Abstract
The aging proteostasis decline manifests in a failure of aging cells and organisms to properly respond to proteotoxic challenges. This proteostasis collapse has long been considered a hallmark of aging in nematodes, and has recently been shown to occur also in human cells upon entry to senescence, opening the way to exploring the phenomenon in the broader context of human aging. Cellular senescence is part of the normal human physiology of aging, with senescent cell accumulation as a prominent feature of aged tissues. Being highly resistant to cell death, senescent cells, as they accumulate, become pro-inflammatory and promote disease. Here we discuss the causes of human senescence proteostasis decline, in view of the current literature on nematodes, on the one hand, and senescence, on the other hand. We review two major aspects of the phenomenon: (1) the decline in transcriptional activation of stress-response pathways, and (2) impairments in proteasome function. We further outline potential underlying mechanisms of transcriptional proteostasis decline, focusing on reduced chromatin dynamics and compromised nuclear integrity. Finally, we discuss potential strategies for reinforcing proteostasis as a means to improve organismal health and address the relationship to senolytics.
Keywords: Protein homeostasis, Proteostasis, Aging, Senescence, HSF1, ATF6, Heat shock response, HSR, UPR
The proteostasis network, the major regulator of cellular protein homeostasis (i.e. proteostasis), is composed of molecular chaperones protecting cells from protein misfolding and aggregation, and protein clearance pathways such as the proteasome and autophagy systems [1]. A sharp increase in the prevalence of many neurodegenerative diseases whose hallmark is protein misfolding and aggregation, is observed with age, including Parkinson's disease, Alzheimer's disease, and others [2]. An impaired cellular stress response during aging has long been thought to contribute to age-related diseases [[2], [3], [4]]. This is in line with the notion that the function of the proteostasis network deteriorates with age, a phenomenon termed “proteostasis collapse” [5,6]. This phenomenon manifests in a decline in the ability of aging cells and organisms to properly induce stress response pathways upon proteotoxic challenges, and a deterioration in the capacity of the proteostasis network to handle protein misfolding and aggregation with age [[5], [6], [7], [8]]. A decade since its initial discovery in nematodes [6], the aging proteostasis collapse has been demonstrated to be an organism-level phenomenon, which begins upon entry into adulthood, and to be closely related to the organism's reproductive capacity [[9], [10], [11]]. It has further been shown to be modulated by different cues and signals in a non-autonomous fashion [7,8]. As we have recently reported, proteostasis decline also occurs in human senescent cells [12]. Together with other reports indicating physiological relevance [[13], [14], [15], [16]], it is becoming increasingly clear that the phenomenon of the aging proteostasis decline extends to humans.
The decline in proteostasis with age could be a consequence of increased burden on the proteostasis regulatory network due to the accumulation of misfolded and damaged proteins throughout an organism's life, or, conversely, a programmed, regulatory event activated upon aging that impairs the ability of the network to properly respond to stress, or a combination of both [7,8]. Human fibroblasts immediately after entry into senescence show no basal difference in chaperone expression in the absence of stress, but when subjected to heat shock, their ability to induce chaperones is diminished [12]. Further support comes from the finding that chaperone levels in aged human brains bear a distinct signature, where some chaperones were either basally reduced or induced compared to young brains [17]. Interestingly, the group of aged-brain-reduced chaperones showed a significant proteostasis decline behavior in senescent human fibroblasts [12]. This may suggest that the same chaperones that are subject to a programmed regulatory decline are also eventually reduced with age, perhaps due to a combination of programmed decline and a continuous accumulation of damaged proteins, which leads to chronic stress. Thus, with the decline in proteostasis generating a conceptual link between aging and misfolding diseases, a deep understanding of the phenomenon, including its causes and consequences, is crucial for improving the health of aging human beings.
In this review, we discuss possible causes of the aging proteostasis decline phenomenon, with a specific focus on human senescence, and present some strategies for proteostasis manipulation with an outlook for improving the health of aging humans.
1. Proteostasis decline in nematodes and humans
Senescence, originally discovered by Hayflick et al. [20], denotes the entry of dividing cells into a state of irreversible growth arrest [18,19]. While the term was initially used to describe a finite division capacity of cultured human cells [20], it was later found that cells in the human body can enter senescence either after exhausting their replicative lifespan, due to telomere shortening, or in response to various stresses, be it oxidative stress [21], unresolved DNA damage [22], oncogene activation [23] or other insults [18]. In addition to exhibiting permanent cell cycle arrest, senescent cells have distinct characteristics, including specific metabolic changes [24] and a senescence-associated pro-inflammatory secretory phenotype, also known as SASP [25].
Senescence is closely tied to aging in humans. Senescent cells accumulate in aged tissues [26], and although highly resistant to cell death [27], their accumulation with aging is associated with various adverse effects, such as inflammation and the promotion of disease [28]. Thus, proteostasis decline in senescent cells could be a major contributor to the exacerbation of the toxic environment in aged tissues, as well as be directly involved in the development of protein misfolding diseases.
While senescence is part of the human aging process, which is substantially different from the aging of the invertebrate nematode, the decline in proteostasis seems to be common to both organisms. Nevertheless, some of the molecular characteristics of the aging proteostasis decline differ between the two systems. While proteostasis collapse in nematodes can be modulated by external signals in a non-autonomous manner [4,5], whether or not it naturally occurs in a cell-autonomous fashion is yet to be resolved. In senescent human fibroblasts, proteostasis decline does occur in an intrinsically cell-autonomous manner [12], implying that at least part of the phenomenon is regulated [8]. Whether proteostasis decline in human senescence can be delayed or altered in response to external cues remains to be explored.
The human senescence proteostasis decline is manifested in the impaired transcriptional induction, upon heat stress, of both the Heat Shock Response (HSR), responsible for cytosolic misfolding, and the Unfolded Protein Response (UPR) of the ER [12]. In nematodes, too, a strong, sharp reduction in both HSR and UPR functions was observed upon early adulthood [9,10], modulated by the suppression of HSF1-mediated transcription of stress response genes [9,10]. Moreover, HSF1 DNA binding has been shown to be hampered both in senescent human fibroblasts [29,30] and in aging nematodes [9].
Some molecular features, however, are different between the two organisms. While the nuclear localization of both HSF1 and ATF6 was found to be deficient in senescent human cells under heat stress [12], the nuclear localization of HSF1 was shown to be intact in aging nematodes [9]. There are also differences between the stress sensing capabilities of the two organisms. Senescent human fibroblasts exhibit enhanced stress sensing, manifested by exacerbated XBP1-splicing following heat shock [12]. Conversely, in nematodes, UPR sensing by the IRE1-XBP1 axis fails completely upon aging [31,32].
Proteasomal function in both states of stress and recovery was diminished in human fibroblasts upon entry into senescence [12], with proteasome basal function and levels shown to further deteriorate with cellular age (further discussed below). In C. elegans, too, proteasomal activity deteriorates with aging [33], while the induction of proteasomal activity was shown to extend the nematode's lifespan [34].
In terms of chromatin landscape, an increase in the H3K27me3 repressive chromatin modification was shown to be a major feature underlying nematode proteostasis collapse (see more below) [9]. In human senescence, the role of chromatin modifications in proteostasis decline has yet to be studied, but it seems that H3K27me3 cannot explain the phenomenon, as senescent chromatin demonstrates a much more complex orchestration, as discussed below (see Box 1 for an introduction to chromatin modifications).
Box 1. Chromatin modifications.
