Abstract
Osteoarthritis (OA) is a chronic joint disease that generally occurs worldwide with pain and disability. The progression is slow, and it is mostly diagnosed midlife and often disturbs the knees, hips, feet, hands, and spine. Sex, age, obesity, occupation, and hereditary factors are risk factors that increase the opportunity for OA. Physical examinations involving X-rays and MRI, joint fluid analysis and blood tests are common tools for the diagnosis of OA. Interventions including exercise, manual therapy, lifestyle modification, and medication can help relieve pain and maintain mobility in the affected joints, yet none of the therapies enables the promotion of regeneration of degenerated tissues. Mesenchymal stem cells (MSCs) are a promising source for the treatment of OA due to their multipotency for differentiation into chondrocytes and their ability to modulate the immune system. Herein, we review the pathogenesis and treatment of OA and address the current status of MSCs as a novel potential therapeutic agent in OA treatment.
Keywords: Mesenchymal stem cell, cell regeneration, osteoarthritis, immune system
Introduction
Osteoarthritis (OA) is a common chronic disease and accounts for major physical pain and disability in older adults. It is assumed to be the fourth leading cause of disability in the world in 2020 [1]. OA consistently influences the knees, hips, hands, feet, and spine [2]. The knee is the most frequently affected site and accounts for almost 85% of the burden of OA worldwide, followed by the hand and hip [3-5]. The particular syndromes of OA encompass chronic pain, stiffness, mobility restriction, and joint tenderness [6]. A number of risk factors such as female sex, age, obesity, genetic factors, and Oxidative stress increase the chances of developing OA [7]. It is growing more prevalent today because of the combined factors of aging, obesity and the increasing numbers of damaged joints, and an estimated 250 million people are affected by this syndrome [1].
The structural variations in OA include articular cartilage, subchondral bone, ligaments, synovium, and periarticular muscles. The articular cartilage defect is the most obvious syndrome of OA, which is caused by degeneration of the extracellular matrix [8,9]. People diagnosed with OA suffer physical weakness, mental pressure and impaired quality of daily life [10]. Currently, both nonpharmacological methods and pharmacological methods are applied to treat OA. Nonpharmacological methods, including self-management, regular exercise, and weight control, are highly recommended and are regarded as first-line treatments for OA [11,12]. Pharmacological methods recommended in the guidelines are paracetamol and NSAIDs, which are often used when nonpharmacological methods are not able to relieve pain and reduce disability. Patients with hip and knee OA who do not respond to topical analgesics are recommended to take intra-articular corticosteroids [11]. Duloxetine is a serotonin and norepinephrine reuptake inhibitor, which is recommended in some guidelines to reduce the severe pain of OA [13]. New treatments, such as nerve growth factor (NGF) antibodies, have been evaluated and have shown positive results in reducing pain in patients with hip and knee OA [14]. Surgical options such as joint replacement surgery, knee osteotomy, and knee joint distraction are either recommended for patients with late-stage OA or young and energetic patients with moderate radiographic severity in OA [15-17]. However, the above treatments are designed to reduce pain and improve the mobility of joints instead of promoting the regeneration of damaged articular cartilage. The regenerative treatments are intended to repair and replace the injured cells and tissues with new ones. As a regenerative cell therapy of OA, mesenchymal stem cells (MSCs) have the potential of self-renewal and differentiation into cartilage and the capability of immune modulation. A number of preclinical and clinical studies have confirmed the potential for MSCs as a novel therapeutic strategy for the treatment of OA. In this review, we provide an extensive review of the pathogenesis and treatment of OA and emphasize the therapeutic features of bone marrow MSCs (BM-MSCs), adipose tissue-derived MSCs (AD-MSCs), and umbilical cord-derived MSCs (UC-MSCs) in OA treatment (Figure 1).
Figure 1.
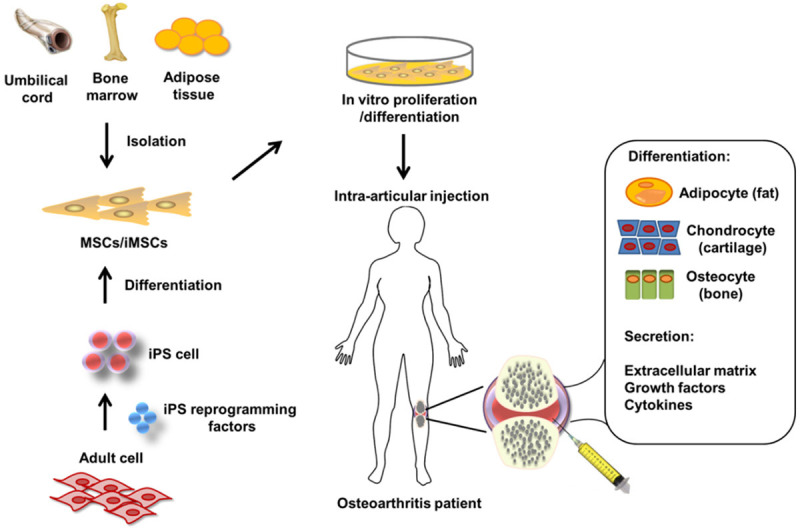
Schematic picture of mesenchymal stem cell applications in the treatment of osteoarthritis. The MSCs can be isolated from bone marrow adipose tissue and umbilical cord or induced from iPS cells. Intra-articular injection of MSCs is followed by in vitro proliferation or differentiation. Patients with OA are treated through bone regeneration and cartilage repair.
Pathogenesis
OA is not an inactive degenerative disease; on the contrary, it is a dynamic disease caused by the imbalance between restoration and destruction of joints [18]. The pathogenesis of OA is complicated and can be described from different perspectives. We will address the pathogenesis of OA through environmental, inflammatory and metabolic aspects.
OA has become more prevalent in recent decades, and the increase in environmental changes is the main cause. Obesity, metabolic syndrome, dietary changes, and physical inactivity are four major environmental factors in OA pathogenesis [19]. Obesity is recognized as a dominant risk factor, especially for knee OA, because of the excess weight loading pressure on weight-bearing joints [20,21]. The abnormal loading of cartilage primarily causes damage to the cartilage matrix of collagen fibrils and proteoglycans and then triggers chondrocyte surface mechanoreceptors, which further trigger the MAPK or NF-κB intracellular signaling pathway and the production of proinflammatory and catabolic mediators [22,23]. There is evidence that adipose tissue produces and releases cytokines such as IL-1, IL-6, IL-8, IFNγ, TNF and leptin during bearing, which have been proven to play a role in promoting inflammation and initiating OA [24,25].
Metabolic syndrome is a cluster of cardiometabolic factors, such as central adiposity, dyslipidemia, impaired fasting glucose levels and hypertension, which follow obesity and are closely associated with cardiovascular disease and type 2 diabetes [26]. Evidence shows that type 2 diabetes can change the structure of the extracellular matrix, and the enrichment of advanced glycation end products can trigger the signal to chondrocytes to finally cause the degradation of cartilage matrix [27].
The obvious change in diets in recent decades has resulted in an increased intake of oxidant foods and proinflammatory omega-6 fatty acids [28]. Although the direct contribution of dietary changes to OA is still controversial, the evidence indicates that a higher fiber intake reduces knee pain in patients with OA [29].
Physical inactivity is another main risk factor for OA, by which people lose the ability to maintain normal joint capacity [30]. Disuse experiments in animals demonstrate a decrease in cartilage layers and cartilage proteoglycan and injury of subchondral bone in joint tissues [31,32].
