Abstract
Coronavirus disease of 2019 (COVID-19) is the clinical manifestation of the respiratory infection caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). While primarily recognized as a respiratory disease, it is clear that COVID-19 is systemic illness impacting multiple organ systems. One defining clinical feature of COVID-19 has been the high incidence of thrombotic events. The underlying processes and risk factors for the occurrence of thrombotic events in COVID-19 remain inadequately understood. While severe bacterial, viral, or fungal infections are well recognized to activate the coagulation system, COVID-19-associated coagulopathy is likely to have unique mechanistic features. Inflammatory-driven processes are likely primary drivers of coagulopathy in COVID-19, but the exact mechanisms linking inflammation to dysregulated hemostasis and thrombosis are yet to be delineated. Cumulative findings of microvascular thrombosis has raised question if the endothelium and microvasculature should be a point of investigative focus. von Willebrand factor (VWF) and its protease, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS-13), play important role in the maintenance of microvascular hemostasis. In inflammatory conditions, imbalanced VWF-ADAMTS-13 characterized by elevated VWF levels and inhibited and/or reduced activity of ADAMTS-13 has been reported. Also, an imbalance between ADAMTS-13 activity and VWF antigen is associated with organ dysfunction and death in patients with systemic inflammation. A thorough understanding of VWF-ADAMTS-13 interactions during early and advanced phases of COVID-19 could help better define the pathophysiology, guide thromboprophylaxis and treatment, and improve clinical prognosis.
Keywords: COVID-19, thrombosis, inflammation, ADAMTS-13, von Willebrand factor
Introduction
Coronavirus disease of 2019 (COVID-19) is a respiratory illness caused by a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). SARS-CoV-2 is an enveloped, positive-sense single-stranded ribonucleic acid virus belonging to the Coronaviridae family. 1 The COVID-19 outbreak started in Wuhan, China, in late 2019 and rapidly spread to rest of the world. On March 11, 2020, the World Health Organization declared COVID-19 outbreak as pandemic. As of June 24, 2020, the global number of COVID-19 cases stood at 9.26 million with 478,000 deaths (Source: Johns Hopkins Coronavirus Resource Center, https://coronavirus.jhu.edu/ ). Disease course is markedly different between individuals while some are completely asymptomatic, others develop mild symptoms including mild fever, loss of taste or smell, dry cough, sore throat, shortness of breath, and myalgia. 2 3 4 In susceptible individuals, the disease progresses to pneumonia, hypoxemia, acute respiratory distress, and multiorgan dysfunction that may lead to death. 3 The predominance of asymptomatic or mild infections has contributed to the rapid spread of COVID-19 compared with earlier coronavirus outbreaks of SARS and Middle East respiratory syndrome in 2002 and 2012, respectively. 4 5
Consumptive Coagulopathy and the High Incidence of Thrombosis in COVID-19 Patients
Altered coagulation is a common feature of acute systemic diseases, specifically to those affecting primarily the respiratory system. Based on studies in patients with acute respiratory distress syndrome (ARDS), the coexistence of disseminated intravascular coagulation (DIC) with subsequent consumption of procoagulation proteins and platelets has been consistently described. 6 This in turn leads to the formation of microthrombi in the vascular bed of organs resulting from excess coagulation byproducts and suppression of endogenous anticoagulation factors. 7 The coexistence of consumptive coagulopathy and thrombosis are the result of a common pathologic pathway; however, the exact mechanisms that tilts the balance toward thrombosis in COVID-19 are less well understood. 8 In this sense, some features of the coagulopathy associated with COVID-19 may be not unique to this disease; however, the magnitude of the thrombotic response and its impact on mortality suggests the presence of additional mechanisms, beyond what is known for similar respiratory acute inflammatory diseases.
Several studies have linked coagulation abnormalities to severe COVID-19 illness 9 10 ( Table 1 ). In a study evaluating 449 severe COVID-19 patients, Tang et al 11 reported positive correlation of 28-day mortality with fibrin degradation product (FDP), D-dimers and prothrombin time (PT), and negative correlation with platelet count. Laboratory parameters were recorded at the time of onset of severe COVID-19 in the study. In an earlier study comprising 183 patients, Tang et al 12 reported elevated D-dimer levels and FDP levels and prolonged PT and activated partial thromboplastin times (aPTTs) at the time of admission in nonsurvivors compared with survivors. In the same study, significantly lower levels of fibrinogen and antithrombin levels were observed during the late hospitalization in nonsurvivors. Huang et al 13 reported higher D-dimers and prolonged PT at the time of admission in intensive care unit (ICU) patients compared with non-ICU patients in a study of 41 patients. Wang et al 14 reported elevated PT in a study of 138 patients. In the same study, elevated levels of D-dimers were found in ICU patients compared with non-ICU patients as well as in survivors compared with nonsurvivors in a subgroup of patients with a definitive outcome. In a study of 94 COVID-19 patients, Han et al 15 reported lower antithrombin and higher D-dimers, FDP, and fibrinogen levels compared with healthy controls. Zhou et al 16 reported an association of elevated D-dimers with in-hospital death in a study of 191 patients. Also, elevated PT and decreased platelet counts were observed in nonsurvivors compared with survivors. Elevated levels of D-dimers were reported by Richardson et al 17 among 5,700 patients in the New York City area. Ranucci et al 18 reported a procoagulant profile in 16 patients characterized by increased clot strength by viscoelastography, elevated D-dimer levels, and hyperfibrinogenemia. A meta-analysis of 9 studies encompassing 1,779 patients with severe disease has identified significantly lower platelet counts. 19 A subgroup analysis based on survival has identified even lower platelet counts in nonsurvivors in this study. Llitjos et al 20 and Helms et al 7 reported elevated D-dimer and fibrinogen levels in 26 and 150 ICU-admitted patients, respectively. Overall, elevated PT, increased D-dimer and fibrinogen levels, and thrombocytopenia are frequently reported in COVID-19 patients. However, bleeding events requiring therapeutic intervention are not reported.
Table 1. Studies (multiple patients) reporting abnormal coagulopathy in COVID-19.
