Highlights
-
•
Oxidative potential (OP) of PM2.5 from Canadian cities was studied using a new acellular chemical assay.
-
•
Redox potential of simulated lung fluid was highly associated with all physiological OP indicators.
-
•
Traffic emissions had the highest OP, followed by industrial emissions and crustal matter.
-
•
OP was strongly associated with black carbon and transition metals, especially Cu, Fe, Mn, and Ti.
-
•
Organic ligand complexation and aerosol pH were associated with metal solubility and OP.
Keywords: Urban air pollution, Inhalation toxicity, PM2.5 redox activity, Aerosol pH, Metal-ligand complexation
Abstract
Air pollution is a major environmental health risk and it contributes to respiratory and cardiovascular diseases and excess mortality worldwide. The adverse health effects have been associated with the inhalation of fine particulate matter (PM2.5) and induction of respiratory oxidative stress. In this work, we quantified the oxidative potential (OP) of PM2.5 from several Canadian cities (Toronto, Hamilton, Montreal, Vancouver) using a recently developed bioanalytical method which measures the oxidation of lung antioxidants, glutathione, cysteine, and ascorbic acid, the formation of glutathione disulfide and cystine, and the related redox potential (RP) in a simulated epithelial lining fluid (SELF). We evaluated the application of empirical SELF RP as a new metric for aerosol OP. We further investigated how PM2.5 chemical composition and OP are related across various emission source sectors and whether these features are linked to specific properties of aerosol aqueous phase, such as pH and metal-ligand complexation. The OP indicators including SELF RP were strongly correlated among each other, indicating that the empirical RP could be used as a reliable metric in future studies. OP based on ascorbic acid showed dependency on the emission source sectors, most likely due to variation in the solubility of Fe. Traffic emissions resulted in the highest OP, followed by industrial emissions and resuspended crustal matter. OP presented low correlation with PM2.5 concentrations, low-moderate correlation with the aerosol organic matter, and moderate-strong association with black carbon and transition metals across the sites. We did not find strong association between the concentration of biomass burning tracers and OP. Copper was the only metal that showed high association with OP across all sites, whereas the correlation with other metals, such as iron, manganese, and titanium, showed clear dependency on the source sectors. The aerosol pH correlated negatively with ambient temperature and positively with biomass burning tracers and the levels of nitrate, ammonium, and aerosol liquid water content. The solubility of Fe was associated with sulfate and aerosol pH at most sites, suggesting the involvement of proton-mediated dissolution pathway, while this was not visible at the site influenced by industrial emission, most likely due to the abundance of pyrogenic Fe. The effect of metal-ligand complexation on the solubility of transition metals, in particular Fe, was clearly observed at all sites, whereas a combined effect with aerosol pH, and a subsequent impact on OP, was only seen at the traffic site in Toronto. The enhanced solubility of Fe due to proton- and ligand-mediated dissolution pathways and subsequent formation of reactive oxygen species may in part explain the health effects of PM2.5 seen in previous epidemiological studies.
1. Introduction
Poor air quality is a major environmental health risk, as it contributes to excess mortality from cardiovascular and respiratory diseases. Morbidity and mortality related to air pollution are mainly associated with fine particulate matter (PM2.5, i.e. particulate matter with aerodynamic diameter < 2.5 μm; WHO: Ambient air pollution: a global assessment of exposure and burden of disease, World Health Organization., 2016, Landrigan et al., 2018, Lelieveld et al., 2018, Lelieveld et al., 2019a, Lelieveld et al., 2019b), as the small size of these particles allows them to deposit in the deep lung (Oberdörster et al., 2005). The prominent sources of PM2.5 are fossil fuel and biomass combustion, industry, agriculture, and resuspension of dust; in densely populated regions, such as urban settings, significant excess death rates can be associated with fossil fuel use and emission from traffic, industry, and power generation (Lelieveld et al., 2019a, Lelieveld et al., 2019b). The underlying biochemical mechanisms behind the health effects of air pollution are not fully understood; however, increasing evidence suggests that one important pathway follows the PM-induced oxidative stress that causes respiratory inflammation with subsequent adverse health impacts (Pope et al., 2011, Hoek et al., 2013, Kelly and Fussell, 2017, Shiraiwa et al., 2017, Landrigan et al., 2018, Münzel et al., 2018, Lelieveld et al., 2019a). Oxidative stress related to inhalation of PM2.5 follows molecular pathways that lead to the formation of reactive oxygen species (ROS; e.g. H2O2, ·O2–, ·OH) upon the reaction of PM2.5 redox-active constituents (e.g. transition metals, quinones) with antioxidants in the epithelial lining fluid (ELF; Lakey et al., 2016). Therefore, the ability of PM-bound chemicals to deplete the levels of antioxidants and to form ROS in ELF (i.e. oxidative potential or OP) has been widely adopted as a metric to characterize the oxidative burden of ambient aerosols (Shiraiwa et al., 2017, Bates et al., 2019).
One challenge in studying aerosol OP is the diversity of available OP indicators and molecular or cellular endpoints that they represent in the context of air pollution toxicology. In the absence of coherent inter-laboratory studies, there is no consensus about the best acellular/chemical indicators which associate with cellular endpoints and toxicological outcomes (Øvrevik, 2019). OP is often determined using acellular assays which employ a simulated-ELF (SELF) containing inorganic salts, one or more molecular probes, and sometimes surfactant lipids and proteins (Calas et al., 2017, Shahpoury et al., 2019). Dithiothreitol (DTT) and ascorbic acid (AA) are the most common molecular probes used with such assays (Bates et al., 2019). While AA is an antioxidant found in ELF, DTT is only used as a surrogate to simulate the lung antioxidants, and there are concerns regarding its low reactivity towards metals, in particular Fe, which is an abundant metal in ambient aerosols (Ayres et al., 2008, Charrier and Anastasio, 2012, Xiong et al., 2017). Glutathione (GSH), a major lung antioxidant, has so far been used less frequently (Weichenthal et al., 2016a, Weichenthal et al., 2019, Crobeddu et al., 2017, Crobeddu et al., 2020, Shahpoury et al., 2019, Gao et al., 2020), possibly due to the complexity of methods for separation and analysis of GSH and its oxidation product glutathione disulfide (GSSG). However, in a recent study, we developed an OP method which made it possible to include thiol antioxidants and their oxidation products in characterizing the aerosol OP with considerably less challenges, including cysteine (CSH) and cystine (CSSC) which were not considered in the past (Shahpoury et al., 2019). In that study, we introduced for the first time the SELF theoretical oxidation–reduction (redox) potential as an alternative physiological metric to characterize the OP of ambient particulate matter. This is because redox potential reflects the chemical dynamics of antioxidant redox couples, as opposed to single antioxidants in SELF. However, a gap in knowledge remains as to whether an empirical redox potential (RP) of SELF reflects the aerosol OP and can be practically used as an alternative new indicator. Such metric would transform the OP testing practice and allow quantification to be more readily available based on a uniform technique, thus overcoming the current challenge related to the lack of coherency in OP techniques. This would make it possible to use and compare in a coherent way the results from various studies that aim at linking the aerosol OP with air pollution health outcomes across the world.
To this date, the studies about aerosol OP have focused on temporal and spatial (between or within cities) distribution of OP, and some other studies have focused on linking OP to specific emission sources (Bates et al., 2019 and references therein) or establishing predictive regression models based on the concentrations of PM2.5 constituents (Weber et al., 2018, Weichenthal et al., 2019). However, achieving a universal empirical model linking OP with site-specific aerosol constituents or sources is highly challenging because OP depends not only on the concentrations of redox-active species in the aerosol, but also on factors that influence the diversity and extent of redox reactions and formation of ROS in the lung. For instance, the oxidation state of metals, e.g. Fe(III) vs. Fe(II), the interaction of aerosol organic and inorganic phase (e.g. metal–ligand complexation), and the acidity of aerosol aqueous phase and its liquid water content (LWC) can influence metal solubility (Nico et al., 2009, Fang et al., 2017a, Wong et al., 2019, Wong et al., 2020, Freedman et al., 2019, Tapparo et al., 2020). The changes in metal solubility could in turn affect aerosol redox activity and oxidative burden. These aerosol characteristics are dynamic and site-specific, as they are influenced by emission source types and atmospheric processing. Considering the adverse health outcomes that have been associated to ambient PM2.5 and its OP in previous epidemiological studies (Weichenthal et al., 2016a, Weichenthal et al., 2016b, Weichenthal et al., 2016c, Cohen et al., 2017, Burnett et al., 2018), and the important role of trace metals in regulating the oxidative burden of PM2.5, it is paramount to understand if aerosol parameters that enhance PM-bound metal solubility (e.g. the presence of organic ligands, aerosol acidity and LWC) in turn affect physiological OP indicators, and if the diversity of emission sources plays a role in these processes. To the best of our knowledge, previous studies have not considered these parameters in the context of PM2.5 inhalation toxicity. This is because such considerations require extensive chemical characterization of both ambient particulate matter and the gas phase alongside meteorological parameters, which are rarely available collectively, even less so with high spatial and temporal resolution. In order to overcome this challenge, for the first time in this study, we took advantage of the Canadian National Air Pollution Surveillance (NAPS) program, which encompasses a large network of air pollution surveillance stations across Canada, and performs comprehensive aerosol chemical speciation. The NAPS sites are systematically positioned and well characterized to reflect dominant emission sources, land use, and population classification. The specific and unique aims of the current study were to (1) examine the OP of PM2.5 from four Canadian urban locations that are well characterized in terms of emission sources, aerosol chemical composition, and meteorological parameters using our recently developed OP method, (2) investigate for the first time if the empirical and theoretical RP of SELF associate with OP indicators based on the lung antioxidants following reaction with PM2.5 and evaluate the use of empirical RP as an alternative OP metric, (3) study the association of physiological OP indicators, among which RP for the first time, with PM2.5 composition, including transition metals, and (4) investigate if organic ligands, aerosol pH, and LWC regulate the PM2.5 OP through their effect on metal solubility.
2. Materials and methods
2.1. Study locations
PM2.5 samples were collected from the NAPS sites in Toronto, Hamilton, Montreal, and Vancouver (Table 1). The sampling sites are classified by the NAPS network based on the source type, urbanization, neighborhood population, and local land use (Table 1).
Table 1.
Details of study locations.
| Site code | Coordinates | Source sector | n | PM2.5 concentration min–max (μg m−3) | |
|---|---|---|---|---|---|
| Torontoa | 60438 | 43.711, −79.543 | T, LU, P6, C | 25 | 10–31.4 |
| Hamilton | 60512 | 43.258, −79.862 | PS, LU, P5, R | 31 | 13.5–26.8 |
| Montreal | 50129 | 45.652, −73.574 | PE, LU, P4, R | 21 | 13.4–28.5 |
| Vancouvera | 100141 | 49.260, −123.078 | T, LU, P6, R | 25 | 1.1–27 |
Near-road site; site type (T: transportation influence, PS: point-source influence, PE: general population exposure); urbanization (LU: large urban area); neighborhood population residing within 4-km of the site (P4: 50,000–99,999, P5: 100,000–149,999, P6: ≥ 150,000); local land use classification (C: commercial, R: residential). n is the number of samples analyzed for OP from each site.
According to the NAPS classification framework, all four sites classify as large urban areas. The sampling sites in Toronto and Vancouver are classified as near-road sites and are influenced primarily by transportation. While Hamilton is influenced by a point source (i.e. industrial activities), Montreal is considered a general population exposure site, and potentially influenced by regionally transported air masses and residential heating activities during colder periods. For the purpose of this study, we performed additional source identification using Principal Component Analysis (PCA; see Section 2.6.1). In terms of local land use, apart from the site in Toronto, which is classified as commercial, the rest of the sites are considered residential. With respect to population, the sites in Toronto and Vancouver are classified as highly populated (≥150,000), whereas Hamilton and Montreal are in the mid-population range (Table 1). As shown by the wind roses in Fig. S1 in the Supplementary Information (SI), all four sites were exposed to northeasterly (~80%) and northerly (~30%) winds for the sampling dates considered for OP analysis, with Hamilton and Montreal experiencing relatively low wind velocities (mean ≤ 1.7 m s−1) compared to Toronto (3.9 m s−1) and Vancouver (8.1 m s−1). The site in Vancouver in particular experienced relatively high wind velocities (6–24 m s−1) for the majority of sampling events regardless of wind direction, whereas in Toronto this only occurred for < 20% of the events (6–12 m s−1) and mainly from northeasterly direction (Fig. S1).
2.2. Sample collection
The samples were collected on 47-mm i.d. polytetrafluoroethylene (PTFE) filters using Dichotomous samplers (flowrate: 15 L min−1; Partisol, 2000i-D, Thermo Scientific, Waltham, USA), alongside SUPER SASS-Plus sequential speciation samplers (flowrate: 10 L min−1; Met One Instruments, Inc., Grants Pass, USA). The speciation sampler was equipped with three (i.e. A, B and C) ChemComb cartridges (Thermo Scientific) which were used for various chemical analyses. Cartridge A contained a pre-baked quartz fiber filter (QA) that collected PM2.5 for the analysis of organic carbon (OC) and elemental carbon (EC). Cartridge B had a PTFE filter followed by a pre-baked quartz back-up filter (QB). Cartridge C contained two honeycomb glass denuders used for scrubbing acidic (HNO3, HONO, and SO2) and basic (NH3) gases, followed by two cassettes containing PTFE and Nylon filter. The latter was used to capture volatile nitrate (Dabek-Zlotorzynska et al., 2011). At each site, 24-hr samples were collected once every three days between 2016 and 2017, resulting in the sampled air volumes of 21.6 and 14.4 m3 with Dichotomous and speciation sampler, respectively. Given the specificity of emission sources at the sites, between 21 and 31 samples were selected from each site for the OP analysis (Table 1). The samples were selected to include two groups, each representative of the cold and warm periods at each site, with the cold period covering October to March with typical temperatures ≤ 15 °C and the warm period covering April to September with temperatures > 15 °C. In addition, the samples from Vancouver were chosen to reflect two distinct periods of high and low biomass burning activity. A 1-cm2 filter punch from each QA filter was used to perform the OP assay; this represents 1 m3 of the sampled air for each sample. Lab blanks consisted of pre-baked quartz fiber filters that were not used for air sampling.
2.3. Aerosol speciation methods
The details of aerosol speciation methods were previously described by Dabek-Zlotorzynska et al., 2011, Dabek-Zlotorzynska et al., 2019. The samples were analyzed for OC and EC, total and water-soluble elements, organic and inorganic ions, biomass burning markers, and gaseous species including NH3 and HNO3. PM2.5 samples collected on PTFE filters were analyzed for 22 elements using nondestructive energy dispersive X–ray fluorescence spectrometry (ED-XRF, Epsilon 5, Malvern Panalytical Inc., Malvern, Montreal, QC, Canada). The samples were subsequently extracted using microwave-assisted acid digestion and analyzed for 25 trace elements by inductively coupled plasma mass spectrometry (ICP-MS; Agilent Technologies, Wilmington, DE, USA) to obtain the near-total concentrations (Celo et al., 2010, Dabek-Zlotorzynska et al., 2019). QA and QB quartz filters were analyzed for PM2.5 OC and EC contents using a thermal/optical carbon analyzer (DRI Model 2001, Atmoslytic Inc., Calabasas, CA, USA). The concentrations of positive OC artifacts were measured using QB filters and were subtracted from the OC concentrations measured with the directly exposed QA filters. The PM2.5 samples collected on PTFE filter downstream of the denuders were extracted using deionized water and analyzed for water-soluble anions and cations, organic acids and biomass burning tracers (monosaccharide anhydrides: levoglucosan, mannosan, and galactosan) and biogenic emission tracers (polyols: arabitol and mannitol) using ion chromatography (IC, Thermo Scientific, Sunnyvale, CA, USA). The PM2.5 aqueous extracts were also analyzed for water-soluble trace metals using ICP-MS. The analytical method limits of quantification (LOQ) were determined using mean + three standard deviation (SD) of the concentrations in blanks (n = 12). Where analyte concentrations in samples exceeded the LOQs, the mean blank concentrations were subtracted from the sample concentrations. More information about PM2.5 speciation and the individual target analytes can be found in Section S1.