In OA, we can see alterations in cellular metabolism, including an increase in the production of antianabolic, procatabolic and proinflammatory factors [33]. The source of energy metabolism in chondrocytes in OA converts from oxidative phosphorylation to anaerobic glycolysis [34,35]. The alterations in mitochondrial structure and dynamics in chondrocytes lead to mitochondrial metabolism impairment and oxidative damage by enrichment of ROS [36]. Damage to mitochondria reduces AMPK activity, downregulates SIRT1 and decreases PGC1α expression, which is a master regulator of mitochondrial biosynthesis [37]. The synovium is inflamed in the early and late stages of OA, and it produces cytokines, ROS, NO, prostaglandin E2 and neuropeptides that alter cellular metabolism and disturb the balance between cartilage matrix degradation and restoration [38,39]. By understanding the cellular metabolism molecules that are altered in the progression of OA in more detail, we can more precisely enable the design of OA targeting therapy.
Inflammation plays an important role in the destruction of cartilage, and proinflammatory cytokines such as IL-1β, IL-6, and TNF-α are essential in the pathogenesis and progression of OA [40]. IL-1β is elevated in the development of OA, induces the release of MMPs and inhibits the expression of ECM components in OA cartilage in which IL-6 synergistically increases inflammation [41,42]. TNF-α is upregulated in OA in joint tissues and synovial fluid, which suppresses the expression of proteoglycan and ECM components in chondrocytes and stimulates the expression of proinflammatory and procatabolic mediators such as MMP-1, IL-6, MCP-1, and NO [43-46].
Several signaling pathways have been proven to play a key role in the destruction of cartilage. It has been identified that the Notch signaling pathway is activated and that the signaling components are upregulated in humans and a mouse model of OA [47,48]. However, activation of Notch was proven to help prevent articular cartilage destruction in another study [49]. HIF-2 acts as a central catabolic regulator by stimulating key mediators such as MMPs, ADAMTS4, NOS2, and PTGS2 in the cartilage destruction of OA [50]. The NF-κB signaling pathway is activated in the development of OA, which induces MMPs and ADAMTSs and inhibits type II collagen and aggrecan expression [51]. Subchondral bone is another tissue affected by catabolic mediators in the progression of OA. Several signaling pathways have been identified to contribute to this progression, including Wnt, TGF-β/BMP, and MAPK signaling, among which Wnt/β-catenin signaling is a key regulator of bone remodeling. β-Catenin is overexpressed in patients with knee OA. Wnt/β-catenin signaling in articular chondrocytes in adult mice is activated in the development of OA [52,53].
Treatment of osteoarthritis
The principal aims for OA treatment are reducing pain, slowing the progression of joint destruction and maintaining and even improving mobility function [54]. Numerous nonpharmacological and pharmacological therapies are currently applied to relieve pain and maintain the function of joint tissues in the treatment of OA. In addition to traditional medication, a number of novel therapeutic strategies are being examined and developed. In addition, combinational therapies are commonly used in OA treatment to maximize the effects beyond what a single application can reach [55].
Nonpharmacological therapy
Nonpharmacological strategies are recognized as first-line treatments for OA and should be considered first before pharmacological interventions and surgical options [56]. Self-management, regular exercise, strength training, and weight control are highly recommended to reduce pain and improve the mobility function of joints [57]. Combinational management of exercise and weight control has been demonstrated to reduce knee OA syndrome in patients with excess weight and obesity [58,59].
Pharmacological therapy
Pharmacological strategies are often applied when nonpharmacologic interventions are not able to relieve pain and reduce disability. Traditional medicine, such as NSAIDs, is constantly applied in OA treatment. NSAIDs suppress Cox-1 and Cox-2 activity in the periphery by decreasing prostaglandin synthesis and reducing pain [60]. However, NSAIDs are associated with an increased risk of gastrointestinal disorders and cardiovascular diseases [61]. Acetaminophen is a first-line therapy used to treat moderate OA pain. Acetaminophen reduces Cox-1 and Cox-2 activity in the periphery, suppresses serotonergic neuronal and L-arginine/NO pathways and stimulates the cannabinoid system in the central antinociception process [62]. However, the use of acetaminophen is limited because of its limited efficacy and severe toxicity [63,64]. Duloxetine is a serotonin-norepinephrine reuptake inhibitor approved by the FDA to relieve pain and improve function in patients with knee OA [65]. Capsaicin is a natural compound obtained from chilies and is also a local analgesic [66]. Both duloxetine and capsaicin are effective adjunct treatments to other treatments [67]. Glucosamine and chondroitin are extracted from animals, and their combination is effective in reducing joint swelling and pain, increasing flexibility and improving function by stimulating proteoglycan synthesis in articular cartilage [68]. Intra-articular steroids only show effectiveness in short-term trials up to 2 weeks but not in long-term trials [69]. Exogenous hyaluronic acid (HA) is a natural joint fluid that can stimulate the synthesis of endogenous HA and proteoglycans in chondrocytes, therefore suppressing cartilage destruction and promoting regeneration [70]. Platelet-rich plasma (PRP) was used in tissue repair and showed pain alleviation and functional improvement that were sustained for 6 months [71].
In addition to traditional medication, a number of biological therapeutic agents are being explored and evaluated for OA treatment. Tanezumab and fasinumab are well-studied anti-NGF antibodies for knee and hip OA treatment, and evidence shows their preponderance in efficacy compared to placebo [72]. Several antibodies against other targets, such as the anti-TNF antibody adalimumab and the anti-IL-6 antibody tocilizumab, are being tested to reduce pain in OA. Several small molecules targeting GPCRs and ion channels are also under investigation [73].
However, the above treatments are designed for pain relief and functional improvement instead of regeneration of damaged articular cartilage or inflammation alleviation. In recent years, new therapeutic strategies for OA treatment have been intended to regenerate joint tissues and manage inflammation. Cell- or stem cell-based therapy has been developed as a new approach in regenerative medication. Autologous chondrocyte implantation (ACI) is an FDA-approved cell therapy for OA treatment. However, the unexpected dedifferentiation and joint invasiveness during harvest limited the application, and long-term trials are needed to confirm the efficacy [74,75]. Mesenchymal stem cells (MSCs) capable of releasing the restrictions of ACI have been broadly explored as novel therapeutic agents in the treatment of OA. Hereinafter, we highlight the therapeutic features and recent progress of MSCs in OA treatment.
Mesenchymal stem cell-based therapy for osteoarthritis
As multipotent cells extensively distributed in the bone marrow, periosteum, trabecular bone, fat pad tissue, synovial membrane, and several other tissues, MSCs have great potential in promoting the regeneration of chondrocytes and differentiation into cartilage [76]. MSCs were first isolated from bone marrow and later from other tissues, such as adipose tissue, placenta, umbilical cord, cord blood, dental pulp, and amniotic fluid [76]. Among the tissues, bone marrow and adipose tissue are major sources for therapeutic MSCs. MSCs from different tissues are capable of differentiating into cartilage [77].
Different sources of MSCs feature different characteristics and have their own advantages and disadvantages. With respect to the MSC content in the tissue, the umbilical cord (UC-MSCs) has the highest, followed by the amniotic fluid and fat [78]. With respect to MSC proliferative capacity, umbilical cord and amniotic-derived MSCs have definite advantages, followed by fat and bone marrow (BM-MSCs). In terms of immunomodulatory capacity, umbilical cord, amnion, and adipose tissue-derived MSCs (AD-MSCs) have superior immune regulation over bone marrow MSCs, while placental MSCs have the lowest immunomodulatory capacity. When compared to cytokine secretion profiles, umbilical cord MSCs secrete more cell growth factor than bone marrow MSCs [77]. The advantages and disadvantages of different sources of stem cells are summarized in Table 1.
Table 1.