| Study | Type of study, number of patients | Findings/Significance |
|---|---|---|
| Clinical features of COVID-19 patients, coagulation parameters included | ||
| Huang et al 13 | Prospective, 41 patients | Prothrombin time and D-dimer levels on admission were higher in patients that required ICU treatment |
| Zhou et al 16 | Retrospective, 191 COVID-19 patients | Increased D-dimer on admission is associated with poor prognosis |
| Guan et al 44 | Retrospective, 1,099 COVID-19 patients | Thrombocytopenia in 36.2% |
| Goyal et al 36 | Retrospective, 393 COVID-19 patients | Thrombocytopenia in 27% |
| Zhu et al 45 | Meta-analysis | Elevated D-dimer in ∼37.2% of patients |
| Studies on coagulation parameters | ||
| Ranucci et al 18 | Prospective, 16 ARDS COVID-19 patients | Patients showed a procoagulant profile (clot strength, platelet, fibrinogen, D-dimers, hyperfibrinogenemia) |
| Tang et al 12 | Retrospective, 183 COVID-19 patients | Nonsurvivors had significantly higher D-dimer and fibrin degradation product (FDP) levels, longer prothrombin time, and activated partial thromboplastin time compared with survivors on admission. 71.4% of nonsurvivors and 0.6% survivors met the criteria of DIC during their hospital stay |
| Lippi et al 19 | Meta-analysis | Low platelet count associated with increased risk of severe disease and mortality in patients with COVID-19 |
| Zhang et al 29 | Retrospective, 343 COVID-19 patients | Patients with D-dimer levels ≥2.0 µg/mL had a higher incidence of mortality when comparing to those who with D-dimer levels < 2.0 µg/mL |
| Escher et al 108 109 | Case study, 1 patient and 3 more in the follow-up publication | Continual increase of D-dimers, elevated FVIII activity, and normal platelet counts |
| Bowles et al 112 | 216 COVID-19 patients 34 tested for lupus anticoagulant |
91% of patients tested positive for lupus anticoagulant. All lupus anticoagulant-positive specimens had a prolonged aPTT. Increased aPTT should not be a reason to withhold anticoagulation therapy |
| Lorenzo-Villalba et al 115 | Case reports, 3 patients | Severe thrombocytopenia during COVID-19 infection associated with either cutaneous purpura or mucosal bleeding |
| Yin et al 116 | Retrospective, 449 COVID-19 and 104 non-COVID severe pneumonia | Patients with severe pneumonia induced by SARS-CoV-2 had higher platelet count than those induced by non-SARS-CoV-2. Patients infected by SARS-CoV-2 may benefit from anticoagulant treatment, if they have markedly elevated D-dimer |
| Tabatabai et al 48 | Case series, 10 patients | Elevated FVIII activity and low normal antithrombin and functional protein C activity |
| Thrombosis in the COVID-19 patients | ||
| Middeldorp et al 24 | Retrospective, 198 patients | The cumulative incidences of VTE at 7, 14, and 21 days were 16%, 33%, and 42%, respectively. VTE was higher in the ICU and was associated with death |
| Nahum et al 25 | Prospective, 34 patients | Deep vein thrombosis was found in 22 patients (65%) at admission and in 27 patients (79%) when the venous ultrasonograms performed 48 hours after ICU admission were included. D-dimers and fibrinogen were also increased |
| Cui et al 26 | Retrospective, 81 severe COVID-19 patients | Incidence of VTE at 25%. D-dimer increase has a predictive value |
| Klok et al 22 | Retrospective, 184 patients, no control group | 31% cumulative incidence of symptomatic acute pulmonary embolism (PE), deep vein thrombosis, ischemic stroke, myocardial infarction, or systemic arterial embolism in COVID-19 patients |
| Zhang et al 27 | Prospective, 281 ICU COVID-19 patients | Cumulative incidence of VTE at 28 days was 9.55%, despite all patients receiving thromboprophylaxis |
| Demelo-Rodríguez et al 117 | Prospective, 156 COVID-19 patients | D-dimer levels > 1,570 ng/mL were associated with asymptomatic DVT |
| Grandmaison et al 118 | Cross-sectional study, 58 COVID-19 patients, 29 in the ICU and 29 in the medicine ward | In the ICU, VTEs were found in 17 (58.6%) of the 29 patients In the medicine ward, VTEs were found in 6 (20.7%) patients |
| Fraissé et al 119 | Retrospective, 92 ICU COVID-19 patients | High rate of thrombotic events (TEs) in ICU COVID-19 patients highlighting the necessity for thromboprophylaxis and TE screening. Hemorrhagic events (HEs) were also observed in patients on full-dose anticoagulation |
| Jian et al 114 | Retrospective, 3,218 COVID-19 patients | Acute stroke was the most common neuroimaging finding, present in 1.1% of hospitalized COVID-19 patients |
| Desborough et al 121 | Retrospective, 66 patients | 10 patients had at least one proven episode of thromboembolism. Major bleeding occurred in seven cases |
| Akel et al 122 | Case reports, 6 patients | Patients did not have any hypercoagulable risk factors yet presented with pulmonary embolism |
| Kashi et al 123 | Case reports, 7 patients | Arterial thrombosis |
| Lax et al 124 | Prospective autopsy study, 11 deceased COVID-19 patients | Death may be caused by the thrombosis observed in segmental and subsegmental pulmonary arterial vessels despite the use of prophylactic anticoagulation |
| Thomas et al 125 | Retrospective, 63 COVID-19 patients | High thrombotic risk in patients with COVID-19 |
| Gomez-Arbelaez et al 126 | Case reports, 4 patients | Aortic thrombosis and associated ischemic complications in patients with severe SARS-CoV-2 infection |
| Anticoagulation treatment in COVID-19 patients | ||
| Tang et al 11 | Retrospective, 449 severe COVID-19 patients, 99 received heparin | Anticoagulant therapy is associated with better prognosis in severe COVID-19 patients with sepsis induced coagulopathy or markedly elevated D-dimer |
| Wang et al 28 | 3 case reports | Treatment with tissue plasminogen activator lead to improvement in the respiratory status |
| Ayerbe et al 127 | 2,075 COVID-19 patients, admitted in 17 hospitals in Spain | Heparin had been used in 1,734 patients. Heparin was associated with lower mortality |
| Wang et al 128 | Retrospective, 1,099 COVID-19 patients | High risk of venous thromboembolism, also high risk of bleeding |
| Artifoni et al 129 | Retrospective, 62 patients | 16 patients developed VTE, 7 patients developed PE Very high negative predictive value of baseline D-dimer level for VTE and PE |
| Russo et al 130 | Retrospective, 192 COVID-19 patients | Preadmission antithrombotic therapy, both antiplatelet and anticoagulant, does not seem to show a protective effect in severe forms of COVID-19 with ARDS at presentation and rapidly evolving toward death |
| Link between SARS-CoV-2 and thrombosis | ||
| Ackermann et al 21 | 7 lung autopsies from COVID-19 patients and 7 from ARDS | Vascular angiogenesis distinguished the pulmonary pathobiology of COVID-19 from that of equally severe influenza virus infection |
| Maier et al 131 | Case studies 15 COVID-19 patients with hyperviscosity |
Possible causal relationship between hyperviscosity and thrombotic complications in COVID-19 |
| Huisman et al 105 | 12 COVID-19 patients | Low ADAMTS-13 activity, increased VWF levels and factor VIII levels |
| Galeano-Valle et al 111 | Prospective study, 24 patients | Prevalence of antiphospholipid antibodies in COVID-19 and venous thrombosis was low |
| Magro et al 132 | Case reports, 5 severe COVID-19 cases | Procoagulant state is associated with systemic complement activation |
Abbreviations: ADAMTS-13, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; aPTT, activated partial thromboplastin time; ARDS, acute respiratory distress syndrome; COVID-19, coronavirus disease of 2019; DIC, disseminated intravascular coagulation; DVT, deep vein thrombosis; FVIII, factor VIII; ICU, intensive care unit; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; VTE, venous thromboembolism; VWF, von Willebrand factor.
