Abstract
Aims
Rate adaptation of the action potential ensures spatial heterogeneities in conduction across the myocardium are minimized at different heart rates providing a protective mechanism against ventricular fibrillation (VF) and sudden cardiac death (SCD), which can be quantified by the ventricular conduction stability (V-CoS) test previously described. We tested the hypothesis that patients with a history of aborted SCD due to an underlying channelopathy or cardiomyopathy have a reduced capacity to maintain uniform activation following exercise.
Methods and results
Sixty individuals, with (n = 28) and without (n = 32) previous aborted-SCD event underwent electro-cardiographic imaging recordings following exercise treadmill test. These included 25 Brugada syndrome, 13 hypertrophic cardiomyopathy, 12 idiopathic VF, and 10 healthy controls. Data were inputted into the V-CoS programme to calculate a V-CoS score that indicate the percentage of ventricle that showed no significant change in ventricular activation, with a lower score indicating the development of greater conduction heterogeneity. The SCD group, compared to those without, had a lower median (interquartile range) V-CoS score at peak exertion [92.8% (89.8–96.3%) vs. 97.3% (94.9–99.1%); P < 0.01] and 2 min into recovery [95.2% (91.1–97.2%) vs. 98.9% (96.9–99.5%); P < 0.01]. No significant difference was observable later into recovery at 5 or 10 min. Using the lowest median V-CoS scores obtained during the entire recovery period post-exertion, SCD survivors had a significantly lower score than those without for each of the different underlying aetiologies.
Conclusion
Data from this pilot study demonstrate the potential use of this technique in risk stratification for the inherited cardiac conditions.
Keywords: Sudden cardiac death, Ventricular conduction stability, Risk stratification, Idiopathic ventricular fibrillation, Brugada syndrome, Hypertrophic cardiomyopathy
What’s new?
Survivors of aborted sudden cardiac arrest develop greater spatial heterogeneities in conduction and repolarization than those without following modulation in autonomic tone and heart rate.
We assess the ventricular conduction stability (V-CoS) test in its ability to discriminate between individual at high risk and low risk of future ventricular arrhythmias in a range of inherited cardiac conditions (ICCs).
The V-CoS test detects abnormalities in cardiac conduction following exercise and has the potential to improve on current risk stratification techniques in the ICCs.
Introduction
Arrhythmogenicity in the inherited cardiac conditions (ICCs) begins with the inheritance or spontaneous acquisition of one or several genetic mutations. These consequentially translate into channelopathies or cardiomyopathies that impair the propagation and physiological rate-adaptive responses of the action potential, affecting the recovery of excitability across the ventricles which predict ventricular fibrillation (VF).1–4 This may be further modulated by heart rate or changes in autonomic tone which have previously been observed to play a critical role in the development of arrhythmias in the ICCs.5–8
We developed and described the ventricular conduction stability (V-CoS) test previously as a method to quantify the alterations in whole heart activation patterns following exercise in vivo.9 We previously demonstrated the utility of this test as a potential marker of risk, although it remained unclear if this could be applied as an adjunctive discriminator for the different ICCs and those with an idiopathic cause of VF. In this study, we validate the V-CoS test in a larger cohort of patients with different ICCs and test the hypothesis that SCD survivors, irrespective of the underlying genetic abnormality, have an abnormal rate-adaptive response resulting in greater spatial heterogeneities in conduction developing following exertion than those without a previous history of aborted SCD.
Methods
Study population
For this study, patients with and without a previous aborted sudden cardiac death (SCD) or equivalent event with different ICCs were recruited. Patients meeting criteria for a diagnosis of Brugada syndrome (BrS), hypertrophic cardiomyopathy (HCM), or an idiopathic cause ventricular fibrillation/tachycardia (iVF/VT) were identified and enrolled.7 Patients with structurally normal hearts with no history of syncope or family history of sudden death/ICC undergoing electrophysiological studies and required electro-cardiographic imaging (ECGi) mapping for ventricular ectopy or supra-ventricular arrhythmias were also studied as a control group. Individuals with an aborted SCD or equivalent event were defined as those requiring resuscitation following a cardiac arrest or an appropriate shock from their ICD.
Study protocol
Enrolled patients had the non-invasive ECGi vest fitted and secured, before undergoing exercise treadmill and a non-contrast computed tomography (CT) chest on the same day. The Bruce protocol was employed and stopped when maximal exertion was achieved. This was defined as reaching and sustaining maximum target heart rate adjusted for age, or cessation owing to fatigue after achieving a minimum of 85% of their maximum target heart rate. Patients were immediately returned to the supine position where ECGi recordings were then performed over a 10-min recovery period, to eliminate interference, movement, and motion artefact. Subjects were excluded from analysis if they were unable to perform or complete exercise treadmill test protocol on the day of ECGi recording. The study protocol was reviewed and approved by the National Research Ethics Committee—London (ref: 14/LO/1318).
