Abstract
Aims
Thermal injury to the oesophagus is an important cause of life-threatening complication after ablation for atrial fibrillation (AF). Thermal protection of the oesophageal lumen by infusing cold liquid reduces thermal injury to a limited extent. We tested the ability of a more powerful method of oesophageal temperature control to reduce the incidence of thermal injury.
Methods and results
A single-centre, prospective, double-blinded randomized trial was used to investigate the ability of the ensoETM device to protect the oesophagus from thermal injury. This device was compared in a 1:1 randomization with a control group of standard practice utilizing a single-point temperature probe. In the protected group, the device maintained the luminal temperature at 4°C during radiofrequency (RF) ablation for AF under general anaesthesia. Endoscopic examination was performed at 7 days post-ablation and oesophageal injury was scored. The patient and the endoscopist were blinded to the randomization. We recruited 188 patients, of whom 120 underwent endoscopy. Thermal injury to the mucosa was significantly more common in the control group than in those receiving oesophageal protection (12/60 vs. 2/60; P = 0.008), with a trend toward reduction in gastroparesis (6/60 vs. 2/60, P = 0.27). There was no difference between groups in the duration of RF or in the force applied (P value range= 0.2–0.9). Procedure duration and fluoroscopy duration were similar (P = 0.97, P = 0.91, respectively).
Conclusion
Thermal protection of the oesophagus significantly reduces ablation-related thermal injury compared with standard care. This method of oesophageal protection is safe and does not compromise the efficacy or efficiency of the ablation procedure.
Keywords: Atrial fibrillation, Catheter ablation, Oesophagus, Atrio-oesophageal fistula, Gastroparesis, Temperature control
What’s new?
The ensoETM device is routinely used in critical care to control body temperature by warming or cooling the lumen of the oesophagus and stomach in the range 4–42°C.
Previous studies on oesophageal cooling by infusion of water during ablation for atrial fibrillation have shown evidence of protection despite the limited heat-extraction achievable by this method.
In a double-blind randomized trial, patients whose oesophageal temperature was maintained at 4°C during ablation for atrial fibrillation suffered fewer thermal injuries to the oesophagus than patients receiving standard treatment.
Oesophageal protection by the ensoETM device did not slow the procedure or reduce its effectiveness.
Introduction
Although its incidence is less than one per thousand cases, atrio-oesophageal fistula is among the most common lethal complications of ablation performed for atrial fibrillation (AF) or left atrial tachycardia.1 Both radiofrequency (RF) ablation and cryoablation have been associated with this complication.1,2 Mild degrees of thermal injury to the oesophagus can be seen on endoscopy after ablation in up to 47% of patients,3 and the incidence of these mild lesions correlate with the risk of fistula. Symptomatic alterations to gastric motility from thermal injury to the peri-oesophageal neural plexus occur between 5% and 74% of cases.4,5 Many strategies have been proposed to lower the risk to the oesophagus, but none has shown consistent evidence of effectiveness.
The ensoETM device (Attune Medical, Chicago, IL, USA) is routinely used to control body temperature in patients who are prone to hypothermia or hyperpyrexia in an intensive care setting, or whose body temperature must be lowered to protect an injured brain.6,7 As it does so by warming or cooling the lumen of the oesophagus and stomach, we hypothesized that it might protect the oesophagus from localized thermal injury by controlling the local temperature.
Methods
Trial design
The IMPACT study was an investigator-initiated single-centre, prospective, double-blind randomized controlled trial (Figure 1). The study cohort consisted of adult patients receiving AF ablation under general anaesthesia. Recruitment ran from February 2019 to January 2020. The study was approved by the London-Stanmore Research Ethics Committee (IRAS ID 253844, NIHR CPMS ID 40619) and registered on ClinicalTrials.gov NCT03819946.
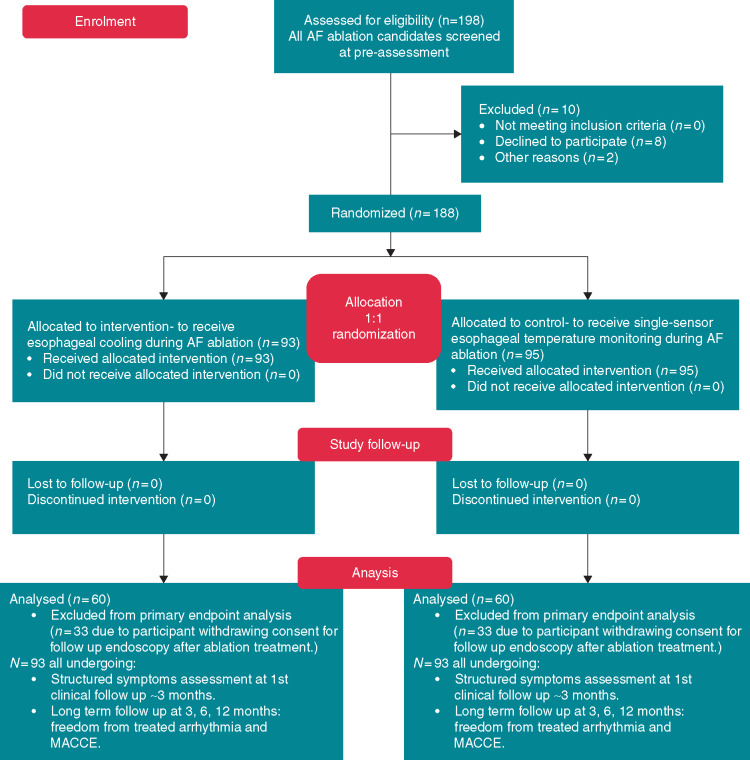
Figure 1.
