Abstract
Background
High respiratory drive in mechanically ventilated patients with spontaneous breathing effort may cause excessive lung stress and strain and muscle loading. Therefore, it is important to have a reliable estimate of respiratory effort to guarantee lung and diaphragm protective mechanical ventilation. Recently, a novel non-invasive method was found to detect excessive dynamic transpulmonary driving pressure (∆PL) and respiratory muscle pressure (Pmus) with reasonable accuracy. During the Coronavirus disease 2019 (COVID-19) pandemic, it was impossible to obtain the gold standard for respiratory effort, esophageal manometry, in every patient. Therefore, we investigated whether this novel non-invasive method could also be applied in COVID-19 patients.
Methods
∆PL and Pmus were derived from esophageal manometry in COVID-19 patients. In addition, ∆PL and Pmus were computed from the occlusion pressure (∆Pocc) obtained during an expiratory occlusion maneuver. Measured and computed ∆PL and Pmus were compared and discriminative performance for excessive ∆PL and Pmus was assessed. The relation between occlusion pressure and respiratory effort was also assessed.
Results
Thirteen patients were included. Patients had a low dynamic lung compliance [24 (20–31) mL/cmH2O], high ∆PL (25 ± 6 cmH2O) and high Pmus (16 ± 7 cmH2O). Low agreement was found between measured and computed ∆PL and Pmus. Excessive ∆PL > 20 cmH2O and Pmus > 15 cmH2O were accurately detected (area under the receiver operating curve (AUROC) 1.00 [95% confidence interval (CI), 1.00–1.00], sensitivity 100% (95% CI, 72–100%) and specificity 100% (95% CI, 16–100%) and AUROC 0.98 (95% CI, 0.90–1.00), sensitivity 100% (95% CI, 54–100%) and specificity 86% (95% CI, 42–100%), respectively). Respiratory effort calculated per minute was highly correlated with ∆Pocc (for esophageal pressure time product per minute (PTPes/min) r2 = 0.73; P = 0.0002 and work of breathing (WOB) r2 = 0.85; P < 0.0001).
Conclusions
∆PL and Pmus can be computed from an expiratory occlusion maneuver and can predict excessive ∆PL and Pmus in patients with COVID-19 with high accuracy.
Keywords: Coronavirus disease 2019, Respiratory monitoring, Occlusion pressure, Dynamic transpulmonary pressure, Respiratory muscle pressure, Respiratory effort
Background
Maintaining spontaneous breathing effort in mechanically ventilated patients limits respiratory muscle disuse and atrophy [1–4]. Too high respiratory effort may lead to excessive lung stress and strain causing lung injury on one hand. On the other hand, it may lead to excessive muscle loading causing muscle injury (mainly diaphragm injury) leading to muscle dysfunction [5]. High respiratory drive and effort frequently exist in critically ill patients, mainly due to insufficient ventilator assistance and sedation, but evidence also suggests biological predisposition (e.g., pulmonary and systemic inflammation, lung mechanical heterogeneity) plays a role as well. Therefore, it is important to have a reliable estimate of respiratory effort to enable lung and diaphragm protective mechanical ventilation [6–8].
The gold standard to obtain respiratory effort is esophageal manometry. This technique is minimally invasive, requires appropriate equipment and expertise, and can be time consuming. Other monitoring techniques or parameters only reflect respiratory drive (P0.1 and electrical activity of the diaphragm) or muscle loading (diaphragm ultrasound) and provide only limited information about lung stress and strain (plateau pressure and driving pressure) [7]. Recently, Bertoni et al. [9] demonstrated that dynamic transpulmonary driving pressure (∆PL) and respiratory muscle pressure (Pmus) can be estimated from the maximal decline in airway pressure (Paw) from positive end-expiratory pressure (PEEP) during an expiratory occlusion maneuver (∆Pocc). Direct estimates of ∆PL and Pmus were unreliable, excessive ∆PL and Pmus, however, could be predicted with reasonable accuracy.
Coronavirus disease 2019 (COVID-19) is a new type of lung disease [10–12] originating from Wuhan, China, in December 2019. Because of the sheer number of mechanically ventilated patients with severe lung disease, it was impossible to measure esophageal pressure to assess respiratory mechanics in every patient. Therefore, we estimated ∆PL and Pmus according to Bertoni et al. [9] in every COVID-19 patient with spontaneous breathing effort as part of standard patient care. If computed ∆PL and/or Pmus were excessive (i.e., higher than Pmus 13–15 cmH2O and ∆PL 16–17 cmH2O), or if patients received prolonged mechanical ventilation with no progress (i.e., ≥14 days) or if patients remained hypercapnic (PaCO2 ≥ 60 mmHg), respiratory mechanics was assessed by esophageal manometry for clinical purposes.