2.4. Acellular oxidative potential assay
2.4.1. Sample preparation
The samples were prepared following the method previously described by Shahpoury et al., (2019). The SELF in this study was made of phosphate buffered saline (PBS, Bio-Performance, Sigma Aldrich) containing NaCl, KCl, and Na2HPO4 and a mixture of GSH, CSH, and AA (200 μM each). For each sample, 2.5 mL of SELF was transferred to a pre-cleaned 8-mL low-density polyethylene bottle (Nalgene, Thermo Scientific), and a 1-cm2 filter punch containing PM2.5 was added to SELF, ensuring that the sample was submerged. The samples were incubated at 37 °C while shaken gently (at 60 rpm) in an incubator-shaker (Benchmark Scientific, Sayreville, USA) for 150 min, together with a reference SELF, a 1-cm2 blank quartz filter in SELF, and a positive control containing 500 nmol Cu(NO3)2 in SELF. Following the incubation, a 300-μL aliquot (n = 3) of each sample was transferred to a 1.5-mL centrifuge tube, added with 100 μL of 100 mM n-ethylmaleimide, mixed for 5 sec using a vortex mixer, and allowed to react at room temperature for 1 min. Subsequently, 200 μL of a mixture containing 2% sulfosalicylic acid and 2 mM ethylenediaminetetraacetic acid was added to the samples, and the samples were vortexed for 5 sec and later centrifuged at 10,000 g for 6 min. Finally, 5 μL of the supernatant from each sample was transferred to a push-filter vial with PTFE membrane (0.2 μm pore size; Whatman, Pittsburgh, USA) containing 495 μL of 20:80 methanol-H2O and used for instrumental analysis. Further details about the analytical method can be found in Shahpoury et al., (2019).
2.4.2. Instrumental analysis
The analysis of antioxidants was carried out using an ultra-high performance liquid chromatograph (UPLC, Acquity, Waters, Milford, USA) interfaced to a triple-quadruple mass spectrometer (TQ-S MS/MS, Xevo, Waters) in electrospray ionization (in the negative mode for AA and in the positive mode for GSH, GSSG, CSH, and CSSC). The target substances were separated using a HSS T3 UPLC column (3 mm, 50 mm, 1.8 μm; Waters), thermostated at 40 °C, with the mobile phase flow rate of 0.4 mL min−1. The mobile phases A and B were made of H2O and acetonitrile (LC-MS grade, Optima, Fisher Scientific), respectively, each containing 0.1% formic acid. For the target compounds in the negative mode, the mobile phase gradient started at 20% phase B, ramped to 100% B from 0.3 to 0.5 min and held for 2.5 min, then decreased to 20% B from 3 to 3.1 min and held for 2.9 min. For substances in the positive mode, the gradient started at 1% phase B, ramped to 50% B from 0.1 to 0.3 min and held for 1.7 min, followed by an increase to 100% B over 0.1 min and a hold time of 1 min, followed by a decrease to 1% B and re-equilibration time of 2.8 min. This resulted in the analytical time of 6 min in either negative or positive mode. The MS/MS parameters used were as follows: source temperature of 120 °C, capillary voltage of 1.5 kV, nitrogen and desolvation gas flow rates of 150 and 900 L h−1, desolvation temperature of 500 °C, and collision gas flow rate of 0.15 mL min−1 (Shahpoury et al., 2019). The antioxidant quantification was done using the external calibration method with 6-point linear calibration curves ranging from 1 to 250 pg μl−1. The chromatographic data analysis and quantification were performed using MassLynx software (Waters).
2.4.3. Measurement of redox potential
The RP of SELF was measured following the 150 min incubation time. The measurement was performed using a platinum oxidation–reduction potential sensor with ARGENTHAL (Ag/AgCl) reference system (InLab Redox Micro, Mettler Toledo, Giessen, Germany), connected to a SevenCompact pH meter (Mettler Toledo). The details related to handling procedures and use of redox sensors with aqueous solutions, which we followed in this work, have been previously described (Nordstrom and Wilde, 2005, Copeland and Lytle, 2014, Striggow, B.: Field measurement of oxidation-reduction potential (ORP), U.S. Environmental Protection Agency, Athens, GA., 2017). Prior to the sample measurements, the electrode performance was verified using a redox buffer (220 mV, pH 7; Mettler Toledo) and values of 220 ± 10 mV were considered acceptable. Prior to each measurement, the outside wall of the glass sensor was rinsed with copious amount of LC-MS grade water. 450 μL of SELF (pH 7.4) was transferred to a 750 μL plastic vial, immediately following the incubation of samples. The redox sensor was placed inside the vial, making sure that the tip of the sensor with the platinum ring was completely submerged without touching the bottom or the inside wall of the vial. The measurement (n = 3) was carried out after 30 sec under standard stabilization criteria at room temperature (21 °C). The reading typically took up to 2 min to stabilize. This depended on the magnitude of redox potential – i.e. the reading for samples with relatively high potential stabilized within seconds.
2.5. Estimation of aerosol pH
A direct measurement of aerosol pH is not possible (Pye et al., 2020), and the most reliable estimates can be obtained through “thermodynamic analysis” of ambient observations (Guo et al., 2015, Hennigan et al., 2015, Pye et al., 2020). The approach entails the application of a thermodynamic model to observations of the major inorganic species in the gas and particulate phase that affect the water content and ionic composition in the aqueous phase present in the aerosol. An overview of the work on aerosol pH to this date can be found in Pye et al. (2020).
In this study, we applied the extensively used ISORROPIA-II (version 2.3) aerosol thermodynamic model (Fountoukis and Nenes, 2007; http://isorropia.epfl.ch) to obtain the equilibrium concentration of H+ and LWC in the aerosol, and calculated the pH using the “pHF” definition of Pye et al. (2020), Eq. (1):
| (1) |
where γH+ is the activity coefficient of the hydronium ion, H+ (assumed unity), Haq+ is its concentration (mol L-1) in the aerosol aqueous phase, Hair+ (μg m−3) is the concentration of H+ per volume of air, and Wi (μg m−3) is the particle water concentration associated with the aerosol inorganic species. We neglected the contribution of organic species to the water uptake, water activity and pH, given that they induce a secondary effect on pH (between 0.15 and 0.30 units; Guo et al., 2015, Song et al., 2018, Vasilakos et al., 2018, Battaglia et al., 2019). More information is provided in Section S2.
2.6. Data analysis
The PM2.5 mass was reconstructed following the method adopted from Dabek-Zlotorzynska et al., (2019) and detailed in Table S1. The loss rates of antioxidants and formation rates of oxidation products were determined using the difference in the concentrations of the target compounds in the reference SELF and SELF samples containing PM2.5, taking into account the incubation time, i.e. (RSHREF - RSHPM)/incubation time (min), and (RSSRPM - RSSRREF)/incubation time, where RSH and RSSR denote the concentrations (nmol) of thiol antioxidant and its oxidation product, respectively. The oxidation rates of AA (OPAA) were calculated using the same approach. The OP results are presented as the antioxidant loss rates (nmol min−1 m−3), their oxidation product formation rates (nmol min−1 m−3), as well as estimated (Eh) and measured (RP) redox potential (mV). Eh was calculated using the measured concentrations of CSH/CSSC and GSH/GSSG redox pairs and a form of Nernst equation (Jones et al., 2000, Iyer et al., 2009), Eqs. (2), (3):
| (2) |
| (3) |
Statistical analysis and graphical presentation were performed using Openair R package (Carslaw and Ropkins, 2012) and Origin software (OriginLab Corporation, Northampton, MA, USA). The association of OP indicators and aerosol constituents was examined using the non-parametric Spearman rank correlation (rs), whereas the spatial–temporal differences of OP indicators and PM2.5 constituents were examined using the non-parametric Mann–Whitney test (alpha = 0.05).
2.6.1. Principal component analysis
The emission sources at the study locations were characterized using PCA with the SPSS software. The PCA was performed on complete datasets spanning the years 2016 and 2017 and encompassed 240 samples from each site, including the samples used for OP analysis. In this analysis, we included 19 PM2.5 species that were found to be strong markers and showed distinct source signatures across urban and background sites in previous studies conducted within the NAPS program, including primary and secondary sources (Sofowote et al., 2015a, Sofowote et al., 2015b). We only considered chemical species that were found in >60% of the samples at each site. These were EC, OC, oxalate, sulfate (SO4), nitrate (NO3), ammonium (NH4), Cu, Sn, Zn, Fe, Mn, Pb, Si, Al, Ca, K, and levoglucosan. Na and Cl (representing sea salt) were initially selected for source analysis but were later removed from the PCA, as they were found in <60% of the samples. We used near-total metal data obtained from ICP-MS analysis and the crustal matter data (Si, Al, Ca) from XRF analysis. Identification of outliers was performed using a single iteration of Grubb’s test and the values that were found to be outliers were not included in the PCA analysis. The missing values were subject to pairwise deletion. EC and OC are typically related to primary combustion sources (e.g. tailpipe emission). SO4, NO3, and NH4 are related to secondary sources, i.e. atmospheric formation; however, nitrogen oxides (NOx) and ammonia (NH3), the precursors for NO3 and NH4, are also emitted from biomass burning (Andreae, 2019). Oxalate is associated with aged combustion aerosols and often correlates with SO4 (Carlton et al., 2007); Cu, Sn, and Zn are related to breakdown of motor vehicle brake pads and lubricants. The clustering of Fe, Mn, and Zn has been associated with metallurgical (ferrous smelting) emissions, while Pb is related to non-ferrous industrial emissions (Sofowote et al., 2015a). It must be noted that, in some of the previous studies, Fe and Mn were associated with primary traffic emissions (abrasion of brake pads) or crustal elements (Almeida et al., 2005, Cesari et al., 2018, Dabek-Zlotorzynska et al., 2019). Si, Al, and Ca are specifically related to crustal matter and dust resuspension. Potassium has been used as tracer of biomass burning but it is also emitted from other sources (e.g. soil dust, sea salt, fertilizer use); hence, we also included levoglucosan, which is a more specific tracer for biomass burning (Zhang et al., 2010).
The PCA was performed using orthogonal transformation and Varimax rotation, and the number of principal components/factors was determined based on eigenvalues greater than unity. Only factor loadings >0.45 were retained for further analysis. After determining the number of principal components, the overall score for each factor was calculated as the sum of the products of factor score coefficients (Table S4a-d) multiplied by the concentrations of individual species in each sample (Dabek-Zlotorzynska et al., 2019). The factor scores were subject to a single iteration of Grubb’s test in order to identify the outliers, and the samples containing outliers were not included in further analysis. Subsequently, the contribution of each factor to PM2.5 mass concentrations was estimated using multiple linear regression (MLR) analysis. Only factors that were deemed significant (p < 0.05) in MLR analysis were included when estimating the factor contributions to PM2.5 mass (Table S4a-d).
3. Results and discussion
3.1. Sources and composition of PM2.5
PM2.5 concentrations were 10–31.4 μg m−3 in Toronto (mean ± standard deviation (SD): 17.3 ± 6.2), 13.5–26.8 μg m−3 in Hamilton (16.8 ± 3.3), 13.4–28.5 μg m−3 in Montreal (17.6 ± 4.6), and 1.1–27 μg m−3 in Vancouver (8.9 ± 4.8) for the samples considered for OP analysis. Despite being a near-road site, Vancouver had considerably lower minimum and mean values, compared to the other sites, where we found similar PM2.5 concentrations. The results of the PCA analysis are shown in Table S4a-d in the SI. Accordingly, at Toronto site, traffic-related emissions from brake lining wear and tailpipe emissions explained 27% of the total variance in the dataset. This was followed by crustal matter, aged combustion aerosols, and biomass burning, each contributing ~ 17% to the total variance. The MLR analysis showed that, in the cold period, aged combustion aerosols contributed 48% and biomass burning 17% to PM2.5 mass concentrations, whereas in the warm period, aged combustion aerosols contributed 60% and primary traffic emissions 17% to the PM2.5 mass. In Vancouver, the primary traffic emissions explained 37% of the variance, followed by biomass burning, aged combustion aerosols, and crustal matter, each explaining ~ 14% of the total variance. The primary traffic emissions contributed 54% to PM2.5 mass in the cold period, whereas, in the warm period, biomass burning and chemically aged aerosols contributed 40 and 23% to PM2.5 mass, respectively. The observation of biomass burning source in the warm period in Vancouver is largely associated with seasonal wildfires that are common in the surrounding regions. Overall, the PCA results for Toronto and Vancouver are consistent with the near-road nature of these sites and their classification under NAPS program (Table 1).
In Hamilton, industrial/metallurgical emissions and to some degree primary traffic emissions (mainly from brake wear) explained 30% of the total variability. This was followed by aged combustion aerosols (20%), and biomass burning and crustal matter (15% each). In the cold period, aged combustion aerosols and biomass burning contributed 48 and 26% to the PM2.5 mass, while, in the warm period, a large portion of PM2.5 mass (92%) was attributed to chemically aged combustion aerosols. In Montreal, crustal material explained 35% of the total variance, followed by biomass burning (23%) and chemically aged combustion aerosols (18%). In the cold period, aged combustion aerosols contributed 55% to PM2.5 mass, followed by crustal material (11%), and biomass burning (9%), whereas, in the warm period, aged combustion aerosols contributed 66% to the PM2.5 mass and other sources did not make a significant contribution (p > 0.05). These results are consistent with the characteristics of this site, as it is away from point emission sources.
