Abstract
This study was designed to investigate the effects of the aging process on peripheral and central auditory functions in adults with normal hearing. In this study, 149 participants with normal hearing were divided into four groups: aged 20-29, 30-39, 40-49 and 50-59 years for statistical purposes. Electrocochleography (EcochG), transient evoked otoacoustic emissions (TEOAE), Mandarin Hearing in Noise Test (MHINT) and the Gap Detection Test (GDT) were used. Our study found: (1) MHINT is significantly associated with aging (left ear R2=0.29, right ear R2=0.35). (2) TEOAE amplitude, TEOAE contralateral acoustic stimulation (CS) amplitude, EcochG action potential (AP), EcochG AP latency, EcochG summating potential (SP) and GDT progressively declined with age. (3) The EcochG SP/AP has no statistically significant difference among different age groups. (4) The peripheral auditory function of the right ear declines more slowly than that of the left ear. (5) Hypofunction of the central auditory system accelerates after age 40. The results demonstrate: (1) The age-related decline in the ability of speech recognition in a noisy environment may be the most sensitive indicator that reflects auditory function. (2) The decline of central auditory function is independent of peripheral auditory function, according to the auditory characteristics of the right ear. (3) Auditory function needs to be assessed individually to allow early prevention before age 40.
Keywords: Aging, central auditory function, peripheral auditory function
Introduction
Age-related hearing impairment has become the third most common disabling disease around the globe [1]. The Epidemiology of Hearing Loss Study (EHLS), in America, reported a prevalence of hearing loss (>20 dB at 0.5 to 4 kHz) of 94% among the whole cohort of 3430 participants (average age 62.3 years) [2]. Even more seriously, a significant number of patients seeking audiological treatment have normal hearing thresholds but report perceptual difficulties in noisy situations [3].
The problem of age-related hearing impairment is becoming increasingly severe along with the aging of the global population. According to a 2015 United Nations report, the number of people aged ≥60 worldwide will reach almost 2.1 billion in the next 35 years [4]. Aging, which is associated with neurodegeneration and dementia, affects every organ in the body, including the ears [5]. It can lead to neuronal atrophy and cognitive deterioration [3,6,7].
Studies in recent years have shown that cochlear synapse lesions develop earlier than hair cell damage [8]. This will cause abnormal conditions in frequency processing, intensity and temporal coding [9]. Researchers have found that 12% of the population with a normal hearing threshold complain of speech comprehension difficulties [10], termed the hidden hearing loss [11]. Pure-tone thresholds are clinically used to evaluate hearing level. However, this is limited by only reflecting the function of outer hair cells; it fails to screen for the situation of hidden hearing loss [12].
The relationship between central auditory function and peripheral auditory function is still controversial [3]. Some studies have proposed that the aging process of the central auditory system is independent of the peripheral auditory organs [13]. Some have found that central auditory decline is secondary to the decline of the cochlea [14]. We aimed to explore the characteristics of auditory aging using common peripheral and central auditory examinations. We investigated whether the central auditory function is secondary to peripheral or independent. Electrocochleography (EcochG) and transient evoked otoacoustic emissions (TEOAE) are common non-traumatic electrophysiological examination methods for clinical use and can reflect the functional state of different sites of the peripheral auditory pathway. In addition, we used psychoacoustic methods such as the Mandarin Hearing in Noise Test (MHINT) and Gap Detection Test (GDT) to reveal central auditory function.
Our research targets were the population with normal hearing thresholds, excluding people whose peripheral and central auditory systems have suffered organic damage. We probed into the relationship between the peripheral and central auditory systems’ hypofunction with age, and the critical age after which the deterioration of auditory function accelerates.
Materials and methods
Participants
A total of 149 participants were chosen for this study, with 72 males and 77 females, 22-59 years old. The average age and age range of the participants in each experimental group were: (1) 20-29 years group (mean age: 25.7 years); (2) 30-39 years group (mean age: 33 years); (3) 40-49 years group (mean age: 44 years); (4) 50-59 years group (mean age: 53.7 years). Mandarin Chinese was the participants’ primary language.
The participants completed an extensive case history questionnaire during their first testing visit. Subsections of this questionnaire included: family history, handedness, medical history, noise exposure history, difficulty hearing in a variety of settings, and tinnitus.
Their otological histories indicated that they were clear of such factors as drug toxicity, long-term noise exposure or ear infections.
Auditory screening
Pure-tone audiometry was performed in a soundproof booth with background noise under 35 dB (A), using a clinical audiometer (Madsen Astera, Denmark) with standard TDH39 headphones, at octave frequencies of 0.25, 0.5, 1, 2, 3, 4, 6, and 8 kHz. The values of the hearing threshold for frequencies from 0.25 to 8 kHz were computed according to the ISO 7029: 2017 age-related pure-tone hearing threshold standard and the average hearing threshold index specified by World Health Organization (WHO). All participants had pure-tone thresholds of 25 dB hearing level (HL) or better for standard audiometric frequencies up to 8 kHz.
TEOAE (without contralateral acoustic stimulation)
Bilateral TEOAE amplitudes were recorded from each ear. The eliciting stimuli were conventional, nonlinear clicks of 80-microsecond duration, delivered at a peak-equivalent sound pressure level (peSPL) of 80 dB. The average signal-to-noise ratio of the four frequency bands (i.e. 1, 2, 3, 4 kilo (k) Hz) exceeded 3 dB.
Contralateral suppression of TEOAE
TEOAE recordings were evoked with 60 dB peSPL linear click stimuli at a rate of 19.3/s under two conditions, with and without a 50 dB HL contralateral white noise suppressor, by inserting earphones (Etymotic Research Model ER-3A, US). Responses were averaged to 2048 sweeps. In the study, contralateral acoustic stimulation (CS) amplitude was defined as follows: CS amplitude = TEOAE level without contralateral acoustic stimulation - the TEOAE level with contralateral acoustic stimulation. The intensity of the contralateral stimulation was less than that presented to the ear in which otoacoustic emissions (OAE) were being recorded, and less than that needed to create the middle-ear reflex.
EcochG
Stimulus generation and data acquisition were processed by Intelligent Hearing System Inc. (IHS4225O, US). Ear canals were prepared by scrubbing with a cotton swab. Electrode gel was applied on the silver ball electrode before insertion. The impedance between pairs of electrodes was <3 kΩ, acoustic stimuli were 100-µs clicks delivered at 90 dB HL at a rate of 7.1/s. Electrical responses were amplified 100,000× with a 10-3000 Hz passband filter. Up to 512 sweeps were averaged.
The summating potential (SP) and action potential (AP) peaks were defined as the difference between peak and baseline by two observers (one blinded to the experimental groups).
MHINT
The HINT is a speech recognition in noise test that simulates hearing situations similar to everyday life, and is available in several languages, including Mandarin. The MHINT was tested by the BLIMP software system developed by Beijing Tongren Hospital affiliated to Capital Medical University (China) and the House Ear Institute (US). The MHINT comprises 12 lists, each with 20 sentences. Each MHINT sentence includes 10 monosyllabic words. The sentences are short, phonemically balanced, easy to understand, and with the same degree of difficulty. All test signals were presented at 65 dB SPL and were delivered monaurally through Sennheiser HD580 headphones. The participant repeated the sentence, then the audiologist selected the correct or wrong button and began the next sentence. The intensity was automatically adjusted according to the accuracy. The test is an adaptive test that measures the reception threshold for sentences in noise and uses an adaptive protocol to determine the signal to noise ratio (SNR) at which the participant recognizes 100% of the sentences. After the participant has listened to the 20 short sentences, the BLIMP test software finally calculates the SNR. The initial SNR is set to 0 dB and two sentences are used as an adaptation test before each test. The left and right ears are tested separately, and the selected vocabulary cannot be repeated.
