Abstract
Objective
Physically active individuals show greater conditioned pain modulation (CPM) compared with less active individuals. Understanding the effects of acute exercise on CPM may allow for a more targeted use of exercise in the management of pain. This study investigated the effects of acute isometric exercise on CPM. In addition, the between-session and within-session reliability of CPM was investigated.
Design
Experimental, randomized crossover study.
Setting
Laboratory at Marquette University.
Subjects
Thirty healthy adults (19.3±1.5 years, 15 males).
Methods
Subjects underwent CPM testing before and after isometric exercise (knee extension, 30% maximum voluntary contraction for three minutes) and quiet rest in two separate experimental sessions. Pressure pain thresholds (PPTs) at the quadriceps and upper trapezius muscles were assessed before, during, and after ice water immersions.
Results
PPTs increased during ice water immersion (i.e., CPM), and quadriceps PPT increased after exercise (P < 0.05). CPM decreased similarly following exercise and quiet rest (P > 0.05). CPM within-session reliability was fair to good (intraclass correlation coefficient [ICC] = 0.43–0.70), and the between-session reliability was poor (ICC = 0.20–0.35). Due to the variability in the systemic exercise-induced hypoalgesia (EIH) response, participants were divided into systemic EIH responders (N = 9) and nonresponders (N = 21). EIH responders experienced attenuated CPM following exercise (P = 0.03), whereas the nonresponders showed no significant change (P > 0.05).
Conclusions
Isometric exercise decreased CPM in individuals who reported systemic EIH, suggesting activation of shared mechanisms between CPM and systemic EIH responses. These results may improve the understanding of increased pain after exercise in patients with chronic pain and potentially attenuated CPM.
Keywords: Exercise, CPM, Pain, Hypoalgesia, Pressure Pain, Reliability
Introduction
Conditioned pain modulation (CPM) and exercise-induced hypoalgesia (EIH) have similar manifestations in humans, including systemic hypoalgesia in pain-free individuals [1–3], interaction with the opioid systems [4–7], and impaired responses in patients with chronic pain [8,9]. Furthermore, CPM, which is often described as “pain inhibits pain,” may contribute to EIH [2]. Specifically, exercise may act as a painful conditioning stimulus, thereby activating descending inhibitory pathways, resulting in systemic hypoalgesia [10,11]. This is supported in young healthy adults, in whom greater hypoalgesia was observed following painful aerobic or isometric exercise compared with nonpainful exercise [12,13]. Moreover, CPM has been shown to predict EIH in young and old healthy individuals [2] and in patients with chronic musculoskeletal pain [8]. In individuals with knee osteoarthritis (OA), those with normal CPM responses experienced EIH, whereas individuals with abnormal CPM did not experience EIH [9].
Physical activity level and body composition may contribute to both EIH and CPM. For instance, physically active individuals show a greater CPM response compared with their less active counterparts [2,14,15], and EIH is less in adolescents with greater sedentary bouts [16]. In relation to body composition, CPM efficiency was related to lean mass in adolescents [14], and adolescents with higher total body lean mass experience greater EIH [16]. Thus, similar contributing factors, such as physical activity and body composition, influence how people respond to a potentially noxious stimulus (i.e., exercise or a conditioning stimulus).
Acute isometric exercise has been shown to reduce central pain facilitatory mechanisms (i.e., temporal summation of pain) [17]; however, to our knowledge, no study has investigated the effect of acute isometric exercise on central pain inhibitory mechanisms (i.e., CPM). Previous research has shown that stimulation of the motor cortex, via transcranial direct current stimulation, enhances CPM in healthy men [18]. Accordingly, activation of the motor cortex occurs with exercise and may enhance the CPM response.
Initially, CPM was used to quantify efficiency of descending pain inhibition in healthy and clinical populations [19]. This technique has progressed to predict nonpharmacological treatment responses [2,3,8] and identify how treatments impact endogenous pain modulation. Therefore, repetitive CPM testing is frequently done within and between sessions. The reliability of CPM depends on the parameters of stimulation, study methodology, and study population [20]. Research is ongoing to identify if CPM reliability is consistent across these parameters.
The primary aim of this study was to investigate both the local (quadriceps) and systemic (upper trapezius) effects of lower extremity isometric exercise on the CPM response in young healthy individuals. Moreover, the experimental design allowed for investigation of the between- and within-session reliability of CPM. Because physical activity and anthropometrics may influence CPM and EIH, these measures were also included. It was hypothesized that 1) isometric exercise would enhance the CPM response in young healthy individuals and 2) CPM would have fair to good between- and within-session reliability.
Methods
Subjects
Thirty young healthy and pain-free men and women (mean age = 19.3 ±1.5 years, 15 females) completed the study. Individuals were excluded from the study if they presented with the following: 1) acute or chronic pain, 2) mental health disorder, 3) history of traumatic injury or neurological disorder, 4) inability to tolerate ice water (e.g., Reynaud’s disease or cold urticaria), or 5) contraindication to exercise. Screening done via the phone eliminated two potential participants. On the days of testing, participants were asked to refrain from exercise. The protocol was approved by the Institutional Review Board at Marquette University.
Experimental Design
Participants completed one familiarization session and two randomized and counterbalanced experimental sessions (isometric exercise or quiet rest) that were separated by one week. During the familiarization session, subjects signed a written informed consent, completed body composition testing (dual-energy x-ray absorptiometry [DXA] scan), and were familiarized to the experimental procedures and the pressure pain device. Because the performance of maximal voluntary isometric contractions (MVIC) may influence pain perception in young adults [13,21], MVIC force was determined at the end of the familiarization session. Specifically, three MVICs were performed with the right knee extensor muscles with one-minute rest between contractions. Participants were given verbal encouragement to achieve maximal force. The highest value was used to calculate the submaximal (30% MVIC) target force in the exercise session.