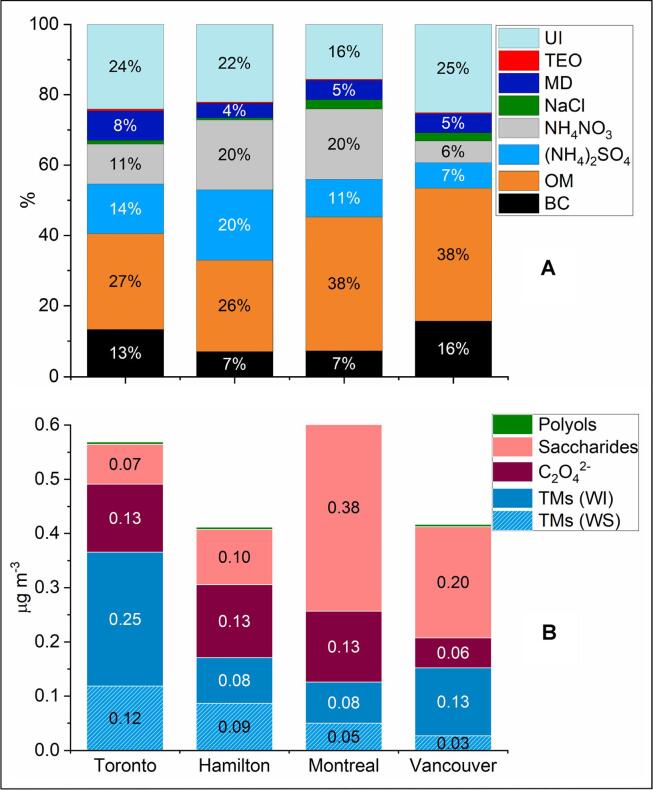
Fig. 1 shows mass reconstruction for PM2.5 samples that were used for OP analysis. The PM2.5 from near-road sites Toronto and Vancouver was dominated by OM (27–38% of the PM2.5 mass), followed by BC (13–16%), (NH4)2SO4 (7–14%), NH4NO3 (6–11%), and mineral dust (5–8%). In Hamilton and Montreal, OM remained the dominant component (26–38%), followed by the inorganic salts (NH4)2SO4 (11–20%) and NH4NO3 (20%), BC (7%), and mineral dust (4–5%). The lowest contributions to PM2.5 mass were from NaCl (<3%) and trace element oxides (≤0.5%) across the sites. Further details about the mass concentrations of these species across the sites can be found in the SI (Section S3, Table S2a-b, and Fig. S2a-b).
Fig. 1.
PM2.5 compositions across the study locations; BC: black carbon, OM: organic matter, MD: mineral dust (i.e. Si, Ca, Fe, K, Ti), TEO: trace element oxides (i.e. V, Mn, Ni, Cu, Zn, As, Pb, Se, Sr, Cr), UI: unidentified chemicals, TMs: transition metals, WS: water-soluble, WI: water-insoluble, saccharides (i.e. monosaccharide anhydrides/biomass burning tracers): levoglucosan, mannosan, galactosan, and polyols (biogenic emission tracers): arabitol and mannitol. For simplicity, (NH4)2SO4 represents all ammonium sulfate species considered in mass reconstruction, including (NH4)3H(SO4)2 and NH4HSO4. Note that the contributions of NaCl and polyols are ≤ 3%, while those of TEO are ≤ 1%.
PM2.5 from Toronto had the highest levels of transition metals (median ∑12 metals: 0.32 μg m−3) compared to the other three sites (0.10–0.13 μg m−3, Fig. 1b and Table S2a-b; the figure includes 12 transition metals). This is mostly related to the site proximity to a high-density flow of traffic, which could release metal particles due to the abrasion of vehicle brake pads, tailpipe emission, as well as road dust resuspension. Fe was the dominant transition metal at all sites, contributing 82–87% to the near-total metals at Hamilton and Montreal, and relatively higher (90–93%) at the near-road sites Toronto and Vancouver. Cu was the second abundant transition metal in Vancouver samples (4%), followed by Ti, Zn, and Mn (1–3%), whereas at all other sites Zn was the second abundant transition metal (2–7%), followed by Ti, Cu, and Mn (1–5%). The water-soluble transition metals contributed between 33 and 50% to the near-total transition metal concentrations at Toronto, Hamilton, and Montreal sites (median ∑12 water-soluble metals: 0.04–0.11 μg m−3), whereas they only contributed 16% at Vancouver site (0.02 μg m−3, Fig. 1b and S2b). The water-soluble concentrations were dominated by Fe (40–60%), followed by Zn (27–39%), Cu (6–16%), and Mn (3–5%).
Biomass burning was previously shown to be a major contributor to intrinsic OP based on the DTT assay (Verma et al., 2015). In the present study, the highest values of PM2.5-bound monosaccharides were found in Vancouver and Montreal (median ∑3 monosaccharides: 162.8 and 303.9 ng m−3, respectively; Table S2a-b, Fig. 1b), suggesting a higher influence of biomass burning aerosols at these sites, compared to Toronto and Hamilton (28.5 and 68.9 ng m−3, respectively).
The individual monosaccharides demonstrated similar concentration profiles across the study locations – on average, levoglucosan dominated the profiles, contributing between 76% (in Vancouver) and 85% (in Montreal) to ∑3 monosaccharides, followed by mannosan (11–19%) and galactosan (4–7%). Polyols are often used as tracers of primary biogenic emission and they were previously used to probe the contribution of biogenic sources to the intrinsic OP of aerosols (Weber et al., 2018, Calas et al., 2019). In the present study, polyols (i.e. arabitol and mannitol) showed relatively low concentrations across the sites (median ∑2 polyols: 3.4–4.3 ng m−3). On average, mannitol dominated the polyol concentration profiles in Toronto, Vancouver, and Hamilton (60–64%), whereas, in Montreal, arabitol and mannitol had similar contributions.
3.1.1. Parameters influencing metal solubility
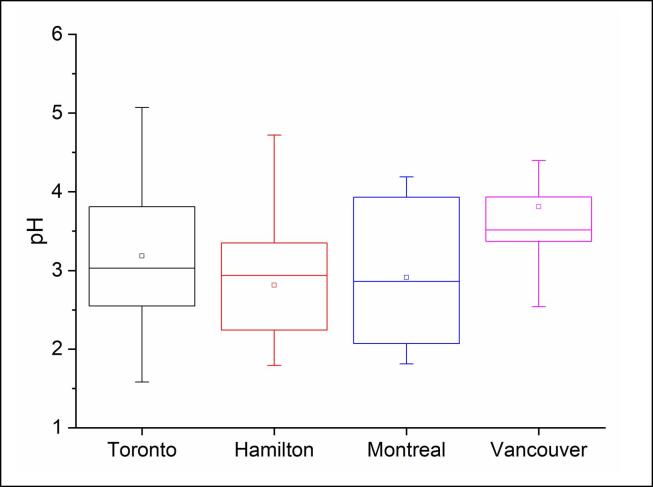
As discussed in Section 3.1, Fe dominated both near-total and water-soluble metals and it demonstrated variable water-solubility (i.e. % water-soluble/near-total) at different sites, ranging from 27 to 35% in Toronto, Hamilton, and Montreal samples, and 8% in Vancouver (Fig. S3). Hence, here we discuss the parameters that may have contributed to its water-solubility. The water-solubility of metals, in particular Fe, is influenced by various parameters, such as the presence of organic ligands as well as the pH in the aerosol aqueous phase (i.e. ligand- vs. proton-mediated dissolution), the aerosol LWC, and the nature of emission sources, e.g. pyrogenic Fe emission (Panias et al., 1996, Zhou et al., 2015, Myriokefalitakis et al., 2015, Ito et al., 2019, Tao and Murphy, 2019, Tapparo et al., 2020, Wong et al., 2020). For instance, oxalic acid can bind to trivalent Fe and convert it to bivalent Fe (the water-soluble form); this process is enhanced under visible and ultraviolet light. In a previous study, a decline in the diurnal levels of oxalate after sunrise was associated with the photolysis of Fe(III)-oxalate complexes in the aerosol aqueous phase, converting Fe(III) to Fe(II) (Zhou et al., 2015). The dissolution of Fe through proton- and ligand-mediated pathways in the aerosol aqueous phase is enhanced at pH < 3; this would be more pronounced with pH < 2 (Tao and Murphy, 2019). Fig. 1b shows that the median levels of oxalate (C2O42-, the deprotonated form of oxalic acid) were relatively high in Toronto, Hamilton, and Montreal PM2.5 samples (median: 0.10–0.14 μg m−3) compared to Vancouver (0.03 μg m−3); this may in part explain the higher water solubility of Fe at these sites. In addition, the aerosol pH, estimated using ISORROPIA II model, covered a relatively small interquartile range (3.4–3.9) in Vancouver compared to the other sites (Toronto 2.5–3.9, Hamilton 2.2–3.4, Montreal 2–4), and its median value was also 0.48–0.65 units higher in Vancouver than in the other sites, i.e. 3.51 compared to 2.86–3.03 (Fig. 2).
Fig. 2.
The pH of aerosol aqueous phase across the study locations estimated using ISORROPIA model.
The elevated aerosol pH combined with relatively low LWC and oxalate levels in Vancouver PM2.5 could indeed limit the amount of water-soluble Fe in these samples (Fig. S3, Table S3), whereas the opposite patterns seen in Hamilton and Montreal would partly explain the relatively high Fe dissolution at these sites (Wong et al., 2020). Although the study site in Vancouver is a near-road location, which is typically influenced by traffic emission, the related samples were chosen in such way that they also reflect two periods of high and low biomass burning activity in order to examine the effect of such activity on the PM2.5 oxidative burden. This choice of samples may have contributed to the higher pH range (Fig. 2). In a previous study, the pH of PM1 associated with biomass-burning was found to be 1–1.5 units higher than the pH associated with other emission sources, e.g. mineral dust, marine, and continental air mass (Bougiatioti et al., 2016). The elevated mean and median pH at Vancouver site can be explained by a combination of factors: (1) high relative humidity (median: 84% compared to 66–77% at other sites, Table S3) and low temperature (median: 10.5 °C compared to 13.1–18.8 °C), leading to a high aerosol moisture uptake, low H+/LWC, and higher pH values (Bougiatioti et al., 2016, Nah et al., 2018); (2) the high abundance of NH3+ combined with noticeably low concentrations of SO42- (median: 0.42 μg m−3 compared to 1.4–2.3 μg m−3 at the other sites, Table S3), indicating that NH3 could readily react with HNO3 to form NH4NO3 aerosols; and (3) the relatively low seasonal variation of pH at Vancouver compared to the other study locations (Fig. 2), especially those at Montreal site. In the latter, the coefficients of variation for temperature and relative humidity were 139 and 24%, respectively, compared to Vancouver, where these were 65 and 12% (Table S3).
3.2. Oxidative potential of PM2.5
3.2.1. Inter-correlation of oxidative potential indicators
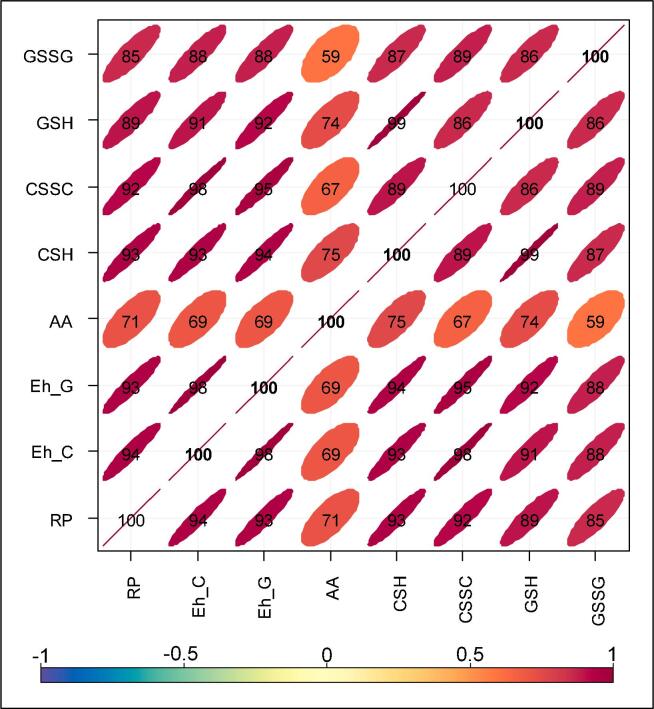
The relationships between the OP indicators in this study are shown as Spearman rank correlation heatmaps in Fig. 3 and S4. RP represents the overall redox state of the SELF influenced by all redox-active species in the system (this parameter was only measured for Toronto, Hamilton, and Montreal samples).
Fig. 3.
Heatmap showing Spearman correlation between oxidative potential indicators; this includes all measurements across four sites. Eh_G and Eh_C are the estimated SELF redox potentials based on GSH/GSSG (i.e. EhGSH-GSSG) and CSH/CSSC (i.e. EhCSH-CSSC) redox pair. AA, CSH, and GSH denote loss rates of these antioxidants (i.e. OPAA, OPCSH, and OPGSH; nmol min−1 m−3), and CSSC and GSSG denote the formation rates of the two oxidation products (nmol min−1 m−3). Correlations are shown as coefficients × 100 and as color-coded ellipses. The latter can be seen as the visual illustration of scatterplots, with high positive correlations appearing as narrow ellipses at 45°and low correlations appearing as ovals.
As shown in the figures, RP was highly correlated with all other target OP indicators, namely the loss rates of of CSH (rs = 0.91–0.96) and GSH (0.81–0.91), and formation rates of CSSC (0.91–0.95) and GSSG (0.78–0.85) and, consequently, the magnitude of EhCSH-CSSC (0.93–0.98) and EhGSH-GSSG (0.90–0.98). While RP presented high correlation with EhGSH-GSSG in all cases (Fig. S4), it occasionally showed higher correlation with GSH than GSSG; this confirms our previous findings that Eh, as opposed to the oxidation rates of individual antioxidants or formation rates of their oxidation products, is a better indicator for OP because it considers the dynamics of redox pairs in the SELF (Shahpoury et al., 2019). The stoichiometric ratios of CSH/CSSC and GSH/GSSG redox pairs were found to be different across the study locations. The mean CSH/CSSC ratios were 6.6 ± 1.3, 5.7 ± 1.4, 7.6 ± 1.4, and 5.3 ± 0.9 in Toronto, Hamilton, Montreal, and Vancouver, respectively, whereas these ratios for GSH/GSSG pair were 2.5 ± 0.7, 2.0 ± 0.5, 2.2 ± 0.7, and 1.8 ± 0.5, respectively. The values for GSH/GSSG ratio reflect the anticipated stoichiometry of 2 RSH → 1 RSSR, and they are in the same range that we previously found with the standard reference PM samples (Shahpoury et al., 2019). One explanation for the deviation from 2 → 1 stoichiometry that was seen with CSH/CSSC, and to a small degree with GSH/GSSG, is the formation of complexes between deprotonated RSH and organic constituents of PM, such as glutathionylated quinone species, prior to the formation of RSSR (Song and Buettner, 2010).