The body surface electrogram (EGM) and reconstructed unipolar epicardial EGM signal data obtained over this period was subsequently extracted from the ECGi system and analysed with the V-CoS programme (Figure 1). The torso, ventricles, tricuspid and mitral valve, and left anterior descending artery geometry were also segmented from the cardiac CT scan using the EcVUE user interface within the ECGi system software. Data encoding the torso and ventricular shell, valves, and coronary arteries was also extracted and processed with the V-CoS programme.
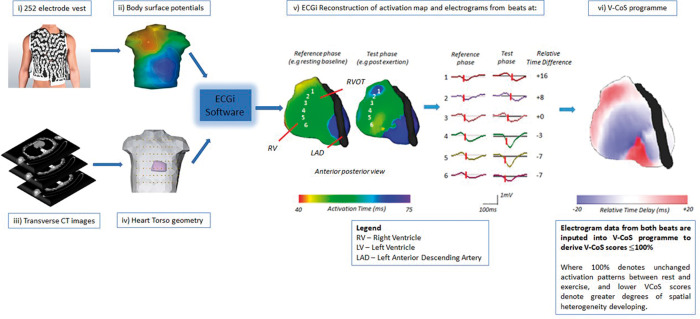
Figure 1.
Body surface potential data are obtained from the EcVue 252 electrode vest and are combined with heart-torso geometry obtained from patient’s CT thorax (i–iv). ECGi reconstructs >1200 unipolar electrograms over the cardiac surface and three-dimensional activation maps of beats obtained at peak exertion and the reference baseline following exercise treadmill testing (v). Electrogram data are inputted into the V-CoS programme that produces a score (0–100%) to indicate the development of spatial heterogeneities in conduction (vi). CT, computed tomography; ECGi, electro-cardiographic imaging; LAD, left anterior descending artery; LV, left ventricle; RV, right ventricle; V-CoS, ventricular conduction stability.
Electro-cardiographic imaging and signal processing with the ventricular conduction stability test
The EcVUE system (Medtronic Inc., USA) was used for ECGi processing. This involved body surface potential data obtained via a 252-electrode vest which was combined with patient-specific heart-torso geometry derived from a thoracic CT scan (Figure 1and Supplementary material online, Figure S1). Using inverse solution mathematical algorithms, the ECGi system reconstructed epicardial unipolar EGMs and panoramic activation maps over a single sinus beat which were visualized on a digitized image of the patient’s heart on EcVUE system user interface.
The V-CoS programme has been described in detail previously.9 Briefly, the programme allows the rapid and automated comparison of ventricular EGM data and activation patterns between two different beats—one from a reference phase (e.g. resting baseline) and the other from a test phase (e.g. peak exertion). Differences in local activation timings (LATs) between the two phases were calculated for every EGM with a spatial point over the heart surface or mesh created by the ECGi system (fuller description under Supplementary material online, Methods).
To provide a measure of conduction stability or a surrogate measure of an appropriate rate-adaptive response, a V-CoS score was automatically derived. This indicated the percentage of epicardial EGMs across the ventricular surface where no significant changes in LAT (<10 ms) occurred between the reference and test phases. A higher percentage or score denoted greater conduction stability or a normal rate adaptive mechanism. Examples of reconstructed ECGi activation and V-CoS maps for each subgroup of study patients are shown in Supplementary material online, Figure S2.
Data analysis
Electrograms were analysed at peak exertion (defined as within a minute of cessation of exercise), and during recovery at 2, 5, and 10 min. Calculation of V-CoS scores was determined with reference to the end of the 10-min recovery period and were based on detecting differences or changes in LAT at each point on the ventricular surface that was in excess of 10 ms as previously described.
Receiver operating characteristic (ROC) analysis and graphs were calculated to assess the diagnostic performance of the V-CoS test, with the area under the curve expressed as the C-statistic. This was based on the minimum V-CoS score achieved by each individual at all test phases. The Youden index, defined as sensitivity + specificity − 1.00, was then calculated for all points on the ROC curve.10 The maximum value of the index was used as the criterion for selecting a cut-off point or threshold to denote optimal sensitivity and specificity.