Consort 2010 flow diagram for the IMPACT study. All patients attending for left atrial ablation by participating physicians were considered. Almost all agreed to participate, but 36% subsequently declined to return for their scheduled endoscopy at 1 week post-ablation.
Study population
All adult patients attending or already listed for radiofrequency ablation for AF or LAT under general anaesthesia by participating electrophysiologists at our centre were screened during pre-assessment. Patients who did not consent to undergo post-procedure endoscopy were excluded. Participants were randomly assigned in a 1:1 design (via electronic randomization www.sealedenvelope.com) to either receive thermal protection with the ensoETM device or standard care consisting of the use of a single sensor temperature probe. Patients were blinded to the treatment assignment.
Protected group: the ensoETM device
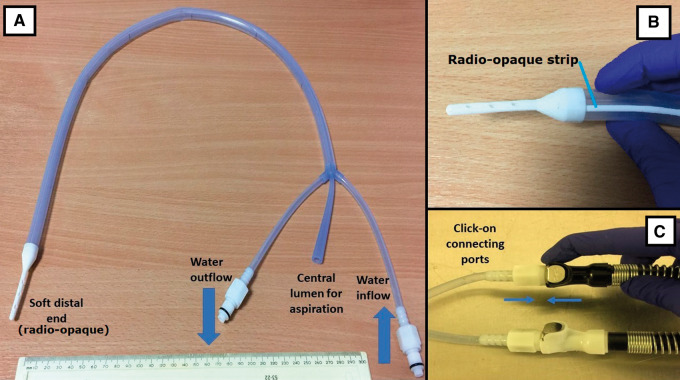
The ensoETM device is a medical-grade silicone tube (dimensions: 75 cm in length and 1.2 cm outer diameter; Figure 2) through which distilled water is pumped in a closed-loop irrigation system: no water enters the gastrointestinal tract of the patient. There is an additional inner lumen that can be used for gastric aspiration like a standard nasogastric tube. The non-patient end of the device is connected to a Blanketrol III mobile console (Gentherm Medical, Cincinnati, OH, USA) that pumps the water and controls its temperature. The ensoETM device maintains the water at a thermostat-controlled set temperature chosen by the operator between 4°C and 42°C. During operation, the water volume in the tubing is 55 mL and it flows at 2.4 L/min exerting a maximum pressure of 103 kPa.
Figure 2.
The ensoETM device. The ensoETM device (A and B) and the connectors to the Blanketrol III mobile console (C). The white elements at the tip of the ensoETM and in a strip along its length are radio-opaque.
Protected group
Patients assigned to receive oesophageal protection underwent preparation for ablation in the standard fashion with transoesophageal echocardiography performed as soon as anaesthesia was induced. After using transoesophageal echocardiography to guide transseptal puncture, the probe was withdrawn and an ensoETM probe was introduced in its place, connected to a Blanketrol III, mobile console.8 The position was confirmed radiographically, aiming to place the distal end of the device below the diaphragm (Figure 3). Before beginning ablation on the posterior part of the left atrium, the probe was set to cooling mode at 4°C for at least 10 min. Cooling continued until ablation was complete. External body temperature was recorded throughout with a temperature probe placed in the axilla or nasopharynx.
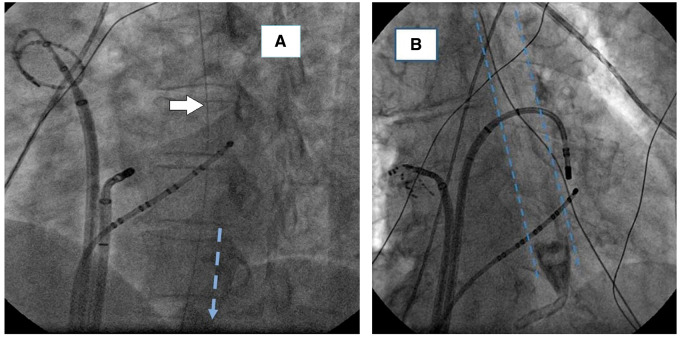
Figure 3.
Radiological images of the device during use. (A) a right anterior oblique view of a device at the correct level of placement. Only the radio-opaque strip is clearly seen (white arrow). (B) a device positioned with the radio-opaque tip above the level of the diaphragm. It was advanced an additional 10 cm before ablation commenced.
Control group
A single-sensor temperature probe (Level 1® Oesophageal Temperature Probe, Smiths Medical, Minneapolis, MN, USA, was used) was placed in the oesophagus by the attending anaesthetist and adjusted approximately to the site of ablation. Adjustment of the position of the probe during ablation was performed by the anaesthetist under the direction of the operating electrophysiologist with the objective of keeping the tip of the probe as close as possible to the site of ablation whenever the site was within 1 cm of the oesophagus. RF delivery was suspended if the temperature indicated by the probe exceeded 38°C and RF was not resumed at that location until the temperature fell below 37°C. To avoid delay, operators often moved to a location distant from the oesophagus to continue work while waiting for the temperature to fall.
Body temperature management and anaesthesia
In all cases, standard anaesthetic methods were used, with intravenous induction using propofol and a non-depolarizing muscle relaxant followed by endotracheal intubation and maintenance with a volatile anaesthetic. Whole body warming was with a heated air-blanket (Bair Hugger™, 3M, St. Paul, MN, USA). The settings on the warming blanket were controlled by the anaesthetist to maintain a body temperature of >35–37°C throughout the procedure. The anaesthetist used the oesophageal temperature probe to judge this in the control patients, axillary, nasal, or auricular temperature in study patients.
