Abstract
This is the first study to show a stepwise increase in venous thrombotic events according to COVID-19 coagulopathy (COVID-19-associated hemostatic abnormalities [CAHA]) staging and lung injuries assessed by chest computed tomography. Excess mortality and/or transfer to intensive care unit according to CAHA staging.
Keywords: COVID-19, lung injuries, thrombosis, venous thromboembolism, D-dimer, CAHA
The relevance of coronavirus disease 2019 (COVID-19) coagulation abnormalities are being increasingly recognized as a substantial proportion of patients develop thromboembolic complications. 1 2 3 4 Recently, Thachil et al 5 proposed a new staging classification characterizing COVID-19-associated hemostatic abnormalities (CAHA). The authors hypothesized that this staging system may enhance risk stratification in COVID-19 patients and help guiding both antithrombotic treatments and allocating scarce resources in intensive care units (ICUs) at the time of the COVID-19 pandemic.
We identified consecutive COVID-19 patients admitted to non-ICU departments and available D-dimer measurements at Strasbourg University Hospital from February 25 to April 19, 2020. COVID-19 was confirmed by a positive result of a reverse transcriptase-polymerase chain reaction assay of a specimen collected on a nasopharyngeal swab. Patients with findings typical of COVID-19 at chest computed tomography (CT) (i.e., bilateral and peripheral ground glass opacities and/or alveolar consolidations) and for whom testing for the COVID-19 virus was either inconclusive or could not be performed, were considered as confirmed COVID-19 cases after careful reviewing by a multidisciplinary team. All patients were on thromboprophylaxis. Patients were categorized according to a simplified proposed CAHA classification 5 into three stages (independent, not additive) depending on the D-dimer level peak value (highest D-dimer levels during the hospital stay)—stage 1: D-dimer less than threefold above normal; stage 2: D-dimer three- to sixfold above normal; and stage 3: D-dimer greater than sixfold above normal. Platelets and activated partial thromboplastin time (aPTT) values were not taken into account. Lesions severity at CT was classified following the European Society of Radiology/European Society of Thoracic Imaging guidelines 6 as minimal (stage 1 < 10%), moderate (stage 2 = 10–25%), severe (stage 3 = 25–50%), and critical (stage 4 > 50%). The primary outcome was a confirmed diagnosis of any venous thromboembolic event including acute pulmonary embolism (APE) and deep vein thrombosis (DVT). Patients with suspected APE had a CT pulmonary angiography performed based on clinical suspicion (either clinical, echocardiographic, or laboratory parameters). DVT imaging test was performed in subjects with signs or symptoms of DVT. The secondary outcome was the composite of death or transfer to ICU. The present study was approved by the Research Ethics Committee of Strasbourg Hospital (authorization CE-2020–57) and waived the need of informed consent.
A total of 150 consecutive patients with confirmed COVID-19 infection, thromboprophylaxis, and available D-dimers kinetics were admitted to general wards between February 25 and April 19, 2020. According to the staging scheme, 72 (48%) patients were in stage 1, 34 (22.7%) patients were in stage 2, and 44 (29.3%) patients were in stage 3 ( Table 1 ). Patients in more advanced stages presented with higher venous thromboembolism (VTE) risk evaluated via the Padua Prediction Score and the IMPROVE score on admission to hospital. According to the staging scheme, baseline aPTT, leukocytes, and platelets peak were similar among the three hemostatic groups. Patients in more advanced stages had higher C-reactive protein (CRP) and fibrinogen peak ( Fig. 1 ). At least one chest CT was available in 139 patients (92.7 of the global cohort). Chest CT severity score was significantly associated with higher CAHA stages. Similarly, higher oxygen requirements were evidenced with more advanced stages. Likewise, a stepwise increase in lactate levels that paralleled CAHA stages was evidenced. The incidence of VTE was 1.4% in stage 1, 5.9% in stage 2, and 31.8% in stage 3 ( p < 0.001). APE was diagnosed in 15 patients (10%) and there was a significant stepwise increase in events according to CAHA staging ( p < 0.001). VTE was diagnosed in median 8 (3–13) days after admission and 15 (12–22) days after COVID-19-related symptoms onset. The composite of death or transfer to ICU occurred in 59 patients (39.3%). The incidence of the secondary outcome was 18.1% in stage 1, 50% in stage 2, and 65.9% in stage 3 ( p < 0.001). The rate of this composite outcome was threefold higher in CAHA stage 2 and 3 compared with stage 1, but mortality did not differ significantly among the groups.
