Abstract
The aim of this integrative literature review was to evaluate the efficacy and safety of Metformin as an add-on therapy to insulin in poorly controlled overweight adolescents with type 1 diabetes mellitus. The research problem centered on providing optimum disease management during the most critical growth period and reducing the potential for cardiovascular-related morbidity and mortality in the future. The findings suggested that Metformin, in conjunction with insulin therapy, helped patients to achieve better metabolic control. The quality of metabolic control varied between studies according to differences in study design, exclusion and inclusion criteria, and methods. Adjunctive Metformin therapy has a positive effect on diabetes management, treatment, and prevention of cardiovascular-related complications with a minimal risk of side effects. Suggestions for further exploration of the research results and clinical implications are included in the review.
Keywords: Children with type 1 diabetes and metformin, Adjunctive therapy to insulin in adolescents with type 1 diabetes, Metformin and type 1 diabetes
An autoimmune reaction can prevent the body from producing insulin and cause type 1 diabetes mellitus (T1DM) [1]. According to the Centers for Disease Control and Prevention (CDC), 34.2 million people were diagnosed with diabetes, which is also the 7th leading cause of death in the United States [1]. In 2015, there were 193,000 of children and adolescents younger than 20-year-old diagnosed with diabetes [2].
The incidence of T1DM among children and adolescents has consistently increased over the years; meanwhile, the prevalence varies across different ethnicities. The reported rate at age 0 to 19 with T1DM is 27.0% for White, 19.0% for African American, 14.8% for Hispanic, 9.7% for Asian/Pacific Islander, and 6.5% for American Indian [2]. Children and adolescents with T1DM may experience symptoms such as frequent urination, abnormal thirst, unexplained weight loss, frequent exhaustion, bedwetting, vaginal yeast infection, sores that heal slowly, dry and/or itchy skin, losing feeling in feet or having tingling in feet, blurry eyesight [3].
Undiagnosed T1DM in children and adolescents can lead to medical emergencies such as diabetic ketoacidosis (DKA), severe hypoglycemia, coma or even death [4]. Patients suffering from DKA emergencies require close monitoring/management in the intensive care unit.
1. Clinical problem
Challenges with managing T1DM in the adolescent population may arise because of shifting hormones and physiologic changes, as the physiological changes of puberty have adverse implications on diabetes control. According to Chowdhury [5], insulin resistance tends to increase during the period of puberty. As the lean body mass of adolescents’ increases, the glycemic control worsens, causing an increase in insulin requirements [5]. In addition, excessive secretion of growth hormones leads to increased ketogenesis [5]. Moreover, excessive weight gain may increase complications stemming from diabetes and intensify insulin resistance. Girls with excessive adiposity have a greater risk of developing hyperandrogenism or polycystic ovary syndrome phenotype, which in turn increases the risk of cardiovascular disease [5]. To combat these issues and help with the overall treatment of T1DM in adolescents, healthcare providers may prescribe Metformin as an add-on therapy to insulin in the treatment of adolescents with poorly controlled T1DM.
1.1. PICOT question
PICOT (Population; Intevention; Comparison; Outcome; Time) format is used in nursing for summarizing research question(s) aimed at exploring the effect of therapy. In adolescents with type 1 diabetes mellitus (P), what is the effect of Metformin as an add-on therapy to insulin (I) compared to standard insulin therapy (C) on disease management, treatment, and prevention of cardiovascular-related complications (O) over the course of 12 months (T)?
1.2. Background and significance
Adolescent patients with T1DM require specialized care from an endocrinologist. The usual form of therapy for T1DM is glycemic control using insulin therapy. According to ADA, the goal of insulin therapy is to mimic normal insulin secretion patterns using a combination of both short-acting and long-acting insulin to treat T1DM [6].
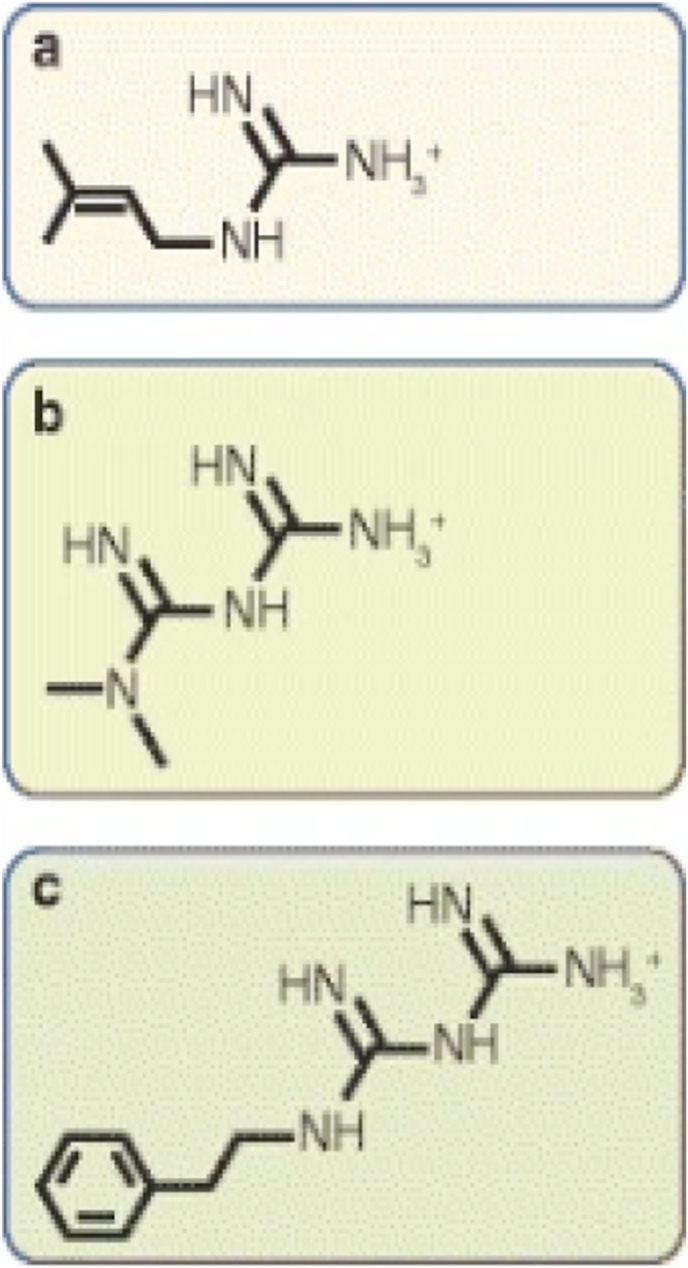
Metformin is the first line drug in the treatment of type 2 diabetes mellitus (T2DM) [7]. Metformin works on the liver by decreasing glucose output and increasing glucose uptake [7]. A previous study found Metformin as adjunctive therapy to insulin to be effective at enhancing glycemic control, preventing extreme body weight changes, minimizing hypoglycemia occurrences, and lowering the total insulin dose by 15–25% in T2DM (Lund et al., 2009, as cited in Ref. [7]. Although Metformin is primarily used to treat T2DM, results indicated that it can be beneficial when added to insulin in the treatment of T1DM. Metformin as an add-on therapy to insulin in adolescents with T1DM has been shown to decrease HbA1C level (Abdelghaffar & Attia, 2009, as cited in Ref. [7]. The chemical structure of Metformin is shown in Fig. 1.
Fig. 1.
From: The mechanisms of action of metformin
Chemical structures of galegine, metformin and phenformin. Metformin and phenformin are synthetic derivatives of galegine. Chemically, (a) galegine (also known as isoprenylguanidine), is an isoprenyl derivative of guanidine, while (b) metformin (dimethylbiguanide) and (c) phenformin (phenethylbiguanide) are biguanides containing two coupled molecules of guanidine with additional substitutions.
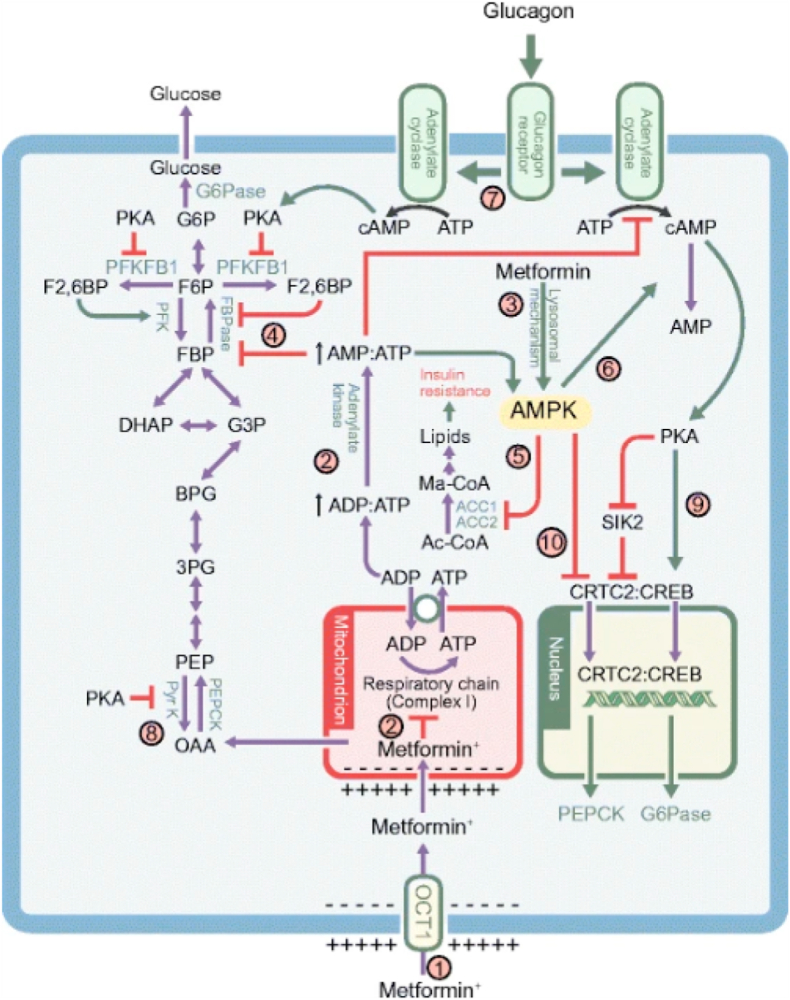
The acting mechanisms of Metformin [8] are portrayed in Fig. 2 and Fig. 3.
