Abstract
Aims
The aim of this study was to determine electrocardiographic (ECG) criteria predicting abnormal infrahissian conduction in patients with myotonic dystrophy type 1 (DM1), as these criteria could be used to identify the need for an electrophysiological study (EPS).
Methods and results
A retrospective multicentre study was conducted including DM1-affected individuals who underwent EPS between 2007 and 2018. For each individual, EPS indication, His-ventricle (HV) interval, resting ECG parameters prior to EPS, left ventricular ejection fraction (LVEF), neurological status, and DM1 DNA analysis results were collected. Electrocardiographic parameters of patients with a normal HV interval were compared with ECG parameters of patients with a prolonged HV interval. Logistic regression was performed to determine predictors for a prolonged HV interval of ≥70 ms on EPS and diagnostic accuracy of ECG parameters was ascertained. Among 100 DM1-affected individuals undergoing EPS, 47 had a prolonged HV interval. The sole presence of a PR interval >200 ms [odds ratio (OR) 8.45, confidence interval (CI) 2.64–27.04] or a QRS complex >120 ms (OR 9.91, CI 3.53–27.80) on ECG were independent predictors of a prolonged HV interval. The combination of both parameters had a positive predictive value of 78% for delayed infrahissian conduction on EPS. His-ventricle interval was independent of DM1 genetic mutation size, neuromuscular status, and LVEF.
Conclusion
The combination of a prolonged PR interval and widened QRS complex on ECG accurately predicts abnormal infrahissian conduction on EPS in patients with DM1. These ECG parameters could be used as a screening tool to determine the need for referral to a specialized multidisciplinary neuromuscular team with EPS capacity.
Keywords: Myotonic dystrophy, Neuromuscular disease, Electrophysiology, Electrocardiogram
What’s new?
The presence of specific electrocardiographic (ECG) conduction abnormalities (PR interval >200 ms or QRS complex >120 ms) are independent predictors of having a prolonged His-ventricle (HV) interval of ≥70 ms on an electrophysiological study (EPS), in patients with the neuromuscular disease myotonic dystrophy type 1 (DM1).
The current study is the first to describe the predictive value of distinct conduction abnormalities, or a combination of specific ECG parameters, for the presence of HV conduction delay in DM1.
When combining individual ECG parameters (PR >200 ms and QRS>120 ms), the positive predictive value for abnormal infrahissian conduction on EPS is 78% in DM1 patients.
The combination of a prolonged PR interval and widened QRS complex on ECG could be used as a simple screening tool for local DM1 management and could help determine the need for referral to a specialized multidisciplinary neuromuscular team with EPS capacity.
Introduction
Myotonic dystrophy type 1 (DM1; also known as Steinert disease) is an autosomal dominantly inherited neuromuscular disease affecting patients of all ages. The genetic basis of DM1 lies in a cytosine–thymine–guanine (CTG) repeat expansion on chromosome 19, with the number of repeats being correlated to disease severity.1 While the main characteristics of DM1 consist of muscle weakness and myotonia (inability to relax muscles), most of the affected individuals die as a result of systemic complications.2 Cardiac involvement is, after pneumonia, the second most frequent cause of mortality.2
Cardiac involvement in DM1 is thought to be the result of myocardial fibrosis and fatty infiltration, leading to conduction disturbances, arrhythmias, and cardiomyopathy.3 On 12-lead electrocardiography (ECG), frequently observed conduction disorders are first degree atrioventricular (AV) blocks (PR interval > 200 ms), ventricular conduction delay (QRS complex >120 ms), and prolonged QTc intervals.4 While conduction disorders in DM1 are generally slowly progressive, faster deterioration has been observed as well.5 In case of progression into higher degree AV block or development of ventricular arrhythmias, these manifestations could lead to sudden cardiac death.
Since conduction disturbances in DM1 are frequently asymptomatic, follow-up of DM1 patients should include regular screening. Despite the need for screening protocols, evidence-based consensus guidelines for the timing, extent and frequency of DM1 cardiac follow-up are lacking. Currently, most centres perform regular ECGs, 24 h rhythm monitoring (Holter) and echocardiographic evaluations. In case of progressive conduction disorders on ECG, or clinical symptomatology such as palpitations, dizziness, or (pre)syncope, an invasive electrophysiological study (EPS) can be considered.