GDT
This test evaluates temporal resolution by using a three-interval forced-choice program. The stimulus pairs were presented from a computer, using MATLAB software (version 7.0). Each trial consists of a series of three 1000 ms presentations of white noise with 1, 2 and 4 kHz frequency ranges. One has a silent gap inserted (the gap varying in size from 20 to 1 ms). Three buttons were presented to the participant, who was asked to select the interval containing the gap. Gap subtests were presented in a random order to minimize presentation order effects. Each test started at 20 ms, and was followed by a decreasing sequence (in 2 ms steps) until the first incorrect answer was recorded. Subsequently, a two-down, one-up procedure was adopted (with gap step size of 1 ms) until the correct answer was recorded [15]. The GDT threshold is the shortest time interval for which the participant correctly detects a gap [16].
Statistical analysis
The differences of each indicator among age groups were analyzed and performed using one-way ANOVO (SAS software 9.4). The correlations between age and each indicator were performed by linear regression (SPSS 25). All figures were produced by Graphpad Prism 7.0. For all statistical analysis, results were considered significant when P<0.05.
Results
TEOAE
TEOAE amplitude in quiet (without contralateral acoustic stimulation)
The TEOAE amplitude in quiet of both ears decreased with age. We took the average of the amplitudes at 1 kHz, 2 kHz, 3 kHz, and 4 kHz for statistical analysis, and it showed statistically significant differences among the various age groups (Supplementary Table 1), and linearly related to age at both ears (P<0.05, Table 1; Figure 1). According to the histogram, from age 30 to age 50, hearing declined slowly; after 50, the deterioration accelerated, especially at right ear (Figure 1).
Table 1.
TEOAE amplitude distribution according to age group
| TEOAE amplitude | Age group | ||||
|---|---|---|---|---|---|
|
| |||||
| 20-29 years | 30-39 years | 40-49 years | 50-59 years | ||
| Left ear | N | 35 | 41 | 40 | 31 |
| Mean (SD) | -1.66 (4.83) | -4.26 (6.52) | -4.84 (5.84) | -6.12 (4.56) | |
| Median | -0.95 | -4.89 | -5.74 | -5.88 | |
| Min~Max | -13.48~7.70 | -14.20~9.43 | -13.00~13.03 | -13.88~4.58 | |
| Right ear | N | 36 | 42 | 40 | 31 |
| Mean (SD) | -0.98 (5.59) | -2.38 (6.17) | -2.50 (5.86) | -5.16 (5.05) | |
| Median | -2.05 | -3.66 | -2.96 | -5.40 | |
| Min~Max | -11.88~13.05 | -12.35~11.20 | -11.40~11.93 | -11.73~6.25 | |
TEOAE, transient evoked otoacoustic emissions; N, number; SD, standard deviation.
Figure 1.
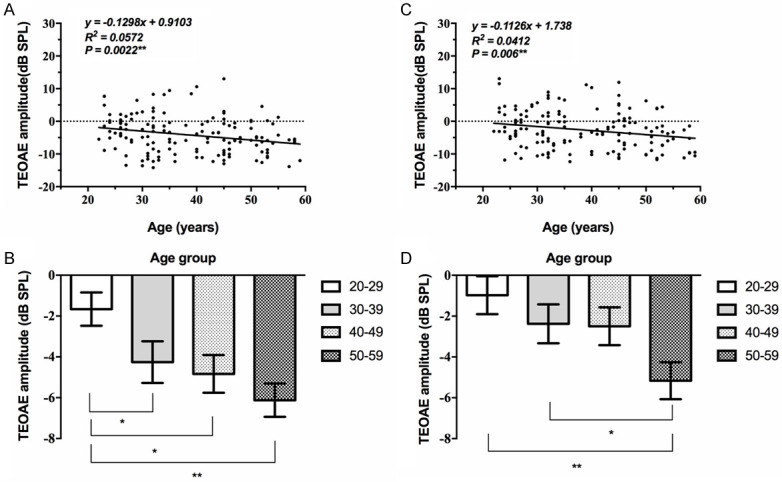
Correlation between TEOAE amplitude and age. The TEOAE amplitude in quiet of both ears decreased with age, and showed a significant correlation (**, P<0.01). A, B: The TEOAE amplitude in quiet at the left ear. All age groups showed a deteriorating trend according to the histogram. C, D: The TEOAE amplitude in quiet at the right ear. All age groups showed a deteriorating trend according to the histogram (*, P<0.05; **, P<0.01).
Age effect on TEOAE contralateral acoustic stimulation (CS)
The TEOAE CS amplitude progressively decreased with age under the stimulation of 1 kHz, 2 kHz, 3 kHz and 4 kHz frequencies. We averaged the values at four frequencies. The differences among the age groups were statistically significant and linearly related to age for the left ear (P<0.05, Table 2; Figure 2). The differences among 20-29 years group and 50-59 years group for right ear were statistically significant (Supplementary Table 2). While the correlation between TEOAE CS amplitude of the right ear and age is not statistically significant (P>0.05, Figure 2).
Table 2.
TEOAE CS amplitude distribution according to age group
| TEOAE CS amplitude | Age group | ||||
|---|---|---|---|---|---|
|
| |||||
| 20-29 years | 30-39 years | 40-49 years | 50-59 years | ||
| Left ear | N | 35 | 41 | 40 | 31 |
| Mean (SD) | 2.34 (1.20) | 1.76 (1.62) | 1.29 (1.60) | 0.95 (1.01) | |
| Median | 2.35 | 1.62 | 0.90 | 0.90 | |
| Min~Max | 0.33~5.00 | -0.60~5.98 | -1.23~6.00 | -1.08~3.65 | |
| Right ear | N | 36 | 42 | 40 | 31 |
| Mean (SD) | 2.19 (1.67) | 1.69 (1.33) | 1.83 (1.44) | 1.43 (1.41) | |
| Median | 1.95 | 1.51 | 1.35 | 1.15 | |
| Min~Max | 0.03~6.40 | -1.10~5.00 | -0.73~4.95 | -0.83~4.93 | |
TEOAE, transient evoked otoacoustic emissions; CS, contralateral acoustic stimulation; N, number; SD, standard deviation.
Figure 2.
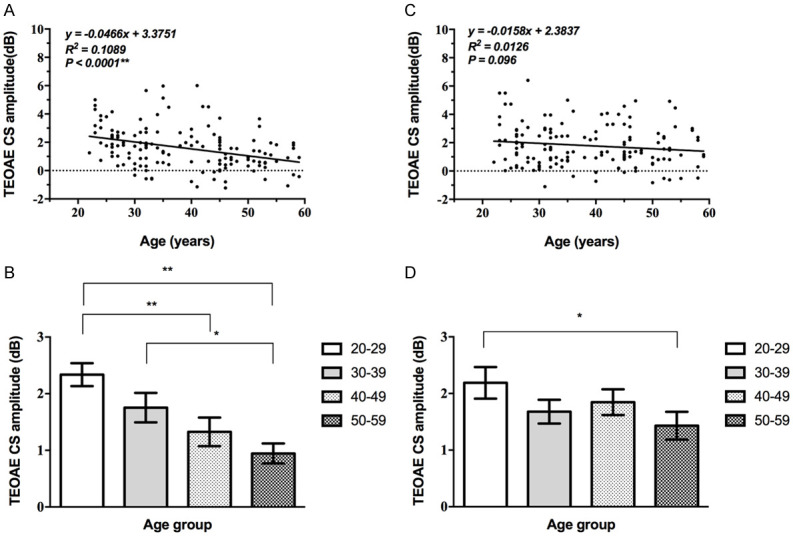
Correlation between TEOAE CS amplitude and age. A, B: The TEOAE CS amplitude of the left ear showed a significant correlation with age (**, P<0.01). C, D: The correlation between that of the right ear and age is not statistically significant (P>0.05).