Multiple studies have reported a higher incidence of thrombotic events, particularly pulmonary embolism, as a frequent complication in COVID-19 patients ( Table 1 ). Llitjos et al 20 reported overall rate of 69% venous thromboembolism (VTE) in severe COVID-19 patients admitted to ICU. In this study, VTE incidence was found to be significantly higher in patients treated with prophylactic anticoagulation compared with those treated with therapeutic anticoagulation. Helms et al 7 reported 64 clinically relevant thrombotic complications in 150 ICU-admitted patients. Importantly, the incidence of thrombotic complications in COVID-19 ARDS patients was significantly higher than non-COVID-19 ARDS patients in this study. Ackermann et al 21 compared lung sections of COVID-19 patients with those died from ARDS secondary to influenza A (H1N1) infection and found relatively higher: (1) endothelial cell injury, (2) alveolar microthrombi (ninefold), and (3) intussusceptive angiogenesis in COVID-19 lung sections. Similarly, higher incidence of thromboembolic complications in ICU-admitted COVID-19 patients was also reported by Klok et al (31%), 22 Lodigiani et al (27.6%), 23 Middeldorp et al (47%), 24 Nahum et al (79%), 25 and Cui et al (25%). 26 For comparison, in a study by Zhang et al, the reported cumulative incidence of VTE in ICU-admitted patients receiving guideline-recommended thromboprophylaxis was 9.55% (95% confidence interval: 6.55–13.81). 27
A high incidence of DIC diagnosed by D-dimer, fibrinogen, and antithrombin III levels has become a focus for the initiation of anticoagulation therapy in severe COVID-19 patients, 28 with some studies relying on D-dimers alone. 11 29 A retrospective analysis of 183 patients performed by Tang et al 12 suggested that more than 70% of severe COVID-19 patients who succumb to the infection demonstrate increased risk of thrombosis, further this group suggests that all of these patients meet the International Society on Thrombosis and Haemostasis definition of DIC. Subsequently, Tang et al 11 reported an equivalent 28-day mortality rate (30%) in 99 patients receiving low molecular weight or unfractionated heparin for 7 days compared with 350 nonheparin treated patients or those receiving a less than 7-day course of therapy. A case series reported by Wang et al 28 detailed the use and outcome following tissue plasminogen activator (tPA) in three patients with ARDS and coagulopathy consistent with DIC. Intravenous dosing with tPA indicated a potential benefit in each of the three cases of COVID-19. However, this study also warns of both unrelated effects and high risk of severe bleeding secondary to off-label tPA use. Several of the studies in coagulopathic COVID-19 patients suspected of DIC rely heavily on analysis of fibrin degradation and D-dimer levels, which are expected to be increased during DIC, arterial and venous thromboses, strokes, and thrombotic microangiopathies. 30 However, D-dimers are a nonspecific indicator of thrombosis in severe COVID-19 patients with pulmonary injury. Fibrin accumulation and lysis continuously occur during nonthrombotic inflammation as well as tissue necrosis, and therefore, significant D-dimer elevations also accumulate during cancers 31 and infections, consistent with inflammatory processes that coincide with the progression of severe COVID-19-related macrophage activation syndrome. 32 Therefore, we suggest that more comprehensive and robust assays be used to evaluate changes in hemostasis. For example, to date the use of thrombin, plasmin, or simultaneous thrombin/plasmin generation assays have not been reported within the context of hemostasis management of COVID-19 patients. Since their introduction thrombin and plasmin generation assays have been highly informative regarding the assessment of hemorrhage, coagulation, and fibrinolysis. 33 34 Assessment of impairment of these systems would provide a useful and appropriate guidance needed for and monitoring of therapeutic interventions in the unique coagulopathies associated with COVID-19. 33 34 Because patients are often on unfractionated or low molecular weight heparin and plasminogen activator inhibitor 1, von Willebrand factor (VWF), plasminogen, fibrinogen, and factor VIII are all reported to be elevated in SARS infection, 35 and therefore careful modification of these assays may be warranted to optimize the concentrations of added tPA, tissue factor, and thrombomodulin.
These studies present a heterogeneous picture that is difficult to evaluate in the aggregate. Inclusion criteria for patients varied across these studies, making direct comparisons between the studies difficult. Further, the studies used different regimens of thromboprophylaxis, which could impact outcomes. In some studies, a high proportion of patients were still hospitalized at the end of the reporting period; conclusions and clinical courses therefore were based on incomplete information, and completion of these patients' clinical course could alter the final conclusions. The picture of coagulopathy in COVID-19 is complex. Specific, sensitive, and temporal assessments of coagulation and fibrinolysis should be established and further work is needed to untangle the roles of the host inflammatory response, preexisting thrombotic risk, and prehospitalization pharmacologic regimens in the optimal management of coagulopathy in the setting of COVID-19.
Inflammation, Liver Injury, and Hypoxia in COVID-19 Patients
The risk of hospitalization, morbidity, and mortality from COVID-19 is highest for older patients with preexisting conditions such as hypertension, diabetes, cardiovascular disease, and obesity. 13 14 16 17 36 37 A common theme of all these comorbidities is their association with vascular inflammation and endothelial dysfunction. 38 39 Proinflammatory conditions affect hemostasis by blocking of fibrinolysis and induction of prothrombotic conditions through activation of endothelial cells and innate immune cells via release of several factors including tissue factor, VWF, and neutrophil extracellular traps (NETs) that promote thrombosis. 40 Induction of proinflammatory conditions was reported in the pathophysiology of several viral diseases including influenza and SARS. 41 Increased inflammation is commonly observed in COVID-19 patients, while severe cases are characterized by immune dysregulation and hyperinflammation, with a markedly increased serum interleukin (IL)-6. 42 Cytokine release syndrome has also been reported in COVID-19 patients and correlates with adverse clinical outcomes. 43 The presence of several inflammatory markers such as C-reactive protein, procalcitonin, ferritin, and fibrinogen are often reported in COVID-19 patients 13 14 16 17 36 37 44 45 46 47 48 ( Table 2 ). Further, multiple studies reported elevated levels of the proinflammatory cytokine IL-6 in severe cases of COVID-19 16 37 42 47 49 50 51 52 53 ( Table 2 ). A concurrent increase in the levels of anti-inflammatory cytokine IL-10, probably in response to overwhelming systemic inflammation, was also observed in several studies. The role of IL-6, in particular, is considered central in the pathogenesis of COVID-19 complications, 54 and therefore tocilizumab, an IL-6 inhibitor, is being used in ongoing clinical trials to prevent catastrophic inflammation. 55 56 57 58
Table 2. Studies reporting elevated inflammatory markers in COVID-19.
| Study | Patient group (number of patients) comparison | Elevated inflammatory markers |
|---|---|---|
| Huang et al 13 | ICU (13) vs. non-ICU (28) | Procalcitonin, IL-1β, IFN-γ, IP10, and MCP1 |
| Wang et al 14 | ICU (36) vs. non-ICU (102) | Procalcitonin |
| Zhou et al 16 | Nonsurvivor (54) vs. survivor (137) | Procalcitonin, ferritin, and IL-6 |
| Richardson et al 17 | Relative to reference range (3066) | Procalcitonin, ferritin, and CRP |
| Ruan et al 37 | Nonsurvivor (68) vs. survivor (82) | CRP and IL-6 |
| Giamarellos-Bourboulis et al 42 | Dysregulated (21) vs. intermediate state (26) of immune activation | CRP and IL-6 |
| Chen et al 47 | Severe (≥9) vs. moderate (≥7) | CRP, ferritin, IL-6, and TNF-α |
| Han et al 49 | COVID-19 patients (102) vs. controls (45) | CRP, IL-6, TNF-α, and IFN-γ |
| Du et al 50 | Mild pneumonia (124) vs. no pneumonia (54) (pediatric patients) | Procalcitonin, IL-6, TNF-α, and IFN-γ |
| Wang et al 52 |
SpO
2
≥90% (≥ 36) vs.
SpO 2 < 90% (≥7) |
CRP and IL-6 |
| Tan et al 53 | Severe (25) vs. mild/moderate 31) | CRP and IL-6 |
| Tabatabai et al 48 | Relative to reference range (10) | Fibrinogen, CRP, and ferritin |
Abbreviations: COVID-19, coronavirus disease of 2019; CRP, C-reactive protein; ICU, intensive care unit; IFN-γ, interferon-γ; IL-1β, interleukin-1β; IL-6, Interluekin-6; IP-10, interferon-γ induced protein 10; MCP-1, monocyte chemotactic protein-1; SpO 2 , blood oxygen saturation level; TNF-α, tumor necrosis factor-α.
Liver injury during COVID-19 infections was described in multiple studies, including elevated levels of alanine aminotransferase, aspartate aminotransferase, and bilirubin. 14 16 17 36 44 47 The liver is the primary source of plasma proteins, particularly those involved in hemostasis. Thus, the occurrence of liver injury may contribute further to derangements of key hemostasis proteins and contributes to coagulopathy. 59 Similarly, hypoxemia observed in COVID-19 patients induces prothrombotic conditions through upregulation of plasminogen activator inhibitor and stimulation of endothelial synthesis of procoagulants, including tissue factor and VWF. 60 61 62 63 Thus, multiple clinical characteristics observed in COVID-19 patients contribute to altered coagulation and lead to increased incidence of thrombosis. However, the early onset of coagulopathy—before systemic organic effects occur—suggests proinflammatory conditions as the primary driving cause of thrombotic events in COVID-19 patients.