Assessment of sudden cardiac death risk with conventional risk stratification techniques
In patients with BrS, a prior history of syncope and the presence of a spontaneous Type I BrS pattern as defined previously was ascertained at enrolment,16 or before the SCD event in survivors to ascertain the predictive value of these risk markers. Individuals were then grouped into three categories according to the presence these factors. High risk—denoting the presence of both syncope and spontaneous type I pattern; intermediate risk—the presence of either syncope or spontaneous Type I pattern; low risk—the absence of either.
For patients with HCM, the ESC 5-year SCD risk score was calculated for each individual.11 This was performed retrospectively in study patients 16 years or older based on data obtained on initial evaluation. To assess the ESC risk score’s predictive value in those who first presented with an aborted SCD event to our institute, a prior history of syncope was considered present if occurred before their presenting SCD event. All other clinical parameters were obtained based on subsequent work up. High-risk individuals were deemed as those having a 5-year risk of >6%; intermediate risk—4 to 6%; low risk— <4%.12
Statistical analysis
Normality testing was performed using the D'Agostino–Pearson test. For non-normally distributed variables, the Kruskal–Wallis test (or Friedman’s test for repeated measures) was used for comparison of three of more groups. For post hoc analysis, the Mann–Whitney test was employed for comparison between two groups with Dunn’s correction where multiple comparisons were required. Statistical analysis was performed using GraphPad PRISM v5 (GraphPad Software Inc., USA), and a P value of <0.05 was considered significant.
Results
Study group characteristics
The V-CoS test was applied to 28 patients with a previously aborted SCD event (mean age 40 ± 11 years, 24 males) and 32 patients without a previous SCD event (mean age 44 ± 12 years, 21 males) who underwent exercise treadmill testing with the ECGi vest. The SCD group comprised of 12 patients with an idiopathic cause of sustained VF/VT, 10 with BrS, and 6 with HCM. In the non-SCD group, 15 BrS patients, 7 HCM patients, and 10 control patients with structurally normal hearts. A summary of clinical characteristics of these different subgroups is summarized in Supplementary material online, Tables S1–S4. In three patients (with previous aborted SCD), anti-arrhythmic therapy was not discontinued prior to the study protocol for clinical reasons.
Sudden cardiac death vs. non-sudden cardiac death group
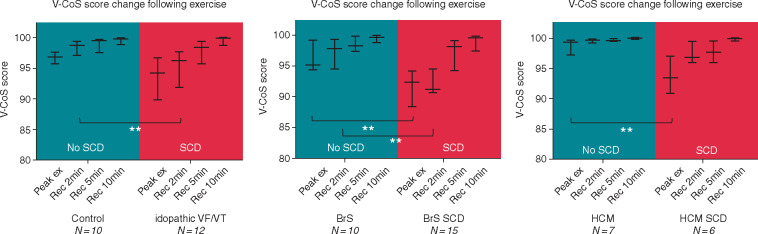
Following peak exertion in both groups, a gradual increase in V-CoS scores (median, interquartile range) could be observed over the 10-min recovery period [non-SCD group: 97.3% (94.9–99.1%) to 99.8% (99.1–100%), P < 0.001; SCD group: 92.8% (89.8–96.3%) to 99.8% (98.7–100%), P < 0.001] (Figure 2). In the early recovery period, V-CoS scores were observed to be significantly lower in the SCD than non-SCD group immediately following peak exertion [92.8% (89.8–96.3%) vs. 97.3% (94.9–99.1%), P = 0.03] and at 2 min [95.2% (91.1–97.2%) vs. 98.9% (96.9–99.5%), P < 0.01]. No significant differences between the groups could be observed by 5 and 10 min into recovery (Table 1). There were no significant differences in heart rate between groups at each stage of recovery (Table 1).
Figure 2.
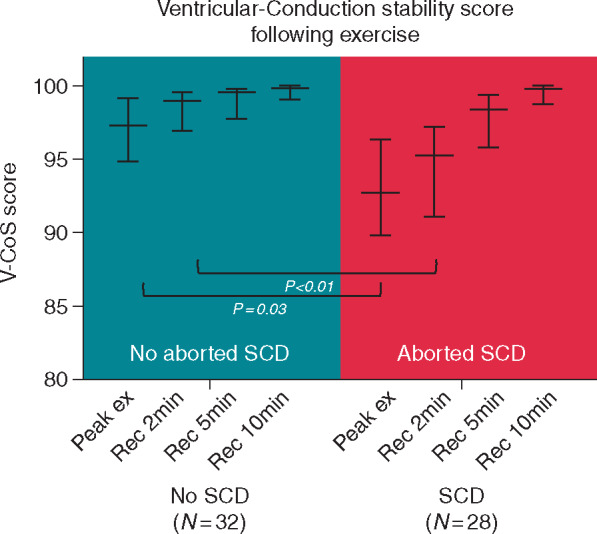
Median and interquartile V-CoS scores following exertion in SCD and non-SCD groups. SCD, sudden cardiac death; V-CoS, ventricular conduction stability.