Fig. 2.
From: The mechanisms of action of metformin.
The multiple mechanism via which metformin affects liver metabolism. Note that the possible effect of metformin on mitochondrial glycerophosphate dehydrogenase [7] has not been included. (1) Uptake of metformin into hepatocytes is catalysed by the organic cation transporter-1 (OCT1) [11]. Being positively charged, the drug accumulates in cells and, further, in the mitochondria because of the membrane potentials across the plasma membrane and the mitochondrial inner membrane [14]. (2) Metformin inhibits Complex I, preventing mitochondrial ATP production and, thus, increasing cytoplasmic ADP:ATP and AMP:ATP ratios (the latter by displacement of the adenylate kinase reaction); these changes activate AMPK
[17]. (3) Alternatively, AMPK may be activated by a lysosomal mechanism, not shown in detail here but requiring Axin and late endosomal/lysosomal adaptor, MAPK and mTOR activator 1 (LAMTOR1) [27]. (4) Increases in AMP:ATP ratio also inhibit fructose-1,6-bisphoshatase (FBPase), resulting in the acute inhibition of gluconeogenesis [30], while also inhibiting adenylate cyclase and lowering cAMP production [32]. (5) Activated AMPK phosphorylates the ACC1 and ACC2 isoforms of ACC, inhibiting fat synthesis and promoting fat oxidation instead, thus reducing hepatic lipid stores and enhancing hepatic insulin sensitivity [34]. (6) AMPK also phosphorylates and activates the cAMP-specific 3′,5′-cyclic phosphodiesterase 4B (PDE4B), thus lowering cAMP by another mechanism [33]. (7) Glucagon-induced increases in cAMP activate cAMP-dependent protein kinase A (PKA), causing a switch from glycolysis to gluconeogenesis via phosphorylation and inactivation of PFKFB1, causing a decrease in fructose-2,6-bisphosphate (F2,6BP), an allosteric activator of phosphofructokinase (PFK) and inhibitor of fructose-1,6-bisphosphatase (FBPase). (8) PKA also phosphorylates and inactivates the liver isoform of the glycolytic enzyme pyruvate kinase (Pyr K) an (9) phosphorylates the transcription factor cAMP response element binding protein (CREB), thus inducing transcription of the genes encoding the gluconeogenic enzymes PEPCK and G6Pase. (10) Phosphorylation of CREB-regulated transcriptional co-activatoer-2 (CRTC2) by AMPK, or by AMPK-related kinases such as salt-inducible kinase 2 (SIK2), causes CRTC2 to be retained in the cytoplasm, antagonising the effects of PKA on the transcription of PEPCK and G6Pase [61, 62]. PKA inhibits SIK2 by direct phosphorylation at multiple site [62]. Ac-CoA, acetyl-CoA; BPG, 1,3-bisphosphoglycerate; DHAP, dihydroxyacetone phosphate; FBP, fructose 1,6-bisphosphate; F6P, fructose 6-phosphate; G3P, glyceraldehyde 3-phosphate; G6P, glucose 6-phosphate; Ma-CoA, malonyl-CoA; OAA,oxaloacetate; PEP, phosphoneolpyruvate; 3PG. 3-phosphoglycerate.
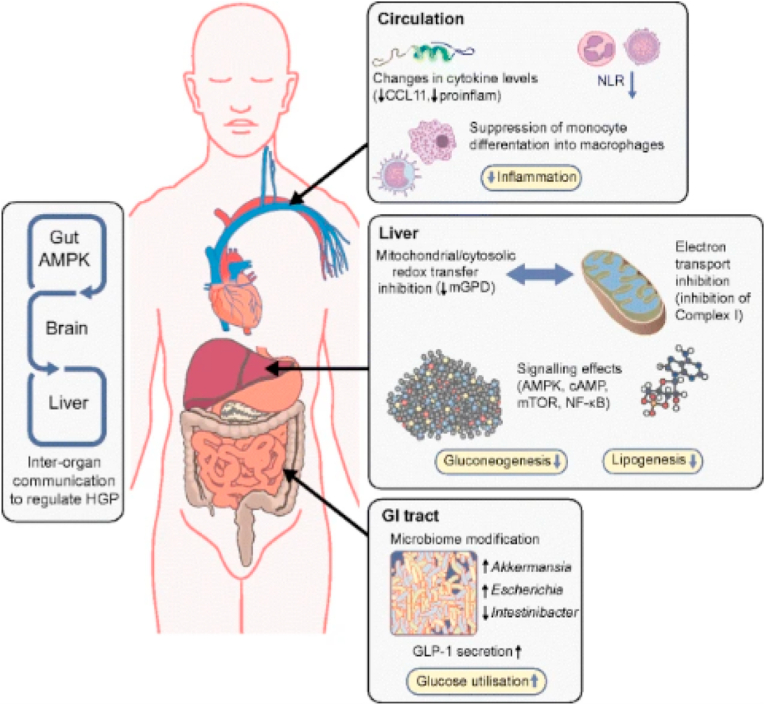
Fig. 3.
From: The mechanisms of action of metformin.
Actions of metformin on metabolism and inflammation. Responses to metformin in the blood, liver and intestines are shown schematically.In the blood, in observational studies, NLR is suppressed in humans with type 2 diabetes, whilst in randomised placebo-controlled trials, cytokines, including C–C motif chemokine 11(CCL11, also known as eotaxin-1), are also shown to be suppressed with metformin treatment. Other results indicate effects of this drug on monocytes and macrophages, affecting monocyte differentiation into macrophages and proinflammatory (proinflam) cytokine secretion. In the intestines, gut metabolism, incretin (GLP-1) secretion and the microbiome are modified upon metformin use. Further, there is evidence for gut-mediated mechanism for metformin action via gut-brain-liver crosstalk, which indirectly regulates hepatic glucose output. In the liver, metformin decreases lipogenesis and gluconeogenesis, as a result of its impact on molecular signalling and on mitochondrial function. HGP; hepatic glucose production; mTOR, mammalian target of rapamycin.
2. Methods
A search of extant literature was performed to achieve the main objective and to draw conclusions based on an all-inclusive knowledge base. The databases searched were Medline via EBSCO (2015–present), CINAHL via EBSCO (2015-present), PubMed Central (2015–present). The search was conducted in October 2020. Keywords employed in this search included children with type 1 diabetes and Metformin, adjunctive therapy to insulin in adolescents with type 1 diabetes, and Metformin and type 1 diabetes. Medical subject headings and Boolean operators were used to focus the search.
Inclusion criteria were set as follows: peer-reviewed articles in the English language with a publication date restriction for the last five years. No age limitations were set because some researchers recruited participants of various ages to provide a heterogeneous sample, thereby ensuring the generalizability of findings. Exclusion criteria extended to systematic and literature reviews. Preference was given to articles with the highest levels of evidence, such as randomized-controlled trials (Level II evidence) and well-designed controlled clinical trials without randomization, that is, quasi-experimental studies (Level III evidence). Studies met the inclusion criteria if they provided substantial evidence of clinical outcome assessment(s) and demonstrated measurable clinical benefits, including but not limited to cardio protective function. Guided by the inclusion and exclusion criteria, 10 articles were chosen.
3. Results
This integrative review included assessment of the rigor, strength, and significance of 10 research articles using a critical appraisal tool [9]. Eight of the 10 articles were quantitative randomized control studies, one was a quasi-experimental design, and one a pilot study. Most of the studies were done in a single center and a few in multicenter settings. The fact that some of the studies were done in single center settings granted advantages that improved consistency, ensured extensive data collection, and provided higher quality assurance. According to Unverzagt et al. [10], single-center trials usually provide larger treatment effects compared to multicenter trials. Two studies were conducted outside of the United States, one in Poland and one in Iran. Eight studies took place in the United States.
The results of these studies largely supported Metformin as adjunctive therapy. Most of the studies (8/10) favored the use of Metformin in addition to insulin therapy. A minority of studies (2/10) identified gastrointestinal events, severe hypoglycemia, lactic acidosis, and diabetic ketoacidosis as secondary outcomes. Some studies did not have control groups, which limited opportunities to compare results [11,12]. These articles were included in this review because authors were able to conduct a study without randomization, which presented possibilities for different interpretations of results. One of these studies had a very small T1DM cohort (n=39); however, none of the patients reported serious side effects [11].
Two studies noted a small sample size as a limitation [11,13], and in one of the studies low enrollment was attributed to strict inclusion criteria [14]. Adjunctive Metformin therapy was not recommended in one study due to its side-effects [15]. Bias control was not clear in a few of the studies, although they featured control groups and larger samples [16,17]. Although the researchers utilized randomization as a gold standard to minimize and/or mitigate bias, all biases might not have been eliminated completely in some studies. Accordingly, Sverdlov and Rosenberg et al. [18] suggested employing more advanced randomization techniques, such as permuted block design, the maximal procedure, and the Hadamard randomization to ensure high quality results. Both Burchardt et al. [17] and Bjornstad et al. [16] were convinced of the reproducibility and repeatability of their studies. Overall, these studies had carefully implemented randomization designs, structured treatment, and intervention programs to address the crucial aspects of the illness, along with larger sample sizes; all of these variables helped strengthen the validity and reliability of the results.
