Abstract
OBJECTIVE
Obstructive sleep apnea (OSA) is a potential cardiovascular risk. We aimed to investigate the association of OSA with heart rhythm disorders and prognosis in elderly patients with new-onset acute myocardial infarction (AMI).
METHODS
We prospectively enrolled 252 AMI elderly patients (mean age, 68.5 ± 6.9 years) who were undergoing revascularization and completed a sleep study during their hospitalization. All subjects were categorized into non-OSA (apnea–hypopnea index (AHI) < 15, n = 130) and OSA (AHI ≥ 15, n = 122) groups based on the AHI. The changes in the autonomic nervous system, incidence of arrhythmia during nocturnal sleep, and major adverse cardiovascular and cerebrovascular events (MACCEs) were compared between the groups.
RESULTS
The mean AHI value in all AMI patients was 22.8 ± 10.9. OSA patients showed higher levels of body mass index and peak high-sensitivity C-reactive protein and lower levels of minimum nocturnal oxygen saturation (MinSaO2), as well as greater proportion of multivessel coronary artery disease (all P < 0.05). The OSA group also showed significant increases in heart rate variability and heart rate turbulence onset (both P < 0.05) and higher incidence of arrhythmia (including sinus, atrial, and ventricular in origin). At a median follow-up of 6 months (mean 0.8–1.6 years), OSA (AHI ≥ 15) combined with hypoxia (MinSaO 2 ≤ 80%) was independently associated with the incidence of MACCEs (hazard ratio [HR]: 4.536; 95% confidence interval [CI]: 1.461−14.084,P = 0.009) after adjusting for traditional risk factors.
CONCLUSIONS
OSA and OSA-induced hypoxia may correlate with the severity of myocardial infarction, increase the occurrence of heart rhythm disorders in elderly subacute MI patients, and worsen their short-term poor outcomes.
Obstructive sleep apnea (OSA) is an increasingly prevalent sleep disorder. A body of evidence has shown that OSA may increase the risk of hypertension,[1] ischemic heart disease,[2]heart failure,[3] cardiac arrhythmia,[4, 5] and mortality for cardiovascular diseases.[6, 7] Although the relationship between OSA and long-term cardiovascular disease is well established, the association between OSA and cardiovascular outcomes following acute coronary syndrome is controversial.[6–9] Recent studies have observed a potential protective effect of OSA in cases of myocardial infarction (MI), which is attributed to chronic intermittent hypoxia resulting in ischemic preconditioning of the myocardium.[10-12] Advanced old age was related to high prevalence of OSA, as well as poor in-hospital prognosis and recurrence of acute MI (AMI) in China.[13, 14]However, the relationship between OSA and AMI remains unclear in elderly patients. Our study aimed to investigate the link of OSA with autonomic nervous system function changes and occurrence of heart rhythm disorders in elderly subacute MI patients, as well as the major adverse cardiac and cerebrovascular events (MACCEs) during a 6-month follow-up.
METHODS
Patients Characteristics
We prospectively enrolled 252 consecutive elderly patients (≥ 60 years, mean age: 68.5 ± 6.9 years) admitted because of AMI to the Critical Cardiac Care Unit in Ruijin hospital affiliated with Shanghai Jiaotong University School of Medicine (Shanghai, China) between March 2018 and March 2020. The diagnoses of AMI were made according to the Fourth Universal Definition of Myocardial Infarction,[15] including ST-elevation myocardial infarction (STEMI) and non-STEMI (NSTEMI).
The exclusion criteria were as follows: patients who (1) had undergone treatment for OSA; (2) had moderate or severe pulmonary diseases such as chronic obstructive pulmonary disease, bronchiectasis, or pulmonary embolism; (3) used medications affecting mental health and the nervous system within the last three months; (4) had severe maxillofacial deformities.
Basic data such as blood biochemical indicators, inflammatory markers, and echocardiography of the patients were obtained. The study was approved by the Institutional Research Ethics Committee of Ruijin hospital affiliated with Shanghai Jiaotong University School of Medicine, and written informed consent was obtained from each patient.
Clinical Procedure and Data Collection
Baseline characteristic data, including of sex, age, body mass index (BMI), smoking, history of hypertension, diabetes mellitus, ischemic stroke, renal dysfunction, and snoring, were collected. Each patient was closely managed in our cardiac intensive care unit according to the European Society of Cardiology guidelines on STEMI (2017) or NSTEMI (2015).[16, 17] All patients underwent regular laboratory tests on the 2nd day and echocardiography within 3 days after admission. The following data were collected: serum or plasma levels of glucose and lipids, liver and renal function, electrolytes and peak values of high-sensitivity C-reactive protein (hs-CRP), N-terminal of the prohormone brain natriuretic peptide (NT-proBNP), and biomarkers of myocardial injury (including creatine kinase-MB [CK-MB] and troponin I).
