Abstract
Background
Acute heart failure is a life-threatening clinical condition. Levosimendan is an effective inotropic agent used to maintain cardiac output, but its usage is limited by the lack of evidence in patients with severely abnormal renal function. Therefore, we analyzed data of patients with acute heart failure with and without abnormal renal function to examine the effects of levosimendan.
Methods
We performed this retrospective cohort study using data from the Chang Gung Research Database (CGRD) of Chang Gung Memorial Hospital (CGMH). Patients admitted for heart failure with LVEF ≤ 40% between January 2013 and December 2018 who received levosimendan or dobutamine in the critical cardiac care units (CCU) were identified. Patients with extracorporeal membrane oxygenation (ECMO) were excluded. Outcomes of interest were mortality at 30, 90, and 180 days after the cohort entry date.
Results
There were no significant differences in mortality rate at 30, 90, and 180 days after the cohort entry date between the levosimendan and dobutamine groups, or between subgroups of patients with an estimated glomerular filtration rate (eGFR) ≥ 30 mL/min/1.73 m2 and eGFR < 30 mL/min/1.73 m2 or on dialysis. The results were consistent before and after propensity score matching.
Conclusions
Levosimendan did not increase short- or long-term mortality rates in critical patients with acute heart failure and reduced ejection fraction compared to dobutamine, regardless of their renal function. An eGFR less than 30 mL/min/1.73 m2 was not necessarily considered a contraindication for levosimendan in these patients.
Keywords: Levosimendan, Dobutamine, Heart failure, Renal failure
Background
Acute heart failure (AHF) is a life-threatening condition defined as a status of acute-onset or rapidly worsening heart failure symptoms. The clinical spectrum of heart failure is wide, ranging from mild pulmonary congestion to cardiogenic shock. Patients with AHF and low cardiac output are at a higher risk of morbidity and mortality [1]. For critical patients with AHF and systemic hypoperfusion, inotropic agents or mechanical cardiac support devices are used to maintain hemodynamic stability. Medications including vasopressors and sympathomimetic inotropes such as norepinephrine, dopamine, dobutamine, and levosimendan are frequently used to treat AHF with reduced LVEF to improve cardiac output. Levosimendan is a Ca2+-sensitizer of the myocardium, which induces vasodilatation and increases cardiac output [2–4]. It is particularly effective in patients with acute decompensated heart failure by improving left ventricular ejection fraction (LVEF), decreasing B-type natriuretic peptide levels, improving renal function, and decreasing the need for renal replacement therapy in different settings [5]. Previous studies have demonstrated a comparable effect on improving cardiac output in heart failure patients to dobutamine, and it may result in a greater improvement in glomerular filtration rate than dobutamine in patients with cardiorenal syndrome [6–12]. However, evidence supporting the use of levosimendan in patients with severe renal dysfunction (i.e., an estimated glomerular filtration rate (eGFR) of < 30 mL/min/1.73 m2) is limited, particularly in patients with AHF and low cardiac output. In this study, we hypothesized that severe renal dysfunction may frequently be complicated with AHF, and that levosimendan may be beneficial in these patients. Therefore, the aim of this study was to analyze patients with AHF and reduced LVEF with or without abnormal renal function to examine the effects of levosimendan.
Methods
Data source
We performed this retrospective cohort study using data from the Chang Gung Research Database (CGRD) of Chang Gung Memorial Hospital (CGMH), which includes two medical centers, two regional hospitals, and three district hospitals around Taiwan. The total numbers of outpatient, emergency room and inpatient visits in 2018 in Taiwan were 420,283, 20,252 and 3172 per day, respectively [13]. Among the hospitals in Taiwan, CGMH has the largest volume of both inpatient (12.4%) and outpatient (21.2%) services in Taiwan. The CGRD comprises patient data derived from initial electronic medical records, and it was established for administrative and insurance purposes for CGMH. Disease diagnoses were coded using International Classification of Disease, Ninth Revision, Clinical Modification codes before 2016, and International Classification of Disease, Tenth Revision codes thereafter. Previous studies have provided additional detailed information on the CGRD [14, 15]. The Institutional Review Board of the Chang Gung Medical Foundation approved this study.