VWF-ADAMTS-13 in Hemostasis and Thrombosis
VWF and its cleaving protease, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS-13), play an important role in hemostasis particularly within the microvasculature. 64 VWF is a large multimeric glycoprotein primarily expressed by endothelial cells and platelets. Endothelial cells show both basal secretion and regulated release of VWF stored in Weibel–Palade bodies in response to various stimuli. On the other hand, platelets secrete VWF stored in α-granules only upon activation. 65 ADAMTS-13 is expressed both by hepatic stellate cells and endothelial cells; the relative contribution of hepatic and microvascular expression is not clear. 66 ADAMTS-13 regulates the biological activity of VWF by cleaving prothrombotic ultra-large VWF multimers (> 10,000 kDa) secreted from endothelial cells into hemostatically active high molecular weight multimers (< 10,000 kDa) under shear stress conditions. 67 Severe deficiency of ADAMTS-13 results in accumulation of ultra-large VWF multimers leading to microvascular thrombosis and consumptive thrombocytopenia, a condition termed thrombotic thrombocytopenic purpura (TTP). 64 In the event of vascular injury, VWF facilitates binding of platelets to subendothelium through its interactions with glycoprotein Ib and collagen, thereby inducing thrombus formation. 64 A reciprocal relationship exists between VWF and ADAMTS-13 levels where elevated circulatory VWF antigen levels are associated with concomitant decrease in ADAMTS-13 activity and vice versa. 68 69 70 Abnormal VWF-ADAMTS-13 ratios are implicated in arterial thrombosis, 71 ischemic stroke, 72 73 pediatric stroke, 74 and perioperative thrombosis in infants. 75 In addition, abnormal VWF/ADAMTS-13 metabolism has been positively associated with myocardial infarction in young women. 76 It is worth highlighting that in the case of perioperative thrombosis, elevated VWF even in the absence of significant deficiency of ADAMTS-13 was associated with thrombosis. 75 Severe hypoxia and acidosis likely caused a higher increase in VWF during cardiac surgery and were at higher risk of thrombosis. 75
Elevated levels of VWF are found in several inflammatory and metabolic disorders including diabetes, obesity, and sickle cell disease. 77 In patients with systemic inflammatory response syndrome, active VWF predicted 28-day mortality. 78 VWF is an acute-phase response protein released by activated endothelial cells in response to inflammatory stimuli. 77 Inflammatory cytokines, IL-8 and tumor necrosis factor-α induced the release of VWF from human umbilical vein endothelial cells. 79 VWF released in inflammation binds to NETs released from activated neutrophils and recruits platelets and leukocytes to promote thrombosis. 77 ADAMTS-13 deficiency in inflammatory conditions was demonstrated to promote VWF-dependent leukocyte adhesion and extravasation in mice. 80
In patients with systemic inflammation, ADAMTS-13 activity decreases proportional to the inflammatory response; an imbalance between ADAMTS-13 activity and VWF antigen is associated with organ dysfunction and death. 81 82 Dysregulated host response to infection including inflammation can result in septic shock. In septic shock, ADAMTS-13 activity was significantly lower 83 84 85 and elevated ratio of VWF propeptide (VWFpp) that is secreted along with ultra-large VWF multimers in to blood stream and ADAMTS-13 was associated with disease severity. 86 In patients with DIC, ADAMTS-13 activity decreased with DIC score 87 and VWFpp/ADAMTS-13 ratio was significantly elevated in nonsurvivors compared with survivors. 88 An interesting observation is that smoking, which is associated with adverse outcomes in COVID-19 patients, 89 was also found to be associated with decreased plasma ADAMTS-13 levels in a study of 3,244 individuals. 90 Increased expression of angiotensin-converting enzyme 2, the entry receptor for SARS-CoV-2, in the small airway epithelia of smokers was suggested as the potential mechanism for increased risk of severe COVID-19 in smokers. 91 Smoking is also associated with increased inflammatory markers. 92
The imbalance between ADAMTS-13 and VWF in heightened inflammation could be a result of inhibition and/or deficiency of ADAMTS-13 activity. 93 The inhibition of VWF cleavage by ADAMTS-13 in inflammatory conditions was suggested to be mediated by several mechanisms: (1) thrombospondin-1 released from α-granules of activated platelets by binding to the A2-A3 domain of VWF 94 95 ; (2) α-defensins released from neutrophils by binding to the A2 domain of VWF 96 ; and (3) oxidation of Met 1606 residue in the ADAMTS-13 cleavage site of VWF. 97 Moreover, nonphysiological high concentrations of IL-6 have been shown to inhibit cleavage of VWF by ADAMTS-13 in vitro under shear flow conditions. 79 Granulocyte elastases, plasmin, and thrombin that are elevated in inflammatory conditions lower ADAMTS-13 activity through its proteolytic cleavage. 98 99
VWF-ADAMTS-13 Interactions in COVID-19
Despite playing an important role in the maintenance of hemostasis and the occurrence of micro- and macrovascular thrombosis, VWF-ADAMTS-13 interactions have not received much investigative attention in the evaluation of COVID-19 pathophysiology, specifically in relation to elevated incidence of VTE. Importantly, reduced ADAMTS-13 activity has been shown to correlate with increased inflammation in multiple systems, 100 101 102 while IL-6 has been shown to inhibit the cleavage of ultra-large VWF strings by ADAMTS-13 under flowing conditions. 79 103 The authors could find only five studies evaluating both VWF and ADAMTS-13 levels in COVID-19 patients in literature 104 105 106 107 108 ( Table 3 ). Majority of these studies reported lower ADAMTS-13 activity concurrent with higher VWF in COVID-19 patients. 104 105 106 107 In one of these studies, Bazzan et al 104 reported lower ADAMTS-13 levels in 88 COVID-19 patients compared with healthy controls (48.71 ± 18.7% vs. healthy control, 108 ± 9.1%; normal value 60–130%). Within patient cohort, lower ADAMTS-13 and higher VWF levels were found in nonsurvivors (9/88) compared with survivors. Further, lower than 30% ADAMTS-13 activity were significantly associated with mortality in survivor analysis. Huisman et al 105 observed low ADAMTS-13 activity levels (0.48 ± 0.14 IU/mL against a reference range of 0.61–1.31) in parallel with elevated VWF antigen and activity (∼ fourfold) in 12 ICU-admitted patients. A similar reduction in ADAMTS-13 and increased VWF levels was also reported by Adam et al 106 and Latimer et al 107 in 4 adult and 1 pediatric patients, respectively. On the other hand, Escher et al 108 observed normal to lower-normal ADAMTS-13 levels concurrently with > 2.5-fold increase in VWF antigen and activity in 3 ICU-admitted patients. Two other studies 7 109 reported VWF measurements alone, observing > threefold increase in both VWF antigen and activity. From the limited number of studies so far, it appears that COVID-19 infection may be characterized by markedly elevated VWF levels and below normal ADAMTS-13 activity. However, the current literature is limited by the small number of studies and variable timing of VWF/ADAMTS-13 measurements in relation to disease onset. Further evaluation of VWF and ADAMTS-13 interactions in large patient cohorts are warranted to more confidently understand their contributions to COVID-19 pathogenesis.
Table 3. Studies reporting ADAMTS-13 and VWF levels in COVID-19.
| Study | Patient group (number of patients) comparison | Findings/Significance |
|---|---|---|
| Bazzan et al 104 | Nonsurvivor (9) vs. survivor (79) | Lower ADAMTS-13 and elevated VWF levels in nonsurvivors compared with survivors. After survival analysis, lower than 30% ADAMTS-13 levels were significantly associated with higher mortality |
| Huisman et al 105 | Relative to reference range (12) | Lower ADAMTS-13 and elevated VWF levels |
| Adam et al 106 | Relative to reference range (4) | Lower ADAMTS-13 and elevated VWF levels |
| Latimer et al 107 | Relative to reference range (1 pediatric patient) | Lower ADAMTS-13 and elevated VWF levels |
| Escher et al 108 109 | Case study, 1 patient and 3 more in the follow-up publication | Massive elevation of VWF and normal to lower-normal ADAMTS-13 activity. COVID-19 coagulopathy may be a distinct entity of highly prothrombotic alterations most probably an endothelial disease |
| Helms et al 7 | Relative to reference range (150) | Elevated VWF levels |
Abbreviations: ADAMTS-13, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; COVID-19, coronavirus disease of 2019; VWF, von Willebrand factor.