Overnight Sleep Study
Every patient underwent an overnight sleep study within 7 days after MI (from 21: 00 to 7: 00 the next day). The sleep study was performed using portable type III sleep monitors (Philips-Respironics Stardust II Sleep Recorder, Bend, OR, USA). Patients refrained from caffeine, sedative, or hypnotic drug intake 1 day before their sleep study. The diagnoses of OSA were made according to the Guideline of the American College of Physicians (2013).[18] Oral and nasal airflow and blood oxygen saturation from nocturnal pulse oximetry were traced. The apnea–hypopnea index (AHI, number of apnea or hypopnea events per hour during sleep) was measured as the main parameter. Data obtained from monitoring were subjected to automatic computer analysis followed by manual correction. Based on the AHI values, the patients were categorized into two groups: the non-OSA group (AHI < 15/h) and OSA (AHI ≥ 15/h) group.
Heart Rate Variability (HRV) and Heart Rate Turbulence (HRT) Analysis
Twenty-four-hour (from 8:00 to 8:00 the next day) electrocardiographic (ECG) recordings were acquired with the Labtech EC-12H-channel Holter ECG system and analyzed with the Cardiospy analysis software (Labtech Kft., Debrecen, Hungary). Holter monitoring and the sleep study were carried out in each patient on the same day. The examinations were analyzed by one physician who had no insight into the patient’s clinical data and the results of sleep study.
The HRV and HRT were automatically calculated by the Labtech system. To properly prepare the ECG for subsequent analysis, the editing of the automatic record was verified visually. The HRV was assessed in frequency parameters and consisted of the following: (1) very-low-frequency power (VLF, frequency band: 0.003−0.04 Hz), serving as an indicator of sympathetic neural activity; (2) low-frequency power (LF, frequency band: 0.04−0.15 Hz), reflecting sympathetic dominance, with a larger amplitude indicating higher sympathetic neural tension; (3) high-frequency power (HF, frequency band: 0.15−0.4 Hz), being related to vagal neural activity; (4) the LF/HF ratio, as a measure of the sympatho-vagal balance. HRT indicators included the turbulence onset (TO) and turbulence slope (TS). TO was defined as the percentage difference between the heart rate immediately following premature ventricular complex and the heart rate immediately preceding premature ventricular complex, while the TS was defined as the steepest slope of the linear regression line for each sequence of five consecutive normal intervals in the local tachogram. HRT analysis was considered normal when TO < 0 and TS > 2.5 ms/RR interval.
Regarding arrythmias, sinus bradycardia and tachycardia were defined as the average heart rate being lower than 60 beats/min and higher than 100 beats/min in 24 h, respectively. Atrial arrythmias included premature atrial contractions, atrial tachycardia, and atrial fibrillation or atrial flutter. Ventricular arrythmias included multifocal premature ventricular contractions and ventricular tachycardias. Atrioventricular block (AVB) included all three degrees of AVB.
Major Adverse Cardiac and Cerebrovascular Events (MACCE) and Follow-up
All patients were monitored and followed up for a minimum of 6 months. MI outpatient clinic visits were scheduled for all patients at 1, 3, 6, and 12 months. MACCEs were defined as composite events of cardiovascular death, MI, stroke, ischemia-driven revascularization, or hospitalization for unstable angina or heart failure.
Statistical Methods
Statistical analyses were performed with SPSS 22.0 (IBM Corp., Armonk, NY, USA). A P‐value below 0.05 was considered statistically significant. Continuous variables are shown as means ± SD or medians (interquartile range) and categorical variables as counts and proportions (%). The variable distribution was verified using Lilliefors and W-Shapiro–Wilk tests. Given that the quantitative variables were not normally distributed, the Mann–Whitney U test was used for further analyses. Univariate analysis was used to determine the clinical parameters related to OSA. Outcomes of MACCEs were estimated using logistic regression for binary outcomes. Potential risk factors, including age, sex, BMI, smoking, comorbid conditions, Syntax score, left ventricular fraction, serum glucose, low-density lipoprotein (LDL)-C, peak levels of hsCRP, biomarkers of myocardial ischemia and NT-proBNP as well as OSA (AHI ≥ 15) and hypoxia (minimum nocturnal oxygen saturation (MinSaO2) ≤ 80%), were adjusted in the multivariable analysis. Adjusted hazard ratios (HR) are reported together with their 95% confidence intervals (CI). Kaplan–Meier MACCE-free survival analysis was performed using the log-rank (Manel–Cox) chi-square test.