Chromatin modifications play a major role in the regulation of gene expression by controlling the access of the transcription machinery, as well as transcriptional regulators, to gene promoters [35]. Different histone modifications facilitate the compacting or opening of DNA, leading to changes in the levels of gene expression, and non-linear effects of different combinations of histone modifications can generate a wide variety of chromatin states, giving rise to the histone code hypothesis [127]. Hundreds of histone modifications have been discovered so far [128]. The most well-known histone modifications include H3K4me3 and H3K27ac near transcription start sites of actively transcribed genes, as well as H3K9me3 and H3K27me3, which are associated with gene repression [129]. Additional types of histone modification changes were described in the context of senescence [43,[47], [48], [49], [50], [51],53], many of which with as-yet-unknown function.
Alt-text: Box 1
In summary, while the phenomenon itself is conserved between nematodes and humans, the molecular characteristics seem to somewhat diverge.
2. Proteostasis decline – potential underlying mechanisms
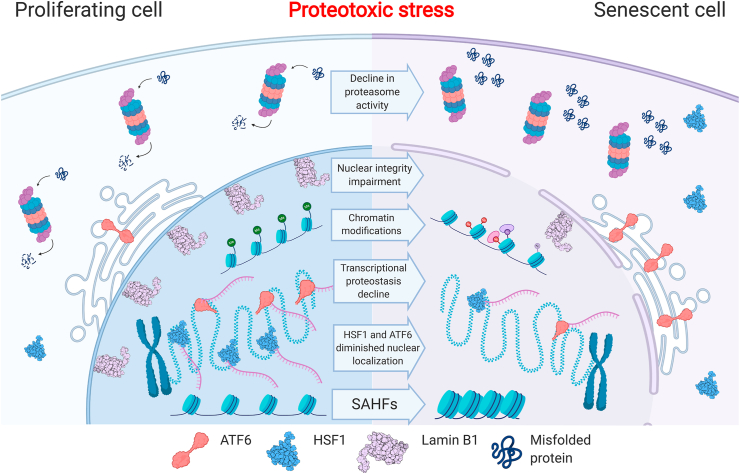
The data above portrays a complex picture of aging-related proteostasis failure, with several potential causes for the impaired transcriptional activation of the HSR and UPR in human senescence. In this section, we discuss some of the underlying causes of the aging proteostasis decline in humans, in view of what is known in the field for nematodes, on one hand, and in the senescence field, on the other hand. Two potential, not mutually exclusive causes are explored: the rise of a chromatin state that hinders the dynamics and the opening of chromatin regions, and impaired nuclear integrity, which would lead to improper transcription factor nuclear entry or nuclear retention upon stress (Fig. 1).
Fig. 1.
Human senescence proteostasis decline – molecular features. Proteostasis decline in replicative senescence involves several impairments, including: decline in proteasomal activity, deterioration in the activation of transcriptional stress responses, and diminished nuclear localization of the HSF1 (structure taken from PDB, ID: 5HDG) and ATF6 stress-response transcription factors. Additional changes in the senescent cell chromatin and nucleus that are suggested here to be potentially involved in mediating the proteostasis decline are: formation of senescence-associated heterochromatin foci (SAHF); wide-spread changes in the chromatin modification landscape; and impairment in nuclear integrity, including loss of Lamin B1 (structure taken from PDB, ID: 2KPW).
2.1. Underlying mechanisms of the senescence proteostasis decline – the chromatin hypothesis
Labbadia and Morimoto have found that the H3K27me3 histone modification, a repressive chromatin mark [35] (see Box 1 for an introduction to chromatin modifications), is increased in several HSR promoters in aged nematodes, with a concomitant reduction in the expression of H3K27 demethylase jmjd-3.1 [9]. Interestingly, forced expression of jmjd-3.1 was found to maintain a proper induction of HSR genes in aging nematodes [9] and to increase the nematode lifespan [36]. Therefore, in aged nematodes, a direct link was demonstrated between diminished proteostasis at the level of transcription and a repressive chromatin state in HSR promoters.
This mode of regulation seems to be a characteristic of aged chromatin also in other organisms. For example, a reduction in the H3K4me3 and H3K36me3 activation histone marks and an increase in the H3K9me3 repressive histone mark was noted in aged flies [37]. In killifish, a global upregulation of the H3K27me3 repressive histone mark has likewise been observed upon aging [38]. While not showing causality, these evidences may support the notion that a more repressive chromatin state at chaperone gene loci specifically, or perhaps also more globally, underlies the deteriorated induction of the HSR and UPR during aging.
2.1.1. Global chromatin changes in senescent cells
It is becoming increasingly clear that the chromatin landscape of senescent cells in mammals is very different from that of the pre-senescent state [39]. One of the earliest known hallmarks of senescent human fibroblasts is the Senescence-Associated Heterochromatin Foci (SAHF), regions with highly dense heterochromatin and repressive chromatin marks. These foci are linked to the constitutive repression of proliferation genes [40]. SAHF contain chromatin regions that were euchromatic in pre-senescent cells (reviewed in Ref. [39]). Their core is enriched with the H3K9me3 repressive mark, surrounded by a H3K27me3-enriched ring, which moves away from the nuclear envelope [41] and shows low Lamin B1 association [42]. A global increase in the repressive histone modifications H3K27me3 and H4K20me3 and the loss of the H3K4me2 and H3K4me3 activation marks has been observed by Chicas et al. in senescent fibroblasts, both under conditions of oncogene-induced senescence and in replicative senescence [43]. Furthermore, the levels of the H4K20me3 repressive mark have been shown to increase in aged rats [44,45]. More broadly, a general decrease in the histone content of senescent and aged cells has been reported [46], while other histone variants, such as H3.3, were shown to be upregulated in senescence [47]. Additional global changes characteristic of senescent cells include a significant upregulation of the H4K16ac chromatin mark, as observed in senescent fibroblasts, and its presence in the promoters of senescent-expressed genes regardless of their expression level [47].
Other studies have demonstrated vast modulations in the chromatin landscape in senescence, supportive of location-specific changes. Shah et al. found that while the fraction of both the H3K4me3 activating mark and the H3K27me3 repressive mark remain unchanged in senescent human fibroblasts, their patterns are drastically reorganized [48]. Additionally, H4K20me3 accumulation near genes that are suppressed in senescence was described in association with SAHF [49], while H3K9me3-enriched loci tend to form dense heterochromatic regions in both oncogene- and replicative-induced senescent fibroblasts [50]. It has recently been found that senescent fibroblasts contain specific chromatin regions rich with various types of acetylated histones, and that these form senescence-specific enhancers that drive senescence gene expression programs [51].
Thus, various types of global and local chromatin changes have been shown to occur upon entry of cells to senescence, which characterize the chromatin landscape of senescent cells [52]. The possibility that global chromatin modifications render the chromatin in an overall less dynamic state, which globally influences dynamic gene expression programs in general, including the HSR and UPR, remains to be explored.