Study design
Patients admitted for heart failure with decreased EF (defined as LVEF ≤ 40% by echocardiography) between January 2013 and December 2018 who received levosimendan or dobutamine in the critical cardiac care units (CCU) were identified from the CGRD. Patients who received levosimendan during the hospital stay were classified as the levosimendan group, regardless of whether they received dobutamine or other inotropic agents prior to the administration of levosimendan. All patients were prescribed inotropes because of heart failure with low cardiac output and systemic hypoperfusion (i.e., cardiogenic shock). Patients in the levosimendan group received continuous intravenous levosimendan at a dose of 0.05–0.2 µg per kilogram of body weight per minute for 72 h (without a loading dose). The date of cohort entry in the levosimendan group was defined as the day of the first levosimendan infusion during the hospital stay. To eliminate immortal time bias, the cohort entry date of the patients in the dobutamine group was assigned based on their counterparts in the levosimendan group [33]. Patients in the levosimendan group were matched with those in the dobutamine group by sex, age, eGFR, and LVEF. We excluded patients with extracorporeal membrane oxygenation (ECMO). The remaining patients were further classified according to their renal function using plasma creatinine and Modification of Diet in Renal Disease (MDRD) eGFR [16].
Covariates and outcomes
We analyzed the following covariates: age, sex, LVEF, eGFR, inotropic agent use during the admission, acute myocardial infarction (AMI) or percutaneous coronary intervention (PCI) during admission, length of intensive care unit stay, hospital days, comorbidities (atrial fibrillation, diabetes mellitus, hypertension and dyslipidemia), medications (aspirin, clopidogrel, ticagrelor, beta-blockers, angiotensin-converting enzyme inhibitors (ACEis), angiotensin II receptor blockers (ARBs), mineralocorticoid receptor antagonists (MRAs), digoxin, amiodarone and ivabradine), and baseline laboratory data (hemoglobin, alanine aminotransferase, B-type natriuretic peptide, HCO3, total bilirubin, blood urea nitrogen, sodium, potassium, platelets, hematocrit, white blood count, albumin, lactate and international normalized ratio). All patients underwent transthoracic echocardiography in the emergency department or after they had been admitted to a CCU. We followed the guidelines of the American Society of Echocardiography and used the biplane method to calculate the LVEF. The patients were defined as having a comorbidity if they had at least two outpatient diagnoses or one inpatient diagnosis for that comorbidity prior to the cohort entry date. Medication and laboratory data for 3 months prior to the cohort entry date were extracted. The outcomes of interest were mortality at 30, 90, and 180 days after the cohort entry date. We also evaluated in-hospital mortality after dosing. Patient follow-up was censored 6 months after the cohort entry date, last visit at CGMH, date of death, or December 31, 2018, whichever occurred first.
Statistical analysis
We evaluated differences in baseline characteristics between groups using standardized differences (STDs), where an absolute value of > 0.2 was considered to be a substantial difference. We performed propensity score matching (PSM) based on the propensity score derived from multivariable logistic regression to balance the two groups. Each patient in the levosimendan group was matched to two counterparts in the dobutamine group, if possible. Propensity scores were calculated using age, sex, LVEF, eGFR, use of inotropic agents (dopamine, norepinephrine, and epinephrine), AMI, myocarditis and mechanical ventilation during the index admission, intra-aortic balloon pumping, Sequential Organ Failure Assessment (SOFA) score and Acute Physiology and Chronic Health Evaluation III (APACHE III) score. PSM was performed separately for those with an eGFR ≥ 30 mL/min/1.73 m2 and those with an eGFR < 30 mL/min/1.73 m2 or dialysis. A univariate Cox proportional hazard model was used to compare the mortality rates between groups. Statistical significance was set at a two-sided P value of < 0.05, and no adjustment of multiple testing (multiplicity) was made in this study. We used SAS (version 9.4; SAS Institute, Cary, NC, USA) to perform all statistical analyses.