RESULTS
Clinical Characteristics
The baseline clinical characteristics of the two OSA groups are presented in Table 1. Among the 252 patients, 130 patients had no OSA (mean age: 68.9 ± 8.0 years, male/female = 85/45) and 122 had OSA (mean age: 68.4 ± 6.7 years, male/female = 106/16), with AHI values of 8.6 ± 4.7 and 34.2 ± 13.6, respectively (P < 0.001). There was no significant difference in age, sex, history of hypertension, diabetes or renal dysfunction, cardiac function, and peak levels of myocardial injury biomarkers or NT-proBNP between the two groups.
1. Comparison of baseline characteristics between non-OSA and OSA group.
| Baseline Parameters | Non-OSA (n = 130) | OSA (n = 122) | P-value |
| Data are presented as mean ± SD or n (%). ACEI: angiotensin converting enzyme inhibitor; ARB: angiotensin receptor blocker; BMI: body mass index; CK-MB: creatine kinase isoenzyme MB; MinSaO2: Minimum nocturnal oxygen saturation; NSTEMI: non-ST-elevation myocardial infarction; PCI: percutaneous coronary intervention; STEMI: ST-elevation myocardial infarction. | |||
| Age, yrs | 68.9 ± 8.0 | 68.4 ± 6.7 | 0.407 |
| Male | 85 (78.7%) | 106 (87.5%) | 0.061 |
| BMI, kg/m2 | 24.1 ± 3.1 | 25.8 ± 3.6 | < 0.001 |
| Hypertension | 49 (45.4%) | 78 (54.2%) | 0.167 |
| Diabetes mellitus | 46 (42.6%) | 59 (41.0%) | 0.796 |
| STEMI | 57 (52.8%) | 85 (59.0%) | 0.322 |
| Alanine transaminase | 25.85 ± 14.27 | 26.29 ± 16.16 | 0.822 |
| Fasting plasma glucose | 6.21 ± 2.20 | 6.40 ± 2.18 | 0.486 |
| Creatinine | 77.18 ± 20.38 | 80.51 ± 19.96 | 0.194 |
| Triglycerides | 1.56 ± 0.81 | 1.57 ± 0.96 | 0.931 |
| Total cholesterol | 4.43 ± 1.29 | 4.73 ± 1.36 | 0.074 |
| High-density lipoproteins | 1.12 ± 0.28 | 1.10 ± 0.24 | 0.679 |
| Low-density lipoproteins | 2.73 ± 1.05 | 3.09 ± 1.27 | 0.019 |
| Left ventricular ejection fraction | 58.54% ± 7.78% | 57.38% ± 7.10% | 0.221 |
| Peak HsCRP, mg/L | 23.99 ± 10.91 | 52.81 ± 27.0 | 0.031 |
| Peak CK-MB, ng/mL | 147.69 ± 197.65 | 176.10 ± 246.65 | 0.326 |
| Peak TnI, ng/mL | 49.23 ± 84.80 | 57.43 ± 86.17 | 0.452 |
| Peak NT-proBNP, pg/mL | 1376.1 ± 1470.7 | 1470.6 ± 1694.4 | 0.643 |
| Triple-vessel disease | 27 (25.0%) | 54 (37.5%) | 0.035 |
| Emermency PCI | 106 (98.1%) | 143 (99.3%) | 0.578 |
| Syntax score | 17.83 ± 11.89 | 19.57 ± 12.60 | 0.572 |
| Asprin | 77 (71.3%) | 107 (74.3%) | 0.594 |
| Clopidogrel | 107 (99.1%) | 142 (98.6%) | 0.945 |
| Statins | 95 (88.0%) | 125 (86.8%) | 0.785 |
| ACEI/ARB | 76 (70.4%) | 106 (73.6%) | 0.571 |
| Beta-blocker | 91 (84.3%) | 134 (93.1%) | 0.025 |
| Calcium channel blocker | 9 (8.3%) | 21 (14.6%) | 0.129 |
| Apnea–hypopnea index | 8.6 ± 4.7 | 34.2 ± 13.6 | < 0.001 |
| Minimum nocturnal oxygen saturation level (MinSaO2), % | 86.0 ± 4.5 | 79.7 ± 8.7 | < 0.001 |
| MinSaO2 ≤ 80% | 5 (4.6%) | 51 (41.8%) | < 0.001 |
| Percentage of time with MinSaO2< 90% | 12.6 ± 1.8 | 18.1 ± 2.7 | < 0.001 |
However, the OSA group has significantly higher BMI (25.8 ± 3.6 vs. 24.1 ± 3.1 kg/m2), peak hs-CRP levels (52.81 ± 27.0 vs. 23.99 ± 10.91 mg/L), lower MinSaO2 (79.7 ± 8.7% vs. 86.0 ± 4.5%), and a higher proportion of hypoxia with MinSaO2≤ 80% (41.8% vs. 4.3%) compared to the non-OSA group (all P< 0.05). Although no difference was found in the percentage of STEMI or Killip Class between groups, OSA appeared to be associated with the severity of coronary disease, as more OSA patients had multivessel coronary artery disease and were prescribed beta-blockers (31.5%vs. 24.5% and 93.1% vs. 84.3%, both P < 0.05).