Table 1. Baseline characteristics, extent of COVID-19 disease on chest CT and outcomes according to a simplified CAHA classification.
| Stage 1 D-dimers < 3 ULN ( n = 72) |
Stage 2 D-dimers 3–6 ULN ( n = 34) |
Stage 3 D-dimers > 6 ULN ( n = 44) |
p -Value | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age, y | 58 ± 15 | 63 ± 15 | 62 ± 15 | 0.198 |
| Male, n (%) | 37 (51.4) | 18 (52.9) | 32 (72.7) | 0.062 |
| BMI (kg/m 2 ) | 27 ± 5 | 28 ± 4 | 28 ± 5 | 0.697 |
| Scoring systems for estimating risk of venous thromboembolism | ||||
| Padua score | 2.9 ± 1.8 | 3.7 ± 1.8 | 4.0 ± 1.8 | 0.004 |
| Padua score > 4 | 28 (38.9) | 21 (61.8) | 31 (70.5) | 0.002 |
| IMPROVE score | 0.80 ± 1.04 | 1.47 ± 1.13 | 1.65 ± 1.16 | < 0.001 |
| Laboratory findings | ||||
| D-dimers admission, ng/mL | 733 ± 335 | 1,617 ± 660 | 4,588 ± 4,403 | < 0.001 |
| D-dimers peak, ng/mL (peak values observed 4.2 ± 4.3 d after admission) |
814 ± 369 | 2,165 ± 448 | 8,531 ± 5,326 | < 0.001 |
| CRP admission, mg/L (peak values observed 3 ± 3.4 d after admission) |
85 ± 76 | 99 ± 65 | 143 ± 89 | 0.001 |
| CRP peak, mg/L | 122 ± 85 | 145 ± 88 | 178 ± 102 | < 0.001 |
| Fibrinogen peak, g/L (peak values observed 5 ± 3.9 d after admission) |
6.7 ± 1.6 | 7.3 ± 1.7 | 8.2 ± 2.1 | 0.006 |
| Blood lactate, mmol/L | 0.97 ± 0.29 | 1.22 ± 0.70 | 1.37 ± 0.60 | 0.007 |
| aPTT admission, s | 1.14 ± 0.23 | 1.09 ± 0.11 | 1.23 ± 0.53 | 0.237 |
| Platelets admission, ×10 9 /L | 215 ± 88 | 212 ± 79 | 242 ± 79 | 0.243 |
| Platelets peak, ×10 9 /L | 394 ± 187 | 391 ± 142 | 439 ± 165 | 0.335 |
| Thromboprophylaxis during hospitalization | ||||
|
Standard-dose anticoagulant thromboprophylaxis,
n
(%)
Enoxaparin 40 Mg/0.4 mL Prophylactic-dose UFH Fondaparinux 2.5 |
45 (62.5) 44 (61.1) 0 (0) 1 (1.4) |
23 (67.6) 23 (67.6) 0 (0) 0 (0) |
23 (52.3) 11 (25) 2 (4.5) 0 (0) |
0.351 |
|
Intermediate dose anticoagulant thromboprophylaxis,
n
(%)
Enoxaparin 40 Mg/0.4 mL BID |
13 (18.1) 13 (18.1) |
6 (17.6) 6(17.6) |
8 (18.2) 8 (18.2) |
0.998 |
| Therapeutic dose anticoagulant thromboprophylaxis, n (%) | 14 (19.4) | 5 (14.7) | 1 (2.6) | 0.245 |
| Extent of COVID-19 disease on chest CT | ||||
| Stage 1, n (%) | 10 (14.7) | 2 (6.3) | 1 (2.6) | 0.001 |
| Stage 2–3, n (%) | 49 (72.1) | 24 (75) | 20 (51.3) | |
| Stage 4, n (%) | 9 (13.2) | 6 (18.6) | 18 (46.2) | |
| Oxygen | ||||
| Oxygen demand (maximal oxygen flow rate, L/min) | 4.5 ± 0.4 | 6.9 ± 4.7 | 8.4 ± 5.1 | < 0.001 |
| Outcomes | ||||
| Venous thromboembolic event, n (%) | 1 (1.4) | 2 (5.9) | 14 (31.8) | < 0.001 |
| Acute pulmonary embolism, n (%) | 1 (1.4) | 2 (5.9) | 12 (27.3) | < 0.001 |
| Deep vein thrombosis, n (%) | 0 (0) | 0 (0) | 2 (4.5) | 0.087 |
| Transfer to ICU or in-hospital death, n (%) | 13 (18.1) | 17 (50) | 29 (65.9) | < 0.001 |
| In-hospital death, n (%) | 1 (1.4) | 1 (2.9) | 6 (6.8) | 0.284 |
| ICU transfer, n (%) | 13 (18.1) | 16 (47.1) | 29 (65.9) | < 0.001 |
| Discharge alive, n (%) | 64 (97) | 24 (85.7) | 33 (86.8) | 0.087 |
Abbreviations: aPTT, activated partial thromboplastin time; BID, twice daily; BMI, body mass index; CRP, C-reactive protein; CT, computed tomography; ICU, intensive care unit; UFH, unfractionated heparin; ULN, upper limit of normal.