Table 1.
Summary of median (interquartile range) V-CoS scores and heart rate at each stage of recovery post-exertion
| Non-SCD (n = 32) | SCD (n = 28) | P-value* | |
|---|---|---|---|
| V-CoS scores | |||
| Peak exertion | 97.3% (94.9–99.1%) | 92.8% (89.8–96.3%) | P < 0.05 |
| Recovery 2 min | 98.9% (96.9–99.5%) | 95.2% (91.1–97.2%) | P < 0.01 |
| Recovery 5 min | 99.5% (97.8–99.8%) | 98.4% (95.8–99.3%) | P = NS |
| Recovery 10 min | 99.8% (99.1–100%) | 99.8% (98.7–100%) | P = NS |
| Heart rate | |||
| Peak exertion | 137 (120–153) | 138 (129–155) | P = NS |
| Recovery 2 min | 94 (88–108) | 101 (90–112) | P = NS |
| Recovery 5 min | 90 (80–99) | 91 (84–96) | P = NS |
| Recovery 10 min | 88 (78–95) | 87 (83–98) | P = NS |
NS, not significant; SCD, sudden cardiac death; V-CoS, ventricular conduction stability.
Dunn’s multiple comparison test was applied.
Ventricular conduction stability scoring within the different subgroups
Subgroup analysis was also performed between SCD and non-SCD patients according to underlying aetiology. A similar pattern of recovery of V-CoS scores in SCD and non-SCD patients could be observed in all three subgroups: (i) iVF/VT vs. controls, (ii) BrS-SCD vs. BrS, and (iii) HCM-SCD vs. HCM (Figure 3and Supplementary material online, Figure S3).
Figure 3.
Trend of V-CoS scores post-exertion in SCD and non-SCD patients in the different subgroups of patients (i) iVF/VT, (ii) BrS, and (iii) HCM. Median and interquartile ranges values are shown. *P < 0.05, **P < 0.01, ***P < 0.001. BrS, Brugada syndrome; HCM, hypertrophic cardiomyopathy; iVF, idiopathic ventricular fibrillation; SCD, sudden cardiac death; V-CoS, ventricular conduction stability; VT, ventricular tachycardia.
Those with previous aborted SCD events were found to have a lower V-CoS score than non-SCD patients in the early stages of recovery for all three subgroups. In the first subgroup, iVF/VT survivors had lower median scores than controls following peak exertion (94.2% vs. 96.8%, P = 0.06) with a significant difference on recovery at 2 min (96.3% vs. 98.8%, P = 0.006). No significant differences were observed at 5 and 10 min. In the BrS group, a significant difference between SCD and non-SCD patients was also observed immediately following peak exertion (92.3% vs. 95.1%, P = 0.009) and at 2 min post-recovery (91.3% vs. 97.8%, P = 0.001) but not at the other stages. In the HCM group, a significant difference between the SCD and non-SCD patients was only observed following peak exertion (93.4% vs. 99.3%, P = 0.0047).
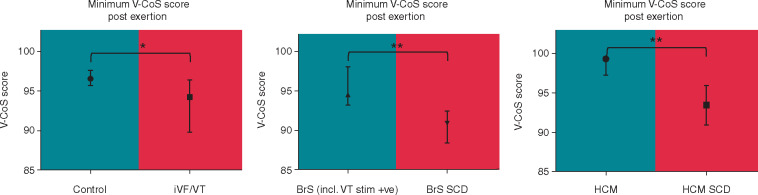
As the time point during recovery at which conduction abnormalities occurred could have varied for the different ICCs and between individuals, we analysed the lowest V-CoS scores obtained throughout the whole recovery period for each person and pooled their scores according to their subgroups (Figure 4 and Supplementary material online, Figure S4). The median minimal V-CoS scores post-exertion were found to be significantly lower in patients with a previously aborted SCD event than those without for all three subgroups (iVF vs. controls: 94.2% vs. 96.5%, P = 0.03) (BrS-SCD vs. BrS: 90.9% vs. 94.5%, P = 0.004) (HCM-SCD vs. HCM: 93.4% vs. 99.2%, P = 0.004). Although the differences were statistically significant, it was observed that there was a degree of overlap between the iVF subjects and controls (Supplementary material online, Figure S4), highlighting the likely heterogeneity in the electrophysiological substrate of the iVF group.