HRV and HRT with OSA
Compared to the non-OSA group, the OSA group showed significant increases in heart rate variability, including significantly higher VLF (12.7 ± 3.0 vs. 9.2 ± 2.5), LF (20.7 ± 7.5 vs. 13.4 ± 8.5), LF/HF ratio (1.8 ± 0.7 vs. 1.4 ± 0.5), and TS (0.04 ± 0.01 vs. −0.04 ± 0.01, all P < 0.05). Furthermore, the HF, (9.0 ± 3.5 vs. 12.2 ± 4.7) and TO (4.2 ± 1.5 vs. 7.6 ± 3.1) were significantly decreased in the OSA group (all P<0.05, the details are shown inTable 2).
2. Comparison of the HRV and HRT data between the two groups.
| HRV and HRT parameters | Non-OSA (n = 130) | OSA (n = 122) |
| * P < 0.05, difference between OSA and non-OSA group. HF: high-frequency power; HRT: heart rate turbulence; HRV: heart rate variability; LF: low-frequency power; OSA: obstructive sleep apnea; TO: turbulence onset; TS: turbulence slope; VLF: very-low-frequency power. | ||
| VLF, Hz | 9.2 ± 2.5 | 12.7 ± 3.0* |
| LF, Hz | 13.4 ± 8.5 | 20.7 ± 7.5* |
| HF, Hz | 12.2 ± 4.7 | 9.0 ± 3.5* |
| LF/HF | 1.4 ± 0.5 | 1.8 ± 0.7* |
| TO | −0.04 ± 0.01 | 0.04 ± 0.01* |
| TS | 7.6 ± 3.1 | 4.2 ± 1.5* |
Comparison of the Incidence of Arrhythmia Between Two Groups
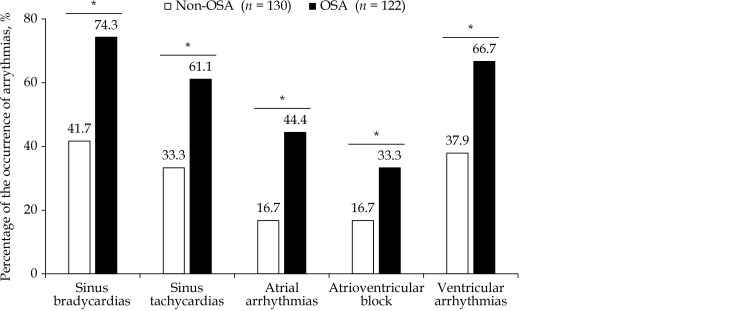
The incidence of arrhythmia is shown in Figure 1. In summary, the OSA group showed almost two-fold higher incidences of sinus bradycardias, sinus tachycardias, AVB, and ventricular arrhythmia than the non-OSA group (all P< 0.05). In addition, the occurrence of atrial arrythmias was almost three-fold higher in the OSA than in the non-OSA group (44.4%vs. 16.7%, P < 0.05).
1.
The difference of the proportions of arrythmias in subacute MI patients between non-OSA and OSA groups.
*P < 0.05, different between OSA and Non-OSA groups. MI: Myocardial infarction; OSA: obstructive sleep apnea.
The Follow-up Prognosis and OSA-induced Hypoxia
The median follow-up time was 0.8 (0.6−1.8) years. There was a significant difference in the occurrence of MACCEs during the follow-up between the two groups (P < 0.01). In the OSA group, MACCEs occurred in 24 patients (19.7%), including 2 cardiac death (1.6%), 2 non-fatal myocardial infarction (1.6%), 2 heart failure (1.6%), 10 re-hospitalizations because of unstable angina (8.2%), 5 revascularization (4.1%), and 3 non-fatal stroke (2.5%). In the non-OSA group, MACCEs occurred in 14 patients (10.7%), including 1 cardiac death (0.8%), 1 non-fatal myocardial infarction (0.8%), 2 heart failure (1.5%), 6 re-hospitalizations because of unstable angina (4.6%), 3 re-vascularization (2.3%), and 1 non-fatal stroke (0.8%).