2.1.2. Examining the potential link between specific chromatin changes and proteostasis decline in human senescence
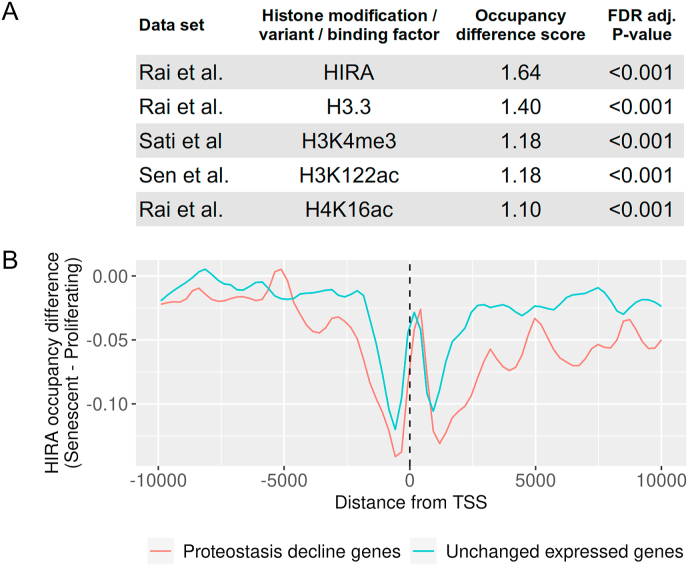
Conceptually, changes in the chromatin landscape of senescent cells could lead to transcriptional proteostasis decline, either due to global effects which would render the chromatin in an overall less dynamic state, or through specific local changes in HSR and UPR promoter regions. If the global chromatin landscape becomes more repressive, it could produce a general effect on the dynamics of chromatin and on additional pathways requiring dynamic changes, including a variety of stress responses. Alternatively, chromatin changes specific to HSR and UPR gene loci could lead to loss of local dynamics, making these promoters less prone to activation. We therefore wished to probed for possible links between chromatin changes in specific regions and transcriptional proteostasis decline. We were interested to ascertain whether we could detect chromatin changes specific to the promoters of genes previously shown to exhibit proteostasis decline in senescent cells; namely, genes that upon heat shock are robustly induced in young fibroblasts but their induction was diminished in senescent cells [12]. We analyzed 16 CHIP-seq datasets from a number of studies, including histone modifications, histone variants (H2A.Z and H3.3), and chromatin binding proteins (CBP, p300, HIRA) from human fibroblasts undergoing replicative senescence [43,[47], [48], [49], [50], [51],53]. We examined the changes between proliferating and senescent CHIP-seq profiles in the promoter regions of proteostasis-decline genes, and compared these changes to randomized sets of genes with similar basal expression levels in young and senescent cells that did not show a change in transcription levels upon stress (termed unchanged expressed genes, Fig. 2A).
Fig. 2.
Survey of senescence chromatin marks reveals significant changes specific to the promoters of human proteostasis-decline genes. (A) The occupancy difference score was calculated as follows: The average density profile of each chromatin mark/chromatin binding factor in the promoters ( ±10 kb around the Transcription Start Site, TSS) of the group of proteostasis-decline genes under senescence conditions was compared to that obtained under proliferating conditions, using Euclidean distance. The proteostasis-decline gene group was previously defined by Sabath et al. [12]. To assess specificity, the same score was calculated for 10,000 random sets of unchanged expressed genes, i.e., genes with a basal expression that is similar to that of the proteostasis-decline gene group but that had not shown a change in expression upon heat shock. The final occupancy difference score was defined as the ratio between the proteostasis-decline genes' score and the average of the unchanged expressed genes' scores. p-values were calculated by comparing the Euclidian distance of the proteostasis-decline group to the randomly sampled sets' distances, followed by FDR adjustment. We note that this score did not identify all types of differences; e.g., if the changes in occupancy between senescence and proliferation for the proteostasis-decline genes showed enrichment/depletion that are inverse from the genome background. Here, however, we verified that none of the analyzed profiles showed this behavior. Five statistically significant chromatin marks/binding factors, from the CHIP-seq datasets by Rai et al. [35], Sati et al. [38] and Sen et al. [39], were found to be significantly specific and are presented in table (B) Relative occupancy profiles (i.e., the difference between the average density profile of senescent cells and proliferating cells). Of the HIRA histone chaperone in the promoter region of the senescence proteostasis-decline genes (red) and an average of the profiles of 10,000 random sets of unchanged expressed genes (blue), defined as in (A). The senescent cells’ profiles show a significant depletion in HIRA binding compared to the rest of the promoters of similarly-expressed genes.
On the one hand, our examination of the H3K27me3 histone mark, shown to be induced in the promoters of HSR genes in aged nematodes [9], found no specific change in the promoters of proteostasis-decline genes of proliferating cells compared to senescent cells. On the other hand, five datasets, HIRA, H3.3, H4K16ac, H3K4me3 and H3K122ac, differed between the senescent and proliferating state specifically in the proteostasis-decline gene group (Fig. 2A), with HIRA exhibiting the most pronounced change.
The histone chaperone HIRA [54], is suggested to facilitate the deposition of variants of histone H3.3 and histone H4 into chromatin in a DNA-replication-independent manner in senescent fibroblasts [47]. Interestingly, the promoters of proteostasis-decline genes feature a major reduction in HIRA binding in senescent cells (Fig. 2B). While Rai et al. have suggested a partial correlation between HIRA binding and H3.3 [47], we did not observe such a correlation in the proteostasis-decline gene group. As HIRA is suggested to facilitate dynamic chromatin in senescent cells, less HIRA occupancy at proteostasis-decline gene promoter regions is perhaps indicative of a chromatin state that is less dynamic and that disfavors rapid activation upon external stimuli. Such a chromatin environment would deny HSF1, ATF6 and perhaps other stress transcription factors full access to their target promoters, while excess transcription factor molecules would easily exit the nucleus, thereby resulting in a diminished capacity for HSR and UPR transcriptional activation (Fig. 1). In support of this notion, HIRA was shown to facilitate the dynamic activation of innate immune-response genes upon viral infection [55]. Nevertheless, additional studies are required in order to elucidate the potential role of HIRA, as well as other chromatin modifications, and combinations thereof, in the possible loss of chromatin dynamics at proteostasis-decline gene loci.
2.1.3. Transposon and repeat derepression in senescence
Chromatin reorganization has been linked to aberrant transposon and repeat expression in senescence and aging. Increased transposon activation during aging was demonstrated in various invertebrates, including in nematodes [56] and in fly brains [57], while transposon silencing was shown to increase the fruit fly lifespan [58]. Retrotransposons, which are usually located in highly repressed regions of the genome and have very low expression levels in non-stressed proliferating cells, were significantly induced in senescent human fibroblasts [59]. Likewise, the activation of retrotransposons is greater in aging mouse tissues [60]. Accordingly, derepression of LINE1 retrotransposons was shown in senescent human and mouse fibroblasts, as well as in aged mice [61].
The derepression of satellite repeats, too, has been shown to occur after entry to senescence in both mouse and human cells [60,62]. Interestingly, HSF1's localization to a few (1–4) distinct nuclear foci upon heat stress was described in several human cell types [63], including in young fibroblasts [12], and has been demonstrated to occur at satellite III regions [64]. In senescence, however, aside from reduced nuclear localization, HSF1's distribution to distinct foci is greatly impaired, with many disorganized foci appearing [12]. Intriguingly, an inverse correlation was demonstrated between the number of HSF1 foci and chaperone activation in response to stress [65]. This further substantiates the link between multiple HSF1 disorganized foci in senescent cells and senescence HSR proteostasis decline.
The expression of transposons and satellite repeats during senescence would jointly pose another layer of misregulation, which, together with an altered chromatin landscape, could affect HSF1 function, in particular, and perhaps also transcription factor accessibility and dynamic redistribution, in general, thereby contributing to the transcriptional proteostasis decline.
It, therefore, seems that the chromatin landscape of senescent cells is highly altered, with large regions of newly-identified combinations of histone marks, whose function is not completely clear, highly repressed SAHF regions, and aberrant transposon and repeat expression (Fig. 1). Future studies of various types of modifications, and their ability to dynamically change in response to stress will illuminate their potential role in the inaccessibility of stress-response regions to their corresponding transcription factors, be it HSF1, ATF6 or others, and in the deteriorated ability of senescent cells to initiate transcriptional stress responses.