Characteristics of different types of stem cells
| MSCs source | Advantages | Disadvantages |
|---|---|---|
| Bone marrow | Easy accessibility | Relatively low cell growth rate |
| High multilineage differentiation | ||
| Relatively adequate clinical trials | ||
| Adipose tissue | Easy accessibility | Reduced differentiation capability |
| Greater number of colonies formation | Inadequacy in clinical trials | |
| Superior immunomodulatory capacity | ||
| Umbilical cord blood stem cells | Longer culture times | Relatively few colonies formation |
| Higher proliferation capacity | Relatively low yield | |
| Higher anti-inflammatory effects | Inadequacy in clinical trials |
The desirable characteristics of MSCs as cell-based therapeutic agents are their ability to stimulate cartilage formation, vascularization, anti-inflammation, and immunoregulation [79]. A great number of studies have evaluated the potential of MSCs in cartilage tissue regeneration both in vitro and in animal models [80]. Studies have not been restricted to animal models in recent years. A number of clinical trials have demonstrated the potential efficacy of MSCs derived from bone marrow, adipose tissue, and umbilical cord blood in the treatment of OA. The data are summarized in Table 2.
Table 2.
Clinical studies of MSCs for osteoporosis treatment
| Cell types | Study design | Outcomes | References |
|---|---|---|---|
| BM-MSCs | Autologous culture-expanded BM-MSCs with collagen gel embedded were transplanted into OA knee with articular cartilage defect in the medial femoral condyle | White soft tissue formation, better arthroscopic and histological grading score | [81] |
| BM-MSCs | Intra-articular injection of cultured autologous BM-MSCs with hyaluronic in varus knees with cartilage defects underwent HTO and microfracture | Better Tegner, Lysholm, IKDC, and MOCART scores | [82] |
| BM-MSCs | Intra-articular injection of 50 × 106 or 150 × 106 allogeneic BM-MSCs into OA knees 7-10 days after the meniscectomy | No ectopic tissue formation, no serious adverse effects, increased meniscal volume, reduction in pain | [83] |
| BM-MSCs | Intra-articular injection of hyaluronic acid together with 10 × 106 or 100 × 106 cultured autologous BM-MSCs into OA knees | No adverse effects, improved VAS WOMAC scores in both low and high doses | [84] |
| AD-MSCs | Intra-articular injection of autologous AD-MSCs with dose escalation: low dose (2 × 106 cells), medium dose (10 × 106), and high dose (50 × 106) to patients with symptomatic and severe knee OA | No serious adverse events, improved WOMAC pain and function subscores | [85] |
| AD-MSCs | Patients with symptomatic knee cartilage defects were Intra-articular injected of AD-MSCs with fibrin glue and MFX treatment | Better signal intensity for repair tissue, improved KOOS pain and symptom subscores, no significant differences in daily activity, sports and recreation and quality of life | [86] |
| AD-MSCs | Intra-articular injection of autologous AD-MSCs into OA knees | No serious adverse events, improved WOMAC score, no significant change of cartilage defect | [87] |
| UC-MSCs | Intra-articular injection of 2-3 × 107 UC-MSCs once a month for 2 times into OA knee | Pain and swelling incidences after injection, no recurrence of knee pain during follow up, better Lysholm, WOMAC, and SF-36 scale scores | [88] |
| UC-MSCs | Intra-articular injection of 5-7 × 107 UC-MSCs once into OA knee | No difference in biochemical parameters, pain reduction, recovered daily activities | [89] |
| UCB-MSCs | Implantation of UCB-MSCs mixture in the drill holes of cartilage defect site of OA knee | Improved IKDC, WOMAC, and VAS score and mean OAS | [90] |
| Patients with varus or valgus deformity greater than 5 degrees were treated simultaneously with osteotomy |
BM-MSCs for osteoarthritis therapy
BM-MSCs are the most widely used source of therapeutic MSCs because of their simple accessibility, fast cell proliferation, long-term sustainment of differentiation capacity and reduced immunological exclusion [91]. The first clinical study of BM-MSC transplantation for articular cartilage defect treatment was conducted almost 20 years ago. The trial recruited 24 patients with knee OA who underwent a high tibial osteotomy, and half of them received autologous BM-MSC transplantation with the other half as controls. After 42 weeks of transplantation, metachromasia was observed in almost all areas of the sampled tissue, and hyaline cartilage-like tissue was partially observed. The arthroscopic and histological grading score was better in the cell-transplanted group than in the cell-free control group, suggesting the suitability of BM-MSC transplantation for OA treatment [81]. Another 2-year follow-up autologous BM-MSC transplantation clinical trial was conducted in 56 patients with unicompartmental knee OA who underwent high tibial osteotomy and microfracture. The cell-recipient group received better Tegner, Lysholm, International Knee Documentation Committee (IKDC) and Magnetic Resonance Observation of Cartilage Repair Tissue (MOCART) scores, indicating the effectiveness of autologous BM-MSC transplantation for OA treatment [82]. In another trial, 55 patients who underwent a partial medial meniscectomy were recruited and received two different doses of allogeneic BM-MSCs in the treatment groups. After 2 years of follow-up, no clinically adverse effects were identified, and the cell-recipient group had increased meniscal volume and a significant reduction in pain based on visual analog scale (VAS) evaluation [83]. A phase I/II multicenter randomized clinical trial demonstrated the long-term effect of two different doses of BM-MSC transplantation versus HA in 30 patients with knee OA. After 4 years of follow-up, the cell-recipient group, especially the high-dose group, received better VAS and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC)scores, indicating the therapeutic potential of BM-MSCs in long-term clinical and functional improvement in knee OA [84]. Another 5-year long-term trial of autologous BM-MSC transplantation in 4 patients with moderate to severe knee OA showed similar results. All parameters, such as the walking time, stair climbing, gelling pain, patella crepitus, flection contracture, and VAS, were improved 6 months after treatment, and they were still better at baseline after 5 years, although gradual decline occurred [92].
AD-MSCs for osteoarthritis therapy
AD-MSCs are another attractive cell source of therapeutic MSCs because of their greater proliferation and differentiation potential than BM-MSCs [93]. Several clinical trials in recent years have demonstrated the effectiveness of AD-MSCs in therapeutic use. In a phase I dose-escalation trial, 18 patients with symptomatic and severe knee OA were transplanted with autologous AD-MSCs in three doses: low dose (2 × 106 cells), medium dose (10 × 106 cells), and high dose (50 × 106 cells). Six-month follow-up showed no clinically adverse effects, and patients treated with low-dose AD-MSCs experienced significant pain relief and functional improvement [85]. This study indicated the safety, tolerance and initial effectiveness of AD-MSCs in knee OA treatment; however, larger, more rigorous long-term trials are needed for further evaluation. Another clinical trial was conducted to evaluate the efficacy of AD-MSCs with microfracture treatment versus microfracture alone in 80 patients aged 18 to 50 years with moderate to severe knee OA. After 24 months of follow-up, the AD-MSC-treated group showed better signal intensity for tissue repair and KOOS pain and symptom subscores but no significant differences in other subscores, such as daily living activity, sports and recreation and quality of life, indicating the potential of AD-MSCs in tissue repair and pain relief [86]. Recently, a phase IIb randomized, placebo-controlled clinical trial of AD-MSCs for the treatment of knee OA was conducted in 24 patients. Six months of follow-up provided safety, increased WOMAC score, improvement in function and reduction of pain in the AD-MSC-treated group, suggesting the potential of AD-MSCs in therapeutic use [87].