A secondary mechanism potentially contributing to ADAMTS-13 deficiency relates to the antiphospholipid antibody generation during SARS-CoV-2 infection. 7 110 111 112 Antiphospholipid antibodies have been inconsistently reported in all cases of COVID 19, 7 111 112 but strongly associated to prolong aPTT as reported by Bowles et al. 112 Patients with antiphospholipid syndrome have been found to have abnormal ADAMTS-13 plasmatic activity further increasing the risk of thrombosis. 113 The exact mechanisms by which antiphospholipid antibodies interfere with ADAMTS-13 cleaving activity are unclear. We speculate that antiphospholipid antibodies generated during active SARS-CoV-2 infection can potentially bind the spacer domain of ADAMTS-13 interfering with the recognition and proteolysis of VWF. Such a mechanism is similar to the binding of autoantibodies against ADAMTS-13 present in TTP resulting in clinical thrombosis. 114
Based on the limited available data, we propose a mechanistic model in which: (1) SARS-CoV-2 causes endothelial activation and damage leading to overwhelming VWF release and (2) proinflammatory mediators or antibodies during the severe phase of COVID-19 result in reduced cleavage of high molecular weight VWF by ADAMTS-13, ultimately leading to thrombosis, see Fig. 1 . This concept should be confirmed by large patient cohorts that encompass mild and severe clinical courses of COVID-19 disease. A mechanistic understanding of thrombosis during COVID-19 infection is greatly needed to better guide thromboprophylaxis and treatment. The extent to which VWF-ADAMTS-13 interactions contribute to the pathophysiology of COVID-19 should be an important investigative focus.
Fig. 1.
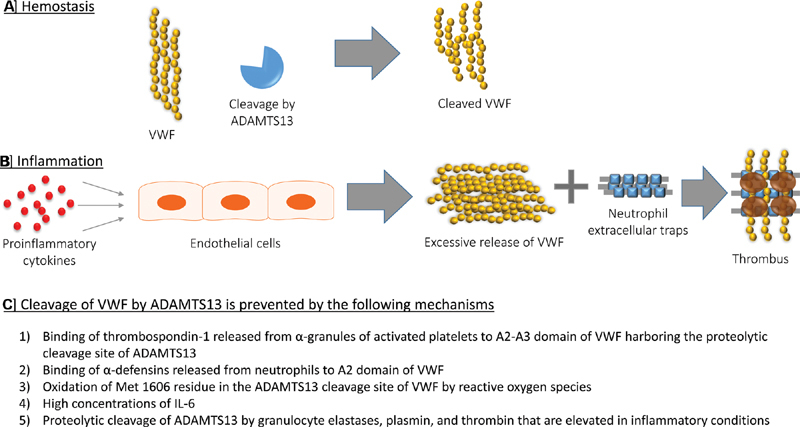
von Willebrand factor (VWF)-a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS-13) metabolism in inflammation. ( A ) During normal homeostasis, ADAMTS-13 regulates the activity of VWF by cleaving prothrombotic ultra-large VWF multimers released from endothelial cells in to hemostatically active high molecular weight multimers. ( B ) In inflammatory disorders, proinflammatory cytokines (e.g., interleukin [IL]-8 and tumor necrosis factor [TNF]-α) stimulate excess release of VWF stored in Weibel–Palade bodies of endothelial cells. VWF interacts with neutrophil extracellular traps (NETs) released from neutrophils to provide a scaffold for platelet adhesion and thrombus formation. ( C ) In inflammation, cleavage of VWF by ADAMT-S13 is prevented by multiple mechanisms that either inhibit or reduce the proteolytic activity of ADAMTS-13.
Funding Statement
Funding This work was partly supported by funds from the Hemostasis Branch/Division of Plasma Protein Therapeutics/Office of Tissues and Advanced Therapies/Center for Biologics Evaluation and Research of the U.S. Food and Drug Administration. This research was also supported by the Intramural Research Program of the National Library of Medicine at the NIH.
Footnotes
Conflict of interest None declared.
References
- 1.Lu R, Zhao X, Li J.Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding Lancet 2020395(10224):565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention . The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China [in Chinese] Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(02):145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Singhal T. A review of coronavirus disease-2019 (COVID-19) Indian J Pediatr. 2020;87(04):281–286. doi: 10.1007/s12098-020-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rothe C, Schunk M, Sothmann P. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382(10):970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petrosillo N, Viceconte G, Ergonul O, Ippolito G, Petersen E. COVID-19, SARS and MERS: are they closely related? Clin Microbiol Infect. 2020;26(06):729–734. doi: 10.1016/j.cmi.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Japanese Association for Acute Medicine (JAAM) Focused Outcomes Research in Emergency Care in Acute Respiratory Distress Syndrome, Sepsis and Trauma (FORECAST) Study Group . Gando S, Fujishima S, Saitoh D. The significance of disseminated intravascular coagulation on multiple organ dysfunction during the early stage of acute respiratory distress syndrome. Thromb Res. 2020;191:15–21. doi: 10.1016/j.thromres.2020.03.023. [DOI] [PubMed] [Google Scholar]
- 7.CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis) . Helms J, Tacquard C, Severac F. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(06):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boral B M, Williams D J, Boral L I. Disseminated intravascular coagulation. Am J Clin Pathol. 2016;146(06):670–680. doi: 10.1093/ajcp/aqw195. [DOI] [PubMed] [Google Scholar]
- 9.Willyard C.Coronavirus blood-clot mystery intensifies Nature 2020581(7808):250. [DOI] [PubMed] [Google Scholar]
- 10.Thachil J, Tang N, Gando S. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(05):1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(05):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(04):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang C, Wang Y, Li X.Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China Lancet 2020395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang D, Hu B, Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han H, Yang L, Liu R. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58(07):1116–1120. doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- 16.Zhou F, Yu T, Du R.Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study Lancet 2020395(10229):1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.and the Northwell COVID-19 Research Consortium . Richardson S, Hirsch J S, Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ranucci M, Ballotta A, Di Dedda U. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost. 2020;18(07):1747–1751. doi: 10.1111/jth.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lippi G, Plebani M, Henry B M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Llitjos J F, Leclerc M, Chochois C. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. 2020;18(07):1743–1746. doi: 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ackermann M, Verleden S E, Kuehnel M. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(02):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klok F A, Kruip M JHA, van der Meer N JM. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Humanitas COVID-19 Task Force . Lodigiani C, Iapichino G, Carenzo L. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Middeldorp S, Coppens M, van Haaps T F.Incidence of venous thromboembolism in hospitalized patients with COVID-19J Thromb Haemost2020 [DOI] [PMC free article] [PubMed]
- 25.Nahum J, Morichau-Beauchant T, Daviaud F. Venous thrombosis among critically ill patients with coronavirus disease 2019 (COVID-19) JAMA Netw Open. 2020;3(05):e2010478. doi: 10.1001/jamanetworkopen.2020.10478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(06):1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang C, Zhang Z, Mi J. The cumulative venous thromboembolism incidence and risk factors in intensive care patients receiving the guideline-recommended thromboprophylaxis. Medicine (Baltimore) 2019;98(23):e15833. doi: 10.1097/MD.0000000000015833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J, Hajizadeh N, Moore E E. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a case series. J Thromb Haemost. 2020;18(07):1752–1755. doi: 10.1111/jth.14828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang L, Yan X, Fan Q. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost. 2020;18(06):1324–1329. doi: 10.1111/jth.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Urban K, Kirley K, Stevermer J J. PURLs: it's time to use an age-based approach to D-dimer. J Fam Pract. 2014;63(03):155–158. [PMC free article] [PubMed] [Google Scholar]