The aim of this paper is to describe respiratory mechanics in mechanically ventilated COVID-19 patients with spontaneous breathing effort, to compute ∆PL and Pmus from ∆Pocc and assess the discriminative performance for excessive ∆PL and Pmus, and to assess the relation between ∆Pocc and respiratory effort.
Methods
Study population
Dynamic transpulmonary driving pressure and respiratory muscle pressure were assessed in COVID-19 patients admitted to the Intensive Care Unit of the Radboud University Medical Center according to Bertoni et al. [9] as follows:
computed ∆PL = (peak Paw − PEEP)—2/3 × ∆Pocc.
computed Pmus = − 3/4 × ∆Pocc.
If patients had high respiratory effort and/or high dynamic transpulmonary driving pressure (i.e., computed Pmus 13–15 cmH2O and ∆PL 16–17 cmH2O or higher), prolonged mechanical ventilation without clinical progress (i.e., ≥ 14 days) or remained hypercapnic (PaCO2 ≥ 60 mmHg), esophageal manometry was obtained as part of our standard clinical protocol. Patients or their legal representatives were informed about the measurements.
Study protocol
This was an observational study. All patients were ventilated with a Servo-i/u ventilator (Getinge, Sölna, Sweden). Ventilator settings were set by the treating intensivist. Patients received a nasogastric catheter with esophageal balloon [Cooper (Cooper Surgical, Trumbull, USA) or Neurovent (NeuroVent Research Inc, Toronto, Canada)] to obtain esophageal pressure (Pes). Catheter position was validated using the dynamic occlusion test [13]. A total of 3–4 manual expiratory occlusions (lasting ~ 1–2 s) were performed during a 10–15 min recording per patient. After the recordings, ventilator settings or sedation strategies were adjusted, if deemed necessary, in accordance with the treating intensivist. Being an observational study, the effect of different ventilator settings or sedatives was not investigated.
Data acquisition
Ventilator flow and airway pressure (Paw) were obtained (sample frequency 100 Hz) by connecting a RS-232 cable via the serial port of the Servo-i/u to a dedicated measurement set-up using Servotracker software (Servotracker release 4.2, Getinge, Sölna, Sweden). The esophageal balloon (i.e., Pes) and a T-piece connected to the expiration port of Servo-i/u (i.e., Paw) were coupled to pressure transducers and acquired (sample frequency 100 Hz) using a dedicated measurement set-up (Biopac MP160, BIOPAC Inc., USA). Signals were synchronized offline based on Paw tracings that were acquired using both software programs. Brief manual expiratory occlusions (lasting ~ 1–2 s) were performed to enable offline synchronization. Data were processed and analyzed offline using Matlab R2018a (Mathworks, Natick, MA, USA).
Signal analysis
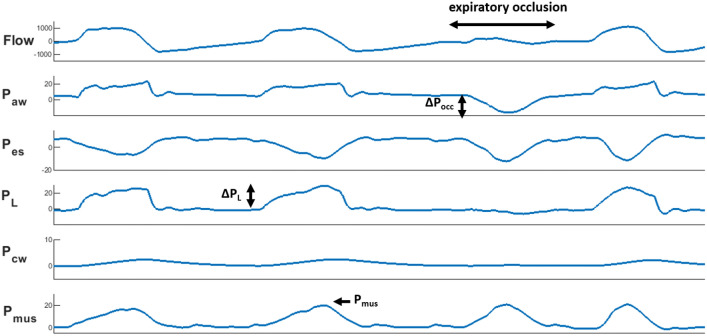
The occlusion pressure (∆Pocc) was defined as the maximal deflection in Paw from positive end-expiratory pressure (PEEP) during an expiratory occlusion maneuver (Fig. 1). The decrease in Pes during the first 100 ms of this maneuver was computed as P0.1. Transpulmonary pressure (PL) was determined by subtracting Pes from Paw. Dynamic transpulmonary driving pressure (∆PL) was computed from onset to peak during inspiration. Dynamic lung compliance (Cdyn) was calculated as tidal volume divided by the increase in PL between points of zero flow. Chest wall elastance (Ecw) was estimated based on predicted vital capacity [9, 14], from this chest wall elastic recoil pressure (Pcw) was computed as the product of tidal volume and Ecw. The pressure generated by the respiratory muscles (Pmus) was calculated as Pcw minus Pes. The integral of the product of Pmus and tidal volume represents work of breathing (WOB), calculated per liter and per minute. The integral of Pmus over time is defined as esophageal pressure–time product (PTPes), calculated per breath and per minute [14, 15].
Fig. 1.