The correlation of RP with OPAA (the depletion rate of AA) was somewhat different from those with the other OP indicators discussed above: while RP showed high correlation with OPAA in Hamilton (rs = 0.81), it demonstrated relatively moderate correlation with OPAA in Toronto and Montreal (rs = 0.65–0.72; Fig. S4). We found similar patterns between EhCSH-CSSC, EhGSH-GSSG and OPAA across the three sites (Fig. S4). Combining all samples from Toronto, Hamilton, and Montreal, we found high association of RP, EhCSH-CSSC and EhGSH-GSSG (rs = 0.93–0.98) and moderate association with OPAA (rs = 0.69–0.71, Fig. 3). These observations imply that the SELF overall redox state was primarily influenced by the redox state of CSH/CSSC and GSH/GSSG pairs, but they may also hint at a key difference between the sites in terms of PM2.5 components and their influence on OPAA (Fig. 3 and S4). AA was shown to be reactive to both Fe and Cu, along with quinones, whereas GSH was shown to have a relatively low reactivity towards Fe (Ayres et al., 2008). For instance, a near-road site such as the one in Toronto is typically influenced by freshly emitted aerosols with distinct physical and chemical characteristics, e.g. externally mixed and in the sub-micron size range. More importantly, here PM2.5 could be enriched in trivalent Fe oxides (water-insoluble form) that are emitted from vehicle brake abrasion (Dabek-Zlotorzynska et al., 2019). Conversely, due to the influence of industrial emission, PM2.5 from Hamilton could contain a higher fraction of pyrogenic water-soluble Fe in the form of Fe(II)SO4 (Ito et al., 2019). As shown in Table S2a-b and Fig. S3, PM2.5 from Toronto has a lower fraction of water-soluble Fe (23%), compared to Hamilton (37%). Such difference is also reflected in the total fraction of water-soluble transition metals between the two sites, i.e. 37 and 52%, respectively. Overall, the results discussed here clearly demonstrate that the empirical RP evaluated for the first time in this study is a reliable indicator for consistent quantification of PM2.5 OP, as it corresponds closely with OP endpoints based on important lung antioxidants and their redox pairs. This is a promising development because, being a straightforward method, it allows uniform future efforts for characterization of ambient particle OP at a larger global scale and in coordination with epidemiological studies in order to better understand the relations between emission sources, aerosol chemical composition and oxidative burden, and health outcomes.
3.2.2. Spatial-temporal distribution of oxidative potential and links with emission sources sectors
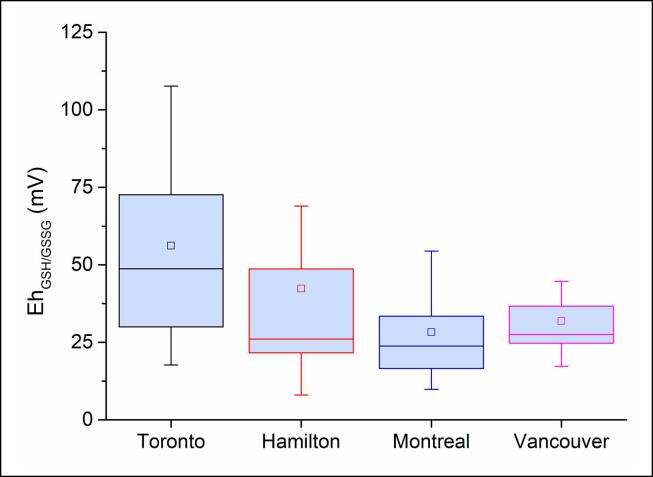
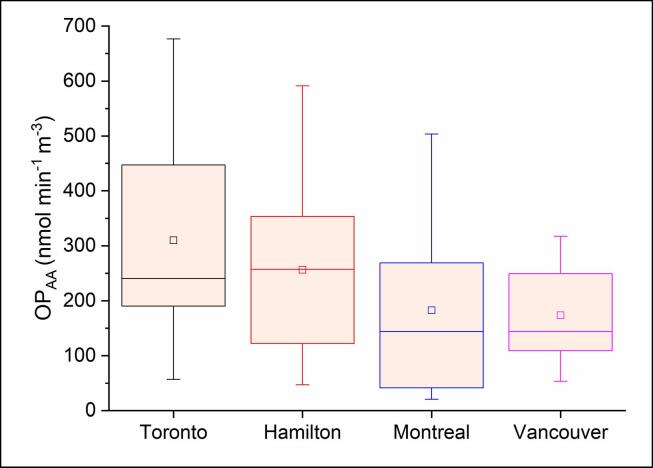
The distribution of OP across the study locations is shown in Fig. 4, Fig. 5 and Table S5. OP is discussed in terms of EhGSH-GSSG (mV, the redox state of SELF estimated based on GSH/GSSG redox pair) as well as OPAA (nmol min−1 m−3). We do not discuss EhCSH-CSSC or RP here because, as indicated in Section 3.2.1, they highly correlate with EhGSH-GSSG (Figs. 3 and S4; see Table S5 for the values related to all OP indicators). As shown in Fig. 4, EhGSH-GSSG covered a relatively larger range in Toronto (18–108 mV, median: 49 mV), followed by narrower ranges in Hamilton (8–69 mV, median: 26), Montreal (10–54 mV, median: 24), and Vancouver (17–45 mV, median: 27). As indicated by the 25th-75th percentile thresholds in the figure, the majority of OP data at these sites fell in the range 30–73, 21–49, 16–34, 24–37 mV, respectively. These correspond to mean GSH loss rates (i.e. OPGSH) of 499 ± 232, 223 ± 130, 193 ± 104, and 194 ± 67 nmol min−1 m−3 (Table S5). The mean EhGSH-GSSG was significantly higher in Toronto (p ≤ 0.01) compared to other sites, whereas there was no statistical difference between these values at other sites (p > 0.05). OPAA followed a similar pattern with mean values of 310 ± 200, 256 ± 153, 183 ± 149, and 174 ± 93 nmol min−1 m−3, respectively (Fig. 5, Table S5); however, these values were not statistically different across the sites (p > 0.05).
Fig. 4.
SELF redox potential based on GSH/GSSG pair following reaction with PM2.5.
Fig. 5.
PM2.5 oxidative potential presented as the loss rate of ascorbic acid in SELF.
It is interesting to note that the mean OPGSH was 11 to 38% higher than OPAA in Toronto, Montreal, and Vancouver, in accordance with our previous results (Shahpoury et al., 2019), but we found an opposite pattern in Hamilton with mean OPAA being 13% higher than OPGSH (Table S5). This may be attributed to the presence of bivalent and water-soluble Fe complexes in PM2.5 from Hamilton which could readily react with AA (see Section 3.2.1). Although EhGSH-GSSG showed higher ranges in the warm season in Toronto and Hamilton and opposite patterns in Montreal and Vancouver (Fig. S5a), the mean EhGSH-GSSG values between warm and cold periods at each location were not statistically different (Mann–Whitney test: p > 0.05). Similarly, OPAA did not show a temporal variation at any site (p > 0.05; Fig. S5b). Having larger sample sizes could improve the statistical significance. We found that mean EhGSH-GSSG from Toronto samples (55 ± 29 mV) in the warm period was significantly higher (p < 0.05) than the mean values from the other sites in either warm or cold period (20 ± 8 to 31 ± 8 mV). In addition, the mean EhGSH-GSSG from Montreal in the warm period was statistically lower than the mean values in Toronto cold period, Hamilton warm period, and Vancouver in either cold or warm period (p < 0.05). As was noted in Section 3.1.1, the Vancouver samples were selected in such way to reflect two distinct periods of high and low biomass burning activity (levoglucosan concentrations of 205.1 ± 91.9 and 18.5 ± 8.9 ng m−3, respectively). We found that the mean EhGSH-GSSG was 21% higher (p < 0.05) in the period with high biomass burning activity, i.e. 31 ± 7 vs. 24 ± 6 mV. This coincided with significantly higher levels of near-total Cu (8.6 ± 2.2 and 6.4 ± 1.7 ng m−3, p < 0.05) which, among other metals, showed the highest association with OP at this site (rs = 0.77; see Section 3.3.1).
When it comes to explaining the contribution of emission sources to PM2.5 OP, one must note that not all sources that contribute to PM2.5 mass also contribute directly to OP. For instance, the secondary inorganic aerosols (e.g. ammonium sulfates or nitrate) make up a large fraction of PM2.5 mass but they do not contribute directly to OP. The sources that are expected to contribute directly to OP (e.g. metal nanoparticles from traffic sources; Gonet and Maher, 2019) do not correlate with PM2.5 mass (de Jesus et al., 2019). In addition, various sources will have overlapping contributions to OP, which cannot be differentiated; for instance, the combination of organic aerosols and secondary inorganic aerosols would synergistically affect the solubility and, consequently, the redox-activity of metallic particles and OP of PM2.5.
Overall, out of the varied emission sources taken into consideration across the four sites in this study, our results show that the emissions that have a major impact on the oxidative burden of PM2.5 are traffic-related (i.e. at the near-road Toronto site). These indeed have a greater impact on OP than the emissions from industrial activities and resuspended crustal matter (i.e. at the other sites). This supports the previous findings related to the OP of PM2.5 from across Toronto (Weichenthal et al., 2019).
Previous epidemiological studies conducted across various cities in Ontario, Canada, indicated that OPGSH was associated with cause-specific mortality, in particular due to lung cancer. Similarly, OPGSH was found associated with myocardial infarction and emergency room visits for respiratory illness (Weichenthal et al., 2016a, Weichenthal et al., 2016b, Weichenthal et al., 2016c). In terms of OP relation to population exposure in the vicinity of the study locations, our results suggest that exposure to redox-active aerosol components is more significant at Toronto and Hamilton sites. This is explained by the relatively high mean OP found at these sites and the site classification in terms of population density (large population centers, i.e. > 150,000 and 100,000–150,000, respectively, residing within 4 km of the study location; Table 1). The population exposure at the sites in Montreal (residential site, with population of 50,000–100,000) and Vancouver (population > 150,000) is relatively less significant, taking into account the relatively low mean OP found at these sites. The exposure at Toronto site could have additional significance in that, being a near-road site, we can anticipate a higher presence of ultrafine (<100 nm diameter) particles (UFPs) in its vicinity. These UFPs are known to be Fe-rich and consist mainly of magnetite (a mixture of ferrous and ferric oxides), maghemite, and hematite from both combustion and brake-wear emissions, along with transition metals such as Cu, Ni, Ti, and Co (Gonet and Maher, 2019, Maher et al., 2020). This could potentially translate in a high particle deposition in the deep lung and a high reactive particle surface which may exacerbate the aerosol oxidative burden and related adverse effects such as oxidation of lipids, proteins, and DNA, and cellular and tissue damage (Oberdörster et al., 2005, Riediker et al., 2019). The particle number concentration and lung deposited particle surface related to submicron and UFPs were previously associated with mortality due to cardiovascular diseases (Hennig et al., 2018). The UFPs are also known to translocate to organs other than their point of entry in the respiratory system, either by entering the blood stream, or directly via olfactory nerve system. For instance, direct penetration of air pollution nanoparticles was reported in post-mortem human brain, heart, and placenta tissues (Maher et al., 2016, Calderón-Garcidueñas et al., 2019, Liu et al., 2021). Post-mortem brain tissues from clinically healthy individuals who were consistently exposed to high levels of air pollution through their life showed high levels of metallic nanoparticles, including magnetite, together with characteristic indicators of Alzheimer’s disease pathogenesis, inflammation, oxidative stress, and DNA signaling impairment (Calderón-Garcidueñas et al., 2008a, Calderón-Garcidueñas et al., 2008b, Maher et al., 2016). In contrast to the traffic site in Toronto, we can expect an opposite picture at Montreal site due to the fact that it is away from point sources and is mainly influenced by wind-blown crustal matter and dust. Although resuspended dust is one of the main sources of urban PM, a large fraction of such particles resides in the super-micron size range, and submicron PM makes up around 10–15% of the particle mass (Gonet and Maher, 2019). This implies that the inhalation bioaccessibility of metals and organic species associated with such particles would be limited because of their relatively low deposition in the lung’s alveolar region (Oberdörster et al., 2005). A higher spatial resolution of the sampling sites will allow a more comprehensive picture of the population exposure to redox-active species across these urban areas (Weichenthal et al., 2019).
3.3. Oxidative potential association with PM2.5 chemical composition
The relationships between OP indicators and the chemical composition of PM2.5 were investigated using Spearman correlation analysis, and the results are presented in Fig. 6, Fig. 7 and S6. For PM2.5-bound transition metals, we considered both their near-total and water-soluble concentrations. In addition, the effects of aerosol pH and metal–ligand complexation on transition metal water-solubility and the potential influence of these parameters on OP were investigated.
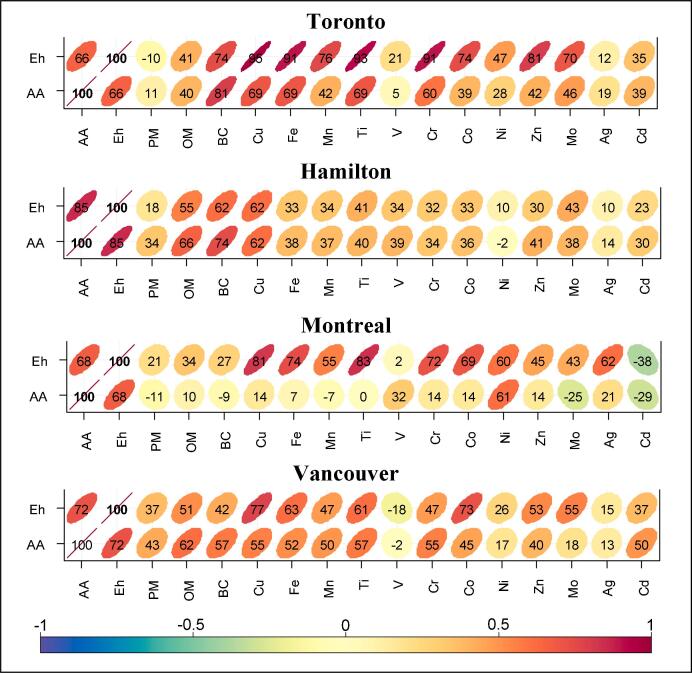
Fig. 6.
Spearman correlation heatmap of OP indicators EhGSH-GSSG (mV) and OPAA (nmol min−1 m−3) with total concentrations of PM2.5 constituents. Correlations are shown as coefficients × 100 and as color-coded ellipses.
Fig. 7.
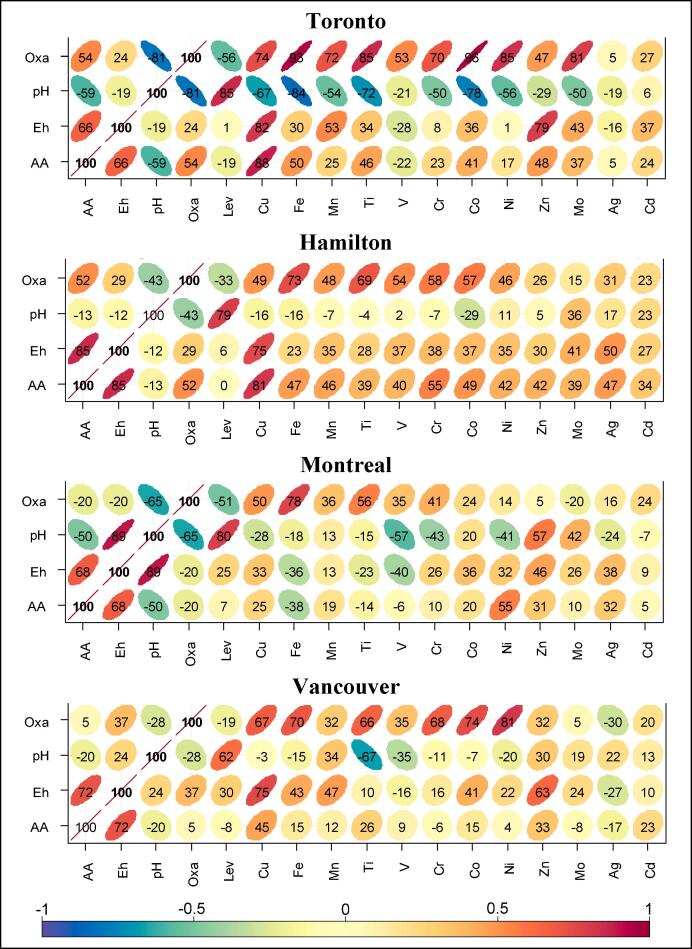
Spearman correlation heatmap of OP indicators EhGSH-GSSG (mV) and OPAA (nmol min−1 m−3), aerosol pH, and oxalate (Oxa) with the concentrations of water-soluble PM2.5 constituents (Lev: levoglucosan). Correlations are shown as coefficients × 100 and as color-coded ellipses.
