The levels of D-dimers have been found to be associated with death in patients with COVID-19 since the first reports. 1 D-Dimer and inflammatory parameters are frequently used as a marker of COVID-19 severity, driving decisions such as the use and dosage of anticoagulation and anti-inflammatory drugs. 2 3 However, a meta-analysis of prediction models of COVID-19 developed so far suggested that none, including those based on biomarkers, can be recommended for clinical practice. 4 Among other methodological issues, available studies did not mention the timing of the biomarker assessment, used only baseline measurements or only the latest available ones, or included participants with incomplete follow-up. 4
To the best of our knowledge, only few authors explored the dynamics of early changes of D-dimer levels and other inflammatory biomarkers in hospitalized patients with COVID-19 and their potential suitability for risk assessment. 5 6 In the present retrospective analysis, we studied the dynamic changes in D-dimer and C-reactive protein (CRP) levels in survivors versus nonsurvivors admitted with laboratory- and imaging-confirmed COVID-19 pneumonia at a single high-volume center since the start of the outbreak and followed until discharge or death.
Patients were admitted to a university hospital (Humanitas Clinical and Research Hospital, Rozzano, Milan, Italy) from February to April 2020. 7 For this analysis, we focused only on the temporal course of D-dimer and CRP, as these biomarkers were most frequently used for patient monitoring after early descriptions of their potential prognostic value in COVID-19. 4 We refrained from analysis of venous thromboembolic (VTE) events, because this topic has been extensively addressed 7 8 and because D-dimer levels often guided VTE imaging, which might have led to erroneous risk estimates.
For both markers, we first identified the highest value measured over the entire hospitalization. We then evaluated the in-hospital course of the marker by calculating two velocities of increase (slopes): the average daily increase from the lowest of all previously measured values to the highest value ( lowest to highest daily increase ) and the average daily increase from the latest of all previously measured values to the highest value ( latest to highest daily increase ). Both were expressed as a continuous variable (unit increase/day). A total of 2,896 daily D-dimer measurements and 4,606 CRP measurements were performed in 8,478 patient-days of follow-up. Values below the limit of detection (LOD) were imputed as LOD/√2. 9 Specifically, 279 out of 2,896 (9.6%) D-dimer values were below the LOD of 200 ng/mL and 21/4,606 (0.5%) CRP values below 0.8 mg/dL. All measurements were conducted in the hospital's laboratory. Categorical variables were described as frequencies (percentages), continuous nonnormally distributed values with median and interquartile range (IQR). We described the overall trend of each marker in survivors and nonsurvivors using smoothed lines generated by locally estimated scatterplot smoothing (LOESS) and assessed the association between both types of daily increase of each marker and patient outcome using the Wilcoxon rank-sum test. To explore whether the association of each slope with death varied in patients with lower or higher biomarker values at baseline, we used multivariable regression models, including the biomarker value at baseline, either slope, and their interaction term, as covariates.
The analysis included 577 patients with median age of 67 (IQR: 56–77) years, of whom 386 (67%) were men. The median follow-up from admission to discharge or death was 10 (IQR: 7–17) days. Of the 577 patients, 144 (25%) died, of whom 117 (82%) in a general hospital ward and the remaining in an intensive care unit. Half of the deaths occurred by day 7 (IQR: 4–12). The LOESS lines describing the trends of both markers suggested an overall increase, especially for D-dimer, until the median day of death ( Fig. 1 ). The association analysis showed that, in nonsurvivors, both the daily increase from the lowest to the highest D-dimer level and the daily increase from the latest to the highest D-dimer level were more than twice as high as those among survivors (median: 69, IQR: 21–200 vs. 150, IQR: 13–814 ng/mL/d, p = 0.017; and median: 74, IQR: 18–297 vs. 185, IQR: 12–1,088 ng/mL/d, p = 0.033; Table 1 ). The lowest to highest and latest to highest daily increases in CRP were also higher in nonsurvivors (median: 2.9, IQR: 1.7–4.9 vs. 1.8, IQR: 0.9–3.3 mg/dL/d in survivors, p < 0.001; and median: 3.1, IQR: 1.4–6.0 vs. 1.7, IQR: 0.7–3.8 mg/dL/d in nonsurvivors, p ≤ 0.001; Table 1 ). For both parameters, the baseline and the highest observed levels were also consistently higher in nonsurvivors. The lowest to highest increase in D-dimer was more strongly associated with death in patients with lower D-dimer at baseline (independent odds ratio [OR] and 95% confidence interval [CI] vs. death of D-dimer at baseline: OR: 1.08, 95% CI: 1.02–1.16 per 250 mg/dL increase; of D-dimer lowest to highest increase: OR: 1.18, 95% CI: 1.09–1.30 per 250 mg/dL/d increase; of the interaction term: OR: 0.99, 95% CI: 0.98–0.99). This was not seen in the logistic regression model for the latest to highest increase in D-dimer, in which only the slope was associated with death, or in the model for both CRP slopes, in which both higher slope and higher baseline values were independently associated with death (data not shown).
