Abstract
Oxaliplatin (OXA), as a third-generation platinum anticancer drug, is a treatment drug for gastric cancer (GC). However, OXA resistance has become the main reason for OXA treatment failure. Serine beta-lactamase-like protein (LACTB), acts as a mitochondrial protein, can affect multiple cancer processes. Here, we aimed to investigate the function and mechanism of LACTB in OXA-resistant GC. After LACTB overexpression or autophagy activator (RAPA) treatment, cell proliferation, reactive oxygen species (ROS), apoptosis, mitochondrial dysfunction were evaluated through CCK-8 assay, Edu staining, flow cytometry and immunofluorescence assay. Moreover, DNA double-stranded damage and autophagy-related proteins were examined via western blot. We revealed that LACTB was downregulated in OXA-resistant MGC-803 cells, and overexpression of LACTB reduced the resistance of GC cells to OXA. Besides, our results uncovered that overexpression of LACTB induced apoptosis, reduced the mitochondrial membrane potential (MMP) and accelerated ROS accumulation in OXA-resistant MGC-803 (MGC-803/OXA) cells. Meanwhile, we verified that overexpression of LACTB decreased glucose uptake and ATP synthesis, induced mitochondria and DNA damages, and inhibited autophagy of MGC-803/OXA cells. Furthermore, our results certified that RAPA could weaken the function of LACTB on apoptosis and mitochondrial morphology and function in OXA-resistant MGC-803 cells with OXA treatment. Therefore, we demonstrated that LACTB could attenuate the resistance of MGC-803/OXA cells to OXA through autophagy-mediated mitochondrial morphological changes, mitochondrial dysfunction, and apoptosis, suggesting that LACTB, functions as a suppressor, is conducive to the therapy of OXA-resistant GC.
Keywords: Gastric cancer, serine beta-lactamase-like protein, oxaliplatin, apoptosis, autophagy, mitochondrial dysfunction
Introduction
Gastric cancer (GC) is the second malignant tumor in China, seriously endangering human health [1]. At present, chemotherapy occupies the vital status in the comprehensive treatment of GC, so the effective rate of chemotherapy is one of the crucial factors affecting the prognosis of GC patients [2]. Oxaliplatin (OXA) is the third-generation derivative of platinum complex anticancer drugs and has no obvious cross-resistance to cisplatin [3]. OXA has been widely applied to treat tumors derived from digestive system, especially GC [4]. Despite the great progress in the development of various anticancer therapies, OXA resistance in the treatment of GC has become increasingly apparent [5]. Therefore, to explore the mechanism of OXA resistance has become one of the key issues in the therapy of GC. Currently, the mechanisms of OXA resistance in GC are very complex, involving multiple factors such as the microenvironment associated with tumor growth, cell mitochondria, cell membrane, cell protein and signal transduction, etc [6,7]. However, the critical factors that determine the OXA resistance of GC are still largely unclear.
Serine beta-lactamase-like protein (LACTB) is a mitochondrial intermembrane protein derived from the penicillin binding proteins (PBPs)/β-lactamase family [8]. LACTB is involved in the synthesis of peptidoglycan and has crucial effects on mitochondrial structure and function [9]. Researchers discovered that LACTB could form a fibrous mitochondrial membrane in the intermembrane space, which affects the conversion of phosphatidylserine to phosphatidyl ethanolamine, and directly or indirectly regulates mitochondrial phospholipid metabolism [10,11]. Meanwhile, studies revealed that mitochondrial phospholipid metabolism was closely related to cell proliferation and tumor formation, suggesting that LACTB can be involved in tumor proliferation and differentiation [12]. Numerous studies have also proved that LACTB was in connection with various types of cancers, such as glioma, colon, breast and liver cancer, etc [13-16]. Besides, LACTB can reduce the growth and promote the apoptosis of tumor cells [17]. Therefore, LACTB might be a promising molecule in prediction, recurrence and survival of GC. However, the associative roles and potential mechanisms of LACTB in OXA-resistant GC stemness remains unrevealed.
Autophagy, as a protective mechanism, can play a protective role by regulating organelle (such as mitochondria) and protein functions in the case of normal or pathological aging [18,19]. A number of researches have confirmed that selective degradation of mitochondria by autophagy has significant effect in the physiological process of disease including cancers [20,21]. For example, autophagy has become a molecular target for cancer therapy [22]; autophagy has been proven to contribute significantly to tumor microenvironment and tumor metastasis [23]. Besides, researches proved that autophagy, as a bridge between cancer cells and chemotherapy drugs, can affect the sensitivity of cancer cells to chemotherapy drugs and even lead to multi-drug resistance [24,25]. Studies also showed that autophagy can play vital roles in drug resistance by removing abnormal proteins, organelles, and ROS in a variety of tumor models [26,27]. However, whether LACTB, a mitochondrial protein, can affect mitochondrial function by regulating autophagy pathway in OXA-resistant GC has not been clearly clarified.
In this study, we investigated whether LACTB could play an essential role in OXA-resistant GC. Besides, we explored the influences of LACTB on apoptosis and mitochondrial functions in OXA-resistant GC. Moreover, we further verified the regulatory effect of LACTB on autophagy in OXA-resistant GC. Therefore, our study might propose a new reference index for the diagnosis and treatment of OXA-resistant GC.
Materials and methods
Cell culture
GES-1 cells (Cat.No. MJ-1235) and MGC-803 cells (Cat.No. TC-hxbz-026) were offered by ATCC. Both the two cells were cultured in DMEM medium with high glucose (Procell; Cat.No. PM150210) containing 10% fetal bovine serum (Gibco, USA) and 1% Penicillin/streptomycin (Solarbio, Cat.No. P1400) at 37°C in a cell incubator containing 5% CO2.
Construction of OXA-resistant cells
We induced MGC-803 cells by intermittent dosing. MGC-803 cells in logarithmic growth stage were incubated with complete medium containing a starting dose of 0.5 mol/L OXA (Aladdin, Cat.No. 0124003) for 24 hrs. After washing with PBS, the OXA-containing medium was replaced with a normal medium until the cells returned to growth. The MGC-803 cells were repeatedly given the OXA-containing medium. After cell tolerance, the OXA concentration was gradually increased until they could tolerate 10 mol/L of OXA. OXA-resistant MGC-803 cells were named as “MGC-803/OXA” cells.
Plasmid construction and transfection
Controlled plasmid (pcDNA 3.0) and pcDNA 3.0-LACTB were gained from Hanbio (Shanghai, China). MGC-803/OXA cells (1 × 105 cells/well) were inoculated with into 6-well plates before transfection 24 hrs until the cell fusion rate reached approximately 80%. And then the cells were transfected with 4 μg pcDNA 3.0 and 4 μg pcDNA 3.0-LACTB using Lipofectamine-3000 (Invitrogen; Cat.No. 100022049) based on the experimental instructions. After 8 hrs of cell culture, the cell culture medium was replaced with normal culture medium. Besides, cells were treated with 4 μmol OXA or 100 nM autophagy activator RAPA (Solarbio, Cat.NO. R8150), respectively.