3.3.1. Oxidative potential and PM2.5 total constituents
As shown in Fig. S6, combining the data from four sites, the total concentrations of PM2.5 constituents had noticeably higher association with EhGSH-GSSG than with OPAA. Accordingly, Cu, Fe, Ti, and Cr had the highest correlation with EhGSG/GSSG (rs = 0.64–0.76). The OP indicators showed low-moderate correlation with PM2.5 concentrations at individual sites (rs ≤ 0.43, Fig. 6) or with pooled samples (r ≤ 0.2, Fig. S6).
This was mainly due to the low-moderate correlation of redox-active transition metals with PM2.5 (rs ≤ 0.57). The correlations between OP indicators and near-total transition metals were not uniform across the study locations. Cu showed moderate to high correlation with at least one OP indicator across all sites (Fig. 6); it had high association with EhGSH-GSSG at Toronto, Vancouver, and Montreal sites (rs = 0.77–0.95), but presented low to moderate correlation with OPAA at these sites (rs = 0.14–0.69). This picture was somewhat different with Hamilton samples, where Cu was moderately correlated with both OP indicators (rs = 0.62).
PCA results suggest that Cu had a mixed industrial (metallurgical) and transport-related origin in Hamilton. While Cu appeared to be the most important metal associated with OP indicators at Hamilton and Vancouver (Fig. 6), other transition metals such as Fe, Ti, and Cr also showed high association with EhGSH-GSSG at Toronto (rs = 0.91–0.93) and Montreal (rs = 0.72–0.83) sites. OM was moderately correlated with OPAA (rs = 0.62–0.66) and EhGSH-GSSG (rs = 0.51–0.55) in Hamilton and Vancouver, while it demonstrated low correlation with the OP indicators at the other two sites (rs ≤ 0.41, Fig. 6). This observation suggests that OPAA is slightly more sensitive to changes in PM2.5 organic content than is EhGSH-GSSG. More importantly, considering the relatively high mass mixing ratio of organic matter in our samples (Fig. 1a), these results indicate that the majority of oxidative activity in our samples may have resided in PM2.5 constituents with smaller mass mixing ratios (e.g. the trace element oxides which contributed < 0.5% to PM2.5 mass). This disagrees with some previous studies where the organic fraction was found to have a large contribution to the OP (Calas et al., 2018, Calas et al., 2019).
Performing this analysis with redox-active organic species, e.g. quinones or organic peroxides, may improve the results for the correlation of organic phase with OP indicators. In Liu et al., (2020), using the same OP assay applied here, we found that a simulated chemical aging of α-pinene and toluene up to 10 days resulted in an increase in the sample peroxide content and OP. Our current data indicate that, despite the large contribution to PM2.5 mass (see Section 3.1 and Table S4a-d), the secondary organic aerosols did not make a large contribution to OP across the sites. This is indicated by the low to moderate correlations of oxalate, a marker for secondary combustion aerosols, with OP indicators (rs ≤ 0.54; see section 3.3.2). We also estimated the contributions of primary vs. secondary organic carbon (i.e. POC vs. SOC) to total organic carbon using the OC/EC minimum ratio method (SOC = OC – (OC/EC)min EC; POC = OC – SOC; Pio et al., 2011, Cesari et al., 2018). The (OC/EC)min was calculated for both cold and warm seasons across the sites; they were 0.3, 0.3, 0.7, and 1.4 at Toronto, Vancouver, Hamilton, and Montreal, respectively, in the cold period, and 0.3, 0.4, 1, and 1.9, respectively, in the warm period. On average, 76–78% of OC at the near-road sites, Toronto and Vancouver, were made of SOC, whereas this contribution at Hamilton and Montreal was 52–66%. The correlations between OP indicators and SOC were found to be low to moderate (rs = 0.26–0.55; not shown in Fig. 6). Interestingly, at Toronto site, the correlations of OP indicators with POC were noticeably high (rs ≤ 0.83). This is consistent with the site characteristics as this is dominated by primary traffic emissions (see section 3.1); we did not observe such distinct features at the other sites. In a previous study based on the DTT assay, volume-normalized OPDTT was shown to have a better correlation with PM2.5 SOC than with POC (Chirizzi et al., 2017), and with OM than with BC. The correlation between OPDTT and OM was higher than those seen with various OP indicators in the present study, and hints at an inherent difference in the nature of chemical assays used in the two studies. This indicates that DTT could have preferential sensitivity to aerosol organic fraction. In fact, Ayres et al., (2008) noted that DTT’s response is primarily related to organic components, as it is insensitive to the presence of metals and H2O2, while Charrier et al. (2012) showed that DTT is significantly more sensitive to equimolar concentrations of quinones than transition metals.
The high correlations found between BC and OP indicators in Toronto and Hamilton (rs = ≥0.81) reflect the nature of emission sources at these sites, the former being a near-road site, and the latter being influenced by point-source industrial emission (<10 km from the source), and reiterate the importance of such emission sources in deriving the oxidative burden of air mixture in urban air (Weichenthal et al., 2019). About 90% of traffic-related exhaust emissions consists of carbonaceous materials, including soot or BC (Gonet and Maher, 2019). BC is made of graphene sheets and it is not anticipated to have redox activity on its own; however, BC can act as carrier for redox active species, such as quinones or metals that are adsorbed onto the graphene structure (McWhinney et al., 2013, Antiñolo et al., 2015). BC produced from diesel engines is in the size range 10–60 nm and is often accompanied by Fe, Cu, Mg, Ca, and Zn (Gonet and Maher, 2019). The indirect redox-activity of BC is expected to be more pronounced at near-source study locations, such as our study site in Toronto, where particles are externally mixed, dominated by UFPs, and not coated with redox-inactive organic species, leaving the redox-active species (e.g. Cu, Fe) exposed and available for reaction with the lung antioxidants. As shown in Fig. 6, there was low correlation between BC and OP indicators at Montreal site (rs = <0.27) and moderate correlation between these variables in Vancouver (rs < 0.57). The samples from these two sites were influenced by biomass burning activity, as indicated by relatively high levels of levoglucosan at these sites (Table S2a-b) and the results of the PCA analysis (Table S4b and d). Biomass burning is the dominant source of BC worldwide (Andreae, 2019). Considering that BC showed similar concentration ranges across our study sites (Table S2a-b), the low-moderate association of OP indicators with BC at Montreal and Vancouver sites and high correlation found in Toronto and Hamilton suggest structural differences in redox-activity of BC associated with biomass burning and traffic emissions. This may point at low abundance of adsorbed redox-active substances that could co-emit with black carbon through biomass combustion.
One important factor which influences the reactivity of aerosols is the aerosol size distribution (Oberdörster et al., 2005, Fang et al., 2017a, Fang et al., 2017b, Costabile et al., 2020). Some of the redox-active organic species (e.g. quinones) were previously found enriched in PM0.5 with the mass median diameters in ultrafine range (Kitanovski et al., 2020, Lammel et al., 2020). As previously noted, aerosols near traffic sources are dominated by submicron and ultrafine particles, including BC and metal particles, with high abundance of ferromagnetic iron oxides consisting mainly of magnetite, maghemite, hematite, and metallic Fe, alongside other transition and heavy metals such as Cu, Mn, Cr, Zn, Ni, and Pb (Gonet and Maher, 2019, Maher et al., 2020). Fe was the dominant transition metal in the present study, and contributed up to 93% to the total metal concentrations, in particular at the near-road sites. The Fe-containing particles in urban environment originate from a variety of sources; mainly gasoline and diesel exhaust, brake and tire wear, and resuspension of crustal matter, but also from urban rail system, aircraft and shipping emissions. The emissions from brake wear was found to be a major source of magnetite particles in near-road sites, accounting for up to 85% of the total airborne magnetite mass, compared to ≤12% for emissions from diesel and gasoline exhaust (Gonet et al., 2021). UFPs make > 90% of the total particle number in brake-dust emissions (Garg et al., 2000, Verma et al., 2016), where the particle composition is dominated by Fe (>50%) alongside other elements including Ba, Sb, Ti, Cr, Zn, Cd, Mo, Sn, Cu, Pb (Gonet and Maher, 2019). The Fe-rich particles typically appear as nanospheres (i.e. spherical or rounded shapes) and are found with various arrangements, including primary Fe nanoparticles from homogenous nucleation, clusters of the primary nanoparticles, carbon clusters with adsorbed primary Fe nanoparticles, and mixture of Fe and carbon clusters (Miller et al., 2007, Gonet and Maher, 2019).
Solid UFPs such as soot and metal particles from exhaust and brake-wear emissions represent particularly high risks at near-road sites because of the high particle reactive surface area which could enhance oxidative response in the lung and cardiovascular system, for instance, due to higher particle deposition in the deep lung and by increasing the oxidation of ELF antioxidants and cellular components. One must note that the presence of organic coating and its thickness can modify the surface reactivity and oxidative burden of otherwise reactive UFPs, as we demonstrated with reactive Ti and Ce nanoparticles in a previous study, i.e. decrease in OP with increase in coating thickness (Liu et al., 2020). Although still important from a toxicological standpoint, other sources, such as emission from tire and road surface wear and resuspension of road dust, could represent a relatively lower risk factor, due to the nature of their dominant components and the size distribution of particles emitted from these sources. For instance, a large fraction (>70%) of tire debris can be made of rubber, textile, and carbon black with relatively small contribution (~16%) from metals (e.g. Fe, Zn, Ti, Al, Si, Ca, K, Mg; Milani et al., 2004, Gonet and Maher, 2019). In terms of mass-size distribution, PM > 10 μm contributes to ~ 70% and PM2.5 to < 5% of total tire emission mass (Kupiainen et al., 2005). Moreover, resuspended road dust is dominated by super-micron particles (~90% of PM10), which are known to have a relatively low deposition in the lung alveolar region compared to UFPs.
The relatively high correlations found between OP indicators, BC, and transition metals at Toronto near-road site could well be related to the abundance of surface reactive and metallic UFPs. This could explain the weak association of PM2.5 mass with transition metals and OP indicators found at this site in the present study. This is because the particle number concentration, typically dominated by UFPs, is weakly correlated with PM2.5 mass (de Jesus et al., 2019). This weak correlation is related to the nature of emission sources: while the number concentration is mainly affected by immediate sources (exhaust emission, brake wear) and new particle formation, PM2.5 mass is influenced by local meteorological conditions and regional transport, including contribution from secondary aerosols (de Jesus et al., 2019). Further field and laboratory studies using size-resolved aerosols are imperative in order to better understand the causal relation between UFPs, the oxidative burden of air mixture and related health effects across various emission source sectors.
3.3.2. Oxidative potential association with PM2.5 water-soluble constituents
The Spearman correlation of water-soluble PM2.5 species and OP indicators at individual sites as well as pooled samples are shown in Fig. 7 and S6. Taking the example of Toronto and Hamilton, Cu had higher near-total concentrations at the former site (11.8 ± 6.8 vs. 5.5 ± 3.8 ng m−3, Table S2a-b); regardless, it showed consistently high correlation with OP indicators at both sites (rs: 0.75–0.88; Fig. 7). This may not be surprising if we consider that, irrespective of its oxidation state, i.e. Cu(I or II), Cu has a relatively high water-solubility in the aerosol aqueous phase (Tapparo et al., 2020; Fig. S3), which would enhance its redox activity in SELF. In fact, as shown in Fig. S6, when all samples across four sites were included in the correlation analysis, the water-soluble Cu had the highest association with OP indicators, while this was not obvious with the other metals. For metals with limited water-solubility (e.g. Fe), it is not merely the aerosol-bound concentration that affects OP; other parameters such as metal dissolution due to organic ligand–metal interactions, aerosol pH and LWC (Panias et al., 1996, Zhou et al., 2015, Fang et al., 2017a, Tapparo et al., 2020, Wong et al., 2020), and the transition metal oxidation state (Nico et al., 2009) could play roles in the way metals influence the aerosol redox activity. The Spearman correlation analysis showed that the aerosol pH in our study had moderate to strong negative correlation with ambient temperature (rs = −0.69 to −0.94), and positive correlation with aerosol LWC (rs = 0.52–0.80; Fig. S7) and NO3 (rs = 0.63–0.77) across the sites and with NH4 at Montreal (rs = 0.72) site. The effect of LWC on pH was most evident with Hamilton samples (rs = 0.80), which also showed a high correlation of LWC with the ambient relative humidity (rs = 0.90), whereas the effect of temperature on pH was more evident at the other sites (Fig. S7). The effect of NO3 on pH, on the other hand, was evenly distributed across all sites.
As shown in Fig. 7, the strongest relationships between the aerosol pH, oxalate levels, metal solubility, and OP indicators were found in Toronto, where pH showed the highest association with the levels of water-soluble Fe, followed by Co, Ti, and Mn. Similarly, oxalate was highly correlated with Fe and Co (rs = 0.93–0.96), followed by Ti, Ni, Mo, Cu, Mn, and Cr (rs = 0.70–0.85). Both pH and oxalate had moderate correlation with OPAA (rs = -0.59 and 0.54, respectively) and relatively low correlation with EhGSH-GSSG (rs = −0.19 and 0.24, respectively). This may be attributed to the higher reactivity of Fe towards AA compared to GSH (Ayres et al., 2008), which is also reflected in the moderate correlation of water-soluble Fe with OPAA (rs = 0.50) and a low correlation with Eh (rs = 0.30, Fig. 7). Regardless, we must note that the actual effect of soluble Fe may not project itself in the response of antioxidants, as described above, but rather in the formation of OH radical from H2O2 through Fenton-like reactions in ELF (Lakey et al., 2016, Shiraiwa et al., 2017, Fang et al., 2019).
