Abstract
Introduction
Varenicline reduces smoking satisfaction during the pre-cessation run-in period, which may contribute to extinction of cravings and smoking behavior. Research indicates that efficacy is enhanced when the run-in period is increased from 1 to 4 weeks, providing a longer extinction opportunity. We hypothesized that efficacy could be further enhanced by harnessing basic and applied research on extinction. We developed a pre-cessation extinction-facilitating intervention and tested its feasibility in a pilot trial.
Methods
The facilitated extinction (FE) intervention comprised brief counseling and workbook-recommending strategies to maximize extinction processes during the run-in, including instructions to smoke at a normal rate across contexts and cues, and use of an extinction cue to enhance generalization. Participants were randomly assigned to one of three varenicline interventions: standard (1-week run-in), extended (4-week run-in), and extended + FE. Interventions were delivered prior to the target quit date (TQD). Assessments were conducted in weeks 1 and 4 pre-TQD and 1 and 3 months post-TQD, with focus on feasibility indices.
Results
Recruitment and retention goals were met (N = 58). Treatment satisfaction was high across groups. The majority of FE participants adhered to instructions and maintained their usual smoking rate during the run-in period. Greater decreases in craving and smoking satisfaction were observed among participants in both extended groups versus the standard group (p < .005).
Conclusions
Feasibility was demonstrated. Participants adhered to the FE intervention, thereby optimizing the number and variety of extinction trials. Findings support testing the novel FE smoking cessation intervention in a fully powered trial.
Implications
This study expands the research on the clinical benefits of extending the pre-cessation run-in period of varenicline. It introduces the hypothesis that further benefit might be achieved by translating basic behavioral research, as well as cue-exposure research and therapy for other disorders, to improve the extinction and generalization processes thought to underlie much of varenicline’s effect. A FE intervention was developed and found acceptable to smokers and feasible to implement in a research setting. The study sets the stage for a subsequent randomized controlled trial.
Introduction
The development of medications for treating tobacco dependence has contributed to the reduction in smoking prevalence over the past 3 decades. One such medication is varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist. In theory, the agonist effects of varenicline reduce nicotine withdrawal symptoms and cravings to smoke, while the antagonist effects block satisfaction and reinforcement from smoking. Whereas there is substantial evidence for the agonistic effects and clinical efficacy of varenicline via multiple randomized controlled trials (RCTs),1–5 empirical support for the antagonistic effects remains limited and has been much slower to emerge. Of those few studies, retrospective self-report data from the varenicline RCTs suggest that varenicline reduced self-reported smoking satisfaction compared to placebo.1,2,6 Experimental studies have reported similar findings when self-reported smoking reward was assessed immediately after smoking among those taking varenicline.7,8
Varenicline’s effect on reducing smoking satisfaction—or nicotine’s reward potency—has an important clinical implication. Namely, during the pre-cessation run-in period, cigarettes should become progressively less rewarding and reinforcing to smokers, which in turn, should contribute to the extinction of cue-provoked cravings and smoking behavior. One study provides support for this expectation concerning extinction: reduction of cue-provoked cravings in a laboratory-based test appeared only after the 12–15 day varenicline run-in, as compared to placebo7 (although see Gass et al.9 for contradictory results). These results suggest that the extinction process must be given sufficient time to progress before conditioned cravings decline. In addition, these results are consistent with the findings that pre-cessation use of nicotine replacement therapy, bupropion, nicotinic antagonists (mecamylamine), and de-nicotinized (placebo) cigarettes appear to improve cessation outcomes.10–15 The presumed mechanisms of action underlying this effect are extinction of operant and classical conditioning. That is, as smokers use cigarettes without receiving their usual reinforcement (i.e., satisfaction), their unreinforced smoking behavior is extinguished. Importantly, this extinction, which is well known to be highly context-dependent, is occurring in the smoker’s natural environment and in response to daily life events. Moreover, cues associated with smoking are also extinguished when they no longer are paired with nicotine effects, as suggested by Brandon et al.7 These cues can serve as both discriminative stimuli for operant conditioning and as conditioned stimuli that evoke conditioned responses, including cravings to smoke.
An implication of the extinction model is that the efficacy of varenicline might be enhanced if smokers were given a longer pre-cessation opportunity to engage extinction processes prior to quitting. There are data to support this thesis. Two studies have found that a 4-week run-in period produced greater smoking reduction and short-term abstinence compared to the standard run-in period of 1 week,10,16 and another reported that reductions in smoking rate and cravings appeared only after 10 days of varenicline use.17 One study that allowed patients to choose when to quit smoking after initiating varenicline use found that patients tended to choose a run-in period more than twice as long as the standard 7 days, and that the odds ratios versus placebo were among the highest of published clinical trials.18
Extinction-based therapies (aka “cue exposure therapies”) for tobacco smoking and other substance use have long been considered a logical component of treatment, given the significant role of conditioning in the development and maintenance of these behaviors.19 However, their success to date has been limited,20 partly because of difficulties in generalizing extinction from the clinic into the real world.21 The advantage of allowing extinction to take place naturally during pre-cessation smoking while reinforcement/reward is blocked by varenicline is that extinction occurs in the smokers’ natural environment across multiple settings (and with extinction trials more distributed in time than is the case in the therapist’s office). Thus, the generalization barrier is greatly reduced. However, one key disadvantage to this naturalistic extinction approach is that the extinction trials are likely to be inconsistent and haphazard across time and contexts—not necessarily in the optimal manner to maximize extinction. In addition, the reduction in smoking that is often observed with initiation of varenicline could diminish the extinction effect by reducing the overall number of extinction trials. Thus, such naturalistic extinction would be expected to occur inconsistently, both across and within individual smokers.
