Abstract
Aims
This study sought to compare mice bred to preferentially consume high amounts of alcohol (crossed-high alcohol preferring, cHAP) to c57BL/6 (C57) mice using a chronic-binge ethanol ingestion model to induce alcoholic liver disease (ALD).
Methods
Male C57 and cHAP mice were randomized to a Lieber-DeCarli control (LDC) diet, Lieber-DeCarli 5% (v/v) ethanol (LDE) diet or free-choice between 10% (v/v) ethanol in drinking water (EtOH-DW) and DW. After 4 weeks mice were gavaged with either 9 g/kg maltose-dextrin (LDC+MD) or 5 g/kg EtOH (LDE+Binge, EtOH-DW+Binge). Nine hours later tissue and serum were collected and analyzed.
Results
cHAP mice on EtOH-DW consumed significantly more ethanol than cHAP or C57 mice maintained on LDE. However, cHAP and C57 mice on the LDE+Binge regiment had greater hepatosteatosis and overall degree of liver injury compared to EtOH-DW+Binge. Changes in pro-inflammatory gene expression was more pronounced in cHAP mice than C57 mice. Analysis of liver enzymes revealed a robust induction of CYP2E1 in C57 and cHAP mice maintained on EtOH-DW+Binge or LDE+Binge. However, while C57 mice exhibited higher basal hepatic glutathione than cHAP mice, these mice appeared more susceptible to oxidative stress following LDE+Binge than cHAP counterparts.
Conclusions
Despite cHAP mice consuming more total ethanol prior to gavage when maintained on EtOH-DW, LDE followed by gavage created a more severe model of ALD in both C57 and cHAP mice. These data suggest factors other than total amount of alcohol consumed affect degree of ALD development in the chronic-binge model in cHAP mice.
Short Summary
cHAP mice voluntarily consume high amounts of ethanol and exhibited hepatic injury when subject to chronic-binge ethanol feeding with the Lieber-DeCarli diet. However, hepatic injury was reduced in cHAP mice in a chronic-binge model following voluntary high ethanol consumption in drinking water.
Keywords: Ethanol, Liver, crossed high alcohol preferring, Hepatic steatosis, CYP2E1
INTRODUCTION
Alcoholic liver disease (ALD) is a global health burden and refers to a disease spectrum ranging from hepatomegaly and simple fatty liver (hepatic steatosis), to more severe pathologies such as alcoholic steatohepatitis and hepatic cirrhosis (Gao and Bataller, 2011; McKillop et al., 2016). Within the USA ~50% of the population consume ethanol and ~38 million people are estimated to engage in binge drinking behavior (Shukla et al., 2013). Uncorrected, chronic high ethanol consumption (>30 g/day over 10 years) can progress to liver fibrosis and cirrhosis, hepatic cirrhosis representing the most common underlying pathology in which hepatocellular carcinoma (HCC) arises (Corrao et al., 2004; McKillop et al., 2016).
Numerous animal models have been employed to mimic human ALD with varying degrees of success (Brandon-Warner et al., 2012a). Baboons maintained on ethanol in drinking-water develop ALD through all stages of progression over 3–4 years, and most closely resemble human ALD pathogenesis (Lieber et al., 1985). Rodent models of ALD offer an alternative to primates, but are not without limitations. Mice and rats have an inherent aversion to ethanol but will, to varying degrees, consume ethanol for its nutritional value. Under free choice, most rodent models stop consuming ethanol when blood-acetaldehyde levels rise, and rodents exhibit higher basal metabolism and ethanol catabolism than humans (Brandon-Warner et al., 2012a). Thus, achieving blood-alcohol content (BAC) sufficient to induce liver injury requires modifications to ethanol regimens (Chester et al., 2003).
At the simplest level, increased ethanol consumption is achieved by restricting liquid availability with drinking water (DW) containing ethanol (EtOH-DW). While EtOH-DW is straightforward and allows normal food intake, the aversion most rodents have to ethanol means that to create sufficient BACs to affect liver pathology alternating higher with lower EtOH-DW levels is required (Yip-Schneider et al., 2011). An alternative to EtOH-DW is the Lieber-DeCarli liquid diet in which a pair-fed isocaloric control diet (LDC), or an ethanol-containing diet, wherein calories provided by sucrose are replaced by 5% ethanol (LDE), is employed (Lieber and DeCarli, 1982). While LDE reproducibly induces hepatic steatosis, it is labor intensive (requiring daily diet preparation of LDE and control [no alchohol] diets (LDC) and pair-matched feeding) and costly (in terms of both diets per se, and the requirement for specialized feeding tubes and cages) compared to EtOH-DW. Moreover, progression to more severe ALD with LDE does not occur without a secondary hepatic stress (Karaa et al., 2008). To achieve more significant hepatic injury the Tsukamoto-French (TF) model (employing intragastric ethanol infusion) was developed, and produces marked elevation of liver enzymes, steatosis and mild fibrosis (Tsukamoto et al., 1985). However, the TF model requires surgical expertise and extensive animal monitoring (Kono et al., 2000; de la M Hall et al., 2001), factors that can limit adoption.
Alternative rodent models have been explored to mimic human ALD. A mouse model of chronic ethanol feeding (10–28 days LDE), followed by single binge administration, termed the ‘NIAAA chronic-binge model’, is characterized by marked elevation of serum transaminases and macrovesicular steatosis (Bertola et al., 2013; Xu et al., 2015). These results are in contrast to LDE (4 weeks) alone, which results in mild steatosis and a moderate elevation of serum transaminases (Petrasek et al., 2012). Rodent models of voluntary, high ethanol consumption have also been explored in an attempt to model human ethanol consumption (Grahame et al., 1999; Kampov-Polevoy et al., 2000). Voluntary ethanol consumption has potential benefits over involuntary approaches, including reduced labor (daily diet preparation and feeding tube change out), increased ethanol consumption over time, cost (the absence of specialized diets, feeding tubes and cages), and/or surgical skills. Previous studies report ethanol preferring rats provided long-term (18 months), voluntary access to 10% (v/v) EtOH-DW develop HCC in the absence of cirrhosis (Yip-Schneider et al., 2011). Crossed high alcohol preferring (cHAP) mice have also been developed through initial selective breeding of high alcohol preferring lines from an eight-way inbred strain cross, which includes C57Bl/6J among seven other strains (Grahame et al., 1999). The crossed, or cHAP line was further selectively bred for high drinking from a cross of two HAP lines and drinks more than either parent line (Oberlin et al., 2011). The cHAP mice voluntarily consume high amounts of ethanol (~25 g/kg/d), achieve mean BACs ≥ 250 mg/dl, and exhibit patterns of consumption similar to humans, with increased intake over time (Matson and Grahame, 2013). However, a 4-week regimen of voluntary 10% EtOH-DW failed to produce significant liver pathology in cHAP mice (Matson et al., 2013).