Quantitative real-time PCR (qRT-PCR) assay
Total RNAs were extracted from the treated MGC-803 and MGC-803/OXA cells by applying TRIzol reagent (Invitrogen). After detection with NanoDro 2000c (Thermo Scientific, USA), 1 μg RNAs were utilized as template to synthetize cDNAs by applying the PrimeScript RT Reagent (Takara). Subsequently, the levels of all genes were confirmed SYBR Premix Taq kit (Takara) on an ABI 7300 Real-Time system (Applied Biosystems). 2-ΔΔCt method was utilized to quantitatively analyze the expressions of genes.
Western blot assay
Total proteins in the treated MGC-803 and MGC-803/OXA cells were extracted with the RIPA reagent (Sigma) including protease inhibitor. After the protein concentration was determined with BCA kit (Thermo Scientific), 30 μg protein was electrodeposited using 10% SDS-PAGE and then transferred onto the nitrocellulose membrane (Sigma). After incubation with 5% skim milk, the specific primary antibodies were utilized to incubate the membranes at 4°C overnight. Afterwards, the membranes were hatched with HRP-labeled secondary antibodies (Promega) for 1 h. The membranes were treated with the ECL reagent (Pierce) and exposed to the X-ray film. Grayscale values are analyzed using Quantity-one image analysis software. The primary antibodies included LACTB (Abcam; ab151624), pSer4/Ser8 RPA2 (Abcam; ab243866), RPA2 (Abcam; ab10359), γH2AX (Abcam; ab11175), LC3I/II (Abcam; ab63817), Beclin-1 (Abcam; ab62557), P62 (Abcam; ab155686) and GAPDH (Abcam; ab8245). GAPDH was used as the internal.
CCK-8 assay
The treated MGC-803/OXA cells were collected at the logarithmic stage to adjust the concentration of cell suspension. 100 µL cells (1 × 105 cells/well) were divided into 96 well plate. After transfected with 0.4 μg LACTB plasmids, all the cells were treated with 1, 2, 4, 6, 12 μM OXA for 48 hrs. Then the cells in each well were added 15 µL CCK-8 solution (Dojindo, Japan) and cultured for 4 hrs. The absorbance value (A) was measured on the microplate (Perkin Elmer, Switzerland; Cat.No. 6005290) at 450 nm.
Edu staining
MGC-803/OXA cells in each group were inoculated in 96-well plates with 8000 cells per well. After 48 hrs of incubation, all cells were labeled with EdU (ribobio, Guangzhou, China), and cell proliferation was tested according to the instruction of the kit. The results were observed using a fluorescence microscope.
Flow cytometry for reactive oxygen species (ROS)
As displayed in previous study [28], DCFH-DA fluorescent probe (Beyotime, Cat.No. S0033) was applied to determine the level of ROS. The treated MGC-803/OXA cells (1 × 106 cells) were treated with 2.5 mmol/L DCFH-DA for 25 mins at 37°C. After washing and collecting, cells were handled with red fluorescence. The fluorescence intensity was examined by FACS Calibur flow cytometer (BD Biosciences).
Flow cytometry analysis for apoptosis
The treated MGC-803/OXA cells were harvested and 6 × 107 cells were suspended with 100 μl 1 × binding buffer. And then cells were double stained with Annexin V-FITC/PI (BD Biosciences) for 15 mins in the dark. Finally, cell apoptosis was determined using a flow cytometer (BD Biosciences) and the data was calculated by appling a ModFit LT 2.0 software.
Flow cytometry analysis for MMP
The treated cells (5 × 105 cells) in each group were treated with 1 μL 1.25 mol/L Rh123 probe for 30 mins and dyed with 500 μL JC-1 staining solution (MultiSciences) for 30 mins at 37°C. The fluorescence was evaluated by flow cytometry (BD Biosciences) in the FL1 channel (525 nm).
Measure of glucose uptake
According to the kit instructions, glucose uptake was analyzed using Glucose Uptake Kit (Abcam; ab136955).
Determination of ATP concentration
Based on the kit instructions, ATP concentration was monitored using ATP Assay Kit (Abcam; ab83355).
Mito-tracker green staining
Treated cells from each group were collected and washed twice with PBS. All cells were fixed with 3.7% formaldehyde for 10 mins and washed three times with PBS containing 0.1% Triton X-100 (Thermo Fisher Scientific). The Mito-Tracker Green probes (Byotime; Cat.no. C2006) were diluted by with DMEM at a 1:5000 ratio. 1 mL diluent was added to the fixed cells and the cells were incubated at room temperature for 40 mins out of light. The results were observed under a fluorescence microscope (Olympus, Japan; Cat.No. IX73).
Transmission electron microscope (TEM)
The treated MGC-803/OXA cells were collected and fixed with 2% glutaraldehyde. And then the mixture was dropped onto EM grids. After drying, the samples were stained with 1% uranyl acetate, and the grids were measured with HT7700 TEM (Hitachi, Tokyo, Japan).
Immunofluorescence (IF) assay
The right amount of t MGC-803/OXA cells after processing were inoculated on the cover glass and incubated overnight at 37°C. After fixed with 4% paraformaldehyde for 30 mins, cells were permeated 0.1% Triton X-100 (Thermo Fisher Scientific) for 10 mins. The cells were sealed in the blocking solution for 1 h at room temperature, and stayed in LC3B antibody (Abcam, ab51520) overnight at 4°C. Next day, the Goat Anti-Rabbit IgG H&L (Alexa Fluor® 488) (Abcam, ab150077) was applied to incubate cells at room temperature for 2 hrs without light. Finally, cells were stained with 4’, 6 diamidino 2 phenylindole (DAPI; Sigma, Cat.No. D8417) for 10 mins. The images were taken under fluorescence microscope (Olympus, Japan; Cat.No. IX73).
Statistical analysis
The data in this study were expressed as the means ± standard deviation (SD) of three repeated experiments. And the data were estimated by SPSS (version 23.0, Inc., Chicago, IL, USA) and GraphPad software (Ver. Prism 7) using the analysis of variance (ANOVA) with Turkey-Kramer multiple comparisons test. P < 0.05 was considered statistically significant.
Results
LACTB was lowly expressed in OXA-resistant MGC-803 cells
To determine whether LACTB has significant effect in OXA-resistant GC cells, we firstly examined the expression change of LACTB in MGC-803 cells. As displayed in Figure 1A and 1B, the mRNA and protein expression levels of LACTB were remarkably downregulated in MGC-803 cells relative to human normal gastric epithelial cells (GES-1), meanwhile, its expression was prominently reduced in OXA-resistant MGC-803 cells versus MGC-803 cells. Hence, we proved that LACTB expression exhibited a remarkable decrease in MGC-803/OXA cells.