Proton pump inhibitors
All participants in this study received proton pump inhibitors (PPI) initiated immediately post-ablation for a period of 8 weeks in accordance with standard practice at our centre.
Method of RF ablation
Catheter ablation was performed using irrigated contact force sensing catheters (STSF or Qdot Micro, Biosense Webster, Johnson and Johnson, Diamond Bar, CA, USA) with a 3D mapping system (Carto® version 6 and 7, Biosense Webster) with Ablation Index (AI) technology. A double trans-septal approach was used, and additional mapping was done using a Lasso-Nav or Pentaray mapping catheter (Biosense Webster). Lesions in the anterior part of the left atrium were created at 40 W with an AI target of 450–500; posterior lesions were at 30 W with an AI target of 350–400.
Endoscopy
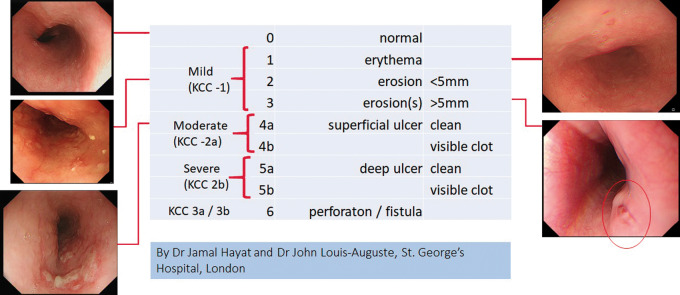
All patients were invited to attend for endoscopy at 7 days after ablation. Endoscopy was performed by one of two senior endoscopists following a standardized protocol with detailed inspection of the anterior wall of the mid-oesophagus. The patient and the endoscopist were blinded to the treatment assignment of the patient. Any lesions observed were categorized according to a scoring system which was devised for the study and represents a refinement of the Kansas City Classification, with a greater degree of gradation in the moderate range of lesion severity that we expected to find (Figure 4). The stomach was inspected for evidence of dysmotility. Gastroparesis was defined as the presence of food material in the stomach after >8 h of fasting.
Figure 4.
The St. George’s Modified Scoring System. For the purposes of the trial, we designed a modified system for categorizing endoscopically detected iatrogenic thermal mucosal lesions. It is more finely graded than the Kansas City Classification (KCC), the classes of which are shown in comparison.
Assessment of symptoms
Patients underwent a structured assessment for upper GI symptoms according to the Gastro-Oesophageal Reflux Disease (GERDQ) questionnaire; the Gastroparesis Cardinal Symptoms Index (GCSI) questionnaire was used to probe for typical features of gastric dysmotility. Specific supplementary questions were added to quantify post-procedural chest pain and the overall unpleasantness of the patient experience on a scale of severity from 0 to 10 (10 = most severe or worst experience). Administration of the questionnaires was at the first clinical follow-up post-ablation at 1–3 months post-ablation by members of the research study without access to the randomization of the participants.
Arrhythmia follow-up
Patients were reviewed at 3- and 6-month post-ablation and underwent ambulatory ECG monitoring between these visits; 12-month follow-up will occur. Standard ECG monitoring devices were used for follow-up, including 12-lead ECG at all follow-up visits in all patients, data download from implantable loop recorders, pacemakers, and defibrillators when available, ambulatory ECG of ≥24 h in all other patients. Any occurrence of atrial tachyarrhythmia at >3 months post-ablation was considered to represent a failure of the procedure.
Power calculation
Based on a pre-study clinical estimate of 15% incidence of lesions in the control group from prior local experience and literature from elsewhere, and an incidence of ∼1% in the study group based on our own pre-trial use of the device, we calculated a sample size of 120 with endoscopic assessment to achieve a study power of 0.80 to answer the hypothesis.
Endpoints
The primary endpoint of the study was the incidence of endoscopically detected oesophageal mucosal lesions and/or gastroparesis. Secondary endpoints included the presence of post-procedure symptoms revealed by the validated questionnaires GERD-Q and GCSI, the incidence of procedural complications and subsequent major adverse cardiovascular cerebrovascular events (MACCE), and the technical and clinical success of the procedure and indices of the difficulty of the procedure.
Technical success was defined as isolation of the pulmonary veins and proven block across any other line of lesions delivered; clinical success was also measured as freedom from the treated arrhythmia at >3 months after ablation. Major adverse cardiovascular cerebrovascular event was defined as MI/CVA, all-cause mortality, vascular trauma needing surgery, cardiac tamponade, atrio-oesophageal fistula, and hospital acquired infection. The duration of the procedure and duration of fluoroscopy was documented in all cases, as well as ablation delivery parameters including total RF ablation time, power, force, FTI, and combined AI.
Statistical analysis
Analysis was performed with IBM SPSS statistical software (Version 22.0, IBM SPSS Statistics, NY, USA) using Student’s t-test, χ2 test, and Fisher's exact test as appropriate.
Ethical approval
Ethically approved by the London-Stanmore Research Ethical Committee (IRAS ID 253844).
Results
Patients were recruited from 22 February 2019 to 13 January 2020. More than 90% of patients screened for recruitment agreed to participate, but 36.2% of recruited patients subsequently expressed unwillingness or inability to return for endoscopy. Drop-out from endoscopic follow-up occurred equally in the protected and control groups. A total of 188 participants were recruited (89 protected and 99 control) of whom 120 (60 protected and 60 control) underwent endoscopic examination.