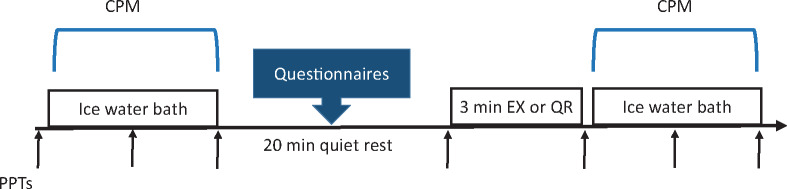
During the experimental sessions (Figure 1), CPM was assessed before and after isometric exercise or quiet rest. In both sessions, 20 minutes of quiet rest separated the first CPM assessment and initiation of exercise or quiet rest, as previous studies have shown that the conditioning effects of pain return to baseline within 15 minutes [19]. Participants also completed the pain catastrophizing scale (PCS) [22] and international physical activity questionnaire (IPAQ) [23] during the quiet rest in the first and second experimental sessions, respectively. These measures were collected to assess their potential influence on CPM and EIH.
Figure 1.
Study design of the experimental sessions. “↑”= PPTs at the quadriceps and upper trapezius muscles; CPM = conditioned pain modulation; EX = exercise; PPT = pressure pain threshold; QR = quiet rest.
Conditioned Pain Modulation
Pressure pain thresholds (PPTs) were measured at the right upper trapezius and right quadriceps muscles (test stimuli) before, during (after 20 seconds), and after submersion of the left foot in a noxious ice water (0°C ± 1°C) bath (conditioning stimulus). Participants were instructed to keep their foot in the ice water bath until the PPTs were completed, at which point they removed their foot from the ice water bath. During foot submersion, foot pain intensity was measured at 20 seconds using a 0–10 numerical rating scale (NRS) with the following anchors: 0 = “no pain” and 10 = “worst pain” [24], followed by PPT measurements. Immediately after foot removal from the ice water bath, peak pain intensity was measured.
Exercise
Participants performed a submaximal (30% MVIC) isometric contraction of the right knee extensor muscles that was held for three minutes while seated upright on the edge of a plinth table. The hips and knees were positioned at 90° while the right foot was unsupported and aligned with the plinth table’s metal leg. A handheld dynamometer (Commander Echo Muscle Testing Dynamometer, JTech Medical, Midvale, UT, USA) was stabilized using Velcro straps to the leg of the plinth and around the participant’s leg (above the malleolus). Two stabilizing straps were placed over the thighs, one distal to the hip joint and the other proximal to the knee joint. Subjects were instructed to fold their arms across their chest and to extend their knee while pushing against the Velcro strap attached to the dynamometer. During the performance of the submaximal isometric contraction, participants were instructed to match the target force as displayed on the wireless portable monitor (Commander Echo Console, JTech Medical, Midvale, UT, USA) while receiving verbal encouragement to maintain the force. All participants maintained the force for the entire three minutes. Participants were asked to rate their perceived exertion using a 0–10 scale with the following anchors: 0 = “nothing at all” and 10 = “very very strong” and pain intensity in the leg in relation to the muscle contraction using the NRS at the beginning of the contraction, midway (1.5 minutes), and at the end of the contraction (3 minutes).
Pressure Pain Thresholds
During each experimental session, PPTs were measured a total of seven times at the quadriceps and upper trapezius muscles with a handheld algometer (Algomed, Medoc Ltd), three times with each of the two CPM protocols (before, during, and after ice) and one immediately before quiet rest or exercise (20 minutes after the first CPM protocol) (Figure 1). For the PPTs, a 1-cm2 rubber tip was used with a ramp protocol at a rate of 50 kPa/sec. Subjects were instructed to press a timing device when the pressure first changed to pain, which was electronically recorded in kilopascals. To minimize exposure time to ice water, two PPT trials were recorded at each site, with a 10-second interstimulus interval, and the two trials were averaged at each measurement site for further analysis. At the beginning of each experimental session, the order for the sites (upper trapezius and quadriceps) was randomized and counterbalanced and kept consistent throughout the session. PPTs were recorded with the participant seated upright in a chair with their knees and hips at 90°. The sites were located and marked as follows: the quadriceps muscle site was located midway between the anterior superior iliac spine and the patella, while the upper trapezius muscle site was located midway between the C7 spinous process and the lateral tip of the acromion [25].
Body Composition
Body composition was measured using a total body scanner (Lunar iDXA, GE Healthcare, Madison, WI, USA). Scan analyses were performed using enCore software (version 14.10, GE Healthcare) to obtain the following outcome measures: body mass index (BMI), total body fat (%), android fat (%), gynoid fat (%), android/gynoid (A/G) ratio, leg fat (%), leg lean (lbs), and visceral fat mass (lbs).
Statistical Analysis
Data were analyzed using the IBM Statistical Package for Social Sciences (SPSS version 23, Armonk, NY, USA) and reported as mean ± SD in the text and tables and mean ± SEM in the figures. Normality was checked using the Kolmogorov-Smirnov test. Outliers were tested with the Grubbs test and removed when significant.
Conditioned Pain Modulation at Baseline
A repeated-measures analysis of variance (ANOVA; session [exercise and quiet rest] × site [quadriceps and upper trapezius] × time [before, during, and after ice]) was performed to determine if PPTs increased at the upper trapezius and quadriceps muscle during and/or after the baseline ice water bath performed in the two experimental sessions. In addition, a repeated-measures ANOVA was done comparing the relative change in CPM at baseline between sessions (quiet rest and exercise) at each site (upper trapezius and quadriceps). Relative change was calculated while the foot was submerged in ice water: CPMduring ice = ([PPT during ice – PPT pre-ice]/PPT pre-ice) and immediately following removal of the foot from ice water: CPMafter ice = ([PPT after ice – PPT pre-ice]/PPT pre-ice). This analysis was repeated with sex as a between-subject factor to examine sex differences in CPM at baseline. To identify potential differences in peak pain intensity of the ice water bath and the total time of foot submersion in the ice during CPM protocols, paired t tests or the Wilcoxon signed rank test for non–normally distributed data were done as appropriate.
Exercise-Induced Hypoalgesia
To identify potential changes in PPT following quiet rest and exercise (i.e., EIH), a repeated-measures ANOVA was performed (session [exercise and quiet rest] × site [quadriceps and upper trapezius] × time [PPTs pre- and immediately post-rest and exercise]). This analysis was repeated with sex as a between-subject factor to identify potential sex differences.