Flow and pressure tracings showing an expiratory occlusion maneuver. From top to bottom: flow (in mL/s), airway pressure (Paw), esophageal pressure (Pes), transpulmonary pressure (PL) (Paw – Pes), chest wall elastic recoil pressure (Pcw) (tidal volume × estimated chest wall elastance) and respiratory muscle pressure (Pmus) (Pcw – Pes) (pressures in cmH2O). During an expiratory occlusion maneuver the patient inhales against a closed valve, resulting in a decrease in airway pressure. The maximal deflection in Paw from positive end-expiratory pressure is defined as occlusion pressure (∆Pocc). From this ∆PL and Pmus were computed and compared with true dynamic lung stress (increase in PL from onset to peak during inspiration) and true respiratory effort (peak Pmus during inspiration)
Data obtained during expiratory occlusion maneuvers were averaged. Data were analyzed on a breath-by-breath basis and averaged over at least a 4-min period free of artifacts or esophageal contractions. Only recordings where ∆Pes/∆Pocc was between 0.8 and 1.2 were included in the analysis.
Statistical analysis
Normality was tested and data are presented accordingly as mean ± standard deviation (SD) or as median [interquartile range (IQR)]. Measured and computed ∆PL and Pmus were compared using Bland–Altman analysis. Receiver operating characteristic curve analysis was performed and sensitivity and specificity were computed to assess the accuracy of computed ∆PL and Pmus to detect excessive ∆PL > 20 cmH2O and Pmus > 10 and > 15 cmH2O. Linear regression analysis was performed to assess the relationship between ∆Pocc and respiratory effort. For all tests a two-tailed P < 0.05 was considered statistically significant. Statistical analyses were performed with Prism 5 (Graphad software, San Diego, USA).
Results
Patient characteristics
Esophageal manometry was obtained in 15 COVID-19 patients between April and July 2020. Two patients were excluded from analysis due to incorrect ∆Pes/∆Pocc. Patient characteristics at time of measurement are shown in Table 1. In general, patients were 61 ± 9 years old, had high PaCO2 (63 ± 17 mmHg) and received prolonged mechanical ventilation (41 ± 32 days). Respiratory failure was the main problem.
Table 1.
Patient characteristics
| Subject | Age | Gender | Main medical history | Position | PaO2/FiO2 ratio | pH | PaCO2 (mmHg) | RASS | Days of MV on study day |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 62 | M | – | P | 175 | 7.26 | 74 | − 5 | 17 |
| 2 | 71 | M | Asthma, ABPA | S | 216 | 7.43 | 59 | − 4 | 4 |
| 3 | 69 | M | 2 × PCI | S | 116 | 7.25 | 94 | − 4 | 22 |
| 4 | 73 | M | COPD Gold II | S | 262 | 7.22 | 64 | − 3 | 38 |
| 5 | 51 | M | Waldeström disease | S | 156 | 7.36 | 57 | − 4 | 14 |
| 6 | 49 | M | – | P | 78 | 7.42 | 47 | − 5 | 6 |
| 7 | 66 | M | OSAS, asthma | S | 182 | 7.47 | 40 | + 1 | 42 |
| 8 | 63 | M | Hypertension, obesity | S | 118 | 7.31 | 97 | − 4 | 46 |
| 9 | 53 | M | Hodgkin | P | 168 | 7.42 | 47 | − 4 | 21 |
| 10 | 47 | F | Hypertension | S | 336 | 7.42 | 47 | 0 | 66 |
| 11 | 62 | M | – | s-L | 251 | 7.41 | 63 | 0 | 80 |
| 12 | 69 | M | CABG | S | 155 | 7.38 | 74 | 0 | 109 |
| 13 | 63 | M | OSAS, hypertension | S | 148 | 7.33 | 62 | − 1 | 74 |
ABPA allergic bronchopulmonary aspergillosis, AKI acute kidney injury, CABG coronary artery bypass grafting, COPD chronic obstructive pulmonary disease, OSAS obstructive sleep apnea syndrome, FiO2 fraction of inspired oxygen, MV mechanical ventilation, P prone, PaCO2 partial pressure of carbon dioxide in arterial blood, PaO2 partial pressure of oxygen in arterial blood, PCI percutaneous coronary intervention, S supine, s-L semi-lateral due to decubitus
Respiratory parameters are shown in Table 2. Only in patient 7 it was not possible to analyze a 4-min period due to continuous esophageal contractions. Patients had a low Cdyn [24 (20–31) mL/cmH2O], high ∆PL (25 ± 6 cmH2O) and high Pmus (16 ± 7 cmH2O).
Table 2.