Figure 1.
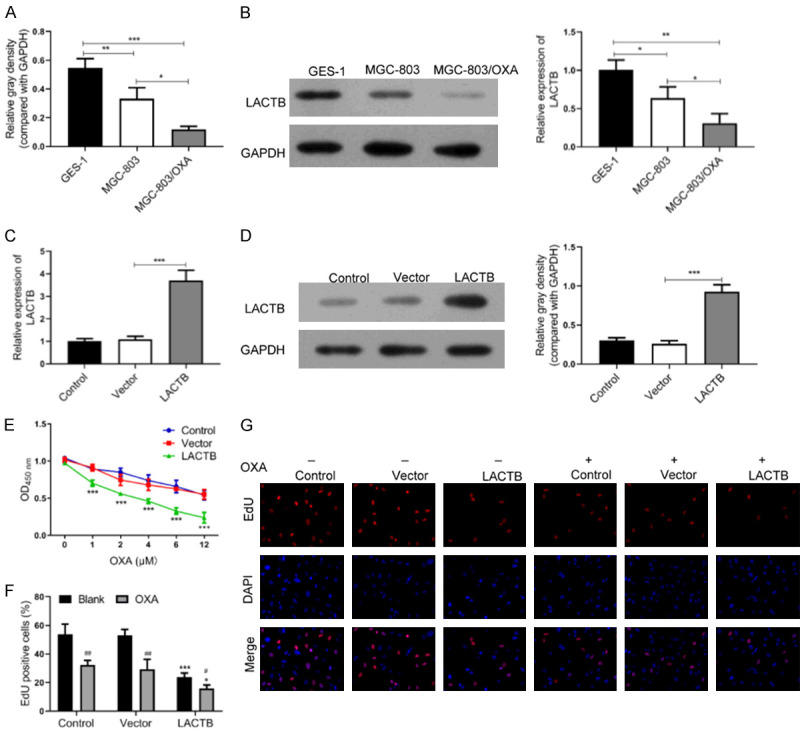
Overexpression of LACTB repressed proliferation of OXA-resistant MGC-803 cells. A. The mRNA level of LACTB was examined by qRT-PCR analysis in GES-1, MGC-803 and MGC-803/OXA cells, respectively. *P < 0.05; **P < 0.01, ***P < 0.001. B. The protein level of LACTB was determined by western blotting analysis in GES-1, MGC-803 and MGC-803/OXA cells, respectively. *P < 0.05; **P < 0.01. C, D. qRT-PCR and western blot assays were applied to confirm the transfection effect of pcDNA3.0 LACTB plasmid in MGC-803/OXA cells. ***P < 0.001. E-G. CCK-8 assay and Edu staining were utilized to examine the effect of LACTB overexpression on the proliferation of MGC-803/OXA cells. *P < 0.05, ***P < 0.001 vs. vector group. #P < 0.05 vs. blank group.
Overexpression of LACTB repressed proliferation of OXA-resistant MGC-803 cells
To investigate the functional effects of LACTB on OXA-resistant MGC-803 cells, LACTB plasmid was transfected into MGC-803/OXA cells. And the transfection effect of LACTB was confirmed by qRT-PCR and western blot assays. As exhibited in Figure 1C and 1D, the results uncovered that LACTB has been significantly overexpressed in OXA-resistant MGC-803 cells, suggesting the good transfection effect of LACTB. Besides, we further determined the influence of LACTB overexpression on the proliferative capability of MGC-803/OXA cells. The data from CCK-8 assay exhibited that overexpression of LACTB prominently decreased the proliferation of MGC-803/OXA cells, and enhanced the sensitivity of MGC-803/OXA to OXA; meanwhile, the ability of cell proliferation significantly decreased with the increase of OXA concentration. Based on the results, we chose 4 μM OXA for further study, which was close to IC50 (Figure 1E). Besides, Edu staining also revealed that LACTB overexpression could result in a prominent decrease in the proliferation of OXA-resistant MGC-803, and overexpression of LACTB could decrease the tolerance of MGC-803/OXA cells to OXA (Figure 1F and 1G). In general, our results implied that LACTB has a noteworthy inhibitory effect on MGC-803/OXA cell proliferation, suggesting that LACTB overexpression can significantly inhibit the tolerance of MGC-803/OXA cells to OXA.
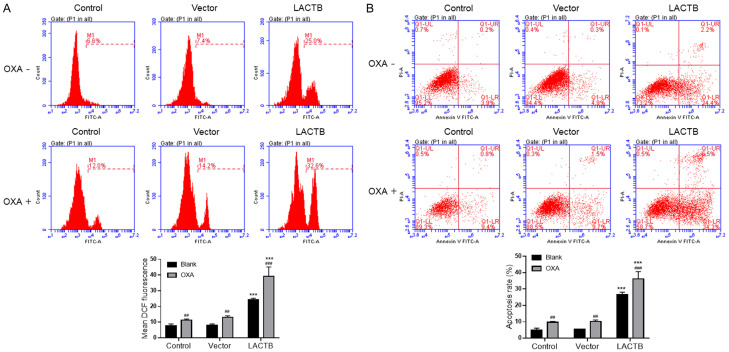
Overexpression of LACTB enhanced OXA-induced ROS and apoptosis-promoting of OXA-resistant MGC-803 cells
Next, more experiments were carried out to deeply explore the antiproliferation effects of LACTB in OXA-resistant MGC-803 cells through flow cytometry. Firstly, we demonstrated that the intracellular level of ROS was notably elevated in LACTB overexpression group compared with vector transfection group (Figure 2A). Secondly, the results disclosed that the apoptotic rates of MGC-803/OXA cells were prominently increased in LACTB overexpression group relative to vector transfection group (Figure 2B). More importantly, we found that overexpression of LACTB could enhance the ROS level and apoptotic rates increased dramatically in MGC-803/OXA cells with OXA treatment. Overall, we suggested that LACTB could increase ROS accumulation and accelerate apoptosis of OXA-resistant MGC-803 cells.
Figure 2.
Overexpression of LACTB enhanced OXA-induced ROS and apoptosis-promoting of OXA-resistant MGC-803 cells. A. After LACTB overexpression in MGC-803/OXA cells, the intracellular level of ROS was examined using DCFH-DA fluorescent probe. B. The apoptosis level of MGC-803/OXA cells after LACTB overexpression was analyzed by flow cytometry, and respective results were also exhibited. ***P < 0.001 vs. control group; ##P < 0.01, ###P < 0.001 vs. blank group.