2.2. Underlying mechanisms of the senescence proteostasis decline – nuclear integrity
Nuclear integrity has also been shown to deteriorate in senescence and aging. This is indeed the case with respect to the lamins, the major architectural proteins of the animal cell nucleus. Their disfunction is tightly linked to aging, as well as to senescence [66]. Lamin B1, for instance, shows decreased expression upon senescence [67,68]. Its loss has major consequences for nuclear and genome organization [36]; it is involved in the formation of SAHF and their translocation from the nuclear lamina [30], and plays a role in massive rearrangements related to the generation of large-scale H3K27me3-rich “mesas” [36]. Lamin B1 loss can also lead to premature senescence [49,50] and is considered one of the key events driving senescent nuclear phenotypes. In C. elegans, too, nuclear lamins plays a role in aging-dependent changes in nuclear organization, with a reduction in lamin levels leading to a shortened nematode lifespan [69]. Lamin A/C is likewise implicated in senescence. Hutchinson–Gilford Progeria Syndrome (HGPS), characterized by premature aging and senescence, is caused by Lamin A splice-isoform mutations that lead to a dominant negative function of Lamin A/C, with various consequences for nuclear structure and chromatin organization (reviewed in Refs. [66,70]). The regulation of lamins during the normal course of aging shows parallel features to HGPS phenotypes (reviewed in Ref. [39]). Specifically, cells from old individuals showed aberrant Lamin A/C localization and marked depletion of Lamin A/C from the nucleoplasm, reminiscent of the hallmark phenotype of HGPS [45].
Another feature of senescent cells is blebs of chromatin positive for the H3K27me3 histone mark, as well as for gamma-H2AX, an indicator of damaged DNA, located in the cytoplasm [71]. These blebs represent a nuclear-autophagy (nucleo-phagy) mechanism [72] and have been shown to enhance inflammation in senescent and aged cells [73,74].
In addition to the compromised integrity of the nuclear envelope itself, nuclear import and export mechanisms (also known as nuclear-cytoplasmic shuttling) are also impaired in aging and senescence [75]. Fibroblasts from aged individuals display a reduction in several nuclear import factors, resulting in lower transport activity [76]. Moreover, excessive nuclear export activity has been demonstrated in HGPS [77]. Nuclear shuttling regulation is known to play an important role in the regulation and maintenance of protein homeostasis. Stress-induced changes in nuclear shuttling are involved in maintaining proteostasis under conditions of proteotoxic stress, including the rapid relocation of cytosolic HSP70 chaperones to the nucleus [78]. Additionally, nucleocytoplasmic transport is disrupted in neurodegenerative diseases involving protein aggregates, such as amyotrophic lateral sclerosis (ALS) and others [79]. Therefore, a deterioration in nuclear-cytoplasmic shuttling could serve as a contributing factor to the senescent proteostasis decline. Many transcription factors utilize cytoplasmic-nuclear shuttling as a key step in their activation cycle, including HSF1 [80]. HSF1 has been shown to utilize importin alpha/beta to translocate to the nucleus during stress [81]. As the nuclear localization of HSF1 and ATF6 is diminished in stressed senescent cells [12], it is possible that deficient nuclear import and excessive nuclear export, together with a compromised nuclear envelope, could lead to nuclear deficiency of these transcription factors in senescent cells.
Thus, nuclear integrity defects, and changes in nuclear-cytoplasmic shuttling, could have direct consequences to senescence proteostasis decline. Future studies would unravel the contribution of deteriorated nuclear integrity and import/export mechanisms to compromised transcription factor localization as part of the transcriptional proteostasis decline.
2.3. Proteasome and protein-clearance dysfunction in senescence and aging
Proteasome function has been shown to decline in senescence and aging [82,83]. Upon entering senescence, WI-38 fibroblasts have a basal proteasome function similar to that of young fibroblasts, but once subjected to heat stress, their proteasome function becomes impaired and cannot recover [12]. Importantly, at this stage, the decline is not accompanied by downregulation at the proteasome subunit levels [12]. Further, it was shown that basal proteasome activity declines significantly in late-passage senescent human fibroblasts, again without being accompanied by diminished proteasome levels [84,85]. At later stages, however, WI-38 fibroblasts do show a significant downregulation of several proteasome beta subunits [86]. Thus, it appears that fibroblasts’ transition to senescence is accompanied by a gradual deterioration in proteasome function, whereby at first its function upon proteotoxic challenges is diminished, then its basal activity deteriorates, and finally proteasome levels are downregulated.
Interestingly, partial inhibition of the proteasome in young cells was shown to induce a senescence-like phenotype [87]. Treating WI-38 fibroblasts with prolonged non-lethal doses of proteasome inhibitors induced an irreversible growth-arrest, senescent-like morphology and up-regulated the senescence biomarker beta-galactosidase [86,87]. Therefore, while proteasome impairments are considered a characteristic of the aging proteostasis decline, it is also possible that they are, at least in part, responsible for the proteostasis decline observed in senescent cells.
It has also been reported that proteasome impairments upon senescence are followed by a significant accumulation of oxidized, cross-linked and ubiquitinated proteins [85,86]. Further research showed a connection between proteasome dysfunction during senescence and mitochondrial dysregulation, including involvement in the increased production of reactive oxygen species [88], a typical characteristic of senescent cells and tissues [89]. This could suggest that the decline in proteasome activity in senescence both contributes to the accumulation of damaged proteins, which burdens the proteostasis network, and leads to a rise in oxidized protein levels, further impairing proteostasis.
As opposed to senescent cells, embryonic stem cells, which are naturally immortal, sustain high levels of proteasome activity, regulated by the 19S proteasome subunit PSMD11 [90]. In nematodes, the homolog of human PSMD11, RPN-6, confers resistance to proteotoxic stress and increases longevity [34]. In D. melanogaster, too, germline tissues feature increased proteasome activity, which does not decline with aging, unlike in somatic tissues [82]. Interestingly, proteasome activity did not decline in aging germline-ablated nematodes [34], which display delayed proteostasis collapse. These findings in flies and nematodes have been proposed to support the disposable soma theory of aging [82,91,92], which claims that a regulatory mechanism that preferentially allocates resources to the germline, to the detriment of the somatic tissues, might give an evolutionary advantage to the organism.
Enhancement of autophagy, another major protein clearance mechanism in eukaryotes [93], has been mainly considered beneficial to aging. In particular, the stimulation of autophagy by genetic or pharmacological means increased the lifespan of many organisms (reviewed in Ref. [94]), while inhibition of autophagy led to premature aging in nematodes [95] and fruit flies [96]. In mice, tissue-specific autophagy defects prompted a variety of aging-related phenotypes, such as sarcopenia [97]. Additionally, caloric restriction, a well-known lifespan-increasing intervention in many organisms [98,99], is thought to function via autophagy induction, with autophagy inhibition reliably preventing the anti-aging effects of caloric restriction [100]. The general notion has been that, similarly to proteasomal function, autophagy deteriorates with age [101]. Studies in nematodes and fruit flies have shown a decrease in autophagy activity with age (reviewed in Refs. [5,101]). Similarly, studies of autophagy in liver cells of aging rodents have indicated decreased autophagy activity [5,101]. Lysosomal activity was also shown to decline in human BJ fibroblasts that underwent replicative senescence [85]. These evidences support the notion that autophagic functions deteriorate with age, which may contribute to aging proteostasis decline. Yet, lysosomal activity remains unchanged or increases when BJ fibroblasts undergo stress-induced senescence [102], and increases in IMR90 fibroblasts upon oncogene-induced senescence [103]. Furthermore, another study has demonstrated induced autophagy activity in senescent IMR90 fibroblasts, as well as in aging mouse brains [104]. To resolve these discrepant findings, it has been suggested that the role of autophagy in senescence might depend on the type of senescence, i.e., stress- or oncogene-induced vs. replicative, and the stage of senescence, i.e., early vs. late stage [105,106]. Thus, while there is a consensus about the deterioration in proteasome activity and function in senescence and aging, studies portray a complex relationship between autophagy and senescence.