There was no defining characteristic in the endoscopy drop-out cohort who had similar baseline characteristics and procedure characteristics to those who attended for endoscopy (Supplementary material online, Table S1). A variety of reasons for endoscopy drop out were cited, mostly relating to the logistical difficulty of attending another hospital appointment but also including a need to spend more time at home to recover from the ablation. Because the primary endpoints of the study were the endoscopic findings, the results are quoted in this manuscript for the 120 patients who completed the entire study protocol including endoscopy. All 188 patients remain under follow-up: their data are available in Supplementary material online, Tables.
Patient and procedure characteristics
The baseline characteristics of the groups were well matched (Table 1). The use of oesophageal cooling was not associated with any difference in the procedure duration, nor was there any evidence that its use made the accomplishment of procedural endpoints more difficult. The duration of RF delivery, including RF delivery to the posterior wall was similar in the protected and the control groups (Table 2). All veins were isolated in all subjects in both groups, and all veins remained isolated at the end of the procedure.
Table 1.
Patient and procedure characteristics
| Patient and procedure characteristics | Protected (n = 60) | Control (n = 60) | P-value |
|---|---|---|---|
| Male | n = 36 (60%) | n = 37 (61.7%) | 0.85 |
| Mean age (years) | 65 ± 10 | 65 ± 9 | 0.9 |
| Left atrial diameter (cm) | 4.1 ± 0.9 | 4.2 ± 0.6 | 0.48 |
| LV ejection fraction (%) | 55 ± 9 | 52 ± 8 | 0.24 |
| BMI (kg/m2) | 28.5 ± 5.3 | 29.8 ± 6.98 | 0.25 |
| Paroxysmal AF, first time ablation | n = 27 (45%) | n = 30 (50%) | 0.71 |
| Persistent AF, first time ablation | n = 24 (40%) | n = 20 (33.4%) | 0.57 |
| Repeat left atrial ablation | n = 8 (13.3%) | n = 9 (15%) | 0.79 |
| Left atrial tachycardia | n = 1 (1.7%) | n = 1 (1.7%) | 1 |
AF, atrial fibrillation; BMI, body mass index; LV, left ventricular.
Table 2.
Procedural metrics and follow-up
| Procedure metrics | Protected (n = 60) | Control (n = 60) | P-value |
|---|---|---|---|
| Procedure duration (min) | 186 ± 47 | 187 ± 48 | 0.9 |
| Fluoroscopy duration (min) | 10.9 ± 7.3 | 11.1 ± 7.4 | 0.88 |
| Total RF time (s) | 2066 ± 1062 | 2315 ± 1053 | 0.20 |
| RF duration (posterior wall only), median (IQR) | 13.1 (10.3–17.5) | 12.1 (9.1–15.8) | <0.001 (z score: 4.073524) |
| AI values (posterior wall only), median (IQR) | 379.7 (362.8–406.7) | 377.2 (360–406.2) | 0.57 (z score: 0.570419) |
| Achievement of PV isolation | 60/60 (100%) | 60/60 (100%) | 1 |
| First pass PV isolation achieved (first time ablations cases) | 45/51 (88.2%) | 42/50 (84%) | 0.58 |
| Reconnection of PV during waiting period or adenosine test (first time ablations only) | 1/51 (1.9%) | 7/50 (14.9%) | 0.03 |
| Posterior wall isolation (persistent or recurrent cases only) | 16/23 | 19/23 | 0.49 |
| Acute complications | 1 | 2 | 0.56 |
| Hospital stay >1 night | 1 | 1 | 1 |
| MACE—within 3 months | 0 | 0 | 1 |
| MACE—within 6 months | 1 | 0 | 0.31 |
| Arrhythmia recurrence during follow-up | 2 | 3 | 0.65 |
| Re-admission to hospital | 1 | 2 | 0.56 |
| Re-ablation since index procedure | 0 | 0 | 1 |
| Moderate to severe grade symptoms as graded by GCSI (>19–45), GERD-Q (7–18) or chest pain scale (1–10) on 1st outpatient follow-up | 2 | 2 | 1 |
| All positive symptoms recorded from GCSI, GERD-Q, or chest pain scale during 1st outpatient follow-up | 5 | 10 | 0.27 |
GCSI, Gastroparesis Cardinal Symptom Index; GERD-Q, Gastro-oesophageal Reflux Disease Questionnaire; IQR, interquartile range; MACE, major adverse cardiovascular event; PV, pulmonary vein; RF, radiofrequency.
Linear ablation lesion sets across the roof of the left atrium, the posterior wall of the left atrium and the posterior mitral isthmus were each attempted at similar proportions of the protected cases and in the control group with a similar rate of success in both. The total number of lesions and the duration of RF delivery required to achieve procedural endpoints was similar in the control and the protected group, both among those who received PVI only and amongst those receiving more extensive lesion sets (Table 2). Because it is our policy to isolate the posterior wall of the left atrium in patients who return for repeat ablation and are found to have prior isolation of three or more veins did some ablation in the posterior left atrium in all trial patients. Of the 23 patients, 16 had enduring posterior wall isolation in the protected group compared with 19/23 patients in the control group (P = 0.49; Table 2). No temperature rise above 43°C was recorded in the oesophagus of any patient.