The aim of this study was to determine whether the cheaper, less labor-intensive cHAP mouse model, when subjected to an NIAAA chronic-binge ethanol regimen, demonstrated equivalent ALD to pair-matched c57BL/6 (C57) mice. Additionally, because cHAP mice exhibit escalating ethanol consumption over time, consume ethanol in parallel with normal dietary intake, and exhibit higher levels of daily ethanol consumption than C57 mice maintained on LDE, this model may provide an additional rodent model to study the effects of ethanol on hepatic pathology that more closely parallels human patterns of alcohol consumption.
MATERIALS AND METHODS
Animals and assurances
Male C57BL/6J (C57) mice were purchased from Jackson Laboratories (Bar Harbor, ME). A breeding colony of cHAP mice was established at CMC from founders provided by IUPUI (Indianapolis, IN) (Matson et al., 2013). All studies were approved by the Institutional Animal Care and Use Committee (CMC) and conformed to the NIH Guidelines for Care and Use of Laboratory Animals.
Materials
Lieber-DeCarli-EtOH diets (LDE; 5% (v/v) ethanol), isocaloric pair-matched liquid control diets (LDC), and maltose-dextrin were purchased from Bio-Serv (Flemington, NJ). A standard rodent chow diet (Teklad Rodent Diet #8604) was purchased from Envigo (Cambs, UK). An ethanol detection kit was purchased from Biovision (Milpitas, CA). Assays to measure alanine transaminase (ALT) and aspartate transaminase (AST) were purchased from Bioo Scientific (Austin, TX). Nicotinamide adenine dinucleotide phosphate (NADPH), 4-nitrophenol, 4-nitrocatechol, perchloric acid and sodium hydroxide were purchased from Sigma-Aldrich (St. Louis, MO). Assay kits to measure triglycerides, thiobarbituric acid reactive species (TBARS) and glutathione (GSH) were purchased from Cayman Chemical (Ann Arbor, MI). Monoclonal antibodies to perform immunohistochemistry (IHC) for detection of neutrophils (rat anti-mouse Ly6G/-GC [NIMP-R14], Cat. # ab2557) and macrophages (anti-F4/80 [CI:A3-1], Cat. # ab6640) were purchased from Abcam (Cambridge, MA). Antibodies against alcohol dehydrogenase (ADH, Cat. #22,750) and acetaldehyde dehydrogenase (ALDH, Cat. # 166,362) were purchased from Santa Cruz Biotechnology Inc. (Dallas, TX), and an antibody against cytochrome P450 2E1 (CYP2E1, Cat. # AB1252) was purchased from Millipore (Billerica, MA).
Feeding models
Five-week old male C57 and cHAP mice were randomized to receive either LDC, LDE or free choice between DW and 10% (v/v) ethanol in DW (EtOH-DW). Mice in LDC and LDE groups were acclimatized to liquid diets for 5 days with ad libitum access to the LDC diet prior to being randomized for LDC or LDE for a further 4 weeks. LDC mice were pair-matched for LDC availability to an amount of diet equal to the mean amount of LDE consumed. Mice in the EtOH-DW group were provided with two water bottles, one containing 10% (v/v) ethanol in DW, the other DW alone. Water bottles were changed out every 2 days with freshly prepared EtOH-DW or DW. Throughout the study animals were maintained on a standard rodent chow diet (Teklad Rodent Diet #8604). Liquid diet (LDE) and voluntary ethanol ingestion (EtOH-DW) consumption was measured daily, and body weights measured twice weekly. After 4 weeks, mice received either a single gavage of 5 g/kg ethanol (LDE+Binge and EtOH-DW+Binge) or 9 g/kg maltose dextrin (LDC+MD group).
Necropsy
At experiment completion mice were anesthetized (isoflurane by inhalation) and sacrificed by exsanguination. Livers were excised, weighed and representative liver sections (4–6 mm) taken from the left, right, median and anterior lobes. Tissue was either snap-frozen in liquid nitrogen or fixed in 10% neutral buffered formalin (24 h) for processing and analysis (Brandon-Warner et al., 2012a).
Enzyme analysis and histology
Serum was separated by centrifugation (1800×g, 4°C) and ALT/AST measured as per the manufacturer’s instructions. Hepatic lipid peroxidation was measured in liver tissue homogenates using a TBARS kit. Hepatic GSH levels were determined by colorimetric analysis of liver tissue lysates (collected in a 2-(N-morpholino) ethanesulfonic acid buffer) using a GSH assay kit.
CYP2E1 activity was determining by measuring oxidation of 4-nitrophenol to 4-nitrocatechol as previously reported (Brandon-Warner et al., 2010). Briefly, ≈50 mg of liver tissue was homogenized in 1 M KH2PO4 buffer and protein concentration determined and equalized. Homogenates (25 mg/ml) were then incubated with 20 mM 4-nitrophenol and 1 mM NADPH for 30 min (37°C). The reaction was terminated by deproteination with 60% (v/v) 1 N perchloric acid to a final volume of 1%. Samples were centrifuged for 10 minutes at 10,000×g, supernatants removed, 10μl of 10 M NaOH added to each sample, and samples read on a spectrophotometer (540 nm). CYP2E1 activity was determined by calculation against a standard curve of known 4-nitrocatechol concentrations.
Multiple liver lobes were sectioned (6 μm) and stained with Mayer’s hematoxylin and eosin (H&E), or Picrosirius red (Brandon-Warner et al., 2012a). Representative sections (5 fields/section) were blind-scored for steatosis, necrosis, inflammation and fibrosis using a modified scoring scale (0–4) (Morgan et al., 2002; Brandon-Warner et al., 2012b), and a total liver injury score (TLIS) calculated (Brandon-Warner et al., 2012a).
RT-qPCR
Total hepatic RNA was reverse-transcribed to cDNA using an Improm-II reverse transcription system (Promega; Madison, WI). Quantitative RT-PCR (qRT-PCR) was performed on 50 ng cDNA using an iQ SYBR Green Supermix (Thermo Fisher Scientific, Grand Island, NY) and gene-specific oligonucleotide primers (Supplementary Table 1). Relative mRNA levels for inflammatory genes (interleukin (IL) −6, −10, −17A, −22 and tumor necrosis factor-α (TNF-α)), and fibrotic genes (Type1α2 collagen (Col(I)α2), α-smooth muscle actin (α-SMA), and transforming growth factor-β (TGFβ)) were calculated and normalized to glyceraldehyde-3-phosphate dehydrogenase.