Conditioned Pain Modulation After Exercise and Quiet Rest
To investigate the effect of exercise on the CPM response, relative change in CPM following quiet rest and exercise was analyzed using a repeated-measures ANOVA (session [exercise and quiet rest] × site [quadriceps and upper trapezius] × time [CPM performed pre- and post-exercise or quiet rest]). Because there was considerable variability in systemic but not local EIH, EIH responders and nonresponders at the upper trapezius muscle were categorized based on the PPT minimum detectable change (42.7 kPa) in a healthy pain-free population with a nonpharmacological intervention [26]. Subjects who had an increase in PPT greater than 42.7 kPa at the upper trapezius muscle after exercise compared with pre-exercise were placed in the EIH responders group (N = 9). Changes in CPM at the upper trapezius following quiet rest and exercise were analyzed using repeated-measures ANOVA with EIH response (responders and nonresponders) as a between-subject factor (time × session × EIH response). When a significant effect was found, post hoc analyses were done using paired t tests. Independent t tests or Mann-Whitney U tests for non–normally distributed data were performed between the groups (EIH responders or nonresponders) to identify potential differences in characteristics.
Within- and Between-Session Reliability of CPM
To examine the reliability of CPM between sessions, repeated-measures ANOVAs were done comparing the relative change in CPM at baseline at each site. Within the quiet rest session, relative change in CPM was compared using a repeated-measures ANOVA (time [pre- and post-rest] × site [quadriceps and upper trapezius]). Intraclass correlations (ICCs) on the bases of absolute agreement were computed for relative change in CPMduring ice between sessions (pre–session 1 and session 2) and within the quiet rest session for each site with 95% confidence interval (CI).
Correlations
To determine potential factors that influenced CPM and or EIH, Pearson correlations or Spearman correlations for non–normally distributed data were calculated between the relative changes in CPM and EIH, body composition measures, pain catastrophizing (PCS), and self-reported physical activity (IPAQ). In addition, Spearman correlations were performed between the relative changes in CPM or EIH and the pain intensity induced by the ice or exercise, respectively. Because the absolute change in CPM was not normally distributed, all the analyses were performed using the relative change in CPM. For statistical significance, a P value ≤0.05 was used initially (i.e., for repeated-measures ANOVA); however, a more rigorous alpha level was selected (P ≤ 0.01) to minimize type I and II errors with multiple group comparisons (i.e., post hoc analyses) and multiple correlations [27,28].
Results
Participant Characteristics
A summary of the subject characteristics is found in Table 1. According to body mass index (BMI) classification, eight participants (26%) were overweight and 22 participants (73%) were normal weight. The individuals’ self-reported physical activity levels were categorized as either moderate or vigorous; no participants reported low physical activity level. The majority of pain catastrophizing scores were considered normal as well; four participants had a score greater than 30. The following variables were non–normally distributed, and therefore nonparametric tests were used: PCS scores, physical activity scores, pain intensity scores during ice water submersion, duration of ice water bath submersion, A/G ratio, and visceral fat mass (lbs). One outlier was identified and removed from the variable CPMafter ice at the quadriceps muscle.
Table 1.
Participant characteristics
| All Participants (N = 30) | Systemic EIH Responders (N = 9, 30%) | Systemic EIH Nonresponders (N = 21, 70%) | P Value | |
|---|---|---|---|---|
| Age, y | 19.3 ± 1.5 | 19.7 ± 1.3 | 19.8 ± 1.6 | 0.803 |
| Females, % | N = 15 (50%) | N = 4 (44%) | N = 11 (52%) | 0.695 |
| Exercise | ||||
| MVC | 368.5 ± 107.9 | 399.2 ± 143.1 | 355.4 ± 90 | 0.414 |
| Peak pain | 3.8 ± 2.5 | 3.4 ± 2.1 | 4.0 ± 2.6 | 0.571 |
| Peak RPE | 5.5 ± 2.1 | 4.7 ± 2.3 | 5.8 ± 1.9 | 0.242 |
| Weight status and body composition | ||||
| BMI | 23.0 ± 3.1 | 22.2 ± 3.2 | 23.3 ± 3.0 | 0.230 |
| Total body fat, % | 24.3 ± 6.8 | 23.1 ± 7.2 | 24.8 ± 6.8 | 0.554 |
| Android fat, % | 22.8 ± 8.4 | 22.0 ± 7.9 | 23.2 ± 8.7 | 0.733 |
| Gynoid fat, % | 26.5 ± 8.6 | 25.0 ± 9.6 | 27.2 ± 8.4 | 0.533 |
| Android/gynoid ratio | 0.86 ± 0.2 | 0.89 ± 0.13 | 0.85 ± 0.22 | 0.213 |
| Leg fat, % | 25.6 ± 8.2 | 24.2 ± 8.7 | 26.1 ± 8.1 | 0.573 |
| Leg lean, lbs | 19.2 ± 4.5 | 19.3 ± 4.8 | 19.1 ± 4.5 | 0.906 |
| Visceral fat mass, lbs | 0.35 ± 0.38 | 0.30 ± 0.25 | 0.37 ± 0.42 | 0.982 |
| Physical activity | ||||
| IPAQ total walking MET, minutes/wk | 1,495.1 ± 1,011.0 | 1,827.8 ± 853.9 | 1,352.5 ± 1,057.9 | 0.245 |
| IPAQ total moderate MET, minutes/wk | 674.6 ± 1,506.7 | 550.0 ± 867.5 | 728.0 ± 1,726.5 | 0.772 |
| IPAQ total vigorous MET, minutes/wk | 1,900.0 ± 1,665.0 | 1,680.0 ± 1,570.3 | 1,994.2 ± 1,732.7 | 0.617 |
| IPAQ MET, minutes/wk | 4,069.7 ± 2,963.8 | 4,057.8 ± 3,033.4 | 4,074.9 ± 3,009.4 | 0.989 |
| IPAQ total sitting, minutes/wk | 2,991.0 ± 1,124.1 | 2,503.3 ± 959.8 | 3,200.0 ± 1,144.9 | 0.122 |
| Pain catastrophizing | ||||
| PCS total | 18.1 ± 10.1 | 21.0 ± 12.8 | 16.8 ± 8.8 | 0.699 |
| PCS helplessness | 6.6 ± 5.0 | 6.2 ± 2.8 | 6.7 ± 5.7 | 0.792 |
| PCS magnification | 4.1 ± 2.7 | 3.4 ± 2.1 | 4.4 ± 2.9 | 0.345 |
| PCS rumination | 7.3 ± 4.1 | 6.4 ± 2.9 | 7.7 ± 4.5 | 0.554 |
There were no significant differences between systemic EIH responders and nonresponders.
