EZH2, LSD1 | Overexpressed AGAP2-AS1 increases proliferation, migration, invasion, and tumorigenesis | EZH2 and LSD1/LATS2 and KLF2/H3K27 trimethylation or H3K4me2 demethylation | [49] | |
| Breast cancer | Trastuzumab-resistant SKBR-3 and BT474 cells | Myd88, HIF-1α | Increased AGAP2-AS1 facilitates tumor growth and trastuzumab resistance, and inhibits apoptosis | SP1/AGAP2-AS1/Myd88/NF-κB signaling pathway | [39] | |
| Breast cancer | SKBR-3 and BT474 cells | AGAP2-AS1 expression is increased in trastuzumab resistant cells | [22] | |||
| Exosome AGAP2-AS1 expression was upregulated by hnRNPA2B1 overexpression | ||||||
| Breast cancer | Increased lncRNA AGAP2-AS1 promotes trastuzumab resistance | [39] | ||||
| CRC | DLD-1, SW480 | miR-497 | FGFR1 | AGAP2-AS1 promotes proliferation, migration and invasion and inhibited apoptosis | [23] | |
| AGAP2-AS1 promotes G1/M phase cell cycle arrest and increased gemcitabine sensitivity | ||||||
| CRC | SW620, HT-29 and HCT8 | miR-4668-3p | SRSF1 | Upregulated AGAP2-AS1 promotes cell proliferation, migration and EMT process | RREB1/AGAP2-AS1/miR-4668-3p/SRSF1 | |
| PC | HPDE6-C7, AsPC-1, SW1990, PANC-1, BxPC-3 | EZH2 | AGAP2-AS1 promotes proliferation and apoptosis, migration and invasion | AGAP2-AS1/EZH2/ANKRD1 and ANGPTL4 axis | [29] | |
| GC | BGC823, AGS cells | EZH2, LSD1 | AGAP2-AS1 promotes proliferation, cell cycle arrest, cell migration, invasion, and tumorigenesis | SP1/AGAP2-AS1/EZH2 and LSD1/P21, E-cadherin | [18] | |
| EC | KYSE70, KYSE-510, and EC9706) and human immortalized esophageal epithelial cells | miR-195-5p | FOSL1 | AGAP2-AS1 promotes proliferation, invasion, and migration as well as tumor growth | AGAP2-AS1/miR-195-5p/FOSL1 | [24] |
| Regulated cell cycle, and apoptosis | ||||||
| HCC | LO2, Hep3B, HCCLM3, Huh7, MHCC-97H, SMMC-7721 | miR-16-5p | ANXA11, AKT | AGAP2-AS1 promotes proliferation, migration, invasion, EMT, and inhibited apoptosis | AGAP2-AS1/miR-16-5p/ANXA11/AKT | [19] |
| Overexpression of AGAP2-AS1 increases HIF-1α expression |
Figure 4.
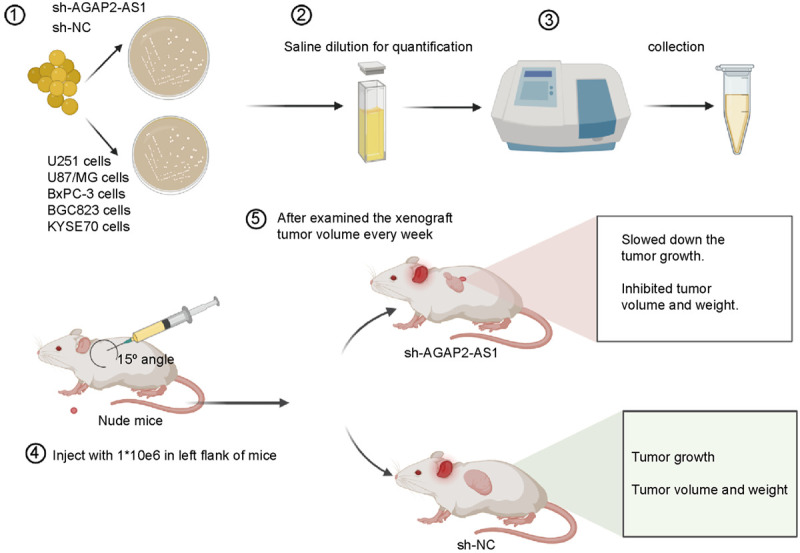
The lncRNA AGAP2-AS1 knockdown cells are transferred into xenograft. The knockdown AGAP2-AS1 slows down tumor growth, inhibits tumor volume and weight compared with normal control xenograft model.