Immunohistochemistry
Sections were prepared and IHC performed on formalin-fixed, paraffin-embedded liver tissue using antibodies specific against either Ly6G/-GC (neutrophil marker) or F4/80 (macrophage marker).
Immunoblotting
Liver tissue (≈80–100 mg) was homogenized and sonicated in radioimmunoprecipitation assay buffer and resolved by SDS-PAGE. Following transfer to nitrocellulose, membranes were stained with Ponceau S stain to confirm equal protein loading. Immunoblot was performed using antibodies against ADH, ALDH or CYP2E1 (1:1000 dilution), densitometric analysis performed, and protein expression corrected to Ponceau S staining/loading (Yip-Schneider et al., 2011).
Triglyceride Assay
Liver tissue (≈100 mg) was homogenized in 250 μl standard diluent assay reagent in the presence of protease inhibitors, and triglyceride content determined using a commercial kit as per the manufacturer’s protocol.
Statistics
Data are presented as mean ± SEM with n = 7 animals per experimental group. Two-way ANOVA was performed with Bonferroni post hoc test using Prism-GraphPad software (La Jolla, CA). P < 0.05 was considered significant.
RESULTS
Ethanol consumption and phenotype characteristics
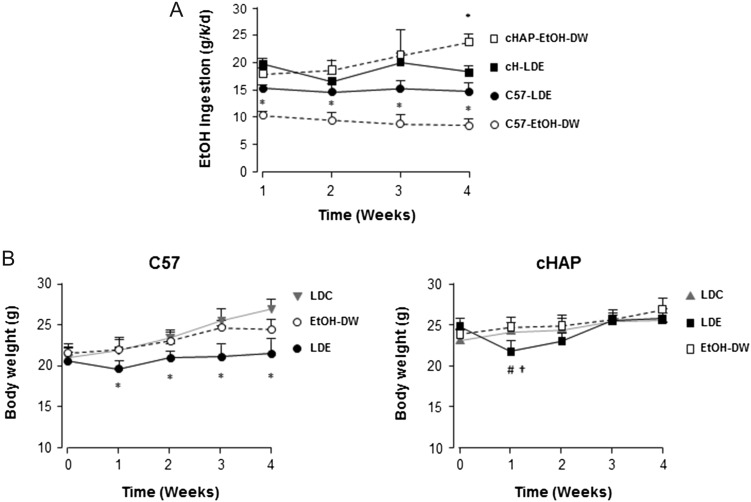
C57 mice maintained on the EtOH-DW regimen consumed significantly less ethanol compared to pair-matched C57-LDE mice and cHAP mice maintained on either LDE or EtOH-DW (Fig. 1A, *P < 0.05 C57 EtOH-DW versus all other groups). Within the cHAP groups, mice consumed approximately equal amounts of ethanol for LDE versus EtOH-DW regimens after the first week (Fig. 1A). However, while those on the LDE diet consumed a relatively constant amount of ethanol over the following 3 weeks of the study, those on the EtOH-DW option consumed increasing amounts of ethanol as the study progressed, such that by the end of the 4-week experimental period, cHAP EtOH-DW mice were consuming significantly more ethanol than all other animals (Fig. 1A, *P < 0.05 cHAP EtOH-DW versus all other groups). A two-way ANOVA demonstrated differences in ethanol consumption were attributable to strain. Analysis of BAC at euthanasia demonstrated significantly elevated BAC in C57 LDE+Binge animals compared to all other experimental groups. (Table 1, *P < 0.05 C57 LDE+Binge versus all other groups). BAC was significantly higher in cHAP mice maintained on LDE+Binge compared to EtOH-DW+Binge (Table 1, †P < 0.05 cHAP LDE+Binge versus EtOH-DW+Binge)
Fig. 1.
Ethanol consumption and effect on body weight in different mouse strains. (A) Weekly ethanol consumption for C57 (C57) and cHAP mice on Lieber-DeCarli control liquid diet (LDC), Lieber-DeCarli ethanol liquid diet (LDE) or free choice between 10% ethanol (v/v) in DW and DW (EtOH-DW). *P < 0.05 versus all other groups. (B) Weight change of C57 (left) and cHAP (right) mice over the experimental time course following initiation on ethanol feeding regimens. *P < 0.05 versus all other groups, #P < 0.05 strain-matched LDE versus LDC, †P < 0.05 strain-matched LDE versus EtOH-DW.
Table 1.