We hypothesized that the extinction process would be optimized by incorporating advances in extinction theory, as well as by adapting methods from both basic and applied research on exposure therapy for other disorders.21–23 A key component of enhancing extinction is to sustain the pre-cessation smoking rate until the quit date, thereby maximizing the number of potential extinction trials (smoking with minimal reinforcement). Discouraging smokers from reducing their smoking may appear counter-intuitive, but in the context of pre-cessation varenicline use, smoking reduction represents reduction of potential extinction trials.
The current research had two primary objectives. The first was to develop an intervention designed to accompany an extended (4-week) run-in period with the goal of facilitating the extinction process and enhancing the efficacy of varenicline via empirically based strategies that are relatively easy to implement and communicate to patients. The second objective was to test the feasibility of implementing the novel intervention in the context of a clinical trial of extended pre-cessation varenicline. Feasibility includes evaluation of demand, practicality, and acceptability of the intervention. We hypothesized that the novel intervention would be acceptable to participants, feasible to implement, and provide satisfactory adherence. Although the study was not powered to test clinical outcomes, we also piloted research components (e.g., assessment procedures) of a future planned RCT.
Methods
Interventions
Eligible participants were randomly assigned to one of three interventions: standard Varenicline (SV), extended Varenicline (EV), or extended Varenicline plus facilitated extinction (FE). Consistent with the standard dosing regimen, participants in the SV condition received a 1-week supply of varenicline that was initiated 1 week prior to the target quit date (TQD). Participants in the EV condition received a 4-week supply of varenicline prior to TQD. Participants in the FE condition received the same varenicline regimen as the EV condition, plus the FE intervention and workbook described below, to enhance the extinction process over the 4 weeks prior to TQD. All participants received an 11-week supply of varenicline for the post-TQD period.
Varenicline dose titration in all three interventions used the following schedule: 0.5 mg on days 1–3, 0.5 mg BID dosing on days 4–7, and 1 mg BID dosing through the remainder of the study. All participants were instructed to continue to smoke until the TQD and to record their cigarette consumption for the duration of the study. Only participants in the FE condition were explicitly instructed not to reduce their cigarette consumption. All participants received 15 minutes of basic cognitive-behavioral smoking cessation counseling based on the 4th “A” (Assist) of the “5 A’s” recommended by the USPHS Clinical Practice Guidelines,24 during the week prior to TQD.
FE Intervention
The FE intervention, including a workbook, was developed by the study team with expertise in pre-cessation varenicline and extinction theory and application. It was pre-tested in a group of five treatment-seeking smokers to evaluate treatment administration and acceptability, as well as comprehension and appeal of the workbook. The intervention was well received, but we identified some relatively minor areas for improvement. For example, some smokers had difficulties maintaining their usual smoking rate during the run-in period, as instructed. As this was a key aspect of the intervention, we incorporated additional strategies to improve adherence to smoking maintenance, including a workbook vignette addressing this issue, and providing therapists with the flexibility to adjust the smoking goal to make it more attainable for participants who had a difficult time maintaining their baseline-smoking rate.
The FE intervention was delivered over four brief weekly counseling sessions by PhD-level therapists during the 4 weeks leading up the TQD. It employed the following theoretically based techniques: (1) The primary technique was to encourage and remind participants to continue to smoke at their normal rate during the extended pre-quit run-in period, in order to maximize the opportunity for extinction. (2) Self-monitoring was used to help participants maintain their usual daily smoking rate. (3) Participants were educated about the extinction process, based on findings that extinction is enhanced when patients hold positive expectancies about the extinction process, and when they are explicitly aware of the diminished reinforcement.25 (4) Therapists reminded and encouraged participants to smoke across a variety of contexts and cues (both common daily cues and rarer cues) during the run-in period, based on the evidence that extinction does not generalize well across contexts.26–28 (5) Participants were encouraged to smoke during negative affect states, and, if necessary, to use imagery to create such opportunities to smoke, because negative affect state are among the most potent cues for smoking relapse.29,30 (6) Participants were instructed to ensure that compound stimuli (i.e., multiple smoking cues) were present on a subset of smoking occasions, based on evidence that compound conditioned stimuli produce more potent responding and more complete and persistent extinction.31 (7) Lastly, participants were provided an extinction retrieval cue in the form of a wristband, based on research indicating that the maintenance and generalizability of extinction (across time and contexts) are enhanced by the use of such a cue.28,32 An extinction cue can serve to trigger the individual’s memory of the extinction trials and can be used in any environment, thus helping to extend the extinction of cravings beyond the quit day. Participants were instructed to wear the wristband only while smoking during the last week of the run-in period and to wear it all the time for at least 1 month after they quit smoking. The intervention workbook, titled CountDown: Preparing to Quit Smoking with Varenicline, and written at a 5–6th grade level, contained a combination of didactic information and interactive exercises designed to reinforce the counseling techniques described above.