During EPS, His-ventricle (HV) intervals of ≥70 ms are considered an indication for prophylactic pacemaker (PM) implantation in DM1 patients, in order to protect against bradycardia (class of recommendation I, level of evidence B-NR).6–8 While an EPS is the diagnostic test of choice for additional assessment of the cardiac conduction system in DM1, strict indications for performing EPS in DM1 remain unclear.6,7,9 Moreover, as cardiac follow-up in DM1 is frequently carried out in hospitals without DM1 expertise, or even by a neurologist through the performance of annual ECGs, the establishment of clear cardiac management guidelines would be of great value in the daily care of patients with DM1.
In this study, we therefore aim to determine ECG criteria predicting abnormal infrahissian conduction (HV ≥70 ms) in patients with DM1, in order to identify criteria for performing EPS.
Methods
Study population
A retrospective multicentre study was conducted at the Maastricht University Medical Center+ (MUMC+), Maastricht, The Netherlands and Radboud University Medical Center (Radboudumc), Nijmegen, The Netherlands. The MUMC+ and Radboudumc together form the national Myotonic Dystrophy Expertise Center in The Netherlands. The Dutch DM1 patient registry (MYODRAFT study) was used to identify DM1-affected individuals who underwent EPS between 2007 and 2018 at one of both centres. For each individual, the following data were collected: reason for EPS, HV interval, resting ECG parameters prior to EPS, possible PM implantation reason and date, neurological assessment consisting of muscular impairment rating scale (MIRS) score, and DM1 DNA analysis results consisting of the CTG repeat size. Data were compared between patients with a normal HV interval (HV <70 ms) on EPS, and patients with a prolonged HV interval (HV ≥70 ms) on EPS.
Data were collected as part of the Dutch DM1 patient registry (MYODRAFT study) for which written informed consent was obtained. The study was conducted in accordance with the Declaration of Helsinki, and the research protocol was approved by the institutional Medical Ethics Committee (METC 16-4-001, approved on 18 March 2016). All clinical measurements were carried out as part of routine clinical care.
Cardiac assessment
At each yearly visit of DM1 patients, history taking, physical examination, and resting ECG were performed. The presence of cardiac symptoms (palpitations, dyspnoea, dizziness, and syncope) was assessed. Echocardiogram was performed every 3 years, and the last known left ventricular ejection fraction (LVEF) was collected. Holter registration was performed every other year. In case of (progressive) conduction disorders on resting ECG, conduction disorders on Holter monitoring, or clinical symptomatology [palpitations, dizziness, or (pre)syncope], an EPS was performed. The decision whether to perform an EPS was always left to the discretion of the electrophysiologist, and was performed independent of inclusion in the DM1 observational registry.
Electrophysiological study
Electrophysiological study was performed under local anaesthesia via the right femoral vein. A bolus of 2500 IE of Heparin was administered as thromboembolic prophylaxis. Next, a quadripolar electrophysiological catheter was used to map the His-bundle region. The HV-interval was measured with the catheter in a proximal His-position. The HV-interval was defined as the time interval between onset of the His-potential to the onset of the QRS complex on the surface ECG. A HV-interval of ≥70 ms was considered abnormal and considered an indication for PM implantation according to recommendations for DM1 patients.6,8
Electrocardiogram
The last recorded baseline 12-lead ECG, performed at annual cardiac screening in DM1 or at interval check-up due to cardiac complaints, prior to EPS, was collected. All ECGs were evaluated by a qualified electrophysiologist for the following parameters: cardiac rhythm, heart rate in beats per minute, heart axis, PR interval in ms, categorical assessment of AV conduction [normal PR interval (PR ≤200 ms) or prolonged PR interval (PR >200 ms)], and further categorized into 1st degree, 2nd degree Wenckebach, 2nd degree Mobitz II, and 3rd degree AV block. A PR interval of >240 ms was considered a separate category as this has been linked to sudden cardiac death in DM1.10 Furthermore, QRS duration was assessed in ms, and a categorical assessment of QRS duration and morphology was made (narrow in case of QRS ≤120 ms or widened in case of QRS >120 ms). If the QRS complex was widened it was further classified into left anterior hemi block (LAHB), right bundle branch block (RBBB), left bundle branch block (LBBB), or intra-ventricular conduction delay (IVCD). QTc time was evaluated in ms, and categorically assessed as normal, or abnormal in case of QTc ≥450 ms in men or ≥460 ms in women.