Respiratory parameters
| Subject | PS (cmH2O) | PEEP (cmH2O) | RR (#/min) | Vt/IBW (mL) | ∆Pocc (cmH2O) | P0.1 (cmH2O) | Cdyn (mL/cmH2O) | ∆PL (cmH2O) | ∆PL, computed (cmH2O) | Pmus (cmH2O) | Pmus, computed (cmH2O) | WOB (J/L) | WOB (J/min) | PTPes breath (cmH2O*s) |
PTPes/min (cmH2O × s × min−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 20 | 8 | 45 | 5.0 | 7 | 4.5 | 21 | 23 | 26 | 9 | 5 | 0.6 | 9.8 | 1.9 | 85 |
| 2 | 14 | 7 | 20 | 7.8 | 24 | 6.2 | 26 | 30 | 31 | 20 | 18 | 1.5 | 17.9 | 8.6 | 176 |
| 3 | 20 | 12 | 23 | 5.8 | 13 | 2.3 | 19 | 24 | 29 | 8 | 9 | 0.3 | 2.6 | 1.6 | 38 |
| 4 | 14 | 10 | 28 | 6.1 | 6 | 2.5 | 40 | 20 | 19 | 9 | 4 | 0.6 | 7.5 | 3.0 | 83 |
| 5 | 8 | 10 | 30 | 6.0 | 25 | 5.4 | 31 | 29 | 27 | 25 | 19 | 1.7 | 25.9 | 8.0 | 239 |
| 6 | 5 | 11 | 24 | 6.2 | 21 | 2.3 | 32 | 23 | 22 | 20 | 16 | 1.4 | 16.4 | 9.4 | 230 |
| 7 | 8 | 8 | 41 | 7.7 | 45 | 6.6 | 19 | 33 | 40 | 29 | 34 | 1.7 | 37.5 | 9.4 | 381 |
| 8 | 16 | 10 | 29 | 5.5 | 18 | 6.0 | 27 | 29 | 31 | 16 | 13 | 1.2 | 13.5 | 4.6 | 134 |
| 9 | 16 | 8 | 28 | 6.2 | 18 | 2.5 | 20 | 29 | 31 | 15 | 13 | 0.7 | 8.6 | 4.4 | 125 |
| 10 | 2 | 6 | 33 | 5.1 | 9 | 2.2 | 51 | 11 | 11 | 9 | 6 | 0.7 | 6.5 | 3.8 | 126 |
| 11 | 13 | 5 | 35 | 6.0 | 22 | 5.2 | 21 | 29 | 32 | 17 | 16 | 1.3 | 19.2 | 7.8 | 271 |
| 12 | 10 | 5 | 28 | 5.1 | 12 | 3.5 | 24 | 21 | 20 | 12 | 9 | 0.7 | 7.9 | 6.0 | 167 |
| 13 | 12 | 10 | 24 | 5.5 | 11 | 1.9 | 20 | 24 | 20 | 13 | 8 | 0.8 | 7.9 | 7.8 | 188 |
Cdyn dynamic lung compliance, IBW ideal body weight, P0.1 decline in esophageal pressure during the first 100 ms of an expiratory occlusion maneuver, PEEP positive end-expiratory pressure, ∆PL dynamic transpulmonary driving pressure, Pmus respiratory muscle pressure, ∆Pocc occlusion pressure, PS pressure support, PTPes pressure time product of esophageal pressure, RR respiratory rate, WOB work of breathing
Computed ∆PL and Pmus
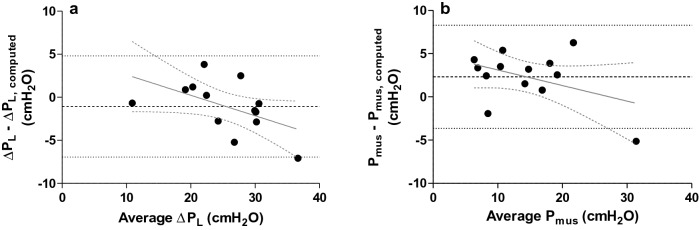
Bland–Altman analysis showed low bias, but wide limits of agreement between measured and computed ∆PL [− 1.1 ± 5.9 cmH2O (bias ± 95% limits of agreement)] (Fig. 2a). Bias between measured and computed Pmus was higher and limits of agreement were equally wide (2.3 ± 6.0 cmH2O) (Fig. 2b). This means there is poor agreement between measured and computed ∆PL and Pmus.
Fig. 2.