Participants
Daily cigarette smokers were recruited from the community for “a smoking cessation intervention study.” The following inclusion criteria were used: (1) ≥18 years of age; (2) smoked ≥10 cigarettes/day for the past year; (3) expired-air carbon monoxide (CO) >8 ppm; (4) medically eligible to receive varenicline; (5) scored ≥5 on the Contemplation Ladder,33 a measure of motivation to quit smoking; and (6) able to speak and read English. Exclusion criteria included: (1) pregnant or breastfeeding; (2) renal dysfunction; (3) history of seizures; (4) medically at risk in the judgment of the study physician; (5) previous use of varenicline; (6) use of other smoking cessation medications within the past 3 months; or (7) current psychiatric disorder, including depression, bipolar, psychotic disorders, or substance/alcohol use disorder, as determined by a psychiatric screener, the Mini International Neuropsychiatric Interview (MINI).34
Procedure
This study was approved by the Institutional Review Board, and all participants provided informed consent. Following a preliminary telephone screening, participants completed an extensive in-person screening evaluation session that included questionnaires, the MINI, expired-air carbon monoxide, a pregnancy test for women of child-bearing age, and a medical exam conducted by a nurse practitioner and reviewed by the study physician. Participants who met all inclusion criteria were scheduled for the first laboratory assessment session and randomly assigned to receive one of three interventions described above. Randomization was stratified by gender and employed a variable block randomization strategy.
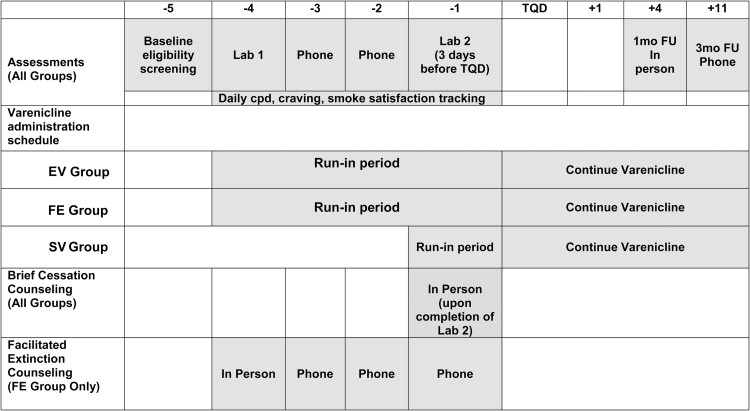
The timeline of the study elements is shown in Figure 1. The first laboratory assessment consisted of questionnaires and a cue-reactivity test (see Figure 1). The second laboratory assessment was scheduled during the fourth week after the first laboratory assessment, immediately preceding the TQD. It involved completion of questionnaires, another cue-reactivity test, and 10 minutes of basic cognitive-behavioral smoking cessation counseling. All participants were instructed to record their daily cigarette consumption, cravings, and smoking satisfaction during the four weeks leading up to the TQD, and to report these data during brief weekly telephone assessments. Participants in the SV group completed the first laboratory assessment and waited for three weeks before receiving and initiating their medication. This aspect of the study design provided a standardized 4-week timeframe indexed to the TQD, allowing for direct comparisons between the three groups on relevant variables collected in the field and laboratory. The 1-month post-TQD follow-up assessment was conducted in person, and the 3-month follow-up assessment was conducted by telephone.
Figure 1.
Schedule of study procedures and progression by week. EV = extended Varenicline; FE = facilitated extinction; SV = standard Varenicline; TQD = target quit date.
Participants were paid $40 for completing the first laboratory assessment, $60 for completing the second laboratory assessment, and $20 for completing each follow-up assessment. Participants were able to earn up to an additional $50 by complying with study procedures (e.g., keeping daily records).
Of the 86 treatment-seeking smokers who provided informed consent, 62 met eligibility criteria and were enrolled. One participant declined to participate. The remaining 23 participants met exclusion criteria, including substance use disorder (n = 9), psychiatric disorder (n = 4), medical contraindication (n = 6), too few cigarettes smoked per day (n = 4). After the exclusion of participants who did not receive treatment due to factors unrelated to the study (see Accrual section), the final sample comprised 58 participants.
Measures
Feasibility Measures
Three feasibility indices were assessed: demand, practicality, and acceptability. Demand was quantified using accrual rates and recruitment costs per participant. Practicality encompassed the degree to which the piloted elements of the planned RCT were carried out successfully, including recruitment, screening, treatment, and assessments. Acceptability of the intervention was measured using the Client Satistfaction Questionnaire (CSQ),35 as well as the rate of adherence to behavioral and pharmacological aspects of the intervention, based on participant and therapist responses to questionnaires developed for this study. The questionnaires asked respondents to evaluate and report completion of specific aspects of the FE intervention, such as helpfulness of information and instructions and ability to complete interactive exercises.
Smoking-Related Measures
Baseline measures included a demographic and smoking history questionnaire, and the Fagerström Test for Nicotine Dependence.24 Cigarettes per day (CPD), cravings to smoke, and smoking satisfaction were recorded daily on a 10-point Likert scale. All participants received brief weekly telephone calls to record daily cigarette consumption, cravings to smoke, response to smoking cigarettes, and varenicline-related side effects, during the 4 weeks leading up the TQD. Additional self-report measures were included in the feasibility trial but are not the focus in the current report.
Cue-Reactivity Assessment
Cue-reactivity testing assessed the effect of varenicline and FE techniques on cue-provoked craving. An established picture-viewing paradigm was used to assess cue reactivity.7,36 Twelve (12) smoking-related and 12 neutral control images were randomly presented to each participant while craving measures were obtained. Smoking cues included photos that have elicited substantial craving reports in our prior research.37,38 Neutral cues consisted of pictures from the International Affective Picture System (IAPS),39 and included objects, people, and situations that have been rated as neither pleasant, unpleasant, or arousing. Following picture offset, smoking craving ratings were obtained on a visual-analog scale. With respect to this feasibility study, the goal was to test the administration of these procedures in anticipation of a future RCT.
Follow-up Assessments
At one month post-TQD, current smoking status, smoking rate, and varenicline use were assessed using the Timeline Follow Back (TLFB) procedure.40 Participant’s expired-air carbon monoxide (CO) levels were measured to verify abstinence. Evaluation of the intervention was also assessed. Participants in the FE condition were asked about continued usage of the extinction cue wristband. A similar assessment was completed via telephone at 3 months post-TQD.