UC-MSCs for osteoarthritis therapy
UC-MSCs have recently been recognized as another source for stem cell therapy because of their advantages of a higher proliferation capacity, active differentiation ability, and superior immunomodulatory capacity compared with BM-MSCs [77,94]. A number of clinical trials have been conducted for consideration of UC-MSCs in the treatment of OA. A safety and efficacy study of UC-MSC transplantation with follow-up for 6 months was conducted in 36 patients with moderate or severe knee OA. The results showed that the cell treatment group provided better Lysholm, WOMAC and SF-36 scale scores than the control group. The cell treatment group demonstrated higher incidences of pain and swelling than the control group after injection, but no recurrence of knee pain was identified during follow-up, indicating the therapeutic potential of UC-MSCs in joint improvement [88]. Similar results were obtained from another clinical trial in which 3 patients with knee OA received 5-7 × 107 UC-MSCs by intra-articular injection. After a 3-month follow-up, the 3 patients showed pain relief and recovery of daily activities ability with no biochemical change observed pre- and posttreatment [89]. Recently, a clinical trial of human umbilical cord blood-derived mesenchymal stem cell (hUCB-MSC) application was conducted in patients with knee OA with full-thickness cartilage defects. A review of 64 patients who underwent second-look arthroscopic surgery 1 year after treatment of hUCB-MSCs showed that IKDC, WOMAC, VAS score and Oswestry Arthroscopy Score (OAS) were improved compared with baseline, indicating the potential of hUCB-MSCs as a therapeutic option for pain relief and functional improvement in OA treatment [90].
To date, several clinical trials have proven the safety and potential efficacy of BM-MSCs, AD-MSCs, and UC-MSCs in the treatment of OA. However, we must admit that some of the trials were conducted with limited samples, without rigorous controls and with relatively short-term follow-ups. Clinical trials with larger samples, more rigorous controls, more careful handling, and long-term follow-ups are needed for further evaluations. Although it has not been highlighted in this review, other considerations should be taken into account to enhance the efficacy in clinical trials. For example, the cell density, the time and location for MSC transplantation, and pretreatment of MSCs by inflammatory cytokines should be carefully evaluated in both preclinical and clinical studies.
Pretreatment of MSCs by a low-level laser to improve therapeutic efficacy
Low-level laser therapy (LLLT) is a therapy that applies low-level lasers or light-emitting diodes (LEDs) to manage cell functions and has been widely acknowledged as a regenerative medical tool in tissue regeneration, improvement in wound healing, reduction in pain, and recovery of functional diseases [95,96]. Although the molecular mechanism of LLLT in accelerating tissue regeneration is not yet clear, its ability to promote cell proliferation in diverse cell types, such as endothelial cells [97], fibroblasts [98], osteoblast-like cells [99], and mesenchymal stem cells [100,101], has been confirmed. There is evidence that accelerated cell proliferation is associated with increased synthesis of growth factors, NO, ROS, ATP, RNA and DNA after irradiation [102].
Although MSCs can be obtained from many sources as mentioned before and different sources of MSCs feature distinct characteristics, one of the common properties of MSCs is the relatively low yield and decreased proliferative capability in vitro. This property will inevitably hamper the progress of MSCs to preclinical and clinical trials and practical use. Recent progress has demonstrated the role of LLLT in promoting the cell growth, proliferation and differentiation of MSCs, and the in vivo and in vitro effects of different protocols of LLLT on MSCs are summarized in Table 3.
Table 3.
In vivo and in vitro effects of different protocols of low-level laser therapy on mesenchymal stem cells
| Cell types | In vitro/in vivo | Wavelength (nm) | Power density (W/cm2), energy density (J/cm2) | Results | References |
|---|---|---|---|---|---|
| BM-MSCs | In vivo | 804 | 10 mW/cm2 | Reduced infarct size and ventricular dilatation, increased c-kit+ cells in the infarcted area | [103] |
| 1 J/cm2 | |||||
| BM-MSCs | In vitro | 630 | 5, 15 mW/cm2 | Enhanced growth of MSCs, increased colony-forming unit fibroblasts | [104] |
| 2, 4 J/cm2 | |||||
| BM-MSCs | In vitro | 1064 | 0.25 W/cm2 | Promotion of BM-MSCs proliferation and osteogenesis at densities of 2 and 4 J/cm2 | [105] |
| 2, 4, 8, 16 J/cm2 | |||||
| BM-MSCs | In vitro | 810 | 2, 3, 4, 6 J/cm2 | Enhanced differentiation of BM-MSCs into neuron and osteoblast in the range of 2-6 J/cm2, increased BM-MSCs proliferation (except for 6 J/cm2) | [106] |
| AD-MSCs | In vivo | 632.8 | 17.0 mW | Enhanced wound healing, regeneration of skin appendages, enhanced survival of AD-MSCs, increased secretion of growth factors | [107] |
| 1.2 J/cm2 | |||||
| AD-MSCs | In vitro | 636 | 5 J/cm2 | No induction of differentiation over 72 h, increased cell viability, and proliferation | [108] |
| AD-MSCs | In vitro | 636 | 5 J/cm2 | Increased viability and proliferation of AD-MSCs when cultured with EGF | [109] |
| UC-MSCs | In vitro | 635, 808 | 20 mW/cm2 | Higher proliferation rate in the 635 nm, no induction of cell proliferation at 808 nm, increased CAT, tGPx, and SOD activity, improved UC-MSCs function and increased antioxidant activity | [110] |
| 12 J/cm2 | |||||
| UC-MSCs | In vitro | 620 | 2 J/cm2 | Higher proliferation rate, higher osteogenic differentiation | [111] |
| DP-MSCs | In vitro | 660 | 30 mW | Increased cell proliferation at 1 J/cm2, no significant changes in cell viability | [112] |
| 0.5, 1 J/cm2 |
BM-MSCs: bone marrow mesenchymal stem cells; AD-MSCs: adipose tissue-derived mesenchymal stem cells; UC-MSCs: umbilical cord mesenchymal stem cells; DP-MSCs: dental pulp mesenchymal stem cells.
The effects of LLLT on MSCs demonstrated the potential of LLLT as a useful tool in the promotion of cell proliferation and differentiation of MSCs, leading to wound healing, osteogenesis and cell function improvement. The results can be influenced by protocols with different parameters, such as the light spectrum, wavelength, power and energy density, cell types and cell numbers used in the experiments. In future studies, the parameters of LLLT and MSCs should be evaluated more carefully, and more in vitro and in vivo studies should be carried out in MSCs from different cell types.
Conclusions
OA is becoming increasingly popular in modern society. Environmental factors and metabolic and inflammatory factors are highlighted in the pathogenesis of OA. Both nonpharmacological and pharmacological strategies are reviewed in the treatment of OA. The main goals of traditional medication and biological agents in OA treatment are relief of pain and slowing down or halting of the progress of OA. Stem cell-based therapy is of great interest in regenerative medicine because of its potential in the regeneration of new cartilage and strong immunoregulatory capacity. Positive results in preclinical and clinical trials have demonstrated that MSC-based agents are a promising strategy in the treatment of OA. However, the limitations and risks of MSC-based therapy should be realized and treated carefully. First, we have an inadequate understanding of MSCs regarding their physiological properties and cellular functions in vitro and in vivo, including the mechanisms of immune modulation and the role of key mediators in differentiation. Second, the therapeutic efficacy of MSCs in preclinical and clinical studies is influenced by many factors, such as the cell culture conditions, the cell density, the time and location for MSC transplantation and the pretreatment of MSCs by LLLT or inflammatory cytokines. These factors should be elucidated more cautiously in future studies to maximize the potential and minimize the adverse effects of MSCs in OA treatment. Third, since MSCs are multipotent cells with a high capability of cell proliferation and differentiation and both autologous and allogeneic MSCs have been applied in several studies, the risks regarding the tumorigenesis potential and serious unwanted immunoregulatory responses in preclinical and clinical trials should be further considered.
Despite the current limitations and risks, cell therapy, especially MSC-based therapy, is becoming an encouraging approach to regenerative medicine in the treatment of OA. The positive effects of pretreatment with LLLT on MSCs indicate that the combination of MSC-based therapy with other treatments might achieve better efficacy in regenerative medicine.
Acknowledgements
This work was financially supported by National key research and development plan (2017YFB0403801 and 2018YFB0407200), Applied and Basic Research Fund of Yunnan Province (2019FB097), and Academician workstation (2017IC051), Breeding Plan of Shandong Provincial Qingchuang Research Team (2019); Yunnan Provincial Department of Science and Technology-Kunming Medical University Joint Special Project on Applied Basic Research (2019FE001(-111); Yunnan health training project of high level talents (D-2018005).