Bland–Altman plots with regression lines in which measured and computed dynamic transpulmonary driving pressure (∆PL) (a) and respiratory muscle pressure (Pmus) (b) are compared. Computed ∆PL overestimates measured ∆PL (a) (− 1.1 ± 5.9 cmH2O (bias ± 95% limits of agreement), while computed Pmus underestimates measured Pmus (b) (2.3 ± 6.0 cmH2O). Limits of agreement are large for both parameters. There was no significant trend in differences (for ∆PL r2 = 0.27; P = 0.06 and for Pmus r2 = 0.18; P = 0.15)
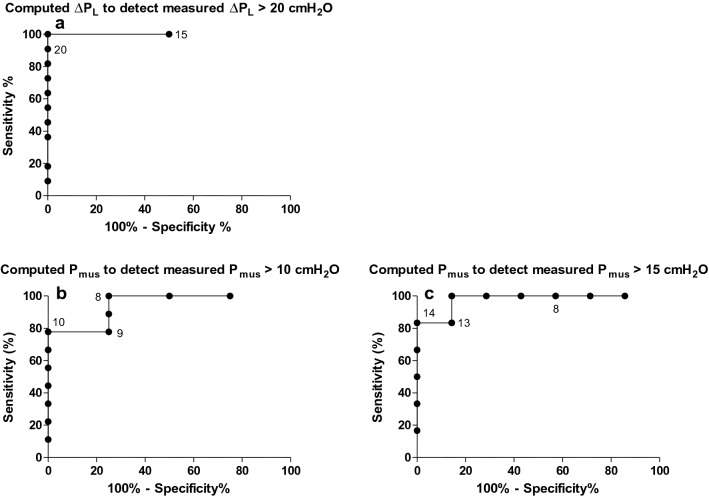
Receiver operating characteristic curve analysis was performed to assess the discriminative performance to predict excessive dynamic lung stress and respiratory effort (Fig. 3; Table 3). Excessive ∆PL > 20 cmH2O was accurately predicted by computed ∆PL > 19 cmH2O [with area under the receiver operating curve (AUROC) 1.00 (95% confidence interval (CI), 1.00–1.00), sensitivity 100% (95% CI, 72–100%) and specificity 100% (95% CI, 16–100%)]. Discriminative performance for Pmus > 10 cmH2O was only moderate, but was acceptable for Pmus > 15 cmH2O with computed Pmus > 13 cmH2O [with AUROC 0.98 (95% CI, 0.90–1.00), sensitivity 100% (95% CI, 54–100%) and specificity 86% (95% CI, 42–100%)] (Fig. 3).
Fig. 3.
Receiver operating characteristic curves (ROC) showing the discriminative performance of computed transpulmonary driving pressure (∆PL) to detect measured excessive (> 20 cmH2O) ∆PL [area under the ROC (AUROC) 1.00 (95% confidence interval (CI), 1.00–1.00)] (a) and computed respiratory muscle pressure (Pmus) to detect measured Pmus > 10 cmH2O [AUROC 0.94 (95% CI, 0.81–1.00)] (b) and 15 cmH2O (AUROC 0.98 (95% CI, 0.90–1.00)] (c). Threshold values are shown as points on the curves
Table 3.
Discriminative performance
| Parameter | Threshold measured value | Threshold computed value for excessive value | Area under receiver operating characteristic curve (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|---|---|
| Excessive dynamic lung stress | ∆PL > 20 cmH2O | Computed ∆PL > 18 cmH2O | 1.00 (1.00–1.00) | 100% (72–100%) | 50% (1–99%) |
| Computed ∆PL > 19 cmH2O | 100% (72–100%) | 100% (16–100%) | |||
| Computed ∆PL > 20 cmH2O | 91% (59–100%) | 100% (16–100%) | |||
| Excessive respiratory effort | Pmus > 10 cmH2O | Computed Pmus > 8 cmH2O | 0.94 (0.81–1.00) | 100% (66–100%) | 75% (19–99%) |
| Computed Pmus > 9 cmH2O | 78% (40–100%) | 75% (19–99%) | |||
| Computed Pmus > 10 cmH2O | 78% (40–97%) | 100% (40–100%) | |||
| Pmus > 15 cmH2O | Computed Pmus > 13 cmH2O | 0.98 (0.90–1.00) | 100% (54–100%) | 86% (42–100%) | |
| Computed Pmus > 14 cmH2O | 83% (36–100%) | 100% (59–100%) | |||
| Computed Pmus > 15 cmH2O | 83% (36–100%) | 100% (59–100%) |
∆PL dynamic transpulmonary driving pressure, Pmus respiratory muscle pressure
∆Pocc and respiratory effort
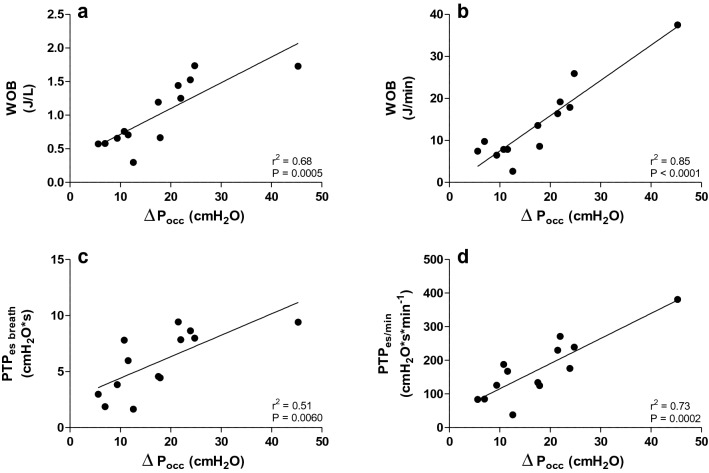
∆Pocc was correlated with respiratory effort (Fig. 4). Only moderate correlations were found between ∆Pocc and PTPes breath (r2 = 0.51; P = 0.0060) and WOB (calculated per liter) (r2 = 0.68; P = 0.0005). Respiratory effort calculated per minute showed much better correlations with ∆Pocc (for PTPes/min r2 = 0.73; P = 0.0002 and WOB r2 = 0.85; P < 0.0001).