BMI=body mass index; IPAQ=International Physical Activity Questionnaire; PCS=Pain Catastrophizing Scale; RPE=rate of perceived exertion.
Conditioned Pain Modulation at Baseline
All subjects completed all the CPM protocols, except two subjects who removed their foot from the ice water before completing the test. These subjects, however, kept their foot in the ice water for at least 20 seconds and completed all PPT assessments. The analyses of CPM were done with and without these subjects, which did not affect the results. Subjects reported moderate to severe peak pain intensity (NRS = 6.6 ± 1.8) during submersion of the foot in the ice water bath. Peak pain intensity during foot submersion in ice decreased significantly between sessions (session 1: 7.0 ± 1.0; session 2: 6.4 ± 1.7; P = 0.01) but was similar within sessions (P > 0.05). The average duration for submersion of the foot in ice water was 99.7 ± 24.5 seconds. This was dependent on PPT duration for each subject and was similar across all CPM protocols (P > 0.05).
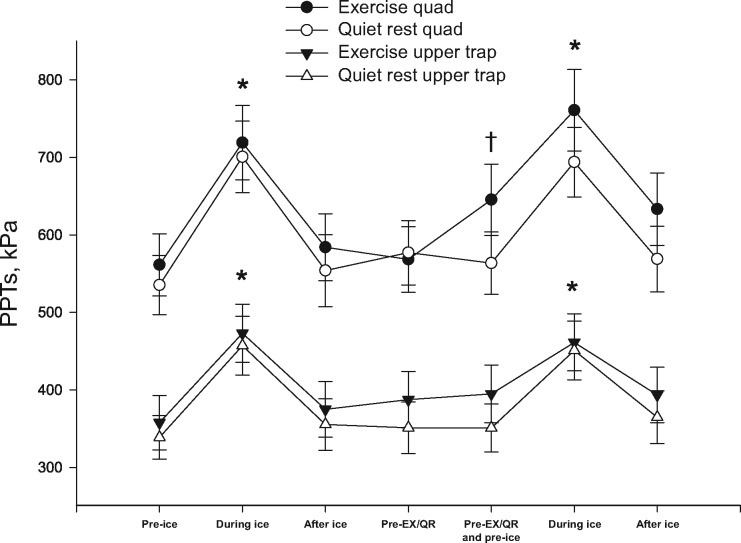
Results of the analysis for baseline CPM demonstrated a site × time interaction (F(2, 28) = 3.526, P < 0.05, ηp2 = 0.201). Post hoc analysis showed that while the foot was submerged in the ice water bath (CPMduring ice), there was an increase in PPTs at the quadriceps muscle and upper trapezius (P < 0.001), which signifies CPM (Figure 2). The majority of subjects reported CPMduring ice (28/30). Immediately following removal of the foot from the ice water bath (CPMafter ice), PPTs were not significantly different from baseline at the quadriceps and upper trapezius muscles (P > 0.05). In addition, PPTs were higher at the quadriceps muscle compared with the upper trapezius muscle (P < 0.001) (Figure 2); however, CPMduring ice had similar relative changes between the two sites (P > 0.05) (Figure 3). No other interactions were found (P > 0.05). When analyses were repeated with sex as a between-subject factor, no main effects of sex or interactions were found (P > 0.05). Pain intensity at 20 seconds, peak pain intensity during the ice water bath, and duration of ice water bath submersion were not related to the relative change in CPM in all protocols at both sites (P > 0.05).
Figure 2.
Pressure pain thresholds (kPa) at the quadriceps muscle and the upper trapezius muscle during the exercise session and the quiet rest session. Significantly different compared with pre-ice (*) and significantly different compared with pre-exercise (†). Data are presented as mean ± SEM. EX = exercise; QR = quiet rest; PPT = pressure pain threshold.
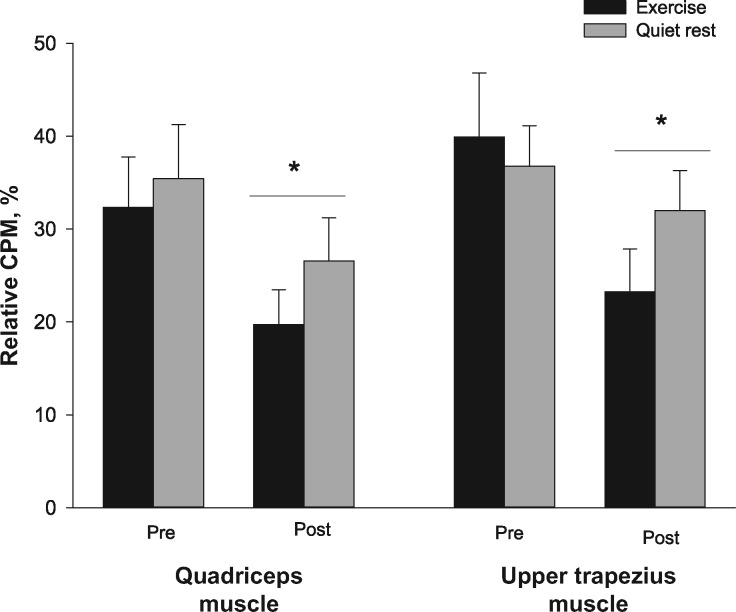
Figure 3.
Relative change in conditioned pain modulation at the quadriceps muscle and the upper trapezius muscle before and after exercise or quiet rest. Significantly different compared with pre-exercise or quiet rest (*). Data are presented as mean ± SEM. CPM = conditioned pain modulation.
Exercise-Induced Hypoalgesia
During exercise, subjects reported no pain (NRS = 0.0 ± 0.3) at the beginning of the isometric contraction, minimal pain (NRS = 2.2 ± 1.9) at the midpoint, and moderate pain (NRS = 3.8 ± 2.5) at the end. Likewise, subjects reported “very weak” exertion (RPE = 1.6 ± 1.5) at the beginning of the isometric contraction, “somewhat strong” exertion (RPE = 4.1 ± 1.5) at the midpoint, and “strong” exertion (RPE = 5.5 ± 2.1) at the end.