Fig. 1.
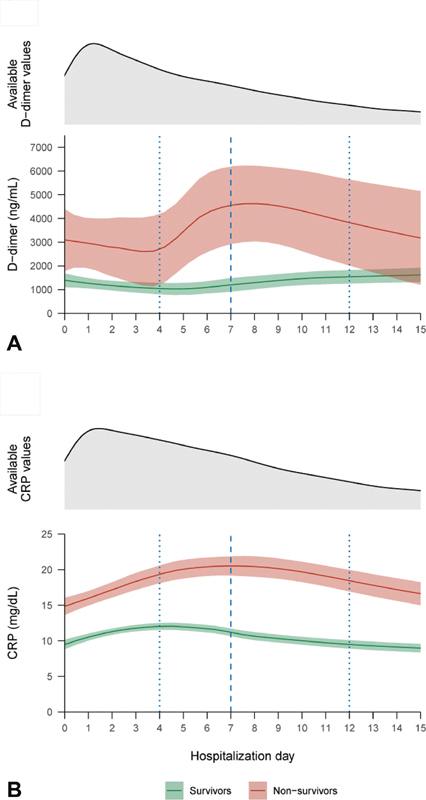
In-hospital trends of D-dimer and C-reactive protein levels in surviving and nonsurviving patients with COVID-19 . Density plots of the distribution of available values and locally estimated smoothed lines (with 95% confidence Intervals) of the in-hospital course of D-dimer ( A ) and C-reactive protein ( B ) in 577 patients with COVID-19 pneumonia with complete follow-up, stratified by outcome (433 survivors and 144 nonsurvivors). The dashed blue line and the dotted blue line indicate the median and the interquartile range, respectively, of the time to death in nonsurvivors. CRP, C-reactive protein.
Table 1. Course of blood coagulation and inflammation markers and in-hospital outcome in 577 patients with COVID-19.
| Available | Total ( N = 577) |
Survivors ( N = 433) |
Nonsurvivors ( N = 144) |
p -Value a | |
|---|---|---|---|---|---|
| Men, n (%) | 577/577 | 386 (67) | 290 (67) | 96 (67) | 0.9 |
| Age, y | 577/577 | 67 (56, 77) | 62 (52, 71) | 77 (72, 85) | <0.001 |
| Intensive care unit, n (%) | 576/577 | 98 (17) | 72 (17) | 26 (18) | 0.8 |
| D-Dimer | |||||
| At baseline, ng/mL | 491/553 b | 435 (268, 864) | 375 (242, 681) | 814 (386, 2230) | <0.001 |
| Highest, ng/mL | 553/553 b | 872 (386, 2,456) | 772 (341, 1,860) | 1844 (678, 4,988) | <0.001 |
| Lowest to highest increase, ng/mL per day | 381/399 c | 79 (21, 249) | 69 (21, 200) | 150 (13, 814) | 0.017 |
| Latest to highest increase, ng/mL per day | 381/399 c | 88 (17, 391) | 74 (18, 297) | 185 (12, 1,088) | 0.033 |
| C-Reactive protein | |||||
| At baseline, mg/dL | 553/574 b | 8 (3, 15) | 7 (3, 13) | 13 (7, 21) | <0.001 |
| Highest, mg/dL | 574/574 b | 15 (7, 24) | 13 (6, 21) | 22 (15, 30) | <0.001 |
| Lowest to highest increase, mg/dL per day | 398/406 c | 2.0 (1.0, 3.7) | 1.8 (0.9, 3.3) | 2.9 (1.7, 4.9) | <0.001 |
| Latest to highest increase, mg/dL per day | 398/406 c | 1.9 (0.8, 4.6) | 1.7 (0.7, 3.8) | 3.1 (1.4, 6.0) | <0.001 |
Note: All continuous values (age in years and biomarker concentrations in ng/mL or mg/dL) are reported as median (interquartile range). Lowest to highest increase is defined as the average daily increase from the lowest measured value preceding the highest measured value to the highest value itself. Latest to highest increase is defined as the average daily increase from the last measured value before the highest measured value to the highest value itself.