Fig. 4.
Relation between occlusion pressure (∆Pocc) and respiratory effort. ∆Pocc is moderately correlated with work of breathing (WOB) calculated per liter (a) and pressure time product of esophageal pressure per breath (PTPes breath) (c). Higher correlations were found between ∆Pocc and respiratory effort when respiratory effort was calculated per minute (b, d)
Discussion
We demonstrate that the mechanically ventilated COVID-19 patients with spontaneous breathing effort included in this study received prolonged mechanical ventilation, had a low dynamic lung compliance, high dynamic transpulmonary driving pressures and high respiratory effort. Dynamic transpulmonary driving pressure and respiratory muscle pressure were estimated from the maximal decline in airway pressure from PEEP during an expiratory occlusion maneuver. Computed ∆PL and Pmus are unreliable for direct estimates of ∆PL and Pmus derived from esophageal manometry, as analysis showed poor agreement between computed and measured values. However, they can predict excessive ∆PL (> 20 cmH2O) and Pmus (> 15 cmH2O) with high sensitivity and specificity. The occlusion pressure is highly correlated with respiratory effort per minute.
Dynamic lung stress and respiratory effort
Maintaining spontaneous breathing effort during mechanical ventilation has become increasingly important in recent years, due to accumulating evidence for over-assistance myotrauma not only during controlled mechanical ventilation, but also during high levels of pressure support ventilation [1–5]. Too high respiratory effort, however, can also cause lung and/or diaphragm injury. This might not be that obvious when relying only on plateau and driving pressures on the ventilator screen. The pressure generated by the respiratory muscles (i.e., Pmus) might in fact be quite high and thus the pleural pressure (i.e., Pes) quite negative, despite high levels of pressure support. Indirect evidence suggests that high Pmus may cause load-induced muscle injury and dysfunction [5, 6]. Negative pleural pressures in an already injured lung increase transpulmonary pressures and thus lung stress and strain and worsen vascular leakage [i.e., patient-self inflicted lung injury (P-SILI)] [16]. In our study, patients had a relatively high Pmus and PaCO2. Apparently, they were not able to increase Pmus to achieve a normal PaCO2. Patients had a high respiratory frequency, but this was insufficient in most patients to meet ventilatory demands as they had high dead space ventilation reflecting severe gas exchange disorders (Additional file 1: Table S1) [17]. ∆Pocc was only moderately correlated with PTPes breath and WOB (J/L), but highly correlated when respiratory effort was multiplied with respiratory frequency [i.e., PTPes/min and WOB (J/min)]. Telias et al. [18] observed something similar for P0.1, which correlated better with respiratory effort per minute as compared to respiratory effort per breath. Together, the data from our study and the study of Telias et al. [18] suggest that in response to high respiratory drive critically ill patients increase respiratory frequency rather than tidal volume, probably due to a combination of respiratory muscle weakness and decreased lung compliance, limiting the ability to increase effort per breath [7, 19, 20].
Clinical implications
Bertoni et al. [9] provided a novel non-invasive method to compute ∆PL and Pmus from ∆Pocc in mechanically ventilated patients with spontaneous breathing effort. We demonstrated that this novel method can also be applied in COVID-19 patients. In accordance with Bertoni et al. [9], computed ∆PL and Pmus cannot directly replace ∆PL and Pmus derived from esophageal manometry. In the external validation cohort they found reasonable discriminative performance for ∆PL > 15 cmH2O and Pmus > 10 cmH2O. In this study, we were able to show that computed values can also be used to predict excessive ∆PL (> 20 cmH2O) and Pmus (> 15 cmH2O). This is very useful when it is not feasible to perform esophageal manometry for various reasons.
COVID-19 patients have severely injured lungs and are prone to high respiratory effort, necessitating close monitoring to enable lung and diaphragm protective ventilation [6, 8]. If computed ∆PL and/or Pmus are/is excessively high, one can decide to measure esophageal pressures. If that is not feasible, ventilator settings should be changed followed by appropriate sedation to keep computed ∆PL and Pmus within the clinically acceptable range based on most recent studies and reviews [6, 8, 9]. Excessive sedation, however, can lead to insufficient respiratory effort (i.e., diminished ∆Pocc) and increased patient ventilator asynchronies [8].