Results
Sample Characteristics
Descriptive statistics for baseline demographic and smoking-related variables are presented by treatment group in Table 1. The three groups differed in percentage of minority participants (χ2(2, N = 58) = 10.10, p < .006). Follow-up analyses showed that the EV group had a lower percentage of minority participants than did the SV group (χ2(1, N = 39) = 9.73, p < .002). There were no other significant differences between groups on baseline variables.
Table 1.
Participant Characteristics by Condition
| Group | ||||
|---|---|---|---|---|
| SV (n = 20) | EV (n = 19) | FE (n = 19) | Total (N = 58) | |
| Gender: n | ||||
| Male | 11 | 10 | 12 | 33 |
| Female | 9 | 9 | 7 | 25 |
| Age, tears: M (SD) | 51.9 (11.0) | 48.4 (11.4) | 51.2 (10.0) | 50.5 (10.8) |
| Race: n | ||||
| White | 10 | 17a | 13 | 40 |
| African American | 9 | 1a | 5 | 15 |
| Other | 1 | 0 | 1 | 2 |
| Hispanic: n | 3 | 2 | 1 | 6 |
| Education: n | ||||
| High school or less | 7 | 1 | 6 | 14 |
| Some college/tech school | 10 | 13 | 12 | 35 |
| College or post-college | 3 | 5 | 1 | 9 |
| Income: n | ||||
| <$40 K | 11 | 11 | 13 | 35 |
| $40 K+ | 9 | 8 | 6 | 23 |
| Smoking-related: M (SD)b | ||||
| # Cigarettes/day | 19.6 (8.9) | 19.5 (6.3) | 20.7 (6.9) | 19.9 (7.3) |
| # Years smoked daily | 31.5 (13.2) | 28.6 (10.9) | 27.4 (13.3) | 29.2 (12.4) |
| # Lifetime quit attempts | 2.5 (2.28) | 6.7 (12.86) | 12.1 (23.75) | 6.9 (15.42) |
| # Quit attempts in last year | 1.8 (2.80) | 2.0 (2.94) | 1.9 (2.82) | 1.9 (2.81) |
| Pre-quit FTND total (range: 1–10) | 5.2 (2.1) | 4.9 (2.0) | 5.2 (2.6) | 5.1 (2.2) |
EV = extended Varenicline; FE = facilitated extinction; FTND = Fagerström Test for Nicotine Dependence; M = mean; SD = standard deviation; SV = standard Varenicline.
aSignificant difference between EV and SV.
bThe following smoking-related measures were missing 1–3 observations within 1 or more groups: # Years smoked daily; # Lifetime quit attempts; # Quit attempts in last year.
Feasibility Outcomes
Demand
Accrual
A total of 383 people were pre-screened for eligibility by telephone. Of those, 204 (53%) met initial eligibility. Reasons for ineligibility included: past varenicline and/or recent cessation medication use (50%); current substance use and/or psychiatric problems (23%); too few cigarettes smoked per day (13%); medical contraindications (10%); not motivated to quit (3%); did not speak English (0.5%); same household as another participant (0.5%). Of the 204 participants who met initial eligibility by telephone, 48 declined to participate, 70 either failed to show or cancelled without rescheduling, and 86 attended the baseline screening session.
Of participants who attended the baseline screening, 62 met full eligibility criteria and were enrolled in the study. One participant failed to attend the first laboratory assessment session before randomization was revealed and was removed from the study. Three other participants discontinued the study and did not receive the treatment due to factors unrelated to the study (i.e., cancer diagnosis, moved from the area, and automobile accident) and were not included in the study analyses. In the final sample, there were 20 participants in the SV condition and 19 each in the EV and FE conditions.
Cost
Recruitment costs, largely for advertisements in local papers, averaged $82.10 per participant enrolled.
Practicality
All elements of the feasibility study, including recruitment and retention, were completed as planned. Ninety-eight percent of those enrolled in the study completed the first laboratory assessment and 87% completed the second laboratory assessment. As noted above, four participants were discontinued from the study early and did not receive treatment. In addition, three participants who received treatment lost contact with the study before the end of treatment and TQD. Hence, seven participants (11%; three from FE, two each from EV and SV) of the 62 consented and enrolled, did not complete the treatment protocol. With regard to follow-up retention, 79% completed the 1-month follow-up; and 81% completed the 3-month follow-up. Excluding those who were dropped from the study prior to the quit day, 89% completed the 1-month follow-up, and 91% completed the 3-month follow-up.
Acceptability
Client Satisfaction
Forty-nine participants completed the CSQ 1-month post treatment. Overall, satisfaction with the program was very high, with mean CSQ scores of 30 (scale of 8–32) for each treatment group, with no differences across groups. All participants rated the quality of service received as “mostly good” or “very good,” and reported that they “generally” or “definitely” received the kind of service that they wanted. All but one participant said the program met “most” or “almost all” of their needs. All but four participants were “mostly satisfied” or “very satisfied” with the amount of help received. Most (96%) of respondents reported that the program helped them quit smoking and stay smoke free. All indicated that they would recommend the program to a friend. A majority of participants (76%) provided positive comments about the treatment program. However, four participants expressed a need for additional assistance following the TQD.
