Abstract
Background
Niemann-Pick disease type C (NP-C) is a neurodegenerative disease for which only palliative treatment exists, and only miglustat is effective in stabilizing neurological manifestations of NP-C. Ketogenic dietary therapies (KDT) are successfully used in patients with seizure disorders, including those associated with various inherited metabolic diseases (IMD), to reduce seizure frequency and medication requirement as well as to confer neuroprotection. Since patients with NP-C suffer pharmacorefractory seizures associated with ongoing neurodegeneration, KDT might be beneficial. The concomitant use of miglustat and KDT in patients with NP-C has not been reported.
Case presentation
We describe our experience in a now 17-year-old female with NP-C manifest early in childhood who has been successfully and continuously treated with miglustat and KDT in a palliative care setting for 3y. Although the neurodegeneration of NP-C progressed, she benefited from a reduction in seizure activity, fewer hospital stays related to seizure exacerbation, and increased alertness.
Conclusion
KDT could be safely deployed in our patient with NP-C, in whom its effects have been beneficial. Generally KDT is demonstratedly efficacious in patients with epilepsy and IMD. It reduces seizure activity and medication requirements and confers neuroprotection. Intracellular cholesterol trafficking and regulation of cholesterol biosynthesis are impaired in NP-C, which may prompt caution with respect to dietary lipid intake.
Keywords: Ketogenic diet, Ketogenic dietary therapy, Lysosomal storage disease, Niemann-Pick disease type C, Palliative care
Highlights
-
•
Niemann-Pick disease type C (NP-C) is a neurodegenerative disease.
-
•
There is no specific causal treatment.
-
•
We are the first to report safe and successful use of KDT in combination with miglustat in a patient with NP-C.
-
•
In our patient, KDT has led to improvement in quality of life due to reduction of seizure activity and hospital stays.
-
•
Neurodegenerative progression ultimately could not be stopped; further research is needed to clarify how KDT can contribute to the care of patients with NP-C.
-
•
Neurodegenerative progression ultimately could not be stopped.
1. Introduction
Niemann-Pick disease type C (NP-C) is a rare neurovisceral lysosomal disorder with autosomal recessive inheritance caused by mutations in either NPC1 (95%) or NPC2 (~4%) [1].
NP-C is characterized by abnormal lysosomal accumulation of unesterified cholesterol and glycolipids that results in progressive neurological deterioration, severe loss of quality of life, and premature death [2]. The only available drug effective in stabilizing neurological manifestations is miglustat [[2], [3], [4], [5]].
Yet NP-C is an invariably progressive disease and therapy is limited to delaying deterioration. Ketogenic dietary therapies (KDT) are successfully used in patients with seizure disorders and various inherited metabolic diseases (IMD) that conduce to seizure disorders. They reduce seizure activity and medication requirements and protect neurologic function [6,7]. Although dietary carbohydrate modification and restriction in patients with NP-C is discussed to ameliorate the gastrointestinal side effects associated with miglustat therapy [8], the concomitant use of miglustat and KDT in patients with NP-C is not described. In a case series where the long-term effects of intrathecal 2-Hydroxypropyl-β-Cyclodextrin treatment was evaluated, one patient who was on KDT prior to diagnosis and miglustat therapy was included who did not show any apparent benefit of KDT [9].
2. Case report
We describe our experience in attending a now 17-year-old female with NP-C manifest aged 3y that has been successfully and continuously treated with miglustat and KDT in a palliative care setting for 3y at this writing. This therapy reduced seizure frequency and hospital stays and improved patient alertness.
2.1. Initial findings and diagnosis
Our patient was born at term after an uneventful second pregnancy to non-consanguineous Caucasian parents. The first pregnancy ended in spontaneous abortion. The mother has epilepsy treated with carbamazepine.
The parents reported motor and language developmental delay apparent at age 3y. The patient was seen in our clinic first at age 5y; no diagnosis was assigned. Behavioral disturbances began at age 9y, with self-harming and injury to others, ataxia, dysphagia, and repetitive seizures. This prompted re-assessment. Bilateral oculomotor palsy was found, with exotropia. Abdominal sonography showed mesenteric lymphadenopathy and hepatosplenomegaly. A lysosomal storage disease was suspected. Filipin staining of skin fibroblasts found accumulation of unesterified cholesterol in the endosomal/lysosomal compartment, permitting the diagnosis of NP-C. The patient proved to harbor biallelic mutations in NPC1 (c.1421C>T/p.Pro474Leu, known [10], and c.3289_3291del/p.Asp1097del).
2.2. Treatment and clinical course
Anti-epileptic treatment with levetiracetam (LEV; initial target dose, 30 mg/kg/d) was initiated empirically at age 9y, when seizures began. Immediately after definitive diagnosis (still at age 9y), substrate reduction therapy with miglustat was initiated (400 mg/m2 body surface area/d). Emesis and diarrhoea were mild. After 2mo, seizures worsened and expressive speech was entirely lost. LEV therapy was thus increased stagewise (dose achieved, 50 mg/kg/d).