Limitations
This study has some limitations. First, the relatively small sample size. However, many physiological studies with critically ill patients are limited in sample size. For example, the external validation cohort in the study by Bertoni et al. [9] only included 12 patients. Second, there is a selection bias. Only patients with computed high respiratory effort and/or high dynamic transpulmonary driving pressure, prolonged mechanical ventilation and/or who were hypercapnic, were included in the study. Therefore, we found relatively high measured ∆PL and Pmus. Third, limitations in measured ∆PL and Pmus. ∆PL is the dynamic transpulmonary driving pressure, therefore it may overestimate lung stress due to the resistance component. Some studies suggest to perform an end-inspiratory occlusion maneuver in the presence of spontaneous breathing activity to obtain semi-static pressure measurements [21, 22]. For Pmus calculations the chest wall elastance was estimated based on predicted vital capacity. Bertoni et al. [9] demonstrated that predicted values approximated measured values of chest wall elastance.
Conclusions
In mechanically ventilated COVID-19 patients with spontaneous breathing effort ∆PL and Pmus can be computed from an expiratory occlusion maneuver. Computed ∆PL and Pmus cannot replace ∆PL and Pmus derived from esophageal manometry, but they can predict excessive ∆PL and Pmus with high accuracy. The occlusion pressure is highly correlated with respiratory effort per minute.
Supplementary Information
Additional file 1: Table S1 Dead space ventilation.
Acknowledgements
Not applicable.
Abbreviations
- ABPA
Allergic bronchopulmonary aspergillosis
- AUROC
Area under the receiver operating characteristic curve
- CABG
Coronary artery bypass grafting
- Cdyn
Dynamic lung compliance
- CI
Confidence interval
- COPD
Chronic obstructive pulmonary disease
- COVID-19
Coronavirus disease 2019
- Ecw
Chest wall elastance
- FiO2
Fraction of inspired oxygen
- IQR
Interquartile range
- OSAS
Obstructive sleep apnea syndrome
- MV
Mechanical ventilation
- P0.1
Decline in esophageal pressure during the first 100 ms of an expiratory occlusion maneuver
- PaCO2
Partial pressure of carbon dioxide in arterial blood
- PaO2
Partial pressure of oxygen in arterial blood
- Paw
Airway pressure
- PCI
Percutaneous coronary intervention
- Pcw
Chest wall elastic recoil pressure
- PEEP
Positive end-expiratory pressure
- Pes
Esophageal pressure
- (∆)PL
(Dynamic) transpulmonary driving pressure
- Pmus
Respiratory muscle pressure
- Pocc
Occlusion pressure
- PS
Pressure support
- PTPes
Pressure time product of esophageal pressure
- RR
Respiratory rate
- SD
Standard deviation
- WOB
Work of breathing
Authors’ contributions
Data acquisition: LR; data analysis: LR; data interpretation: all authors; manuscript drafting and revising: all authors. All authors read and approved the final manuscript.
Funding
There was no financial funding.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Due to standard patient care and the urgent need to gain knowledge about this new lung disease, informed consent was deemed unnecessary, but also not feasible in most cases.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13613-021-00821-9.
References
- 1.Goligher EC, Dres M, Fan E, Rubenfeld GD, Scales DC, Herridge MS, et al. Mechanical ventilation-induced diaphragm atrophy strongly impacts clinical outcomes. Am J Respir Crit Care Med. 2018;197(2):204–213. doi: 10.1164/rccm.201703-0536OC. [DOI] [PubMed] [Google Scholar]
- 2.Goligher EC, Fan E, Herridge MS, Murray A, Vorona S, Brace D, et al. Evolution of diaphragm thickness during mechanical ventilation. Impact of inspiratory effort. Am J Respir Crit Care Med. 2015;192(9):1080–1088. doi: 10.1164/rccm.201503-0620OC. [DOI] [PubMed] [Google Scholar]
- 3.Hooijman PE, Beishuizen A, Witt CC, de Waard MC, Girbes AR, Spoelstra-de Man AM, et al. Diaphragm muscle fiber weakness and ubiquitin-proteasome activation in critically ill patients. Am J Respir Crit Care Med. 2015;191(10):1126–1138. doi: 10.1164/rccm.201412-2214OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaber S, Petrof BJ, Jung B, Chanques G, Berthet JP, Rabuel C, et al. Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am J Respir Crit Care Med. 2011;183(3):364–371. doi: 10.1164/rccm.201004-0670OC. [DOI] [PubMed] [Google Scholar]