Conclusions
Cancers have become the second leading cause of death worldwide. Despite progress, many difficulties remain in early detection, targeted therapy, and precision medicine. Recently, changes in lncRNA regulation has been the most common RNA modification detected in human cancers and may be a potential trigger for the pathogenesis of solid malignancies. LncRNA AGAP2-AS1 is a well-characterized cancer-related RNA that is expressed at significantly higher levels in tumors compared with normal tissues. Reporters also validated that AGAP2-AS1 has an oncogenic role and diverse functions, such as promoting proliferation, migration, invasion, EMT, and drug resistance in cell lines and in vivo. Upregulated AGAP2-AS1 was involved in multiple pathology processes through banding with miRNAs (e.g., miR-15a/b-5p, miR-4668-3p, miR-195-5p, miR-16-5p). The lncRNAs band with miRNAs to form a loop, leading to enhanced expression of the target genes, thereby enhancing malignancy activities.
Overall, the current research suggests that lncRNA-AGAP2-AS1 may function as a potential biomarker for cancer diagnosis and prognosis. Further exploration is needed to understand the potential of AGAP2-AS1-based diagnostic methods and therapies in the clinical setting.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81790631), and Zhejiang University Academic Award for Outstanding Doctoral Candidates (2020052).
Disclosure of conflict of interest
None.
References
- 1.Wender RC, Brawley OW, Fedewa SA, Gansler T, Smith RA. A blueprint for cancer screening and early detection: advancing screening’s contribution to cancer control. CA Cancer J Clin. 2019;69:50–79. doi: 10.3322/caac.21550. [DOI] [PubMed] [Google Scholar]
- 2.Cortes J, Perez-García JM, Llombart-Cussac A, Curigliano G, El Saghir NS, Cardoso F, Barrios CH, Wagle S, Roman J, Harbeck N, Eniu A, Kaufman PA, Tabernero J, García-Estévez L, Schmid P, Arribas J. Enhancing global access to cancer medicines. CA Cancer J Clin. 2020;70:105–124. doi: 10.3322/caac.21597. [DOI] [PubMed] [Google Scholar]
- 3.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 4.Zheng J, Zhou Y, Li Y, Xu DP, Li S, Li HB. Spices for prevention and treatment of cancers. Nutrients. 2016;8:495. doi: 10.3390/nu8080495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bakhoum SF, Cantley LC. The multifaceted role of chromosomal instability in cancer and its microenvironment. Cell. 2018;174:1347–1360. doi: 10.1016/j.cell.2018.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turajlic S, Sottoriva A, Graham T, Swanton C. Resolving genetic heterogeneity in cancer. Nat Rev Genet. 2019;20:404–416. doi: 10.1038/s41576-019-0114-6. [DOI] [PubMed] [Google Scholar]
- 7.Sansregret L, Vanhaesebroeck B, Swanton C. Determinants and clinical implications of chromosomal instability in cancer. Nat Rev Clin Oncol. 2018;15:139–150. doi: 10.1038/nrclinonc.2017.198. [DOI] [PubMed] [Google Scholar]