Neurological assessment
As standard of care, DM1-affected individuals visit the neurology outpatient clinic each year to determine disease progression and muscle status. In order to define neuromuscular progression at the time of EPS, MIRS scores of the same year were collected. The MIRS score is a disease-specific ordinal five-point rating scale, based on manual muscle testing of 11 muscle groups.11 Myotonic dystrophy type 1-affected individuals with a MIRS score of 1–3, indicating distal muscle weakness, were categorized as having a low MIRS score. Patients with a MIRS score of 4 or 5, indicating proximal muscle weakness, were categorized as having a high MIRS score.
DNA analysis
DNA analysis took place at DM1 diagnosis. All CTG-repeat lengths were determined by analysing DNA extracted from peripheral blood samples through polymerase chain reaction, followed by fragment length analysis and Southern blot analysis.
Data analysis
Statistical analysis was performed using IBM SPSS statistics software version 24 (SPSS Inc., Chicago, IL, USA). The distribution of continuous variables was assessed for normality using Shapiro–Wilk test or Kolmogorov–Smirnov when appropriate and was visually evaluated by inspection of histograms and standardized normal probability plots. Continuous variables are expressed as mean ± standard deviation (SD) or as median with interquartile range (IQR) in case of skewness. Categorical variables are expressed as counts (percentages). Differences between groups were compared using the χ2 test or Fisher’s exact test (categorical data) and the unpaired Student’s t-test or the Mann–Whitney U test (continuous variables).
Univariable binary logistic regression using predefined variables was performed to identify predictors for the presence of a prolonged HV interval of ≥70 ms on EPS. Selected variables consisted of age, gender, PR interval >200 ms on ECG, QRS complex >120 ms on ECG, and having a high MIRS score (MIRS 4–5). Selection of these variables was based on literature and clinical experience of a qualified electrophysiologist with DM1 expertise.5,12 Variables with P < 0.20 on univariable analysis were considered important and were included in the multivariable logistic regression analysis for identification of independent predictors, presented as odds ratios (ORs) with confidence intervals (CIs). Diagnostic accuracy was determined by calculation of sensitivity, specificity, positive predictive values (PPV), negative predictive values (NPV), and by drawing receiver operating characteristic (ROC) curves. Area under the curve (AUC) is presented with corresponding CI. P-values of <0.05 were considered statistically significant.
Results
Study population
A total of 100 patients underwent EPS between 2007 and 2018, of which 90 underwent EPS in the Maastricht University Medical Center+, Maastricht, The Netherlands, and 10 underwent EPS in Radboud University Medical Center, Nijmegen, The Netherlands. Reasons for performing EPS, which were most frequently conduction disturbances on the 12-lead ECG, are displayed in Table 1. Median age of the study population was 49 years old (41–56). Other baseline characteristics of patients are presented in Table 2.
Table 1.
Reasons for performing EPS
| Total (n = 100) | |
|---|---|
| PR interval >200 ms and QRS complex >120 ms on resting ECG | 46 |
| PR interval ≥200 ms on resting ECG | 26 |
| QRS complex >120 ms on resting ECG | 10 |
| Conduction delay on Holter monitoring (with normal ECG) | 6 |
| Conduction delay on Holter monitoring (with abnormal ECG) | 3 |
| Other ECG abnormalities on resting ECG | 5 |
| PR interval >200 ms on resting ECG and cardiac complaints | 2 |
| Recurrent cardiac complaints with normal resting ECG | 2 |
EPS, electrophysiological study; ECG, electrocardiogram.
Table 2.