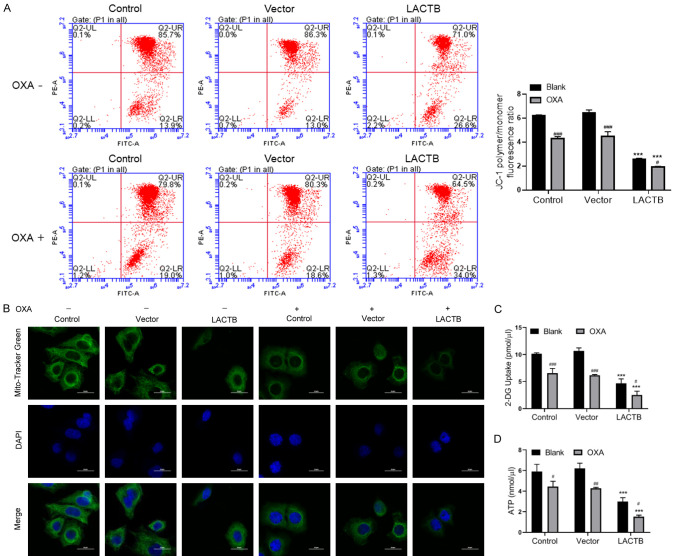
Upregulation of LACTB reduced OXA-induced mitochondrial depolarization, glucose uptake and ATP synthesis of OXA-resistant MGC-803 cells
And then the data also manifested that mitochondrial membrane potential was observably reduced in LACTB overexpressed MGC-803/OXA cells relative to that in vector-transfected cells, especially in OXA treatment group (Figure 3A). In addition, the experimental results of IF assay exhibited that in the control group, the morphology of mitochondria was normal and filamentous; in the LACTB overexpression group, the mitochondria of MGC-803/OXA cells were broken and swollen, forming vacuoles and aggregation. Meanwhile, we discovered that the mitochondrial fluorescence intensity was obviously decreased in LACTB overexpressed MGC-803/OXA cells relative to vector transfected cells. Besides, we revealed that overexpression of LACTB could aggravate the damage of mitochondria in MGC-803/OXA cells with OXA treatment (Figure 3B). Mitochondria, as an energy factory of cells, can convert glucose and other major energy sources into ATP. In the following study, we further confirmed the changes of mitochondrial structure, glucose uptake and ATP synthesis in MGC-803/OXA cells. Our results first uncovered that overexpression of LACTB could significantly reduce glucose uptake and ATP content in MGC-803/OXA cells, especially in OXA treatment group (Figure 3C and 3D). Therefore, we testified that LACTB could notably reduce mitochondrial depolarization and inhibit glucose uptake and ATP synthesis of OXA-resistant MGC-803 cells.
Figure 3.
Upregulation of LACTB reduced OXA-induced mitochondrial depolarization, glucose uptake and ATP synthesis of OXA-resistant MGC-803 cells. A. After LACTB overexpression, MGC-803/OXA cells were stained with JC-1 to evaluate mitochondrial depolarization by flow cytometry. B. The degree of mitochondria damage was examined by IF assay. Magnification 200 ×, Scale bars = 20 μm. C, D. The levels of 2-DG uptake and ATP were determined using the respective kits in LACTB overexpressed MGC-803/OXA cells. ***P < 0.001 vs. control group; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. blank group.
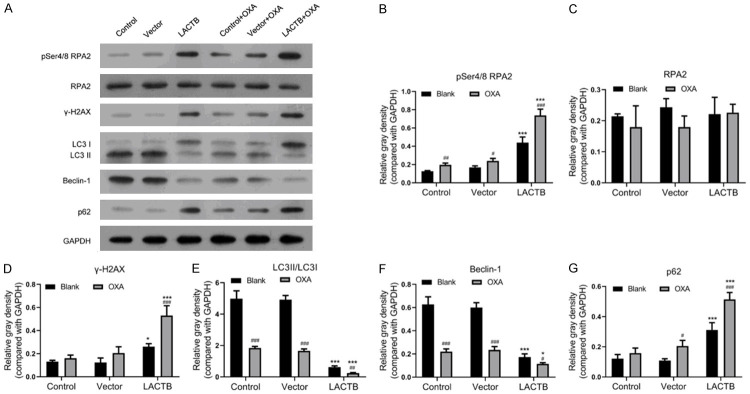
Overexpression of LACTB enhanced OXA-induced DNA damage and inhibition of autophagy of OXA-resistant MGC-803 cells
Subsequently, we further analyzed the effects of LACTB overexpression on DNA damage and autophagy related proteins. The results of western blot assay demonstrated that upregulation of LACTB led to the marked increases of pSer4/Ser8 RPA2, γH2AX and P62 expressions, and prominent reductions of LC3II/I and Beclin-1 expressions in MGC-803/OXA cells; simultaneously, we also found that overexpression of LACTB could enhance the degree of change in the expression of these proteins in MGC-803/OXA cells with OXA treatment (Figure 4). Therefore, we certified that over expression of LACTB could accelerate DNA damage and prevent autophagy of OXA-resistant MGC-803 cells.
Figure 4.
Overexpression of LACTB induced DNA damage and inhibited autophagy of OXA-resistant MGC-803 cells. A. Western blot assay was applied to examine the protein levels of pSer4/Ser8 RPA2, RPA2, γH2AX, LC3I/II, Beclin-1 and P62 in LACTB overexpressed MGC-803/OXA cells. B-G. And the relative expressions were calculated according to the gray values of each group. *P < 0.05, ***P < 0.001 vs. vector group; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. blank group.
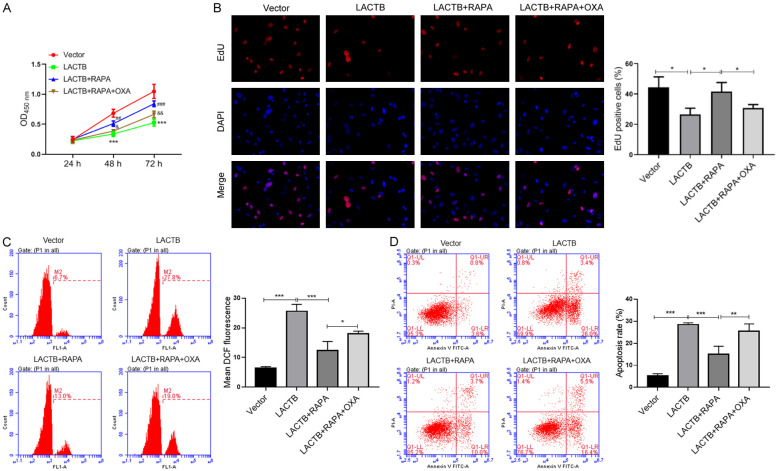
RAPA reversed the effects of LACTB overexpression on the proliferation, ROS production and apoptosis of MGC-803/OXA cells
Since LACTB had a significant inhibitory effect on autophagy, we then verified whether the autophagy pathway was involved in the impacts of LACTB on the proliferation, ROS production and apoptosis of OXA-resistant MGC-803 cells. As the experiments revealed that the inhibition of LACTB overexpression on MGC-803/OXA cell proliferation was reversed when additional added autophagy activator (RAPA); meanwhile, we discovered that OXA treatment then notably reduced the regulatory effect of RAPA on the proliferation of MGC-803/OXA cells (Figure 5A and 5B). In addition, our data displayed that RAPA could signally reduce the intracellular level of ROS in LACTB-overexpressed MGC-803/OXA cells, while OXA could further attenuate the reduction of LACTB on ROS production in MGC-803/OXA cells (Figure 5C). In the meantime, our results also disclosed that RAPA could weaken the promoting effect of LACTB overexpression on the apoptosis of MGC-803/OXA cells, which also could be reversed by OXA processing (Figure 5D). On the whole, we verified that LACTB can affect GC cell proliferation, ROS production and apoptosis by regulating autophagy.