Some researchers investigated the clinical implications of the adjunctive therapy on patients’ cardio-vascular functioning [16,14,19,20] in T1DM patients. These studies reported high rates of participant retention regardless of their sample size. The results indicated improvements in cardiovascular functioning from the therapy.
Researchers also tested the impact of the adjunctive therapy on glycemic control [15] and observed the effects of dual therapy on metabolic control in a small sample of patients with T1DM [13]. Their results showed that the therapies improved target areas which are displayed in Table 2. In addition, Ziaee et al. [13] utilized adjusted indirect comparisons of Metformin and acarbose, the only reliable method recommended by the U.S. Food and Drug Administration [21]. The adjusted indirect comparison is a statistical method that allows researchers to retain the randomization of the original groups [21]. In adjusted indirect comparison, researchers analyze the size of the effect between two treatments in relation to a common comparator, which sheds light on the relationship between the two treatments [21]. Analysis revealed that Metformin had a greater treatment effect in comparison with the treatment effect of acarbose on decreasing fasting blood sugar level and HbA1C level. In essence, both insulin sensitizers, Metformin and acarbose, provided enhanced metabolic control.
Table 2.
Quantitative description of the improvement in metabolism and cardiovascular benefits.
| Author(s) and Date | Glycemic Benefit | Insulin | Triglycerides, HDL and LDL | Cardiovascular | BMI | BP | Insulin Resistance |
|---|---|---|---|---|---|---|---|
| Ahmed et al. [19] | HbA1c remained unchanged | Insulin dose reduced in Metformin group (p < 0.001) | An inverse correlation between changes in PACs number and triglycerides (r = −0.6; p = 0.001) in Metformin group | Metformin improved cEPCs, PACs, CFU-Hill’s colonies number, cECs and PACs adhesion (p < 0.05-all variables) to levels seen in healthy volunteers. A 75% rise in cEPCs number in type 1 diabetes patients equates to the reclassification of patients into a lower CVD risk group with approximate Hazard Ratio for CVD death of 0.77 (23% reduction) | Unchanged | Unchanged | |
| Anderson et al. [20] | Metformin had a beneficial effect on HbA1c at 3 months (P = 0.001) and difference in adjusted HbA1c between groups during 12 months was 1.0%; 95% CI 0.4, 1.5 (10.9 mmol/mol; 95% CI 4.4, 16.4), P = 0.001).There was a significant benefit in adjusted (age, sex) HbA1c at 3 months for the metformin group (8.4%; 95% CI 8.0, 8.8) (68 mmol/mol; 95% CI 64, 73) vs placebo group (9.3%; 95% CI 9.0, 9.7) (78 mmol/mol; 95% CI 75, 83) (P = 0.001) | Insulin dose reduced by 0.2 U/kg/d (95% CI 0.1, 0.3, P = 0.001) during 12 months, with effects from 3 months | No significant effect | No significant effect on IMT; Vascular smooth muscle function (GTN) [flow-mediated dilatation/glyceryl trinitrate–mediated dilatation (GTN)] improved, independent of glycosylated hemoglobin (HbA1c), by 3.3% units [95% confidence interval (CI) 0.3, 6.3, P = 0.03] | No significant effect | No significant effect | Enhanced insulin sensitivity.Estimated insulin sensitivity as calculated previously remained 0.2 U higher during 12 months (95% CI 0.06, 0.34, P = 0.005) in the Metformin group compared with placebo |
| Bjornstad et al. [16] | No significant changes in HbA1c | Greater reductions in total daily insulin dose (−6.4 ± 2.5 U/d versus −0.06 ± 2.6 U/d; P = 0.09) and total daily insulin dose per 1 kg (−0.07 ± 0.04 U·kg−1 d−1 versus −0.02 ± 0.04 U·kg−1 d−1; P = 0.31) were observed in the Metformin group compared with the placebo group | No significant changes | The Metformin group had reduced AA WSS MAX (−0.3 ± 0.4 dyne/cm2 versus 1.5 ± 0.5 dyne/cm2; P = 0.03), AA pulse wave velocity (−1.1 ± 1.20 m/s versus 4.1 ± 1.6 m/s; P = 0.04), and far-wall diastolic carotid intima-media thickness (−0.04 ± 0.01 mm versus −0.00 ± 0.01 mm; P = 0.049) versus placebo. No significant improvements were observed for brachial distensibility Significant reduction of mean IMT in the common carotid artery on Metformin(0.6 ± 0.1 cm vs 0.53 ± 0.1 cm; P = 0.002) |
Adolescents with T1DM in the Metformin versus placebo group had reduced weight (−0.5 ± 0.5 kg versus 1.6 ± 0.5 kg; P = 0.004), BMI (−0.2 ± 0.15 kg/m2 versus 0.4 ± 0.15 kg/m2; P = 0.005), and fat mass (−0.7 ± 0.3 kg versus 0.6 ± 0.4 kg; P = 0.01) | No significant change in systolic blood pressure (SBP) | Improved IR as measured by gold-standard hyperinsulinemic euglycemic clamp. Adolescents with T1DM in the Metformin versus placebo group had improved glucose infusion rate/insulin (12.2 ± 3.2 [mg·kg−1 min−1]/μIU/μL versus −2.4 ± 3.6 [mg·kg−1 min−1]/μIU/μL, P = 0.005; 18.6 ± 4.8 [mg·lean kg−1 min−1]/μIU/μL versus −3.4 ± 5.6 [mg·lean kg−1 min−1]/μIU/μL, P = 0.005) |
| Burchardt et al. [17] | Type 1 diabetes on adjunctive Metformin therapy had improved glycemic control. A significant improvement in fasting glucose levels (177.9 ± 65.7 mg/dl vs 123.8 ± 38.3 mg/dl; P < 0.001), postprandial glycemia (197.7 ± 57.6 mg/dl vs 133.9 ± 44.4 mg/dl; P < 0.001), average glycemia (162.8 ± 36.1 mg/dl vs 134.6 ± 19.8 mg/dl; P < 0.001) and HbA1c (8.6% ± 1.8% vs 7.6% ± 1.2%; 70 ±[–3.8] mmol/mol vs 60 ±[–10.4] mmol/mol; P < 0.001) | After 6 months of adjunctive Metformin therapy a significant increase in the levels of CEL (117.1 ± 33 pg/ml vs 118.2 ± 33 pg/ml; P < 0.001), Lp-PLA2 (82.4 ± 45.6 pg/ml vs 83.4 ± 45.6 pg/ml; P < 0.001), and a reduction in the levels of glycated LDL (1.5 ± 1.6 mg/dl vs 1.0 ± 0.5 mg/dl; P = 0.006) and triglycerides (130.1 ± 72 mg/dl vs 105.5 ± 65.2 mg/dl; P < 0.001) were observed. A trend towards increased HDL cholesterol levels (56 ± 12.6 mg/dl vs 60.9 ± 12.9 mg/dl; P = 0.07) was observed | Weight reduction (90 ± 16 kg vs 87 ± 15 kg, P = 0.054) | Enhanced tissue sensitivity to insulin | |||
| Cree-Green et al. [14] | Change in HbA1c (%) in placebo group (N = 16) 0.37 + 0.59; in Metformin group (N = 19) 0.15 + 1.24; adjusted p value 0.562 | No significant differences between groups were observed | Change in BMI z score in placebo group (N = 16) 0.02 + 0.08, in Metformin group (N = 19) −0.04 + 0.10; adjusted p value 0.041 (p < 0.05–0.01) | Improvements in whole-body IR and peripheral muscle IR: whole-body IR measured by hyperinsulinemic-euglycemic clamp [change in glucose infusion rate 1.3 (0.1, 2.4) mg/kg/min, P = 0.03] and peripheral IR [change in metabolic clearance rate 0.923 (20.002, 1.867) dL/kg/min, P = 0.05] | |||
| Libman et al. [15] | At 13-week follow-up, reduction in HbA1c was greater with Metformin (−0.2%) than placebo (0.1%; mean difference, −0.3% [95% CI, −0.6% to 0.0%]; P = .02). However, this differential effect was not sustained at 26-week follow up when mean change in HbA1c from baseline was 0.2% in each group (mean difference, 0% [95% CI, −0.3% to 0.3%]; P = .92) | At 26-week follow-up, total daily insulin per kg of body weight was reduced by at least 25% from baseline among 23% (16) of participants in the metformin group vs 1% (1) of participants in the placebo group (mean difference, 21% [95% CI, 11% to 32%]; P = .003) | No significant differences between groups were observed | A potential decrease in the risk of CVD and other complications as a result of improved insulin sensitivity, a significant decrease in BMI, and body composition | 24% (17) of participants in the Metformin group and 7% (5) of participants in the placebo group had a reduction in BMI z score of 10% or greater from baseline to 26 weeks (mean difference, 17% [95% CI, 5% to 29%]; P = .01) | No significant differences between groups were observed | Changes in body weight composition and insulin requirements may have improved insulin sensitivity |
| Nadeau et al. [22] | Girls in the Metformin group had lower HbA1c at 3 months (9.79 ± 1.67–9.39 ± 1.93%, p = 0.06), but boys did not (9.36 ± 0.88–9.32 ± 1.29%, p = 0.9). The overweight/obese participants in the metformin group had a significantly lower HbA1c at 3 months (9.42 ± 1.06–8.78 ± 1.17%, p = 0.03), and a lower HbA1c at 6 months (9.42 ± 1.06–8.84 ± 1.09%, p = 0.08), whereas normal weight participants had no significant HbA1c changes from baseline | In the metformin group a significant decrease in daily insulin dose in U and U/kg between 0 and 3 months (U, p < 0.03; U/kg, p = 0.035) as well as between 0 and 6 months (U, p < 0.05; U/kg p = 0.014) | No changes in lipids at 6 months, lipids were not measured at the 3-month visit | A significant decrease in BMI z-score within the Metformin group between baseline and 3 months (p = 0.02) and between baseline and 6 months (p = 0.01). A significant decrease in waist circumference within the Metformin group between baseline and 3 months (p = 0.003) and between baseline and 6 months (p < 0.02). Among overweight/obese participants, waist circumferences tended to decrease from at 3 months (84.9 ± 6.5–82.9 ± 5.0 cm, p = 0.12) to at 6 months (84.9 ± 6.5–83.2 ± 5.3 cm, p = 0.06), as did BMI z-score at 6 months (1.45 ± 0.14–1.25 ± 0.33, p = 0.07). Similarly, waist circumference among normal weight participants decreased significantly at 3 months (73.6 ± 7.5–71.8 ± 7.4 cm, p = 0.01), and tended to do so at 6 months (73.6 ± 7.5–72.7 ± 6.8, p = 0.12), as did BMI z-score (0.49 ± 0.53 at 0 months–0.33 ± 0.61 at 3 months, p < 0.05; 0.41 ± 0.47 at 6 months, p = 0.10) | A significant increase in systolic blood pressure was noted in the placebo group at 3 months compared to baseline (p = 0.03) | Improved insulin sensitivity as evidenced by a significant decrease in insulin dose | |
| Setoodeh et al. [12] | HbA1c level was significantly reduced during the study (p < 0.001) and following a 12 months period | Dosage of insulin significantly decreased (p < 0.001) | Serum lipid was decreased (p = 0.7) before (95.7 ± 22.8) after (94.8 ± 30.1) | Weight and BMI were increased (p < 0.001) | The insulin sensitivity was not measured directly.The evidence indicating the decrease of HbA1c as well as insulin level could be considered as an indicator for the improvement of insulin sensitivity in cases who received Metformin | ||