- 31.Schäfer M, Werner S. Cancer as an overhealing wound: an old hypothesis revisited. Nat Rev Mol Cell Biol. 2008;9(08):628–638. doi: 10.1038/nrm2455. [DOI] [PubMed] [Google Scholar]
- 32.Merad M, Martin J C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20(06):355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hemker H C, Giesen P, Al Dieri R. Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol Haemost Thromb. 2003;33(01):4–15. doi: 10.1159/000071636. [DOI] [PubMed] [Google Scholar]
- 34.Simpson M L, Goldenberg N A, Jacobson L J, Bombardier C G, Hathaway W E, Manco-Johnson M J. Simultaneous thrombin and plasmin generation capacities in normal and abnormal states of coagulation and fibrinolysis in children and adults. Thromb Res. 2011;127(04):317–323. doi: 10.1016/j.thromres.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 35.Wu Y P, Wei R, Liu Z H. Analysis of thrombotic factors in severe acute respiratory syndrome (SARS) patients. Thromb Haemost. 2006;96(01):100–101. doi: 10.1160/TH05-12-0827. [DOI] [PubMed] [Google Scholar]
- 36.Goyal P, Choi J J, Pinheiro L C. Clinical characteristics of Covid-19 in New York City. N Engl J Med. 2020;382(24):2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(05):846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petrie J R, Guzik T J, Touyz R M. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can J Cardiol. 2018;34(05):575–584. doi: 10.1016/j.cjca.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Milan-Mattos J C, Anibal F F, Perseguini N M. Effects of natural aging and gender on pro-inflammatory markers. Braz J Med Biol Res. 2019;52(09):e8392. doi: 10.1590/1414-431X20198392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2013;13(01):34–45. doi: 10.1038/nri3345. [DOI] [PubMed] [Google Scholar]
- 41.Jamilloux Y, Henry T, Belot A. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun Rev. 2020;19(07):102567. doi: 10.1016/j.autrev.2020.102567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giamarellos-Bourboulis E J, Netea M G, Rovina N. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27(06):992–1.0E6. doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang C, Wu Z, Li J W, Zhao H, Wang G Q. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55(05):105954. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.China Medical Treatment Expert Group for Covid-19 . Guan W J, Ni Z Y, Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu J, Ji P, Pang J. Clinical characteristics of 3,062 COVID-19 patients: a meta-analysis. J Med Virol. 2020 doi: 10.1002/jmv.25884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Connors J M, Levy J H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen G, Wu D, Guo W. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(05):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tabatabai A, Rabin J, Menaker J. Factor VIII and functional protein C activity in critically ill patients with coronavirus disease 2019: a case series. A A Pract. 2020;14(07):e01236. doi: 10.1213/XAA.0000000000001236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han H, Ma Q, Li C. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect. 2020;9(01):1123–1130. doi: 10.1080/22221751.2020.1770129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Du H, Dong X, Zhang J J.Clinical characteristics of 182 pediatric COVID-19 patients with different severities and allergic statusAllergy2020 [DOI] [PMC free article] [PubMed]
- 51.Liu J, Li S, Liu J. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2020:ciaa272. doi: 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tan M, Liu Y, Zhou R. Immunopathological characteristics of coronavirus disease 2019 cases in Guangzhou, China. Immunology. 2020;160(03):261–268. doi: 10.1111/imm.13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen X, Zhao B, Qu Y. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. Clin Infect Dis. 2020:ciaa449. doi: 10.1093/cid/ciaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tocilizumab in COVID-19 Pneumonia (TOCIVID-19)Accessed July 29, 2020 at:https://ClinicalTrials.gov/show/NCT04317092
- 56.Tocilizumab for Prevention of Respiratory Failure in Patients With Severe COVID-19 InfectionAccessed July 29, 2020 at:https://ClinicalTrials.gov/show/NCT04377659
- 57.Efficacy of Early Administration of Tocilizumab in COVID-19 PatientsAccessed July 29, 2020 at:https://ClinicalTrials.gov/show/NCT04346355
- 58.Tocilizumab in the Treatment of Coronavirus Induced Disease (COVID-19)Accessed July 29, 2020 at:https://ClinicalTrials.gov/show/NCT04335071
- 59.Premkumar M, Saxena P, Rangegowda D. Coagulation failure is associated with bleeding events and clinical outcome during systemic inflammatory response and sepsis in acute-on-chronic liver failure: an observational cohort study. Liver Int. 2019;39(04):694–704. doi: 10.1111/liv.14034. [DOI] [PubMed] [Google Scholar]
- 60.Mojiri A, Nakhaii-Nejad M, Phan W L. Hypoxia results in upregulation and de novo activation of von Willebrand factor expression in lung endothelial cells. Arterioscler Thromb Vasc Biol. 2013;33(06):1329–1338. doi: 10.1161/ATVBAHA.113.301359. [DOI] [PubMed] [Google Scholar]
- 61.Matsuura Y, Yamashita A, Iwakiri T. Vascular wall hypoxia promotes arterial thrombus formation via augmentation of vascular thrombogenicity. Thromb Haemost. 2015;114(01):158–172. doi: 10.1160/TH14-09-0794. [DOI] [PubMed] [Google Scholar]
- 62.Ogawa S, Clauss M, Kuwabara K. Hypoxia induces endothelial cell synthesis of membrane-associated proteins. Proc Natl Acad Sci U S A. 1991;88(21):9897–9901. doi: 10.1073/pnas.88.21.9897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fearns C, Loskutoff D J. Induction of plasminogen activator inhibitor 1 gene expression in murine liver by lipopolysaccharide. Cellular localization and role of endogenous tumor necrosis factor-alpha. Am J Pathol. 1997;150(02):579–590. [PMC free article] [PubMed] [Google Scholar]
- 64.Katneni U K, Ibla J C, Hunt R, Schiller T, Kimchi-Sarfaty C. von Willebrand factor/ADAMTS-13 interactions at birth: implications for thrombosis in the neonatal period. J Thromb Haemost. 2019;17(03):429–440. doi: 10.1111/jth.14374. [DOI] [PubMed] [Google Scholar]
- 65.Bryckaert M, Rosa J P, Denis C V, Lenting P J. Of von Willebrand factor and platelets. Cell Mol Life Sci. 2015;72(02):307–326. doi: 10.1007/s00018-014-1743-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zheng X L. ADAMTS13 and von Willebrand factor in thrombotic thrombocytopenic purpura. Annu Rev Med. 2015;66:211–225. doi: 10.1146/annurev-med-061813-013241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stockschlaeder M, Schneppenheim R, Budde U. Update on von Willebrand factor multimers: focus on high-molecular-weight multimers and their role in hemostasis. Blood Coagul Fibrinolysis. 2014;25(03):206–216. doi: 10.1097/MBC.0000000000000065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mannucci P M, Capoferri C, Canciani M T. Plasma levels of von Willebrand factor regulate ADAMTS-13, its major cleaving protease. Br J Haematol. 2004;126(02):213–218. doi: 10.1111/j.1365-2141.2004.05009.x. [DOI] [PubMed] [Google Scholar]
- 69.Reiter R A, Knöbl P, Varadi K, Turecek P L. Changes in von Willebrand factor-cleaving protease (ADAMTS13) activity after infusion of desmopressin. Blood. 2003;101(03):946–948. doi: 10.1182/blood-2002-03-0814. [DOI] [PubMed] [Google Scholar]