Figure 5.
RAPA reversed the effects of LACTB overexpression on the proliferation, ROS production and apoptosis of MGC-803/OXA cells. After transfection with LACTB plasmid, MGC-803 cells were treated with RAPA or/and OXA, respectively. A. CCK-8 assay was carried out to assess the proliferation changes of MGC-803/OXA cells in each group. ***P < 0.001 vs. vector group; ##P < 0.001, ###P < 0.001 vs. LACTB group; &P < 0.05, &&P < 0.01 vs. LACTB+RAPA group. B. EdU staining was utilized to detect the proliferation changes of MGC-803/OXA cells in each group. C. DCFH-DA fluorescent probe was utilized to confirm the intracellular level of ROS. D. Flow cytometry was applied to analyze the apoptosis of MGC-803/OXA cells. *P < 0.05; **P < 0.01, ***P < 0.001.
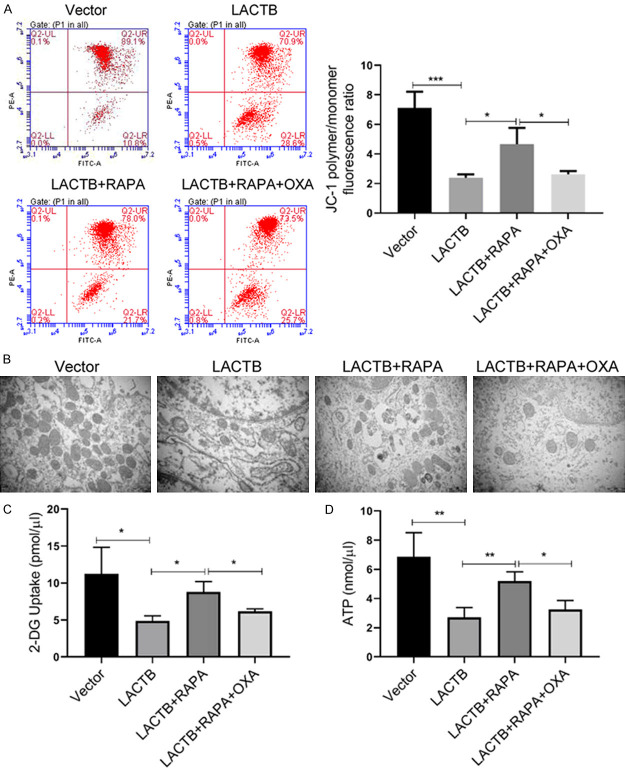
LACTB suppressed mitochondrial depolarization, glucose uptake and ATP synthesis, and reduced mitochondria of MGC-803/OXA cells by autophagy pathway
Furthermore, we investigated whether autophagy pathway also plays a key role in the influences of LACTB on mitochondrial dysfunction, glucose uptake and ATP synthesis of MGC-803/OXA cells. Our data displayed that the suppression of LACTB on mitochondrial depolarization could be reversed by RAPA in MGC-803/OXA cells, and OXA could attenuate the protective effects of RAPA on the mitochondria in MGC-803/OXA cells (Figure 6A). Subsequently, our results from TEM also uncovered that overexpression of LACTB notably reduced the number of mitochondria and damaged their morphology in MGC-803/OXA cells, while the addition of RAPA can increase the number of mitochondria in cells and reduce mitochondrial damage to some extent, and OXA also could weaken the increase of mitochondria induced by RAPA in MGC-803/OXA cells (Figure 6B). Simultaneously, we certified that RAPA markedly attenuated the decreases of glucose uptake and ATP content, which were mediated by LACTB overexpression in MGC-803/OXA cells, and OXA reduced the elevation of glucose uptake and ATP mediated by RAPA in MGC-803/OXA cells (Figure 6C and 6D). Taken together, we suggested that LACTB promoted the dysfunction of mitochondrial and energy synthesis in OXA-resistant MGC-803 cells by inhibition of autophagy.
Figure 6.
LACTB suppressed mitochondrial depolarization, glucose uptake and ATP synthesis, and reduced mitochondria of MGC-803/OXA cells by autophagy pathway. A. The mitochondrial depolarization was examined using flow cytometry with JC-1 staining. B. Mitochondrial damage of MGC-803/OXA cells was evaluated by TEM after treatment with LACTB plasmid, RAPA or/and OXA. C. D. Respective kits were used to determine the levels of 2-DG uptake and ATP in the treated MGC-803/OXA cells. *P < 0.05, **P < 0.01, ***P < 0.001.
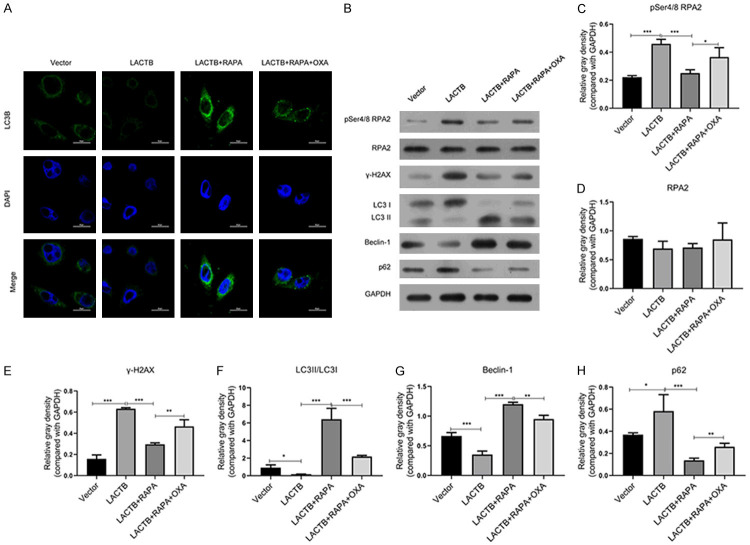
LACTB accelerated OXA-induced DNA damage by inhibiting autophagy pathway in MGC-803/OXA cells
Moreover, we further verified that autophagy pathway was closely related with the regulatory effect of LACTB on DNA damage in OXA-resistant MGC-803 cells. The data displayed that LACTB memorably suppressed LC3B expression, this suppression was then weakened by RAPA, and OXA intervention then reduced LC3B expression mediated by RAPA in MGC-803/OXA cells (Figure 7A). Secondly, the results of western blotting uncovered that RAPA could memorably reverse the upregulations of pSer4/Ser8, γH2AX and P62 expressions, and the downregulations of LC3II/I and Beclin-1 expressions, which were mediated by LACTB overexpression, then OXA treatment could prevent the regulatory effects of RAPA on the DNA damage-related proteins in MGC-803/OXA cells (P < 0.05, P < 0.01, P < 0.001, Figure 7B). These findings indicated that RAPA reversed the inductive effect of LACTB on DNA damage of MGC-803/OXA cells by inducing autophagy.