At age 10y exotropia and hand-eye-coordination improved. Over the next 2y, however, neurodegenerative worsening was volatile, with emergence of swallowing difficulties that led to malnutrition. A gastrostomy thus was placed at age 12y and an overnight partial continuous feeding regimen (1/3 of estimated total energy expenditure = 500 kcal) was established. At the same time, the patient lost ability to walk. Alternating patterns of high- and low- (1–2 seizures a day) seizure frequency (respectively 20 seizures/h and 1–2/d) characterized the patient's 12th and 13th years of life. At age 13y gastrointestinal manifestations emerged (chronic constipation, successfully treated with soluble fibre). At age 14y exacerbations of seizures led to escalation of anti-epileptic treatment. Buccal midazolam (10 mg) was used as rescue medication for seizures lasting longer than 5 min. The patient's LEV was enhanced (dose achieved, 70 mg/kg/d) and complemented with clobazam (CLB; 0.15 mg/kg/d). Yet neurological deterioration progressed, in particular seizure severity and seizure frequency.
After 3mo of unsatisfactory drug therapy, we re-considered the therapeutic options. Expecting more side effects without improvements if another anti-epileptic drug was begun, we decided to initiate a classical KDT with a ketogenic ratio of 4:1 whilst continuing the drug regimen then in place.
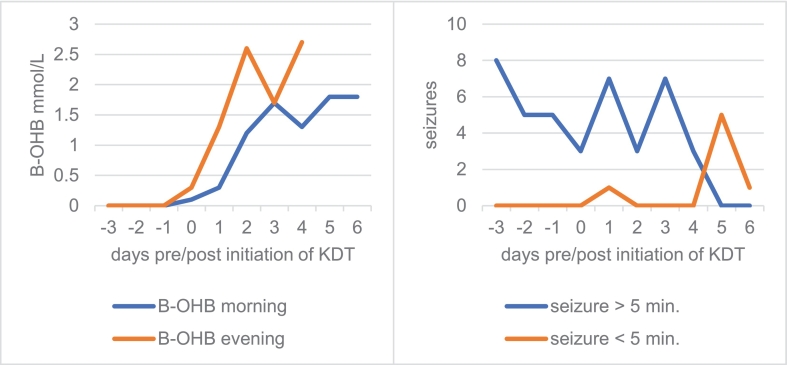
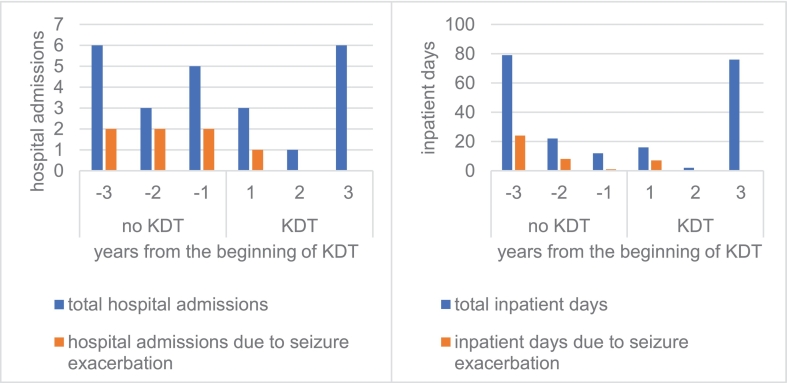
Enteral feeding via gastrostomy facilitated this, using a ketogenic formula (fat to non-fat 4:1). KDT is traditionally introduced by increasing the ketogenic ratio stepwise. We replaced one-third of calories/d until full tolerance was achieved (1560 kcal/d, 45 kcal/kg). Within 5d whole blood β-hydroxybutyrate (B-OHB) levels rose to 2.7 mmol/L (Fig. 1). Duration and frequency of seizures fell (Fig. 1) and alertness increased. Although neurodegeneration was thought to progress, with persistent and worsening signs, during the first 3y of KDT, average hospital admissions and inpatient days were fewer within 3y under KDT than during the 3y before (10 vs. 14 and 94 vs. 113 respectively).
Fig. 1.
Ketosis (serum B-OHB levels) and seizure frequency during KDT.
As electroencephalography (EEG) was stressful for our patient and little clinical benefit was expected, it was limited to a minimum. EEG 10mo prior to KDT showed multifocal as well as generalized epileptogenic activity as typically seen in patients with Lennox-Gastaut syndrome. After initiation of KDT, little changed yet stabilized epileptogenic activity was observed. Changes included slowing of background rhythm and continuous disorganization of sleep related elements. Generalized seizures were solely identified by an increased activity from 6 to 8/s to 14-30/s for 30 to 120 s.