Multivariate Logistic Regression Analysis
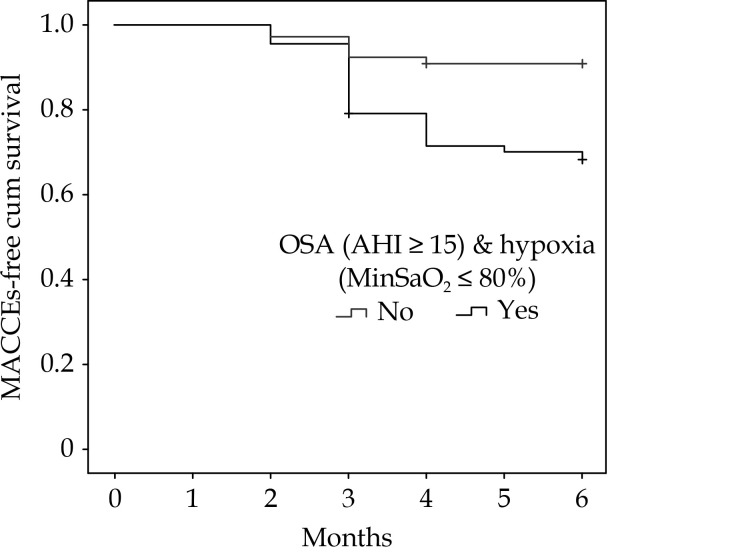
Univariate analysis showed that OSA severity was correlated with age, BMI, and MinSaO2. Multivariate logistic regression analysis showed that MACCEs were independently correlated with heart failure, Syntax score, CK-MB peak levels after adjusting for age, BMI, sex, smoking, chronic kidney diseases, hypertension, diabetes, and LDL levels; furthermore, OSA (AHI ≥ 15) combined with OSA-induced hypoxia (MinSaO2≤ 80%) was independently associated with the incidence of MACCEs (HR: 4.536; 95% CI: 1.461–14.084, P = 0.009) after adjusting for heart failure, Syntax score and CK-MB peak levels (Table 3). Kaplan–Meier survival analysis showed that the MACCE-free survival in patients with both AHI ≥ 15 and MinSaO2 ≤ 80% was significantly poorer than that in the comparative patients with AHI < 15 or MinSaO 2> 80% from the 3rd month during the follow-up (Figure 2, log-rank chi-square test, P = 0.011).
3. Multiple logistic regression analysis of risk factors for 6-Month MACCEs in AMI patients.
| Risk factors for analysis | P-value | Hazard Ratio | 95% CI |
| AMI: acute myocardial infarction; CKD: chronic kidney disease; CK-MB: creatine kinase isoenzyme MB; HF: heart failure; MACCEs: major adverse cardiac and cerebrovascular events, defined as a composite of cardiovascular death, myocardial infarction, stroke, ischemia-driven revascularization, or hospitalization for unstable angina or heart failure; MinSaO2: minimum nocturnal oxygen saturation; OSA: obstructive sleep apnea. | |||
| Traditional risk factors for MACCEs | |||
| BMI | 0.211 | 1.128 | 0.932−1.305 |
| Age | 0.145 | 1.043 | 0.986−1.364 |
| Gender (Female/Male) | 0.812 | 1.252 | 0.195−8.031 |
| Smoking | 0.221 | 0.485 | 0.152−1.544 |
| Hypertension | 0.938 | 0.952 | 0.283−3.209 |
| Diabetes mellitus | 0.383 | 0.558 | 0.151−2.034 |
| CKD | 0.106 | 4.001 | 0.746−20.751 |
| HF | 0.014 | 5.345 | 1.411−20.243 |
| CK-MB peak level, ng/mL | 0.042 | 0.996 | 0.993−1.000 |
| Troponin I peak level, ng/mL | 0.888 | 1.000 | 0.994−1.005 |
| hsCRP peak level, mg/L | 0.223 | 1.948 | 0.666−5.692 |
| NT-proBNP peak level, pg/mL | 0.661 | 1.368 | 0.336−5.757 |
| Low-density lipoproteins | 0.872 | 0.954 | 0.539−1.689 |
| Syntax score | 0.023 | 1.051 | 1.008−1.189 |
| OSA and MACCEs after adjusting significant traditional risk factors | |||
| CK-MB peak levels, ng/mL | 0.040 | 0.997 | 0.995−1.000 |
| HF | 0.002 | 5.938 | 1.944−18.141 |
| Syntax score | 0.005 | 1.070 | 1.021−1.120 |
| AHI ≥ 15 & MinSaO 2 ≤ 80% | 0.009 | 4.536 | 1.461−14.083 |
2.