Disclosure of conflict of interest
None.
Abbreviations
- OA
Osteoarthritis
- MSCs
Mesenchymal stem cells
- NGF
nerve growth factor
- BM-MSCs
bone marrow MSCs
- AD-MSCs
adipose tissue derived MSCs
- UC-MSCs
umbilical cord derived MSCs
- hUCB-MSCs
human umbilical cord blood-derived mesenchymal stem cells
- OAS
Oswestry Arthroscopy Score
- LLLT
Low-level laser therapy
- LEDs
light-emitting diodes
References
- 1.Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet (London, England) 2019;393:1745–1759. doi: 10.1016/S0140-6736(19)30417-9. [DOI] [PubMed] [Google Scholar]
- 2.Lambova SN, Muller-Ladner U. Osteoarthritis - current insights in pathogenesis, diagnosis and treatment. Curr Rheumatol Rev. 2018;14:91–97. doi: 10.2174/157339711402180706144757. [DOI] [PubMed] [Google Scholar]
- 3.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prieto-Alhambra D, Judge A, Javaid MK, Cooper C, Diez-Perez A, Arden NK. Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: influences of age, gender and osteoarthritis affecting other joints. Ann Rheum Dis. 2014;73:1659–1664. doi: 10.1136/annrheumdis-2013-203355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turkiewicz A, Petersson IF, Bjork J, Hawker G, Dahlberg LE, Lohmander LS, Englund M. Current and future impact of osteoarthritis on health care: a population-based study with projections to year 2032. Osteoarthr Cartilage. 2014;22:1826–1832. doi: 10.1016/j.joca.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 6.Abhishek A, Doherty M. Diagnosis and clinical presentation of osteoarthritis. Rheum Dis Clin N Am. 2013;39:45–66. doi: 10.1016/j.rdc.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Krasnokutsky S, Samuels J, Abramson SB. Osteoarthritis in 2007. Bull NYU Hosp Jt Dis. 2007;65:222–228. [PubMed] [Google Scholar]
- 8.Jo H, Park JS, Kim EM, Jung MY, Lee SH, Seong SC, Park SC, Kim HJ, Lee MC. The in vitro effects of dehydroepiandrosterone on human osteoarthritic chondrocytes. Osteoarthr Cartilage. 2003;11:585–594. doi: 10.1016/s1063-4584(03)00094-3. [DOI] [PubMed] [Google Scholar]
- 9.Wilson JF. To stop osteoarthritis, fixing cartilage may not be enough. Ann Intern Med. 2007;147:437–439. doi: 10.7326/0003-4819-147-6-200709180-00024. [DOI] [PubMed] [Google Scholar]
- 10.Arden N, Nevitt MC. Osteoarthritis: epidemiology. Best Pract Res Cl Rh. 2006;20:3–25. doi: 10.1016/j.berh.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Nelson AE, Allen KD, Golightly YM, Goode AP, Jordan JM. A systematic review of recommendations and guidelines for the management of osteoarthritis: the chronic osteoarthritis management initiative of the U. S. bone and joint initiative. Semin Arthritis Rheum. 2014;43:701–712. doi: 10.1016/j.semarthrit.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Block JA. Osteoarthritis: OA guidelines: improving care or merely codifying practice? Nat Rev Rheumatol. 2014;10:324–326. doi: 10.1038/nrrheum.2014.61. [DOI] [PubMed] [Google Scholar]
- 13.Wang ZY, Shi SY, Li SJ, Chen F, Chen H, Lin HZ, Lin JM. Efficacy and safety of duloxetine on osteoarthritis knee pain: a meta-analysis of randomized controlled trials. Pain Med. 2015;16:1373–1385. doi: 10.1111/pme.12800. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Li J, Li R, Wang H, Yang J, Xu J, Zha Z. Efficacy and safety of tanezumab on osteoarthritis knee and hip pains: a meta-analysis of randomized controlled trials. Pain Med. 2017;18:374–385. doi: 10.1093/pm/pnw262. [DOI] [PubMed] [Google Scholar]
- 15.Higashi H, Barendregt JJ. Cost-effectiveness of total hip and knee replacements for the Australian population with osteoarthritis: discrete-event simulation model. PLoS One. 2011;6:e25403. doi: 10.1371/journal.pone.0025403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JH, Kim HJ, Lee DH. Survival of opening versus closing wedge high tibial osteotomy: a meta-analysis. Sci Rep. 2017;7:7296–7303. doi: 10.1038/s41598-017-07856-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Woude JAD, Wiegant K, van Heerwaarden RJ, Spruijt S, van Roermund PM, Custers RJH, Mastbergen SC, Lafeber FPJG. Knee joint distraction compared with high tibial osteotomy: a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2017;25:876–886. doi: 10.1007/s00167-016-4131-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu K, Robbins SR, McDougall JJ. Osteoarthritis: the genesis of pain. Rheumatology (Oxford) 2018;57:iv43–iv50. doi: 10.1093/rheumatology/kex419. [DOI] [PubMed] [Google Scholar]
- 19.Berenbaum F, Wallace IJ, Lieberman DE, Felson DT. Modern-day environmental factors in the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2018;14:674–681. doi: 10.1038/s41584-018-0073-x. [DOI] [PubMed] [Google Scholar]
- 20.Felson DT. Epidemiology of hip and knee osteoarthritis. Epidemiol Rev. 1988;10:1–28. doi: 10.1093/oxfordjournals.epirev.a036019. [DOI] [PubMed] [Google Scholar]
- 21.Wearing SC, Hennig EM, Byrne NM, Steele JR, Hills AP. Musculoskeletal disorders associated with obesity: a biomechanical perspective. Obes Rev. 2006;7:239–250. doi: 10.1111/j.1467-789X.2006.00251.x. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez-Adams J, Leddy HA, McNulty AL, O’Conor CJ, Guilak F. The mechanobiology of articular cartilage: bearing the burden of osteoarthritis. Curr Rheumatol Rep. 2014;16:451–460. doi: 10.1007/s11926-014-0451-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Houard X, Goldring MB, Berenbaum F. Homeostatic mechanisms in articular cartilage and role of inflammation in osteoarthritis. Curr Rheumatol Rep. 2013;15:375–385. doi: 10.1007/s11926-013-0375-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542:177–185. doi: 10.1038/nature21363. [DOI] [PubMed] [Google Scholar]
- 25.Berenbaum F, Eymard F, Houard X. Osteoarthritis, inflammation and obesity. Curr Opin Rheumatol. 2013;25:114–118. doi: 10.1097/BOR.0b013e32835a9414. [DOI] [PubMed] [Google Scholar]
- 26.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 27.Steenvoorden MM, Huizinga TW, Verzijl N, Bank RA, Ronday HK, Luning HA, Lafeber FP, Toes RE, DeGroot J. Activation of receptor for advanced glycation end products in osteoarthritis leads to increased stimulation of chondrocytes and synoviocytes. Arthritis Rheum. 2006;54:253–263. doi: 10.1002/art.21523. [DOI] [PubMed] [Google Scholar]
- 28.Simopoulos AP. An increase in the Omega-6/Omega-3 fatty acid ratio increases the risk for obesity. Nutrients. 2016;8:128–145. doi: 10.3390/nu8030128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dai Z, Lu N, Niu J, Felson DT, Zhang Y. Dietary fiber intake in relation to knee pain trajectory. Arthrit Care Res. 2017;69:1331–1339. doi: 10.1002/acr.23158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slemenda C, Brandt KD, Heilman DK, Mazzuca S, Braunstein EM, Katz BP, Wolinsky FD. Quadriceps weakness and osteoarthritis of the knee. Ann Intern Med. 1997;127:97–104. doi: 10.7326/0003-4819-127-2-199707150-00001. [DOI] [PubMed] [Google Scholar]