Figure 7.
LACTB resulted in OXA-induced DNA damage by inhibiting autophagy pathway in MGC-803/OXA cells. A. IF assay was performed to assess the degree of LC3B. Magnification 200 ×, Scale bars = 20 μm. B. Markers of DNA double-stranded damage and autophagy were examined by Western blot assay in MGC-803/OXA cells after treatment with LACTB plasmid, RAPA or/and OXA. C-H. The relative gray density was counted by comparison with GAPDH. *P < 0.05; **P < 0.01, ***P < 0.001.
Discussion
GC is one of high incidence and mortality of malignant tumors [29]. At present, OXA, as one of the widely used drugs in GC, the resistance is often one of the main reasons for the failure of GC treatment [30]. Therefore, it has become a research hotspot to explore the mechanism of OXA resistance and actively search for mechanisms to reverse OXA resistance. In order to further investigate the mechanism of GC resistance, the MGC-803/OXA cells were first established in this study. Meanwhile, we discovered that LACTB was significantly downregulated in OXA-resistant MGC-803 cells, suggesting that LACTB might be a potential target for the OXA resistance inhibition in GC.
LACTB, as a mitochondrial membrane protein, can suppress cell growth, differentiation, and tumor formation [10]. For example, LACTB could prevent colorectal cancer progression by weakening MDM2-mediated p53 or inhibiting epithelial-to-mesenchymal transition (EMT) [14,31]; LACTB as a target gene of miR-374a could participate in the metastasis progression of breast cancer [32]; silence of LACTB could accelerate hepatocellular carcinoma progression and predict a poor prognosis [13]; LACTB could suppress proliferation and invasion of glioma cells [15]. In our study, we further certified that LACTB could repress proliferation and promote apoptosis of MGC-803/OXA cells, and enhanced the tolerance of MGC-803 to different concentrations of OXA.
Mitochondria are the primary sites of energy metabolism in cells [33,34]. ATP production is the main energy source of cell life [35]. Researches showed that mitochondrial damage can result in the release of ROS or apoptotic factors, which can cause cell damage or induce apoptosis [36,37]. Studies suggested that mitochondrial dysfunction was closely associated with GC [38-40]. For instance, mitochondrial dysfunction could enhance the cisplatin resistance in GC [38]; Genipin could prevent the process of GC through regulating mitochondrial dysfunction [39]; VCPA could sensitize GC to doxorubicin-induced apoptosis by mitochondrial dysfunction [40]. Therefore, it is crucial to timely remove the damaged mitochondria and maintain the normal function and number of mitochondria. In our study, our results disclosed that overexpression of LACTB could induce mitochondrial damage and suppress glucose uptake and ATP synthesis, and LACTB also could reduce the resistance of MGC-803/OXA to OXA.
At present, the related studies also revealed that the changes in DNA damage repair process are the main possible mechanism of platinum and other chemotherapy drug resistance [41]. Study proved that RPA2 phosphorylation is one of the earliest responses to DNA replication stagnation or DNA damage [42]. γH2AX is a marker for a double strand break in DNA [43]. Elevated levels of phosphorylation in γH2AX and RPA2 may indicate DNA damage [44]. In our study, we revealed that LACTB could cause the increases of pSer4/Ser8 RPA2 and γH2AX expressions, suggesting that LACTB could induce DNA damage. Besides, we proved that the induction of LACTB on DNA damage could be weakened by RAPA, suggesting that LACTB could promote DNA damage by autophagy pathway in MGC-803/OXA cells.
Autophagy is a widespread degradation/recycling system in eukaryotic cells [45]. In recent years, it has been also gradually recognized that autophagy can regulate the renewal of peroxide, mitochondria and endoplasmic reticulum, and actively remove the damaged organelles and metabolites in the cytoplasm [46]. The autophagy lysosomal pathway also has vital role in regulating the degradation of the damaged mitochondria and maintaining the metabolic stability of mitochondria [47]. Therefore, autophagy is crucial for maintaining the homeostasis and metabolic balance of cells. A growing body of research has confirmed that autophagy is crucial in the progression of GC [48,49]. At present, the known autophagy related proteins include LC3, Beclin1 and P62 [50]. In the present study, we found that LACTB could increase P62 expression, and decrease LC3II/I and Beclin-1 expressions in MGC-803/OXA cells, suggesting that LACTB could refrain autophagy of OXA-resistant MGC-803 cells, and enhance the tolerance of MGC-803/OXA to different concentrations of OXA. In addition, we found that the effects of LACTB on apoptosis, and mitochondrial morphology and function of OXA-resistant MGC-803 cells could be notably attenuated by autophagy activator (RAPA). In the recent research, LACTB could suppress the development of CRC by promoting autophagy [31]. In contrast to its role in constraining GC progression, autophagy has a complex role in cancer and its function can be regulated by biological factors, for multiple cancer types is a factor [51]. Therefore, we demonstrated that LACTB promoted mitochondrial damage and apoptosis of OXA-resistant MGC-803 cells via inhibiting autophagy.
In conclusion, our findings suggested that LACTB could reduce the resistance of GC cells to OXA by through autophagy-mediated mitochondrial dysfunction and apoptosis, which might contribute to become effective therapeutic measures against OXA resistance in GC.
Acknowledgements
This research was financially supported by the Guizhou Provincial Department of Education Project (YJSCXJH (2019)069) and the Hospital-level project of Guizhou Cancer Hospital (YJ2019019).
Disclosure of conflict of interest
None.