An interesting feature of Toronto samples is the high negative correlation of aerosol pH with oxalate (rs = -0.81) and the positive correlation with levoglucosan (rs = 0.85). Moreover, oxalate had a higher association with water-soluble Fe (rs = 0.93) than did the aerosol pH (rs = −0.84); we found similar patterns with some other transition metals, such as Mn, Ti, and Cr and these features were also evident at the other sites with various intensities (Fig. 7). The negative correlation of pH with oxalate is explained by the negative correlation of pH with ambient temperature and the positive correlation of the latter with oxalate levels, i.e. oxalate formation via secondary processes is higher during warmer periods due to high photochemical activity and this coincides with low aerosol LWC and pH (Fig. S7). The combination of low pH and high oxalate concentrations could lead to high Fe solubility (Tao and Murphy, 2019); this would be more evident in conditions where PM2.5 is rich in Fe(III) oxides, e.g. at Toronto site. In contrast, the low correlation between pH and water-soluble Fe at Hamilton site was likely related to the abundance of pyrogenic Fe (e.g. in the form of Fe(II)SO4, suggested by a high correlation between water-soluble Fe and SO4; rs = 0.86). The presence of bivalent Fe in the PM2.5 metal mixture could enhance the Fe reductive dissolution pathway by reducing the induction period and favoring the autocatalytic dissolution process (Panias et al., 1996). Although the proton-mediated Fe dissolution in the aerosol aqueous phase is most effective at pH < 2 (Fang et al., 2017a, Wong et al., 2020), the influence of pH on metal dissolution should be considered alongside the role of ligands, since the two factors are related in the aerosol aqueous phase. For instance, the complexation of Fe(III) with oxalate in the aerosol becomes important at pH < 3, and this tends to decrease with increase in pH (Tao and Murphy, 2019, Tapparo et al., 2020). One must note that pH also affects the gas-particle partitioning of oxalic acid and oxalate in the aerosol, with > 95% estimated to partition as oxalate in the particulate phase at pH ≥ 3. The ligand-mediated Fe dissolution is expected to be more pronounced during the time of heightened photochemical activity, such as in spring and summer or in the early hours of the day following sunrise; this follows the conversion of Fe(III)-oxalate complex to Fe(II) and CO2 under the ultraviolet and visible light (Panias et al., 1996, Zhou et al., 2015). In fact, our data from Toronto and Hamilton show that the mean concentrations of water-soluble Fe were significantly higher in the warm periods, e.g. 80 ± 32 vs. 28 ± 16 in Toronto, and 53 ± 30 vs. 24 ± 14 in Hamilton, p < 0.01. Although this observation could partly be related to a higher emission of pyrogenic Fe in the warm period, it also points at the enhanced solubility of Fe through proton- and ligand-mediated dissolution pathways.
A recent epidemiological study in the U.S. found that cardiovascular diseases were highly associated with the levels of water-soluble metals, in particular Fe (Ye et al., 2018). Based on this study, Wong et al., (2020) examined the sources and mechanisms behind the temporal changes in water-soluble Fe concentrations, and found noticeable association between changes in SO42-, Fe solubility and PM2.5 mass, suggesting that proton-mediated pathway controlled the Fe solubility. This also led to the hypothesis that the health effects of PM2.5 found in former epidemiological studies might indeed be related to the levels of water-soluble Fe. In the present study, Vancouver was the only site where we found a moderate correlation between PM2.5 mass and water-soluble Fe (rs = 0.62), whereas the other sites showed relatively low correlation (rs ≤ 0.35). However, we found noticeable correlation between Fe water-solubility (i.e. the ratio of water-soluble/near-total Fe), aerosol pH (rs = −0.62 to −0.66), and levels of SO42- (rs = 0.66 to 0.73) in Toronto, Montreal, and Vancouver, which suggests that acid processing contributed to Fe solubility in the aerosol aqueous phase. Interestingly, the correlation coefficients between these parameters were relatively low in Hamilton (rs = -0.22 and 0.32, respectively), which reiterates the pyrogenic nature of Fe at this site. The enhancement in Fe solubility (and other transition metals) through the pathways noted above is expected to facilitate the loss of antioxidants, as we demonstrated here, and the formation of ROS in ELF. These processes may, at least partly, explain the association between water-soluble metals, in particular Fe, and health outcomes seen in Ye et al. (2018).
Fig. 7 shows that the biomass burning tracer levoglucosan had no noticeable association with OP indicators at any study location (rs ≤ 0.30), while it showed strong positive correlation with pH in Toronto, Hamilton, and Montreal (rs = 0.79–0.85) and a moderate correlation in Vancouver. Considering that redox-active quinones are emitted from biomass burning (Shen et al., 2013, Vicente et al., 2016), the lack of correlation between levoglucosan and OP could be related to potentially low mass mixing ratios of quinones compared to transition metals in our samples, rather than the insensitivity of OP assay to such organic species. The assay was previously tested with some prevalent quinones, such as 1,4-naphthoquinone and 9,10-phenanthrenequinone, and was found responsive to these species. The influence of biomass burning emission on increased aerosol pH has been previously reported (Bougiatioti et al., 2016). The periods with biomass burning often coincide with periods of low ambient temperature, leading to enhanced aerosol water uptake and increased LWC, which eventually result in relatively low H+/LWC ratios and high pH values.
Similar to levoglucosan, polyols, the tracers for primary biogenic emission, showed no association with OP indicators (not shown in the figure). This finding contradicts a previous report where the primary biogenic emission showed a noticeable intrinsic OP (Weber et al., 2018); however, that study noted the possibility of an overlap between biogenic and combustion sources. On the contrary, water-soluble Cu had the highest association with at least one OP indicator in Toronto, Hamilton, and Vancouver (rs = 0.75–0.88); specifically, at the near-road sites, the Cu correlation with Eh was followed by moderate to high correlation with Zn (rs = 0.63–0.79), and moderate association with Mn (rs = 0.47–0.53). We did not find any meaningful relationship between water-soluble metals and OP indicators with Montreal samples, which may indicate that the OP in those samples resided in the water-insoluble metal fraction. In fact, a separate correlation analysis between OP indicators and the water-insoluble metals showed high association with the concentrations of Ti, Mn, and Fe (rs = 0.79–0.83). The correlations with water-insoluble metals were more pronounced at Toronto site, especially with Ti, Fe, Cr, Cu, Mo, and Zn (rs = 0.81–0.90), and with Mn, V, and Co (rs = 0.71–0.73). We did not find high correlation with water-insoluble metals at the other two sites.
4. Conclusions and future direction
As we demonstrated in this study, OP based on GSH/GSSG redox pair provided the most consistent results across the sites, regardless of the studied source sectors. Similarly, RP provided a simple and reliable alternative for the OP indicators, while closely reflecting the GSH/GSSG and CSH/CSSC redox states, and promises applications in future studies. Given that GSH is a major antioxidant that takes part in the lung redox reactions, OPGSH or RP in combination with assays that directly measure the formation of ROS in SELF and epithelial cell lines should be the preferred approach when characterizing the oxidative burden of atmospheric aerosols. This approach will allow a better understanding of the correlations, or the lack thereof, between PM2.5 inhalation and oxidative stress, and respiratory-cardiovascular diseases in epidemiological studies. The elevated OP and its high association with transition metals and BC at Toronto near-road site with a high traffic intensity, and the lack of association with PM2.5 mass, indicate that metal nanoparticles from various transport-related sources may play an important role in regulating OP and the associated toxicity outcomes at similar locations. In addition, the apparent difference in the degree of association between OP and BC from traffic and non-traffic emissions hints at key differences in BC chemistry and morphology from various combustion sources. These aspects warrant further investigation in relation to chemistry, redox activity, and toxicity of nanoparticles in Canadian and other urban atmospheres around the world. Given the anticipated decline in exhaust related emissions due to the electrification of transport fleet in the coming years, the future air pollution mitigation strategies should put emphasis on reducing the emission and atmospheric concentrations of metal particles from non-exhaust sources in urban atmosphere.
Although we did not find significant temporal variation in the OP measured via the loss rates of antioxidants, seasonality may play an important role in the formation of ROS and in particular OH radical, due to enhanced solubility of Fe and Fenton reactions, and following an increase in atmospheric photochemical activity and proton- and ligand-mediated dissolution pathways. These processes may be further influenced by the diversity of emission sources and consequently the Fe oxidation state, and could explain the apparent difference that we found between Toronto traffic site and Hamilton industrial site in the degree of associations (higher at Toronto) between ambient temperature, aerosol pH, oxalate level, and water-soluble Fe. Our results indicate that enhancement in Fe solubility due to various dissolution processes could have a moderate effect on the OP quantified based on antioxidants; however, this effect could be much stronger on OH radical formation in ELF. Future laboratory and modelling studies should consider the overlapping effects of organic aerosols and secondary inorganic aerosols and their sources on aerosol aqueous phase processes and solubility of metal nanoparticles, in particular Fe, and how these may contribute to the formation of OH radical in ELF. Such studies should consider SELF formulations that are representative of the physiological ELF and include biomolecules such as lipids and proteins. These considerations are important in elucidating the contribution of various emission sources to aerosol oxidative burden, in explaining the role of OP in the health effects associated with the inhalation of PM2.5, and in defining efficient air quality management strategies.
CRediT authorship contribution statement
Pourya Shahpoury: Conceptualization, Supervision, Methodology, Validation, Investigation, Formal analysis, Visualization, Writing - original draft. Zheng Wei Zhang: Investigation, Formal analysis, Writing - review & editing. Andrea Arangio: Conceptualization, Formal analysis, Validation, Writing - review & editing. Valbona Celo: Methodology, Supervision, Resources, Writing - review & editing. Ewa Dabek-Zlotorzynska: Methodology, Supervision, Resources, Writing - review & editing. Tom Harner: Conceptualization, Supervision, Writing - review & editing. Athanasios Nenes: Methodology, Supervision, Formal analysis, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
We thank Ky Su, Mahmoud Yassine, and Elizabeth Mackay for laboratory support, and Jean-Pierre Charland for managing the NAPS program.
Financial support
This work was funded by the Air Pollution Program of Environment and Climate Change Canada. AN and AA acknowledge support by the project PyroTRACH (ERC-2016-COG) funded from H2020-EU.1.1. - Excellent Science - European Research Council (ERC), project ID 726165 and Swiss Federal funds.
Handling Editor: Adrian Covaci
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2020.106343.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Almeida, S., Pio, C., Freitas, M., Reis, M., Trancoso, M., 2005. Source apportionment of fine and coarse particulate matter in a sub-urban area at the Western European Coast, Atmos. Environ., 39, 3127–3138, doi:10.1016/j.atmosenv.2005.01.048, 2005.
- Andreae M.O. Emission of trace gases and aerosols from biomass burning – an updated assessment. Atmos. Chem. Phys. 2019;19:8523–8546. doi: 10.5194/acp-19-8523-2019. [DOI] [Google Scholar]
- Antiñolo M., Willis M.D., Zhou S., Abbatt J.P.D. Connecting the oxidation of soot to its redox cycling abilities. Nat. Commun. 2015;6:6812. doi: 10.1038/ncomms7812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayres J.G., Borm P., Cassee F.R., Castranova V., Donaldson K., Ghio A., Harrison R.M., Hider R., Kelly F., Kooter I.M., Marano F., Maynard R.L., Mudway I., Nel A., Sioutas C., Smith S., Baeza-Squiban A., Cho A., Duggan S., Froines J. Evaluating the toxicity of airborne particulate matter and nanoparticles by measuring oxidative stress potential – a workshop report and consensus statement. Inhal. Toxicol. 2008;20:75–99. doi: 10.1080/08958370701665517. [DOI] [PubMed] [Google Scholar]
- Bates J.T., Fang T., Verma V., Zeng L., Weber R.J., Tolbert P.E., Abrams J.Y., Sarnat S.E., Klein M., Mulholland J.A., Russell A.G. Review of acellular assays of ambient particulate matter oxidative potential: methods and relationships with composition, sources, and health effects. Environ. Sci. Technol. 2019;53:4003–4019. doi: 10.1021/acs.est.8b03430. [DOI] [PubMed] [Google Scholar]
- Battaglia M.A., Jr., Weber R.J., Nenes A., Hennigan C.J. Effects of water-soluble organic carbon on aerosol pH. Atmos. Chem. Phys. 2019;19:14607–14620. doi: 10.5194/acp-19-14607-2019. [DOI] [Google Scholar]
- Bougiatioti A., Nikolaou P., Stavroulas I., Kouvarakis G., Weber R., Nenes A., Kanakidou M., Mihalopoulos N. Particle water and pH in the eastern Mediterranean: source variability and implications for nutrient availability. Atmos. Chem. Phys. 2016;16:4579–4591. doi: 10.5194/acp-16-4579-2016. [DOI] [Google Scholar]
- Burnett, R., Chen, H., Szyszkowicz, M., Fann, N., Hubbell, B., Pope, C. A., Apte, J. S., Brauer, M., Cohen, A., Weichenthal, S., Coggins, J., Di, Q., Brunekreef, B., Frostad, J., Lim, S. S., Kan, H., Walker, K. D., Thurston, G. D., Hayes, R. B., Lim, C. C., Turner, M. C., Jerrett, M., Krewski, D., Gapstur, S. M., Diver, W. R., Ostro, B., Goldberg, D., Crouse, D. L., Martin, R. V., Peters, P., Pinault, L., Tjepkema, M., van Donkelaar, A., Villeneuve, P. J., Miller, A. B., Yin, P., Zhou, M., Wang, L., Janssen, N. A. H., Marra, M., Atkinson, R. W., Tsang, H., Quoc Thach, T., Cannon, J. B., Allen, R. T., Hart, J. E., Laden, F., Cesaroni, G., Forastiere, F., Weinmayr, G., Jaensch, A., Nagel, G., Concin, H. and Spadaro, J. V.: Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter, Proc. Natl. Acad. Sci., 115(38), 9592–9597, doi:10.1073/pnas.1803222115, 2018. [DOI] [PMC free article] [PubMed]
- Calas, A., Uzu, G., Martins, J. M. F., Voisin, Di., Spadini, L., Lacroix, T., Jaffrezo, J.-L., 2017. The importance of simulated lung fluid (SLF) extractions for a more relevant evaluation of the oxidative potential of particulate matter, Sci. Rep., 7, 11617, doi:10.1038/s41598-017-11979-3, 2017. [DOI] [PMC free article] [PubMed]
- Calas A., Uzu G., Kelly F.J., Houdier S., Martins J.M.F., Thomas F., Molton F., Charron A., Dunster C., Oliete A., Jacob V., Besombes J.-L., Chevrier F., Jaffrezo J.-L. Comparison between five acellular oxidative potential measurement assays performed with detailed chemistry on PM10 samples from the city of Chamonix (France) Atmos. Chem. Phys. 2018;18:7863–7875. doi: 10.5194/acp-18-7863-2018. [DOI] [Google Scholar]
- Calas A., Uzu G., Besombes J.L., Martins J.M.F., Redaelli M., Weber S., Charron A., Albinet A., Chevrier F., Brulfert G., Mesbah B., Favez O., Jaffrezo J.L. Seasonal variations and chemical predictors of oxidative potential (OP) of particulate matter (PM), for seven urban French sites. Atmosphere. 2019;10:698. doi: 10.3390/atmos10110698. [DOI] [Google Scholar]
- Calderón-Garcidueñas L., Solt A.C., Henríquez-Roldán C., Torres-Jardón R., Nuse B., Herritt L., Villarreal-Calderón R., Osnaya N., Stone I., García R., Brooks D.M., González-Maciel A., Reynoso-Robles R., Delgado-Chávez R., Reed W. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid β-42 and α-synuclein in children and young adult. Toxicol. Pathol. 2008;36:289–310. doi: 10.1177/0192623307313011. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L., Villarreal-Calderon R., Valencia-Salazar G., Henríquez-Roldán C., Gutiérrez-Castrellón P., Torres-Jardón R., Osnaya-Brizuela N., Romero L., Torres-Jardón R., Solt A., Reed W. Systemic inflammation, endothelial dysfunction, and activation in clinically healthy children exposed to air pollutants. Inhal. Toxicol. 2008;20:499–506. doi: 10.1080/08958370701864797. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L., González-Maciel A., Mukherjee P.S., Reynoso-Robles R., Pérez-Guillé B., Gayosso-Chávez C., Torres-Jardón R., Cross J.V., Ahmed I.A.M., Karloukovski V.V., Maher B.A. Combustion- and friction-derived magnetic air pollution nanoparticles in human hearts. Environ. Res. 2019;176 doi: 10.1016/j.envres.2019.108567. [DOI] [PubMed] [Google Scholar]
- Carlton A.G., Turpin B.J., Altieri K.E., Seitzinger S., Reff A., Lim H.-J., Ervens B. Atmospheric oxalic acid and SOA production from glyoxal: results of aqueous photooxidation experiments. Atmos. Environ. 2007;41:7588–7602. doi: 10.1016/j.atmosenv.2007.05.035. [DOI] [Google Scholar]
- Carslaw D.C., Ropkins K. Openair - an R package for air quality data analysis. Environ. Model. Softw. 2012;27:52–61. doi: 10.1016/j.envsoft.2011.09.008. [DOI] [Google Scholar]
- Cesari, D., De Benedetto, G. E., Bonasoni, P., Busetto, M., Dinoi, A., Merico, E., Chirizzi, D., Cristofanelli, P., Donateo, A., Grasso, F. M., Marinoni, A., Pennetta, A. and Contini, D.: Seasonal variability of PM2.5 and PM10 composition and sources in an urban background site in Southern Italy, Sci. Total Environ., 612, 202–213, doi:10.1016/j.scitotenv.2017.08.230, 2018. [DOI] [PubMed]
- Celo, V., Dabek-Zlotorzynska, E., Mathieu, D. and Okonskaia, I.: Validation of a simple microwave-assisted acid digestion method using microvessels for analysis of trace elements in atmospheric PM2.5 in monitoring and fingerprinting studies, Open Chem. Biomed. Methods J., 3, 143–152, doi:10.2174/1875038901003010143, 2010.