Primary endpoint analysis—endoscopy findings
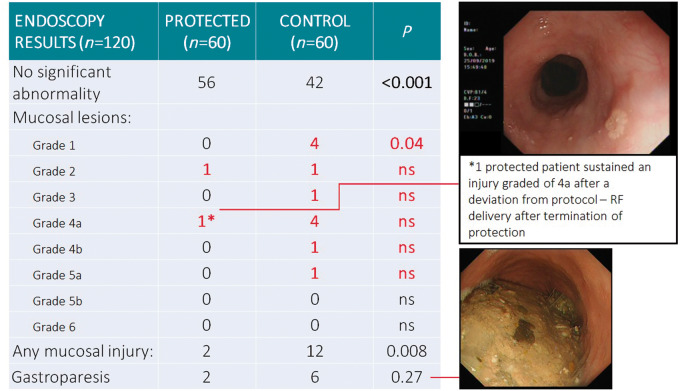
Endoscopy demonstrated significantly fewer thermal injuries in patients protected by the ensoETM compared with the control group (2/60 vs. 12/60, P = 0.008; Figure 5). Gastroparesis was present in 2/60 protected patients, 6/60 patients in the control group (P = 0.27). There were no endoscopy findings consistent with trauma from the ensoETM device or from trans-oesophageal echo. Moderate to severe (Grade 4 or more) lesions occurred in one case in the protected group, six cases of the control group (P = NS).
Figure 5.
Primary endpoints of the IMPACT trial. The endpoints were all derived from endoscopy. Mucosal lesions were significantly less common after ablation carried out with the protection of the ensoETM device, with a trend toward reduction in gastroparesis. The only moderately severe lesion in a protected patient related to a protocol breach.
The only moderately severe lesion in the protected group related to a protocol breach: the patient in question exhibited a recurrence of conduction into the right pulmonary veins as the procedure was finishing, and the operator delivered additional lesions at a site on the posterior margin of the right inferior vein, unaware that the oesophageal cooling had been turned off 20 min earlier. The event was discussed after the protocol breach was noticed, but the patient continued in the study as the intention had been to protect throughout the procedure. The endoscopist was unaware of the treatment assignment or the deviation from protocol. The site of the lesion was in keeping with the delivery in question.
Other than the use of the ensoETM device, we were not able to identify significant predictors of the occurrence of mucosal lesions. They were non-significantly more frequent in patients who received a line of ablation across the posterior wall of the left atrium (Table 3), but they did not follow the expected pattern of higher prevalence in leaner patients, nor did they occur more frequently in patients with prior gastrointestinal complaints including Barrett’s oesophagus.
Table 3.
Factors associated with the occurrence or absence of mucosal lesions detected on endoscopy
| Patients with mucosal lesions (n = 14) | Patients without mucosal lesions (n = 106) | P-value | |
|---|---|---|---|
| Use of ensoETM device | n = 2 | n = 58 | 0.008 |
| Posterior left atrial ablation line | n = 8 | n = 38 | 0.15 |
| Age (years) | 65.5 ± 5.4 SD | 65.2 ± 5.8 | 0.85 |
| Body mass index (kg/m2) | 29.7 ± 5.6 SD | 28.6 ± 5.6 | 0.49 |
| Prior active GI condition | n = 0 | n = 6 | 0.46 |
GI: gastrointestinal.
Secondary endpoints—major adverse cardiovascular cerebrovascular events
There was no significant difference in acute complication rates between the protected and control groups. There were two cases of acute vascular trauma needing intervention with thrombin injection in the control group and one case of additional hospital night stay due to post-procedural bradycardia. There were two cases of extra hospital night stay in the protected group, of which one was planned pre-ablation due to previous adverse reaction to Heparin. The other case was due to a small pericardial effusion that was conservatively managed. There were no acute cases of tamponade, MI, CVA, or death in either group. There was one case of late mortality in the protected group, a patient who died at between 3- and 6-month post-ablation due to progression of pre-existing severe heart failure. All other patients were discharged the day after the procedure and remained well until 6 months or the last available follow-up.
Procedural workflow
Fluoroscopy and procedure duration were similar in both groups. Recovery time was also similar with no difference in hospital night stay between the two groups (Table 2).
Patient symptoms—relationship to protection and endoscopic findings
The combined questions of GERD-Q and GCSI covered a wide range of gastro-oesophageal or gastroparetic symptoms that could occur after an ablation, and the occurrence of chest pain. Results of the questionnaires were available in all patients who reached the 3-month follow-up point. The majority of the study participants did not experience significant gastrointestinal symptoms post-ablation, as scored by the GERD-Q and GCSI questionnaires (Table 2). The presence of mucosal lesions did not correlate strongly with symptoms, confirming previous literature which has shown that many patients who sustain mucosal lesions remain asymptomatic. Those who had signs of gastroparesis on endoscopy were generally symptomatic. The overall symptom burden was similar in the protected and the control groups.
Clinical outcome
Clinical follow-up has been disrupted since late February 2020 due to the COVID-19 pandemic. All patients remain in telephone contact, but attendances for ECG have been suspended. At the time when disruption began, no patient had reached their 12-month follow-up and only 50/120 and 82/120 had reached the 6-month and 3-month assessment (Supplementary material online). Recurrence of AF or AT outside the blanking period was equally common in the protected group vs. the control group (Table 2). Follow-up of the study subjects is ongoing.
Discussion
This prospective double-blind randomized controlled trial demonstrates that endoscopically detected thermal injury to the oesophagus is less frequent when the ensoETM device is used to control the local temperature of the oesophageal lumen during RF ablation for AF. This is the first randomized clinical study to show superiority of this method of oesophageal protection over standard care.