References
- 1.Tan Z. Recent advances in the surgical treatment of advanced gastric cancer: a review. Med Sci Monit. 2019;25:3537–3541. doi: 10.12659/MSM.916475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wagner AD, Syn NL, Moehler M, Grothe W, Yong WP, Tai BC, Ho J, Unverzagt S. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2017;8:CD004064. doi: 10.1002/14651858.CD004064.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fritsch R, Hoeppner J. Oxaliplatin in perioperative chemotherapy for gastric and gastroesophageal junction (GEJ) adenocarcinoma. Expert Rev Gastroenterol Hepatol. 2019;13:285–291. doi: 10.1080/17474124.2019.1573143. [DOI] [PubMed] [Google Scholar]
- 4.Osawa H, Yamada K, Kuroda Y. Trastuzumab combined oxaliplatin and S-1 therapy demonstrated pathological complete response of synchronous liver metastasis of gastric cancer. Cell Mol Med. 2017;3:1. [Google Scholar]
- 5.Petrioli R, Francini E, Cherri S, Marrelli D, Rovello F, Fiaschi AI, Miano ST, Savelli V, Calomino N, Farsi M, Vernillo R, Francini G. Feasibility of modified docetaxel, oxaliplatin, capecitabine followed by capecitabine as maintenance chemotherapy as first-line therapy for patients with metastatic gastric or gastroesophageal cancer. Anticancer Drugs. 2020;31:292–297. doi: 10.1097/CAD.0000000000000877. [DOI] [PubMed] [Google Scholar]
- 6.Namikawa T, Maeda H, Kitagawa H, Oba K, Tsuji A, Yoshikawa T, Kobayashi M, Hanazaki K. Treatment using oxaliplatin and S-1 adjuvant chemotherapy for pathological stage III gastric cancer: a multicenter phase II study (TOSA trial) protocol. BMC Cancer. 2018;18:186. doi: 10.1186/s12885-018-4109-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang N, Liu D, Guo J, Sun Y, Guo T, Zhu X. Molecular mechanism of Poria cocos combined with oxaliplatin on the inhibition of epithelial-mesenchymal transition in gastric cancer cells. Biomed Pharmacother. 2018;102:865–873. doi: 10.1016/j.biopha.2018.03.134. [DOI] [PubMed] [Google Scholar]
- 8.Pettinati I, Brem J, Lee SY, McHugh PJ, Schofield CJ. The chemical biology of human metallo-β-lactamase fold proteins. Trends Biochem Sci. 2016;41:338–355. doi: 10.1016/j.tibs.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polianskyte Z, Peitsaro N, Dapkunas A, Liobikas J, Soliymani R, Lalowski M, Speer O, Seitsonen J, Butcher S, Cereghetti GM, Linder MD, Merckel M, Thompson J, Eriksson O. LACTB is a filament-forming protein localized in mitochondria. Proc Natl Acad Sci U S A. 2009;106:18960–18965. doi: 10.1073/pnas.0906734106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keckesova Z, Donaher JL, De Cock J, Freinkman E, Lingrell S, Bachovchin DA, Bierie B, Tischler V, Noske A, Okondo MC, Reinhardt F, Thiru P, Golub TR, Vance JE, Weinberg RA. LACTB is a tumour suppressor that modulates lipid metabolism and cell state. Nature. 2017;543:681–686. doi: 10.1038/nature21408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cucchi D, Mauro C. LACTB-mediated tumour suppression by increased mitochondrial lipid metabolism. Cell Death Differ. 2017;24:1137–1139. doi: 10.1038/cdd.2017.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith TS, Southan C, Ellington K, Campbell D, Tew DG, Debouck C. Identification, genomic organization, and mRNA expression of LACTB, encoding a serine beta-lactamase-like protein with an amino-terminal transmembrane domain. Genomics. 2001;78:12–14. doi: 10.1006/geno.2001.6643. [DOI] [PubMed] [Google Scholar]
- 13.Xue C, He Y, Zhu W, Chen X, Yu Y, Hu Q, Chen J, Liu L, Ren F, Ren Z, Cui G, Sun R. Low expression of LACTB promotes tumor progression and predicts poor prognosis in hepatocellular carcinoma. Am J Transl Res. 2018;10:4152–4162. [PMC free article] [PubMed] [Google Scholar]
- 14.Zeng K, Chen X, Hu X, Liu X, Xu T, Sun H, Pan Y, He B, Wang S. LACTB, a novel epigenetic silenced tumor suppressor, inhibits colorectal cancer progression by attenuating MDM2-mediated p53 ubiquitination and degradation. Oncogene. 2018;37:5534–5551. doi: 10.1038/s41388-018-0352-7. [DOI] [PubMed] [Google Scholar]
- 15.Li HT, Dong DY, Liu Q, Xu YQ, Chen L. Overexpression of LACTB, a mitochondrial protein that inhibits proliferation and invasion in glioma cells. Oncol Res. 2019;27:423–429. doi: 10.3727/096504017X15030178624579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J, He Y, Yu Y, Chen X, Cui G, Wang W, Zhang X, Luo Y, Li J, Ren F. Upregulation of miR-374a promotes tumor metastasis and progression by downregulating LACTB and predicts unfavorable prognosis in breast cancer. Cancer Med. 2018;7:3351–3362. doi: 10.1002/cam4.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li HT, Dong DY, Liu Q, Xu YQ, Chen L. Overexpression of LACTB, a mitochondrial protein that inhibits proliferation and invasion in glioma cells. Oncol Res. 2019;27:423–429. doi: 10.3727/096504017X15030178624579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin J, Liu Y, Huang L, Tan H. Advances in epigenetic regulation of vascular aging. Rev Cardiovasc Med. 2019;20:19–25. doi: 10.31083/j.rcm.2019.01.3189. [DOI] [PubMed] [Google Scholar]
- 19.Rubinsztein DC, Mariño G, Kroemer G. Autophagy and aging. Cell. 2011;146:682–695. doi: 10.1016/j.cell.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 20.Rabinowitz JD, White E. Autophagy and metabolism. Science. 2010;330:1344–1348. doi: 10.1126/science.1193497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature. 2009;458:1131. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kocaturk NM, Akkoc Y, Kig C, Bayraktar O, Gozuacik D, Kutlu O. Autophagy as a molecular target for cancer treatment. Eur J Pharm Sci. 2019;134:116–137. doi: 10.1016/j.ejps.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 23.Mowers EE, Sharifi MN, Macleod KF. Functions of autophagy in the tumor microenvironment and cancer metastasis. FEBS J. 2018;285:1751–1766. doi: 10.1111/febs.14388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li YJ, Lei YH, Yao N, Wang CR, Hu N, Ye WC, Zhang DM, Chen ZS. Autophagy and multidrug resistance in cancer. Chin J Cancer. 2017;36:52. doi: 10.1186/s40880-017-0219-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sui X, Chen R, Wang Z, Huang Z, Kong N, Zhang M, Han W, Lou F, Yang J, Zhang Q, Wang X, He C, Pan H. Autophagy and chemotherapy resistance: a promising therapeutic target for cancer treatment. Cell Death Dis. 2013;4:e838. doi: 10.1038/cddis.2013.