| Ziaee et al. [13] | Decrease in FBS levels on Metformin before (168.80 ± 19.90) after (113.56 ± 14.90) (p < 0.001). Decrease in HbA1C on Metformin before (8.36 ± 0.80) after (8.02 ± 0.63) (p value 0.143). | A significant decrease in TG levels (p < 0.045) | A significant reduction of systolic (p = 0.02) and diastolic (p = 0.03) blood pressure | ||||
| Zawada et al. [11] | The mean decrease in FBS and HbA1C after treatment with Metformin was significantly higher than treatment with acarbose (p < 0.001). A significant reduction of fasting glucose (p = 0.01), postprandial glucose (p = 0.00002), and mean daily glucose (p = 0.02). There was a reduction in HbA1c (9.3 till 8.9%), but it was not statistically significant | A significant reduction in total daily insulin requirements (p = 0.02) | A significant improvement of triglycerides (p = 0.002) and non-HDL cholesterol (p = 0.01) | A statistically significant reduction in total adipose tissue (4.8 ± 4.0 vs. 2.9 ± 4.4 kg, p = 0.03), waist circumference (96.4 ± 9.5 vs. 89.1 ± 11.1 cm, p = 0.003), and Visceral adiposity index (VAI) (4.1 ± 2.4 vs. 2.5 ± 1.8, p = 0.006) |
Studies with smaller and larger samples experienced obstacles to an almost equal degree. Smaller populations were not diverse because they either did not have a control group or had more female than male participants [11]. The results did not show a great improvement with therapy, so the researchers had to duplicate the study with a broader sample. Another study had a larger sample of overweight and obese T1DM patients, and their 6-month long trial showed that the therapy improved the body mass index (BMI), blood pressure, lipid levels, and insulin sensitivity/resistance, but the researchers also reported occurrences of minor side effects [22].
The researchers did not note the potential for error in one open-label randomized clinical trial that reported weight reduction after six months [17]. A single-center, placebo-controlled study had control groups, but the published results did not include the before and after results for comparison [13]. Studies with small samples may have been limited to some extent because small samples could potentially affect the margin of error; however, the studies under question were adequately powered, which helped researchers to detect significant differences between control and experimental groups [13,19]. In fact, the small sample size in these studies provided more meaningful outcomes by yielding results with a larger effect size.
Nevertheless, because bias could not be ruled out completely in some of the studies, the results of those studies should be scrutinized further before translating into practice. All things considered, these studies were Phase I, II and III trials. While these inquiries achieved their objective, Phase IV trials would provide additional information on the best use of Metformin. See Table 1 below for a list of the 10 articles and their conclusions.
Table 1.
The summary of articles.
| Author(s) and date | Questions, variables, objectives, hypothesis | Design, sample, setting | Findings | Notes |
|---|---|---|---|---|
| Ahmed et al. (2016) | Does adjunctive Metformin use have cardiovascular benefit, without causing hypoglycemic effect in adolescents with TIDM? Independent variables: Sex and age T1DM, Metfomin Dependent variables: Insulin dose HbA1C Lipid profile Lab results Blood glucose BMI Objectives: To assess the impact of Metformin on endothelial cells and endothelial progenitor cells in Type 1 diabetes. Hypothesis: Metformin can help improve endothelial dysfunction and reduce CVD risk. |
Randomized Control Quantitative Improvement Study. 23 participants in the Treatment Group 9 in the Standard Group 23 healthy volunteers Study visit Telephone visit Queen Elizabeth Hospital, Gateshead or the Royal Victoria Infirmary, Newcastle, UK |
After treating the participants with Metformin there was an improvement in CVD, while the levels of HbA1c remained the same. Metformin functions independently of hypoglycemic effect. | For the first time the research showed that in treatment group, in which participants received Metformin, there were changes in the markers of vascular/endothelial damage. CFU-Hill’s colonies and cECs are the markers of CVD risk. Metformin had a favorable effect on these makers. Also, the levels of HbA1c have improved. |
| Anderson et al. (2017) | Will metformin improve the vascular function in children with type 1 diabetes? Will early intervention reduce cardiovascular disease in children with type1 diabetes? Variables: Independent: Ethnicity Sex Age T1DM Variables: Dependent: HbA1c GTN Insulin dose BMI Objectives: To evaluate the effect of Metformin on vascular function in children with type 1 diabetes. Hypothesis: Metformin will improve vascular function in children with type 1 diabetes. |
Quantitative study. Twelve-month double-blind, randomized, placebo-controlled trial. 90 children with type 1 diabetes ages 8 to 18 years old with greater than 50 percentile body mass index. Tertiary pediatric diabetes clinic. |
Metformin improved vascular smooth muscle function and HbA1c, and lowered insulin dose in type 1 diabetes children. No participant experienced severe hypoglycemia which was attributed to the consistency in care. The number of moderate hypoglycemia cases did not increase significantly. The retention rate was high. |
Participants with type 1 diabetes at the onset of adolescence adhered to the therapy and benefitted the most at three months. Their HbA1c improved due to increased insulin sensitivity during 12 months. |
| Bjornstad et al. (2018) | Will Metformin as an adjunctive therapy to insulin improve insulin resistance and vascular dysfunction? What are the clinical implications, since glycemic control is most difficult during adolescence? Independent variables: Age, sex, Tanner stage, Metformin Dependent variables: Insulin dose, blood glucose, urine ketones, BMI, insulin sensitivity surrogate markers, MRI changes of ascending and descending aorta. Objectives: The purpose of the study is to demonstrate the use of Metformin as a safe oral medication, which can improve insulin resistance, BMI and offer potential cardio protective effects long term. Hypothesis: Adolescents with type 1 diabetes have impaired vascular function. Will the addition of Metformin as adjunctive therapy improve insulin resistance and vascular dysfunction? |
Randomized double-blind placebo-control 48 adolescents with type 1 diabetes who went through a baseline comprehensive evaluation of insulin sensitivity, body composition, and vascular health for three months. Children’s Hospital in Colorado |
The current study confirmed that insulin resistance, which was measured by hyperinsulinemic euglycemic clamp, improves in adolescents with type 1 diabetes with the addition of Metformin as adjunctive therapy versus a placebo study. | The current study confirmed that insulin resistance which is measured by hyperinsulinemic euglycemic clamp improves in adolescents with type 1 diabetes with the addition of Metformin as adjunctive therapy when compared to placebo. |
| Burchardt et al. (2016) | Does the addition of Metformin have a positive effect on atherogenic lipid fractions in relation to lipoprotein-associated phospholipase and cholesteryl ester lipase, and improve the intima-media thickness of the common carotid artery in young type 1 diabetics with excess body fat? Independent variables: Age, sex, diagnosis of T1DM Dependent variables: Metformin dose, Insulin dose, FBS, atherogenic lipid fractions in relation to lipoprotein-associated phospholipase, cholesteryl ester lipase, intima-media thickness, BMI Objective: To determine if the addition of Metformin has a positive effect on the cardiovascular risk factors. Hypothesis: The addition of Metformin is able to reduce lipoprotein-associated phospholipase, and cholesteryl ester lipase as well as reduce the intima-media thickness of the common carotid artery in T1DM who are overweight. |
Open-label randomized clinical trial The study included 84 patients (47 men, 37 women) at a mean age of 33.2 years. A total of 42 participants. A total of 42 patients were randomized to the arm receiving adjunctive Metformin at a dose of 1000 ± 500 mg/d (insulin-plus-metformin group). The control group comprised another 42 people randomly assigned to the insulin-only arm (control group, insulin group). |
Young type 1 diabetics who were treated with metformin had a reduction in mean carotid intima-media thickness, which could be due to weight reduction, and there was a decrease in atherogenic glycated LDL levels. This was not seen in patients who were treated with insulin alone. | This is a credible study, which can be reproduced and repeated. The variables should remain the same, and it shows there is a benefit of having Metformin as an adjunctive therapy. One of the limitations of the study is that it does not include the potential for error. |
| Cree-Green et al. (2019) | Insulin resistance increases the risk for cardiovascular disease and mortality in adults. Will metformin decrease the risk of cardiovascular disease caused by insulin resistance? Independent Variables: Sex, Age, Diagnosis of T1DM, Metformin Dependent Variables: Insulin dose, BMI, tissue specific insulin resistance, HbA1c, FBS. Objective: To demonstrate if Metformin as an adjunctive therapy to insulin treatment improves insulin resistance and BMI of youth with type 1 diabetes. Insulin resistance increases the risk for cardiovascular disease and mortality in adults. Will Metformin decrease the risk of cardiovascular disease caused by insulin resistance? Hypothesis: Metformin will decrease the risk of cardiovascular disease caused by insulin resistance. |