Baseline characteristics
| Total (n = 100) | Normal HV time <70 ms group (n = 53) | Prolonged HV time ≥70 ms group (n = 47) | P-Value | |
|---|---|---|---|---|
| Age (years), median (IQR) | 49 (41–56) | 49 (40–57) | 50 (42–56) | 0.931 |
| Male, n (%) | 56 (56) | 32 (60) | 24 (51) | 0.350 |
| CTG repeat size, median (IQR) | 200 (150–200) | 190 (126–200) | 200 (150–200) | 0.209 |
| Cardiac symptoms, n (%) | 20 (20) | 12 (23) | 8 (17) | 0.480 |
| Palpitations | 5 (5) | 4 (8) | 1 (2) | |
| (Near) syncope | 9 (9) | 4 (8) | 5 (11) | |
| Dizziness | 5 (5) | 4 (8) | 1 (2) | |
| Other | 1 (1) | 0 (0) | 1 (2) | |
| High MIRS score (4–5), n (%) | 38 (38) | 17 (32) | 21 (45) | 0.231 |
| Normal ECG, n (%) | 8 (8) | 8 (15) | 0 (0) | 0.006 |
| PR interval >200 ms, n (%) | 72 (72) | 31 (58) | 41 (87) | 0.001 |
| PR interval >240 ms, n (%) | 24 (24) | 10 (19) | 14 (30) | 0.202 |
| QRS >120 ms, n (%) | 59 (59) | 21 (40) | 38 (81) | 0.000 |
| LBBB | 22 | 4 | 18 | |
| RBBB | 11 | 5 | 6 | |
| LAHB | 4 | 2 | 2 | |
| IVCD | 31 | 15 | 16 | |
| PR >200 ms and QRS>120 ms, n (%) | 41 (41) | 9 (17) | 32 (68) | 0.000 |
| PR >200 ms and LBBB, n (%) | 18 (18) | 4 (8) | 14 (30) | 0.004 |
| Prolonged QTc, n (%) | 24 (24) | 10 (19) | 14 (30) | 0.202 |
| LVEF, % (SD) | 56 (8) | 56 (8) | 57 (8) | 0.643 |
HV, His-ventricle; IVCD, intraventricular conduction delay; LBBB, left bundle branch block; LAHB, left anterior hemiblock; LVEF, left ventricular ejection fraction; MIRS, muscular impairment rating scale with high MIRS scores (4–5) indicating extensive muscle weakness; RBBB, right bundle branch block.
Of the 100 DM1-affected individuals undergoing EPS, 47 (47%) had a prolonged HV interval of ≥70 ms. In the group of DM1-affected individuals with a prolonged HV time, there was a higher frequency of prolonged PR intervals (87% vs. 58%, P = 0.001, Table 2) and a higher frequency of prolonged QRS duration (81% vs. 40%, P < 0.001, Table 2) on 12-lead ECG. In our cohort, prolonged PR intervals of >240 ms were uncommon in patients with a prolonged HV interval (30%). Yet, the combination of a prolonged PR and widened QRS complex was more frequently observed in the group of individuals with a prolonged HV interval at EPS (68% vs. 17%, P < 0.001, Table 2). Specifically, the combination of a prolonged PR interval and the presence of LBBB was more common in individuals with a prolonged HV interval (30% vs. 8%, P = 0.004, Table 2).
All 47 patients with HV intervals of ≥70 ms were referred for direct PM implantation after EPS. In the group of patients with normal HV intervals (<70 ms), 10 of the 53 patients required PM implantation during follow-up. Reasons for PM implantation during follow-up consisted of deterioration of conduction delay on ECG or recurrent syncope symptoms. EPS was not repeated in these cases.
Eight patients undergoing EPS had a normal resting ECG and underwent the procedure due to cardiac complaints or conduction abnormalities on Holter registration. All of these eight patients had a normal HV interval (Table 2).
There was no statistical difference in age (49 vs. 50 years, P = 0.931), CTG repeat size (190 vs. 200 repeats, P = 0.209), and frequency of high MIRS scores (32% vs. 45%, P = 0.231) between individuals with a normal or prolonged HV time (Table 2). Moreover, there was no relationship between LVEF and HV time, as LVEF was comparable between both groups (56% vs. 57%, P = 0.643).