EcochG
To assess cochlear neural function, we used ear-canal electrodes to measure the AP and the SP. By taking the ratio between SP and AP in our participants, we could eliminate some of the variability in human electrophysiology that arose from inter-subject differences in head size, electrode contact, etc. [17,18].
EcochG AP amplitude changes with age
For the left ear, the EcochG AP amplitude decreased gradually and there were statistically significant differences among the age groups (Supplementary Table 3) and linearly related to age (P<0.05, Table 3; Figure 3), while that of the right ear fluctuated among all age groups with an overall downward trend, the correlation between that of the right ear and age is not statistically significant (P>0.05, Figure 3).
Table 3.
EcochG AP amplitude distribution according to age group
| EcochG AP amplitude | Age group | ||||
|---|---|---|---|---|---|
|
| |||||
| 20-29 years | 30-39 years | 40-49 years | 50-59 years | ||
| Left ear | N | 36 | 40 | 39 | 31 |
| Mean (SD) | 1.18 (0.71) | 0.88 (0.39) | 0.76 (0.45) | 0.65 (0.37) | |
| Median | 0.97 | 0.8 | 0.68 | 0.58 | |
| Min~Max | 0.24~3.04 | 0.11~1.82 | 0.20~2.90 | 0.14~1.98 | |
| Right ear | N | 34 | 41 | 40 | 31 |
| Mean (SD) | 0.88 (0.64) | 0.96 (0.67) | 0.69 (0.24) | 0.80 (0.50) | |
| Median | 0.79 | 0.78 | 0.68 | 0.66 | |
| Min~Max | 0.17~3.37 | 0.20~3.27 | 0.22~1.24 | 0.31~2.29 | |
EcochG, Electrocochleography; AP, action potential; N, number; SD, standard deviation.
Figure 3.
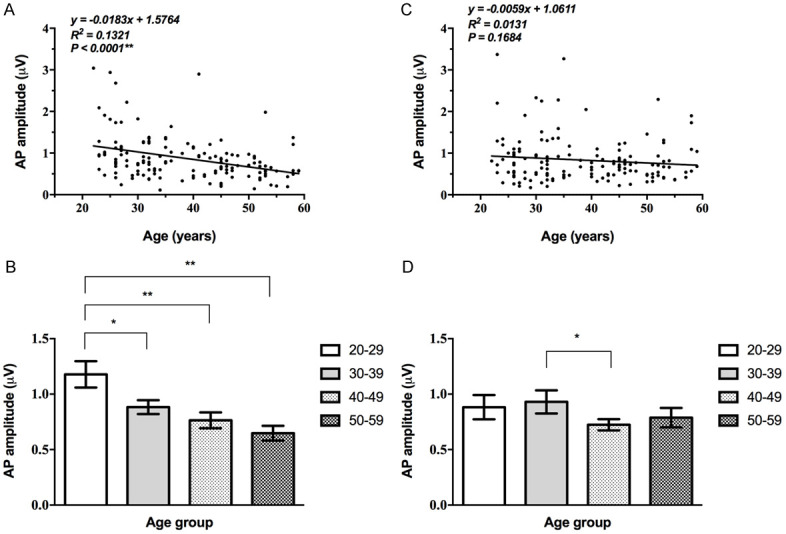
Correlation between EcochG AP amplitude and age. A, B: The EcochG AP of the left ear progressively decreased with age, and showed a significant correlation (**, P<0.01). C, D: The correlation between that of the right ear and age is not statistically significant (P>0.05).
EcochG AP latency changes with age
The differences of EcochG AP latency among the age groups were statistically significant for the left ear and linearly related to age (P<0.05, Table 4) while the latency of the right ear fluctuated among all age groups (Supplementary Table 4). The correlation between that of the right ear and age is not statistically significant (P>0.05, Figure 4).
Table 4.
EcochG AP latency distribution according to age group
| EcochG AP Latency | Age group | ||||
|---|---|---|---|---|---|
|
| |||||
| 20-29 years | 30-39 years | 40-49 years | 50-59 years | ||
| Left ear | N | 35 | 40 | 39 | 31 |
| Mean (SD) | 1.58 (0.12) | 1.59 (0.17) | 1.62 (0.13) | 1.70 (0.18) | |
| Median | 1.53 | 1.58 | 1.65 | 1.68 | |
| Min~Max | 1.38~1.92 | 1.37~2.10 | 1.38~1.85 | 1.27~2.13 | |
| Right ear | N | 34 | 41 | 40 | 31 |
| Mean (SD) | 1.58 (0.11) | 1.61 (0.12) | 1.58 (0.12) | 1.62 (0.27) | |
| Median | 1.57 | 1.60 | 1.58 | 1.67 | |
| Min~Max | 1.38~1.95 | 1.38~1.85 | 1.35~1.90 | 0.47~2.00 | |
EcochG, Electrocochleography; AP, action potential; N, number; SD, standard deviation.
Figure 4.
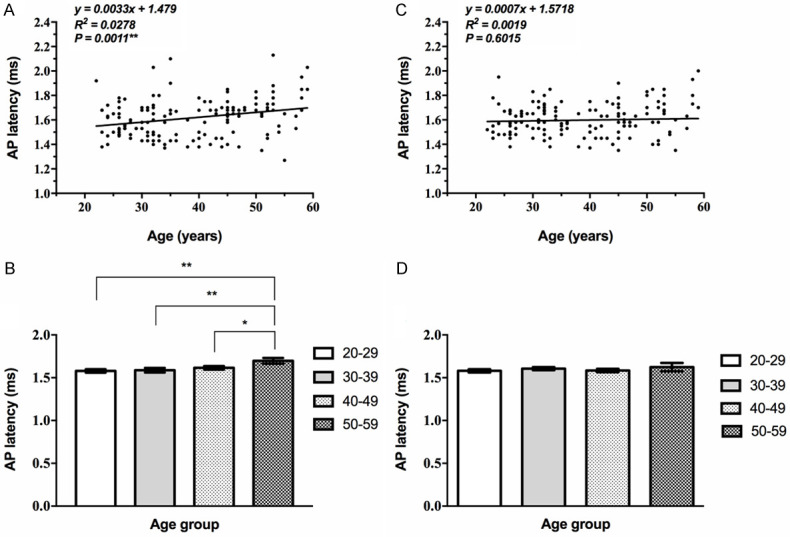
Correlation between EcochG AP latency and age. A, B: The EcochG AP latency of the left ear extended with age, and showed a significant correlation (**, P<0.01). C, D: The correlation between that of the right ear and age is not statistically significant (P>0.05).
EcochG SP amplitude changes with age
The EcochG SP amplitude of the left ear decreased progressively with age (P<0.05, Table 5) and linearly related to age. While that of the right ear fluctuated among all age groups. The average of the 50-59 group was higher than that of the 40-year-olds but lower than those of the other two groups (Supplementary Table 5). The correlation between that of the right ear and age is not statistically significant (P>0.05, Figure 5).
Table 5.
EcochG SP amplitude distribution according to age group
| EcochG SP amplitude | Age group | ||||
|---|---|---|---|---|---|
|
| |||||
| 20-29 years | 30-39 years | 40-49 years | 50-59 years | ||
| Left ear | N | 36 | 40 | 39 | 31 |
| Mean (SD) | 0.28 (0.03) | 0.21 (0.02) | 0.16 (0.01) | 0.20 (0.01) | |
| Median | 0.26 | 0.19 | 0.13 | 0.13 | |
| Min~Max | 0.05~0.75 | 0.04~0.48 | 0.05~0.41 | 0.04~0.75 | |
| Right ear | N | 34 | 41 | 40 | 31 |
| Mean (SD) | 0.23 (0.04) | 0.23 (0.03) | 0.15 (0.07) | 0.19 (0.16) | |
| Median | 0.18 | 0.16 | 0.12 | 0.14 | |
| Min~Max | 0.04~1.2 | 0.05~0.82 | 0.05~0.32 | 0.07~0.62 | |
EcochG, Electrocochleography; SP, summating potential; N, number; SD, standard deviation.