Multi-center Double-blind, placebo-controlled, randomized trial. participants ages 12–20 with an HbA1c of 7.5–9.9, a BMI being in the 85th percentile, total daily insulin dosage of 0.8 units/kg/day, and self-monitor blood glucose three or more times a day. Participants per site: University of Colorado Anschutz Medical Campus had a total of 11 participants; Yale University had 6 participate, Nemours Children’s Specialty Care included 3 participants; Indiana University included 5; University of Minnesota had 3; University of Iowa, 6; Children’s Hospital of Pittsburgh, 3. This yielded a total of 37 participants. |
The change in insulin sensitivity favored metformin in regards to whole body insulin resistance and peripheral insulin resistance. Metformin did not impact insulin suppression of endogenous glucose release. Adipose insulin resistance was not assessable with traditional methods in this insulin resistant population. Metformin appears to improve whole body peripheral insulin resistance in youth who are overweight or considered obese. | A major limitation of this study is the small sample size, this does now allow for evaluation of effect on sex, ethnicity, or differing insulin regimens. Secondary study measures, such as change in GDR is close to being significant but suggests a possibility of type 2 error because the sample size is too small. The inclusion criteria was strict in terms of HbA1c, and weight which limits the population of type 1 diabetics to certain parameters. |
| Libman et al. (2015) | Will Metformin as an adjunct to insulin improve the glycemic control among overweight and obese adolescents with type 1 diabetes? Variables Independent: Sex, Age, Diagnosis of T1DM, Metformin Variables Dependent: BMI Daily insulin dose Body fat Weight gain HbA1c levels Objectives: To assess the efficacy and safety of metformin as an adjunct to insulin in treating overweight adolescents with type 1 diabetes. Hypothesis: Metformin improves glycemic control in overweight or obese adolescents with type 1 diabetes. |
Quantitative study, 6 months, multi-center, placebo-controlled, randomized clinical trial. 140 adolescents aged 12 to 19 years with type 1 diabetes and BMI of 94th percentile along with HbA1c of 8.8%. Referred from 26 different pediatric endocrinology clinics. |
Metformin did not help overweight adolescents with type 1 diabetes during the six months of treatment. It only increased a risk for gastrointestinal adverse events. | Participants experienced hypoglycemia after 6 weeks of starting metformin. They had their insulin dose adjusted during that time. There were no episodes of lactic acidosis, which is consistent with other reports of Metformin use in youth. |
| Nadeau et al. (2015) | Will a low dose of Metformin improve BMI and insulin sensitivity in adolescents with type 1 diabetes mellitus? Independent Variables Metformin Age Height Dependent: Variables BMI HbA1c Insulin dose Blood pressure Fasting lipids Waist circumference Objectives: To determine the effects of low dose Metformin in adolescents with type 1 diabetes mellitus. Hypothesis: Metformin will improve metabolic parameters in adolescent with type 1 diabetes mellitus. |
Randomized, double -blinded, placebo-controlled trial. 74 pubertal adolescents, ages 13 to 20 years old with type 1 diabetes mellitus. Participants were recruited from the Barbara Davis Center for Childhood Diabetes and the Denver metro area |
Low dose Metformin improves BMI and insulin sensitivity in adolescents with type 1 diabetes mellitus. | The results show a positive impact of Metformin in T1DM youth on insulin sensitivity, BMI and body composition. That promises more options in treatments of CVD and other complications. Metformin helped both, the overweight and normal weight groups, so future studies will need to assess the impact of Metformin on T1DM-associated complications. |
| Setoodeh et al. (2017) | Will Metformin reduce HbA1c and insulin as an adjunct therapy in adolescents with type 1 diabetes? Independent Variable: Metformin T1DM diagnosis Dependent Variables: BMI Weight HbA1c Triglyceride and cholesterol levels Insulin dose Objectives: To investigate the effect of Metformin as an adjunct therapy in adolescents with poorly controlled type 1 diabetes. Hypothesis Metformin as an adjunct therapy decreases insulin resistance in adolescent population by increasing sensitivity to the insulin. |
Quasi-experimental (an uncontrolled before and after) study. 29 patients were analyzed from ages 10–17 with type 1 diabetes mellitus. Endocrinology clinic of children’s medical center of Tehran University of Medical Science in Iran. |
Adjunct therapy reduced HbA1c level and insulin dose in adolescents with type 1 diabetes mellitus after 12 months of treatment. | Prior to this study, Metformin as adjunct therapy was tested on type 2 diabetes patients with successful outcomes. This study results show that type 1 diabetes patients can benefit from it as well. In a 12-month period, Metformin used in combination with insulin lowered insulin resistance in type 1 diabetes participants. |
| Zawada et al. (2015) | Does Metformin as a concomitant therapy have an effect on anthropometric parameters such as visceral adiposity, and body fat accumulation in patients with type 1 diabetes who are treated with insulin therapy? Independent Variable: Age, sex, Diagnosis of T1DM, Metformin Dependent Variable: Insulin dose, weight, body fat percentage, waist circumference, urine ketones. Objective The purpose of this study was to evaluate the effect of Metformin as a concomitant therapy on anthropometric parameters such as visceral adiposity, and body fat accumulation in patients with type 1 diabetes who were treated with insulin therapy. Hypothesis Metformin treatment might reduce fat accumulation and improve metabolic control as well as decrease the incidence of insulin resistance and insulin dosage. Weight gain and obesity are possible complications of insulin therapy. Failure to reduce insulin dose during remission may cause undesirable clinical and biochemical consequences. This can cause increased appetite, decreased insulin sensitivity and cause weight gain. |
Pilot prospective study had a group of 45 patients enrolled (28 women, and 11 men) but only 39 people participated. The study group consisted of type 1 diabetics with increased body fat measured according to the WHO criteria, with age and gender considered. The body fat was assessed using electrical impedance. These patients were hospitalized in the Department of Internal Medicine and Diabetology, Poznań University of Medical Science, in 2010–2011. They were enrolled in a pilot group of Metformin Therapy in a Type 1 Diabetes Mellitus program which was registered as a clinical trial. | In the group of patients treated with Metformin, a significant improvement in lipid profile was observed. The Diabetes Treatment Satisfaction Questionnaire results after 6 months showed a significant increase in satisfaction with treatment. There was no significant change in incidence of acetonuria. There was a reduction in body fat and waist circumference. They were able to observe a reduction in daily insulin requirement, fasting, postprandial and mean daily glucose. The VAI reduced as well, showing an improvement in triglycerides and non-HDL. | The limitations in this study is that there were no control group. They didn’t-test the patients against those using insulin alone, and the patients using insulin and metformin adjunctively. While the results seem to be clinically significant, the size of the group was small and primarily female. This study can be duplicated using a broader demographic of patients. The article shows that adjunctive therapy with Metformin did have a positive impact on fat accumulation and metabolic control. |
| Ziaee et al. (2017) | Does the use of adjunctive Metformin to insulin, or a combination of Metformin and acarbose therapy provide metabolic control and decrease insulin requirements? Independent Variable: Age, sex, diagnosis of T1DM, Tanner stage, Metfomin, Acarbose. Dependent Variables: FBS, Post-Prandial Blood glucose, insulin dose, HbA1c, Cholesterol levels, triglycerides, LDL, HDL Objective: The aim of this study was to compare adjunctive therapy of Metformin and acarbose in patients with type 1 diabetes. Hypothesis The use of adjunctive Metformin to insulin therapy, or dual Metformin and acarbose therapy will provide metabolic control and decrease insulin requirements. |
A single-center, placebo-controlled study, participants were referred from their endocrinologist’s office. The participants met the age criteria of: 15–25 years, puberty stage of 2–5 Tanner, at least three-year history of diabetes, HbA1C 7–11 (within the recent six months). 40 participants, all of them remained in the program. | The results of this experiment, show that the addition of acarbose to Metformin type 1 diabetics who are primarily controlled with insulin is well tolerated and improves metabolic control. The use of the dual therapy had shown a decrease in fasting blood glucose and HbA1C, which had a remarkable effect on 2-h post prandial levels, triglycerides, cholesterol level, LDL and regular insulin use. | The results of the study favored the use of Metformin and acarbose as adjunctive therapy. The results also showed a clinical difference in the metabolic parameters. To enhance his study the sample size should be larger, and the age group of participants should be broadened. The study has placebo-control groups, but it did not publish before and after results for comparison. |
4. Discussion and recommendations
The results of the 10 research articles were clinically significant and, taken together, presented a compelling argument in favor of using Metformin as an add-on therapy to insulin in poorly controlled overweight adolescents with T1DM in order to reduce the risk of CVD. The majority of studies (6/10) demonstrated that Metformin leads to improvements in insulin resistance (IR) [16,[14], [15], [17],20,22]. The American Heart Association asserted that IR is a major predictor of cardiovascular morbidity and mortality [23]. Therefore, prevention and treatment of IR are of paramount importance because the longer adolescents are exposed to IR, the greater their risk of developing CVD.