- 31.Nomura M, Sakitani N, Iwasawa H, Kohara Y, Takano S, Wakimoto Y, Kuroki H, Moriyama H. Thinning of articular cartilage after joint unloading or immobilization. An experimental investigation of the pathogenesis in mice. Osteoarthr Cartilage. 2017;25:727–736. doi: 10.1016/j.joca.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 32.Campbell TM, Reilly K, Laneuville O, Uhthoff H, Trudel G. Bone replaces articular cartilage in the rat knee joint after prolonged immobilization. Bone. 2018;106:42–51. doi: 10.1016/j.bone.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 33.Loeser RF. Aging and osteoarthritis. Curr Opin Rheumatol. 2011;23:492–496. doi: 10.1097/BOR.0b013e3283494005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lotz M, Loeser RF. Effects of aging on articular cartilage homeostasis. Bone. 2012;51:241–248. doi: 10.1016/j.bone.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bolduc JA, Collins JA, Loeser RF. Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic Biol Med. 2019;132:73–82. doi: 10.1016/j.freeradbiomed.2018.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loeser RF, Collins JA, Diekman BO. Ageing and the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12:412–420. doi: 10.1038/nrrheum.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, Zhao X, Lotz M, Terkeltaub R, Liu-Bryan R. Mitochondrial biogenesis is impaired in osteoarthritis chondrocytes but reversible via peroxisome proliferator-activated receptor gamma coactivator 1alpha. Arthritis Rheumatol. 2015;67:2141–2153. doi: 10.1002/art.39182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scanzello CR, Goldring SR. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012;51:249–257. doi: 10.1016/j.bone.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mathiessen A, Conaghan PG. Synovitis in osteoarthritis: current understanding with therapeutic implications. Arthritis Res Ther. 2017;19:18–27. doi: 10.1186/s13075-017-1229-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Larsson S, Englund M, Struglics A, Lohmander LS. Interleukin-6 and tumor necrosis factor alpha in synovial fluid are associated with progression of radiographic knee osteoarthritis in subjects with previous meniscectomy. Osteoarthr Cartilage. 2015;23:1906–1914. doi: 10.1016/j.joca.2015.05.035. [DOI] [PubMed] [Google Scholar]
- 41.Dayer JM. The process of identifying and understanding cytokines: from basic studies to treating rheumatic diseases. Best Pract Res Clin Rheumatol. 2004;18:31–45. doi: 10.1016/j.berh.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 42.Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 43.Wojdasiewicz P, Poniatowski LA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014;2014:561459. doi: 10.1155/2014/561459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Q, Yang QL, Wu W. Graphene-based steganographic aptasensor for information computing and monitoring toxins of biofilm in food. Front Microbiol. 2020;10:3139. doi: 10.3389/fmicb.2019.03139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salzmann GM, Ossendorff R, Gilat R, Cole BJ. Autologous minced cartilage implantation for treatment of chondral and osteochondral lesions in the knee joint: an overview. Cartilage. 2020:1947603520942952. doi: 10.1177/1947603520942952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Artuzi FE, Puricelli E, Baraldi CE, Quevedo AS, Ponzoni D. Reduction of osteoarthritis severity in the temporomandibular joint of rabbits treated with chondroitin sulfate and glucosamine. PLoS One. 2020;15:e0231734. doi: 10.1371/journal.pone.0231734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hosaka Y, Saito T, Sugita S, Hikata T, Kobayashi H, Fukai A, Taniguchi Y, Hirata M, Akiyama H, Chung UI, Kawaguchi H. Notch signaling in chondrocytes modulates endochondral ossification and osteoarthritis development. Proc Natl Acad Sci U S A. 2013;110:1875–1880. doi: 10.1073/pnas.1207458110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sugita S, Hosaka Y, Okada K, Mori D, Yano F, Kobayashi H, Taniguchi Y, Mori Y, Okuma T, Chang SH, Kawata M, Taketomi S, Chikuda H, Akiyama H, Kageyama R, Chung UI, Tanaka S, Kawaguchi H, Ohba S, Saito T. Transcription factor Hes1 modulates osteoarthritis development in cooperation with calcium/calmodulin-dependent protein kinase 2. Proc Natl Acad Sci U S A. 2015;112:3080–3085. doi: 10.1073/pnas.1419699112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Z, Chen J, Mirando AJ, Wang C, Zuscik MJ, O’Keefe RJ, Hilton MJ. A dual role for NOTCH signaling in joint cartilage maintenance and osteoarthritis. Sci Signal. 2015;8:Ra71. doi: 10.1126/scisignal.aaa3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang S, Kim J, Ryu JH, Oh H, Chun CH, Kim BJ, Min BH, Chun JS. Hypoxia-inducible factor-2alpha is a catabolic regulator of osteoarthritic cartilage destruction. Nat Med. 2010;16:687–693. doi: 10.1038/nm.2153. [DOI] [PubMed] [Google Scholar]
- 51.Firner S, Zaucke F, Michael J, Dargel J, Schiwy-Bochat KH, Heilig J, Rothschild MA, Eysel P, Brüggemann GP, Niehoff A. Extracellular distribution of collagen ii and perifibrillar adapter proteins in healthy and osteoarthritic human knee joint cartilage. J Histochem Cytochem. 2017;65:593–606. doi: 10.1369/0022155417729154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013;19:179–192. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- 53.Tao R, Xu XB, Sun C, Wang YH, Wang ST, Liu ZB, Zhai LL, Cheng HB, Xiao M, Zhang DM. KPNA2 interacts with P65 to modulate catabolic events in osteoarthritis. Exp Mol Pathol. 2015;99:245–252. doi: 10.1016/j.yexmp.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 54.Cohen E, Lee YC. A mechanism-based approach to the management of osteoarthritis pain. Curr Osteoporos Rep. 2015;13:399–406. doi: 10.1007/s11914-015-0291-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bhatia D, Bejarano T, Novo M. Current interventions in the management of knee osteoarthritis. J Pharm Bioallied Sci. 2013;5:30–38. doi: 10.4103/0975-7406.106561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Geenen R, Overman CL, Christensen R, Åsenlöf P, Capela S, Huisinga KL, Husebø MEP, Köke AJA, Paskins Z, Pitsillidou IA, Savel C, Austin J, Hassett AL, Severijns G, Stoffer-Marx M, Vlaeyen JWS, Fernández-de-Las-Peñas C, Ryan SJ, Bergman S. EULAR recommendations for the health professional’s approach to pain management in inflammatory arthritis and osteoarthritis. Ann Rheum Dis. 2018;77:797–807. doi: 10.1136/annrheumdis-2017-212662. [DOI] [PubMed] [Google Scholar]
- 57.Fransen M, McConnell S, Harmer AR, Van der Esch M, Simic M, Bennell KL. Exercise for osteoarthritis of the knee: a Cochrane systematic review. Br J Sport Med. 2015;49:1554–7. doi: 10.1136/bjsports-2015-095424. [DOI] [PubMed] [Google Scholar]