Analysis of tissue and serum markers of ALD
| C57 | cHAP | |||||
|---|---|---|---|---|---|---|
| LDC+MD | LDE+B | EtOH-DW+B | LDC+MD | LDE+B | EtOH-DW+B | |
| BAC (mM) | ND | 81.2 ± 9.9*# | 8.5 ± 3.9# | ND | 13.1 ± 2.3#†‡ | 6.5 ± 1.4# |
| TBARS (μM) | 12.8 ± 2.0 | 22.0 ± 5.6* | 12.2 ± 0.9 | 9.4 ± 0.9‡ | 9.3 ± 0.9‡ | 11.3 ± 0.6 |
| GSH (μM) | 20.7 ± 1.1* | 11.5 ± 1.6# | 13.9 ± 0.5# | 14.2 ± 1.4‡ | 11.5 ± 0.9# | 13.1 ± 0.8 |
| Liver:BW | 0.036 ± 0.002 | 0.040 ± 0.002 | 0.040 ± 0.002 | 0.037 ± 0.002 | 0.044 ± 0.003# | 0.048 ± 0.003† |
| ALT (IU/l) | 21.3 ± 4.9 | 108.7 ± 23.1#† | 48.2 ± 6.7 | 30.3 ± 4.6 | 119.6 ± 18.7#† | 59.0 ± 22.7 |
| AST (IU/l) | 114.5 ± 32.4 | 300.4 ± 74.2#† | 136.1 ± 33.2 | 143.3 ± 32.4 | 259.0 ± 35.7#† | 169.7 ± 40.7 |
| TLIS | 0.5 ± 0.1 | 2.7 ± 0.4#† | 1.5 ± 0.3# | 0.4 ± 0.3 | 2.9 ± 0.1#† | 0.9 ± 0.4 |
BW, body weight; ALT, alanine aminotransferase; AST, aspartate aminotransferase. *P < 0.05 versus all other groups, #P < 0.05 strain-matched versus LDC+MD, †P < 0.05 strain-matched LDE+B versus EtOH-DW+B, ‡P < 0.05 cHAP versus pair-matched C57. ND, not detected. Serum or tissue was collected from c57BL/6 (C57) and cHAP mice 4 weeks after maintenance of a control Lieber-DeCarli diet + maltose-dextrin (LDC+MD), ethanol containing Lieber-DeCarli diet + binge (LDE+B) or free choice between 10% ethanol in DW and DW + binge (EtOH-DW+B)
Analysis of tissue lipid peroxidation (malondialdehyde [MDA], reported as thiobarbituric acid reactive substances [TBARS]) demonstrated significantly increased TBARS levels in C57 mice maintained on the LDE+Binge regimen compared to all other animals (Table 1, *P < 0.05 C57 EtOH-DW+Binge versus all other groups). Comparing cHAP to C57 mice, TBARS levels were significantly lower in cHAP mice maintained on either LDC+MD or LDE+Binge regimens compared to pair-matched C57 mice (Table 1, ‡P < 0.05 cHAP versus treatment-matched C57). Conversely, there was no difference in TBARS between cHAP and C57 mice maintained on EtOH-DW+Binge (Table 1). Measurement of GSH revealed significantly higher GSH levels in C57 LDC+MD mice compared to all other animals (Table 1, *P < 0.05 versus all other groups). Ethanol consumption via LDE+Binge or EtOH-DW+Binge significantly decreased GSH in C57 mice with no difference in GSH levels between those on LDE+Binge versus those on EtOH-DW+Binge regimens (Table 1, #P < 0.05 C57 LDE+Binge and EtOH-DW+Binge versus C57 LDC+MD). In contrast, GSH levels were only significantly reduced in cHAP mice maintained on LDE+Binge regimens versus LDC+MD (Table 1, #P < 0.05 cHAP LDE+Binge versus C57 LDC+MD).
At study initiation, despite age matching, cHAP mice were significantly heavier than C57 counterparts (Fig. 1B). Over the 4-week course of the study differences in body weight between cHAP and C57 mice became indistinguishable for all groups, with the exception of C57 LDE mice, which remained significantly lighter compared to all other animals (Fig. 1B, *P < 0.05, C57 LDE versus all other groups). Of note, Both the C57 and cHAP mice in the LDE group lost weight over the first week of the LDE diet, but thereafter gained weight, the cHAP mice doing so more readily than the C57 mice (Fig. 1B). In contrast, C57 and cHAP mice on the EtOH-DW regimen gained weight in parallel to that of animals in the LDC diet group (Fig. 1B).
At necropsy there was no apparent morbidity or gross hepatic pathology in any of the groups. Following liver removal, no significant differences in liver-body weight (L-BW) ratios were measured within the C57 experimental groups (Table 1). In contrast, L-BW ratios were significantly increased in cHAP mice maintained on LDE+Binge or EtOH-DW+Binge regimens compared to those maintained on LDC+MD (Table 1, #P < 0.05 cHAP LDE+Binge and EtOH-DW+Binge versus cHAP LDC+MD).
Ethanol feeding model determines degree of liver damage
ALT and AST levels were significantly elevated in C57 and cHAP mice maintained on the LDE+Binge diet compared to pair-matched C57 and cHAP maintained on LDC+MD diet or EtOH-DW+Binge regimens (Table 1, #P < 0.05 strain-matched LDE+Binge versus LDC+MD mice, †P < 0.05 strain-matched LDE+Binge versus EtOH-DW+Binge mice).
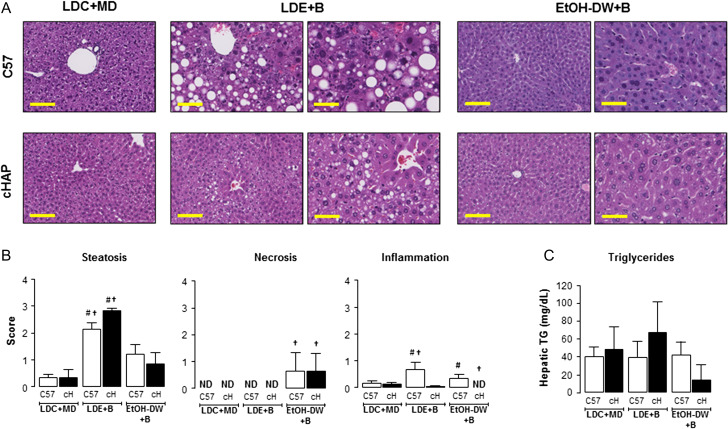
To further characterize differences between strains and feeding regimens, we blind-scored liver sections for steatosis, necrosis, inflammation and fibrosis (Fig. 2 and Supplementary Fig. S1). Both C57 and cHAP mice in the LDE+Binge and EtOH-DW+Binge groups were characterized by higher hepatic steatosis scoring than those maintained on the LDC+MD diet (Fig. 2A and B). However, the steatosis observed in EtOH-DW+Binge mice was characterized by predominantly microvesicular steatosis, while the steatosis in animals maintained on the LDE+Binge diet featured widespread microvesicular and macrovesicular steatosis (Fig. 2A). Both the C57 and cHAP mice in the LDE+Binge group had significantly higher steatotic scoring than strain-matched counterparts maintained on EtOH-DW+Binge (Fig. 2B, #P < 0.05 strain-matched LDE+Binge versus LDC+MD, and †P < 0.05 strain-matched LDE+Binge versus EtOH-DW+Binge). In an attempt to further analyze, the degree of hepatic steatosis we measured total triglycerides in tissue lysates prepared from frozen tissue. Using this approach, despite histological evidence/blind scoring data to the contrary, we failed to detect any significant difference in total hepatic triglycerides between any of the groups, with a wide degree of variability measured between samples within experimental groups (Fig. 2C).
Fig. 2.