Results
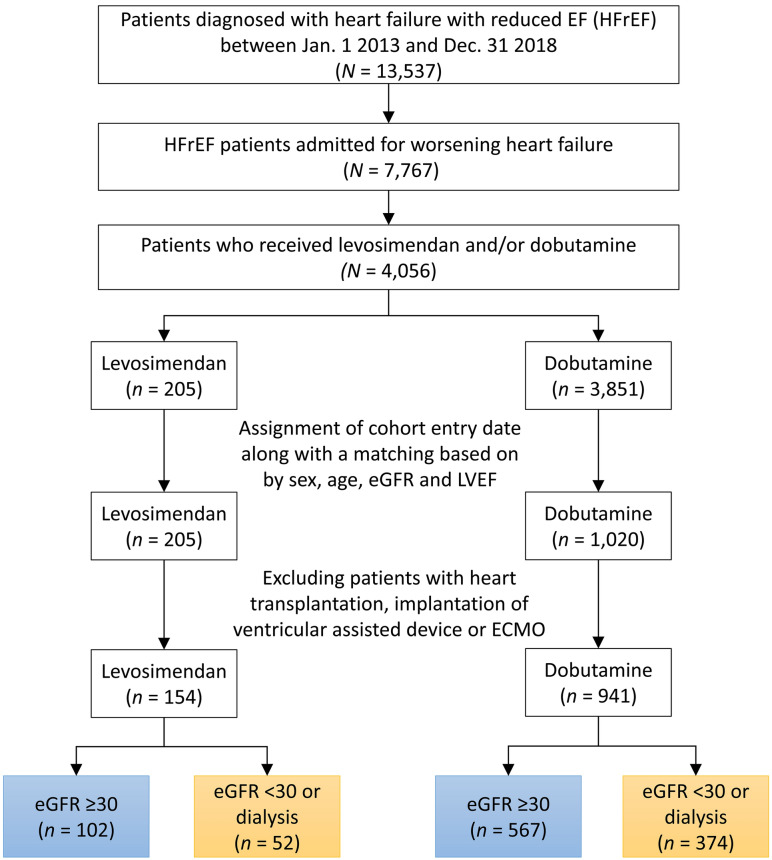
After matching, 102 and 567 patients with eGFR > 30 mL/min/1.73 m2 were classified into the levosimendan and dobutamine groups, respectively, and 52 and 374 patients with eGFR ≤ 30 mL/min/1.73 m2 or dialysis were classified into the levosimendan and dobutamine groups (Fig. 1), respectively.
Fig. 1.
Study flowchart
Baseline characteristics (supplemental digital content)
Before PSM, patients in the levosimendan group were more likely to receive dopamine and less likely to receive epinephrine during hospital stay. The usage rate of norepinephrine was comparable. Myocarditis, AMI, PCI, and IABP were more common in the levosimendan group. After PSM, 63 patients in the levosimendan group with an eGFR ≥ 30 mL/min/1.73 m2 had 2 counterparts and 19 patients had only 1 counterpart, resulting in a total of 145 patients in the dobutamine group. After PSM, 38 patients in the levosimendan group with an eGFR < 30 mL/min/1.73 m2 or dialysis had 2 counterparts and 5 patients had only 1 counterpart, resulting in a total of 81 patients in the dobutamine group. No significant group differences were observed in age, sex, LVEF, eGFR, inotropic agent use, and AMI after PSM (Tables 1, 2).
Table 1.
Baseline characteristics of patients with eGFR ≥ 30 who received levosimendan versus dobutamine alone
| Variable | Valid N | Before PSM‡ | After PSM‡ | ||||
|---|---|---|---|---|---|---|---|
| Levosimendan (n = 102) |
Dobutamine (n = 567) |
STD | Levosimendan (n = 82) |
Dobutamine (n = 145) |
STD | ||
| Age, year* | 669 | 65.8 ± 15.3 | 64.3 ± 15.1 | 0.10 | 65.5 ± 14.7 | 65.7 ± 15.6 | − 0.01 |
| Male* | 669 | 76 (74.5) | 437 (77.1) | − 0.06 | 64 (78.0) | 105 (72.4) | 0.13 |
| LVEF, %* | 669 | 28.7 ± 6.8 | 28.3 ± 7.8 | 0.05 | 27.7 ± 6.7 | 27.7 ± 7.8 | < 0.01 |
| eGFR, mL/min/1.73m2* | 669 | 66.2 ± 31.9 | 65.8 ± 29.1 | 0.01 | 66.6 ± 32.5 | 67.6 ± 29.5 | − 0.03 |
| Inotropic agents during the index admission | |||||||
| Dopamine* | 669 | 24 (23.5) | 67 (11.8) | 0.31 | 15 (18.3) | 24 (16.6) | 0.05 |
| Norepinephrine* | 669 | 15 (14.7) | 87 (15.3) | − 0.02 | 12 (14.6) | 20 (13.8) | 0.02 |
| Epinephrine* | 669 | 8 (7.8) | 112 (19.8) | − 0.35 | 6 (7.3) | 11 (7.6) | − 0.01 |
| AMI during the index admission* | 669 | 35 (34.3) | 85 (15.0) | 0.46 | 20 (24.4) | 37 (25.5) | − 0.03 |
| PCI during the index admission* | 669 | 23 (22.5) | 41 (7.2) | 0.44 | 12 (14.6) | 21 (14.5) | < 0.01 |