Secondary endpoints
The analyses on the secondary endpoints show that controlled active thermal protection of the oesophagus utilizing the ensoETM device is safe, with no difference in MACCE rates or acute complications when compared with the control group cases. Endoscopy did not show any evidence of oesophageal trauma attributable to the ensoETM device. Procedure workflow and acute success were unaffected by the device and at the 6-month time point, there is no effect on the efficacy of the ablation.
Thermal injury to the oesophagus
Lesions of the oesophageal mucosa are common after AF ablation. This is widely recognized for RF and also well documented for those who receive cryoablation. Schemes of classification have been devised to reflect this gradation of prognostic importance. The commonly used Kansas City Classification9 has six levels of severity including zero for normal; our more graded classification has eight levels, providing a higher level of discrimination which we believe to be useful in demonstrating trends in lesion severity as well as incidence.
There is no correct time for endoscopy after ablation. Our trial was unusual among studies of post-ablation injury in choosing a 7-day time point for endoscopy. Most thermal lesions are transient, consisting of erythema that resolves within days. Mechanical injury to the oesophagus from echo probes, temperature probes, or protective devices would be expected to follow a similar time-pattern. Moderately severe lesions appear soon after ablation but heal within weeks. Ulceration of the mucosa is known to be linked to progression towards fistula formation,10,11 a complication that typically presents clinically at between 2 and 4 weeks after ablation.
We chose 7 days as the ideal interval between ablation and endoscopy based on the available literature as the optimum for detecting significant lesions without being overwhelmed by trivial findings. A systematic review showed that with up to 47% incidence of all mucosal lesions at endoscopy performed at 24 h post-procedure,3,12 differentiation between minor lesions and those worthy of attention was difficult; endoscopy performed at an interval of 7–14 days from the time of ablation gave far greater discrimination.10 By performing endoscopy later than most, we may have missed some of the milder lesions and lost statistical power; it also makes our data less easy to compare with previously published work.
We recognize that any single endoscopic assessment has limitations. There is still a risk of falsely declaring a case as ‘all clear’; previous reports show anomalous cases having no mucosal lesions detected at endoscopy 18 days post-ablation but nonetheless progressing to perforation or fistula.5 Despite this limitation, endoscopic examination is still our best diagnostic tool. Computerized tomography has a poorer performance, with far greater numbers of false-negative reports but is a recognized current 1st line diagnostic test for emergent cases of suspected atrio-oesophageal fistula.13
Patient symptoms
This study confirms that those who sustain endoscopically detected mucosal lesions in the oesophagus correlate poorly with patient symptoms. Only those who have signs of gastroparesis on endoscopy also have a clearly defined excess of symptoms as scored by the GCSI questionnaire. Overall, the majority of AF ablation patients recover smoothly with no significant gastrointestinal symptoms or chest pain.
Preventing atrio-oesophageal fistula
Strategies have been tried for the prevention of fistula including the administration of proton-pump inhibitors to facilitate oesophageal healing. This has intuitive appeal but no backing in trial evidence.3,14 It is based on the assumption that gastric acid contributes to the injury that progresses to fistula formation; it is equally likely that bacterial action is the prime driver for this progression and acidity might be regarded as protective in its bactericidal effect.
Mechanical deviation of the oesophagus away from the point of ablation has been investigated extensively. The most common method involves manipulation of the trans-oesophageal echo probe to deviate the oesophagus.15 Dedicated devices have also been studied such as the Balloon Retractor.16 These methods are imperfect and potentially harmful. A recent large multi-centre study showed that trans-oesophageal echo probe insertion and manipulation is an important cause of mortality in general anaesthetic cases undergoing cardiothoracic surgery or cardiac procedures with an incidence of 1 in 3000 and a mortality rate of 40% to those that sustained oesophageal injury.17 Mechanical deviation of the oesophagus by any device could cause similar complications.
Oesophageal temperature measurement is commonly used. For our control group, we adopted the standard approach in our centre: Use of a single-point temperature probe for all cases. There is no proof that this method improves safety compared with ablations performed without oesophageal temperature measurement, and it comes at a cost to efficiency as time is expended in adjusting the position of the probe and waiting for the temperature to return to baseline each time a temperature rise occurs. These factors may have reduced efficiency and perhaps even efficacy in our control group. The effect of oesophageal thermal protection on efficiency and safety might have been different if the control population had not had any temperature probe or if a multi-point probe had been used.
There are considerable differences between the oesophageal temperature probes used in AF ablation. Turagam et al.18 investigated the characteristics of a range of them. Solid shaft and acoustascopes and single-sensor probes and multi-sensor probes were compared in a series of experiments, measuring the thermal response of each probe after immersion in temperature-set water baths. The probes were equilibrated in water bath 1 at 37°C and then quickly transferred to water bath 2 at 45°C. The time taken to register the full extent of the temperature change varied from 6.2 to 19.7s, with the quickest response given by multi-sensor probes, followed by single-sensor probes and with acoustascopes responding least rapidly.
Although the Turagam study did not replicate the exact conditions found in the clinical situation, it confirmed the notion that different probes can give substantially different results, and some could give a false sense of security during ablation. The probes also differ in price and ease of insertion to the correct level. The apparent superiority of multi-sensor probes in detecting temperature rises in this study may also have a paradoxical effect. Increased oesophageal thermal injury was observed after use of multi-sensor probes in one study compared with single-sensor probes,19 probably due to bipolar thermal energy transmission during RF ablation. The probe used in the control group of our study was chosen as an example of the sort that in our experience is most commonly used.
Operator restraint in the power delivered to the left atrial posterior wall was the first strategy shown to reduce the risk of fistula. More recently, limitation in the use of contact force has been shown to help.20 Unfortunately, this conservative methodology risks producing less effective lesion sets, hindering procedural success, and fostering inefficient workflow.