For PPTs, there was a session × site × time interaction (F(1, 29) = 13.203, P = 0.001, ηp2 = 0.313). Post hoc analyses showed that PPTs increased following exercise at the quadriceps muscle (mean = 15 ± 19% change, P < 0.001) and were unchanged following quiet rest (P > 0.05) (Figure 2). At the upper trapezius muscle, no significant differences in PPTs were found (mean = 2 ± 14% change, P > 0.05) following exercise or quiet rest. Due to differences in the EIH response at the upper trapezius muscle, participants were divided into systemic EIH responders (N = 9) and nonresponders (N = 21). The average change in PPTs at the upper trapezius muscle following exercise for EIH responders was 20 ± 9% compared with –5 ± 8% in the nonresponders. When analyses were repeated with sex as a between-subject factor, no main effects of sex or interactions were found (P > 0.05). Neither RPE nor pain intensity at all time points during the exercise was related to EIH at either site (P > 0.05).
Conditioned Pain Modulation After Exercise and Quiet Rest
Following quiet rest and exercise, CPMduring ice decreased at the quadriceps and upper trapezius muscles (F(1, 29) = 13.069, P = 0.001, ηp2 = 0.311); this decrease was similar for the quiet rest and exercise sessions (time × session: P > 0.05, ηp2 = 0.052) and between sites (session × site × time: P > 0.05, ηp2 = 0.037) (Figure 3). At the quadriceps muscle, CPMduring ice decreased following exercise (32% to 19%) and quiet rest (35% to 26%). Similarly, CPMduring ice decreased at the upper trapezius following exercise (40% to 23%) and quiet rest (37% to 32%).
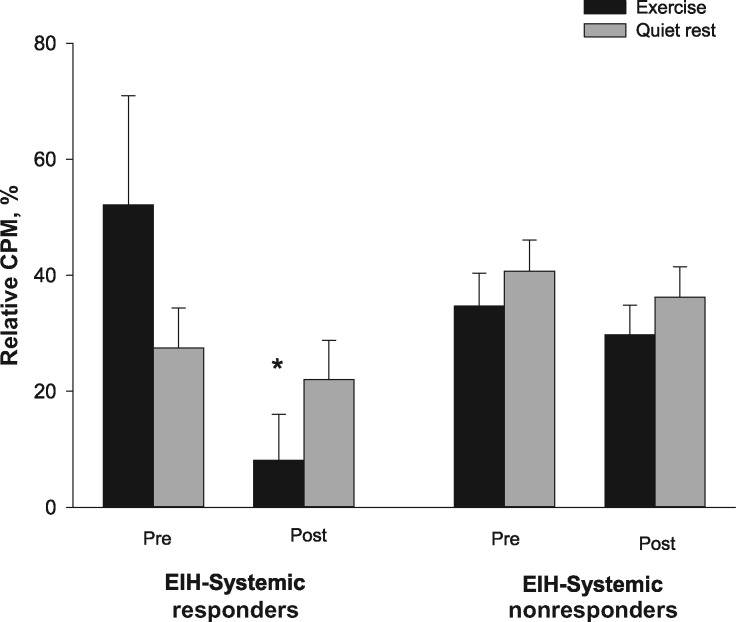
The CPM response was different following exercise compared with quiet rest in systemic EIH responders and nonresponders (time × session × EIH response; P = 0.03, ηp2 = 0.154). Post hoc analyses showed that the EIH responders had a significant decrease in the CPM response following exercise (52% to 8%, P = 0.01) without any change following quiet rest (27% to 22%, P > 0.05) (Figure 4). The EIH nonresponders did not have a significant change in their CPM response following exercise (34% to 29%) or quiet rest (40% to 36%, P > 0.05).
Figure 4.
Relative change in conditioned pain modulation at the upper trapezius muscle before and after exercise or quiet rest for EIH systemic responders and nonresponders. Significantly different compared with pre-exercise (*). Data are presented as mean ± SEM. CPM=conditioned pain modulation.
Within- and Between-Session Reliability of CPM
Results from the ANOVA showed no significant main effects or interactions within or between sessions; the relative change in baseline CPM was similar between the first and second sessions, and the CPM responses were similar within the quiet rest session (P > 0.05). ICC results are shown in Table 2. There was fair to good within-session reliability for CPM during quiet rest and poor reliability when comparing relative change in CPM at baseline between the two sessions.
Table 2.
Reliability values (ICCs) and percent change for CPM within and between sessions
| Percent Change | ICCs (95% CI) | ||
|---|---|---|---|
| Within quiet rest session | CPM Quad trial 1 | 35.4 | 0.707 (0.395 to 0.859) |
| CPM Quad trial 2 | 26.5 | ||
| CPM Upper trap trial 1 | 36.7 | 0.433 (−0.190 to 0.730) | |
| CPM Upper trap trial 2 | 31.9 | ||
| Between sessions | CPM Quad session 1 | 33.4 | 0.208 (−0.715 to 0.628) |
| CPM Quad session 2 | 34.2 | ||
| CPM Upper trap session 1 | 38.6 | 0.350 (−0.401 to 0.694) | |
| CPM Upper trap session 2 | 38.1 |
CPM=conditioned pain modulation; CI=confidence interval; CPM=conditioned pain modulation; ICC=intraclass correlation coefficient.
Correlations
Self-reported physical activity (IPAQ MET-min/wk and IPAQ total walking MET-min/wk) was moderately correlated with EIH at the quadriceps muscle; however, this relationship did not reach statistical significance when correcting for multiple correlations (r = 0.43, P = 0.02, and r = 0.38, P = 0.04, respectively). Similarly, CPMduring ice at the quadriceps after exercise was moderately related to the A/G ratio (r = 0.432, P = 0.02) but failed to reach statistical significance after adjusting for multiple correlations. No other relations were found for pain catastrophizing, physical activity, or body composition with CPM or EIH (P > 0.05).
Discussion
The novel finding of the study was that individuals who reported systemic EIH had a significant decrease in CPM following exercise only, whereas those individuals who had no systemic EIH had no change in CPM following exercise or quiet rest. Thus, activation of descending inhibitory pathways was less following sustained isometric contractions for those individuals with systemic EIH, indicating the possibility of shared mechanisms with CPM. Moreover, this study demonstrated that the decrease in CPM response after exercise and quiet rest was comparable, and the within-session reliability of the CPM protocol used was fair to good. The reliability of CPM between sessions was poor.