Lieber-DeCarli ethanol + binge regimen induces the highest degree of liver injury independent of strain. (A) Representative images of liver sections from C57BL/6 (C57) and cHAP mice stained with hematoxylin and eosin (H&E). Mice were maintained on Lieber-DeCarli control liquid diet + maltose-dextrin (LDC+MD), Lieber-DeCarli ethanol liquid diet + Binge (LDE+B), or free access to 10% (v/v) ethanol in DW + binge (EtOH-DW+B). Yellow bars = 100 μm. (B) Representative sections from C57 and cHAP (cH) mice were blind scored for steatosis, inflammation and necrosis. (C) Triglyceride (TG) levels measured in hepatic tissue lysates. #P < 0.05 strain-matched LDE+B or EtOH-DW+B versus LDC+MD. †P < 0.05 strain-matched LDE+B versus EtOH-DW+B. ND, not detected.
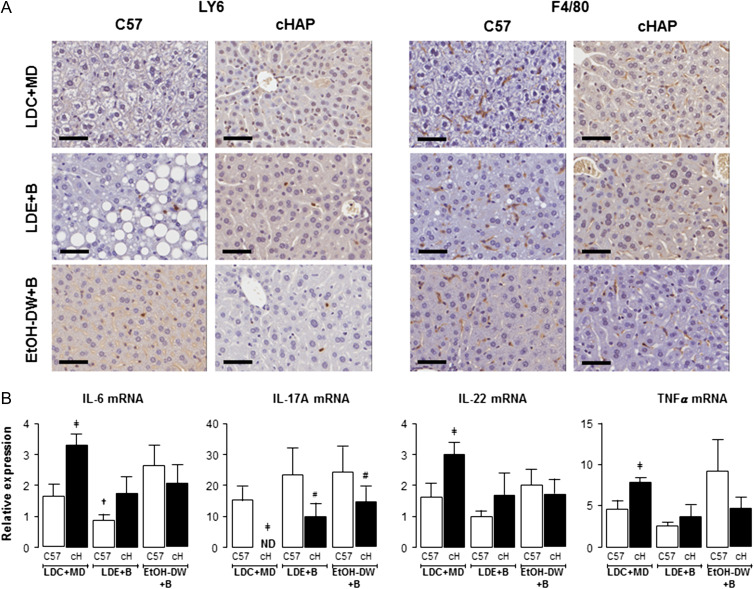
Hepatic necrosis scoring revealed the absence of necrosis in C57 and cHAP LDC+MD and LDE+Binge mice, and a relatively low incidence/level of necrosis in C57 and cHAP mice maintained on EtOH-DW+Binge (Fig. 2B). As with necrosis, inflammatory infiltration scoring was relatively low for all groups, although inflammation scoring revealed significant increases in inflammatory infiltration in C57 mice maintained on LDE+Binge or EtOH-DW+Binge regimens compared to the C57 LDC+MD group (Fig. 2B, #P < 0.05 C57 LDE+Binge versus C57 LDC+MD, †P < 0.05 C57 LDE+Binge versus C57 EtOH-DW+Binge). Because the level of scoring was low (compared to steatotic scoring) we attempted to further address potential changes in inflammation using IHC and qRT-PCR. Using IHC we noted neutrophil staining (Ly6-positive cells) was significantly increased in cHAP (0.70 ± 0.10) and C57 (0.66 ± 0.07) mice maintained on the LDE+Binge regimen compared to LDC+MD (0.06 ± 0.05, C57; 0.12 ± 0.04, cHAP) and EtOH-DW+Binge (0.29 ± 0.10, C57; 0.46 ± 0.07, cHAP) counterparts, with no difference between strains (Fig. 3A). Conversely, there was no observable difference in macrophage staining (F4/80-positive cells) between experimental groups (Fig. 3A).
Fig. 3.
Effect of ethanol feeding on immune response varies depending on strain and ethanol feeding model. (A) Representative images of liver sections from C57BL/6 and cHAP mice following IHC to detect neutrophils (anti-LY6g antibody) and macrophages (anti-F4/80 antibody). Mice were maintained on either Lieber-DeCarli control liquid diet + maltose-dextrin (LDC+MD) (LDC), Lieber-DeCarli ethanol liquid diet+binge (LDE+B) or free access 10% (v/v) ethanol in DW + binge (EtOH-DW+B). Black bars = 50μm. (B) Hepatic IL-6, IL-17A, IL-22, and TNF-α mRNA was measured by qRT-PCR. ‡P < 0.05 cHAP LDC+MD versus C57 LDC+MD, #P < 0.05 strain-matched LDE+B or EtOH-DW+B versus LDC+MD. †P < 0.05 strain-matched LDE+B versus EtOH-DW+B. ND, not detected.
Changes in inflammatory gene expression were assayed by qRT-PCR. For animals maintained on LDC+MD regimens cHAP mice exhibited higher IL-6, IL-22 and TNFα mRNA than c57 counterparts (Fig. 3B, ‡P < 0.05 cHAP LDC+MD versus C57 LDC+MD). However, while readily detectable in C57 mice, we failed to detect IL-17A mRNA in cHAP mice on the LDC+MD regimen (Fig. 3B). For IL-6 mRNA, ethanol feeding plus binge significantly increased expression in C57 EtOH-DW+Binge mice compared to LDE+Binge (Fig. 3B, †P < 0.05 C57 LDE+Binge versus C57 EtOH-DW+Binge). In contrast, LDE+Binge or EtOH-DW+Binge inhibited IL-6 mRNA expression in cHAP mice, although not significantly (Fig. 3B) and ethanol feeding + binge led to IL-17A mRNA expression in cHAP mice (Fig. 3B). For IL-22 mRNA expression, LDE+Binge led to lower expression in C57 mice compared to C57 maintained on EtOH-DW+Binge, although the level was not significantly different between C57 LDE+Binge and C57 LDC+MD mice (Fig. 3B). Finally, ethanol feeding did not significantly change TNFα mRNA expression for either LDE+Binge or EtOH-DW+Binge (Fig. 3B).
Analysis of scoring for hepatic fibrosis (Sirius red staining) failed to detect fibrosis across all groups (Supplementary Fig. S1), and these data were confirmed by a failure to detect changes in fibrotic gene expression (collagen 1α2, αSMA and TGFβ, mRNA) between experimental groups (Supplementary Fig. S1).
Using data from these four parameters (steatosis, necrosis, inflammation and fibrosis) we calculated a TLIS (Brandon-Warner et al., 2012b). These data demonstrated a significantly increased TLIS in C57 mice maintained on the LDE+Binge regimen compared to LDC+MD or EtOH-DW+Binge (Table 1, #P < 0.05 C57 and cHAP LDE+Binge versus LDC+MD, †P < 0.05 C57 and cHAP LDE+Binge versus EtOH-DW+Binge). However, while C57 mice exhibited higher TLIS when maintained on EtOH-DW+Binge compared to LDC+MD animals, the TLIS for cHAP mice maintained on EtOH-DW+Binge was not significantly different to that of LDC+MD cHAP mice (Table 1).