- 5.Goligher EC, Brochard LJ, Reid WD, Fan E, Saarela O, Slutsky AS, et al. Diaphragmatic myotrauma: a mediator of prolonged ventilation and poor patient outcomes in acute respiratory failure. Lancet Respir Med. 2019;7(1):90–98. doi: 10.1016/S2213-2600(18)30366-7. [DOI] [PubMed] [Google Scholar]
- 6.Goligher EC, Dres M, Patel BK, Sahetya SK, Beitler JR, Telias I, et al. Lung and diaphragm-protective ventilation. Am J Respir Crit Care Med. 2020. [DOI] [PMC free article] [PubMed]
- 7.Vaporidi K, Akoumianaki E, Telias I, Goligher EC, Brochard L, Georgopoulos D. Respiratory drive in critically ill patients. Pathophysiology and clinical implications. Am J Respir Crit Care Med. 2020;201(1):20–32. doi: 10.1164/rccm.201903-0596SO. [DOI] [PubMed] [Google Scholar]
- 8.Goligher EC, Jonkman AH, Dianti J, Vaporidi K, Beitler JR, Patel BK, et al. Clinical strategies for implementing lung and diaphragm-protective ventilation: avoiding insufficient and excessive effort. Intensive Care Med. 2020;46(12):2314–2326. doi: 10.1007/s00134-020-06288-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertoni M, Telias I, Urner M, Long M, Del Sorbo L, Fan E, et al. A novel non-invasive method to detect excessively high respiratory effort and dynamic transpulmonary driving pressure during mechanical ventilation. Crit Care. 2019;23(1):346. doi: 10.1186/s13054-019-2617-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gattinoni L, Chiumello D, Caironi P, Busana M, Romitti F, Brazzi L, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46(6):1099–1102. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pickkers P, van der Hoeven H, Citerio G. COVID-19: 10 things I wished I’d known some months ago. Intensive Care Med. 2020;46(7):1449–1452. doi: 10.1007/s00134-020-06098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baydur A, Behrakis PK, Zin WA, Jaeger M, Milic-Emili J. A simple method for assessing the validity of the esophageal balloon technique. Am Rev Respir Dis. 1982;126(5):788–791. doi: 10.1164/arrd.1982.126.5.788. [DOI] [PubMed] [Google Scholar]
- 14.Mauri T, Yoshida T, Bellani G, Goligher EC, Carteaux G, Rittayamai N, et al. Esophageal and transpulmonary pressure in the clinical setting: meaning, usefulness and perspectives. Intensive Care Med. 2016;42(9):1360–1373. doi: 10.1007/s00134-016-4400-x. [DOI] [PubMed] [Google Scholar]
- 15.Akoumianaki E, Maggiore SM, Valenza F, Bellani G, Jubran A, Loring SH, et al. The application of esophageal pressure measurement in patients with respiratory failure. Am J Respir Crit Care Med. 2014;189(5):520–531. doi: 10.1164/rccm.201312-2193CI. [DOI] [PubMed] [Google Scholar]
- 16.Brochard L, Slutsky A, Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med. 2017;195(4):438–442. doi: 10.1164/rccm.201605-1081CP. [DOI] [PubMed] [Google Scholar]
- 17.Doorduin J, Nollet JL, Vugts MP, Roesthuis LH, Akankan F, van der Hoeven JG, et al. Assessment of dead-space ventilation in patients with acute respiratory distress syndrome: a prospective observational study. Crit Care. 2016;20(1):121. doi: 10.1186/s13054-016-1311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Telias I, Junhasavasdikul D, Rittayamai N, Piquilloud L, Chen L, Ferguson ND, et al. Airway occlusion pressure as an estimate of respiratory drive and inspiratory effort during assisted ventilation. Am J Respir Crit Care Med. 2020;201(9):1086–1098. doi: 10.1164/rccm.201907-1425OC. [DOI] [PubMed] [Google Scholar]
- 19.Roussos CS, Macklem PT. Diaphragmatic fatigue in man. J Appl Physiol Respir Environ Exerc Physiol. 1977;43(2):189–197. doi: 10.1152/jappl.1977.43.2.189. [DOI] [PubMed] [Google Scholar]
- 20.Spinelli E, Mauri T, Beitler JR, Pesenti A, Brodie D. Respiratory drive in the acute respiratory distress syndrome: pathophysiology, monitoring, and therapeutic interventions. Intensive Care Med. 2020;46(4):606–618. doi: 10.1007/s00134-020-05942-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bellani G, Grassi A, Sosio S, Foti G. Plateau and driving pressure in the presence of spontaneous breathing. Intensive Care Med. 2019;45(1):97–98. doi: 10.1007/s00134-018-5311-9. [DOI] [PubMed] [Google Scholar]
- 22.Bellani G, Grassi A, Sosio S, Gatti S, Kavanagh BP, Pesenti A, et al. Driving pressure is associated with outcome during assisted ventilation in acute respiratory distress syndrome. Anesthesiology. 2019;131(3):594–604. doi: 10.1097/ALN.0000000000002846. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1 Dead space ventilation.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.