- Charrier J.G., Anastasio C. On dithiothreitol (DTT) as a measure of oxidative potential for ambient particles: evidence for the importance of soluble transition metals. Atmos. Chem. Phys. 2012;12:9321–9333. doi: 10.5194/acp-12-9321-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirizzi, D., Cesari, D., Guascito, M. R., Dinoi, A., Giotta, L., Donateo, A. and Contini, D.: Influence of Saharan dust outbreaks and carbon content on oxidative potential of water-soluble fractions of PM2.5 and PM10, Atmos. Environ., 163, 1-8, doi:10.1016/j.atmosenv.2017.05.021, 2017.
- Cohen A.J., Brauer M., Burnett R., Anderson H.R., Frostad J., Estep K., Balakrishnan K., Brunekreef B., Dandona L., Dandona R., Feigin V., Freedman G., Hubbell B., Jobling A., Kan H., Knibbs L., Liu Y., Martin R., Morawska L., Pope C.A., Shin H., Straif K., Shaddick G., Thomas M., van Dingenen R., van Donkelaar A., Vos T., Murray C.J.L., Forouzanfar M.H. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet. 2017;389(10082):1907–1918. doi: 10.1016/S0140-6736(17)30505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland A., Lytle D.A. Measuring the oxidation–reduction potential of important oxidants in drinking water. J. Am. Water Work. Assoc. 2014;106:10–20. doi: 10.5942/jawwa.2014.106.0002. [DOI] [Google Scholar]
- Costabile F., Gualtieri M., Ancona C., Canepari S., Decesari S. Ultrafine particle features associated with pro-inflammatory and oxidative responses: implications for health studies. Atmosphere. 2020;11:414. doi: 10.3390/atmos11040414. [DOI] [Google Scholar]
- Crobeddu, B., Aragao-Santiago, L., Bui, L.-C., Boland, S. and Baeza Squiban, A.: Oxidative potential of particulate matter 2.5 as predictive indicator of cellular stress, Environ. Pollut., 230, 125–133, doi:10.1016/j.envpol.2017.06.051, 2017. [DOI] [PubMed]
- Crobeddu B., Baudrimont I., Deweirdt J., Sciare J., Badel A., Camproux A.-C., Bui L.C., Baeza-Squiban A. Lung antioxidant depletion: a predictive indicator of cellular stress induced by ambient fine particles. Environ. Sci. Technol. 2020;54:2360–2369. doi: 10.1021/acs.est.9b05990. [DOI] [PubMed] [Google Scholar]
- Dabek-Zlotorzynska, E., Dann, T. F., Kalyani Martinelango, P., Celo, V., Brook, J. R., Mathieu, D., Ding, L. and Austin, C. C.: Canadian National Air Pollution Surveillance (NAPS) PM2.5 speciation program: Methodology and PM2.5 chemical composition for the years 2003–2008, Atmos. Environ., 45, 673–686, doi:10.1016/j.atmosenv.2010.10.024, 2011.
- Dabek-Zlotorzynska, E., Celo, V., Ding, L., Herod, D., Jeong, C. H., Evans, G. and Hilker, N.: Characteristics and sources of PM2.5 and reactive gases near roadways in two metropolitan areas in Canada, Atmos. Environ., 218, 116980, doi:10.1016/j.atmosenv.2019.116980, 2019.
- de Jesus, A. L., Rahman, M. M., Mazaheri, M., Thompson, H., Knibbs, L. D., Jeong, C., Evans, G., Nei, W., Ding, A., Qiao, L., Li, L., Portin, H., Niemi, J. V., Timonen, H., Luoma, K., Petäjä, T., Kulmala, M., Kowalski, M., Peters, A., Cyrys, J., Ferrero, L., Manigrasso, M., Avino, P., Buonano, G., Reche, C., Querol, X., Beddows, D., Harrison, R. M., Sowlat, M. H., Sioutas, C. and Morawska, L.: Ultrafine particles and PM2.5 in the air of cities around the world: Are they representative of each other?, Environ. Int., 129, 118-135, doi:10.1016/j.envint.2019.05.021, 2019. [DOI] [PubMed]
- Fang T., Guo H., Zeng L., Verma V., Nenes A., Weber R.J. Highly acidic ambient particles, soluble metals, and oxidative potential: a link between sulfate and aerosol toxicity. Environ. Sci. Technol. 2017;51:2611–2620. doi: 10.1021/acs.est.6b06151. [DOI] [PubMed] [Google Scholar]
- Fang T., Zeng L., Gao D., Verma V., Stefaniak A.B., Weber R.J. Ambient size distributions and lung deposition of aerosol dithiothreitol-measured oxidative potential: contrast between soluble and insoluble particles. Environ. Sci. Technol. 2017;51:6802–6811. doi: 10.1021/acs.est.7b01536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang T., Lakey P.S.J., Weber R.J., Shiraiwa M. Oxidative potential of particulate matter and generation of reactive oxygen species in epithelial lining fluid. Environ. Sci. Technol. 2019;53:12784–12792. doi: 10.1021/acs.est.9b03823. [DOI] [PubMed] [Google Scholar]
- Fountoukis C., Nenes A. ISORROPIA II: a computationally efficient thermodynamic equilibrium model for K+ −Ca2+ −Mg2+ −NH4+ −Na+ −SO42− −NO3−, –Cl− –H2O. Atmos. Chem. Phys. 2007;7:4639–4659. doi: 10.5194/acp-7-4639-2007. [DOI] [Google Scholar]
- Freedman M.A., Ott E.J.E., Marak K.E. Role of pH in aerosol processes and measurement challenges. J. Phys. Chem. A. 2019;123:1275–1284. doi: 10.1021/acs.jpca.8b10676. [DOI] [PubMed] [Google Scholar]
- Gao D., Godri Pollitt K.J., Mulholland J.A., Russell A.G., Weber R.J. Characterization and comparison of PM2.5 oxidative potential assessed by two acellular assays. Atmos. Chem. Phys. 2020;20:5197–5210. doi: 10.5194/acp-20-5197-2020. [DOI] [Google Scholar]
- Garg B.D., Cadle S.H., Mulawa P.A., Groblicki P.J., Laroo C., Parr G.A. Brake wear particulate matter emissions. Environ. Sci. Technol. 2000;34:4463–4469. doi: 10.1021/es001108h. [DOI] [Google Scholar]
- Gonet T., Maher B.A. Airborne, vehicle-derived Fe-bearing nanoparticles in the urban environment: a review. Environ. Sci. Technol. 2019;53:9970–9991. doi: 10.1021/acs.est.9b01505. [DOI] [PubMed] [Google Scholar]
- Gonet T., Maher B.A., Kukutschová J. Source apportionment of magnetite particles in roadside airborne particulate matter. Sci. Total Environ. 2021;752 doi: 10.1016/j.scitotenv.2020.141828. [DOI] [PubMed] [Google Scholar]
- Guo H., Xu L., Bougiatioti A., Cerully K.M., Capps S.L., Hite J.R., Carlton A.G., Lee S.-H., Bergin M.H., Ng N.L., Nenes A., Weber R.J. Fine-particle water and pH in the southeastern United States. Atmos. Chem. Phys. 2015;15:5211–5228. doi: 10.5194/acp-15-5211-2015. [DOI] [Google Scholar]
- Hennig F., Quass U., Hellack B., Küpper M., Kuhlbusch T.A.J., Stafoggia M., Hoffmann B. Ultrafine and fine particle number and surface area concentrations and daily cause-specific mortality in the Ruhr area, Germany, 2009–2014. Environ. Health Perspect. 2018;126 doi: 10.1289/EHP2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennigan C.J., Izumi J., Sullivan A.P., Weber R.J., Nenes A. a critical evaluation of proxy methods used to estimate the acidity of atmospheric particles. Atmos. Chem. Phys. 2015;15:2775–2790. doi: 10.5194/acp-15-2775-2015. [DOI] [Google Scholar]
- Hoek G., Krishnan R.M., Beelen R., Peters A., Ostro B., Brunekreef B., Kaufman J.D. Long-term air pollution exposure and cardio- respiratory mortality: a review. Environ. Heal. 2013;12:43. doi: 10.1186/1476-069X-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito A., Myriokefalitakis S., Kanakidou M., Mahowald N.M., Scanza R.A., Hamilton D.S., Baker A.R., Jickells T., Sarin M., Bikkina S., Gao Y., Shelley R.U., Buck C.S., Landing W.M., Bowie A.R., Perron M.M.G., Guieu C., Meskhidze N., Johnson M.S., Feng Y., Kok J.F., Nenes A., Duce R.A. Pyrogenic iron: the missing link to high iron solubility in aerosols. Sci. Adv. 2019;5:7671. doi: 10.1126/sciadv.aau7671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer S.S., Jones D.P., Brigham K.L., Rojas M. Oxidation of plasma cysteine/cystine redox state in endotoxin-induced lung injury. Am. J. Respir. Cell Mol. Biol. 2009;40:90–98. doi: 10.1165/rcmb.2007-0447OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D.P., Carlson J.L., Mody V.C., Cai J., Lynn M.J., Sternberg P. Redox state of glutathione in human plasma. Free Radic. Biol. Med. 2000;28:625–635. doi: 10.1016/S0891-5849(99)00275-0. [DOI] [PubMed] [Google Scholar]
- Kelly F.J., Fussell J.C. Role of oxidative stress in cardiovascular disease outcomes following exposure to ambient air pollution. Free Radic. Biol. Med. 2017;110:345–367. doi: 10.1016/j.freeradbiomed.2017.06.019. [DOI] [PubMed] [Google Scholar]
- Kitanovski Z., Shahpoury P., Samara C., Voliotis A., Lammel G. Composition and mass size distribution of nitrated and oxygenated aromatic compounds in ambient particulate matter from southern and central Europe – implications for the origin. Atmos. Chem. Phys. 2020;20:2471–2487. doi: 10.5194/acp-20-2471-2020. [DOI] [Google Scholar]
- Kupiainen K.J., Tervahattu H., Räisänen M., Mäkelä T., Aurela M., Hillamo R. Size and composition of airborne particles from pavement wear, tires, and traction sanding. Environ. Sci. Technol. 2005;39:699–706. doi: 10.1021/es035419e. [DOI] [PubMed] [Google Scholar]
- Lakey P.S.J., Berkemeier T., Tong H., Arangio A.M., Lucas K., Pöschl U., Shiraiwa M. Chemical exposure-response relationship between air pollutants and reactive oxygen species in the human respiratory tract. Sci. Rep. 2016;6:32916. doi: 10.1038/srep32916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel G., Kitanovski Z., Kukučka P., Novák J., Arangio A.M., Codling G.P., Filippi A., Hovorka J., Kuta J., Leoni C., Příbylová P., Prokeš R., Sáňka O., Shahpoury P., Tong H., Wietzoreck M. Oxygenated and Nitrated Polycyclic Aromatic Hydrocarbons in Ambient Air—Levels, Phase Partitioning, Mass Size Distributions, and Inhalation Bioaccessibility. Environ. Sci. Technol. 2020;54:2615–2625. doi: 10.1021/acs.est.9b06820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrigan, P. J., Fuller, R., Acosta, N. J. R., Adeyi, O., Arnold, R., Basu, N. (Nil), Baldé, A. B., Bertollini, R., Bose-O’Reilly, S., Boufford, J. I., Breysse, P. N., Chiles, T., Mahidol, C., Coll-Seck, A. M., Cropper, M. L., Fobil, J., Fuster, V., Greenstone, M., Haines, A., Hanrahan, D., Hunter, D., Khare, M., Krupnick, A., Lanphear, B., Lohani, B., Martin, K., Mathiasen, K. V., McTeer, M. A., Murray, C. J. L., Ndahimananjara, J. D., Perera, F., Potočnik, J., Preker, A. S., Ramesh, J., Rockström, J., Salinas, C., Samson, L. D., Sandilya, K., Sly, P. D., Smith, K. R., Steiner, A., Stewart, R. B., Suk, W. A., van Schayck, O. C. P., Yadama, G. N., Yumkella, K. and Zhong, M.: the Lancet Commission on pollution and health, Lancet, 391, 462-512, doi:10.1016/S0140-6736(17)32345-0, 2018. [DOI] [PubMed]
- Lelieveld J., Haines A., Pozzer A. Age-dependent health risk from ambient air pollution: a modelling and data analysis of childhood mortality in middle-income and low-income countries. Lancet Planet. Heal. 2018;2:292–300. doi: 10.1016/S2542-5196(18)30147-5. [DOI] [PubMed] [Google Scholar]
- Lelieveld J., Klingmüller K., Pozzer A., Pöschl U., Fnais M., Daiber A., Münzel T. Cardiovascular disease burden from ambient air pollution in Europe reassessed using novel hazard ratio functions. Eur. Heart J. 2019;40:1590–1596. doi: 10.1093/eurheartj/ehz135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelieveld J., Klingmüller K., Pozzer A., Burnett R.T., Haines A., Ramanathan V. Effects of fossil fuel and total anthropogenic emission removal on public health and climate. Proc. Natl. Acad. Sci. 2019;116:7192–7197. doi: 10.1073/pnas.1819989116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N.M., Miyashita L., Maher B.A., McPhail G., Jones C.J.P., Barratt B., Thangaratinam S., Karloukovski V., Ahmed I.A., Aslam Z., Grigg J. Evidence for the presence of air pollution nanoparticles in placental tissue cells. Sci. Total Environ. 2021;751 doi: 10.1016/j.scitotenv.2020.142235. [DOI] [PubMed] [Google Scholar]
- Liu Q., Shahpoury P., Liggio J., Harner T., Li K., Lee P., Li S.-M. Understanding the key role of atmospheric processing in determining the oxidative potential of airborne engineered nanoparticles. Environ. Sci. Technol. Lett. 2020;7:7–13. doi: 10.1021/acs.estlett.9b00700. [DOI] [Google Scholar]
- Maher B.A., Ahmed I.A.M., Karloukovski V., MacLaren D.A., Foulds P.G., Allsop D., Mann D.M.A., Torres-Jardón R., Calderon-Garciduenas L. Magnetite pollution nanoparticles in the human brain. Proc. Natl. Acad. Sci. 2016;113:10797–10801. doi: 10.1073/pnas.1605941113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher B.A., González-Maciel A., Reynoso-Robles R., Torres-Jardón R., Calderón-Garcidueñas L. Iron-rich air pollution nanoparticles: an unrecognised environmental risk factor for myocardial mitochondrial dysfunction and cardiac oxidative stress. Environ. Res. 2020;188 doi: 10.1016/j.envres.2020.109816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWhinney R.D., Badali K., Liggio J., Li S.M., Abbatt J.P.D. Filterable redox cycling activity: a comparison between diesel exhaust particles and secondary organic aerosol constituents. Environ. Sci. Technol. 2013;47:3362–3369. doi: 10.1021/es304676x. [DOI] [PubMed] [Google Scholar]
- Milani M., Pucillo F.P., Ballerini M., Camatini M., Gualtieri M., Martino S. First evidence of tyre debris characterization at the nanoscale by focused ion beam. Mater. Charact. 2004;52:283–288. doi: 10.1016/j.matchar.2004.06.001. [DOI] [Google Scholar]
- Miller A., Ahlstrand G., Kittelson D., Zachariah M. The fate of metal (Fe) during diesel combustion: morphology, chemistry, and formation pathways of nanoparticles. Combust. Flame. 2007;149:129–143. doi: 10.1016/j.combustflame.2006.12.005. [DOI] [Google Scholar]
- Münzel T., Gori T., Al-Kindi S., Deanfield J., Lelieveld J., Daiber A., Rajagopalan S. Effects of gaseous and solid constituents of air pollution on endothelial function. Eur. Heart J. 2018;39:3543–3550. doi: 10.1093/eurheartj/ehy481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myriokefalitakis S., Daskalakis N., Mihalopoulos N., Baker A.R., Nenes A., Kanakidou M. Changes in dissolved iron deposition to the oceans driven by human activity: a 3-D global modelling study. Biogeosciences. 2015;12:3973–3992. doi: 10.5194/bg-12-3973-2015. [DOI] [Google Scholar]
- Nah, T., Guo, H., Sullivan, A. P., Chen, Y., Tanner, D. J., Nenes, A., Russell, A., Lee Ng, N., Gregory Huey, L. and Weber, R. J.: Characterization of aerosol composition, aerosol acidity, and organic acid partitioning at an agriculturally intensive rural southeastern US site, Atmos. Chem. Phys., 18, 11471-11491, doi:10.5194/acp-18-11471-2018, 2018.