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang F, Wang BR, Wang YG. Role of autophagy in tumorigenesis, metastasis, targeted therapy and drug resistance of hepatocellular carcinoma. World J Gastroenterol. 2018;24:4643–4651. doi: 10.3748/wjg.v24.i41.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu G, Pei F, Yang F, Li L, Amin AD, Liu S, Buchan JR, Cho WC. Role of autophagy and apoptosis in non-small-cell lung cancer. Int J Mol Sci. 2017;18:367. doi: 10.3390/ijms18020367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen Y, Guo W, Wang Z, Zhang Y, Zhong L, Zhu Y. Protective effects of hydrogen sulfide in hypoxic human umbilical vein endothelial cells: a possible mitochondria-dependent pathway. Int J Mol Sci. 2013;14:13093–13108. doi: 10.3390/ijms140713093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi YJ, Kim N. Gastric cancer and family history. Korean J Intern Med. 2016;31:1042–1053. doi: 10.3904/kjim.2016.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shitara K, Chin K, Yoshikawa T, Katai H, Terashima M, Ito S, Hirao M, Yoshida K, Oki E, Sasako M, Emi Y, Tsujinaka T. Phase II study of adjuvant chemotherapy of S-1 plus oxaliplatin for patients with stage III gastric cancer after D2 gastrectomy. Gastric Cancer. 2017;20:175–181. doi: 10.1007/s10120-015-0581-1. [DOI] [PubMed] [Google Scholar]
- 31.Xu W, Yu M, Qin J, Luo Y, Zhong M. LACTB regulates PIK3R3 to promote autophagy and inhibit EMT and proliferation through the PI3K/AKT/mTOR signaling pathway in colorectal cancer. Cancer Manag Res. 2020;12:5181–5200. doi: 10.2147/CMAR.S250661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang J, He Y, Yu Y, Chen X, Cui G, Wang W, Zhang X, Luo Y, Li J, Ren F, Ren Z, Sun R. Upregulation of miR-374a promotes tumor metastasis and progression by downregulating LACTB and predicts unfavorable prognosis in breast cancer. Cancer Med. 2018;7:3351–3362. doi: 10.1002/cam4.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu PS, Ho PC. Mitochondria: a master regulator in macrophage and T cell immunity. Mitochondrion. 2018;41:45–50. doi: 10.1016/j.mito.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 34.Smith GM, Gallo G. The role of mitochondria in axon development and regeneration. Dev Neurobiol. 2018;78:221–237. doi: 10.1002/dneu.22546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mailhot R, Traviss-Pollard T, Pal R, Butler SJ. Cationic europium complexes for visualizing fluctuations in mitochondrial ATP levels in living cells. Chemistry. 2018;24:10745–10755. doi: 10.1002/chem.201801008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marchi S, Patergnani S, Missiroli S, Morciano G, Rimessi A, Wieckowski MR, Giorgi C, Pinton P. Mitochondrial and endoplasmic reticulum calcium homeostasis and cell death. Cell Calcium. 2018;69:62–72. doi: 10.1016/j.ceca.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 37.Sinha K, Das J, Pal PB, Sil PC. Oxidative stress: the mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch Toxicol. 2013;87:1157–1180. doi: 10.1007/s00204-013-1034-4. [DOI] [PubMed] [Google Scholar]
- 38.Wang SF, Chen MS, Chou YC, Ueng YF, Yin PH, Yeh TS, Lee HC. Mitochondrial dysfunction enhances cisplatin resistance in human gastric cancer cells via the ROS-activated GCN2-eIF2α-ATF4-xCT pathway. Oncotarget. 2016;7:74132–74151. doi: 10.18632/oncotarget.12356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jo MJ, Jeong S, Yun HK, Kim DY, Kim BR, Kim JL, Na YJ, Park SH, Jeong YA, Kim BG, Ashktorab H, Smoot DT, Heo JY, Han J, Lee DH, Oh SC. Genipin induces mitochondrial dysfunction and apoptosis via downregulation of Stat3/mcl-1 pathway in gastric cancer. BMC Cancer. 2019;19:739. doi: 10.1186/s12885-019-5957-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu H, Liu S, Gong J, Liu J, Zhang Q, Leng X, Zhang N, Li Y. VCPA, a novel synthetic derivative of α-tocopheryl succinate, sensitizes human gastric cancer to doxorubicin-induced apoptosis via ROS-dependent mitochondrial dysfunction. Cancer Lett. 2017;393:22–32. doi: 10.1016/j.canlet.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 41.Goldstein M, Kastan MB. The DNA damage response: implications for tumor responses to radiation and chemotherapy. Annu Rev Med. 2015;66:129–143. doi: 10.1146/annurev-med-081313-121208. [DOI] [PubMed] [Google Scholar]
- 42.Zuazua-Villar P, Ganesh A, Phear G, Gagou ME, Meuth M. Extensive RPA2 hyperphosphorylation promotes apoptosis in response to DNA replication stress in CHK1 inhibited cells. Nucleic Acids Res. 2015;43:9776–9787. doi: 10.1093/nar/gkv835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siddiqui MS, Francois M, Fenech MF, Leifert WR. Persistent gammaH2AX: a promising molecular marker of DNA damage and aging. Mutat Res Rev Mutat Res. 2015;766:1–19. doi: 10.1016/j.mrrev.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 44.Ibler AE, ElGhazaly M, Naylor KL, Bulgakova NA, El-Khamisy SF, Humphreys D. Typhoid toxin exhausts the RPA response to DNA replication stress driving senescence and salmonella infection. Nat Commun. 2019;10:4040. doi: 10.1038/s41467-019-12064-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galluzzi L, Baehrecke EH, Ballabio A, Boya P, Bravo-San Pedro JM, Cecconi F, Choi AM, Chu CT, Codogno P, Colombo MI. Molecular definitions of autophagy and related processes. EMBO J. 2017;36:1811–1836. doi: 10.15252/embj.201796697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mowers EE, Sharifi MN, Macleod KF. Autophagy in cancer metastasis. Oncogene. 2017;36:1619. doi: 10.1038/onc.2016.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gomez-Suaga P, Paillusson S, Miller CC. ER-mitochondria signaling regulates autophagy. Autophagy. 2017;13:1250–1251. doi: 10.1080/15548627.2017.1317913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang X, Wu WKK, Gao J, Li Z, Dong B, Lin X, Li Y, Li Y, Gong J, Qi C, Peng Z, Yu J, Shen L. Autophagy inhibition enhances PD-L1 expression in gastric cancer. J Exp Clin Cancer Res. 2019;38:140. doi: 10.1186/s13046-019-1148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cao Y, Luo Y, Zou J, Ouyang J, Cai Z, Zeng X, Ling H, Zeng T. Autophagy and its role in gastric cancer. Clin Chim Acta. 2019;489:10–20. doi: 10.1016/j.cca.2018.11.028. [DOI] [PubMed] [Google Scholar]
- 50.Tanida I, Ueno T, Kominami E. Autophagosome and Phagosome. Springer; 2008. LC3 and Autophagy; pp. 77–88. [DOI] [PubMed] [Google Scholar]
- 51.Kimmelman AC, White E. Autophagy and tumor metabolism. Cell Metab. 2017;25:1037–1043. doi: 10.1016/j.cmet.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]