Conditioned Pain Modulation
In the current study, CPM occurred only when the testing and conditioning stimuli were performed at the same time, which is in agreement with previous studies [1,8,29–31] but not in line with other studies [19,32], or recent recommendations for CPM testing that favor measuring the test stimulus sequential to the conditioning stimulus [33]. The discrepancy in these results could possibly be explained by the location of the conditioning stimulus, as the location in the previous studies [32,34] was the hand while the present study used the foot. The representation of the hand in the brain is larger than the foot, which may have yielded more central activation and a longer-lasting effect compared with the current study [35]. The results of Vaegter et al. [1] support this hypothesis where a higher CPM magnitude was observed during a cold pressor test on the hand compared with the foot.
To our knowledge, this is the first study to report reliability of CPM with foot submersion in a conditioning ice water bath. Despite acceptable within-session reliability (fair to good), CPM decreased following quiet rest. This decrease reflects the mean change in CPM magnitude as a group, whereas ICCs represent the differentiability of the measure between subjects. Thus following quiet rest, CPM decreased, but the rank of subjects between others was relatively the same, yielding an acceptable ICC value.
One approach to attenuate potential changes in CPM magnitude following quiet rest is to increase the duration of the washout period. Valencia et al. [36] found that a repeated assessment of CPM with a washout period of two minutes was not adequate, as CPM magnitude decreased significantly in the second CPM trial, even with good to excellent reliability. Previous studies have been equivocal in relation to the washout period, with ranges from two to 60 minutes [20]. The reliability in these studies was between fair and excellent [34,36,37], but not all studies examined the difference in CPM magnitude following the washout period. Therefore, future studies with repeated CPM assessments should consider a longer washout period.
In the current study, the between-session reliability was poor despite similar magnitude between the two sessions. A recent study by Imai et al. [38] tested the reliability of CPM using different test and conditioning stimuli and concluded that the best between-session reliability was achieved measuring PPTs during hand submersion in ice water (0–4°C, ICC = 0.49). One potential reason for the poor between-session reliability in the current study could be the low temperature (i.e., high intensity) of the conditioning stimulus. Olesen et al. [39] observed poor reliability (ICC = 0.10) when using a conditioning cold water immersion of the hand at 2°C for three minutes in patients with chronic pain. The authors reported that not all patients tolerated the conditioning stimulus, which may have impacted the reliability and was similar to our study, in which two people did not tolerate the ice water bath. Furthermore, a systematic review of the CPM reliability suggested temperatures between 8°C and 12°C of the cold conditioning water for improving repeatability [20]. Thus, these results demonstrate that reliability may be lower when applying a stronger conditioning stimulus (ice water) to a larger surface area (foot vs hand).
The comparable decrease in CPM following exercise and quiet rest suggests that the modulatory effects of pain are not restored following the first CPM exposure, despite PPTs returning to baseline following the washout period. Thus, using a static pain assessment (PPTs) as a restorative marker for a dynamic process (CPM) may not be appropriate. Alternatively, the influence of expectations of a painful response has been shown to affect the CPM magnitude [40], where a higher expectation of the noxious conditioning stimulus results in a lower CPM magnitude. While not measured in this study, it is possible that participants in the current study had a higher expectation for the conditioning stimulus in the second CPM testing, which resulted in a lower CPM magnitude.
Exercise-Induced Hypoalgesia
In the current study, EIH occurred locally at the exercising muscle (quadriceps muscle) and not systemically (upper trapezius muscle). The local effects are in line with previous research showing greater EIH effects at the exercising muscle compared with contralateral or distal sites [1,11]. However, several studies have demonstrated systemic hypoalgesia after isometric exercise [1,17]. One explanation for the lack of systemic hypoalgesia is that baseline CPM testing negatively impacted systemic EIH, potentially due to their shared manifestations. It is possible that CPM is a contributing mechanism to systemic EIH. As CPM was initiated earlier in the session and not enough washout period was provided to restore CPM, systemic EIH was not observed. Not all our data support this explanation as there were no correlations observed between CPM and EIH. Previous research has demonstrated an association between CPM and EIH across the lifespan [2,3,41]. This relation is more consistent when EIH is measured systemically and following exhaustive exercise. However, similar to the current study, Vaegter et al. [1] showed no correlation between CPM and EIH after low-intensity isometric exercise held for three minutes. The relation between CPM and EIH is likely dependent on both the exercise dose and testing site for EIH.
To our knowledge, this is the first study to investigate the effect of isometric exercise on CPM. Because stimulation to the motor cortex enhances CPM, we expected that CPM would be enhanced following exercise. Contrary to our hypothesis, CPM decreased following exercise only in those individuals who had systemic EIH. This is potentially related to 1) a ceiling effect for PPTs and the exercise-induced increase in PPT attenuated the subsequent testing stimulus [42] or 2) systemic hypoalgesia that occurs following exercise is due to CPM. Arendt-Nielsen et al. [43] found that two concurrent painful conditioning stimuli (muscle pain and cold presser pain) had a decreased effect than either stimulus alone. Because the exercise protocol in this study was painful, the increase in PPTs at the upper trapezius muscle following exercise may actually be a CPM protocol, with exercise acting as the conditioning stimulus and PPT the testing stimulus. EIH responders experienced a 20% increase in PPTs following exercise and an additional 8% increase following the ice conditioning stimulus, which is comparable to what they have experienced with the conditioning stimulus alone in the quiet rest session (27%). The nonresponders had only local hypoalgesia (i.e., quadriceps muscle) following exercise; the lack of hypoalgesia systemically (i.e., upper trapezius muscle) suggests that local exercise effects do not influence CPM due to different mechanisms. Previous reports have shown that CPM magnitude is influenced by the intensity of the conditioning stimulus but not by the pain reported during the conditioning stimulus [44]. Likewise, in this study, pain reported during exercise or ice water bath did not influence EIH nor CPM. If exercise produces hypoalgesia via activation of the CPM response, then increasing the exercise intensity (i.e., the conditioning stimulus) should produce greater hypoalgesia.
Emerging evidence has shown that body composition and physical activity may influence EIH and CPM [2,14–16]. This is contrary to the current study in that body composition and self-reported physical activity were not correlated with EIH or CPM. This is similar to a recent study by Black et al. [45] that showed no relation between EIH and physical activity, assessed via accelerometer. These results may be due to the homogenous sample in the current study, as most individuals reported moderate to vigorous physical activity levels and normal to slightly overweight BMI levels. Likewise, the weakly correlated pain catastrophizing scores with neither CPM nor EIH may be due to the relatively normal catastrophizing scores (e.g., only four individuals above 30) observed in this sample.