Figure 4.
Comparison of minimum V-CoS scores obtained post-exertion between SCD and non-SCD patients in the different subgroups. Median and interquartile ranges values are shown. *P < 0.05, **P < 0.01, ***P < 0.001. BrS, Brugada syndrome; HCM, hypertrophic cardiomyopathy; iVF, idiopathic ventricular fibrillation; SCD, sudden cardiac death; V-CoS, ventricular conduction stability; VT, ventricular tachycardia.
Predictive performance of ventricular conduction stability and comparison to current risk stratification
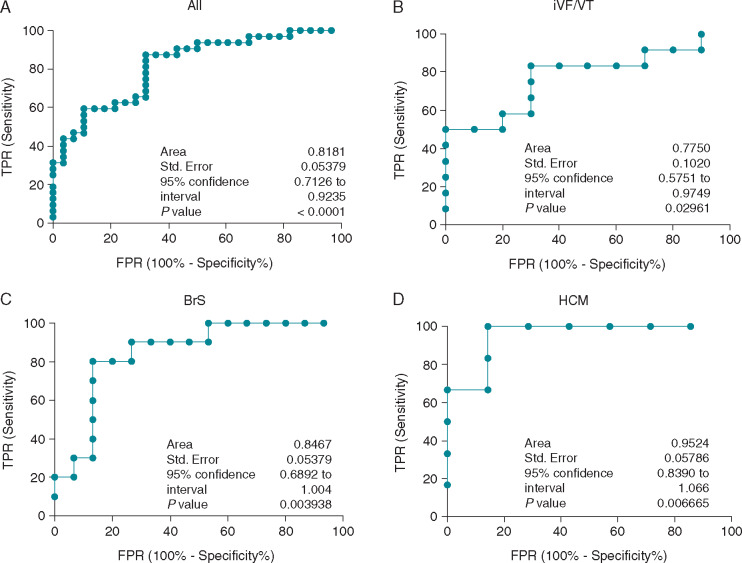
Area under the ROC curve (C-statistic) was 0.82 (standard error ±0.05) when applied to all patients in this study. The C-statistic ranged from 0.79 to 0.93 when it was applied to each of the different subgroups as shown in Figure 5. Based on the Youden index, a cut-off minimum V-CoS score at 93.9% provided a sensitivity and specificity of 87.5% and 67.9%, respectively when applied to all patients. In the BrS group, a cut-off minimum score at 92.3% derived from Youden index, provided a sensitivity and specificity of 80.0% and 86.7%, respectively. In HCM, a threshold at 97.0% provided a sensitivity and specificity at 100% and 85.7%, respectively. A Youden index derived threshold of 96.4% in iVF/VT provided a sensitivity and specificity of 83.3% and 70.0%, respectively.
Figure 5.
ROC curves showing the predictive performance of the V-CoS test when applied to (A) all patients, (B) iVF/VT and structurally normal hearts, (C) Brugada syndrome, and (D) hypertrophic cardiomyopathy. BrS, Brugada syndrome; FPR, false positive rate; HCM, hypertrophic cardiomyopathy; iVF, idiopathic ventricular fibrillation; ROC, receiver operating characteristics; SCD, sudden cardiac death; TPR, true positive rate; V-CoS, ventricular conduction stability; VT, ventricular tachycardia.
As an exploratory study of the small subgroups of BrS and HCM patients, V-CoS testing was compared to conventional markers or risk. In the BrS cohort, conventional risk stratification based on the presence of a spontaneous Type I BrS pattern and syncope identified 2 out of 10 (20%) of the patients with aborted SCD events as high risk. Most of these patients would have been identified as low risk given the absence of these parameters. With V-CoS testing, a threshold of 92.3% identified 80% of the SCD cohort as high risk (Figure 6). In the HCM subgroup, the C-statistic for the V-CoS test (0.95) was 0.14 higher than C-statistic of the ESC risk score calculator (0.81). Using the threshold of 97%, the V-CoS test correctly classified 12 out of the 13 patients (Figure 7).
Figure 6.