- 58.Messier SP, Mihalko SL, Legault C, Miller GD, Nicklas BJ, DeVita P, Beavers DP, Hunter DJ, Lyles MF, Eckstein F, Williamson JD, Carr JJ, Guermazi A, Loeser RF. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA. 2013;310:1263–1273. doi: 10.1001/jama.2013.277669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bliddal H, Leeds AR, Christensen R. Osteoarthritis, obesity and weight loss: evidence, hypotheses and horizons - a scoping review. Obes Rev. 2014;15:578–586. doi: 10.1111/obr.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- 61.Al-Saeed A. Gastrointestinal and cardiovascular risk of nonsteroidal anti-inflammatory drugs. Oman Med J. 2011;26:385–391. doi: 10.5001/omj.2011.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jozwiak-Bebenista M, Nowak JZ. Paracetamol: mechanism of action, applications and safety concern. Acta Pol Pharm. 2014;71:11–23. [PubMed] [Google Scholar]
- 63.Negrini F, Negrini S. Is paracetamol better than placebo for knee and hip osteoarthritis? A Cochrane review summary with commentary. Int J Rheum Dis. 2020;23:595–596. doi: 10.1111/1756-185X.13827. [DOI] [PubMed] [Google Scholar]
- 64.Jaeschke H. Acetaminophen: dose-dependent drug hepatotoxicity and acute liver failure in patients. Digest Dis. 2015;33:464–471. doi: 10.1159/000374090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abou-Raya S, Abou-Raya A, Helmii M. Duloxetine for the management of pain in older adults with knee osteoarthritis: randomised placebo-controlled trial. Age Ageing. 2012;41:646–652. doi: 10.1093/ageing/afs072. [DOI] [PubMed] [Google Scholar]
- 66.Mason L, Moore RA, Derry S, Edwards JE, McQuay HJ. Systematic review of topical capsaicin for the treatment of chronic pain. BMJ. 2004;328:991–994. doi: 10.1136/bmj.38042.506748.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Frakes EP, Risser RC, Ball TD, Hochberg MC, Wohlreich MM. Duloxetine added to oral nonsteroidal anti-inflammatory drugs for treatment of knee pain due to osteoarthritis: results of a randomized, double-blind, placebo-controlled trial. Curr Med Res Opin. 2011;27:2361–2372. doi: 10.1185/03007995.2011.633502. [DOI] [PubMed] [Google Scholar]
- 68.Li SJ, Ma FB, Pang XC, Tang B, Lin LJ. Synthesis of chondroitin sulfate magnesium for osteoarthritis treatment. Carbohyd Polyms. 2019;212:387–394. doi: 10.1016/j.carbpol.2019.02.061. [DOI] [PubMed] [Google Scholar]
- 69.Faundez J, Cotoras P, Irarrazaval S. Are intraarticular steroids effective for knee osteoarthritis? Medwave. 2016;16:e6599. doi: 10.5867/medwave.2016.6599. [DOI] [PubMed] [Google Scholar]
- 70.Gormeli G, Gormeli C, Ataoglu B, Colak C, Aslanturk O, Ertem K. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sport Tr A. 2017;25:958–965. doi: 10.1007/s00167-015-3705-6. [DOI] [PubMed] [Google Scholar]
- 71.Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sport Med. 2013;41:356–364. doi: 10.1177/0363546512471299. [DOI] [PubMed] [Google Scholar]
- 72.Schnitzer TJ, Marks JA. A systematic review of the efficacy and general safety of antibodies to NGF in the treatment of OA of the hip or knee. Osteoarthr Cartilage. 2015;23:S8–17. doi: 10.1016/j.joca.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 73.Miller RE, Block JA, Malfait AM. What is new in pain modification in osteoarthritis? Rheumatology (Oxford) 2018;57:Iv99–Iv107. doi: 10.1093/rheumatology/kex522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mistry H, Connock M, Pink J, Shyangdan D, Clar C, Royle P, Court R, Biant LC, Metcalfe A, Waugh N. Autologous chondrocyte implantation in the knee: systematic review and economic evaluation. Health Technol Asses. 2017;21:1–294. doi: 10.3310/hta21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Medvedeva EV, Grebenik EA, Gornostaeva SN, Telpuhov VI, Lychagin AV, Timashev PS, Chagin AS. Repair of damaged articular cartilage: current approaches and future directions. Int J Mol Sci. 2018;19:2366–2389. doi: 10.3390/ijms19082366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen FH, Rousche KT, Tuan RS. Technology Insight: adult stem cells in cartilage regeneration and tissue engineering. Nat Clin Pract Rheum. 2006;2:373–382. doi: 10.1038/ncprheum0216. [DOI] [PubMed] [Google Scholar]
- 77.Saghahazrati S, Ayatollahi SA, Kobarfard F, Zang BM. The synergistic effect of glucagon-like peptide-1 and chamomile oil on differentiation of mesenchymal stem cells into insulin-producing cells. Cell J. 2020;21:371–378. doi: 10.22074/cellj.2020.6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Somoza RA, Welter JF, Correa D, Caplan AI. Chondrogenic differentiation of mesenchymal stem cells: challenges and unfulfilled expectations. Tissue Eng Part B Rev. 2014;20:596–608. doi: 10.1089/ten.teb.2013.0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hosseini S, Taghiyar L, Safari F, Baghaban Eslaminejad M. Regenerative medicine applications of mesenchymal stem cells. Adv Exp Med Biol. 2018;1089:115–141. doi: 10.1007/5584_2018_213. [DOI] [PubMed] [Google Scholar]
- 80.Kitta T, Kanno Y, Chiba H, Higuchi M, Ouchi M, Togo M, Moriya K, Shinohara N. Benefits and limitations of animal models in partial bladder outlet obstruction for translational research. Int J Urol. 2018;25:36–44. doi: 10.1111/iju.13471. [DOI] [PubMed] [Google Scholar]
- 81.Wakitani S, Imoto K, Yamamoto T, Saito M, Murata N, Yoneda M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis Cartilage. 2002;10:199–206. doi: 10.1053/joca.2001.0504. [DOI] [PubMed] [Google Scholar]
- 82.Wong KL, Lee KB, Tai BC, Law P, Lee EH, Hui JH. Injectable cultured bone marrow-derived mesenchymal stem cells in varus knees with cartilage defects undergoing high tibial osteotomy: a prospective, randomized controlled clinical trial with 2 years’ follow-up. Arthroscopy. 2013;29:2020–2028. doi: 10.1016/j.arthro.2013.09.074. [DOI] [PubMed] [Google Scholar]
- 83.Vangsness CT Jr, Farr J 2nd, Boyd J, Dellaero DT, Mills CR, LeRoux-Williams M. Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy: a randomized, double-blind, controlled study. J Bone Joint Surg Am. 2014;96:90–98. doi: 10.2106/JBJS.M.00058. [DOI] [PubMed] [Google Scholar]
- 84.Lamo-Espinosa JM, Mora G, Blanco JF, Granero-Molto F, Nunez-Cordoba JM, Sanchez-Echenique C, Bondia JM, Aquerreta JD, Andreu EJ, Ornilla E, Villaron EM, Valenti-Azcarate A, Sanchez-Guijo F, Del Canizo MC, Valenti-Nin JR, Prosper F. Intra-articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: multicenter randomized controlled clinical trial (phase I/II) J Transl Med. 2016;14:246–255. doi: 10.1186/s12967-016-0998-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pers YM, Rackwitz L, Ferreira R, Pullig O, Delfour C, Barry F, Sensebe L, Casteilla L, Fleury S, Bourin P, Noel D, Canovas F, Cyteval C, Lisignoli G, Schrauth J, Haddad D, Domergue S, Noeth U, Jorgensen C. Adipose mesenchymal stromal cell-based therapy for severe osteoarthritis of the knee: a phase i dose-escalation trial. Stem Cells Transl Med. 2016;5:847–856. doi: 10.5966/sctm.2015-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Koh YG, Kwon OR, Kim YS, Choi YJ, Tak DH. Adipose-derived mesenchymal stem cells with microfracture versus microfracture alone: 2-year follow-up of a prospective randomized trial. Arthroscopy. 2016;32:97–109. doi: 10.1016/j.arthro.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 87.Lee WS, Kim HJ, Kim KI, Kim GB, Jin W. Intra-articular injection of autologous adipose tissue-derived mesenchymal stem cells for the treatment of knee osteoarthritis: a phase iib, randomized, placebo-controlled clinical trial. Stem Cells Transl Med. 2019;8:504–511. doi: 10.1002/sctm.18-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang Y, Jin W, Liu H, Cui Y, Mao Q, Fei Z, Xiang C. Curative effect of human umbilical cord mesenchymal stem cells by intra-articular injection for degenerative knee osteoarthritis. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2016;30:1472–1477. doi: 10.7507/1002-1892.20160305. [DOI] [PubMed] [Google Scholar]