3. Modulation of proteostasis to improve aging health
The aging proteostasis decline thus involves the broad proteostasis network, from a deterioration in the HSR and UPR to a gradual decline in proteasome and protein clearance functions (Fig. 1). These principles are conserved from nematode to human, including human senescence, and may be a driving force of the human aging phenotype.
While proteostasis decline seems to be a conserved hallmark of aging, it is also necessary to look beyond the important questions regarding the underlying mechanisms, to the potential implications for organismal health. As the lifespan of humans increases, so do aging-related diseases, many of which are proteostasis-related, representing a major health and economic challenge [2]. It is, therefore, pertinent to ask not only how do we increase the human lifespan but also how can we improve the health of the aging human being, or in other words, the human healthspan.
Two potential strategies could be employed in the context of the human proteostasis decline: (1) Reinforcement of the proteostasis network, which would potentially enhance its deteriorated function and aid in the battle against disease-causing protein misfolding and aggregation; or (2) induce the complete breakdown of the proteostasis network, to eliminate the potentially harmful senescent cell.
Reinforcement of the proteostasis network has been the focus of several studies in nematodes. In particular, the aging proteostasis decline has been shown to be controlled by external stimuli in a non-autonomous fashion [5,7,107], primarily by reproductive capacities [7,9,10,108]. Directly boosting the proteostasis network, via, for example, the overexpression of HSF1 or proteasome subunits, has been shown to delay proteostasis decline in C. elegans [6,34]. Other non-autonomous factors, such as various hormones, have likewise been demonstrated to modulate the phenomenon (reviewed in Refs. [5,7]).
Of particular interest are exogenous factors that have been shown to affect the proteostasis capacity during aging. Primarily, dietary restriction has long been known to positively affect organismal lifespan in numerous species, from yeast to primates (reviewed in Refs. [98,99]), and has also been demonstrated to substantially improve proteostasis in aged nematodes [7,109,110]. In humans, caloric restriction was shown to improve proteostasis [111] and reduce markers of senescence [112]. Interestingly, specific dietary supplements have been shown to improve the proteostasis capacity in aged nematodes, including oleic acid [113] and arachidonic acid [110], which was found to modulate HSF1 function [114]. As dietary restrictions extend the lifespan of mammals, the potential of specific dietary supplements or modifications to improve proteostasis during aging in humans is highly appealing, and needs to be further investigated in mammalian organisms.
As senescent cells accumulate in aged tissues, they become harmful. They develop a typical senescence-associated secretory phenotype, SASP, which becomes chronically pro-inflammatory [25,28]. Moreover, senescent cells are highly resilient to various kinds of stress-induced apoptosis [27], which could potentially aggravate their toxic effect on the surrounding tissue. It has been shown that the elimination of senescent cells have a positive influence on aged organismal health [115,116], as well as on age-related diseases such as Alzheimer's disease [117,118]. These observations have led to the new field of senolytic therapeutics, drugs that specifically target and eliminate senescent cells [116,119]. Interestingly, HSP90 inhibitors have been found to have senolytic activity [120,121]. Specifically, Geldanamycin and 17-AAG can selectively kill senescent cells, and other HSP90 inhibitors were shown to be even more specific [120]. This senolytic activity has already been demonstrated in several mouse and human cell types [120,122], as well as in a mouse model of progeria [120].
HSP90 has been shown to also repress the regulatory effects of some retrotransposons (ERVs) on neighboring genes [123] as well as transposon-facilitated mutagenesis [124]. As transposon deregulation was shown to occur in senescence [59,60], as detailed above, other potential mechanisms of the senolytic effect can be hypothesized, where HSP90 inhibition exacerbates an already disturbed transcriptional landscape, leading to increased cell mortality.
Interestingly, HSP90 inhibitors contribute to longevity in aged nematodes through the indirect activation of HSF1 [125,126]. Therefore, it seems that while, in general, HSP90 inhibitors may have beneficial effects on longevity through HSF1-mediated reinforcement of the proteostasis network, HSP90 inhibition specifically in senescent cells can lead to cell death, thereby indirectly contributing to organismal health.
While the study of the causes, consequences and implications of proteostasis decline in human aging is still in its infancy, future research will delineate the proteostasis decline effects on human health, including the interplay between proteostasis reinforcement and proteostatic senolytics.
Author credit statement
Anatoly Meller: Data curation, Formal analysis, writing. Reut Shalgi: Supervision, Conceptualization, writing.
Declaration of competing interest
The authors declare no competing interests.
Acknowledgments
We thank Flonia Levy-Adam, Kinneret Rozales and Amal Younis for their critical reading of the manuscript. A.M. and R.S. have received funding from the European Research Council under the European Union's Horizon 2020 programme Grant 677776.
References
- 1.Hartl F.U., Bracher A., Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475(7356):324–332. doi: 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- 2.Haigis M.C., Yankner B.A. The aging stress response. Mol. Cell. 2010;40(2):333–344. doi: 10.1016/j.molcel.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez-Otin C. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Labbadia J., Morimoto R.I. The biology of proteostasis in aging and disease. Annu. Rev. Biochem. 2015;84:435–464. doi: 10.1146/annurev-biochem-060614-033955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor R.C., Dillin A. Aging as an event of proteostasis collapse. Cold Spring Harb Perspect Biol. 2011;3(5) doi: 10.1101/cshperspect.a004440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ben-Zvi A., Miller E.A., Morimoto R.I. Collapse of proteostasis represents an early molecular event in Caenorhabditis elegans aging. Proc. Natl. Acad. Sci. U. S. A. 2009;106(35):14914–14919. doi: 10.1073/pnas.0902882106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shai N., Shemesh N., Ben-Zvi A. Remodeling of proteostasis upon transition to adulthood is linked to reproduction onset. Curr. Genom. 2014;15(2):122–129. doi: 10.2174/1389202915666140221005023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morimoto R.I. Cell-Nonautonomous regulation of proteostasis in aging and disease. Cold Spring Harb Perspect Biol. 2020;12(4) doi: 10.1101/cshperspect.a034074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Labbadia J., Morimoto R.I. Repression of the heat shock response is a programmed event at the onset of reproduction. Mol. Cell. 2015;59(4):639–650. doi: 10.1016/j.molcel.2015.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shemesh N., Shai N., Ben-Zvi A. Germline stem cell arrest inhibits the collapse of somatic proteostasis early in Caenorhabditis elegans adulthood. Aging Cell. 2013;12(5):814–822. doi: 10.1111/acel.12110. [DOI] [PubMed] [Google Scholar]
- 11.Sala A.J. Embryo integrity regulates maternal proteostasis and stress resilience. Genes Dev. 2020;34(9–10):678–687. doi: 10.1101/gad.335422.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sabath N. Proc Natl Acad Sci U S A; 2020. Cellular proteostasis decline in human senescence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall D.M. Aging reduces adaptive capacity and stress protein expression in the liver after heat stress. J. Appl. Physiol. 1985;89(2):749–759. doi: 10.1152/jappl.2000.89.2.749. 2000. [DOI] [PubMed] [Google Scholar]