Figure 5.
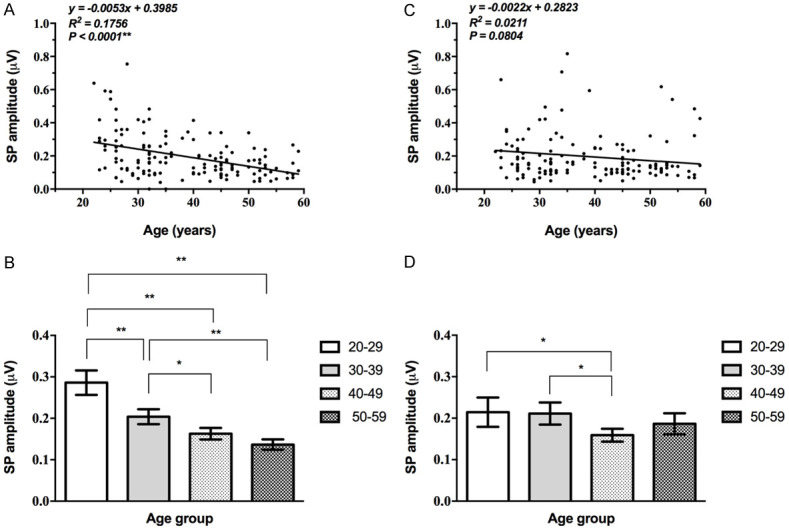
Correlation between EcochG SP amplitude and age. A, B: The EcochG SP of the left ear progressively decreased with age, and showed a significant correlation (**, P<0.01). C, D: The correlation between that of the right ear and age is not statistically significant (P>0.05).
EcochG SP/AP ratio
The SP/AP ratio had no statistically significant correlation with age at both ears (Supplementary Table 6) (P>0.05, Table 6; Figure 6), with an average SP/AP ratio of 0.22-0.27. Six participants had an SP/AP ratio greater than 0.4 in all cases (Table 6).
Table 6.
EcochG SP/AP ratio distribution according to age group
| EcochG SP/AP ratio | Age group | ||||
|---|---|---|---|---|---|
|
| |||||
| 20-29 years | 30-39 years | 40-49 years | 50-59 years | ||
| Left ear | N | 36 | 40 | 39 | 31 |
| Mean (SD) | 0.24 (0.07) | 0.24 (0.07) | 0.22 (0.08) | 0.23 (0.08) | |
| Median | 0.24 | 0.25 | 0.22 | 0.22 | |
| Min~Max | 0.11~0.36 | 0.12~0.37 | 0.07~0.41 | 0.05~0.40 | |
| Right ear | N | 34 | 41 | 40 | 31 |
| Mean (SD) | 0.26 (0.06) | 0.24 (0.06) | 0.22 (0.08) | 0.25 (0.11) | |
| Median | 0.27 | 0.24 | 0.22 | 0.23 | |
| Min~Max | 0.11~0.36 | 0.13~0.37 | 0.10~0.40 | 0.08~0.66 | |
EcochG, Electrocochleography; AP, action potential; SP, summating potential; N, number; SD, standard deviation.
Figure 6.
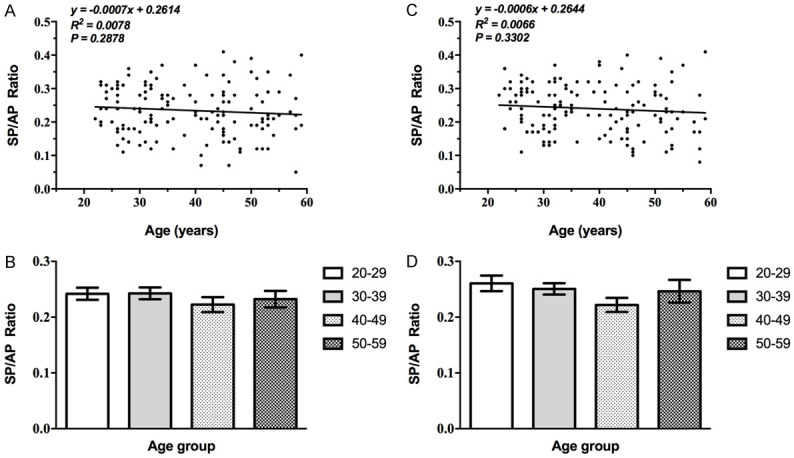
Correlation between EcochG SP/AP ratio and age. A, B: The correlation between the SP/AP ratio of the left ear and age is not statistically significant (P>0.05). C, D: The correlation between that of the right ear and age is not statistically significant (P>0.05).
Age effect on MHINT
The thresholds are presented in dB S/N. Taking the left ear of the 20-29 age group for example, when the participants recognized 100% of the sentences, the MHINT threshold was 0.73±1.00 dB S/N (Table 7). The SNR of both ears showed linearly related to age. Slight differences were observed in the 20-29 and 30-39 year groups, and the SNR in the 40-49 and 50-59 year groups were increasing significantly, showing significant differences from the other two age groups (Supplementary Table 7) (P<0.05, Figure 7).
Table 7.
MHINT SNR distribution according to age group
| MHINT SNR | Age group | ||||
|---|---|---|---|---|---|
|
| |||||
| 20-29 years | 30-39 years | 40-49 years | 50-59 years | ||
| Left ear | N | 36 | 41 | 40 | 31 |
| Mean (SD) | 0.73 (1.00) | 0.53 (1.40) | 2.00 (1.72) | 3.67 (2.23) | |
| Median | 0.82 | 0.86 | 2.22 | 3.80 | |
| Min~Max | -1.80~2.60 | -2.32~3.70 | -2.08~4.98 | 0.22~8.93 | |
| Right ear | N | 36 | 42 | 40 | 31 |
| Mean (SD) | 0.50 (1.53) | 0.73 (1.12) | 2.43 (1.81) | 4.22 (3.06) | |
| Median | 0.63 | 0.96 | 2.44 | 4.04 | |
| Min~Max | -5.52~2.70 | -2.86~2.62 | -0.86~7.05 | -0.58~15.42 | |
MHINT, Mandarin Hearing in Noise Test; SNR, signal to noise ratio; N, number; SD, standard deviation.
Figure 7.
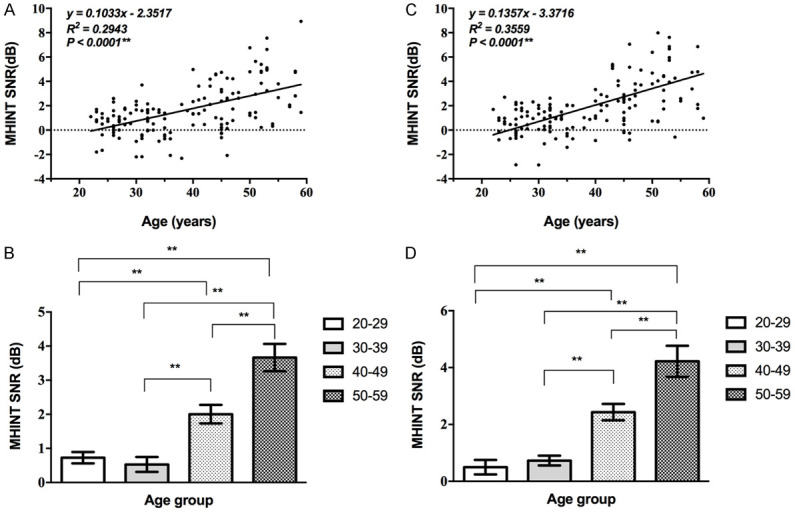
Correlation between MHINT SNR and age. A, B: The SNR of the left ear showed a gradual increase with age (**, P<0.01). Slight differences were observed in the 20-29 and 30-39 year groups, and the SNR in the 40-49 group increased significantly. C, D: The SNR of the right ear showed a gradual increase with age and the trend was consistent with the left ear (**, P<0.01).