Chi-square test for categorical variables and Wilcoxon rank-sum test for continuous variables.
Proportion out of all patients with at least one marker value available.
Proportion out of all patients in whom a slope with zero or positive increase was calculable (at least two values available and the highest value was not the baseline value).
Potential mechanisms for the increase in D-dimer levels in patients with COVID-19 include pulmonary endothelial injury with inflammation-associated deposits of intra-alveolar fibrin, systemic endothelial injury with diffuse thrombosis of smaller vessels 10 or of larger veins, 11 and coagulopathy. 12 13 14 Therefore, the in-hospital course of D-dimer and other coagulation parameters may reflect disease activity in COVID-19 patients. Indeed, the velocity of D-dimer increase may predict VTE in patients with cancer, 15 whereas elevated D-dimer levels during anticoagulation for VTE or after discontinuation may predict recurrence. 16 Our finding further supports the notion that the dynamics of coagulation and inflammatory biomarkers may be considered for integration in clinical risk-assessment models for the management of patients with COVID-19 8 and for use as surrogate outcomes in the early stages of the clinical development of pharmaceuticals for patients with COVID-19, especially in patients with no initial biomarker elevation. In a broader perspective, one cannot exclude that the lack of adequately validated tools to predict deterioration and diagnose VTE in patients with COVID-19 11 17 ultimately affects overall and cause-specific mortality on a global scale. 18 19
This is an exploratory analysis with limitations. First, the associations observed do not imply causality. Second, biomarker measurements were at the discretion of the treating physicians. This may have introduced some biases: patients whose condition was worsening or failing to improve may have undergone more frequent measurements than those whose condition was improving. Accordingly, descending slopes would be less likely to be observed than increasing slopes, and their possible association with survival harder to assess. Therefore, we only considered increasing or zero (constant levels over hospitalization) slopes; still, our results may be only relevant to patients with a stable or worsening condition. Third, the small number of deaths limited formal evaluation of the predictive performance of increasing slopes for death. Likewise, we could not correlate our results with the type, dose, and duration of anticoagulation because of the relatively small number of patients using therapeutic anticoagulation.
In conclusion, higher levels and a higher velocity of increase of D-dimer and CRP levels were observed in nonsurvivors compared with survivors. This suggests that the dynamics of D-dimer and CRP may help to monitor disease activity. Their potential predictive value should be tested in adequately sized studies that implemented a routine measurement of these parameters.
Conflict of Interest None declared.
Authors' Contributions
L.V. analyzed the data. L.V. and S.B. drafted the manuscript. L.V., S.B., and C.L. designed the study. P.F., C.S., and C.L. collected the data. C.L., W.R., N.K., and S.V.K. reviewed and edited the manuscript.