The 3rd year of KDT saw an abrupt rise in hospital admissions and inpatient days that we ascribe to personnel and structural changes in the care facility where the patient lives rather than to a change in her disorder. After initiation of KDT no further hospital admissions due to seizure exacerbation were necessary (Fig. 2). Seizure duration remains <5 min even after 3y on KDT. The patient now, at age 17y, has received KDT for 3y without any identified KDT-associated side effects.
Fig. 2.
Hospital admissions and inpatient days before and after initiation of KDT.
3. Discussion
Concomitant use of miglustat and KDT (“Syner-G”) improves survival in patients with GM1 gangliosidosis and GM2 gangliosidosis (Tay-Sachs disease, Sandhoff disease) [11]. Ketosis may increase miglustat uptake and accumulation in forebrain of the β-hexosaminidase (Hexb) knockout (−/−) murine model of Sandhoff disease [12].
How KDT acts in pharmacoresistant epilepsy is not fully understood. Proposed mechanisms are linked either to direct effects of ketones and of glucose restriction or to interactions with receptors, channels, and metabolic enzymes [13]. KDTs in IMDs are used either to target the underlying metabolic disorder (e.g., glucose transporter type 1 deficiency syndrome) or to target symptoms (e.g., seizures in non-ketotic hyperglycinemia) [14]. They efficaciously reduce seizure activity and medication requirements and confer neuroprotection. One of the hypotheses of underlying pathophysiology in NP-C is that it triggers oxidative stress damage and apoptotic cell death [15]. Chronic ketosis as induced via KDT is thought to limit reactive oxygen species generation and thus might prevent apoptosis [16]. In addition, carbohydrate modification and reduction as derived by KDT could show successful amelioration of gastrointestinal side effects associated with miglustat in two patients with NP-C [8].
Although intracellular cholesterol trafficking and regulation of cholesterol biosynthesis are impaired in NP-C, which may prompt caution with respect to dietary lipid intake, fat-rich KDT can perhaps be safely deployed in patients with NP-C, in whom its effects may be beneficial.
4. Conclusion
In a non-terminal palliative care setting, KDT could temporarily improve quality of life through reduction in seizure activity, increased alertness, and reduction in both hospital admissions and inpatient days in a 14-year-old female patient with NP-C. Our experience may encourage further research into how KDT can be beneficially deployed in NP-C patients.
Synopsis
Over 3y beginning at age 14y in a girl with NP-C, classical KDT combined with miglustat, levetiracetam, and clobazam improved quality of life over that achieved with miglustat, levetiracetam, and clobazam. KDT reduced seizure activity and increased patient alertness.
Details of contribution of individual authors
Alexander Höller is a pediatric dietitian specializing in inborn disorders of metabolism and neuropediatrics.
Daniela Karall, Sabine Scholl-Bürgi, Sara Baumgartner Sigl, and Ursula Albrecht are pediatricians specializing in inborn disorders of metabolism and neuropediatrics.
Thomas Zöggeler, Gabriele Ramoser, and Benoit Bernar are physicians training in pediatrics.
Author who serves as guarantor for the article: Sabine Scholl-Bürgi.
Declaration of Competing Interest
All authors declare that they have no competing interest.
References
- 1.Patterson M.C., Clayton P., Gissen P., Mathieu Anheim, Bauer P., Bonnot O., Dardis A., Dionisi-Vici C., Klünemann H.H., Latour P., Lourenco S.M., Ory D., Parker A., Pocoví M., Strupp A., Vanier M.T., Walterfang M., Marquardt T. Recommendations for the detection and diagnosis of Niemann-Pick disease type C: an update. Neurol. Clin. Pract. 2017;7(6):499–511. doi: 10.1212/CPJ.0000000000000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patterson M.C., Mengel E., Vanier M.T., Moneuse P., Rosenberg D., Pineda M. Treatment outcomes following continuous miglustat therapy in patients with Niemann-Pick disease type C: a final report of the NPC registry. Orphanet J Rare Dis. 2020;25(15(1)):104. doi: 10.1186/s13023-020-01363-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patterson M.C., Vecchio D., Jacklin E., Abel L., Chadha-Boreham H., Luzy C., Giorgino R., Wraith J.E. Long-term miglustat therapy in children with Niemann-Pick disease type C. J. Child Neurol. 2010;25(3):300–305. doi: 10.1177/0883073809344222. [DOI] [PubMed] [Google Scholar]