Voluntary access to ethanol enhances CYP2E1 expression in cHAP EtOH-DW mice
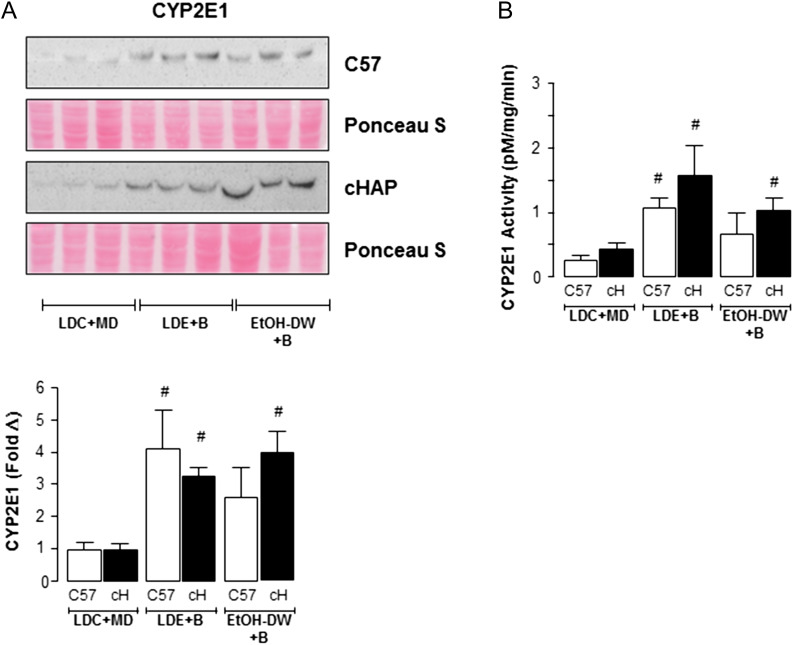
To determine if ethanol consumption affected expression of enzymes associated with ethanol metabolism (alcohol dehydrogenase, ADH; acetaldehyde dehydrogenase, ALDH and cytochrome P4502E1, CYP2E1) were measured by immunoblot. For both C57 and cHAP mice, no significant changes in ADH or ALDH expression were detected when maintained on LDE+Binge or EtOH-DW+Binge regimens compared to LDC+MD mice (Supplementary Fig. S2). For mice maintained on LDE+Binge regimens, significant increases in CYP2E1 protein expression were detected in both C57 and cHAP mice compared to LDC+MD (Fig. 4A, #P < 0.05 strain-matched LDE+Binge versus LDC+MD). In contrast, while CYP2E1 expression was increased in both strains maintained on EtOH-DW+Binge, it was only significantly increased in cHAP EtOH-DW+Binge mice (Fig. 4A, #P < 0.05 cHAP EtOH-DW+Binge versus cHAP LDC+MD). Because expression and activity of CYP2E1 are inducible by ethanol, we analyzed changes in CYP2E1 activity in tissue lysates. These data reflected in Western blot results, whereby both strains of mice exhibited increased CYP2E1 activity when maintained on LDE+Binge or EtOH-DW+Binge regimens, with no differences measured between strains on equivalent ethanol feeding regimens (Fig. 4B, #P < 0.05 strain-matched LDE+Binge or EtOH-DW+Binge versus LDC+MD).
Fig. 4.
Cytochrome P450 2E1 (CYP 2E1) is induced in cHAP but not C57BL/6 (C57) EtOH-DW+binge mice. (A) Representative immunoblots CYP2E1 protein expression in hepatic tissue lysates from C57BL/6 and cHAP mice maintained on either Lieber-DeCarli control liquid diet + maltose-dextrin (LDC+MD), Lieber-DeCarli ethanol liquid diet+binge (LDE+B) or free access 10% (v/v) ethanol in DW + binge (EtOH-DW+B). Densitometric analysis was performed and fold change in expression was determined and corrected using Ponceau S staining of membranes performed following transfer prior to antibody detection. #P < 0.05 strain-matched LDE+B or EtOH-DW+B versus LDC+MD. (B) CYP2E1 activity in tissue lysates expressed as pMol 4-nitrocatechol formation per mg tissue per minute. #P < 0.05 strain-matched LDE+B or EtOH-DW+B versus LDC+MD.
DISCUSSION
The best-characterized experimental rodent model of chronic ethanol feeding is the LDE liquid diet. However, liver damage with this model is restricted to mild steatosis and limited changes in transaminases (Bala et al., 2016). To induce greater liver injury, a single ethanol gavage followed by euthanasia 9 h later has been developed, generating moderate ALT and AST increases, concomitant with marked steatosis (Bertola et al., 2013). Further exacerbation of hepatic steatosis is observed by increasing the LDE period from 10 days to 4 weeks, in the absence of further increases in ALT-AST (Xu et al., 2015). Our data utilizing this approach yielded similar hepatic steatosis and increases in serum transaminases, independent of mouse strain.
By voluntarily consuming increasing amounts of ethanol to achieve high BACs, the cHAP model more closely mimics human patterns of ethanol consumption in alcohol-dependent individuals (Oberlin et al., 2011; Matson et al., 2014). Despite a preference for ethanol, cHAP mice maintained on the LDE liquid diet consumed less ethanol than mice given a choice of DW/EtOH-DW, cHAP mice given DW/EtOH-DW choice consuming amounts of ethanol similar to those previously reported (Matson et al., 2014). Differences in ethanol consumption between cHAP LDE and EtOH-DW mice could be attributed to the percentage of EtOH available; ethanol content in the LDE is 5% (v/v), whereas EtOH-DW have access to 10% EtOH (v/v). As such, satiety from the additional calories in the LDE liquid diet could potentially impair consumption of ethanol in cHAP LDE mice.
The potential of cHAP mice as an experimental ALD model was dampened following initial reports that mice did not exhibit evidence of steatosis or other markers of liver injury (Matson et al., 2013). In discussing these outcomes, the authors speculated saturated fat in the diet of the standard rodent chow used (Purina Laboratory Rodent Diet #5001), and/or epigenetic changes during strain development, may have accounted for lack of liver injury (Matson et al., 2013). This position is corroborated by studies demonstrating a protective role for saturated fats in chronic ethanol-fed rodents in which diminished inflammation and decreased micro- and macro-vesicular steatosis occurs to promote hepatic fatty oxidation (Chen et al., 2015b). Saturated fats may also inhibit the development of ALD by maintaining growth of intestinal microbiota. For example, oral ethanol intake decreases Lactobacillus sp., which maintain gut integrity, and translocation of gut-derived endotoxin plays an integral role in the pathogenesis of ALD (Kirpich et al., 2008). These findings are further supported by studies in which dietary supplementation with saturated long-chain fatty acids, in an intra-gastric model of ethanol consumption, reversed liver injury and alleviated gut microbial dysbiosis (Chen et al., 2015a).