Prolonged His-ventricle interval on electrophysiological study
Logistic regression analysis was performed to assess the impact of pre-defined resting ECG predictors on having a prolonged HV interval of ≥70 ms on EPS (Table 3). The multiple logistic regression model contained two independent variables (PR interval >200 ms and QRS complex >120 ms on ECG). As shown in Table 3, both independent variables made a statistically significant contribution to the model [PR interval >200 ms (OR 8.45, CI 2.64–27.04) and QRS complex >120 ms (OR 9.91, CI 3.53–27.80) on resting ECG].
Table 3.
Binary logistic regression analysis for occurrence of prolonged HV interval on EPS
| Univariate |
Multivariate |
|||||
|---|---|---|---|---|---|---|
| OR | CI | P-Value | OR | CI | P-Value | |
| Age | 1.01 | 0.97–1.05 | 0.59 | |||
| Gender | 0.69 | 0.31–1.52 | 0.35 | |||
| PR interval >200 ms | 4.85 | 1.76–13.40 | 0.002 | 8.45 | 2.64–27.04 | 0.000 |
| QRS complex >120 ms | 6.43 | 2.59–16.01 | 0.000 | 9.91 | 3.53–27.80 | 0.000 |
| High MIRS score (4–5) | 1.67 | 0.72–3.85 | 0.23 | |||
A prolonged HV interval was defined as ≥70 ms on electrophysiological study.
CI, confidence interval; EPS, electrophysiological study; HV, His-ventricle; MIRS, muscular impairment rating scale with high MIRS scores (4–5) indicating extensive muscle weakness; OR, odds ratio.
Diagnostic accuracy
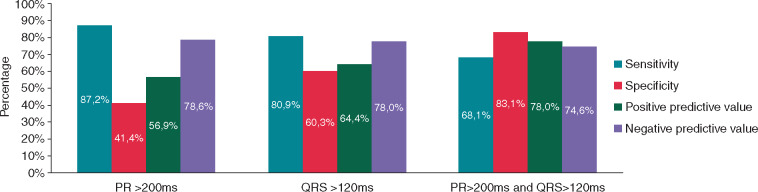
The diagnostic accuracy of three variables predicting a prolonged HV interval on EPS was determined: PR interval >200 ms on resting ECG, QRS complex of >120 ms on resting ECG, and the combination of both (Figure 1). For a prolonged PR interval (>200 ms), the PPV and NPV were 56.9% and 78.6%, respectively. For a widened QRS complex (>120 ms), the PPV and NPV were 64.4% and 78.0%, respectively. The combination of both ECG criteria (PR interval >200 ms and QRS complex >120 ms) had a sensitivity of 68.1% and a specificity of 83.1%, with corresponding PPV and NPV values of 78.0% and 74.6%, respectively. Receiver operating characteristic curve analysis of the combination of ECG criteria (PR interval >200 ms and QRS complex >120 ms) demonstrated an AUC of 0.79 (CI 0.70–0.88).
Figure 1.
Diagnostic accuracy of ECG parameters. The grouped bar chart gives an overview of diagnostic accuracy of specific ECG abnormalities in patients with myotonic dystrophy type 1, for the prediction of abnormal infrahissian conduction (≥70 ms) on electrophysiological study. ECG, electrocardiographic.
Discussion
In a population of 100 individuals with genetically confirmed DM1, we demonstrated that either the presence of a PR interval >200 ms or the presence of a QRS complex >120 ms on ECG were independent predictors of having a prolonged HV interval of ≥70 ms on EPS. When combined, these ECG parameters have a high PPV for the presence of delayed infrahissian conduction (HV ≥70 ms) on EPS.
Prevalence of electrocardiographic abnormalities and prolonged His-ventricle intervals
Electrocardiographic abnormalities are a common phenomenon in DM1, as PR prolongation is described in 28–45% of patients, and widened QRS complexes are observed on 17–20% of surface ECGs.4,10,13 In the current study, ECG abnormalities were even more prevalent and only eight DM1 patients had a normal ECG prior to EPS. This corresponds with the fact that ECG abnormalities were the main reasons for performing EPS in our cohort.