Note: Continuous variables were expressed as mean ± standard deviation and categorical variables as counts and percentages. Continuous variables between all groups were compared using ANOVA. Pearson’s Chi-squared test was used to compare categorical variables. A p -value < 0.05 was considered significant. Calculations were performed using SPSS 17.0 (SPSS Inc., Chicago, IL, United States).
Fig. 1.
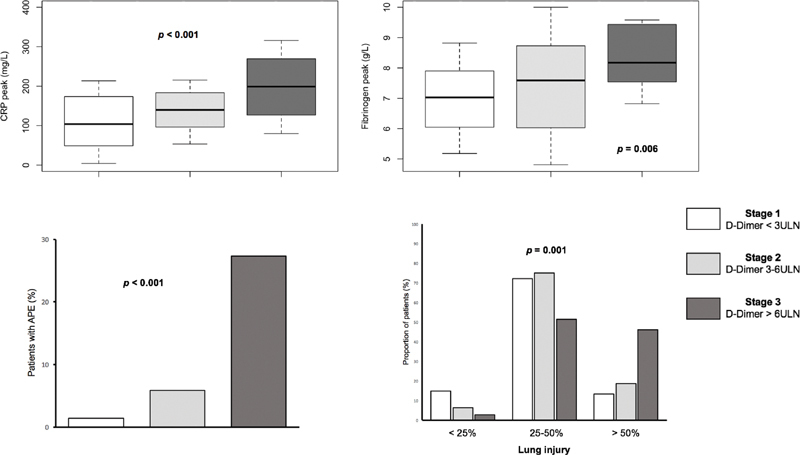
CRP peak, fibrinogen peak, acute pulmonary embolism, and extent of coronavirus disease 2019 (COVID-19) disease on chest CT according to the three stages COVID-19-associated hemostatic abnormalities (CAHA) classification. The extent of COVID-19 disease on chest CT was assessed as pulmonary injuries extension in percentage of the total pulmonary field and classified as minimal (stage 1 < 10%), moderate (stage 2 = 10–25%), severe (stage 3 = 25–50%), and critical (stage 4 > 50%). APE, acute pulmonary embolism; CRP, C-reactive protein; CT, computed tomography; ULN, upper limit of normal.
This study is, to the best of our knowledge, the first investigation specifically designed to assess the prevalence and prognosis impact of the new CAHA staging among COVID-19 patients hospitalized in non-ICU wards. We observed a high rate of CAHA stage 2 and 3 accounting for approximately 50% of the cohort. D-dimer increased levels were reported to be associated with severity of COVID-19 disease 1 7 8 and CAHA stage 2 and 3 patients may undoubtedly benefit from critical care support as suggested by Thachil et al. 5 This staging scheme has the potential to identify the patients who may benefit from early elective ICU transfer versus those who can be managed in wards.
Interestingly, aPTT on admission and platelets on admission or at peak were similar between the three groups of patients and did not appear to provide additional prognostic value. Adding the peak of CRP or fibrinogen in the stage definition may be worth considering to improve its discrimination. Indeed, a stepwise increase in CRP or fibrinogen peaks was evidenced for each increment in the stage of CAHA.
Our study emphasized the interrelationship between the extent of COVID-19 disease on chest CT, oxygen requirement, lactate levels, and a gradual increase in CAHA grading. Recent reports provided important insight into the relationship between hypercoagulability, thromboinflammation, and acute lung injury. 9 10 11 While pulmonary microthrombosis has been reported in small autopsy series, 12 13 no studies have yet been able to correlate chest CT severity score with the extent of COVID-19-induced coagulopathy or PE. Furthermore, we described a gradual increase in VTE occurrence and the composite risk of ICU transfer and short-term mortality with each increment in the staging. The risk of VTE in the short term was increased when CAHA grade was ≥ 2. Hence, this threshold could be used to consider early aggressive strategies 14 including early VTE imaging screening, intermediate dose or therapeutic (full dose) anticoagulation, and critical care support.