Several potential limitations should be taken into consideration. First, a small number of individuals had a systemic EIH response (N = 9), possibly due to the low intensity and short duration of the isometric exercise, thereby limiting the generalizability of the results. Future studies should verify these results following an exercise duration that is known to produce systemic effects (e.g., isometric exercise until task failure or aerobic exercise). In addition, the between-session reliability of CPM was poor. However, this should have minimal effects on our results as we are comparing changes in CPM within session. Finally, the results in the present study are generalizable to young healthy adults only. It is unclear whether individuals with chronic pain would yield similar results.
Despite these limitations, several clinical implications can be drawn from this study. Our results suggest that the systemic effects of exercise activate descending inhibitory pathways, making exercise a good clinical modality in the management of pain. Thus, in individuals with impaired CPM, the systemic effects of exercise may be more variable in producing pain-relieving effects. The local effects, however, do not appear to be mediated by CPM and could be an alternative clinical tool in those conditions with impaired CPM. Finally, our results show the potential benefits of assessing CPM to help guide clinical decision-making. With repeated assessments, an appropriate length of time (e.g., greater than 23 minutes) is necessary for the restoration of CPM. Additional research that includes individuals with chronic pain is essential, including whether this relation between systemic EIH and CPM occurs with exercise training. Understanding these effects in patients will allow for a more targeted use of exercise in the management of pain.
Conclusion
Individuals who experienced EIH systemically had an attenuated CPM response compared with those individuals who only experienced local EIH. The results raise the possibility that there are shared mechanisms between CPM and systemic EIH. In addition, CPM decreased following exercise and quiet rest, which may be due to an insufficient washout period, while the within-session reliability was fair to good and the between-session reliability was poor.
Conflicts of interest: The authors have no conflicts of interest.
References
- 1. Vaegter HB, Handberg G, Graven-Nielsen T.. Similarities between exercise-induced hypoalgesia and conditioned pain modulation in humans. Pain 2014;155(1):158–67. [DOI] [PubMed] [Google Scholar]
- 2. Lemley KJ, Hunter SK, Bement MK.. Conditioned pain modulation predicts exercise-induced hypoalgesia in healthy adults. Med Sci Sports Exerc 2015;47(1):176–84. [DOI] [PubMed] [Google Scholar]
- 3. Stolzman S, Bement MH.. Does exercise decrease pain via conditioned pain modulation in adolescents? Pediatr Phys Ther 2016;28(4):470–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Willer JC, Le Bars D, De Broucker T.. Diffuse noxious inhibitory controls in man: Involvement of an opioidergic link. Eur J Pharmacol 1990;182(2):347–55. [DOI] [PubMed] [Google Scholar]
- 5. Le Bars D, Chitour D, Kraus E, Dickenson AH, Besson JM.. Effect of naloxone upon diffuse noxious inhibitory controls (DNIC) in the rat. Brain Res 1981;204(2):387–402. [DOI] [PubMed] [Google Scholar]
- 6. Smith MA, Yancey DL.. Sensitivity to the effects of opioids in rats with free access to exercise wheels: Mu-opioid tolerance and physical dependence. Psychopharmacology (Berl) 2003;168(4):426–34. [DOI] [PubMed] [Google Scholar]
- 7. Janal MN, Colt EW, Clark WC, Glusman M.. Pain sensitivity, mood and plasma endocrine levels in man following long-distance running: Effects of naloxone. Pain 1984;19(1):13–25. [DOI] [PubMed] [Google Scholar]
- 8. Vaegter HB, Handberg G, Graven-Nielsen T.. Hypoalgesia after exercise and the cold pressor test is reduced in chronic musculoskeletal pain patients with high pain sensitivity. Clin J Pain 2016;32(1):58–69. [DOI] [PubMed] [Google Scholar]
- 9. Fingleton C, Smart K, Doody C.. Exercise-induced hypoalgesia in people with knee osteoarthritis with normal and abnormal conditioned pain modulation. Clin J Pain 2017;33:395–404. [DOI] [PubMed] [Google Scholar]
- 10. Lemley KJ, Drewek B, Hunter SK, Hoeger Bement MK.. Pain relief after isometric exercise is not task-dependent in older men and women. Med Sci Sports Exerc 2014;46(1):185–91. [DOI] [PubMed] [Google Scholar]
- 11. Kosek E, Lundberg L.. Segmental and plurisegmental modulation of pressure pain thresholds during static muscle contractions in healthy individuals. Eur J Pain 2003;7(3):251–8. [DOI] [PubMed] [Google Scholar]
- 12. Ellingson LD, Koltyn KF, Kim JS, Cook DB.. Does exercise induce hypoalgesia through conditioned pain modulation? Psychophysiology 2014;51(3):267–76. [DOI] [PubMed] [Google Scholar]
- 13. Hoeger Bement MK, Dicapo J, Rasiarmos R, Hunter SK.. Dose response of isometric contractions on pain perception in healthy adults. Med Sci Sports Exerc 2008;40(11):1880–9. [DOI] [PubMed] [Google Scholar]
- 14. Stolzman S, Hoeger Bement M.. Lean mass predicts conditioned pain modulation in adolescents across weight status. Eur J Pain 2016;20(6):967–76. [DOI] [PubMed] [Google Scholar]
- 15. Naugle KM, Riley JL 3rd.. Self-reported physical activity predicts pain inhibitory and facilitatory function. Med Sci Sports Exerc 2014;46(3):622–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stolzman S, Danduran M, Hunter SK, Bement MH.. Pain response after maximal aerobic exercise in adolescents across weight status. Med Sci Sports Exerc 2015;47(11):2431–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vaegter HB, Handberg G, Graven-Nielsen T.. Isometric exercises reduce temporal summation of pressure pain in humans. Eur J Pain 2015;19:973–83. [DOI] [PubMed] [Google Scholar]