Intervention Adherence
Three intervention adherence elements were assessed: medication, FE treatment, and smoking rate prior to TQD. The vast majority of participants tolerated the varenicline well and completed the regimen as prescribed. Three of 58 individuals prematurely discontinued varenicline due to side effects. Six individuals missed two or more consecutive doses in the weeks before quit day. At 1-month post TQD, the percent of participants who were still using varenicline was 80% for the FE group, 84% for the EV group, and 80% for the SV group. Reasons for nonadherence included: side effects (n = 3), unrelated medical problems (n = 2), “ran out of medication” (n = 1), “haven’t needed it” (n = 1), “unsure if want to quit” (n = 1), and unknown (n = 1). There was good adherence with taking the medication at the same time each day (>90% at all time points). There were no differences in medication adherence by group assignment.
Therapists reported that participants understood most or all of the treatment content across all four treatment sessions. Ninety-four percent of participants completed “most” or “all” assignments. However, 35% of participants had difficulty identifying rare and combination triggers in one of the sessions. Adherence to wearing the treatment wristband (extinction renewal cue) for five or more days before the quit day was 93%. At 1-month post TQD, 86% of participants reported wearing the wristband for 20 or more days in the past month.
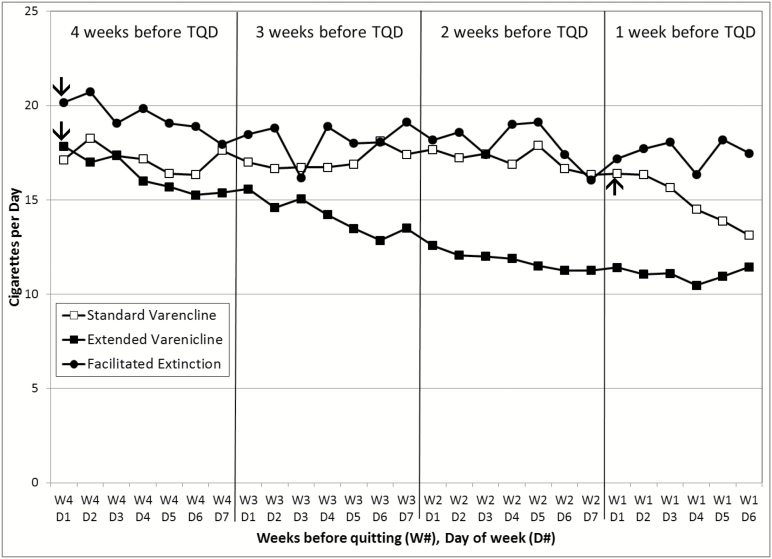
One of the primary goals of the FE intervention was to sustain the baseline-smoking rate during the 4 weeks leading up to the TQD. Figure 2 illustrates the CPD results across the four pre-quit weeks by treatment group. Generalized estimating equations (GEE) were used to compare the first and the last week of the pre-quit period in a model with group, week, day, and their interaction terms as predictors. The week × group interaction was significant, χ2(2) = 8.78, p = .012. The three groups did not differ significantly in the first week (χ2(2) = 2.07, p = .356), but did in the last week (χ2(2) = 8.20, p = .017). Notably, the average CPD during the last week was significantly higher for the FE group (adjusted mean [M] = 17.54, standard error [SE] = 1.43) than for the EV group (adjusted M = 11.27, SE = 1.41, χ2(1) = 7.37, p = .007). Moreover, in the last week of the pre-cessation period, 76.5% of FE participants continued to smoke within 80% of their baseline CPD, and 47.1% continued to smoke within 90% of their baseline CPD. In contrast, only 26.3% and 10.5% of EV participants continued to smoke at 80% and 90% of baseline CPD, respectively. Note in Figure 2 that the SV group showed a decline in smoking during the week immediately preceding the TQD, which coincides with the week of medication initiation in this group.
Figure 2.
Mean cigarettes per day by treatment group during the 4 weeks prior to target quit day. EV = extended Varenicline; FE = facilitated extinction; SV = standard Varenicline; TQD = target quit date. TQD occurred on week 1 (W1), day 7 (D7). Varenicline was started on day 1 of week 4 for the EV and FE conditions and on day 1 of week 1 for the SV condition, as indicated by the arrows.