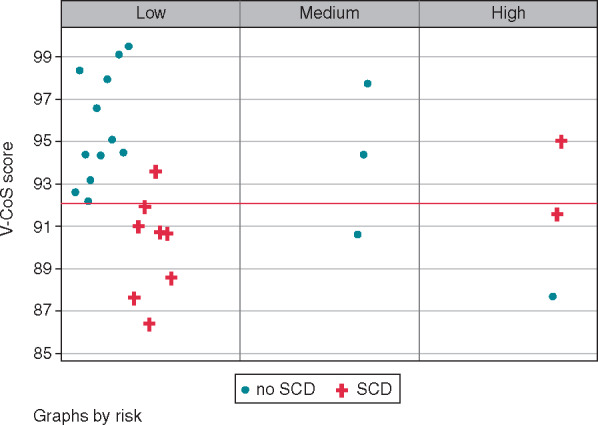
Comparison of V-CoS with conventional risk stratification for BrS. BrS, Brugada syndrome; SCD, sudden cardiac death; V-CoS, ventricular conduction stability.
Figure 7.
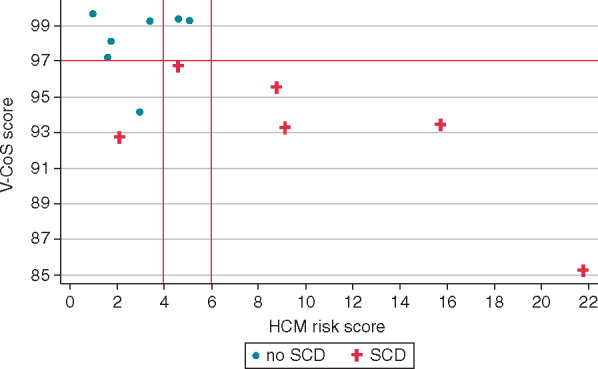
Comparison of V-CoS with conventional risk stratification for HCM. HCM, hypertrophic cardiomyopathy; SCD, sudden cardiac death; V-CoS, ventricular conduction stability.
Discussion
In this study, the V-CoS test was applied to a larger cohort of patients with ICC with previous aborted SCD events, who demonstrated a significantly lower V-CoS score than those without SCD events following an exertional stress test. Interestingly, the same pattern was observed for each of the different ICC subgroups enrolled in this study. This supports the hypothesis that increased spatial changes in activation between phases is associated with increased arrhythmogenic potential regardless of the underlying aetiology.
Previous clinical studies in BrS and HCM patients have demonstrated the existence of spatial heterogeneities in conduction,13–15 and increase in arrhythmogenic potential following exertion.8,16–19 In iVF patients, Peeters et al.20 had previously described the finding of late potentials on signal averaged electrocardiogram in such a cohort, suggesting the presence of regions with slow conduction to support re-entrant arrhythmias. Saumarez et al.4 had also found that local EGMs were wider and more fractionated after pacing at shorter coupling intervals in patients with iVF when compared with unaffected controls, suggesting that intraventricular conduction delay has a role in the arrhythmogenic mechanism in these patients. Whether the development of spatial heterogeneities in conduction is related purely to the effects of heart rate and/or autonomic modulation is currently unclear and will require further investigation.
A degree of similarity or V-CoS score overlap could also be observed between the controls and BrS/HCM individuals without previous aborted SCD events (Supplementary material online, Figures S3 and S4). This would be in keeping with the notion that absence of significant conduction delay or the development of spatial heterogeneities in conduction would be at lower risk of developing re-entrant ventricular arrhythmias even in the presence of an ICC. It was of interest to observe that individuals with HCM (without previous aborted SCD) had similar and overlapping scores to low-risk individuals without structural heart disease. We postulate that this may relate to the degree of underlying fibrosis present and its correlation with V-CoS scores will require further investigation.
As a measure of arrhythmogenic potential, the minimum V-CoS score was considered as the pattern of V-CoS recovery appeared to differ between groups. In the iVF and HCM groups, the lowest V-CoS scores could be primarily observed just after peak exertion in comparison to that seen in the BrS group where a lowered V-CoS score could be observed at 2 min in recovery. This variation in arrhythmogenic potential is in keeping with clinical conservation and reports of ventricular arrhythmias occurring during peak exertion rather than recovery in HCM, and on early recovery of post-exertion in BrS.16,17,19
Based on the Youden’s index employed in the analysis of the ROC curves, there appeared to be different thresholds or cut-offs to indicate the optimal sensitivity and specificity of the V-CoS test. Whilst the interpretation of this is limited by the relatively small numbers in each group, it would suggest that having a common threshold applied to all the different pathologies may dilute the sensitivity/specificity of the test. The effect of this will need to be evaluated in a larger cohort of patients.