- 14.Heydari A.R. Expression of heat shock protein 70 is altered by age and diet at the level of transcription. Mol. Cell Biol. 1993;13(5):2909–2918. doi: 10.1128/mcb.13.5.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kregel K.C., Moseley P.L. Differential effects of exercise and heat stress on liver HSP70 accumulation with aging. J. Appl. Physiol. 1985;80(2):547–551. doi: 10.1152/jappl.1996.80.2.547. 1996. [DOI] [PubMed] [Google Scholar]
- 16.Locke M., Tanguay R.M. Diminished heat shock response in the aged myocardium. Cell Stress Chaperones. 1996;1(4):251–260. doi: 10.1379/1466-1268(1996)001<0251:dhsrit>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brehme M. A chaperome subnetwork safeguards proteostasis in aging and neurodegenerative disease. Cell Rep. 2014;9(3):1135–1150. doi: 10.1016/j.celrep.2014.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharpless N.E., Sherr C.J. Forging a signature of in vivo senescence. Nat. Rev. Canc. 2015;15(7):397–408. doi: 10.1038/nrc3960. [DOI] [PubMed] [Google Scholar]
- 19.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120(4):513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Hayflick L., Moorhead P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 21.Chen J.H., Ozanne S.E., Hales C.N. Methods of cellular senescence induction using oxidative stress. Methods Mol. Biol. 2007;371:179–189. doi: 10.1007/978-1-59745-361-5_14. [DOI] [PubMed] [Google Scholar]
- 22.von Zglinicki T. Human cell senescence as a DNA damage response. Mech. Ageing Dev. 2005;126(1):111–117. doi: 10.1016/j.mad.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 23.Courtois-Cox S., Jones S.L., Cichowski K. Many roads lead to oncogene-induced senescence. Oncogene. 2008;27(20):2801–2809. doi: 10.1038/sj.onc.1210950. [DOI] [PubMed] [Google Scholar]
- 24.Wiley C.D., Campisi J. From ancient pathways to aging cells-connecting metabolism and cellular senescence. Cell Metabol. 2016;23(6):1013–1021. doi: 10.1016/j.cmet.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coppe J.P. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campisi J., d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007;8(9):729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 27.Soto-Gamez A., Quax W.J., Demaria M. Regulation of survival networks in senescent cells: from mechanisms to interventions. J. Mol. Biol. 2019;431(15):2629–2643. doi: 10.1016/j.jmb.2019.05.036. [DOI] [PubMed] [Google Scholar]
- 28.Campisi J. Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Westerheide S.D. Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1. Science. 2009;323(5917):1063–1066. doi: 10.1126/science.1165946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zelin E., Freeman B.C. Lysine deacetylases regulate the heat shock response including the age-associated impairment of HSF1. J. Mol. Biol. 2015;427(7):1644–1654. doi: 10.1016/j.jmb.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor R.C., Dillin A. XBP-1 is a cell-nonautonomous regulator of stress resistance and longevity. Cell. 2013;153(7):1435–1447. doi: 10.1016/j.cell.2013.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor R.C. Aging and the UPR(ER) Brain Res. 2016;1648(Pt B):588–593. doi: 10.1016/j.brainres.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 33.Hamer G., Matilainen O., Holmberg C.I. A photoconvertible reporter of the ubiquitin-proteasome system in vivo. Nat. Methods. 2010;7(6):473–478. doi: 10.1038/nmeth.1460. [DOI] [PubMed] [Google Scholar]
- 34.Vilchez D. RPN-6 determines C. elegans longevity under proteotoxic stress conditions. Nature. 2012;489(7415):263–268. doi: 10.1038/nature11315. [DOI] [PubMed] [Google Scholar]
- 35.Zhou V.W., Goren A., Bernstein B.E. Charting histone modifications and the functional organization of mammalian genomes. Nat. Rev. Genet. 2011;12(1):7–18. doi: 10.1038/nrg2905. [DOI] [PubMed] [Google Scholar]
- 36.Merkwirth C. Two conserved histone demethylases regulate mitochondrial stress-induced longevity. Cell. 2016;165(5):1209–1223. doi: 10.1016/j.cell.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wood J.G. Chromatin remodeling in the aging genome of Drosophila. Aging Cell. 2010;9(6):971–978. doi: 10.1111/j.1474-9726.2010.00624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baumgart M. RNA-seq of the aging brain in the short-lived fish N. furzeri - conserved pathways and novel genes associated with neurogenesis. Aging Cell. 2014;13(6):965–974. doi: 10.1111/acel.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sen P. Epigenetic mechanisms of longevity and aging. Cell. 2016;166(4):822–839. doi: 10.1016/j.cell.2016.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Narita M. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003;113(6):703–716. doi: 10.1016/s0092-8674(03)00401-x. [DOI] [PubMed] [Google Scholar]
- 41.Chandra T. Independence of repressive histone marks and chromatin compaction during senescent heterochromatic layer formation. Mol. Cell. 2012;47(2):203–214. doi: 10.1016/j.molcel.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sadaie M. Redistribution of the Lamin B1 genomic binding profile affects rearrangement of heterochromatic domains and SAHF formation during senescence. Genes Dev. 2013;27(16):1800–1808. doi: 10.1101/gad.217281.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chicas A. H3K4 demethylation by Jarid1a and Jarid1b contributes to retinoblastoma-mediated gene silencing during cellular senescence. Proc. Natl. Acad. Sci. U. S. A. 2012;109(23):8971–8976. doi: 10.1073/pnas.1119836109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sarg B. Postsynthetic trimethylation of histone H4 at lysine 20 in mammalian tissues is associated with aging. J. Biol. Chem. 2002;277(42):39195–39201. doi: 10.1074/jbc.M205166200. [DOI] [PubMed] [Google Scholar]
- 45.Scaffidi P., Misteli T. Lamin A-dependent nuclear defects in human aging. Science. 2006;312(5776):1059–1063. doi: 10.1126/science.1127168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O'Sullivan R.J. Reduced histone biosynthesis and chromatin changes arising from a damage signal at telomeres. Nat. Struct. Mol. Biol. 2010;17(10):1218–1225. doi: 10.1038/nsmb.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rai T.S. HIRA orchestrates a dynamic chromatin landscape in senescence and is required for suppression of neoplasia. Genes Dev. 2014;28(24):2712–2725. doi: 10.1101/gad.247528.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shah P.P. Lamin B1 depletion in senescent cells triggers large-scale changes in gene expression and the chromatin landscape. Genes Dev. 2013;27(16):1787–1799. doi: 10.1101/gad.223834.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nelson D.M. Mapping H4K20me3 onto the chromatin landscape of senescent cells indicates a function in control of cell senescence and tumor suppression through preservation of genetic and epigenetic stability. Genome Biol. 2016;17(1):158. doi: 10.1186/s13059-016-1017-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sati S. 4D genome rewiring during oncogene-induced and replicative senescence. Mol. Cell. 2020;78(3):522–538 e9. doi: 10.1016/j.molcel.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sen P. Histone acetyltransferase p300 induces de novo super-enhancers to drive cellular senescence. Mol. Cell. 2019;73(4):684–698 e8. doi: 10.1016/j.molcel.2019.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang N., Sen P. The senescent cell epigenome. Aging (N Y) 2018;10(11):3590–3609. doi: 10.18632/aging.101617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muniz L. Control of gene expression in senescence through transcriptional read-through of convergent protein-coding genes. Cell Rep. 2017;21(9):2433–2446. doi: 10.1016/j.celrep.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 54.Hammond C.M. Histone chaperone networks shaping chromatin function. Nat. Rev. Mol. Cell Biol. 2017;18(3):141–158. doi: 10.1038/nrm.2016.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McFarlane S. The histone chaperone HIRA promotes the induction of host innate immune defences in response to HSV-1 infection. PLoS Pathog. 2019;15(3) doi: 10.1371/journal.ppat.1007667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lund J. Transcriptional profile of aging in C. elegans. Curr. Biol. 2002;12(18):1566–1573. doi: 10.1016/s0960-9822(02)01146-6. [DOI] [PubMed] [Google Scholar]