Intermediary and Clinical Outcomes
Pre-Quit effects
Peak Craving and Smoking Satisfaction
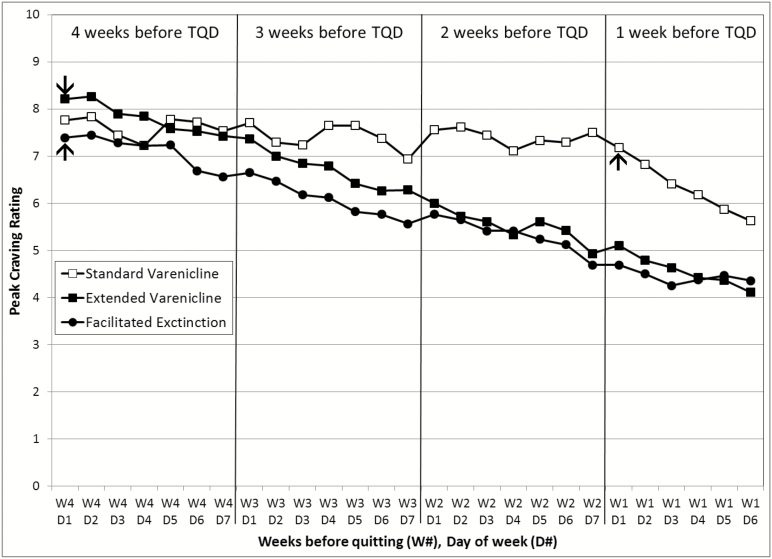
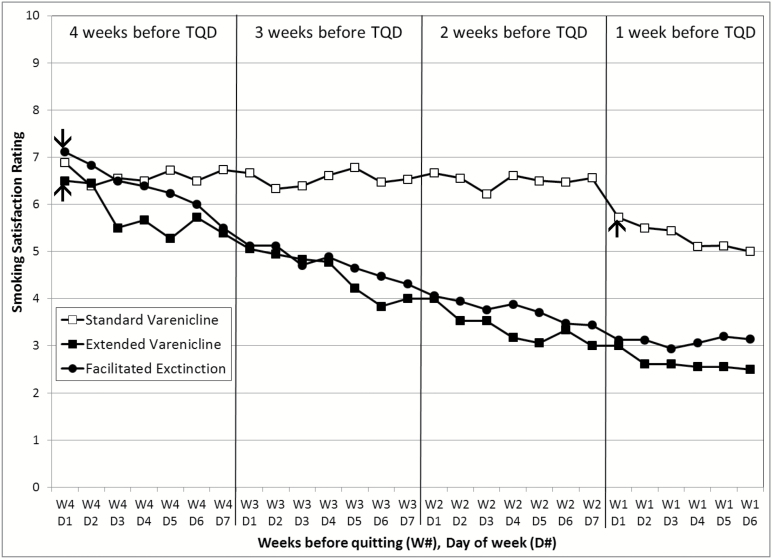
Figure 3 illustrates peak craving ratings across the four pre-quit weeks by treatment group. GEE analyses that compared the first and the last week of the pre-quit period revealed a significant week × group interaction (χ2(2) = 10.76, p = .005). Follow-up analyses showed a significant decrease in the average peak craving from the first to the last week in the EV (χ2(1) = 20.99, p < .0001) and FE (χ2(1) = 11.52, p = .0007) groups, but not the SV group (χ2(1) = 1.42, p = .23), which did not initiate the medication until the start of the last week. A similar pattern emerged for smoking satisfaction ratings (Figure 4). There was a significant week × group interaction (χ2(2) = 16.18, p = .0003), with FE and EV groups exhibiting a greater decrease in smoking satisfaction relative to the SV group.
Figure 3.
Peak craving per day by treatment group during the 4 weeks prior to target quit day. EV = extended Varenicline; FE = facilitated extinction; SV = standard Varenicline; TQD = target quit date. TQD occurred on week 1 (W1), day 7 (D7). Varenicline was started on day 1 of week 4 for the EV and FE conditions and on day 1 of week 1 for the SV condition, as indicated by the arrows.
Figure 4.
Smoking satisfaction per day by treatment group during the 4 weeks prior to target quit day. EV = extended Varenicline; FE = facilitated extinction; SV = standard Varenicline; TQD = target quit date. TQD occurred on week 1 (W1), day 7 (D7). Varenicline was started on day 1 of week 4 for the EV and FE conditions and on day 1 of week 1 for the SV condition, as indicated by the arrows.
Cue Reactivity
Craving ratings in response to smoking cues across the three treatment groups (SV = 18, EV = 19, FE = 17) and 2 laboratory assessments (Lab 1 and Lab 2) were compared using a general linear model, while controlling for neutral cues. Although power to detect differences was limited, Lab 2 smoking cue-reactivity craving ratings were significantly lower than Lab 1 scores [F(2,52) = 26.14, p < .001]. There was no Lab by treatment interaction [F(2,51) = 0.08, p = .92].
Cessation Outcomes
The abstinence rates for the SV, EV, and FE groups (with missing data imputed as smoking) were, respectively, 25%, 37%, and 32%, at 1 month, and were 45%, 58%, and 42% at 3 months post TQD. The abstinence rates for “responders only” (excluding participants who failed to complete the follow-up assessments) were 33%, 37%, and 40% at 1 month, and were 56%, 58%, and 53% at 3 months, for the SV, EV, and FE groups, respectively. Given the small sample size and the associated large confidence intervals ranging from ±19% to ±25%, it is not surprising that analyses of abstinence rates for either approach found no significant group differences in this feasibility study. To put in context, a minimum of 350 participants per group would be needed in a full-scale efficacy trial to detect a group difference of 10% with power >.80.
Discussion
The current study was designed to provide preliminary data regarding the feasibility of a RCT for a novel intervention that may boost the efficacy of pharmacotherapy for the treatment of tobacco dependence. All elements of the pilot feasibility RCT were successfully demonstrated, including demand, practicality, and acceptability. Study completion, retention, and treatment satisfaction were high across all intervention groups. The majority of participants in the novel FE intervention sustained their smoking rate, smoked across a variety of contexts, and used their extinction cue wristband as instructed during the pre-cessation treatment with varenicline, thereby optimizing their theory-based extinction experience. Although we did not find an additional benefit of FE on cue-reactivity or abstinence rates when added to an extended run-in period (EV)—albeit with minimal statistical power—both extended conditions demonstrated reductions in smoking satisfaction and peak craving.
Maintaining participants’ smoking rate during the 4 weeks of pre-cessation varenicline use was a primary goal of the intervention. After the first week on varenicline, smokers tend to reduce their smoking rate due to decreases in craving levels and smoking satisfaction.16 However, in theory, reduced smoking rate also minimizes the number of extinction trials, and consequently the potential success of the extinction process. Hence, the sustained smoking rate in principle was an important process indicator of intervention success in the FE condition.
Based on a number of lessons learned from the current feasibility study, the following points regarding the FE intervention should be considered in any future trial. First, as indicated by several of the participants, it may be helpful to add one or more post-cessation treatment sessions for support after the TQD. Second, in light of the difficulty that some of the participants had with identifying compound and rare cues, instructions for such cues should be clarified and simplified.