When comparing patients with a normal HV interval with patients with a prolonged HV interval, we observed LBBB to be more frequently present (combined with first degree AV block) in DM1 patients with a HV interval ≥70 ms. This observation seems to be in accordance with the 2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients With Bradycardia and Cardiac Conduction Delay, describing that the observation of a LBBB on ECG markedly increases the likelihood of distal conduction disturbances and underlying structural heart disease.8
In the general DM1 population, HV intervals have been reported to be abnormal in 43–54% of cases.7,12,14 When looking at DM1 patients with clinical symptoms and/or conduction disturbances on ECG; however, percentages up to 94% have been described.13,15,16 Since the selection criteria for the performance of an EPS differed among studies and was usually influenced by expert opinion, these results might not be representative for the entire DM1 population.13,15,16
The role of electrocardiographics as a predictor of prolonged His-ventricle intervals
Infrahissian conduction is unstable over time and has the tendency to increase in DM1 patients.5,17 Simultaneously, the number of ECG abnormalities seems to increase with patients’ age, suggesting a time-dependent degenerative process.18 Still, the rate of cardiac progression seems to be variable among DM1 patients.5,17 Previously, ECG abnormalities have been found to be indicative of cardiac conduction system disease and have been linked to autopsy findings, such as cardiac fibrosis, fatty infiltration, and atrophy.10,12,15 As a result, the usefulness of ECGs as a predictive tool to determine the need and appropriate timing for an invasive measure of the conduction system has been previously researched.5,12 A study of 39 consecutive DM1 patients demonstrated that the PPV of an abnormal ECG prior to EPS was 65.2% in predicting a prolonged HV interval.12 In a study evaluating the effect of prophylactic PM implantation in 100 DM1 patients, the PPV of ECG abnormalities prior to EPS was 66%.7 Although the role of ECG abnormalities were taken into account in other EPS studies in DM1,13,14,16 the current study is the first to describe the diagnostic value of distinct and combined ECG parameters, for the presence of a prolonged HV interval.
PR intervals >240 ms have been associated with an increased risk of sudden cardiac death10 and the same PR interval cut-off value is described in the 2018 ACC/AHA/HRS Guidelines as an indicator for possible prophylactic pacing.8 Remarkably, we did not observe PR intervals >240 ms to be more frequently present in patients with a prolonged HV interval. Based on the data in the current study, a cut-off value of 240 ms as an indicator for performance of an EPS may therefore be too high, specifically when other conduction disturbances are already present.
Despite the possible role of ECGs in determining the need for EPS, it has also been reported that DM1 patients with normal ECGs may have infrahissian conduction abnormalities.14,15 In our study, eight patients had a normal resting ECG and all of these patients had HV intervals <70 ms.
Relationship between electrocardiographic abnormalities and myotonic dystrophy type 1 severity
Several studies have suggested a relationship between the size of the CTG repeat expansion and the extent of cardiac involvement, and between the degree of neuromuscular and cardiac involvement in DM1.4 In the current study, however, we did not find a difference in MIRS score and mean CTG repeat size between patients with a normal HV interval and patients with a prolonged HV interval. Moreover, it is known that the length of the CTG repeat is instable and may increase over time in the same individual, making it difficult to correlate CTG repeat size with disease severity at a given time point.19 Importantly, it has also been reported that DM1 patients with small sized CTG repeat expansions are at increased risk of severe cardiac conduction abnormalities, making cardiac follow-up essential for DM1 patients across the entire range of DM1-related CTG repeat lengths.20
Clinical implications
While the 2018 ACC/AHA/HRS Guidelines recommend PM implantation in case of HV intervals of ≥70 ms in neuromuscular patients,8 the 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy merely describe that PM implantation might be considered in case of a prolonged HV interval.9 In neither guideline, recommendations for the timing of EPS are addressed, making it difficult to determine when EPS is necessary in clinical practice. Even though specialized neuromuscular centres are available, practical limitations, such as disabling muscle weakness, travel distance, and lack of motivation in DM1 patients make local cardiac management a necessity. Hence, the utility of ECGs as a fast and accessible screening tool is of great value, especially if specific ECG parameters can be used to determine the need for referral. As demonstrated by the data in this study, the PR interval and QRS complex could play an important role in the assessment of screening ECGs in DM1. Due to the high PPV of the combined parameters, referral to a hospital with a multidisciplinary DM1 team and EPS capacity should be considered when both ECG abnormalities are present. Most likely, such guiding ECG criteria could significantly increase DM1 quality of care, as early PM implantation can protect against complete AV block and sudden cardiac death.6,10
Since the 2018 ACC/AHA/HRS Guidelines also state that the recommendations for other neuromuscular disorders are similar to recommendations in DM1, these screening ECG parameters may be useful in the management of other neuromuscular diseases.8 Nevertheless, it is important to note that the absence of the combination of a prolonged PR interval and widened QRS complex should not indicate that referral to a specialized centre is unnecessary, specifically when symptoms or progressive conduction disorders are present.