- 8.Chen B, Dragomir MP, Fabris L, Bayraktar R, Knutsen E, Liu X, Tang C, Li Y, Shimura T, Ivkovic TC, De Los Santos MC, Anfossi S, Shimizu M, Shah MY, Ling H, Shen P, Multani AS, Pardini B, Burks JK, Katayama H, Reineke LC, Huo L, Syed M, Song S, Ferracin M, Oki E, Fromm B, Ivan C, Bhuvaneshwar K, Gusev Y, Mimori K, Menter D, Sen S, Matsuyama T, Uetake H, Vasilescu C, Kopetz S, Parker-Thornburg J, Taguchi A, Hanash SM, Girnita L, Slaby O, Goel A, Varani G, Gagea M, Li C, Ajani JA, Calin GA. The long noncoding RNA CCAT2 induces chromosomal instability through BOP1 - AURKB signaling. Gastroenterology. 2020;20:35057–5. doi: 10.1053/j.gastro.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rothschild G, Basu U. Lingering questions about enhancer RNA and enhancer transcription-coupled genomic instability. Trends Genet. 2017;33:143–154. doi: 10.1016/j.tig.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huarte M. The emerging role of lncRNAs in cancer. Nat Med. 2015;21:1253–1261. doi: 10.1038/nm.3981. [DOI] [PubMed] [Google Scholar]
- 11.Khorkova O, Hsiao J, Wahlestedt C. Basic biology and therapeutic implications of lncRNA. Adv Drug Deliv Rev. 2015;87:15–24. doi: 10.1016/j.addr.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nair L, Chung H, Basu U. Regulation of long non-coding RNAs and genome dynamics by the RNA surveillance machinery. Nat Rev Mol Cell Biol. 2020;21:123–136. doi: 10.1038/s41580-019-0209-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kopp F, Mendell JT. Functional classification and experimental dissection of long noncoding RNAs. Cell. 2018;172:393–407. doi: 10.1016/j.cell.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ransohoff JD, Wei Y, Khavari PA. The functions and unique features of long intergenic non-coding RNA. Nat Rev Mol Cell Biol. 2018;19:143–157. doi: 10.1038/nrm.2017.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pandya G, Kirtonia A, Sethi G, Pandey AK, Garg M. The implication of long non-coding RNAs in the diagnosis, pathogenesis and drug resistance of pancreatic ductal adenocarcinoma and their possible therapeutic potential. Biochim Biophys Acta Rev Cancer. 2020;1874:188423. doi: 10.1016/j.bbcan.2020.188423. [DOI] [PubMed] [Google Scholar]
- 16.Knoll M, Lodish HF, Sun L. Long non-coding RNAs as regulators of the endocrine system. Nat Rev Endocrinol. 2015;11:151–160. doi: 10.1038/nrendo.2014.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kolenda T, Guglas K, Kopczyńska M, Sobocińska J, Teresiak A, Bliźniak R, Lamperska K. Good or not good: Role of miR-18a in cancer biology. Rep Pract Oncol Radiother. 2020;25:808–819. doi: 10.1016/j.rpor.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qi F, Liu X, Wu H, Yu X, Wei C, Huang X, Ji G, Nie F, Wang K. Long noncoding AGAP2-AS1 is activated by SP1 and promotes cell proliferation and invasion in gastric cancer. J Hematol Oncol. 2017;10:48. doi: 10.1186/s13045-017-0420-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Z, Wang Y, Wang L, Yao B, Sun L, Liu R, Chen T, Niu Y, Tu K, Liu Q. Long non-coding RNA AGAP2-AS1, functioning as a competitive endogenous RNA, upregulates ANXA11 expression by sponging miR-16-5p and promotes proliferation and metastasis in hepatocellular carcinoma. J Exp Clin Cancer Res. 2019;38:194. doi: 10.1186/s13046-019-1188-x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Li H, Guo S, Zhang M, Li L, Wang F, Song B. Long non-coding RNA AGAP2-AS1 accelerates cell proliferation, migration, invasion and the EMT process in colorectal cancer via regulating the miR-4,668-3p/SRSF1 axis. J Gene Med. 2020;22:e3250. doi: 10.1002/jgm.3250. [DOI] [PubMed] [Google Scholar]