The main limitations of the current study are a function of its scope as a feasibility study rather than an efficacy trial. Namely, the sample size was adequate for feasibility testing, but too small for group comparisons. In addition, follow-up was only through 3 months post-TQD, corresponding to the end of varenicline dosing, and shorter than traditional smoking cessation RCTs. Although it may be tempting to interpret the lack of cessation outcome differences across conditions, those statistics were highly unstable and overly influenced by chance events among participants, due to the limited power of this feasibility study. We presented those results in the interest of full disclosure only. The primary difference between the two extended medication conditions was the sustained pre-cessation smoking rate of the FE group relative to the reduced rate of the EV group. A fully powered RCT is needed to test the impact of the greater number of extinction trials experienced by the FE group on the distal cessation outcome. However, it is interesting that both the responder-only and intent-to-treat abstinence rates improved between the 1-month and 3-month follow-up points. Although the opposite trend is typically found in smoking cessation trials, this pattern of rising point-prevalence abstinence rates during the course of varenicline administration has been previously reported,2 indicating a significant delayed-quitting benefit of varenicline.41 This finding is also consistent with the notion of continued extinction occurring when smoking while on this medication. Potential delayed-quitting benefit of additional extinction trials produced by the FE intervention can be tested in a trial with a longer follow-up period. In summary, results from the current study support the feasibility of testing the efficacy of the novel FE smoking cessation intervention in a fully powered clinical trial.
Funding
This study was funded by the James and Esther King Biomedical Research Program Grant #4KB05.
Declaration of Interests
Thomas H. Brandon has served of the Varenicline Advisory Board for Pfizer and consulted on the development of the online behavioral adjuvant for varenicline users. David J. Drobes serves as a paid expert witness in litigation against tobacco companies.
Acknowledgments
Study medication was provided by Pfizer, Inc. via Investigator Initiated Research Agreement #WI176726. The authors thank Paul M. Cinciripini for his consultation on this project. We also thank John Correa and Michelle Kovacs for their work on this project.
References
- 1. Gonzales D, Rennard SI, Nides Met al. ; Varenicline Phase 3 Study Group Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):47–55. [DOI] [PubMed] [Google Scholar]
- 2. Jorenby DE, Hays JT, Rigotti NAet al. ; Varenicline Phase 3 Study Group Efficacy of varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):56–63. [DOI] [PubMed] [Google Scholar]
- 3. Nides M, Oncken C, Gonzales Det al. . Smoking cessation with varenicline, a selective alpha4beta2 nicotinic receptor partial agonist: results from a 7-week, randomized, placebo- and bupropion-controlled trial with 1-year follow-up. Arch Intern Med. 2006;166(15):1561–1568. [DOI] [PubMed] [Google Scholar]
- 4. Oncken C, Gonzales D, Nides Met al. . Efficacy and safety of the novel selective nicotinic acetylcholine receptor partial agonist, varenicline, for smoking cessation. Arch Intern Med. 2006;166(15):1571–1577. [DOI] [PubMed] [Google Scholar]
- 5. Ray LA, Lunny K, Bujarski S, Moallem N, Krull JL, Miotto K. The effects of varenicline on stress-induced and cue-induced craving for cigarettes. Drug Alcohol Depend. 2013;131(1-2):136–142. [DOI] [PubMed] [Google Scholar]
- 6. West R, Baker CL, Cappelleri JC, Bushmakin AG. Effect of varenicline and bupropion SR on craving, nicotine withdrawal symptoms, and rewarding effects of smoking during a quit attempt. Psychopharmacology (Berl). 2008;197(3):371–377. [DOI] [PubMed] [Google Scholar]
- 7. Brandon TH, Drobes DJ, Unrod Met al. . Varenicline effects on craving, cue reactivity, and smoking reward. Psychopharmacology (Berl). 2011;218(2):391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Patterson F, Jepson C, Strasser AAet al. . Varenicline improves mood and cognition during smoking abstinence. Biol Psychiatry. 2009;65(2):144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gass JC, Wray JM, Hawk LW, Mahoney MC, Tiffany ST. Impact of varenicline on cue-specific craving assessed in the natural environment among treatment-seeking smokers. Psychopharmacology (Berl). 2012;223(1):107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hawk LW Jr, Ashare RL, Lohnes SFet al. . The effects of extended pre-quit varenicline treatment on smoking behavior and short-term abstinence: a randomized clinical trial. Clin Pharmacol Ther. 2012;91(2): 172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hawk LW Jr, Ashare RL, Rhodes JD, Oliver JA, Cummings KM, Mahoney MC. Does Extended Pre Quit Bupropion Aid in Extinguishing Smoking Behavior?Nicotine Tob Res. 2015;17(11):1377–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Levin ED, Westman EC, Stein RMet al. . Nicotine skin patch treatment increases abstinence, decreases withdrawal symptoms, and attenuates rewarding effects of smoking. J Clin Psychopharmacol. 1994; 14(1):41–49. [PubMed] [Google Scholar]
- 13. Rose JE, Behm FM, Westman EC, Levin ED, Stein RM, Ripka GV. Mecamylamine combined with nicotine skin patch facilitates smoking cessation beyond nicotine patch treatment alone. Clin Pharmacol Ther. 1994; 56(1):86–99. [DOI] [PubMed] [Google Scholar]