The results of this study raise the question whether PM implantation could take place without prior performance of EPS in the future. While avoiding an invasive measure in a group of vulnerable patients could be beneficial, a PPV of 78% would also cause overtreatment. Thus, in order to consider direct PM implantation based on ECG conduction abnormalities, we believe that the suggested ECG criteria should be validated in a prospective study including patients with normal resting ECGs.
Limitations
The main limitations of this study consist of its retrospective nature and the fact that included DM1-affected individuals had already been selected for EPS by an electrophysiologist, introducing potential selection bias. Consequently, our study may have overestimated the predictive value of ECG parameters, specifically when comparing these results to the general DM1 population without conduction abnormalities on their ECG. Furthermore, there was a difference in the amount of EPS performed at both participating centres. As the decision whether to perform an EPS in DM1 was left to the discretion of an experienced electrophysiologist, this suggests a difference in local opinions, possibly affecting the outcomes of this study. Yet again, these different managing approaches stress the need for specific guidelines in DM1.
Conclusions
This retrospective study demonstrates the predictive value of specific ECG conduction abnormalities in a group of 100 genetically confirmed DM1 patients. The combination of a prolonged PR interval and widened QRS complex on ECG, accurately predicts abnormal infrahissian conduction on EPS. Therefore, these criteria could be used as a screening tool in clinical practice in order to select patients for referral to a multidisciplinary neuromuscular team with EPS capacity. As the presence of a prolonged HV interval was independent of DM1 genetic mutation size, neuromuscular status and last recorded LVEF, ECG abnormalities should be taken seriously in any DM1 patient. Finally, there is a need for DM1-specific consensus guidelines on the timing, extent, and frequency of DM1 cardiac follow-up.
Acknowledgements
We would like to thank Suzanne Philippens and Janneke Schouten for their support in data collection.
Funding
The Dutch DM1 patient registry (MYODRAFT study) was funded by the Prinses Beatrix Spierfonds (project number W.OR15-25).
Conflict of interest: none declared.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Brook JD, McCurrach ME, Harley HG, Buckler AJ, Church D, Aburatani H. et al. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3' end of a transcript encoding a protein kinase family member. Cell 1992;68:799–808. [DOI] [PubMed] [Google Scholar]
- 2. de Die-Smulders C, Howeler CJ, Thijs C, Mirandolle JF, Anten HB, Smeets HJ. et al. Age and causes of death in adult-onset myotonic dystrophy. Brain 1998;121:1557–63. [DOI] [PubMed] [Google Scholar]
- 3. Pelargonio G, Dello RA, Sanna T, De MG, Bellocci F.. Myotonic dystrophy and the heart. Heart 2002;88:665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Petri H, Vissing J, Witting N, Bundgaard H, Kober L.. Cardiac manifestations of myotonic dystrophy type 1. Int J Cardiol 2012;160:82–8. [DOI] [PubMed] [Google Scholar]
- 5. Lallemand B, Clementy N, Bernard-Brunet A, Pierre B, Corcia P, Fauchier L. et al. The evolution of infrahissian conduction time in myotonic dystrophy patients: clinical implications. Heart 2012;98:291–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lazarus A, Varin J, Babuty D, Anselme F, Coste J, Duboc D.. Long-term follow-up of arrhythmias in patients with myotonic dystrophy treated by pacing: a multicenter diagnostic pacemaker study. J Am Coll Cardiol 2002;40:1645–52. [DOI] [PubMed] [Google Scholar]