- 4.Pineda M., Wraith J.E., Mengel E., Sedel F., Hwu W.L., Rohrbach M., Bembi B., Walterfang M., Korenke G.C., Marquard T., Luzy C., Giorgino R., Patterson M.C. Miglustat in patients with Niemann-pick disease type C (NP-C): a multicenter observational retrospective cohort study. Mol. Genet. Metab. 2009;98(3):243–249. doi: 10.1016/j.ymgme.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Wraith J.E., Vecchio D., Jacklin E., Abel L., Chadha-Boreham H., Luzy C., Giorgino R., Patterson M.C. Miglustat in adult and juvenile patients with Niemann-pick disease type C: long-term data from a clinical trial. Mol. Genet. Metab. 2010;99(4):351–357. doi: 10.1016/j.ymgme.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Kossoff E.H., Zupec-Kania B.A., Auvin S., Ballaban-Gil K.R., AGC Bergqvist, Blackford R., Buchhalter J.R., Caraballo R.H., Cross J.H., Dahlin M.G., Donner E.J., Guzel O., Jehle R.S., Klepper J., Kang H.C., Lambrechts D.A., YMC Liu, Nathan J.K., Nordli D.R., Pfeifer H.H., Rho J.M., Scheffer I.E., Sharma S., Stafstrom C.E., Thiele E.A., Turner Z., Vaccarezza M.M., EJTM Van Der Louw, Veggiotti P., Wheless J.W., Wirrell E.C., Charlie Foundation, Matthew’s Friends, Practice Committee of the Child Neurology Society Optimal clinical management of children receiving dietary therapies for epilepsy: Updated recommendations of the International Ketogenic Diet Study Group. Epilepsia Open. 2018;3(2):175–192. doi: 10.1002/epi4.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scholl-Bürgi S., Höller A., Pichler K., Michel M., Haberlandt E., Karall D. Ketogenic diets in patients with inherited metabolic disorders. J. Inherit. Metab. Dis. 2015;38(4):765–773. doi: 10.1007/s10545-015-9872-2. [DOI] [PubMed] [Google Scholar]
- 8.Och U., Fischer T., Marquardt T. Dietary carbohydrate modification in Niemann-Pick Type C. Case series of dietary treatment during miglustat (Zavesca®) therapy. Ernährungs Umschau. 2019;66(03):36–44. [Google Scholar]
- 9.Berry-Kravis E., Chin J., Hoffmann A., Winston A., Stoner R., La Gorio L., Friedmann K., Hernandez M., Ory D.S., Porter F.D., O’Keefe J.A. Long-Term Treatment of Niemann-Pick Type C1 Disease With Intrathecal 2-Hydroxypropyl-β-Cyclodextrin. Pediatr. Neurol. 2018;80:24–34. doi: 10.1016/j.pediatrneurol.2017.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tarugi P., Ballarini G., Bembi B., Battisti C., Palmeri S., Panzani F., Di Leo E., Martini C., Federico A., Calandra S. Niemann-Pick type C disease: mutations of NPC1 gene and evidence of abnormal expression of some mutant alleles in fibroblasts. J. Lipid Res. 2002;43(11) doi: 10.1194/jlr.m200203-jlr200. 1908-1199. [DOI] [PubMed] [Google Scholar]
- 11.Jarnes Utz J.R., Kim S., King K., Ziegler R., Schema L., Redtree E.S., Whitley C.B. Infantile gangliosidoses: mapping a timeline of clinical changes. Mol. Genet. Metab. 2017;121(2):170–179. doi: 10.1016/j.ymgme.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denny C.A., Heinecke K.A., Kim Y.P., Baek R.C., Loh K.S., Butters T.D., Bronson R.T., Platt F.M., Seyfried T.N. Restricted ketogenic diet enhances the therapeutic action of N-butyldeoxynojirimycin towards brain GM2 accumulation in adult Sandhoff disease mice. J. Neurochem. 2010;113(6):1525–1535. doi: 10.1111/j.1471-4159.2010.06733.x. [DOI] [PubMed] [Google Scholar]
- 13.Boison D. New insights into the mechanisms of the ketogenic diet. Curr. Opin. Neurol. 2017;30(2):187–192. doi: 10.1097/WCO.0000000000000432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Höller A., Zöggeler T., Meisinger B., Albrecht U., Karall D., Baumann M., Scholl-Bürgi S. Ketogenic dietary therapy: principles of implementation and application for inborn disorders of metabolism. Monatsschr Kinderheilkd. 2020;168(9):804–814. [Google Scholar]
- 15.Vázquez M.C., Balboa E., Alvarez A.R., Zanlungo S. Oxidative stress: a pathogenic mechanism for Niemann-Pick type C disease. Oxidative Med. Cell. Longev. 2012;2012:205713. doi: 10.1155/2012/205713. (Epub 2012 Jun 5. PMID: 22720116; PMCID: PMC3374944) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simeone T.A., Simeone K.A., Stafstrom C.E., Rho J.M. Do ketone bodies mediate the anti-seizure effects of the ketogenic diet? Neuropharmacology. 2018;133:233–241. doi: 10.1016/j.neuropharm.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]