- 89.Liu C, Yang Y, He G. Efficacy and safety of umbilical cord-mesenchymal stem cells transplantation for treating osteoarthritis. Osteoarthr Cartilage. 2017;25:S389–S389. [Google Scholar]
- 90.Pak JY. Clinical outcomes of human umbilical cord blood derived mesenchymal stem cells application in knee osteoarthritis patients. Osteoarthr Cartilage. 2019;27:S511–S511. [Google Scholar]
- 91.Volarevic V, Gazdic M, Simovic Markovic B, Jovicic N, Djonov V, Arsenijevic N. Mesenchymal stem cell-derived factors: immunomodulatory effects and therapeutic potential. Biofactors. 2017;43:633–644. doi: 10.1002/biof.1374. [DOI] [PubMed] [Google Scholar]
- 92.Davatchi F, Sadeghi Abdollahi B, Mohyeddin M, Nikbin B. Mesenchymal stem cell therapy for knee osteoarthritis: 5 years follow-up of three patients. Int J Rheum Dis. 2016;19:219–225. doi: 10.1111/1756-185X.12670. [DOI] [PubMed] [Google Scholar]
- 93.Chen HT, Lee MJ, Chen CH, Chuang SC, Chang LF, Ho ML, Hung SH, Fu YC, Wang YH, Wang HI, Wang GJ, Kang L, Chang JK. Proliferation and differentiation potential of human adipose-derived mesenchymal stem cells isolated from elderly patients with osteoporotic fractures. J Cell Mol Med. 2012;16:582–593. doi: 10.1111/j.1582-4934.2011.01335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ilic D, Miere C, Lazic E. Umbilical cord blood stem cells: clinical trials in non-hematological disorders. Br Med Bull. 2012;102:43–57. doi: 10.1093/bmb/lds008. [DOI] [PubMed] [Google Scholar]
- 95.Kim J, Kim EH, Lee K, Kim B, Kim Y, Na SH, Yoon YW. Low-level laser irradiation improves motor recovery after contusive spinal cord injury in rats. Tissue Eng Regen Med. 2017;14:57–64. doi: 10.1007/s13770-016-0003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gonçalves ED, Souza PS, Lieberknecht V, Fidelis GS, Barbosa RI, Silveira PC, de Pinho RA, Dutra RC. Low-level laser therapy ameliorates disease progression in a mouse model of multiple sclerosis. Autoimmunity. 2016;49:132–142. doi: 10.3109/08916934.2015.1124425. [DOI] [PubMed] [Google Scholar]
- 97.So KF, Leung MC, Cui Q. Effects of low level laser treatment on the survival of axotomized retinal ganglion cells in adult Hamsters. Neural Regen Res. 2014;9:1863–1869. doi: 10.4103/1673-5374.145337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shingyochi Y, Kanazawa S, Tajima S, Tanaka R, Mizuno H, Tobita M. A low-level carbon dioxide laser promotes fibroblast proliferation and migration through activation of Akt, ERK, and JNK. PLoS One. 2017;12:e0168937. doi: 10.1371/journal.pone.0168937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Abdallah AN, Shamaa AA, El-Tookhy OS. Evaluation of treatment of experimentally induced canine model of multiple sclerosis using laser activated non-expanded adipose derived stem cells. Res Vet Sci. 2019;125:71–81. doi: 10.1016/j.rvsc.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 100.Fekrazad R, Sadeghi Ghuchani M, Eslaminejad MB, Taghiyar L, Kalhori KA, Pedram MS, Shayan AM, Aghdami N, Abrahamse H. The effects of combined low level laser therapy and mesenchymal stem cells on bone regeneration in rabbit calvarial defects. J Photoch Photobio B. 2015;151:180–185. doi: 10.1016/j.jphotobiol.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 101.Pinheiro CCG, de Pinho MC, Aranha AC, Fregnani E, Bueno DF. Low power laser therapy: a strategy to promote the osteogenic differentiation of deciduous dental pulp stem cells from cleft lip and palate patients. Tissue Eng Pt A. 2018;24:569–575. doi: 10.1089/ten.TEA.2017.0115. [DOI] [PubMed] [Google Scholar]
- 102.Bamps M, Dok R, Nuyts S. Low-level laser therapy stimulates proliferation in head and neck squamous cell carcinoma cells. Front Oncol. 2018;8:343–349. doi: 10.3389/fonc.2018.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Abdallah AN, Shamaa AA, El-Tookhy OS. Evaluation of treatment of experimentally induced canine model of multiple sclerosis using laser activated non-expanded adipose derived stem cells. Res Vet Sci. 2019;125:71–81. doi: 10.1016/j.rvsc.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 104.Ruan YR, Kato H, Taguchi Y, Yamauchi N, Umeda M. Irradiation by high-intensity red light-emitting diode enhances human bone marrow mesenchymal stem cells osteogenic differentiation and mineralization through Wnt/beta-catenin signaling pathway. Laser Med Sci. 2021;36:55–65. doi: 10.1007/s10103-020-03002-5. [DOI] [PubMed] [Google Scholar]
- 105.Wang L, Wu F, Liu C, Song Y, Guo J, Yang Y, Qiu Y. Low-level laser irradiation modulates the proliferation and the osteogenic differentiation of bone marrow mesenchymal stem cells under healthy and inflammatory condition. Lasers Med Sci. 2019;34:169–178. doi: 10.1007/s10103-018-2673-8. [DOI] [PubMed] [Google Scholar]
- 106.McColloch A, Liebman C, Liu HL, Cho M. Alterted adipogenesis of human mesenchymal stem cells by photobiomodulation using 1064 nm laser light. Laser Surg Med. 2020 doi: 10.1002/lsm.23278. [DOI] [PubMed] [Google Scholar]
- 107.Andreeva NV, Yusupov VI, Zotov KV, Timashev PS, Belyavsky AV. Effects of infrared laser irradiation on aging of mesenchymal stem cells in culture. Opt Eng. 2020;59:8. [Google Scholar]
- 108.Liao X, Li SH, Xie GH, Xie S, Xiao LL, Song JX, Liu HW. Preconditioning with low-level laser irradiation enhances the therapeutic potential of human adipose-derived stem cells in a mouse model of photoaged skin. Photochem Photobiol. 2018;94:780–790. doi: 10.1111/php.12912. [DOI] [PubMed] [Google Scholar]
- 109.Park IS, Chung PS, Ahn JC. Adipose-derived stem cell spheroid treated with low-level light irradiation accelerates spontaneous angiogenesis in mouse model of hindlimb ischemia. Cytotherapy. 2017;19:1070–1078. doi: 10.1016/j.jcyt.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 110.Chen H, Wang H, Li Y, Liu W, Wang C, Chen Z. Biological effects of low-level laser irradiation on umbilical cord mesenchymal stem cells. AIP Adv. 2016;6:14–26. [Google Scholar]
- 111.Yang D, Yi W, Wang E, Wang M. Effects of light-emitting diode irradiation on the osteogenesis of human umbilical cord mesenchymal stem cells in vitro. Sci Rep. 2016;6:37370. doi: 10.1038/srep37370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zaccara IM, Ginani F, Mota-Filho HG, Henriques AC, Barboza CA. Effect of low-level laser irradiation on proliferation and viability of human dental pulp stem cells. Lasers Med Sci. 2015;30:2259–2264. doi: 10.1007/s10103-015-1803-9. [DOI] [PubMed] [Google Scholar]