The Kaplan–Meier survival curve of OSA (AHI ≥ 15) combined with hypoxia (MinSaO2≤ 80%) on MACCE-free survival in elderly subacute myocardial infarction patients during the 6-month follow-up.
AHI: apnea–hypopnea index; MACCEs: major adverse cardiac and cerebrovascular events; MinSaO2: minimum nocturnal oxygen saturation; OSA: obstructive sleep apnea.
DISCUSSION
Our prospective study showed that OSA and its associated hypoxia may lead to instability of autonomic nervous system function, thus triggering the incidence of ischemia-related arrhythmia or heart rhythm disorders in elderly subacute MI patients and further worsening their poor prognosis.
OSA has been well associated with increased risk of MI, arrythmia, sudden cardiac death, and long-term outcomes of cardiovascular diseases.[3, 4, 6, 7, 10, 19] However, there are limited data on the association between OSA and subacute MI in the elderly. Previous study showed that OSA and myocardial ischemia may interact as both cause and effect. The prevalence of OSA (AHI ≥ 10/h) was two to three-fold higher in patients with a history of MI, and patients with nocturnal onset of MI have a six-fold higher likelihood of having OSA than those with diurnal MI.[20, 21] Conversely, OSA was associated with the development and instability of atherosclerotic plaques and thus may act as a trigger for MI.[22] In our study, we found that approximately 48% of the elderly subacute MI patients were diagnosed with OSA with AHI ≥ 15. Besides the traditional risk factors for OSA such as higher BMI and hs-CRP level, a greater proportion of OSA patients had multivessel coronary artery disease, which supports the hypothesis that OSA may be significantly associated with the severity of myocardial infarction.
OSA-induced hypoxia can lead to nocturnal dysautonomia, characterized by sympatho-adrenal over-activation. Both HRV and HRT are important prognostic indicators for the activity of the autonomic system in cardiovascular diseases, especially in myocardial infarction,[23, 24] while reduced HRT has been proven to be a relatively accurate indicator of autonomic system damage in severe OSA, sudden death, and the prognosis after myocardial infarction.[25, 26 ] Our study found that the OSA group showed significant increases in HRV frequency-domain metrics (including VLF, LF, and the LF/HF ratio) and TO, as well as significant decreases in HF and TS compared with the non-OSA group. Besides, the incidences of sinus, atrial, and ventricular arrhythmia in the OSA group were also significantly increased compared with those in the non-OSA group. These data further support the relationship of OSA with imbalanced autonomic nervous system function and heart rhythm disorders in elderly AMI patients. The detailed mechanisms may be explained by hypoxia-induced inflammatory response, oxidative stress, and hypercoagulability, as well as increased expression of ventricular calcium channels.[27, 28]
The link between OSA and cardiovascular events remains controversial. Recent studies have observed a potential protective effect of OSA in cases of myocardial infarction, and the prescription of continuous positive airway pressure (CPAP) compared with usual care did not result in a positive effect on the incidence of cardiovascular events, which is attributed to chronic intermittent hypoxia resulting in ischemic preconditioning of the myocardium.[10-12, 29] However, the issue remains controversial. Researchers have attempted to determine prognostic parameters of OSA for cardiovascular outcomes and showed that AHI ≥ 15, MinSaO2 ≤ 85%, Epworth Sleepiness Scale scores ≥ 11 and the presence of excessive daytime sleepiness were all independent risk factors of MACCEs during a follow-up of 3–5 years.[30-32] Similarly, our present study demonstrated that OSA (AHI ≥ 15) together with hypoxia (MinSaO2≤ 80%) was independently associated with the incidence of MACCEs during the 6-month follow up (HR = 4.53), and patients with both OSA and severe hypoxia showed poorer MACCE-free survival than those without OSA or hypoxia. Our results further support the hypothesis that OSA-associated apnea can aggravate the severity of myocardial ischemia in elderly subacute MI patients and thus produce adverse effects on the middle-term outcomes during the follow-up.