- 7. Laurent V, Pellieux S, Corcia P, Magro P, Pierre B, Fauchier L. et al. Mortality in myotonic dystrophy patients in the area of prophylactic pacing devices. Int J Cardiol 2011;150:54–8. [DOI] [PubMed] [Google Scholar]
- 8. Kusumoto FM, Schoenfeld MH, Barrett C, Edgerton JR, Ellenbogen KA, Gold MR. et al. ACC/AHA/HRS Guideline on the Evaluation and Management of Patients With Bradycardia and Cardiac Conduction Delay: executive Summary: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines, and the Heart Rhythm Society. J Am Coll Cardiol 2019;74:932–87. [DOI] [PubMed] [Google Scholar]
- 9. Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA. et al. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the task force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Europace 2013;15:1070–118. [DOI] [PubMed] [Google Scholar]
- 10. Groh WJ, Groh MR, Saha C, Kincaid JC, Simmons Z, Ciafaloni E. et al. Electrocardiographic abnormalities and sudden death in myotonic dystrophy type 1. N Engl J Med 2008;358:2688–97. [DOI] [PubMed] [Google Scholar]
- 11. Mathieu J, Boivin H, Meunier D, Gaudreault M, Begin P.. Assessment of a disease-specific muscular impairment rating scale in myotonic dystrophy. Neurology 2001;56:336–40. [DOI] [PubMed] [Google Scholar]
- 12. Babuty D, Fauchier L, Tena-Carbi D, Poret P, Leche J, Raynaud M. et al. Is it possible to identify infrahissian cardiac conduction abnormalities in myotonic dystrophy by non-invasive methods? Heart 1999;82:634–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wahbi K, Meune C, Porcher R, Becane HM, Lazarus A, Laforet P. et al. Electrophysiological study with prophylactic pacing and survival in adults with myotonic dystrophy and conduction system disease. JAMA 2012;307:1292–301. [DOI] [PubMed] [Google Scholar]
- 14. Simeon E, Patier-Dussauge A, Bernard-Brunet A, Clementy N, Gouraud JB, Guyomarch B. et al. Insufficiency of electrocardiogram alone in predicting infrahisian abnormalities in patients with type 1 myotonic dystrophy. Int J Cardiol 2014;172:625–7. [DOI] [PubMed] [Google Scholar]
- 15. Lazarus A, Varin J, Ounnoughene Z, Radvanyi H, Junien C, Coste J. et al. Relationships among electrophysiological findings and clinical status, heart function, and extent of DNA mutation in myotonic dystrophy. Circulation 1999;99:1041–6. [DOI] [PubMed] [Google Scholar]
- 16. Brembilla-Perrot B, Luporsi JD, Louis S, Kaminsky P.. Long-term follow-up of patients with myotonic dystrophy: an electrocardiogram every year is not necessary. Europace 2011;13:251–7. [DOI] [PubMed] [Google Scholar]
- 17. Prystowsky EN, Pritchett EL, Roses AD, Gallagher J.. The natural history of conduction system disease in myotonic muscular dystrophy as determined by serial electrophysiologic studies. Circulation 1979;60:1360–4. [DOI] [PubMed] [Google Scholar]
- 18. Groh WJ, Lowe MR, Zipes DP.. Severity of cardiac conduction involvement and arrhythmias in myotonic dystrophy type 1 correlates with age and CTG repeat length. J Cardiovasc Electrophysiol 2002;13:444–8. [DOI] [PubMed] [Google Scholar]
- 19. Meola G, Cardani R.. Myotonic dystrophies: an update on clinical aspects, genetic, pathology, and molecular pathomechanisms. Biochim Biophys Acta 2015;1852:594–606. [DOI] [PubMed] [Google Scholar]
- 20. Denicourt M, Pham MT, Mathieu J, Breton R.. DM1 patients with small CTG expansions are also at risk of severe conduction abnormalities. J Neuromuscular Diseases 2015;2:99–105. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.