- 14. Rose JE, Behm FM, Westman EC, Kukovich P. Precessation treatment with nicotine skin patch facilitates smoking cessation. Nicotine Tob Res. 2006;8(1):89–101. [DOI] [PubMed] [Google Scholar]
- 15. Rose JE, Herskovic JE, Behm FM, Westman EC. Precessation treatment with nicotine patch significantly increases abstinence rates relative to conventional treatment. Nicotine Tob Res. 2009;11(9):1067–1075. [DOI] [PubMed] [Google Scholar]
- 16. Hajek P, McRobbie HJ, Myers KE, Stapleton J, Dhanji AR. Use of varenicline for 4 weeks before quitting smoking: decrease in ad lib smoking and increase in smoking cessation rates. Arch Intern Med. 2011;171(8):770–777. [DOI] [PubMed] [Google Scholar]
- 17. Ashare RL, Tang KZ, Mesaros AC, Blair IA, Leone F, Strasser AA. Effects of 21 days of varenicline versus placebo on smoking behaviors and urges among non-treatment seeking smokers. J Psychopharmacol. 2012;26(10):1383–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rennard S, Hughes J, Cinciripini PMet al. ; Flexible Quit Date Study Group A randomized placebo-controlled trial of varenicline for smoking cessation allowing flexible quit dates. Nicotine Tob Res. 2012;14(3):343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brandon TH, Vidrine JI, Litvin EB. Relapse and relapse prevention. Annu Rev Clin Psychol. 2007;3(1):257–284. [DOI] [PubMed] [Google Scholar]
- 20. Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97(2):155–167. [DOI] [PubMed] [Google Scholar]
- 21. Bouton ME A learning theory perspective on lapse, relapse, and the maintenance of behavior change. Health Psychol. 2000;19(1S):57–63. [DOI] [PubMed] [Google Scholar]
- 22. Foa EB Prolonged exposure therapy: past, present, and future. Depress Anxiety. 2011;28(12):1043–1047. [DOI] [PubMed] [Google Scholar]
- 23. Laborda MA, McConnel BL, Miller RR. Behavioral techniques to reduce relapse after exposure therapy: applications of studies of experimental extinction. In TR, Schachtman, S, Rielly, eds. Associative Learning and Conditioning Theory: Human and Non-Human Applications. New York, NY: Oxford University Press; 2011:79–103. [Google Scholar]
- 24. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström test for nicotine dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. [DOI] [PubMed] [Google Scholar]
- 25. Hofmann SG Cognitive processes during fear acquisition and extinction in animals and humans: implications for exposure therapy of anxiety disorders. Clin Psychol Rev. 2008;28(2):199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bouton ME Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry. 2002;52(10):976–986. [DOI] [PubMed] [Google Scholar]
- 27. Bouton ME, Todd TP, Vurbic D, Winterbauer NE. Renewal after the extinction of free operant behavior. Learn Behav. 2011;39(1):57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Collins BN, Brandon TH. Effects of extinction context and retrieval cues on alcohol cue reactivity among nonalcoholic drinkers. J Consult Clin Psychol. 2002;70(2):390–397. [PubMed] [Google Scholar]
- 29. Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev. 2004;111(1):33–51. [DOI] [PubMed] [Google Scholar]
- 30. Shiffman S, Balabanis MH, Gwaltney CJet al. . Prediction of lapse from associations between smoking and situational antecedents assessed by ecological momentary assessment. Drug Alcohol Depend. 2007;91(2–3):159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rescorla RA Deepened extinction from compound stimulus presentation. J Exp Psychol Anim Behav Process. 2006;32(2):135–144. [DOI] [PubMed] [Google Scholar]
- 32. Brooks DC, Bouton ME. A retrieval cue for extinction attenuates response recovery (renewal) caused by a return to the conditioning context. Exp Psychol Anim Behav Process. 1994;20(4):366–379. [Google Scholar]
- 33. Biener L, Abrams DB. The Contemplation Ladder: validation of a measure of readiness to consider smoking cessation. Health Psychol. 1991;10(5):360–365. [DOI] [PubMed] [Google Scholar]
- 34. Sheehan DV, Lecrubier Y, Sheehan KHet al. . The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33;quiz 34. [PubMed] [Google Scholar]
- 35. Attkisson CC, Greenfield, TK. Client satisfaction questionnaire-8 and service satisfaction scale-30. In M. E., Maruish, ed. The Use of Psychological Testing for Treatment Planning and Outcome Assessment. Hillsdale, NJ: Lawrence Erlbaum Associates; 1994:402–420 [Google Scholar]
- 36. Drobes DJ Cue reactivity in alcohol and tobacco dependence. Alcohol Clin Exp Res. 2002;26(12):1928–1929. [DOI] [PubMed] [Google Scholar]
- 37. Carter BL, Robinson JD, Lam CYet al. . A psychometric evaluation of cigarette stimuli used in a cue reactivity study. Nicotine Tob Res. 2006;8(3):361–369. [DOI] [PubMed] [Google Scholar]
- 38. Gilbert DG, Rabinovich NE.. International Smoking Images Series (With Neutral Counterparts), (version 1.2 Carbondale). Integrative Neuroscience Laboratory, Department of Psychology, Southern Illinois University; 2003. [Google Scholar]
- 39. Center for the Study of Emotion and Attention The International Affective Picture System [photographic slides]. Gainesville, FL: The Center for Research in Psychophysiology, University of Florida; 1995. [Google Scholar]
- 40. Brown RA, Burgess ES, Sales SD, Whiteley JA, Evans DM, Miller IW. Reliability and validity of a smoking timeline follow-back interview. Psychol Addict Behav. 1998;12:101–112. [Google Scholar]
- 41. Gonzales D, Jorenby DE, Brandon TH, Arteaga C, Lee TC. Immediate versus delayed quitting and rates of relapse among smokers treated successfully with varenicline, bupropion SR or placebo. Addiction. 2010;105(11):2002–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]