- 21.Poulet C, Njock MS, Moermans C, Louis E, Louis R, Malaise M, Guiot J. Exosomal long non-coding RNAs in lung diseases. Int J Mol Sci. 2020;21:3580. doi: 10.3390/ijms21103580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohebi M, Ghafouri-Fard S, Modarressi MH, Dashti S, Zekri A, Kholghi-Oskooei V, Taheri M. Expression analysis of vimentin and the related lncRNA network in breast cancer. Exp Mol Pathol. 2020;115:104439. doi: 10.1016/j.yexmp.2020.104439. [DOI] [PubMed] [Google Scholar]
- 23.Hong S, Yan Z, Song Y, Bi M, Li S. LncRNA AGAP2-AS1 augments cell viability and mobility, and confers gemcitabine resistance by inhibiting miR-497 in colorectal cancer. Aging (Albany NY) 2020;12:5183–5194. doi: 10.18632/aging.102940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen S, Li K, Liu Y, Liu X, Liu B, Ba Y, Xing W. Silencing lncRNA AGAP2-AS1 upregulates miR-195-5p to repress migration and invasion of EC cells via the decrease of FOSL1 expression. Mol Ther Nucleic Acids. 2020;20:331–344. doi: 10.1016/j.omtn.2019.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu Y, Xia X, Jiang Y, Wu D, Wang S, Fu S, Yang N, Zhang Y, Sun L. Down-regulated lncRNA AGAP2-AS1 contributes to pre-eclampsia as a competing endogenous RNA for JDP2 by impairing trophoblastic phenotype. J Cell Mol Med. 2020;24:4557–4568. doi: 10.1111/jcmm.15113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu F, Yin Z, Yang L, Fan J, Xu J, Jin Y, Yu J, Zhang D, Yang G. Smoking induced extracellular vesicles release and their distinct properties in non-small cell lung cancer. J Cancer. 2019;10:3435–3443. doi: 10.7150/jca.30425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen J, Peng X, Dai Y. The long non-coding RNA (lncRNA) AGAP2-AS1 is upregulated in ovarian carcinoma and negatively regulates lncRNA MEG3. Med Sci Monit. 2019;25:4699–4704. doi: 10.12659/MSM.914766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng Z, Chen M, Xing P, Yan X, Xie B. Increased expression of exosomal AGAP2-AS1 (AGAP2 antisense RNA 1) in breast cancer cells inhibits trastuzumab-induced cell cytotoxicity. Med Sci Monit. 2019;25:2211–2220. doi: 10.12659/MSM.915419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hui B, Ji H, Xu Y, Wang J, Ma Z, Zhang C, Wang K, Zhou Y. RREB1-induced upregulation of the lncRNA AGAP2-AS1 regulates the proliferation and migration of pancreatic cancer partly through suppressing ANKRD1 and ANGPTL4. Cell Death Dis. 2019;10:207. doi: 10.1038/s41419-019-1384-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Messemaker TC, Chadli L, Cai G, Goelela VS, Boonstra M, Dorjée AL, Andersen SN, Mikkers HMM, van ‘t Hof P, Mei H, Distler O, Draisma HHM, Johnson ME, Orzechowski NM, Simms RW, Toes REM, Aarbiou J, Huizinga TW, Whitfield ML, DeGroot J, de Vries-Bouwstra J, Kurreeman F. Antisense long non-coding RNAs are deregulated in skin tissue of patients with systemic sclerosis. J Invest Dermatol. 2018;138:826–835. doi: 10.1016/j.jid.2017.09.053. [DOI] [PubMed] [Google Scholar]