Previous studies on oesophageal cooling
Previous small studies have investigated the efficacy of oesophageal cooling during ablation. Although individually inconclusive, taken together, these show clear evidence of protection, but the effect is small.21,22 The small magnitude of the protection is unsurprising, given the limited heat extraction capability of the methods used. Oesophageal cooling using the ensoETM device is simple and easily standardized; it is also a much more effective heat extractor than the methods investigated by previous studies.
Gastric motility
Previous post-ablation endoscopic studies were either focused on mucosal lesions or functional pathology.4,23 Our trial protocol included a detailed assessment of gastric motility as well as the oesophageal mucosa. The IMPACT study shows a non-significant excess of both endoscopically defined delay in gastric emptying and in symptoms related to this condition in the control group compared with patients receiving thermal protection.
Applicability of the method
The use of the ensoETM device in our unit encountered no significant obstacle. The work-flow was smooth and no complication occurred. We had the benefit of the availability of general anaesthesia for all the cases studied and the benefit of a spacious operating room. The console occupies much less space than either the anaesthetic machine or any of the mapping systems that we use, but in a cramped and over-filled lab it might be an unwelcome addition. We do not have direct experience of the use of the device in patients undergoing procedures under conscious sedation; it is feasible to do this, but the safety and acceptability have not been quantified.
The ensoETM device costs less than multi-sensor temperature monitoring devices that are routinely used, though more than the single-point probe used in our control cases. Based on an incidence of atrio-oesophageal fistula of 0.1%, representing the lower end of the range generally reported,1 and assuming that the 83% reduction in mild to moderate thermal lesions seen with the ensoETM in our study is mirrored in the prevention of fistula, we would need to protect 1160 patients to prevent one fistula. The relevance of this cost-benefit calculation will vary between centres.
Limitations
We have not proved that the use of the ensoETM device eliminates the possibility of atrio-oesophageal fistula formation. A far larger trial would be required to answer this critical question.
Operators participating in the study were not and could not have been blinded to the randomization. We were conscious of the risk that a perceived protection might foster recklessness by the operators, so all were exhorted to adhere as closely as possible to their standard methods and lesion sets. The data collected on ablation power and contact force suggests that they did so. This strict adherence to standard methodology may have contributed to the high level of oesophageal protection observed. It should not be interpreted as a licence to abandon all restraint in ablating near the oesophagus.
The control group followed standard practice at our centre: oesophageal protection was offered in the form of a single sensor probe. This is limited in its ability to sense temperature change at the site of greatest risk to the oesophagus with sufficient rapidity to prevent injury. The results found in our control group may not be generalizable to temperature measurement using other measurement devices.
Redo procedures and first-time ablations were included, potentially reducing the power of the study to demonstrate significant differences. The follow-up duration available on these patients is still not sufficient to exclude the possibility of an effect of oesophageal cooling on the formation of thermal lesions in the adjacent atrium. Longer follow-up on this cohort will be needed.
Conclusions
Thermal protection of the oesophagus significantly reduces ablation-related thermal injury compared with standard care. This method of oesophageal protection is safe and does not compromise the efficacy or efficiency of the ablation procedure.
Supplementary material
Supplementary material is available at Europace online.
Funding
This study was funded by the charitable organization ‘Cardiac Risk in the Young’ (CRY). Additional support was provided by Attune Medical, Chicago, IL, USA.
Conflict of interest: L.W.L. has received research support from Attune Medical. M.M.G. has received research funding from Attune Medical and has acted as a consultant and a paid speaker for Boston Scientific and Cook Medical. A.L.: Other—Travel and accommodation support (modest): Abbott. A.B.: Honoraria (modest) Bayer. Other—Educational and Travel Grant (modest): Biosense Webester, Other—Educational Grant (modest): Medtronic. Speaker/Speaker's Bureau (modest) Diachii Sankyo, Pfizer/BMS.
Data availability
Data available upon request to: lleung@sgul.ac.uk.