- Nico P.S., Kumfer B.M., Kennedy I.M., Anastasio C. Redox dynamics of mixed metal (Mn, Cr, and Fe) ultrafine particles. Aerosol Sci. Technol. 2009;43:60–70. doi: 10.1080/02786820802482528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstrom, D. K. and Wilde, F. D.: Reduction-oxidation potential (electrode method), U.S. Geological Survey, Reston, VA., 2005.
- Oberdörster G., Oberdörster E., Oberdörster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Øvrevik J. Oxidative potential versus biological effects: a review on the relevance of cell-free/abiotic assays as predictors of toxicity from airborne particulate matter. Int. J. Mol. Sci. 2019;20:4772. doi: 10.3390/ijms20194772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panias D., Taxiarchou M., Paspaliaris I., Kontopoulos A. Mechanisms of dissolution of iron oxides in aqueous oxalic acid solutions. Hydrometallurgy. 1996;42:257–265. doi: 10.1016/0304-386X(95)00104-O. [DOI] [Google Scholar]
- Pio, C., Cerqueira, M., Harrison, R. M., Nunes, T., Mirante, F., Alves, C., Oliveira, C., Sanchez de la Campa, A., Artíñano, B. and Matos, M.: OC/EC ratio observations in Europe: Re-thinking the approach for apportionment between primary and secondary organic carbon, Atmos. Environ., 45, 6121–6132, doi:https://doi.org/10.1016/j.atmosenv.2011.08.045, 2011.
- Pope C.A., Burnett R.T., Turner M.C., Cohen A., Krewski D., Jerrett M., Gapstur S.M., Thun M.J. Lung cancer and cardiovascular disease mortality associated with ambient air pollution and cigarette smoke: shape of the exposure–response relationships. Environ. Health Perspect. 2011;119:1616–1621. doi: 10.1289/ehp.1103639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pye H.O.T., Nenes A., Alexander B., Ault A.P., Barth M.C., Clegg S.L., Collett J.L., Jr., Fahey K.M., Hennigan C.J., Herrmann H., Kanakidou M., Kelly J.T., Ku I.-T., McNeill V.F., Riemer N., Schaefer T., Shi G., Tilgner A., Walker J.T., Wang T., Weber R., Xing J., Zaveri R.A., Zuend A. The acidity of atmospheric particles and clouds. Atmos. Chem. Phys. 2020;20:4809–4888. doi: 10.5194/acp-20-4809-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riediker M., Zink D., Kreyling W., Oberdörster G., Elder A., Graham U., Lynch I., Duschl A., Ichihara G., Ichihara S., Kobayashi T., Hisanaga N., Umezawa M., Cheng T.-J., Handy R., Gulumian M., Tinkle S., Cassee F. Particle toxicology and health – where are we? Part. Fibre Toxicol. 2019;16:19. doi: 10.1186/s12989-019-0302-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahpoury P., Harner T., Lammel G., Lelieveld S., Tong H., Wilson J. Development of an antioxidant assay to study oxidative potential of airborne particulate matter. Atmos. Meas. Tech. 2019;12:6529–6539. doi: 10.5194/amt-12-6529-2019. [DOI] [Google Scholar]
- Shen G., Tao S., Wei S., Chen Y., Zhang Y., Shen H., Huang Y., Zhu D., Yuan C., Wang H., Wang Y., Pei L., Liao Y., Duan Y., Wang B., Wang R., Lv Y., Li W., Wang X., Zheng X. Field measurement of emission factors of PM, EC, OC, parent, nitro-, and oxy- polycyclic aromatic hydrocarbons for residential briquette, coal cake, and wood in rural Shanxi, China. Environ. Sci. Technol. 2013;47:2998–3005. doi: 10.1021/es304599g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraiwa M., Ueda K., Pozzer A., Lammel G., Kampf C.J., Fushimi A., Enami S., Arangio A.M., Fröhlich-Nowoisky J., Fujitani Y., Furuyama A., Lakey P.S.J., Lelieveld J., Lucas K., Morino Y., Pöschl U., Takahama S., Takami A., Tong H., Weber B., Yoshino A., Sato K. Aerosol health effects from molecular to global scales. Environ. Sci. Technol. 2017;51:13545–13567. doi: 10.1021/acs.est.7b04417. [DOI] [PubMed] [Google Scholar]
- Sofowote, U. M., Su, Y., Dabek-Zlotorzynska, E., Rastogi, A. K., Brook, J. and Hopke, P. K.: Constraining the factor analytical solutions obtained from multiple-year receptor modeling of ambient PM2.5 data from five speciation sites in Ontario, Canada, Atmos. Environ., 108, 151–157, doi:10.1016/j.atmosenv.2015.02.045, 2015a.
- Sofowote, U. M., Su, Y., Dabek-Zlotorzynska, E., Rastogi, A. K., Brook, J. and Hopke, P. K.: Sources and temporal variations of constrained PMF factors obtained from multiple-year receptor modeling of ambient PM2.5 data from five speciation sites in Ontario, Canada, Atmos. Environ., 108, 140–150, doi:10.1016/j.atmosenv.2015.02.055, 2015b.
- Song S., Gao M., Xu W., Shao J., Shi G., Wang S., Wang Y., Sun Y., McElroy M.B. Fine-particle pH for Beijing winter haze as inferred from different thermodynamic equilibrium models. Atmos. Chem. Phys. 2018;18:7423–7438. doi: 10.5194/acp-18-7423-2018. [DOI] [Google Scholar]
- Song Y., Buettner G.R. Thermodynamic and kinetic considerations for the reaction of semiquinone radicals to form superoxide and hydrogen peroxide. Free Radic. Biol. Med. 2010;49:919–962. doi: 10.1016/j.freeradbiomed.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striggow, B.: Field measurement of oxidation-reduction potential (ORP), U.S. Environmental Protection Agency, Athens, GA., 2017.
- Tao, Y. and Murphy, J. G.: the mechanisms responsible for the interactions among oxalate, pH, and Fe dissolution in PM2.5, ACS Earth Sp. Chem., 3, 2259–2265, doi:10.1021/acsearthspacechem.9b00172, 2019.
- Tapparo A., Di Marco V., Badocco D., D’Aronco S., Soldà L., Pastore P., Mahon B.M., Kalberer M., Giorio C. Formation of metal-organic ligand complexes affects solubility of metals in airborne particles at an urban site in the Po valley. Chemosphere. 2020;241 doi: 10.1016/j.chemosphere.2019.125025. [DOI] [PubMed] [Google Scholar]
- Vasilakos P., Russell A., Weber R., Nenes A. Understanding nitrate formation in a world with less sulfate. Atmos. Chem. Phys. 2018;18:12765–12775. doi: 10.5194/acp-18-12765-2018. [DOI] [Google Scholar]
- Verma P.C., Alemani M., Gialanella S., Lutterotti L., Olofsson U., Straffelini G. Wear debris from brake system materials: a multi-analytical characterization approach. Tribol. Int. 2016;94:249–259. doi: 10.1016/j.triboint.2015.08.011. [DOI] [Google Scholar]
- Verma, V., Fang, T., Xu, L., Peltier, R. E., Russell, A. G., Ng, N. L. and Weber, R. J.: Organic aerosols associated with the generation of reactive oxygen species (ROS) by water-soluble PM2.5, Environ. Sci. Technol., 49, 4646-4656, doi:10.1021/es505577w, 2015. [DOI] [PubMed]
- Vicente, E. D., Vicente, A. M., Musa Bandowe, B. A. and Alves, C. A.: Particulate phase emission of parent polycyclic aromatic hydrocarbons (PAHs) and their derivatives (alkyl-PAHs, oxygenated-PAHs, azaarenes and nitrated PAHs) from manually and automatically fired combustion appliances, Air Qual. Atmos. Heal., 9, 653–668, doi:10.1007/s11869-015-0364-1, 2016.
- Weber S., Uzu G., Calas A., Chevrier F., Besombes J.L., Charron A., Salameh D., Ježek I., Moĉnik G., Jaffrezo J.L. An apportionment method for the oxidative potential of atmospheric particulate matter sources: application to a one-year study in Chamonix, France. Atmos. Chem. Phys. 2018;18:9617–9629. doi: 10.5194/acp-18-9617-2018. [DOI] [Google Scholar]
- Weichenthal S., Crouse D.L., Pinault L., Godri-Pollitt K., Lavigne E., Evans G., van Donkelaar A., Martin R.V., Burnett R.T. Oxidative burden of fine particulate air pollution and risk of cause-specific mortality in the Canadian Census Health and Environment Cohort (CanCHEC) Environ. Res. 2016;146:92–99. doi: 10.1016/j.envres.2015.12.013. [DOI] [PubMed] [Google Scholar]
- Weichenthal, S., Lavigne, E., Evans, G., Pollitt, K. and Burnett, R. T.: Ambient PM2.5 and risk of emergency room visits for myocardial infarction: impact of regional PM2.5 oxidative potential: a case-crossover study, Environ. Heal., 15, 46, doi:10.1186/s12940-016-0129-9, 2016b. [DOI] [PMC free article] [PubMed]
- Weichenthal, S. A., Lavigne, E., Evans, G. J., Godri Pollitt, K. J. and Burnett, R. T.: Fine particulate matter and emergency room visits for respiratory illness. Effect modification by oxidative potential, Am. J. Respir. Crit. Care Med., 194, 577–586, doi:10.1164/rccm.201512-2434OC, 2016c. [DOI] [PubMed]
- Weichenthal, S., Shekarrizfard, M., Traub, A., Kulka, R., Al-Rijleh, K., Anowar, S., Evans, G. and Hatzopoulou, M.: Within-City Spatial Variations in Multiple Measures of PM 2.5 Oxidative Potential in Toronto, Canada, Environ. Sci. Technol., 53, 2799–2810, doi:10.1021/acs.est.8b05543, 2019. [DOI] [PubMed]
- WHO: Ambient air pollution: a global assessment of exposure and burden of disease, World Health Organization., 2016.
- Wong J.P.S., Tsagkaraki M., Tsiodra I., Mihalopoulos N., Violaki K., Kanakidou M., Sciare J., Nenes A., Weber R.J. Effects of atmospheric processing on the oxidative potential of biomass burning organic aerosols. Environ. Sci. Technol. 2019;53:6747–6756. doi: 10.1021/acs.est.9b01034. [DOI] [PubMed] [Google Scholar]
- Wong J.P.S., Yang Y., Fang T., Mulholland J.A., Russell A.G., Ebelt S., Nenes A., Weber R.J. Fine particle iron in soils and road dust is modulated by coal-fired power plant sulfur. Environ. Sci. Technol. 2020 doi: 10.1021/acs.est.0c00483. [DOI] [PubMed] [Google Scholar]
- Xiong Q., Yu H., Wang R., Wei J., Verma V. Rethinking dithiothreitol-based particulate matter oxidative potential: measuring dithiothreitol consumption versus reactive oxygen species generation. Environ. Sci. Technol. 2017;51:6507–6514. doi: 10.1021/acs.est.7b01272. [DOI] [PubMed] [Google Scholar]
- Ye, D., Klein, M., Mulholland, J. A., Russell, A. G., Weber, R., Edgerton, E. S., Chang, H. H., Sarnat, J. A., Tolbert, P. E. and Ebelt Sarnat, S.: Estimating Acute Cardiovascular Effects of Ambient PM2.5 Metals, Environ. Health Perspect., 126, 27007, doi:10.1289/EHP2182, 2018. [DOI] [PMC free article] [PubMed]
- Zhang, X., Hecobian, A., Zheng, M., Frank, N. H. and Weber, R. J.: Biomass burning impact on PM2.5 over the southeastern US during 2007: integrating chemically speciated FRM filter measurements, MODIS fire counts and PMF analysis, Atmos. Chem. Phys., 10, 6839–6853, doi:10.5194/acp-10-6839-2010, 2010.
- Zhou Y., Huang X.H., Bian Q., Griffith S.M., Louie P.K.K., Yu J.Z. Sources and atmospheric processes impacting oxalate at a suburban coastal site in Hong Kong: Insights inferred from 1year hourly measurements. J. Geophys. Res. 2015;120:9772–9788. doi: 10.1002/2015JD023531. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.