This study shows that the CAHA staging classification provides incremental prognostic value in non-ICU COVID-19 patients. This staging system may be helpful to identify coronavirus patients at higher risk who could benefit from early VTE imaging screening, intermediate dose or therapeutic (full dose) anticoagulation, and early critical care support. In the subset of patients harboring a stage ≥ 2, active clinical and biological surveillance should be considered.
Funding Statement
Funding None declared.
Conflict of Interest M.O. reports personal fees from Canon Medical Systems Europe, outside the submitted work.
Authors' Contributions
B.M.: drafting of the manuscript and critical revision for important intellectual content; A.T.: data collection and drafting of the manuscript; A.C.: data collection and interpretation; A.C.: data collection and interpretation; K.M.: data collection and interpretation; C.S.: data collection and interpretation; I.L.-L.: data collection and interpretation; L.S.: data collection and interpretation; L.G.: data collection and interpretation; M.O.: data collection, interpretation, and critical revision for important intellectual content; P.O.: interpretation of data and critical revision for important intellectual content; L.J.: drafting of the manuscript, interpretation of data, and critical revision for important intellectual content; O.M.: study conception and design, data collection and interpretation, drafting of the manuscript, and critical revision for important intellectual content. The manuscript has been read and approved for submission by all authors. All the authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
References
- 1.Leonard-Lorant I, Delabranche X, Severac F. Acute pulmonary embolism in COVID-19 patients on CT angiography and relationship to D-dimer levels. Radiology. 2020:201561. doi: 10.1148/radiol.2020201561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spiezia L, Boscolo A, Poletto F. COVID-19-related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost. 2020;120(06):998–1000. doi: 10.1055/s-0040-1710018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prevention Treatment of VTE Associated with COVID-19 Infection Consensus Statement Group . Zhai Z, Li C, Chen Y. Prevention and treatment of venous thromboembolism associated with coronavirus disease 2019 infection: a consensus statement before guidelines. Thromb Haemost. 2020;120(06):937–948. doi: 10.1055/s-0040-1710019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Violi F, Pastori D, Cangemi R, Pignatelli P, Loffredo L. Hypercoagulation and antithrombotic treatment in coronavirus 2019: a new challenge. Thromb Haemost. 2020;120(06):949–956. doi: 10.1055/s-0040-1710317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thachil J, Cushman M, Srivastava A. A proposal for staging COVID-19 coagulopathy. Res Pract Thromb Haemost. 2020;4(05):731–736. doi: 10.1002/rth2.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.European Society of Radiology (ESR) and the European Society of Thoracic Imaging (ESTI) . Revel M P, Parkar A P, Prosch H. COVID-19 patients and the radiology department - advice from the European Society of Radiology (ESR) and the European Society of Thoracic Imaging (ESTI) Eur Radiol. 2020:1–7. doi: 10.1007/s00330-020-06865-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou F, Yu T, Du R.Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study Lancet 2020395(10229):1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lippi G, Favaloro E J. D-dimer is associated with severity of coronavirus disease 2019: a pooled analysis. Thromb Haemost. 2020;120(05):876–878. doi: 10.1055/s-0040-1709650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGonagle D, O'Donnell J S, Sharif K, Emery P, Bridgewood C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet. 2020;2(07):E437–E445. doi: 10.1016/S2665-9913(20)30121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ranucci M, Ballotta A, Di Dedda U. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost. 2020;18(07):1747–1751. doi: 10.1111/jth.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciceri F, Beretta L, Scandroglio A M. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. Crit Care Resusc. 2020;22(02):95–97. doi: 10.51893/2020.2.pov2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dolhnikoff M, Duarte-Neto A N, de Almeida Monteiro R A. Pathological evidence of pulmonary thrombotic phenomena in severe COVID-19. J Thromb Haemost. 2020;18(06):1517–1519. doi: 10.1111/jth.14844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ackermann M, Verleden S E, Kuehnel M. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(02):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Global COVID-19 Thrombosis Collaborative Group . Bikdeli B, Madhavan M V, Gupta A. Pharmacological agents targeting thromboinflammation in COVID-19: review and implications for future research. Thromb Haemost. 2020;120(07):1004–1024. doi: 10.1055/s-0040-1713152. [DOI] [PMC free article] [PubMed] [Google Scholar]