- 31.Amelio I, Bernassola F, Candi E. Emerging roles of long non-coding RNAs in breast cancer biology and management. Semin Cancer Biol. 2020:S1044–579. doi: 10.1016/j.semcancer.2020.06.019. [DOI] [PubMed] [Google Scholar]
- 32.Weng W, Zhang Z, Huang W, Xu X, Wu B, Ye T, Shan Y, Shi K, Lin Z. Identification of a competing endogenous RNA network associated with prognosis of pancreatic adenocarcinoma. Cancer Cell Int. 2020;20:231. doi: 10.1186/s12935-020-01243-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuan L, Xu ZY, Ruan SM, Mo S, Qin JJ, Cheng XD. Long non-coding RNAs towards precision medicine in gastric cancer: early diagnosis, treatment, and drug resistance. Mol Cancer. 2020;19:96. doi: 10.1186/s12943-020-01219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang Z, Zhou JK, Peng Y, He W, Huang C. The role of long noncoding RNAs in hepatocellular carcinoma. Mol Cancer. 2020;19:77. doi: 10.1186/s12943-020-01188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merdrignac A, Papoutsoglou P, Coulouarn C. Long non-coding RNAs in cholangiocarcinoma. Hepatology. 2020;8:31534. doi: 10.1002/hep.31534. [DOI] [PubMed] [Google Scholar]
- 36.Yan Y, Wang Y, Liu Y, Chen T, Zhu Y, Li H, Kong F. Long non-coding RNA AGAP2-AS1/miR-628-5p/PTN axis modulates proliferation, migration, invasion, and apoptosis of glioma cells. Cancer Manag Res. 2020;12:6059–6068. doi: 10.2147/CMAR.S250890. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Zheng Y, Lu S, Xu Y, Zheng J. Long non-coding RNA AGAP2-AS1 promotes the proliferation of glioma cells by sponging miR-15a/b-5p to upregulate the expression of HDGF and activating Wnt/β-catenin signaling pathway. Int J Biol Macromol. 2019;128:521–530. doi: 10.1016/j.ijbiomac.2019.01.121. [DOI] [PubMed] [Google Scholar]
- 38.Tao Y, Tang Y, Yang Z, Wu F, Wang L, Yang L, Lei L, Jing Y, Jiang X, Jin H, Bai Y, Zhang L. Exploration of serum exosomal LncRNA TBILA and AGAP2-AS1 as promising biomarkers for diagnosis of non-small cell lung cancer. Int J Biol Sci. 2020;16:471–482. doi: 10.7150/ijbs.39123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dong H, Wang W, Mo S, Chen R, Zou K, Han J, Zhang F, Hu J. SP1-induced lncRNA AGAP2-AS1 expression promotes chemoresistance of breast cancer by epigenetic regulation of MyD88. J Exp Clin Cancer Res. 2018;37:202. doi: 10.1186/s13046-018-0875-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Shao L, Sun W, Zhang H, Zhang P, Wang Z, Dong W, He L, Zhang T, Qin Y. Long non-coding RNA AGAP2-AS1 increases the invasiveness of papillary thyroid cancer. Aging (Albany NY) 2020;12:18019–18032. doi: 10.18632/aging.103570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xie P, Liu M, Chen F, Wu S, Shao T, Wang W, Xu C, Zhou H. Long non-coding RNA AGAP2-AS1 silencing inhibits PDLIM5 expression impeding prostate cancer progression via up-regulation of microRNA-195-5p. Front Genet. 2020;11:1030. doi: 10.3389/fgene.2020.01030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White K, Connor K, Clerkin J, Murphy BM, Salvucci M, O’Farrell AC, Rehm M, O’Brien D, Prehn JHM, Niclou SP, Lamfers MLM, Verreault M, Idbaih A, Verhaak R, Golebiewska A, Byrne AT. New hints towards a precision medicine strategy for IDH wild-type glioblastoma. Ann Oncol. 2020;31:1679–1692. doi: 10.1016/j.annonc.2020.08.2336. [DOI] [PubMed] [Google Scholar]