- 57.Li W. Activation of transposable elements during aging and neuronal decline in Drosophila. Nat. Neurosci. 2013;16(5):529–531. doi: 10.1038/nn.3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wood J.G. Chromatin-modifying genetic interventions suppress age-associated transposable element activation and extend life span in Drosophila. Proc. Natl. Acad. Sci. U. S. A. 2016;113(40):11277–11282. doi: 10.1073/pnas.1604621113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.De Cecco M. Genomes of replicatively senescent cells undergo global epigenetic changes leading to gene silencing and activation of transposable elements. Aging Cell. 2013;12(2):247–256. doi: 10.1111/acel.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Cecco M. Transposable elements become active and mobile in the genomes of aging mammalian somatic tissues. Aging (N Y) 2013;5(12):867–883. doi: 10.18632/aging.100621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van Meter M. SIRT6 represses LINE1 retrotransposons by ribosylating KAP1 but this repression fails with stress and age. Nat. Commun. 2014;5:5011. doi: 10.1038/ncomms6011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Swanson E.C. Higher-order unfolding of satellite heterochromatin is a consistent and early event in cell senescence. J. Cell Biol. 2013;203(6):929–942. doi: 10.1083/jcb.201306073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jolly C., Usson Y., Morimoto R.I. Rapid and reversible relocalization of heat shock factor 1 within seconds to nuclear stress granules. Proc. Natl. Acad. Sci. U. S. A. 1999;96(12):6769–6774. doi: 10.1073/pnas.96.12.6769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jolly C. In vivo binding of active heat shock transcription factor 1 to human chromosome 9 heterochromatin during stress. J. Cell Biol. 2002;156(5):775–781. doi: 10.1083/jcb.200109018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gaglia G. HSF1 phase transition mediates stress adaptation and cell fate decisions. Nat. Cell Biol. 2020;22(2):151–158. doi: 10.1038/s41556-019-0458-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gruenbaum Y., Foisner R. Lamins: nuclear intermediate filament proteins with fundamental functions in nuclear mechanics and genome regulation. Annu. Rev. Biochem. 2015;84:131–164. doi: 10.1146/annurev-biochem-060614-034115. [DOI] [PubMed] [Google Scholar]
- 67.Freund A. Lamin B1 loss is a senescence-associated biomarker. Mol. Biol. Cell. 2012;23(11):2066–2075. doi: 10.1091/mbc.E11-10-0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shimi T. The role of nuclear lamin B1 in cell proliferation and senescence. Genes Dev. 2011;25(24):2579–2593. doi: 10.1101/gad.179515.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Haithcock E. Age-related changes of nuclear architecture in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U. S. A. 2005;102(46):16690–16695. doi: 10.1073/pnas.0506955102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Burtner C.R., Kennedy B.K. Progeria syndromes and ageing: what is the connection? Nat. Rev. Mol. Cell Biol. 2010;11(8):567–578. doi: 10.1038/nrm2944. [DOI] [PubMed] [Google Scholar]
- 71.Ivanov A. Lysosome-mediated processing of chromatin in senescence. J. Cell Biol. 2013;202(1):129–143. doi: 10.1083/jcb.201212110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dou Z. Autophagy mediates degradation of nuclear lamina. Nature. 2015;527(7576):105–109. doi: 10.1038/nature15548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dou Z. Cytoplasmic chromatin triggers inflammation in senescence and cancer. Nature. 2017;550(7676):402–406. doi: 10.1038/nature24050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lan Y.Y. Extranuclear DNA accumulates in aged cells and contributes to senescence and inflammation. Aging Cell. 2019;18(2) doi: 10.1111/acel.12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martins F. Nuclear envelope dysfunction and its contribution to the aging process. Aging Cell. 2020;19(5):e13143. doi: 10.1111/acel.13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pujol G., Soderqvist H., Radu A. Age-associated reduction of nuclear protein import in human fibroblasts. Biochem. Biophys. Res. Commun. 2002;294(2):354–358. doi: 10.1016/S0006-291X(02)00492-8. [DOI] [PubMed] [Google Scholar]
- 77.Garcia-Aguirre I. Enhanced nuclear protein export in premature aging and rescue of the progeria phenotype by modulation of CRM1 activity. Aging Cell. 2019;18(5) doi: 10.1111/acel.13002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shibata Y., Morimoto R.I. How the nucleus copes with proteotoxic stress. Curr. Biol. 2014;24(10):R463–R474. doi: 10.1016/j.cub.2014.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim H.J., Taylor J.P. Lost in transportation: nucleocytoplasmic transport defects in ALS and other neurodegenerative diseases. Neuron. 2017;96(2):285–297. doi: 10.1016/j.neuron.2017.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Akerfelt M., Morimoto R.I., Sistonen L. Heat shock factors: integrators of cell stress, development and lifespan. Nat. Rev. Mol. Cell Biol. 2010;11(8):545–555. doi: 10.1038/nrm2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vujanac M., Fenaroli A., Zimarino V. Constitutive nuclear import and stress-regulated nucleocytoplasmic shuttling of mammalian heat-shock factor 1. Traffic. 2005;6(3):214–229. doi: 10.1111/j.1600-0854.2005.00266.x. [DOI] [PubMed] [Google Scholar]
- 82.Saez I., Vilchez D. The mechanistic links between proteasome activity, aging and age-related diseases. Curr. Genom. 2014;15(1):38–51. doi: 10.2174/138920291501140306113344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vilchez D., Saez I., Dillin A. The role of protein clearance mechanisms in organismal ageing and age-related diseases. Nat. Commun. 2014;5:5659. doi: 10.1038/ncomms6659. [DOI] [PubMed] [Google Scholar]
- 84.Sitte N. Protein oxidation and degradation during proliferative senescence of human MRC-5 fibroblasts. Free Radic. Biol. Med. 2000;28(5):701–708. doi: 10.1016/s0891-5849(99)00279-8. [DOI] [PubMed] [Google Scholar]
- 85.Sitte N. Protein oxidation and degradation during cellular senescence of human BJ fibroblasts: part I--effects of proliferative senescence. Faseb. J. 2000;14(15):2495–2502. doi: 10.1096/fj.00-0209com. [DOI] [PubMed] [Google Scholar]
- 86.Chondrogianni N. Central role of the proteasome in senescence and survival of human fibroblasts: induction of a senescence-like phenotype upon its inhibition and resistance to stress upon its activation. J. Biol. Chem. 2003;278(30):28026–28037. doi: 10.1074/jbc.M301048200. [DOI] [PubMed] [Google Scholar]
- 87.Torres C., Lewis L., Cristofalo V.J. Proteasome inhibitors shorten replicative life span and induce a senescent-like phenotype of human fibroblasts. J. Cell. Physiol. 2006;207(3):845–853. doi: 10.1002/jcp.20630. [DOI] [PubMed] [Google Scholar]
- 88.Torres C.A., Perez V.I. Proteasome modulates mitochondrial function during cellular senescence. Free Radic. Biol. Med. 2008;44(3):403–414. doi: 10.1016/j.freeradbiomed.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Davalli P. Vol. 2016. Oxid Med Cell Longev; 2016. ROS, cell senescence, and novel molecular mechanisms in aging and age-related diseases; p. 3565127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vilchez D. Increased proteasome activity in human embryonic stem cells is regulated by PSMD11. Nature. 2012;489(7415):304–308. doi: 10.1038/nature11468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kirkwood T.B. Evolution of ageing. Nature. 1977;270(5635):301–304. doi: 10.1038/270301a0. [DOI] [PubMed] [Google Scholar]