Some researchers concluded by a method of deduction that insulin sensitivity improved as a result of reductions in BMI and fat mass along with the reduction of the daily insulin dose in the treatment group that tested Metformin. Two studies [16,14] provided more definitive results in favor of Metformin to improve whole-body IR. The researchers employed a highly reliable measurement tool, such as the hyperinsulinemic-euglycemic clamp, the most reliable means of assessing insulin resistance in humans, as previously established by ADA [24]. The results from several studies (4/10) were promising in terms of vascular repair [16,17,19,20]. The results indicated not only that Metformin can be used to prevent cardiovascular damage in adolescents diagnosed with T1DM, but also it can be incorporated as part of a treatment protocol.
At the same time, glycemic control, another variable instrumental in predicting cardiac outcomes in diabetic patients, remains the cornerstone of disease management and treatment. With this in mind, ADA currently does not recommend using Metformin as an adjunctive therapy to treat T1DM in overweight and obese adolescents [6]. ADA arrived at this recommendation based on findings by Libman et al. [15] and Nadeau et al. [22]; who were not able to determine a glycemic benefit after implementing Metformin treatment. The researchers found no significant change in HbA1C during their studies, which both lasted six months [15,22].
Nevertheless, five of 10 studies indicated that Metformin led to significant improvements in HbA1C, which in turn led to improved glycemic control [11,12,13,17,20]. These later studies were longer in duration (12 months) and had a larger sample size than previous studies. The reasons the remaining studies found no significant changes to HbA1C could have been tied to their inclusion and exclusion criteria; the duration of studies, which ranged from eight weeks to six months; the number of sites; or the number of clinicians adjusting the insulin doses throughout the trial period, any of which could have posed a threat to consistency.
Potentially important results from one study indicated that vascular repair can be accomplished without strict adherence to a tightly controlled HbA1C level with the help of Metformin [19]. These results may offer hope to patients who struggle to achieve the elusive recommended levels of HbA1C. Patients’ attempts to achieve HbA1C of less than 7% has been linked to hypoglycemia; furthermore, all-cause mortality is greater at low (5.6%) and high (11.8%) levels of HbA1C (Schoenaker et al., 2014, as cited in Ref. [19]. Explicating these findings leads to the conclusion that while intensive glycemic control may reduce diabetes complications, it cannot eliminate them completely. Therefore, interventions aimed at decreasing IR might be in order to reduce the risk for developing CVD in patients with T1DM.
Overall, the vast majority of studies (9/10) determined that Metformin therapy is safe in adolescent children; no severe adverse events were reported [16,11,[12], [13], [14],17,19,20,22]. Only minor gastrointestinal side effects were reported in several studies (4/10), and these side effects resolved upon the adjustment of the dose of Metformin [16,14,19,20]. Only one of the studies reported DKA in three participants and severe hypoglycemia in five participants in the treatment group [15]. According to Abubakar et al. [25]; gender is a predisposing factor to side effects; it has a significant effect on pharmacokinetics and pharmacodynamics. For instance, hepatic enzymes in females are more active than hepatic enzymes in males, which can affect drug metabolism [25]. Furthermore, menstruation and race can affect drug action [25]. The aforementioned potential contributing factors were not addressed in the study that reported severe side effects. Therefore, the study would benefit from the analysis of the social and patient related factors in conjunction with the exploration of the genetic predisposition of the participants to side effects by turning to pharmacogenomics. Healthcare providers should perform a causality assessment in all instances of side effects and/or adverse drug reactions. As such, researchers recommend using the following reliable and effective method: Naranjo probability scale [25,26]. Additionally, the trial took place at multiple sites with a variety of clinicians adjusting the doses of Metformin and insulin, which could have produced outcomes inconsistent with other studies [15].
In brief, Metformin provides many benefits; among them are enhanced insulin tissue sensitivity, reduction in total daily insulin dose, reduction in weight, and improvement in cardiac markers, including those responsible for vascular repair and improved glycemic control in some instances. Several researchers demonstrated additional benefits, such as a reduction in the levels of LDL and triglycerides, a significant reduction of the mean carotid intima media thickness (cIMT), and improved vascular smooth muscle function [16,11,12,13,17,19,20]. These results indicated that in the world of medicine, professionals cannot take an all-or-nothing approach and label Metformin as an only good or only bad intervention. Metformin provides an array of benefits as well as side effects. As with any therapy, healthcare providers must weigh and balance benefits against risks for each patient. Table 2 provides a summary of the improvement of symptoms in T1DM adolescents when using a combination of Metformin and insulin as shown by the 10 articles, which is comprised of quantitative description of the improvement in metabolism and cardiovascular benefits. Table 3 provides a summary of side effects of Metformin as reported by the ten studies.
Table 3.
The side effects of Metformin.
| Author(s) and date | Gastrointestinal | Diabetic Ketoacidosis | Lactic Acidosis | Hypoglycemia |
|---|---|---|---|---|
| Ahmed et al. [19] | 5 events | Not reported | Not reported | No major hypoglycemia events; no significant effect of Metformin on minor hypoglycaemic events (% ≤3.9 mmol/l and area under curve 3.9 mmol/l on CGMS: 8.6% vs 13.3%; p = 0.2 and 0.08 vs 0.1; p = 0.5 respectively) |
| Anderson et al. [20] | Metformin group (22), placebo group (14) | Metformin group (2), placebo group (2) | None | Moderate hypoglycemia in Metformin group (40, placebo group (2). No severe hypoglycemia |
| Bjornstad et al. [16] | Nausea, diarrhea, reduced appetite, stomach pain in ten participants in the Metformin group (40%) compared with 2 participants in the placebo group (8%; P < 0.01) | None | None | None |
| Burchardt et al. [17] | None | None | None | None |
| Cree-Green et al. [14] | (5) in the Metformin group | (1) in the Metformin group | None | No severe hypoglycemia |
| Libman et al. [15] | 50 events in the Metformin group (70% [95% CI, 60% to 81%]) and 24 events in the placebo group (35% [95% CI, 23% to 46%]; P < .001) (mean difference, 36% [95% CI, 19% to 51%]; P < .001) | 3 (4% [95% CI, 1% to 9%]) participants in the Metformin group vs 2 (3% [95% CI, 1% to 7%]) participants in the placebo group (mean difference, 1% [95% CI, −16% to 18%]; P ≥ .99) | None | Severe hypoglycemia 5 (7% [95% CI, 1% to 13%]) participants in the metformin group and 0 in the placebo group (mean difference, 7% [95% CI, −9% to 23%]; P = .06) |
| Nadeau et al. [22] | No difference between groups in gastrointestinal symptoms (metformin: nausea 8%, diarrhea 7%, abdominal pain 1% vs. placebo: nausea 8%, diarrhea 6%) or ketones |
None | No severe adverse events | No severe hypoglycemia |
| Setoodeh et al. [12] | Not reported | Not reported | Not reported | None |
| Zawada et al. [11] Ziaee et al. [13] |
(12) temporary gastrointestinal symptoms in the form of bloating, heaviness in the abdomen, and diarrhea (resolved spontaneously after a few days of use and did not constitute a reason to terminate Metformin therapy) The adverse effects (bloating, stomachache, diarrhea and hypoglycemia) were recorded, but they were not reported by the study |
No DKA. Acetonuria in seven patients (17.95%) on the first day of the run-in period before adding Metformin. On the day of control examination, five patients (10.26%) presented acetonuria (p = 0.08) Not reported |
None Not reported |
No hypoglycemia Not reported |
In general, Metformin has a good safety profile in adolescents. The results of the research studies provided substantial evidence for the potential promise of alternative and effective intervention to T1DM management, treatment, and prevention of related complications. Expanding the perspective beyond the rigid HbA1C control will provide a new direction for research and practice.
4.1. Implications for practice
Metformin is administered orally for the purpose of lowering blood glucose in patients. According to Song [27]; pharmacologically, Metformin is a biguanide class of antidiabetic medications. Metformin is known to be well tolerated and has a superior safety profile. Metformin at therapeutic doses has shown to have a very low risk of lactic acidosis as a side effect. Clinical advantages of Metformin’s use include the reduction of hepatic glucose output, an improvement in peripheral IR, and a reduction of the risk of CVD. Metformin does not increase islet insulin secretion, increase the risk of weight gain, or pose additional risks of hypoglycemia [27]. Clinical use of Metformin in the treatment of type 2 diabetes [28] is outlined in Table 4.
Table 4.