The use of conventional surface electrocardiogram (ECG) and ECGi electrophysiological parameters has been previously explored in a group of patients that form part of this study cohort, where SCD survivors possessed the greatest amount of dispersion of repolarization and regional conduction abnormalities following exertion.8 It would stand to reason that the lowest V-CoS scores found in SCD survivors are the result of these electrophysiological perturbations detected by conventional means. As to what degree repolarization abnormalities may also be reflected by this score or the additive effect of other ECGi electrophysiological parameters remains to be investigated.
In comparison to conventional risk stratification techniques for BrS and HCM, V-CoS testing would appear to have greater accuracy to identify individuals at high risk of SCD. In those with idiopathic VF/VT, there is an absence of a formal apriori risk stratification system. In one of our patients with iVF, an ICD had been implanted for primary prevention following a multidisciplinary discussion at our institute. This was made on the basis of ongoing intermittent palpitations, a family history of SCD and having a similar ECG of T-wave inversion in the inferior leads to her deceased brother. All other investigations were normal (including Holter, exercise ECG, and magnetic resonance imaging). Three years later, she received an appropriate shock for VF from her device with no other apparent cardiac abnormality on subsequent clinical work up. Her V-CoS score was 86.5%, below the threshold of 96.4% as defined on ROC analysis.
Study considerations
Given the small numbers within each subgroup, a prospective study involving a much larger group of patients is required to validate these findings before this may be translated into clinical management.
Factors, such as gender and age, may affect sensitivity/specificity of the test although numbers in this study are too small to allow meaningful analysis. Although no significant differences were observed between comparative study groups, the effects of such factors on the sensitivity and specificity of V-CoS testing will be need to be explored in future studies.
We assume ECGi provides a reasonable reflection of epicardial activation patterns based on previous validation work of the system but also acknowledge the limitations in its reconstructive accuracy.9 As we are quantifying magnitude of change within an individual using the same heart-torso geometry data and have algorithms in place to resolve annotation issues that can arise as described previosuly,9 we believe these factors are less of an issue.
We have not tested whether low V-CoS scores observed in the SCD group might be the result of a previous episode of VF or defibrillation. However, this would be unlikely because some patients within the aborted SCD group have relative high V-CoS scores.
Ventricular conduction stability is described as intended to address only timings of activation. We do not know to what extent it is associated with repolarization abnormalities. Direct measures of repolarization may further discriminate those at high risk.
There is beat to beat variability in measurements which could impair the diagnostic ability of V-CoS. However, in a previous study, this variability appeared to be small.9
Finally, autonomic tone can be variable and the reproducibility of this test on a different day is unknown. We acknowledge that the aforementioned factors may theoretically compound variability in measurements of V-CoS scores and requires further study. Whilst this could be explored in future, it would require double radiation because each day’s vest positioning would need its own CT thorax. In summary, these results and the non-invasive nature of the test have important implications for risk stratification but require a large prospective study to validate these findings.
Conclusion
This study demonstrates the potential discriminative ability of the V-CoS test and its application in different types of ICC patients as a tool to measure the electrophysiological substrate that predisposes to VF. The V-CoS test described provides a novel approach to automatically quantify alterations in whole heart activation patterns which may help better identify those with an inheritable cardiac condition and at increased risk of ventricular arrhythmias.
Supplementary material
Supplementary material is available at Europace online.
Funding
This study was supported by a British Heart Foundation Project Grant (PG/15/20/31339). Imperial Innovations holds the patent for the intellectual property of the algorithm for the V-CoS test on behalf of the authors. P.D. Lambiase was supported by the UCL/UCLH Biomedicine NIHR and Barts BRC.
Conflict of interest: none declared.
Data availability
Relevant data on the V-CoS test have been incorporated into the article and its Supplementary material online. Additional data were available on request.