- 43.Pottoo FH, Javed MN, Rahman JU, Abu-Izneid T, Khan FA. Targeted delivery of miRNA based therapeuticals in the clinical management of Glioblastoma Multiforme. Semin Cancer Biol. 2020;14:S1044–579. doi: 10.1016/j.semcancer.2020.04.001. [DOI] [PubMed] [Google Scholar]
- 44.Peng Z, Liu C, Wu M. New insights into long noncoding RNAs and their roles in glioma. Mol Cancer. 2018;17:61. doi: 10.1186/s12943-018-0812-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paulmurugan R, Malhotra M, Massoud TF. The protean world of non-coding RNAs in glioblastoma. J Mol Med (Berl) 2019;97:909–925. doi: 10.1007/s00109-019-01798-6. [DOI] [PubMed] [Google Scholar]
- 46.Tian Y, Zheng Y, Dong X. AGAP2-AS1 serves as an oncogenic lncRNA and prognostic biomarker in glioblastoma multiforme. J Cell Biochem. 2019;120:9056–9062. doi: 10.1002/jcb.28180. [DOI] [PubMed] [Google Scholar]
- 47.Luo W, Li X, Song Z, Zhu X, Zhao S. Long non-coding RNA AGAP2-AS1 exerts oncogenic properties in glioblastoma by epigenetically silencing TFPI2 through EZH2 and LSD1. Aging (Albany NY) 2019;11:3811–3823. doi: 10.18632/aging.102018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peng W, Wang J, Shan B, Peng Z, Dong Y, Shi W, He D, Cheng Y, Zhao W, Zhang C, Li B, Duan C. Diagnostic and prognostic potential of circulating long non-coding RNAs in non small cell lung cancer. Cell Physiol Biochem. 2018;49:816–827. doi: 10.1159/000493043. [DOI] [PubMed] [Google Scholar]
- 49.Li W, Sun M, Zang C, Ma P, He J, Zhang M, Huang Z, Ding Y, Shu Y. Upregulated long non-coding RNA AGAP2-AS1 represses LATS2 and KLF2 expression through interacting with EZH2 and LSD1 in non-small-cell lung cancer cells. Cell Death Dis. 2016;7:e2225. doi: 10.1038/cddis.2016.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fan KJ, Liu Y, Yang B, Tian XD, Li CR, Wang B. Prognostic and diagnostic significance of long non-coding RNA AGAP2-AS1 levels in patients with non-small cell lung cancer. Eur Rev Med Pharmacol Sci. 2017;21:2392–2396. [PubMed] [Google Scholar]
- 51.Peng L, Jiang J, Tang B, Nice EC, Zhang YY, Xie N. Managing therapeutic resistance in breast cancer: from the lncRNAs perspective. Theranostics. 2020;10:10360–10377. doi: 10.7150/thno.49922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Crudele F, Bianchi N, Reali E, Galasso M, Agnoletto C, Volinia S. The network of non-coding RNAs and their molecular targets in breast cancer. Mol Cancer. 2020;19:61. doi: 10.1186/s12943-020-01181-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu L, Zhang Y, Lu J. The roles of long noncoding RNAs in breast cancer metastasis. Cell Death Dis. 2020;11:749. doi: 10.1038/s41419-020-02954-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wei L, Wang X, Lv L, Zheng Y, Zhang N, Yang M. The emerging role of noncoding RNAs in colorectal cancer chemoresistance. Cell Oncol (Dordr) 2019;42:757–768. doi: 10.1007/s13402-019-00466-8. [DOI] [PubMed] [Google Scholar]