- 18. Flood A, Waddington G, Cathcart S.. High-definition transcranial direct current stimulation enhances conditioned pain modulation in healthy volunteers: A randomized trial. J Pain 2016;17(5):600–5. [DOI] [PubMed] [Google Scholar]
- 19. Lewis GN, Rice DA, McNair PJ.. Conditioned pain modulation in populations with chronic pain: A systematic review and meta-analysis. J Pain 2012;13(10):936–44. [DOI] [PubMed] [Google Scholar]
- 20. Kennedy DL, Kemp HI, Ridout D, Yarnitsky D, Rice AS.. Reliability of conditioned pain modulation: A systematic review. Pain 2016;157(11):2410–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Koltyn KF, Trine MR, Stegner AJ, Tobar DA.. Effect of isometric exercise on pain perception and blood pressure in men and women. Med Sci Sports Exerc 2001;33(2):282–90. [DOI] [PubMed] [Google Scholar]
- 22. Sullivan MJL, Bishop SR, Pivik J.. The pain catastrophizing scale: Development and validation. Psychol Assess 1995;7(4):524–32. [Google Scholar]
- 23. Craig C, Marshall A, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003;35(8):1381–95. [DOI] [PubMed] [Google Scholar]
- 24. McCaffery M, Pasero C.. Pain Clinical Manual. St. Louis: Mosby; 1999. [Google Scholar]
- 25. Ekstrom RA, Donatelli RA, Soderberg GL.. Surface electromyographic analysis of exercises for the trapezius and serratus anterior muscles. J Orthop Sports Phys Ther 2003;33(5):247–58. [DOI] [PubMed] [Google Scholar]
- 26. Walton DM, Macdermid JC, Nielson W, et al. Reliability, standard error, and minimum detectable change of clinical pressure pain threshold testing in people with and without acute neck pain. J Orthop Sports Phys Ther 2011;41(9):644–50. [DOI] [PubMed] [Google Scholar]
- 27. Garamszegi L. Comparing effect sizes across variables: Generalization without the need for bonferroni correction. Behav Ecol 2006;17(4):682–7. [Google Scholar]
- 28. Avin KG, Law LA.. Age-related differences in muscle fatigue vary by contraction type: A meta-analysis. Phys Ther 2011;91(8):1153–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kosek E, Ordeberg G.. Lack of pressure pain modulation by heterotopic noxious conditioning stimulation in patients with painful osteoarthritis before, but not following, surgical pain relief. Pain 2000;88(1):69–78. [DOI] [PubMed] [Google Scholar]
- 30. Leffler AS, Hansson P, Kosek E.. Somatosensory perception in a remote pain-free area and function of diffuse noxious inhibitory controls (DNIC) in patients suffering from long-term trapezius myalgia. Eur J Pain 2002;6(2):149–59. [DOI] [PubMed] [Google Scholar]
- 31. Oono Y, Wang K, Svensson P, Arendt-Nielsen L.. Conditioned pain modulation evoked by different intensities of mechanical stimuli applied to the craniofacial region in healthy men and women. J Orofac Pain 2011;25:364–75. [PubMed] [Google Scholar]
- 32. Pud D, Sprecher E, Yarnitsky D.. Homotopic and heterotopic effects of endogenous analgesia in healthy volunteers. Neurosci Lett 2005;380(3):209–13. [DOI] [PubMed] [Google Scholar]
- 33. Yarnitsky D, Bouhassira D, Drewes AM, et al. Recommendations on practice of conditioned pain modulation (CPM) testing. Eur J Pain 2015;19(6):805–6. [DOI] [PubMed] [Google Scholar]
- 34. Lewis GN, Heales L, Rice DA, Rome K, McNair PJ.. Reliability of the conditioned pain modulation paradigm to assess endogenous inhibitory pain pathways. Pain Res Manag 2012;17(2):98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Le Bars D, Dickenson AH, Besson JM.. Diffuse noxious inhibitory controls (DNIC). I. Effects on dorsal horn convergent neurones in the rat. Pain 1979;6(3):283–304. [DOI] [PubMed] [Google Scholar]
- 36. Valencia C, Fillingim RB, Bishop M, et al. Investigation of central pain processing in postoperative shoulder pain and disability. Clin J Pain 2014;30(9):775–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cathcart S, Winefield AH, Rolan P, Lushington K.. Reliability of temporal summation and diffuse noxious inhibitory control. Pain Res Manag 2009;14(6):433–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Imai Y, Petersen KK, Morch CD, Arendt Nielsen L.. Comparing test-retest reliability and magnitude of conditioned pain modulation using different combinations of test and conditioning stimuli. Somatosens Mot Res 2016;33(3–4):169–77. [DOI] [PubMed] [Google Scholar]
- 39. Olesen SS, van Goor H, Bouwense SA, Wilder-Smith OH, Drewes AM.. Reliability of static and dynamic quantitative sensory testing in patients with painful chronic pancreatitis. Reg Anesth Pain Med 2012;37(5):530–6. [DOI] [PubMed] [Google Scholar]
- 40. Larivière M, Goffaux P, Marchand S, Julien N.. Changes in pain perception and descending inhibitory controls start at middle age in healthy adults. Clin J Pain 2007;23(6):506–10. [DOI] [PubMed] [Google Scholar]
- 41. Vaegter HB, Handberg G, Jorgensen MN, Kinly A, Graven-Nielsen T.. Aerobic exercise and cold pressor test induce hypoalgesia in active and inactive men and women. Pain Med 2015;16(5):923–33. [DOI] [PubMed] [Google Scholar]
- 42. Granot M, Weissman-Fogel I, Crispel Y, et al. Determinants of endogenous analgesia magnitude in a diffuse noxious inhibitory control (DNIC) paradigm: Do conditioning stimulus painfulness, gender and personality variables matter? Pain 2008;136(1):142–9. [DOI] [PubMed] [Google Scholar]
- 43. Arendt-Nielsen L, Sluka KA, Nie HL.. Experimental muscle pain impairs descending inhibition. Pain 2008;140(3):465–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nir RR, Granovsky Y, Yarnitsky D, Sprecher E, Granot M.. A psychophysical study of endogenous analgesia: The role of the conditioning pain in the induction and magnitude of conditioned pain modulation. Eur J Pain 2011;15(5):491–7. [DOI] [PubMed] [Google Scholar]
- 45. Black CD, Huber JK, Ellingson LD, et al. Exercise-induced hypoalgesia is not influenced by physical activity type and amount. Med Sci Sports Exerc 2017;49(5):975–82. [DOI] [PubMed] [Google Scholar]