A prospective design and a rather homogeneous group of elderly people are the strengths of our study. However, there are some limitations in our study. First, the sleep studies were performed by using type III portable sleep monitors, which may underestimate the AHI values compared with the gold standard of polysomnography. Second, identifying the prevalence of OSA in the AMI patient population was not a goal of this study, as we only recruit stable subacute MI patients who can tolerate the sleep study, which may conceal the real influence of OSA on more severe patients complicated with heart failure, severe kidney diseases, or infection. Third, the sample size of our prospective, cross-sectional study conducted at a single medical center was relatively small. Large-scale multicenter double-blind prospective clinical studies may be required for further confirmation of the influence of OSA on AMI patients.
In conclusion, OSA-induced hypoxia may correlate with the severity of myocardial infarction, increase the occurrence of heart rhythm disorders in elderly subacute MI patients, and worsen their poor outcomes in the short term.
ACKNOWLEDGEMENTS
The authors are grateful to Dr. Feng-Ru ZHANG and Dr. Jia-An HU for their skilled technical assistance. This work was supported by National Natural Science Youth Fund of China (81100098), Shanghai Municipal Commission of Health and Family Planning for Key Discipline Establishment (2015ZB0503 & 201840083), and Production, Teaching and Research Program for University Teachers in Shanghai (RC20190079). The authors declare that they have no conflict of interest.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
Contributor Information
Wei-Wei QUAN, Email: springqww@163.com.
Zhi-Hong XU, Email: zhihxu@163.com.
References
- 1.Nieto FJ, Young TB, Lind BK, et al Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 2.Sorajja D, Gami AS, Somers VK, Behrenbeck TR, Garcia-Touchard A, Lopez-Jimenez F Independent association between obstructive sleep apnea and subclinical coronary artery disease. Chest. 2008;133:927–933. doi: 10.1378/chest.07-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang H, Parker JD, Newton GE, et al Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol. 2007;49:1625–1631. doi: 10.1016/j.jacc.2006.12.046. [DOI] [PubMed] [Google Scholar]
- 4.Christensen MA, Dixit S, Dewland TA, et al Sleep characteristics that predict atrial fibrillation. Heart Rhythm. 2018;15:1289–1295. doi: 10.1016/j.hrthm.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.May AM, Van Wagoner DR, Mehra R OSA and Cardiac Arrhythmogenesis: Mechanistic Insights. Chest. 2017;151:225–241. doi: 10.1016/j.chest.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jia S, Zhou YJ, Yu Y, et al Obstructive sleep apnea is associated with severity and long-term prognosis of acute coronary syndrome. J Geriatr Cardiol. 2018;15:146–152. doi: 10.11909/j.issn.1671-5411.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mooe T, Franklin KA, Holmstrom K, Rabben T, Wiklund U Sleep-disordered breathing and coronary artery disease: long-term prognosis. Am J Respir Crit Care Med. 2001;164:1910–1913. doi: 10.1164/ajrccm.164.10.2101072. [DOI] [PubMed] [Google Scholar]
- 8.Baumert M, Immanuel SA, Stone KL, et al Composition of nocturnal hypoxaemic burden and its prognostic value for cardiovascular mortality in older community-dwelling men. Eur Heart J. 2020;41:533–541. doi: 10.1093/eurheartj/ehy838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marin JM, Carrizo SJ, Vicente E, Agusti AG Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 10.Mandal S, Kent BD Obstructive sleep apnoea and coronary artery disease. J Thorac Dis. 2018;10:S4212–S4220. doi: 10.21037/jtd.2018.12.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abugroun A, Patel P, Natarajan S, et al Relation of Age to Survival in Patients with Obstructive Sleep Apnea who Develop an Acute Coronary Event (from the National Inpatient Sample) Am J Cardiol. 2020;125:1571–1576. doi: 10.1016/j.amjcard.2020.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Shah N, Redline S, Yaggi HK, et al Obstructive sleep apnea and acute myocardial infarction severity: ischemic preconditioning? Sleep Breath. 2013;17:819–826. doi: 10.1007/s11325-012-0770-7. [DOI] [PubMed] [Google Scholar]