In our study, we utilized a Teklad 8604 diet (in animals maintained on the EtOH-DW regimen), where the majority of calories from fat are mono- and polyunsaturated fats. Despite the change of dietary fat source, cHAP mice on the EtOH-DW+Binge regimen exhibited only mild hepatic microvesicular steatosis compared to moderate micro- and macro-vesicular steatosis observed in mice maintained on the LDE+Binge regimen. An additional consideration to the source of fat when comparing dietary intake between feeding models is the percentage of calories derived from different fat sources. The Teklad 8604 diet derives 14% of calories from fat as compared to 36% of calories from fat in the LDC and LDE feeding regimens. Although dietary fat at these levels are not sufficient to induce hepatic steatosis, variations of the percentage calories from fat in ethanol feeding regimens can influence hepatic triglycerides, and may explain high amounts of ethanol consumption produced only microvesicular steatosis in cHAP EtOH-DW+Binge compared to C57 and cHAP LDE+Binge animals (Lieber and DeCarli, 1989). To overcome this limitation, it would be of interest to replace the rodent chow with a high fat variant, and follow the free access 10% EtOH-DW+Binge regimen.
Patterns of ethanol consumption between the cHAP and C57 mice on the different feeding regimens could also be a source of variability in hepatic injury following gavage. Of note in our study, we only measured sustained BAC levels 9 h after ethanol gavage in C57 mice maintained on the LDE regimen. One explanation for this finding is that during the experimental course liquid diet and EtOH-DW bottles were replaced immediately prior to the end of the light cycle. Previous studies using this approach with C57 mice on LDE liquid diet report peak BAC (~75 mM) 3 h after light cycle end (Filiano et al., 2013). In comparison, cHAP display a delayed but prolonged ethanol consumption pattern when provided free-choice (between DW and EtOH-DW), with peak BAC levels (~42 mM) occurring 6–10 h after light cycle end (Oberlin et al., 2011; Matson et al., 2013). In our study, cHAP mice on LDE had modestly increased ethanol intake compared to C57 LDE mice, while cHAP EtOH-DW mice exhibited significantly greater total daily ethanol consumption after 4 weeks compared to other groups. Given the differences in ethanol consumption patterns and amounts of ethanol consumed between the models and the strains, changing the timing of the gavage by several hours to accommodate a delayed peak in cHAP-BACs may lead to equivalent, or greater, liver injury in cHAP mice compared to C57 counterparts. However, because of the increased capacity for total ethanol consumption in cHAP mice (peak BAC of ≥200 mg/dl are reported (Oberlin et al., 2011; Matson et al., 2013)) this raises the possibility that delaying time of gavage could create lethal BAC levels in cHAP mice, suggesting these studies should be approached with caution. Indeed, a significant limitation to interpreting data from our study is that BAC was only measured 9 hours after bolus ethanol gavage, rather than immediately prior to gavage. Based on our findings, future studies using the cHAP model that address modifications to the time of gavage may thus benefit from optimizing bolus ethanol gavage based on changes in BACs through the light-dark cycle.
Despite robust induction of CYP2E1 expression and activity in cHAP animals maintained on the LDE+Binge or EtOH-DW+Binge regimens, and higher daily intakes during EtOH-DW than LDE in cHAP mice, only cHAP mice maintained on LDE+Binge demonstrated significant hepatic injury. This finding might suggest impaired CYP2E1 activity in cHAP mice, however this was not evidenced in the analysis we performed. These data raise the possibility for other compensatory mechanisms for CYP2E1-mediated oxidative stress, such as non-GSH members of Nrf2-responsive genes, as a means to explain the limited hepatic injury in cHAP-EtOH-DW+Binge animals in the setting of robust levels of ethanol consumption. Alternatively, cHAP mice may have developed a phenotype to defray liver injury during successive generations of selective breeding, or mice acquiring liver pathology while drinking may have had difficulty reproducing. Thus, one hypothesis is that a compensatory mechanism developed through natural selection, which inevitably accompanies selective breeding.
Another interesting observation associated with cHAP mice was the study of inflammation marker mRNAs. Specifically, an increased expression of IL-6, IL-22 and TNFα mRNA was detected in cHAP mice compared to C57 mice for animals maintained on LDC+MD regimens, while IL-17A (a pro-inflammatory cytokine associated with ALD) was undetectable in cHAP-LDC+MD mice. These data suggest selective breeding may have led to altered basal expression of hepatic inflammatory markers in cHAP mice that impacts responses to ethanol feeding. While beyond the scope of this study, it is of further note that chronic ethanol feeding + binge led to IL-17A mRNA expression to become readily detectable in cHAP mice at levels equivalent to those measured in C57 mice. However, because levels of IL-17A were undetectable in cHAP-LDC-MD animals, increases in message may be below the threshold necessary to influence phenotype.
GSH and MDA analysis suggest alternative compensatory mechanisms may exist, elevated oxidative stress only being observed only in C57 mice maintained on the LDE+Binge diet. Although GSH levels were reduced in C57 mice (LDE+Binge and EtOH-DW+Binge regimens compared to LDC-MD animals) no reduction was observed in cHAP mice independent of chronic + binge ethanol regimen. It is interesting to note however, cHAP LDC+MD animals had lower basal GSH levels than C57 LDC+MD mice meaning additional studies to characterize antioxidant defenses in cHAP mice may be of merit to determine whether selective breeding for ethanol preference is also associated with fundamental changes in hepatic antioxidant capacity.
Collectively, our findings suggest that although cHAP mice consume consistently high/sustained levels of ethanol, other factors such as disparities in specific dietary components, differences in the patterns of alcohol consumption (during the feeding-cycle), and timing of gavage relative to peak BAC, alter the degree of liver injury in cHAP versus C57 mice in the NIAAA model of ALD. Additional studies to optimize the NIAAA chronic-binge model in cHAP mice may provide a cheaper, less labor-intensive model than exisiting LDE approaches.