- 55.Okugawa Y, Grady WM, Goel A. Epigenetic alterations in colorectal cancer: emerging biomarkers. Gastroenterology. 2015;149:1204–1225. e1212. doi: 10.1053/j.gastro.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Neve B, Jonckheere N, Vincent A, Van Seuningen I. Epigenetic regulation by lncRNAs: an overview focused on UCA1 in colorectal cancer. Cancers (Basel) 2018;10:440. doi: 10.3390/cancers10110440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun Z, Liu J, Chen C, Zhou Q, Yang S, Wang G, Song J, Li Z, Zhang Z, Xu J, Sun X, Chang Y, Yuan W. The biological effect and clinical application of long noncoding RNAs in colorectal cancer. Cell Physiol Biochem. 2018;46:431–441. doi: 10.1159/000488610. [DOI] [PubMed] [Google Scholar]
- 58.Gao L, Zhao A, Wang X. Upregulation of lncRNA AGAP2-AS1 is an independent predictor of poor survival in patients with clear cell renal carcinoma. Oncol Lett. 2020;19:3993–4001. doi: 10.3892/ol.2020.11484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Csermely P, Kunsic N, Mendik P, Kerestély M, Faragó T, Veres DV, Tompa P. Learning of signaling networks: molecular mechanisms. Trends Biochem Sci. 2020;45:284–294. doi: 10.1016/j.tibs.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 60.Fang Y, Yang C, Yu Z, Li X, Mu Q, Liao G, Yu B. Natural products as LSD1 inhibitors for cancer therapy. Acta Pharm Sin B. 2020 doi: 10.1016/j.apsb.2020.06.007. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li M, Tang Y, Li Q, Xiao M, Yang Y, Wang Y. Mono-ADP-ribosylation of H3R117 traps 5mC hydroxylase TET1 to impair demethylation of tumor suppressor gene TFPI2. Oncogene. 2019;38:3488–3503. doi: 10.1038/s41388-018-0671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barceló C, Etchin J, Mansour MR, Sanda T, Ginesta MM, Sanchez-Arévalo Lobo VJ, Real FX, Capellà G, Estanyol JM, Jaumot M, Look AT, Agell N. Ribonucleoprotein HNRNPA2B1 interacts with and regulates oncogenic KRAS in pancreatic ductal adenocarcinoma cells. Gastroenterology. 2014;147:882–892. e888. doi: 10.1053/j.gastro.2014.06.041. [DOI] [PubMed] [Google Scholar]
- 63.Wang L, Wen M, Cao X. Nuclear hnRNPA2B1 initiates and amplifies the innate immune response to DNA viruses. Science. 2019;365:eaav0758. doi: 10.1126/science.aav0758. [DOI] [PubMed] [Google Scholar]
- 64.Shim MK, Moon Y, Yang S, Kim J, Cho H, Lim S, Yoon HY, Seong JK, Kim K. Cancer-specific drug-drug nanoparticles of pro-apoptotic and cathepsin B-cleavable peptide-conjugated doxorubicin for drug-resistant cancer therapy. Biomaterials. 2020;261:120347. doi: 10.1016/j.biomaterials.2020.120347. [DOI] [PubMed] [Google Scholar]
- 65.Yang WB, Hsu CC, Hsu TI, Liou JP, Chang KY, Chen PY, Liu JJ, Yang ST, Wang JY, Yeh SH, Chen RM, Chang WC, Chuang JY. Increased activation of HDAC1/2/6 and Sp1 underlies therapeutic resistance and tumor growth in glioblastoma. Neuro Oncol. 2020;22:1439–1451. doi: 10.1093/neuonc/noaa103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vizcaíno C, Mansilla S, Portugal J. Sp1 transcription factor: a long-standing target in cancer chemotherapy. Pharmacol Ther. 2015;152:111–124. doi: 10.1016/j.pharmthera.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 67.Lunde PK. WHO’s programme on essential drugs. Background, implementation, present state and prospectives. Dan Med Bull. 1984;31(Suppl 1):23–27. [PubMed] [Google Scholar]