Supplementary Material
References
- 1. Kapur S, Barbhaiya C, Deneke T, Michaud GF.. Esophageal injury and atrioesophageal fistula caused by ablation for atrial fibrillation. Circulation 2017;136:1247–55. [DOI] [PubMed] [Google Scholar]
- 2. Miyazaki S, Nakamura H, Taniguchi H, Takagi T, Iwasawa J, Watanabe T. et al. Esophagus-related complications during second-generation cryoballoon ablation—insight from simultaneous esophageal temperature monitoring from 2 esophageal probes. J Cardiovasc Electrophysiol 2016;27:1038–44. [DOI] [PubMed] [Google Scholar]
- 3. Schmidt M, Nolker G, Marschang H, Gutleben KJ, Schibgilla V, Rittger H. et al. Incidence of oesophageal wall injury post-pulmonary vein antrum isolation for treatment of patients with atrial fibrillation. Europace 2008;10:205–9. [DOI] [PubMed] [Google Scholar]
- 4. Lakkireddy D, Reddy YM, Atkins D, Rajasingh J, Kanmanthareddy A, Olyaee M. et al. Effect of atrial fibrillation ablation on gastric motility: the atrial fibrillation gut study. Circ Arrhythm Electrophysiol 2015;8:531–6. [DOI] [PubMed] [Google Scholar]
- 5. Park SY, Camilleri M, Packer D, Monahan K.. Upper gastrointestinal complications following ablation therapy for atrial fibrillation. Neurogastroenterol Motil 2017;29:e13109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hegazy AF, Lapierre DM, Butler R, Martin J, Althenayan E.. The esophageal cooling device: a new temperature control tool in the intensivist’s arsenal. Heart Lung 2017;46:143–8. [DOI] [PubMed] [Google Scholar]
- 7. Bhatti F, Naiman M, Tsarev A, Kulstad E.. Esophageal temperature management in patients suffering from traumatic brain injury. Ther Hypothermia Temp Manag 2019;9:238–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zagrodzky J, Gallagher MM, Leung LW, Sharkoski T, Santangeli P, Tschabrunn C. et al. Cooling or warming the esophagus to reduce esophageal injury during left atrial ablation in the treatment of atrial fibrillation. J Vis Exp 2020;(157):e60733.doi:10.3791/60733. [DOI] [PubMed] [Google Scholar]
- 9. Yarlagadda B, Deneke T, Turagam M, Dar T, Paleti S, Parikh V. et al. Temporal relationships between esophageal injury type and progression in patients undergoing atrial fibrillation catheter ablation. Heart Rhythm 2019;16:204–12. [DOI] [PubMed] [Google Scholar]
- 10. Keshishian J, Young J, Hill E, Saloum Y, Brady PG.. Esophageal injury following radiofrequency ablation for atrial fibrillation. Gastroenterol Hepatol 2012;8:411–4. [PMC free article] [PubMed] [Google Scholar]
- 11. Doll N, Borger MA, Fabricius A, Stephan S, Gummert J, Mohr FW. et al. Esophageal perforation during left atrial radio-frequency ablation: is the risk too high? J Thorac Cardiovasc Surg 2003;125:836–42. [DOI] [PubMed] [Google Scholar]
- 12. Di Biase L, Saenz LC, Burkhardt DJ, Vacca M, Elayi CS, Barrett CD. et al. Esophageal capsule endoscopy after radiofrequency catheter ablation for atrial fibrillation: documented higher risk of luminal esophageal damage with general anesthesia as compared with conscious sedation. Circ Arrhythm Electrophysiol 2009;2:108–12. [DOI] [PubMed] [Google Scholar]
- 13. Chavez P, Messerli FH, Dominguez AC, Aziz EF, Sichrovsky T, Garcia D. et al. Atrioesophageal fistula following ablation procedures for atrial fibrillation: systematic review of case reports. Open Heart 2015;2:e000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zellerhoff S, Lenze F, Eckardt L.. Prophylactic proton pump inhibition after atrial fibrillation ablation: is there any evidence? Europace 2011;13:1219–21. [DOI] [PubMed] [Google Scholar]
- 15. Herweg B, Johnson N, Postler G, Curtis AB, Barold SS, Ilercil ARZU.. Mechanical esophageal deflection during ablation of atrial fibrillation. Pacing Clin Electrophysiol 2006;29:957–61. [DOI] [PubMed] [Google Scholar]
- 16. Bhardwaj R, Naniwadekar A, Whang W, Mittnacht AJ, Palaniswamy C, Koruth JS. et al. Esophageal deviation during atrial fibrillation ablation. JACC Clin Electrophysiol 2018;4:1020–30. [DOI] [PubMed] [Google Scholar]
- 17. Ramalingam G, Choi SW, Agarwal S, Kunst G, Gill R, Fletcher SN. et al. ; on behalf of the Association of Cardiothoracic Anaesthesia and Critical Care. Complications related to peri-operative transoesophgeal echocardiography—a one-year prospective national audit by the Association of Cardiothoracic Anaesthesia and Critical Care. Anaesthesia 2020;75:21–6. [DOI] [PubMed] [Google Scholar]
- 18. Turagam MK, Miller S, Sharma SP, Prakash P, Gopinathannair R, Lakkireddy P. et al. Differences in transient thermal response of commerical esophageal temperature probes. JACC Clin Electrophysiol 2019;5:1280–8. [DOI] [PubMed] [Google Scholar]
- 19. Carroll BJ, Contreras-Valdes FM, Heist EK, Barrett CD, Danik SB, Ruskin JN. et al. Esophageal temperature probe used during radiofrequency ablation for atrial fibrillation is associated with increased intraluminal temperature detection and increased risk of esophageal injury compared to single‐sensor probe. J Cardiovasc Electrophysiol 2013;24:958–64. [DOI] [PubMed] [Google Scholar]
- 20. Zhang X, Kuang X, Gao X, Xiang H, Wei F, Liu T. et al. RESCUE-AF in patients undergoing atrial fibrillation ablation. Circ Arrhythm Electrophysiol 2019;12:e007044. [DOI] [PubMed] [Google Scholar]
- 21. Leung LW, Gallagher MM, Santangeli P, Tschabrunn C, Guerra JM, Campos B. et al. Esophageal cooling for protection during left atrial ablation: a systematic review and meta-analysis. J Interv Card Electrophysiol 2019. doi:10.1007/s10840-019-00661-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tsuchiya T, Ashikaga K, Nakagawa S, Hayashida K, Kugimiya H.. Atrial fibrillation ablation with esophageal cooling with a cooled water-irrigated intra-esophageal balloon: a pilot study. J Cardiovasc Electrophysiol 2007;18:145–50. [DOI] [PubMed] [Google Scholar]
- 23. Knopp H, Halm U, Lamberts R, Knigge I, Zachaus M, Sommer P. et al. Incidental and ablation-induced findings during upper gastrointestinal endoscopy in patients after ablation of atrial fibrillation: a retrospective study of 425 patients. Heart Rhythm 2014;11:574–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available upon request to: lleung@sgul.ac.uk.