Supplementary Material
SUPPLEMENTARY MATERIAL
Supplementary data are available at Alcohol And Alcoholism online.
FUNDING
This study was funded in part by NIH grant AA015512 [R.L. Bell, IUPUI].
CONFLICT OF INTEREST STATEMENT
None of the authors have any conflicts of interest to declare.
REFERENCES
- Bala S, Csak T, Saha B, et al. (2016) The pro-inflammatory effects of miR-155 promote liver fibrosis and alcohol-induced steatohepatitis. J Hepatol 64:1378–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertola A, Mathews S, Ki SH, et al. (2013) Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat Protoc 8:627–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon-Warner E, Schrum LW, Schmidt CM, et al. (2012. a) Rodent models of alcoholic liver disease: of mice and men. Alcohol 46:715–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon-Warner E, Sugg JA, Schrum LW, et al. (2010) Silibinin inhibits ethanol metabolism and ethanol-dependent cell proliferation in an in vitro model of hepatocellular carcinoma. Cancer Lett 291:120–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon-Warner E, Walling TL, Schrum LW, et al. (2012. b) Chronic ethanol feeding accelerates hepatocellular carcinoma progression in a sex-dependent manner in a mouse model of hepatocarcinogenesis. Alcohol Clin Exp Res 36:641–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Torralba M, Tan J, et al. (2015. a) Supplementation of saturated long-chain fatty acids maintains intestinal eubiosis and reduces ethanol-induced liver injury in mice. Gastroenterology 148:203–214 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YL, Peng HC, Wang XD, et al. (2015. b) Dietary saturated fatty acids reduce hepatic lipid accumulation but induce fibrotic change in alcohol-fed rats. Hepatob Surg Nutr 4:172–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester JA, Lumeng L, Li TK, et al. (2003) High- and low-alcohol-preferring mice show differences in conditioned taste aversion to alcohol. Alcohol Clin Exp Res 27:12–8. [DOI] [PubMed] [Google Scholar]
- Corrao G, Bagnardi V, Zambon A, et al. (2004) A meta-analysis of alcohol consumption and the risk of 15 diseases. Prev Med 38:613–9. [DOI] [PubMed] [Google Scholar]
- de la M Hall P, Lieber CS, Decarli LM, et al. (2001) Models of alcoholic liver disease in rodents: a critical evaluation. Alcohol Clin Exp Res 25:254S–261S. [DOI] [PubMed] [Google Scholar]
- Filiano AN, Millender-Swain T, Johnson R Jr, et al. (2013) Chronic ethanol consumption disrupts the core molecular clock and diurnal rhythms of metabolic genes in the liver without affecting the suprachiasmatic nucleus. PLoS One 8:e71684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Bataller R (2011) Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology 141:1572–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahame NJ, Li TK, Lumeng L (1999) Selective breeding for high and low alcohol preference in mice. Behav Genet 29:47–57. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Matthews DB, Gause L, et al. (2000) P rats develop physical dependence on alcohol via voluntary drinking: changes in seizure thresholds, anxiety, and patterns of alcohol drinking. Alcohol Clin Exp Res 24:278–84. [PubMed] [Google Scholar]
- Karaa A, Thompson KJ, Mckillop IH, et al. (2008) S-adenosyl-L-methionine attenuates oxidative stress and hepatic stellate cell activation in an ethanol-LPS-induced fibrotic rat model. Shock 30:197–205. [DOI] [PubMed] [Google Scholar]
- Kirpich IA, Solovieva NV, Leikhter SN, et al. (2008) Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol 42:675–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono H, Rusyn I, Yin M, et al. (2000) NADPH oxidase-derived free radicals are key oxidants in alcohol-induced liver disease. J Clin Invest 106:867–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber CS, Decarli LM (1982) The feeding of alcohol in liquid diets: two decades of applications and 1982 update. Alcohol Clin Exp Res 6:523–31. [DOI] [PubMed] [Google Scholar]
- Lieber CS, Decarli LM (1989) Liquid diet technique of ethanol administration: 1989 update. Alcohol Alcohol 24:197–211. [PubMed] [Google Scholar]
- Lieber CS, Leo MA, Mak KM, et al. (1985) Choline fails to prevent liver fibrosis in ethanol-fed baboons but causes toxicity. Hepatology 5:561–72. [DOI] [PubMed] [Google Scholar]
- Matson LM, Grahame NJ (2013) Pharmacologically relevant intake during chronic, free-choice drinking rhythms in selectively bred high alcohol-preferring mice. Addict Biol 18:921–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson LM, Kasten CR, Boehm SL II, et al. (2014) Selectively bred crossed high-alcohol-preferring mice drink to intoxication and develop functional tolerance, but not locomotor sensitization during free-choice ethanol access. Alcohol Clin Exp Res 38:267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson L, Liangpunsakul S, Crabb D, et al. (2013) Chronic free-choice drinking in crossed high alcohol preferring mice leads to sustained blood ethanol levels and metabolic tolerance without evidence of liver damage. Alcohol Clin Exp Res 37:194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckillop IH, Schrum LW, Thompson KJ (2016) Alcohol in hepatocarcinogenesis and progression. Hepatic Oncol 3:29–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan K, French SW, Morgan TR (2002) Production of a cytochrome P450 2E1 transgenic mouse and initial evaluation of alcoholic liver damage. Hepatology 36:122–34. [DOI] [PubMed] [Google Scholar]
- Oberlin B, Best C, Matson L, et al. (2011) Derivation and characterization of replicate high- and low-alcohol preferring lines of mice and a high-drinking crossed HAP line. Behav Genet 41:288–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrasek J, Bala S, Csak T, et al. (2012) IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J Clin Invest 122:3479–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla SD, Pruett SB, Szabo G, et al. (2013) Binge ethanol and liver: new molecular developments. Alcohol Clin Exp Res 37:550–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto H, French SW, Benson N, et al. (1985) Severe and progressive steatosis and focal necrosis in rat liver induced by continuous intragastric infusion of ethanol and low fat diet. Hepatology 5:224–32. [DOI] [PubMed] [Google Scholar]
- Xu MJ, Cai Y, Wang H, et al. (2015) Fat-specific protein 27/CIDEC promotes development of alcoholic steatohepatitis in mice and humans. Gastroenterology 149:1030-41 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip-Schneider MT, Doyle CJ, Mckillop IH, et al. (2011) Alcohol induces liver neoplasia in a novel alcohol-preferring rat model. Alcohol Clin Exp Res 35:2216–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.