References
- 1.Zhou F, Yu T, Du R.Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study Lancet 2020395(10229):1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global COVID-19 Thrombosis Collaborative Group . Bikdeli B, Madhavan M V, Gupta A. Pharmacological agents targeting thromboinflammation in COVID-19: review and implications for future research. Thromb Haemost. 2020;120(07):1004–1024. doi: 10.1055/s-0040-1713152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marchandot B, Trimaille A, Curtiaud A. Staging severity of COVID-19 according to hemostatic abnormalities (CAHA Score) Thromb Haemost. 2020;120(12):1716–1719. doi: 10.1055/s-0040-1715836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wynants L, Van Calster B, Collins G S. Prediction models for diagnosis and prognosis of covid-19 infection: systematic review and critical appraisal. BMJ. 2020;369:m1328. doi: 10.1136/bmj.m1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y, Zhao K, Wei H. Dynamic relationship between D-dimer and COVID-19 severity. Br J Haematol. 2020;190(01):e24–e27. doi: 10.1111/bjh.16811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye W, Chen G, Li X. Dynamic changes of D-dimer and neutrophil-lymphocyte count ratio as prognostic biomarkers in COVID-19. Respir Res. 2020;21(01):169. doi: 10.1186/s12931-020-01428-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Humanitas COVID-19 Task Force . Lodigiani C, Iapichino G, Carenzo L. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prevention Treatment of VTE Associated with COVID-19 Infection Consensus Statement Group . Zhai Z, Li C, Chen Y. Prevention and treatment of venous thromboembolism associated with coronavirus disease 2019 infection: a consensus statement before guidelines. Thromb Haemost. 2020;120(06):937–948. doi: 10.1055/s-0040-1710019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hornung R W, Reed L D. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5(01):46–51. [Google Scholar]
- 10.Katneni U K, Alexaki A, Hunt R C. Coagulopathy and thrombosis as a result of severe COVID-19 infection: a microvascular focus. Thromb Haemost. 2020;120(12):1668–1679. doi: 10.1055/s-0040-1715841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roncon L, Zuin M, Barco S. Incidence of acute pulmonary embolism in COVID-19 patients: systematic review and meta-analysis. Eur J Intern Med. 2020;82:29–37. doi: 10.1016/j.ejim.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eriksson O, Hultstrom M, Persson B. Mannose-binding lectin is associated with thrombosis and coagulopathy in critically ill COVID-19 patients. Thromb Haemost. 2020;120(12):1720–1724. doi: 10.1055/s-0040-1715835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Onishi T, Nogami K, Ishihara T. A pathological clarification of sepsis-associated disseminated intravascular coagulation based on comprehensive coagulation and fibrinolysis function. Thromb Haemost. 2020;120(09):1257–1269. doi: 10.1055/s-0040-1713890. [DOI] [PubMed] [Google Scholar]
- 14.Spiezia L, Boscolo A, Poletto F. COVID-19-related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost. 2020;120(06):998–1000. doi: 10.1055/s-0040-1710018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Posch F, Riedl J, Reitter E-M. Dynamic assessment of venous thromboembolism risk in patients with cancer by longitudinal D-dimer analysis: a prospective study. J Thromb Haemost. 2020;18(06):1348–1356. doi: 10.1111/jth.14774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.PROLONG Investigators (FCSA and Italian Federation of Thrombosis Centers) . Legnani C, Palareti G, Cosmi B, Cini M, Tosetto A, Tripodi A. Different cut-off values of quantitative D-dimer methods to predict the risk of venous thromboembolism recurrence: a post-hoc analysis of the PROLONG study. Haematologica. 2008;93(06):900–907. doi: 10.3324/haematol.12320. [DOI] [PubMed] [Google Scholar]
- 17.Collins G S, van Smeden M, Riley R D. COVID-19 prediction models should adhere to methodological and reporting standards. Eur Resp J. 2020;56(03):2.002643E6. doi: 10.1183/13993003.02643-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barco S, Mahmoudpour S H, Valerio L. Trends in mortality related to pulmonary embolism in the European Region, 2000-15: analysis of vital registration data from the WHO Mortality Database. Lancet Respir Med. 2020;8(03):277–287. doi: 10.1016/S2213-2600(19)30354-6. [DOI] [PubMed] [Google Scholar]
- 19.Barco S, Valerio L, Ageno W. Age-sex specific pulmonary embolism-related mortality in the USA and Canada, 2000–18: an analysis of the WHO Mortality Database and of the CDC Multiple Cause of Death database. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30417-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