Supplementary Material
References
- 1. Han J, Moe GK.. Nonuniform recovery of excitability in ventricular muscle. Circ Res 1964;14:44–60. [DOI] [PubMed] [Google Scholar]
- 2. Antzelevitch C. Role of spatial dispersion of repolarization in inherited and acquired sudden cardiac death syndromes. Am J Physiol Heart Circ Physiol 2007;293:2024–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nishii N, Nagase S, Morita H, Kusano KF, Namba T, Miura D. et al. Abnormal restitution property of action potential duration and conduction delay in Brugada syndrome: both repolarization and depolarization abnormalities. Europace 2010;12:544–52. [DOI] [PubMed] [Google Scholar]
- 4. Saumarez RC, Heald S, Gill J, Slade AKB, de Belder M, Walczak F. et al. Primary ventricular fibrillation is associated with increased paced right ventricular electrogram fractionation. Circulation 1995;92:2565–71. [DOI] [PubMed] [Google Scholar]
- 5. Cao JM, Qu Z, Kim YH, Wu TJ, Garfinkel A, Weiss JN. et al. Spatiotemporal heterogeneity in the induction of ventricular fibrillation by rapid pacing: importance of cardiac restitution properties. Circ Res 1999;84:1318–31. [DOI] [PubMed] [Google Scholar]
- 6. Ng GA, Brack KE, Patel VH, Coote JH. Autonomic modulation of electrical restitution, alternans and ventricular fibrillation initiation in the isolated heart. Cardiovasc Res 2007;73:750–60. [DOI] [PubMed] [Google Scholar]
- 7. Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M. et al. ACC/AHA/ESC 2006 Guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines. Europace 2006;8:746–837. [DOI] [PubMed] [Google Scholar]
- 8. Leong KMW, Ng FS, Roney C, Cantwell C, Shun-Shin MJ, Linton NWF. et al. Repolarization abnormalities unmasked with exercise in sudden cardiac death survivors with structurally normal hearts. J Cardiovasc Electrophysiol 2018;29:115–26. [DOI] [PubMed] [Google Scholar]
- 9. Shun-Shin MJ, Leong KMW, Ng FS, Cantwell C, Shun-Shin MJ, Linton N. et al. Ventricular conduction stability test: a method to identify and quantify changes in whole heart activation patterns during physiological stress. Eurpace 2019;21:1422–31. [DOI] [PubMed] [Google Scholar]
- 10. Youden WJ. Index for rating diagnostic tests. Cancer 1950;3:32–5. [DOI] [PubMed] [Google Scholar]
- 11. O'Mahony C, Jichi F, Pavlou M, Monserrat L, Anastasakis A, Rapezzi C. et al. ; for the Hypertrophic Cardiomyopathy Outcomes Investigators. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM Risk-SCD). Eur Heart J 2014;35:2010–20. [DOI] [PubMed] [Google Scholar]
- 12. Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P. et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2733–79. [DOI] [PubMed] [Google Scholar]
- 13. Varnava AM, Elliott PM, Baboonian C, Davison F, Davies MJ, McKenna WJ.. Hypertrophic cardiomyopathy: histopathological features of sudden death in cardiac troponin T disease. Circulation 2001;104:1380–4. [DOI] [PubMed] [Google Scholar]
- 14. Lambiase PD, Ahmed AK, Ciaccio EJ, Brugada E, Lizotte E, Chaubey S. et al. High-density substrate mapping in Brugada syndrome: combined role of conduction and repolarization heterogeneities in arrhythmogenesis. Circulation 2009;120:106–17. [DOI] [PubMed] [Google Scholar]
- 15. Zhang J, Sacher F, Hoffmayer K, O’Hara T, Strom M, Cuculich P. et al. Cardiac electrophysiological substrate underlying the ECG phenotype and electrogram abnormalities in Brugada syndrome patients. Circulation 2015;131:1950–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Papadakis M, Petzer E, Sharma S.. Unmasking of the Brugada phenotype during exercise testing and its association with ventricular arrhythmia on the recovery phase. Heart 2009;95:2022. [DOI] [PubMed] [Google Scholar]
- 17. Elliott PM, Sharma S, Varnava A, Poloniecki J, Rowland E, McKenna WJ.. Survival after cardiac arrest or sustained ventricular tachycardia in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 1999;33:1596–601. [DOI] [PubMed] [Google Scholar]
- 18. Makimoto H, Nakagawa E, Takaki H, Yamada Y, Okamura H, Noda T. et al. Augmented ST-segment elevation during recovery from exercise predicts cardiac events in patients with Brugada syndrome. J Am Coll Cardiol 2010;56:1576–84. [DOI] [PubMed] [Google Scholar]
- 19. Link MS, Bockstall K, Weinstock J, Alsheikh-Ali A, Semsarian C, Estes NAM. et al. Ventricular tachyarrhythmias in patients with hypertrophic cardiomyopathy and defibrillators: triggers, treatment, and implications. J Cardiovasc Electrophysiol 2017;28:531–7. [DOI] [PubMed] [Google Scholar]
- 20. Peeters HAP, Sippensgroenewegen A, Wever EFD, Potse M, Daniels MCG, Grimbergen CA. et al. Electrocardiographic identification of abnormal ventricular depolarization and repolarization in patients with idiopathic ventricular fibrillation. J Am Coll Cardiol 1998;31:1406–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Relevant data on the V-CoS test have been incorporated into the article and its Supplementary material online. Additional data were available on request.