GDT changes with age
The GDT threshold is the shortest time interval at which the participant detects a gap, and is expressed in ms. The GDT threshold increased progressively with age, and the differences of average GDT threshold in each age group in the 1 kHz and 4 kHz regions were statistically significant (P<0.01, Table 8). The differences in the 20-29 and 30-39 year groups were not statistically significant. The gap threshold appreciably increased in the 40-49 and 50-59 year groups. Moreover, we discovered that higher frequency was associated with a lower threshold, and the frequency differences in the 50-59 year group were no longer apparent (Supplementary Table 8) (Figure 8).
Table 8.
GDT distribution according to age group
| GDT | Age group | ||||
|---|---|---|---|---|---|
|
| |||||
| 20-29 years | 30-39 years | 40-49 years | 50-59 years | ||
| 1 kHz | N | 35 | 41 | 40 | 30 |
| Mean (SD) | 6.44 (2.37) | 6.10 (2.13) | 7.19 (2.93) | 8.16 (3.28) | |
| Median | 5.67 | 5.58 | 6.13 | 7.04 | |
| Min~Max | 2.67~14.92 | 3.42~17.25 | 3.33~16.83 | 4.25~19.00 | |
| 2 kHz | N | 35 | 41 | 40 | 30 |
| Mean (SD) | 4.33 (3.15) | 3.99 (0.78) | 4.25 (1.76) | 5.24 (2.58) | |
| Median | 3.83 | 3.83 | 4.04 | 4.21 | |
| Min~Max | 1.92~21.83 | 2.42~5.83 | 2.33~13.50 | 3.08~14.08 | |
| 4 kHz | N | 35 | 41 | 40 | 30 |
| Mean (SD) | 2.83 (0.83) | 3.10 (1.16) | 3.60 (1.91) | 5.60 (4.77) | |
| Median | 2.58 | 2.75 | 2.79 | 3.38 | |
| Min~Max | 1.75~5.75 | 2.17~7.75 | 2.00~9.92 | 2.00~17.33 | |
GDT, Gap Detection Test; N, number; SD, standard deviation.
Figure 8.
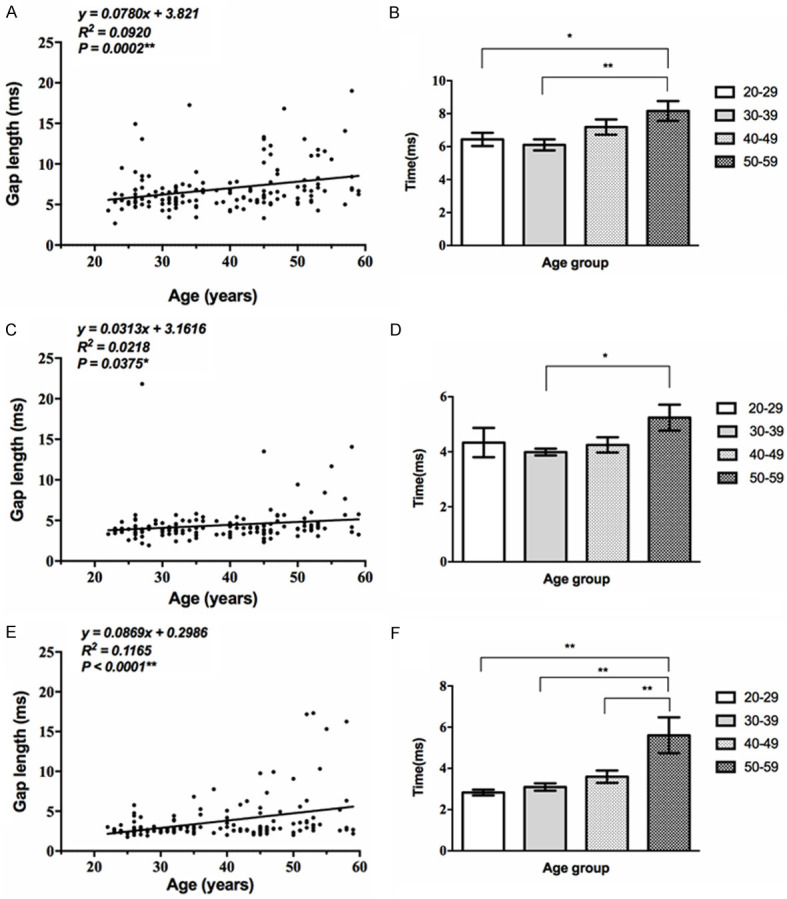
Correlation between GDT and age. A, B: The GDT threshold of 1 kHz increased progressively with age, and the average of each age group showed significant differences (**, P<0.01). The GAP threshold appreciably increased in the 40-49 and 50-59 year groups. C, D: The GDT threshold of 2 kHz increased progressively with age (*, P<0.05). E, F: The GDT threshold of 4 kHz increased progressively with age (**, P<0.01).
Differences between left and right ears
The peripheral auditory functional indicators for the left ear, such as TEOAE CS amplitude and EcochG, declined gradually with age, while such indicators for the right ear fluctuated among the four age groups. The central auditory functional indicators for both ears (e.g. MHINT and GDT) decreased with age.
Discussion
Pure tone hearing threshold tests are commonly used to evaluate the ability of the auditory system in the clinic, but are actually unable to reflect sensitively and comprehensively abnormal conditions in the transmission and processing of sound signals by the auditory system. The participants in this study had normal hearing threshold, and they were not suffering from hearing loss or speech communication difficulties, but their peripheral and central auditory function still decreased with age. The TEOAE amplitude, TEOAE CS amplitude, and EcochG SP/AP gradually decreased, and the MHINT and GDT progressively declined with age and showed a significant decrease after age 40.
In addition, we also found differences in the peripheral hearing loss between the left and right ear. Many studies confirm the hearing advantage of the right ear [19], and the hearing threshold of the right ear is better than that of the left ear [20,21]. We think this may be related to some of the participants’ occupations, such as drivers.
The decline of peripheral and central auditory function with aging
The function of OHCs
TEOAE are bioacoustics sound emissions measured in the ear canal and thought to originate from sound-evoked oscillations of the cochlear outer hair cells (OHCs). The TEOAE level shows the status of the OHC system. It is first necessary to have hearing sensitivity better than 30-35 dB HL and a normally functioning middle ear. Researchers have discovered that the amplitude of OAE is an indicator that is more sensitive than hearing threshold and is able to reflect the status of auditory function [22]. Our research suggested that the function of OHC declined progressively with age in adults with normal hearing.
Afferent nerves
In recent years, studies in non-human animals have found that synaptic degeneration between hair cells and cochlear neurons in noise-induced or aging deafness occurs earlier than the decrease in the number of hair cells [8]. A modest reduction in synapses does not affect the hearing threshold, but can cause hidden hearing loss [17,23]. Experimental animal work has shown that the suprathreshold amplitude of wave I of the auditory brainstem response (ABR), which represents the summed activity of the cochlear nerve fibers, can be diagnostic of cochlear synaptopathy. Wave I in humans, as measured via conventional ABR electrode configurations, is small and variable. We used ear-canal electrodes to measure the AP and SP [24]. EcochG AP is equivalent to the ABR I wave and represents the total potential of cochlear afferent nerve fibers, while SP is dominated by inner hair cell potential. Our result showed that the EcochG AP amplitude, SP amplitude and SP/AP ratio of all age groups with normal hearing thresholds were similar to the standard EcochG findings (mean SP amplitude of 0.27 µV, mean AP amplitude of 1.34 µV, and mean SP/AP ratio of 0.21) [24]. EcochG AP amplitude and SP amplitude decreased with age in our study.