Clinical use of metformin in the treatment of type 2 diabetes
From: Metformin: historical overview.
| Feature | Comment |
|---|---|
| Indicationsa | Monotheraphy or in combination with other glucose-lowering agents including insulin in type 2 diabetes patients inadequately controlled by diet, exercise, and health education |
| Dosage formsb | 500, 850 and 1000 mg standard (IR) tablets (taken with meals); 500, 750 and 1000 mg XR tablets (mostly taken with evening meal); 500 mg/5 ml liquid formulation; 500 mg powder sachets |
| Titration | Increase does slowly; monitor glycaemic control; maximal does is 2550 or 3000 mg/day, depending on country (2000 mg/day in children) |
| Contraindicationsa | Renal and hepatic disease; cardiac or respiratory insufficiency; any hypoxic condition; severe infection; alcohol abuse; history of lactic acidosis; temporarily discontinue during use of i.v. radiographic contrast agents; pregnancy (although safe use is demonstrated in several studies) N.B. Some guidelines have relaxed the renel contraindication an suggest; reduce metformin does in renal impairment if eGFR <60 ml/min/1.73m2(MDR); avoid initiating metformin if eGFR < 45 ml/min/1.73m2; stop metformin if eGFR < 30 ml/min/1.73m2 |
| Side effects | Gastrointestinal symptoms (may include diarrhea) and metallic taste, likely to improve with does reduction and re-titration; may impair absorption of vitamin B12 and folic acid |
| Adverse reactions | Risk of lactic acidosis in patients with a contraindication; hypoglycaemia can occur when taken in combination with another glucose-lowering drug or during alcohol abuse |
| Monitoring | Check for contraindications; check plasma creatinine level of eGFR and haemoglobin periodically; possible interaction with cimetidine therapy |
The information in this table is based on the approved labelling of metformin by the FDA an European Medicines Agency (EMA), and recommendations of the National Institute for Health and Care Excellence (NICE) in the UK.
eGFR, estimated glomerular filtration rate; IR, immediate-release; MDRD, modification of diet in renal disease; XR, extended-release/slow-release.
The exact wording of indications and contraindications varies according to the labelling approved in different countries and regional and national guidelines.
Dose and formulation varies depending on country.
Metformin offers many benefits as well as some potential for side effects, which is true of any therapy. Benefits must be weighed and balanced against risks for any patient. The research indicated that Metformin has a good safety profile for adolescent patients, and the drug has demonstrated promise of an effective and alternative intervention for the management of T1DM and the prevention of related complications [16,11,12,13,17,19,20]. Metformin has shown to decrease CVD and increase the markers of vascular repair and health markers of vascular damage. Metformin also increased circulating endothelial progenitor cells (cEPCs), pro-angiogenic cells (PACs) and decreased circulating endothelial cells (cECs) while maintaining glycemic control and exerting cardioprotective effects (Fadini et al., 2010; Hill et al., 2003; Hörtenhuber et al., 2013; Loomans et al., 2004; Tepper et al., 2002, as cited in Ref. [19]. Metformin has shown a 75% increase in cEPCs number in type 1 diabetics; such a change can reclassify patients into a lower CVD risk group and create an approximate decrease in hazard ratio for CVD death by 23% (Werner et al., 2005, as cited in Ref. [19]. These outcomes suggest avenues of study for future researchers to confirm findings by examining cardiovascular events using large, randomized controls [19].
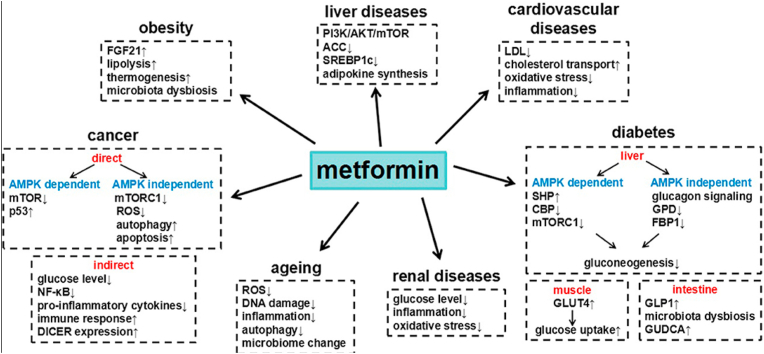
Metformin confers additional benefits, such as a reduction in the levels of LDL and triglycerides, a significant reduction of the mean cIMT, and improved vascular smooth muscle function. Metformin use in T1DM shows improvement in vascular and cardiovascular health without lowering blood glucose concentrations to strict ranges in HbA1C levels [16,11,12,13,17,20]. Intensive glycemic control may reduce diabetes complications, but it cannot completely eliminate all of the risks. Focusing on interventions aimed at reducing IR may offer a benefit such as the reduction of the risk of CVD in patients with T1DM. According to Lv & Guo [29]; Metformin has an array of other benefits. Fig. 4 provides a schematic representation.
Fig. 4.
Metformin and its benefits for various diseases.
From: Metformin and its benefits for various diseases.
The youth who are overweight or obese with T1DM treated with Metformin as adjunctive therapy showed improvement in whole-body and peripheral IR. Adolescents tolerated Metformin well with no severe occurrences of adverse events. The only side effects reported were minor gastrointestinal side effects, which were managed with dosage adjustment [16,14,19,20]. Puberty and the increase of growth hormone levels increases IR, which affects peripheral glucose uptake and has only a small effect on fat metabolism. Typically, the insulin dosage is increased to overcome IR, which can worsen metabolic control during the final stages of puberty [30]; Travers et al., 1995, as cited in Ref. [30].
The addition of Metformin to insulin therapy improved whole-body and peripheral muscle IR [14]. Prevention and treatment of IR is of greatest importance to adolescents, as their risk of CVD increases with their exposure to IR. Metformin has been found to help in the reduction of BMI and fat mass, along with a total insulin dose [16,14]. Metformin may also improve whole-body IR, which can be validated by tests using the hyperinsulinemic-euglycemic clamp, the industry standard for measuring IR [24].
4.2. Implications for further research
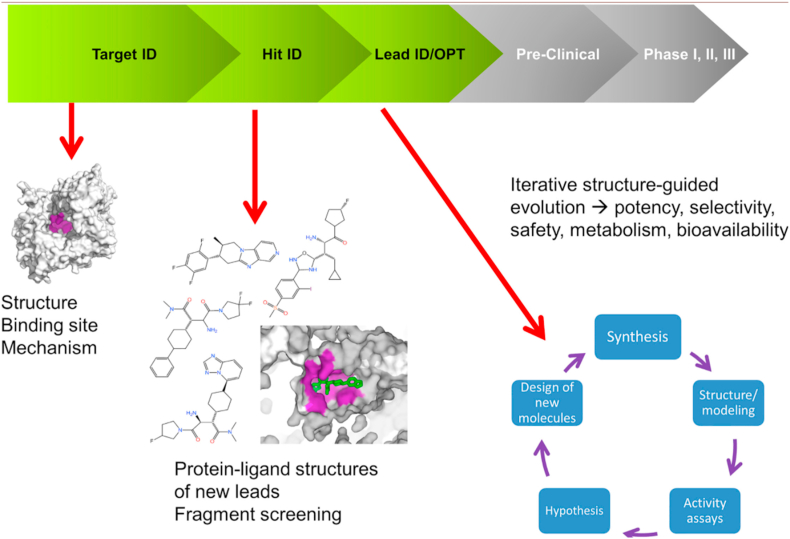
New avenues to explore include the effect of Metformin on a molecular level, which would broaden the understanding of arcane mechanisms of this drug and provide additional encouragement for clinical use of Metformin in treating T1DM. Scientists have only begun to unlock Metformin’s molecular secrets; a lot remains to be discovered. One of such discoveries revealed an indirect effect of Metformin on complex I in the mitochondria of liver cells [31]. Metformin exerts a significant effect on the energy of cells by decreasing cellular respiration and activating AMP-activated protein kinase (AMPK) enzyme [31]. AMPK is known as the “master switch in metabolism” [32]. The activation of the “master switch” leads to suppression of glucose production in the liver and also contributes to glucose uptake into skeletal muscle [31]. According to Van Drie & Tong [33]; Cryogenic Electron Microscopy (cryo-EM) is a powerful novel technology not only for determining mechanism of action of known drugs but also for the development of brand-new compounds. Thus, a detailed structural study utilizing the latest technology such as cryo-EM is of the essence. It would shed light on the direct interaction of Metformin with complex I and other cellular structures, and help put an end to the diabetes epidemic. Future direction for treatment of T1DM comes from none other but cryo-EM. Schematic representation of the drug discovery and development process utilizing cryo-EM [34] is shown in Fig. 5.
Fig. 5.
Future direction for treatment of T1DM (drug discovery and development process).
From: Cryo-EM for small molecules discovery, design, understanding, and application. The areas in the R&D process where structural information can be used are highlighted. In the Target ID space, one structure may be sufficient to identify the target binding site and characterize its mechanism. During the Hit ID stage, a few structures may be required to structurally characterize the lead compounds identified during screening. If a robust structural system is in place, X-ray can be used to run fragment screening campaigns. The heaviest request for structural data happens at the Lead ID/Opt stage, in which several iterations (from compound synthesis to structure/activity to SBDD back to synthesis) may happen in few weeks.
Findings of this integrative literature review may inspire supplementary inquiry into the effect of Metformin on preventing degeneration of brain and nerve tissues, as well as adverse changes in blood vessels resulting from diabetes. In this regard, future research is required to examine the effects of Metformin on a long-term basis.
5. Conclusion
Preliminary studies, including Phase I, II and III trials, showed promise in adding Metformin as an adjunctive therapy to adolescents with T1DM. An array of benefits accrued; Metformin has a good safety profile, and it is a well-tolerated drug in the adolescent population. Accordingly, recommendations include establishing benefit-risk ratios for each patient to tailor the therapy appropriately. The literature review revealed evidence of a promising role of Metformin as an effective adjunctive therapy to T1DM management, treatment and prevention of complications. The results of the research studies suggested a new direction for research and practice, expanding the use of Metformin beyond HbA1C control.