- 70.Reiter R A, Varadi K, Turecek P L, Jilma B, Knöbl P. Changes in ADAMTS13 (von-Willebrand-factor-cleaving protease) activity after induced release of von Willebrand factor during acute systemic inflammation. Thromb Haemost. 2005;93(03):554–558. doi: 10.1160/TH04-08-0467. [DOI] [PubMed] [Google Scholar]
- 71.Sonneveld M A, de Maat M P, Leebeek F W. Von Willebrand factor and ADAMTS13 in arterial thrombosis: a systematic review and meta-analysis. Blood Rev. 2014;28(04):167–178. doi: 10.1016/j.blre.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 72.Bongers T N, de Maat M P, van Goor M L. High von Willebrand factor levels increase the risk of first ischemic stroke: influence of ADAMTS13, inflammation, and genetic variability. Stroke. 2006;37(11):2672–2677. doi: 10.1161/01.STR.0000244767.39962.f7. [DOI] [PubMed] [Google Scholar]
- 73.Sonneveld M A, de Maat M P, Portegies M L. Low ADAMTS13 activity is associated with an increased risk of ischemic stroke. Blood. 2015;126(25):2739–2746. doi: 10.1182/blood-2015-05-643338. [DOI] [PubMed] [Google Scholar]
- 74.Lambers M, Goldenberg N A, Kenet G. Role of reduced ADAMTS13 in arterial ischemic stroke: a pediatric cohort study. Ann Neurol. 2013;73(01):58–64. doi: 10.1002/ana.23735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hunt R, Hoffman C M, Emani S. Elevated preoperative von Willebrand factor is associated with perioperative thrombosis in infants and neonates with congenital heart disease. J Thromb Haemost. 2017;15(12):2306–2316. doi: 10.1111/jth.13860. [DOI] [PubMed] [Google Scholar]
- 76.Andersson H M, Siegerink B, Luken B M. High VWF, low ADAMTS13, and oral contraceptives increase the risk of ischemic stroke and myocardial infarction in young women. Blood. 2012;119(06):1555–1560. doi: 10.1182/blood-2011-09-380618. [DOI] [PubMed] [Google Scholar]
- 77.Gragnano F, Sperlongano S, Golia E. The role of von Willebrand factor in vascular inflammation: from pathogenesis to targeted therapy. Mediators Inflamm. 2017;2017:5.620314E6. doi: 10.1155/2017/5620314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hyseni A, Kemperman H, de Lange D W, Kesecioglu J, de Groot P G, Roest M. Active von Willebrand factor predicts 28-day mortality in patients with systemic inflammatory response syndrome. Blood. 2014;123(14):2153–2156. doi: 10.1182/blood-2013-08-508093. [DOI] [PubMed] [Google Scholar]
- 79.Bernardo A, Ball C, Nolasco L, Moake J F, Dong J F. Effects of inflammatory cytokines on the release and cleavage of the endothelial cell-derived ultralarge von Willebrand factor multimers under flow. Blood. 2004;104(01):100–106. doi: 10.1182/blood-2004-01-0107. [DOI] [PubMed] [Google Scholar]
- 80.Chauhan A K, Kisucka J, Brill A, Walsh M T, Scheiflinger F, Wagner D D. ADAMTS13: a new link between thrombosis and inflammation. J Exp Med. 2008;205(09):2065–2074. doi: 10.1084/jem.20080130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bockmeyer C L, Claus R A, Budde U. Inflammation-associated ADAMTS13 deficiency promotes formation of ultra-large von Willebrand factor. Haematologica. 2008;93(01):137–140. doi: 10.3324/haematol.11677. [DOI] [PubMed] [Google Scholar]
- 82.Reuken P A, Kussmann A, Kiehntopf M. Imbalance of von Willebrand factor and its cleaving protease ADAMTS13 during systemic inflammation superimposed on advanced cirrhosis. Liver Int. 2015;35(01):37–45. doi: 10.1111/liv.12657. [DOI] [PubMed] [Google Scholar]
- 83.Kremer Hovinga J A, Zeerleder S, Kessler P. ADAMTS-13, von Willebrand factor and related parameters in severe sepsis and septic shock. J Thromb Haemost. 2007;5(11):2284–2290. doi: 10.1111/j.1538-7836.2007.02743.x. [DOI] [PubMed] [Google Scholar]
- 84.Bongers T N, Emonts M, de Maat M P. Reduced ADAMTS13 in children with severe meningococcal sepsis is associated with severity and outcome. Thromb Haemost. 2010;103(06):1181–1187. doi: 10.1160/TH09-06-0376. [DOI] [PubMed] [Google Scholar]
- 85.Karim F, Adil S N, Afaq B, Ul Haq A. Deficiency of ADAMTS-13 in pediatric patients with severe sepsis and impact on in-hospital mortality. BMC Pediatr. 2013;13(01):44. doi: 10.1186/1471-2431-13-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fukushima H, Nishio K, Asai H. Ratio of von Willebrand factor propeptide to ADAMTS13 is associated with severity of sepsis. Shock. 2013;39(05):409–414. doi: 10.1097/SHK.0b013e3182908ea7. [DOI] [PubMed] [Google Scholar]
- 87.Hyun J, Kim H K, Kim J E. Correlation between plasma activity of ADAMTS-13 and coagulopathy, and prognosis in disseminated intravascular coagulation. Thromb Res. 2009;124(01):75–79. doi: 10.1016/j.thromres.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 88.Habe K, Wada H, Ito-Habe N. Plasma ADAMTS13, von Willebrand factor (VWF) and VWF propeptide profiles in patients with DIC and related diseases. Thromb Res. 2012;129(05):598–602. doi: 10.1016/j.thromres.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 89.Vardavas C I, Nikitara K. COVID-19 and smoking: a systematic review of the evidence. Tob Induc Dis. 2020;18:20. doi: 10.18332/tid/119324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ma Q, Jacobi P M, Emmer B T. Genetic variants in ADAMTS13 as well as smoking are major determinants of plasma ADAMTS13 levels . Blood Adv. 2017;1(15):1037–1046. doi: 10.1182/bloodadvances.2017005629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Leung J M, Yang C X, Tam A. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur Respir J. 2020;55(05):2.000688E6. doi: 10.1183/13993003.00688-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Barigye O. Smoking and inflammation. PLoS Med. 2005;2(06):e198. [Google Scholar]
- 93.Schwameis M, Schörgenhofer C, Assinger A, Steiner M M, Jilma B. VWF excess and ADAMTS13 deficiency: a unifying pathomechanism linking inflammation to thrombosis in DIC, malaria, and TTP. Thromb Haemost. 2015;113(04):708–718. doi: 10.1160/TH14-09-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang A, Liu F, Dong N. Thrombospondin-1 and ADAMTS13 competitively bind to VWF A2 and A3 domains in vitro. Thromb Res. 2010;126(04):e260–e265. doi: 10.1016/j.thromres.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 95.Bonnefoy A, Daenens K, Feys H B. Thrombospondin-1 controls vascular platelet recruitment and thrombus adherence in mice by protecting (sub)endothelial VWF from cleavage by ADAMTS13. Blood. 2006;107(03):955–964. doi: 10.1182/blood-2004-12-4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pillai V G, Bao J, Zander C B. Human neutrophil peptides inhibit cleavage of von Willebrand factor by ADAMTS13: a potential link of inflammation to TTP. Blood. 2016;128(01):110–119. doi: 10.1182/blood-2015-12-688747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen J, Fu X, Wang Y. Oxidative modification of von Willebrand factor by neutrophil oxidants inhibits its cleavage by ADAMTS13. Blood. 2010;115(03):706–712. doi: 10.1182/blood-2009-03-213967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Crawley J T, Lam J K, Rance J B, Mollica L R, O'Donnell J S, Lane D A. Proteolytic inactivation of ADAMTS13 by thrombin and plasmin. Blood. 2005;105(03):1085–1093. doi: 10.1182/blood-2004-03-1101. [DOI] [PubMed] [Google Scholar]
- 99.Ono T, Mimuro J, Madoiwa S. Severe secondary deficiency of von Willebrand factor-cleaving protease (ADAMTS13) in patients with sepsis-induced disseminated intravascular coagulation: its correlation with development of renal failure. Blood. 2006;107(02):528–534. doi: 10.1182/blood-2005-03-1087. [DOI] [PubMed] [Google Scholar]
- 100.Liu C, Zhao L, Zhao J, Xu Q, Song Y, Wang H. Reduced ADAMTS-13 level negatively correlates with inflammation factors in plasma of acute myeloid leukemia patients. Leuk Res. 2017;53:57–64. doi: 10.1016/j.leukres.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 101.Takaya H, Kawaratani H, Kubo T. Platelet hyperaggregability is associated with decreased ADAMTS13 activity and enhanced endotoxemia in patients with acute cholangitis. Hepatol Res. 2018;48(03):E52–E60. doi: 10.1111/hepr.12926. [DOI] [PubMed] [Google Scholar]