Regulation of efferent nerves
The OHCs are innervated by crossed medial olivocochlear (MOC) efferent neurons originating in the brainstem. The auditory efferent nervous system can be activated by a sound stimulus, and changes in ciliary stiffness affect the mechanical performance of the OHC. In this way, the cochlea is maintained in the optimal mechanical state, which protects the cochlea from intense sound injury and acts as a negative feedback regulator of cochlear functions [11]. The rigid phonemic frequency selectivity and response sensitivity of efferent nerves can help to improve auditory resolution, and to weaken the masking effect of background noise, thus enhancing the ability of the cochlea to extract signals from the noise. One commonly used test of MOC function involves measuring the degree of suppression of an ipsilateral OAE by a contralateral white noise. The OAE CS showed an average reduction of 0.5-2 dB due to the presence of noise in the contralateral ear [25,26]. Some studies have found that MOC is mainly related to the detection rate of OAE, and there is no significant correlation with the OAE amplitude [27]. The relationship between TEOAE CS amplitude and age is still controversial [28,29]. Our research showed that the function of TEOAE CS declined with age, as reported by others [30].
Central auditory function - temporal resolution (GDT)
Temporal resolution is an auditory temporal processing skill that refers to the minimal time required to resolve acoustic events. The temporal signs provide vital information to the nervous system in the perception of speech [31].
It has been found that the gap threshold of young individuals with a normal hearing threshold was about 2.99 ms, and that of older persons with an increased hearing threshold was about 4.23 ms [16]. The age-related decline in temporal resolution may be related to inaccurate phase-locking of auditory neurons in the midbrain. Experiments with aging animals found the neurons that encode the gap durations in the inferior colliculi (IC) was reduced by 50% and showed a slow recovery from previous stimulation. Abnormal expression of γ-aminobutyric acid (GABA) and calcium-binding proteins in the IC is a possible mechanism [32].
Many studies have confirmed that the gap detection threshold increases with age [33]. Our research found that 4 kHz had the lowest average threshold, followed by 2 kHz, and 1 kHz had the highest threshold, suggesting that the human ear can easily distinguish the time interval at high frequency. The GDT is most correlated with age under 1 kHz and 4 kHz stimulation; 2 kHz had no significant correlation with age, indicating that the functions related to speech frequency were likely to decline with age most slowly. The threshold of GDT 1 kHz increased significantly in the 40-year-old group. However, as the time interval occurs only once, individuals are easily distracted, which may interfere with the result.
Central auditory function - MHINT
The HINT can effectively reflect the actual auditory ability [34]. The understanding of speech is a very complex activity that depends on the peripheral hearing mechanisms, central auditory processing and cognition. The standard HINT of young people with a normal hearing threshold is 4.8±1.8 dB SNR [35].
Numerous studies have found that speech recognition ability in noisy environments decreases progressively with age [36,37]. A study on 3,430 individuals with an average age of 62.3 years discovered that only 7% of them had normal SNR (≤6 dB SNR) [2]. The 60-year-old group, therefore, showed a decline in speech recognition.
In this study, we screened a population with normal auditory threshold and found that the MHINT was the most age-related indicator among all the indicators tested (left ear R2=0.29, right ear R2=0.35). The SNR increased gradually with age, and age 40 was the dividing line. The upward trend was gentle before age 40, but more volatile after 40, suggesting that the attenuation rate of central language processing ability accelerated after the age of 40. Middle-aged adults experience declines in both sensory and cognitive processing [38].
Hearing in noise is related to low-SR afferent nerve fibers and MOC, but is also related to spatial and temporal processing deficits in the auditory cortex. The HINT was significantly related to GDT, but GDT was just one of the factors [39,40]. Therefore, the MHINT reflected auditory function more comprehensively than GDT.
In addition, the strength of afferent (brainstem to primary auditory cortices) neural signaling plays a significant role in a signal in noise (SIN) task [41]. Unlike young monkeys, neuronal responses in aged monkeys with normal hearing threshold do not show a sharpening of spatial tuning between the primary auditory cortex (A1) and caudolateral (CL) field [42]. Furthermore, deficits in cognitive function, working memory, speed of processing, and filtering out task-irrelevant stimuli also have an effect [43]. Imaging methods have confirmed the influence of central non-auditory structures on speech comprehension. Functional magnetic resonance imaging (fMRI) found a correlation between attention-related cortical areas of the brain (prefrontal and precuneus regions) and performance on a SIN task [44]. Moreover, aging is associated with damage to white matter tracts caused by axonal loss, which can disrupt the information flow in related neural networks (e.g. working memory; sound perception) [45].
Results from a number of studies suggest that the age-related decline in hearing in noisy environments, often independent of hearing loss [46], is linked to not only a decline in the ability of auditory system (peripheral and central) but also hypofunction of the central non-auditory structures.
The differences of aging effects on peripheral auditory function between left ear and right ear
Our study found that the TEOAE CS amplitude and EcochG AP in the right ear was lower in the 20- and 30-year-old groups than that in the left ear, and the opposite in the 50-year-old group. Except for TEOAE amplitude, the peripheral auditory function of the right ear showed little difference among all age groups. The advantage of the right ear may be due to this slow decline. Studies indicate that the asymmetry of the auditory efferent varies according to handedness. The medial efferent system has been found to be more effective in the right than in the left ear in right-handers while functioning symmetrically in left-handers, aged from 18 to 34 years [47]. All our participants were right-handed. In addition, environmental noise is an important factor. China’s driving laws determine that driver’s left ear is more likely to be exposed to noise, which may cause hearing loss. In the follow-up study, the driving experience and working environment of the participants will be considered.
Peripheral and central auditory function are relatively independent
Although our result predicted the advantage of the peripheral auditory function of the right ear, the central auditory function of the right ear did not appear better than that of the left ear. There was no significant difference between the right ear and left ear for the MHINT SNR, which predicted that hypofunction of central auditory function is independent of peripheral hearing function. This is consistent with other reports [13,48-50]. Aging is accompanied by atrophy of the gray and white matter, resulting in the enlargement of the cerebrospinal fluid space. Age-related changes in the central auditory system occur mostly independently of the changes in the normal aging ear [48]. Peripheral hearing loss has an effect on central auditory under pathological conditions, reduced lateral olivocochlear (LOC) and auditory nerve innervation in ears with observed approximately 1 year after conductive hearing loss [51].
EcochG SP/AP ratio is not a sensitive indicator to judge the aging of cochlear function
The EcochG SP/AP ratio is widely used in the diagnosis of endolymphatic edema [52]. In addition, the SP/AP ratio is used to evaluate noise-induced deafness. In a research, college students were divided into a low-risk group and a high-risk group according to their noise exposure history. They found that the EcochG SP amplitude in the high-risk group increased significantly, accompanied by a decrease in AP amplitude, which led to an increase in the SP/AP ratio (mean value 0.46). Therefore, it was believed that the SP/AP ratio of EcochG can reflect hidden hearing loss, steadily and sensitively [17]. In addition, when healthy college students were exposed to music for 2 hours in the same night club, with temporary threshold shift (TTS) of about 7 dB, 67% of the subjects had an SP/AP ratio greater than 0.4 [53]. Our study found that the SP/AP ratio was relatively stable, with little difference among the various age groups. The ratio showed the trend of an increase with age, yet without statistical significance. This contrasted with the related reports of noise-induced deafness, probably because all the individuals we screened had normal hearing thresholds; 6 out of 149 participants had an SP/AP ratio greater than 0.4. The mean values of the EcochG SP/AP ratio were less than 0.27, which all fell within the normal range and were lower than those reported in the above literature. With the increase of age, SP and AP both decreased, especially in the left ear, which was an important factor that stabilized the SP/AP ratio. It is thus believed that the SP/AP ratio should be mainly used to diagnose diseases, but that it is not a sensitive indicator with which to judge the aging of cochlear function.