CRediT authorship contribution statement
Katsiaryna Sikorskaya: Conceptualization, Methodology, Investigation, Resources, Writing - original draft, Writing - review & editing, Supervision. Iwona Zarzecka: Conceptualization, Investigation, Resources, Writing - original draft, Writing - review & editing. Uzoamaka Ejikeme: Conceptualization, Investigation, Resources, Writing - original draft, Writing - review & editing. Jennifer Russell: Conceptualization, Investigation, Resources, Writing - original draft, Writing - review & editing.
References
- 1.Centers for Disease Control and Prevention What is diabetes? https://www.cdc.gov/diabetes/basics/diabetes.html n.d.-a.
- 2.Centers for Disease Control and Prevention. (n.d.-b). Diabetes in youth. https://www.cdc.gov/diabetes/library/reports/reportcard/diabetes-in-youth-2017.html.
- 3.https://my.clevelandclinic.org/health/diseases/17666-type-1-diabetes-in-children
- 4.American Diabetes Association. (n.d.). https://www.diabetes.org/diabetes/type-1/symptoms.
- 5.Chowdhury S. Puberty and type 1 diabetes. Indian Journal of Endocrinology and Metabolism. 2015;19(7):51. doi: 10.4103/2230-8210.155402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiang J.L., Maahs D.M., Garvey K.C., Hood K.K., Laffel L.M., Weinzimer S.A., Wolfsdorf J.I., Schatz D. Type 1 diabetes in children and adolescents: a position statement by the American Diabetes Association. Diabetes Care. 2018;41(9):2026–2044. doi: 10.2337/dci18-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rojas L., Gomes M. Metformin: an old but still the best treatment for type 2 diabetes. Diabetol Metab Syndrome. 2013;5(1):6. doi: 10.1186/1758-5996-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rena G., Hardie D.G., Pearson R.E. The mechanisms of action of metformin. Diabetologia. 2017;60(9):1577–1585. doi: 10.1007/s00125-017-4342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polit D.F., Beck C.T. 9th ed. Lippincott, Williams & Wilkins; 2018. Essentials of nursing research: appraising evidence for nursing practice. [Google Scholar]
- 10.Unverzagt S., Prondzinsky R., Peinemann F. Single-center trials tend to provide larger treatment effects than multicenter trials: a systematic review. J Clin Epidemiol. 2013;66(11):1271–1280. doi: 10.1016/j.jclinepi.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 11.Zawada A., Naskret D., Burchardt P., Niedzwiecki P., Wierusz-Wysocka B. The improvement of metabolic control after using metformin in patients with type 1 diabetes mellitus and excessive visceral fat tissue treated with intensive insulin therapy—pilot study. Int J Diabetes Dev Ctries. 2015;35(4):400–407. doi: 10.1007/s13410-015-0289-0. [DOI] [Google Scholar]
- 12.Setoodeh A., Didban A., Rabbani A., Sayarifard A., Abbasi F., Sayarifard F., Hoseinzade F. The effect of metformin as an adjunct therapy in adolescents with type 1 diabetes. J Clin Diagn Res. 2017;11(4) doi: 10.7860/JCDR/2017/24901.9725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ziaee A., Esmailzadehha N., Honardoost M. Comparison of adjunctive therapy with metformin and acarbose in patients with type-1 diabetes mellitus. Pakistan Journal of Medical Sciences. 2017;33(3):686–690. doi: 10.12669/pjms.333.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cree-Green M., Bergman B.C., Cengiz E., Fox L.A., Hannon T.S., Miller K., Nathan B., Pyle L., Kahn D., Tansey M., Tichy E., Tsalikian E., Libman I., Nadeau K.J. Metformin improves peripheral insulin sensitivity in youth with type 1 diabetes. J Clin Endocrinol Metabol. 2019;104(8):3265–3278. doi: 10.1210/jc.2019-00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Libman I., Miller K., DiMeglio L., Bethin K., Katz M., Shah A., Simmons J., Haller M., Raman S., Tamborlane W., Coffey J., Saenz A., Beck R., Nadeau K. Effect of metformin added to insulin on glycemic control among overweight/obese adolescents with type 1 diabetes: a randomized clinical trial. J Am Med Assoc: JAMA, J Am Med Assoc. 2015;314(21):2241–2250. doi: 10.1001/jama.2015.16174. [DOI] [PubMed] [Google Scholar]
- 16.Bjornstad P., Schäfer M., Truong U., Cree-Green M., Pyle L., Baumgartner A., Garcia Reyes Y., Maniatis A., Nayak S., Wadwa R.P., Browne L.P., Reusch J., Nadeau K.J. Metformin improves insulin sensitivity and vascular health in youth with type 1 diabetes mellitus. Circulation. 2018;138(25):2895–2907. doi: 10.1161/CIRCULATIONAHA.118.035525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burchardt P., Zawada A., Kaczmarek J., Marcinkaniec J., Wysocki H., Wierusz-Wysocka B., Grzymisławski M., Rzeźniczak J., Zozulińska-Ziółkiewicz D., Naskręt D. Association between adjunctive metformin therapy in young type 1 diabetes patients with excess body fat and reduction of carotid intima-media thickness. Pol Arch Med Wewn. 2016;126(7–8):514–520. doi: 10.20452/pamw.3527. [DOI] [PubMed] [Google Scholar]
- 18.Sverdlov O., Rosenberger W. Randomization in clinical trials: can we eliminate bias? Clin Invest. 2013;3(1):37–47. doi: 10.4155/cli.12.130. [DOI] [Google Scholar]
- 19.Ahmed F., Rider R., Glanville M., Narayanan K., Razvi S., Weaver J. Metformin improves circulating endothelial cells and endothelial progenitor cells in type 1 diabetes: MERIT study. Cardiovasc Diabetol. 2016;15(1):116. doi: 10.1186/s12933-016-0413-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson J., Couper J., Giles L., Leggett C., Gent R., Coppin B., Peña A. Effect of metformin on vascular function in children with type 1 diabetes: a 12-month randomized controlled trial. J Clin Endocrinol Metabol. 2017;102(12):4448–4456. doi: 10.1210/jc.2017-00781. [DOI] [PubMed] [Google Scholar]
- 21.Kim H., Gurrin L., Ademi Z., Liew D. Overview of methods for comparing the efficacies of drugs in the absence of head-to-head clinical trial data. Br J Clin Pharmacol. 2014;77(1):116–121. doi: 10.1111/bcp.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nadeau C., Chow K., Alam S., Lindquist K., Campbell S., McFann K., Klingensmith G., Walravens P. Effects of low dose metformin in adolescents with type I diabetes mellitus: a randomized, double-blinded placebo-controlled study. Pediatr Diabetes. 2015;16(3):196–203. doi: 10.1111/pedi.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maahs D. Cardiovascular disease risk factors in youth with diabetes mellitus: a scientific statement from the American Heart Association. Circulation. 2014;130(17):1532–1558. doi: 10.1161/CIR.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 24.Tam X. Defining insulin resistance from hyperinsulinemic-euglycemic clamps. Diabetes Care. 2012;35(7):1605–1610. doi: 10.2337/dc11-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abubakar A.R., Simbak N.B., Haque M. Adverse drug reactions: predisposing factors, modern classifications and causality assessment. Res J Pharm Technol. 2014;7(9):1091–1098. [Google Scholar]
- 26.Thaker J.S., Sinha S.R., Gogtay J.N., Thatte M.U. Evaluation of inter-rater agreement between three causality assessment methods used in pharmacovigilance. J Pharmacol Pharmacother. 2016;7(1):31–33. doi: 10.4103/0976-500x.179361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song R. Mechanism of metformin: a tale of two sites. Diabetes Care. 2016;39(2):187–189. doi: 10.2337/dci15-0013. [DOI] [PubMed] [Google Scholar]
- 28.Bailey C.J. Metformin: historical overview. Diabetologia. 2017;60(9):1566–1576. doi: 10.1007/s00125-017-4318-z. [DOI] [PubMed] [Google Scholar]
- 29.Lv Z., Guo Y. Metformin and its benefits for various diseases. Front Endocrinol. 2020;11 doi: 10.3389/fendo.2020.00191. 191–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moran A., Jacobs D.R., Steinberger J., Cohen P., Hong C.-P., Prineas R., Sinaiko A.R. Association between the insulin resistance of puberty and the insulin-like growth factor-i/growth hormone axis. J Clin Endocrinol Metabol. 2002;87(10):4817–4820. doi: 10.1210/jc.2002-020517. [DOI] [PubMed] [Google Scholar]
- 31.Spiering M. The mystery of metformin. J Biol Chem. 2019;294(17):6689–6691. doi: 10.1074/jbc.CL119.008628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shomali M. Diabetes treatment in 2025: can scientific advances keep pace with prevalence? Therapeutic Advances in Endocrinology and Metabolism. 2012;3(5):163–173. doi: 10.1177/2042018812465639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Drie H.G., Tong L. Cryo-EM as a powerful tool for drug discovery. Bioorg Med Chem Lett. 2020;30(22) doi: 10.1016/j.bmcl.2020.127524. 127524–127524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scapin G., Potter C.S., Carragher B. Cryo-EM for small molecules discovery, design, understanding, and application. Cell Chemical Biology. 2018;25(11):1318–1325. doi: 10.1016/j.chembiol.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]