- 13.Ng SS, Chan TO, To KW, et al Prevalence of obstructive sleep apnea syndrome and CPAP adherence in the elderly Chinese population. PLoS One. 2015;10:e0119829. doi: 10.1371/journal.pone.0119829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao CF, Li SF, Chen H, Song JX Predictors and in-hospital prognosis of recurrent acute myocardial infarction. J Geriatr Cardiol. 2016;13:836–839. doi: 10.11909/j.issn.1671-5411.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thygesen K, Alpert JS, Jaffe AS, et al Fourth Universal Definition of Myocardial Infarction (2018) Circulation. 2018;138:e618–e651. doi: 10.1161/CIR.0000000000000617. [DOI] [PubMed] [Google Scholar]
- 16.Ibanez B, James S, Agewall S, et al 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 17.Roffi M, Patrono C, Collet JP, et al 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC) Eur Heart J. 2016;37:267–315. doi: 10.1093/eurheartj/ehv320. [DOI] [PubMed] [Google Scholar]
- 18.Qaseem A, Holty JE, Owens DK, et al Management of obstructive sleep apnea in adults: A clinical practice guideline from the American College of Physicians. Ann Intern Med. 2013;159:471–483. doi: 10.7326/0003-4819-159-7-201310010-00704. [DOI] [PubMed] [Google Scholar]
- 19.Gami AS, Howard DE, Olson EJ, Somers VK Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med. 2005;352:1206–1214. doi: 10.1056/NEJMoa041832. [DOI] [PubMed] [Google Scholar]
- 20.Mooe T, Rabben T, Wiklund U, et al Sleep-disordered breathing in men with coronary artery disease. Chest. 1996;109:659–663. doi: 10.1378/chest.109.3.659. [DOI] [PubMed] [Google Scholar]
- 21.Kuniyoshi FH, Garcia-Touchard A, Gami AS, et al Day-night variation of acute myocardial infarction in obstructive sleep apnea. J Am Coll Cardiol. 2008;52:343–346. doi: 10.1016/j.jacc.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Konishi T, Kashiwagi Y, Funayama N, et al Obstructive sleep apnea is associated with increased coronary plaque instability: an optical frequency domain imaging study. Heart Vessels. 2019;34:1266–1279. doi: 10.1007/s00380-019-01363-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nenna A, Lusini M, Spadaccio C, et al Heart rate variability: a new tool to predict complications in adult cardiac surgery. J Geriatr Cardiol. 2017;14:662–668. doi: 10.11909/j.issn.1671-5411.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie JY, Liu WX, Ji L, et al Relationship between inflammatory factors and arrhythmia and heart rate variability in OSAS patients. Eur Rev Med Pharmacol Sci. 2020;24:2037–2053. doi: 10.26355/eurrev_202002_20382. [DOI] [PubMed] [Google Scholar]
- 25.Sulimov V, Okisheva E, Tsaregorodtsev D Non-invasive risk stratification for sudden cardiac death by heart rate turbulence and microvolt T-wave alternans in patients after myocardial infarction. Europace. 2012;14:1786–1792. doi: 10.1093/europace/eus238. [DOI] [PubMed] [Google Scholar]
- 26.Ari H, Ari S, Yazici F, Koca V, Bozat T Cardiac autonomic function and cardiac arrhythmias in patients with obstructive sleep apnea. Turk Kardiyol Dern Ars. 2011;39:292–299. doi: 10.5543/tkda.2011.01045. [DOI] [PubMed] [Google Scholar]
- 27.Huang YS, Chin WC, Guilleminault C, et al Inflammatory Factors: Nonobese Pediatric Obstructive Sleep Apnea and Adenotonsillectomy. J Clin Med. 2020;9:1028. doi: 10.3390/jcm9041028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li R, Wang Y, Yang Z, et al Hypoxia-inducible factor-1alpha regulates the expression of L-type voltage-dependent Ca(2+) channels in PC12 cells under hypoxia. Cell Stress Chaperones. 2015;20:507–516. doi: 10.1007/s12192-015-0575-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanchez-de-la-Torre M, Sanchez-de-la-Torre A, Bertran S, et al Effect of obstructive sleep apnoea and its treatment with continuous positive airway pressure on the prevalence of cardiovascular events in patients with acute coronary syndrome (ISAACC study): a randomised controlled trial. Lancet Respir Med. 2020;8:359–367. doi: 10.1016/S2213-2600(19)30271-1. [DOI] [PubMed] [Google Scholar]
- 30.Xie J, Sert Kuniyoshi FH, Covassin N, et al Nocturnal hypoxemia due to obstructive sleep apnea is an independent predictor of poor prognosis after myocardial infarction. J Am Heart Assoc. 2016;5:e003162. doi: 10.1161/JAHA.115.003162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie J, Sert Kuniyoshi FH, Covassin N, et al Excessive daytime sleepiness independently predicts increased cardiovascular risk after myocardial infarction. J Am Heart Assoc. 2018;7:e007221. doi: 10.1161/JAHA.117.007221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koo CY, Aung AT, Chen Z, et al Sleep apnoea and cardiovascular outcomes after coronary artery bypass grafting. Heart. 2020;106:1495–1502. doi: 10.1136/heartjnl-2019-316118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data to this article can be found online.