Auditory intervention
Our results showed that, except for the TEOAE amplitude, the peripheral auditory function of the right ear had no significant correlation with age. This implied that peripheral hearing loss can be prevented. In addition to avoiding noise exposure, studies have reported that regulation of the MOC may play an important role [54].
Furthermore, our study showed that the MHINT in the right ear was significantly related to age; it is suggested that central auditory function is not completely secondary to peripheral hearing loss. It should be noted that different listening tasks can result in improvements in auditory temporal processing tasks [42]. Auditory expertise, as engendered by musical training, provides both behavioural and neural advantages for processing speech in noise [49]. While aging can result in dramatic physiological and anatomical changes in the central nervous system independent of peripheral auditory function, these appear to be plastic throughout life [42].
Recent evidence suggests that hearing loss may be an early sign of dementia [55,56]. Researchers have suggested that hearing loss may increase the speed of age-related cognitive decline [57]. Therefore, the central auditory function may need to be investigated and treated early in middle age (e.g. at 40 years old).
Conclusion
In our study, the peripheral and central auditory function was shown to decline progressively with age. Central auditory function is not completely secondary to peripheral hearing loss. The 40-year-old group was the key age group for the acceleration of MHINT decline.
Acknowledgements
This study was funded by Elite Program at Shanghai Ninth People’s Hospital (JY201802 to Y.H. and Z.H.), Natural Science Foundation of Shanghai Science and Technology Committee to Z.H. (20ZR1431200) and Shanghai Ninth People’s Hospital to Z.H (YBKA202903). This study was also funded by the Shanghai Key Laboratory of Translational Medicine on Ear and Nose Diseases (14DZ2260300).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Salvi R, Ding D, Jiang H, Chen GD, Greco A, Manohar S, Sun W, Ralli M. Hidden age-related hearing loss and hearing disorders: current knowledge and future directions. Hearing Balance Commun. 2018;16:74–82. doi: 10.1080/21695717.2018.1442282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson RH. Clinical experience with the words-in-noise test on 3430 veterans: comparisons with pure-tone thresholds and word recognition in quiet. J Am Acad Audiol. 2011;22:405–423. doi: 10.3766/jaaa.22.7.3. [DOI] [PubMed] [Google Scholar]
- 3.Gates GA, Mills JH. Presbycusis. Lancet. 2005;366:1111–1120. doi: 10.1016/S0140-6736(05)67423-5. [DOI] [PubMed] [Google Scholar]
- 4.Wyss-Coray T. Ageing, neurodegeneration and brain rejuvenation. Nature. 2016;539:180–186. doi: 10.1038/nature20411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perez P, Bao J. Why do hair cells and spiral ganglion neurons in the cochlea die during aging? Aging Dis. 2011;2:231–241. [PMC free article] [PubMed] [Google Scholar]
- 6.Elobeid A, Libard S, Leino M, Popova SN, Alafuzoff I. Altered proteins in the aging brain. J Neuropathol Exp Neurol. 2016;75:316–325. doi: 10.1093/jnen/nlw002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wongrakpanich S, Petchlorlian A, Rosenzweig A. Sensorineural organs dysfunction and cognitive decline: a review article. Aging Dis. 2016;7:763–769. doi: 10.14336/AD.2016.0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009;29:14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crandell CC. Individual differences in speech recognition ability: implications for hearing aid selection. Ear Hear. 1991;12:100s–108s. doi: 10.1097/00003446-199112001-00003. [DOI] [PubMed] [Google Scholar]
- 10.Tremblay KL, Pinto A, Fischer ME, Klein BE, Klein R, Levy S, Tweed TS, Cruickshanks KJ. Self-reported hearing difficulties among adults with normal audiograms: the beaver dam offspring study. Ear Hear. 2015;36:e290–299. doi: 10.1097/AUD.0000000000000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liberman MC, Liberman LD, Maison SF. Efferent feedback slows cochlear aging. J Neurosci. 2014;34:4599–4607. doi: 10.1523/JNEUROSCI.4923-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kujawa SG, Liberman MC. Synaptopathy in the noise-exposed and aging cochlea: primary neural degeneration in acquired sensorineural hearing loss. Hear Res. 2015;330:191–199. doi: 10.1016/j.heares.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frisina RD. Subcortical neural coding mechanisms for auditory temporal processing. Hear Res. 2001;158:1–27. doi: 10.1016/s0378-5955(01)00296-9. [DOI] [PubMed] [Google Scholar]
- 14.Lin FR, Ferrucci L, An Y, Goh JO, Doshi J, Metter EJ, Davatzikos C, Kraut MA, Resnick SM. Association of hearing impairment with brain volume changes in older adults. Neuroimage. 2014;90:84–92. doi: 10.1016/j.neuroimage.2013.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li B, Guo Y, Yang G, Feng Y, Yin S. Effects of various extents of high-frequency hearing loss on speech recognition and gap detection at low frequencies in patients with sensorineural hearing loss. Neural Plast. 2017;2017:8941537. doi: 10.1155/2017/8941537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoover E, Pasquesi L, Souza P. Comparison of clinical and traditional gap detection tests. J Am Acad Audiol. 2015;26:540–546. doi: 10.3766/jaaa.14088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liberman MC, Epstein MJ, Cleveland SS, Wang H, Maison SF. Toward a differential diagnosis of hidden hearing loss in humans. PLoS One. 2016;11:e0162726. doi: 10.1371/journal.pone.0162726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bharadwaj HM, Mai AR, Simpson JM, Choi I, Heinz MG, Shinn-Cunningham BG. Non-invasive assays of cochlear synaptopathy - candidates and considerations. Neuroscience. 2019;407:53–66. doi: 10.1016/j.neuroscience.2019.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tervaniemi M, Hugdahl K. Lateralization of auditory-cortex functions. Brain Res Brain Res Rev. 2003;43:231–246. doi: 10.1016/j.brainresrev.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Bidelman GM, Bhagat SP. Right-ear advantage drives the link between olivocochlear efferent ‘antimasking’ and speech-in-noise listening benefits. Neuroreport. 2015;26:483–487. doi: 10.1097/WNR.0000000000000376. [DOI] [PubMed] [Google Scholar]
- 21.Khalfa S, Morlet T, Micheyl C, Morgon A, Collet L. Evidence of peripheral hearing asymmetry in humans: clinical implications. Acta Otolaryngol. 1997;117:192–196. doi: 10.3109/00016489709117767. [DOI] [PubMed] [Google Scholar]
- 22.Konomi U, Kanotra S, James AL, Harrison RV. Age related changes to the dynamics of contralateral DPOAE suppression in human subjects. J Otolaryngol Head Neck Surg. 2014;43:15. doi: 10.1186/1916-0216-43-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liberman MC, Kiang NY. Acoustic trauma in cats. Cochlear pathology and auditory-nerve activity. Acta Otolaryngol Suppl. 1978;358:1–63. [PubMed] [Google Scholar]
- 24.Kaf WA, Lewis KM, Yavuz E, Dixon SM, Van Ess M, Jamos AM, Delgado RE. Fast click rate electrocochleography and auditory brainstem response in normal-hearing adults using continuous loop averaging deconvolution. Ear Hear. 2017;38:244–254. doi: 10.1097/AUD.0000000000000381. [DOI] [PubMed] [Google Scholar]