- 102.Takaya H, Yoshiji H, Kawaratani H. Decreased activity of plasma ADAMTS13 are related to enhanced cytokinemia and endotoxemia in patients with acute liver failure. Biomed Rep. 2017;7(03):277–285. doi: 10.3892/br.2017.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen J, Chung D W. Inflammation, von Willebrand factor, and ADAMTS13. Blood. 2018;132(02):141–147. doi: 10.1182/blood-2018-02-769000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bazzan M, Montaruli B, Sciascia S, Cosseddu D, Norbiato C, Roccatello D.Low ADAMTS 13 plasma levels are predictors of mortality in COVID-19 patientsIntern Emerg Med2020 [DOI] [PMC free article] [PubMed]
- 105.Huisman A, Beun R, Sikma M, Westerink J, Kusadasi N.Involvement of ADAMTS13 and von Willebrand factor in thromboembolic events in patients infected with SARS-CoV-2Int J Lab Hematol2020 [DOI] [PMC free article] [PubMed]
- 106.Adam E H, Zacharowski K, Miesbach W. A comprehensive assessment of the coagulation profile in critically ill COVID-19 patients. Thromb Res. 2020;194:42–44. doi: 10.1016/j.thromres.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Latimer G, Corriveau C, DeBiasi R L. Cardiac dysfunction and thrombocytopenia-associated multiple organ failure inflammation phenotype in a severe paediatric case of COVID-19. Lancet Child Adolesc Health. 2020;4(07):552–554. doi: 10.1016/S2352-4642(20)30163-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Escher R, Breakey N, Lämmle B. ADAMTS13 activity, von Willebrand factor, factor VIII and D-dimers in COVID-19 inpatients. Thromb Res. 2020;192:174–175. doi: 10.1016/j.thromres.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Escher R, Breakey N, Lämmle B. Severe COVID-19 infection associated with endothelial activation. Thromb Res. 2020;190:62. doi: 10.1016/j.thromres.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee S J, Kim J E, Han K S, Kim H K. Thrombotic risk of reduced ADAMTS13 activity in patients with antiphospholipid antibodies. Blood Coagul Fibrinolysis. 2016;27(08):907–912. doi: 10.1097/MBC.0000000000000512. [DOI] [PubMed] [Google Scholar]
- 111.Galeano-Valle F, Oblitas C M, Ferreiro-Mazón M M. Antiphospholipid antibodies are not elevated in patients with severe COVID-19 pneumonia and venous thromboembolism. Thromb Res. 2020;192:113–115. doi: 10.1016/j.thromres.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bowles L, Platton S, Yartey N. Lupus anticoagulant and abnormal coagulation tests in patients with Covid-19. N Engl J Med. 2020;383(03):288–290. doi: 10.1056/NEJMc2013656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Austin S K, Starke R D, Lawrie A S, Cohen H, Machin S J, Mackie I J. The VWF/ADAMTS13 axis in the antiphospholipid syndrome: ADAMTS13 antibodies and ADAMTS13 dysfunction. Br J Haematol. 2008;141(04):536–544. doi: 10.1111/j.1365-2141.2008.07074.x. [DOI] [PubMed] [Google Scholar]
- 114.Jian C, Xiao J, Gong L. Gain-of-function ADAMTS13 variants that are resistant to autoantibodies against ADAMTS13 in patients with acquired thrombotic thrombocytopenic purpura. Blood. 2012;119(16):3836–3843. doi: 10.1182/blood-2011-12-399501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lorenzo-Villalba N, Zulfiqar A A, Auburtin M. Thrombocytopenia in the course of COVID-19 infection. Eur J Case Rep Intern Med. 2020;7(06):1702. doi: 10.12890/2020_001702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yin S, Huang M, Li D, Tang N. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J Thromb Thrombolysis. 2020 doi: 10.1007/s11239-020-02105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Demelo-Rodríguez P, Cervilla-Muñoz E, Ordieres-Ortega L. Incidence of asymptomatic deep vein thrombosis in patients with COVID-19 pneumonia and elevated D-dimer levels. Thromb Res. 2020;192:23–26. doi: 10.1016/j.thromres.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Grandmaison G, Andrey A, Périard D. Systematic screening for venous thromboembolic events in COVID-19 pneumonia. TH Open. 2020;4(02):e113–e115. doi: 10.1055/s-0040-1713167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fraissé M, Logre E, Pajot O, Mentec H, Plantefève G, Contou D. Thrombotic and hemorrhagic events in critically ill COVID-19 patients: a French monocenter retrospective study. Crit Care. 2020;24(01):275. doi: 10.1186/s13054-020-03025-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bastiani G, Valle M T. Determination of factor XII in blood products and correction of its deficiency after plasma transfusion in a case [in Italian] Haematologica. 1979;64(05):635–640. [PubMed] [Google Scholar]
- 121.Desborough M JR, Doyle A J, Griffiths A, Retter A, Breen K A, Hunt B J. Image-proven thromboembolism in patients with severe COVID-19 in a tertiary critical care unit in the United Kingdom. Thromb Res. 2020;193:1–4. doi: 10.1016/j.thromres.2020.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Akel T, Qaqa F, Abuarqoub A, Shamoon F. Pulmonary embolism: a complication of COVID 19 infection. Thromb Res. 2020;193:79–82. doi: 10.1016/j.thromres.2020.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kashi M, Jacquin A, Dakhil B. Severe arterial thrombosis associated with Covid-19 infection. Thromb Res. 2020;192:75–77. doi: 10.1016/j.thromres.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lax S F, Skok K, Zechner P. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: results from a prospective, single-center, clinicopathologic case series. Ann Intern Med. 2020;173(05):350–361. doi: 10.7326/M20-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Thomas W, Varley J, Johnston A. Thrombotic complications of patients admitted to intensive care with COVID-19 at a teaching hospital in the United Kingdom. Thromb Res. 2020;191:76–77. doi: 10.1016/j.thromres.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gomez-Arbelaez D, Ibarra-Sanchez G, Garcia-Gutierrez A, Comanges-Yeboles A, Ansuategui-Vicente M, Gonzalez-Fajardo J A. COVID-19-related aortic thrombosis: a report of four cases. Ann Vasc Surg. 2020:S0890-5096(20)30438-6. doi: 10.1016/j.avsg.2020.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ayerbe L, Risco C, Ayis S. The association between treatment with heparin and survival in patients with Covid-19. J Thromb Thrombolysis. 2020;50(02):298–301. doi: 10.1007/s11239-020-02162-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang T, Chen R, Liu C. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haematol. 2020;7(05):e362–e363. doi: 10.1016/S2352-3026(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Artifoni M, Danic G, Gautier G. Systematic assessment of venous thromboembolism in COVID-19 patients receiving thromboprophylaxis: incidence and role of D-dimer as predictive factors. J Thromb Thrombolysis. 2020;50(01):211–216. doi: 10.1007/s11239-020-02146-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Russo V, Di Maio M, Attena E. Clinical impact of pre-admission antithrombotic therapy in hospitalized patients with COVID-19: a multicenter observational study. Pharmacol Res. 2020;159:104965. doi: 10.1016/j.phrs.2020.104965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Maier C L, Truong A D, Auld S C, Polly D M, Tanksley C L, Duncan A.COVID-19-associated hyperviscosity: a link between inflammation and thrombophilia? Lancet 2020395(10239):1758–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Magro C, Mulvey J J, Berlin D. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