- 92.Vilchez D., Simic M.S., Dillin A. Proteostasis and aging of stem cells. Trends Cell Biol. 2014;24(3):161–170. doi: 10.1016/j.tcb.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 93.He C., Klionsky D.J. Regulation mechanisms and signaling pathways of autophagy. Annu. Rev. Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Madeo F. Essential role for autophagy in life span extension. J. Clin. Invest. 2015;125(1):85–93. doi: 10.1172/JCI73946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jia K., Levine B. Autophagy is required for dietary restriction-mediated life span extension in C. elegans. Autophagy. 2007;3(6):597–599. doi: 10.4161/auto.4989. [DOI] [PubMed] [Google Scholar]
- 96.Eisenberg T. Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 2009;11(11):1305–1314. doi: 10.1038/ncb1975. [DOI] [PubMed] [Google Scholar]
- 97.Masiero E. Autophagy is required to maintain muscle mass. Cell Metabol. 2009;10(6):507–515. doi: 10.1016/j.cmet.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 98.Mair W., Dillin A. Aging and survival: the genetics of life span extension by dietary restriction. Annu. Rev. Biochem. 2008;77:727–754. doi: 10.1146/annurev.biochem.77.061206.171059. [DOI] [PubMed] [Google Scholar]
- 99.Fontana L., Partridge L. Promoting health and longevity through diet: from model organisms to humans. Cell. 2015;161(1):106–118. doi: 10.1016/j.cell.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rubinsztein D.C., Marino G., Kroemer G. Autophagy and aging. Cell. 2011;146(5):682–695. doi: 10.1016/j.cell.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 101.Cuervo A.M. Autophagy and aging: keeping that old broom working. Trends Genet. 2008;24(12):604–612. doi: 10.1016/j.tig.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sitte N. Protein oxidation and degradation during cellular senescence of human BJ fibroblasts: part II--aging of nondividing cells. Faseb. J. 2000;14(15):2503–2510. doi: 10.1096/fj.00-0210com. [DOI] [PubMed] [Google Scholar]
- 103.Young A.R. Autophagy mediates the mitotic senescence transition. Genes Dev. 2009;23(7):798–803. doi: 10.1101/gad.519709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gamerdinger M. Protein quality control during aging involves recruitment of the macroautophagy pathway by BAG3. EMBO J. 2009;28(7):889–901. doi: 10.1038/emboj.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gewirtz D.A. Autophagy and senescence: a partnership in search of definition. Autophagy. 2013;9(5):808–812. doi: 10.4161/auto.23922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kwon Y. Autophagy is pro-senescence when seen in close-up, but anti-senescence in long-shot. Mol. Cell. 2017;40(9):607–612. doi: 10.14348/molcells.2017.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Taylor R.C., Berendzen K.M., Dillin A. Systemic stress signalling: understanding the cell non-autonomous control of proteostasis. Nat. Rev. Mol. Cell Biol. 2014;15(3):211–217. doi: 10.1038/nrm3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shemesh N. Uncoupling the trade-off between somatic proteostasis and reproduction in Caenorhabditis elegans models of polyglutamine diseases. Front. Mol. Neurosci. 2017;10:101. doi: 10.3389/fnmol.2017.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shpigel N. Dietary restriction and gonadal signaling differentially regulate post-development quality control functions in Caenorhabditis elegans. Aging Cell. 2019;18(2) doi: 10.1111/acel.12891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shemesh N. Dietary-induced signals that activate the gonadal longevity pathway during development regulate a proteostasis switch in Caenorhabditis elegans adulthood. Front. Mol. Neurosci. 2017;10:254. doi: 10.3389/fnmol.2017.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yang L. Long-term calorie restriction enhances cellular quality-control processes in human skeletal muscle. Cell Rep. 2016;14(3):422–428. doi: 10.1016/j.celrep.2015.12.042. [DOI] [PubMed] [Google Scholar]
- 112.Fontana L. The effects of graded caloric restriction: XII. Comparison of mouse to human impact on cellular senescence in the colon. Aging Cell. 2018;17(3) doi: 10.1111/acel.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Imanikia S. XBP-1 remodels lipid metabolism to extend longevity. Cell Rep. 2019;28(3):581–589 e4. doi: 10.1016/j.celrep.2019.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jurivich D.A. Arachidonate is a potent modulator of human heat shock gene transcription. Proc. Natl. Acad. Sci. U. S. A. 1994;91(6):2280–2284. doi: 10.1073/pnas.91.6.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Baker D.J. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479(7372):232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kirkland J.L., Tchkonia T. Senolytic drugs: from discovery to translation. J. Intern. Med. 2020 Nov;288(5):518–536. doi: 10.1111/joim.13141. Epub 2020 Aug 4. PMID: 32686219; PMCID: PMC7405395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang P. Senolytic therapy alleviates Abeta-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer's disease model. Nat. Neurosci. 2019;22(5):719–728. doi: 10.1038/s41593-019-0372-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bussian T.J. Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature. 2018;562(7728):578–582. doi: 10.1038/s41586-018-0543-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhu Y. The Achilles' heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14(4):644–658. doi: 10.1111/acel.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fuhrmann-Stroissnigg H. Identification of HSP90 inhibitors as a novel class of senolytics. Nat. Commun. 2017;8(1):422. doi: 10.1038/s41467-017-00314-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fuhrmann-Stroissnigg H., Niedernhofer L.J., Robbins P.D. Hsp90 inhibitors as senolytic drugs to extend healthy aging. Cell Cycle. 2018;17(9):1048–1055. doi: 10.1080/15384101.2018.1475828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pungsrinont T. Senolytic compounds control a distinct fate of androgen receptor agonist- and antagonist-induced cellular senescent LNCaP prostate cancer cells. Cell Biosci. 2020;10:59. doi: 10.1186/s13578-020-00422-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hummel B. The evolutionary capacitor HSP90 buffers the regulatory effects of mammalian endogenous retroviruses. Nat. Struct. Mol. Biol. 2017;24(3):234–242. doi: 10.1038/nsmb.3368. [DOI] [PubMed] [Google Scholar]
- 124.Specchia V. Hsp90 prevents phenotypic variation by suppressing the mutagenic activity of transposons. Nature. 2010;463(7281):662–665. doi: 10.1038/nature08739. [DOI] [PubMed] [Google Scholar]
- 125.Fuentealba M. Using the drug-protein interactome to identify anti-ageing compounds for humans. PLoS Comput. Biol. 2019;15(1) doi: 10.1371/journal.pcbi.1006639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Janssens G.E. Transcriptomics-based screening identifies pharmacological inhibition of Hsp90 as a means to defer aging. Cell Rep. 2019;27(2):467–480 e6. doi: 10.1016/j.celrep.2019.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jenuwein T., Allis C.D. Translating the histone code. Science. 2001;293(5532):1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 128.Zhao Y., Garcia B.A. Comprehensive catalog of currently documented histone modifications. Cold Spring Harb Perspect Biol. 2015;7(9):a025064. doi: 10.1101/cshperspect.a025064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kimura H. Histone modifications for human epigenome analysis. J. Hum. Genet. 2013;58(7):439–445. doi: 10.1038/jhg.2013.66. [DOI] [PubMed] [Google Scholar]