- 25.Veuillet E, Collet L, Duclaux R. Effect of contralateral acoustic stimulation on active cochlear micromechanical properties in human subjects: dependence on stimulus variables. J Neurophysiol. 1991;65:724–735. doi: 10.1152/jn.1991.65.3.724. [DOI] [PubMed] [Google Scholar]
- 26.Abdala C, Dhar S. Maturation and aging of the human cochlea: a view through the DPOAE looking glass. J Assoc Res Otolaryngol. 2012;13:403–421. doi: 10.1007/s10162-012-0319-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McFadden D. A speculation about the parallel ear asymmetries and sex differences in hearing sensitivity and otoacoustic emissions. Hear Res. 1993;68:143–151. doi: 10.1016/0378-5955(93)90118-k. [DOI] [PubMed] [Google Scholar]
- 28.Kim S, Frisina DR, Frisina RD. Effects of age on contralateral suppression of distortion product otoacoustic emissions in human listeners with normal hearing. Audiol Neurootol. 2002;7:348–357. doi: 10.1159/000066159. [DOI] [PubMed] [Google Scholar]
- 29.Yilmaz ST, Sennaroglu G, Sennaroglu L, Kose SK. Effect of age on speech recognition in noise and on contralateral transient evoked otoacoustic emission suppression. J Laryngol Otol. 2007;121:1029–1034. doi: 10.1017/S0022215107006883. [DOI] [PubMed] [Google Scholar]
- 30.Morlet T, Goforth L, Hood LJ, Ferber C, Duclaux R, Berlin CI. Development of human cochlear active mechanism asymmetry: involvement of the medial olivocochlear system? Hear Res. 1999;137:179. doi: 10.1016/s0378-5955(99)00155-0. [DOI] [PubMed] [Google Scholar]
- 31.Buonomano DV, Karmarkar UR. How do we tell time? Neuroscientist. 2002;8:42–51. doi: 10.1177/107385840200800109. [DOI] [PubMed] [Google Scholar]
- 32.Walton JP, Frisina RD, O’Neill WE. Age-related alteration in processing of temporal sound features in the auditory midbrain of the CBA mouse. J Neurosci. 1998;18:2764–2776. doi: 10.1523/JNEUROSCI.18-07-02764.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snell KB, Frisina DR. Relationships among age-related differences in gap detection and word recognition. J Acoust Soc Am. 2000;107:1615–1626. doi: 10.1121/1.428446. [DOI] [PubMed] [Google Scholar]
- 34.Theunissen M, Swanepoel de W, Hanekom J. Sentence recognition in noise: variables in compilation and interpretation of tests. Int J Audiol. 2009;48:743–757. doi: 10.3109/14992020903082088. [DOI] [PubMed] [Google Scholar]
- 35.von Gablenz P, Holube I. Hearing loss and speech recognition in the elderly. Laryngorhinootologie. 2017;96:759–764. doi: 10.1055/s-0043-119388. [DOI] [PubMed] [Google Scholar]
- 36.Pronk M, Deeg DJ, Festen JM, Twisk JW, Smits C, Comijs HC, Kramer SE. Decline in older persons’ ability to recognize speech in noise: the influence of demographic, health-related, environmental, and cognitive factors. Ear Hear. 2013;34:722–732. doi: 10.1097/AUD.0b013e3182994eee. [DOI] [PubMed] [Google Scholar]
- 37.Barrenas ML, Wikstrom I. The influence of hearing and age on speech recognition scores in noise in audiological patients and in the general population. Ear Hear. 2000;21:569–577. doi: 10.1097/00003446-200012000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Humes LE. Age-related changes in cognitive and sensory processing: focus on middle-aged adults. Am J Audiol. 2015;24:94–97. doi: 10.1044/2015_AJA-14-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shannon RV, Zeng FG, Kamath V, Wygonski J, Ekelid M. Speech recognition with primarily temporal cues. Science. 1995;270:303–304. doi: 10.1126/science.270.5234.303. [DOI] [PubMed] [Google Scholar]
- 40.Heeke P, Vermiglio AJ, Bulla E, Velappan K, Fang X. The relationship between random Gap detection and hearing in noise test performances. J Am Acad Audiol. 2018;29:948–954. doi: 10.3766/jaaa.18005. [DOI] [PubMed] [Google Scholar]
- 41.Bidelman GM, Price CN, Shen D, Arnott SR, Alain C. Afferent-efferent connectivity between auditory brainstem and cortex accounts for poorer speech-in-noise comprehension in older adults. Hear Res. 2019;382:107795. doi: 10.1016/j.heares.2019.107795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Recanzone G. The effects of aging on auditory cortical function. Hear Res. 2018;366:99–105. doi: 10.1016/j.heares.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alain C, Zendel BR, Hutka S, Bidelman GM. Turning down the noise: the benefit of musical training on the aging auditory brain. Hear Res. 2014;308:162–173. doi: 10.1016/j.heares.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 44.Wong PC, Jin JX, Gunasekera GM, Abel R, Lee ER, Dhar S. Aging and cortical mechanisms of speech perception in noise. Neuropsychologia. 2009;47:693–703. doi: 10.1016/j.neuropsychologia.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alain C, McDonald KL. Age-related differences in neuromagnetic brain activity underlying concurrent sound perception. J Neurosci. 2007;27:1308–1314. doi: 10.1523/JNEUROSCI.5433-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bidelman GM, Davis MK, Pridgen MH. Brainstem-cortical functional connectivity for speech is differentially challenged by noise and reverberation. Hear Res. 2018;367:149–160. doi: 10.1016/j.heares.2018.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khalfa S, Veuillet E, Collet L. Influence of handedness on peripheral auditory asymmetry. Eur J Neurosci. 1998;10:2731–2737. doi: 10.1046/j.1460-9568.1998.00286.x. [DOI] [PubMed] [Google Scholar]
- 48.Ouda L, Profant O, Syka J. Age-related changes in the central auditory system. Cell Tissue Res. 2015;361:337–358. doi: 10.1007/s00441-014-2107-2. [DOI] [PubMed] [Google Scholar]
- 49.Anderson S, Kraus N. Sensory-cognitive interaction in the neural encoding of speech in noise: a review. J Am Acad Audiol. 2010;21:575–585. doi: 10.3766/jaaa.21.9.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burianova J, Ouda L, Profant O, Syka J. Age-related changes in GAD levels in the central auditory system of the rat. Exp Gerontol. 2009;44:161–169. doi: 10.1016/j.exger.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 51.Liberman MC, Liberman LD, Maison SF. Chronic conductive hearing loss leads to cochlear degeneration. PLoS One. 2015;10:e0142341. doi: 10.1371/journal.pone.0142341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Deelen GW, Ruding PR, Smoorenburg GF, Veldman JE, Huizing EH. Electrocochleographic changes in relation to cochlear histopathology in experimental endolymphatic hydrops. Acta Otolaryngol. 1988;105:193–201. doi: 10.3109/00016488809096998. [DOI] [PubMed] [Google Scholar]
- 53.Kim JS, Nam EC, Park SI. Electrocochleography is more sensitive than distortion-product otoacoustic emission test for detecting noise-induced temporary threshold shift. Otolaryngol Head Neck Surg. 2005;133:619–624. doi: 10.1016/j.otohns.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 54.Bidelman GM, Schneider AD, Heitzmann VR, Bhagat SP. Musicianship enhances ipsilateral and contralateral efferent gain control to the cochlea. Hear Res. 2017;344:275–283. doi: 10.1016/j.heares.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 55.Ray M, Dening T, Crosbie B. Dementia and hearing loss: a narrative review. Maturitas. 2019;128:64–69. doi: 10.1016/j.maturitas.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 56.Hardy CJ, Marshall CR, Golden HL, Clark CN, Mummery CJ, Griffiths TD, Bamiou DE, Warren JD. Hearing and dementia. J Neurol. 2016;263:2339–2354. doi: 10.1007/s00415-016-8208-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peracino A. Hearing loss and dementia in the aging population. Audiol Neurootol. 2014;19(Suppl 1):6–9. doi: 10.1159/000371595. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