| Myocarditis during the index admission* | 669 | 6 (5.9) | 8 (1.4) | 0.24 | 3 (3.7) | 2 (1.4) | 0.15 |
| Mechanical ventilator* | 669 | 52 (51.0) | 233 (41.1) | 0.20 | 41 (50.0) | 59 (40.7) | 0.19 |
| IABP* | 669 | 34 (33.3) | 26 (4.6) | 0.79 | 14 (17.1) | 20 (13.8) | 0.09 |
| ICU days | 669 | 7 [4, 11] | 2 [0, 6] | NA | 7 [3, 10] | 3 [0, 7] | NA |
| Admission days | 669 | 19 [15, 36] | 19 [12, 31] | NA | 20 [15, 38] | 18 [12, 29] | NA |
| Risk score* | |||||||
| SOFA | 669 | 6.4 ± 2.0 | 6.8 ± 2.2 | − 0.20 | 6.4 ± 2.0 | 6.2 ± 1.8 | 0.10 |
| APACHE III | 669 | 37.5 ± 17.6 | 32.4 ± 16.2 | 0.31 | 37.3 ± 18.2 | 35.5 ± 19.0 | 0.10 |
| Comorbidities | |||||||
| Atrial fibrillation | 669 | 39 (38.2) | 216 (38.1) | 0.00 | 35 (42.7) | 47 (32.4) | 0.21 |
| Diabetes mellitus | 669 | 44 (43.1) | 266 (46.9) | − 0.08 | 38 (46.3) | 85 (58.6) | − 0.25 |
| Hypertension | 669 | 63 (61.8) | 391 (69.0) | − 0.15 | 51 (62.2) | 107 (73.8) | − 0.25 |
| Dyslipidemia | 669 | 70 (68.6) | 301 (53.1) | 0.32 | 54 (65.9) | 90 (62.1) | 0.08 |
| Baseline medications | |||||||
| Aspirin | 669 | 78 (76.5) | 379 (66.8) | 0.21 | 61 (74.4) | 101 (69.7) | 0.11 |
| Clopidogrel | 669 | 58 (56.9) | 273 (48.1) | 0.18 | 45 (54.9) | 83 (57.2) | − 0.05 |
| Ticagrelor | 669 | 41 (40.2) | 88 (15.5) | 0.57 | 26 (31.7) | 27 (18.6) | 0.31 |
| Beta-blockers | 669 | 97 (95.1) | 474 (83.6) | 0.38 | 79 (96.3) | 122 (84.1) | 0.42 |
| ACEi | 669 | 65 (63.7) | 314 (55.4) | 0.17 | 49 (59.8) | 79 (54.5) | 0.11 |
| ARBs | 669 | 73 (71.6) | 375 (66.1) | 0.12 | 59 (72.0) | 98 (67.6) | 0.10 |
| MRA | 669 | 73 (71.6) | 443 (78.1) | − 0.15 | 61 (74.4) | 111 (76.6) | − 0.05 |
| Digoxin | 669 | 42 (41.2) | 268 (47.3) | − 0.12 | 39 (47.6) | 65 (44.8) | 0.05 |
| Amiodarone | 669 | 72 (70.6) | 298 (52.6) | 0.38 | 60 (73.2) | 60 (41.4) | 0.68 |
| Ivabradine | 669 | 43 (42.2) | 117 (20.6) | 0.48 | 32 (39.0) | 34 (23.4) | 0.34 |
| Baseline laboratory data | |||||||
| Hemoglobin, g/dl | 664 | 12.1 ± 2.5 | 11.8 ± 2.3 | 0.12 | 12.1 ± 2.5 | 11.8 ± 2.3 | 0.13 |
| ALT, U/L | 595 | 37 [23, 85] | 25 [17, 50] | NA | 35 [21, 96] | 26 [18, 56] | NA |
| BNP, pg/mL | 401 |
1451 [840, 3789] |
1494 [772, 2560] |
NA |
1435 [840, 3810] |
1751 [957, 2821] |
NA |
| HCO3, mmol/L | 289 | 23.6 ± 5.8 | 25.9 ± 5.7 | − 0.40 | 23.9 ± 6.4 | 25.2 ± 5.1 | − 0.22 |
| Total bilirubin, mg/dL | 413 | 1.7 ± 2.0 | 1.7 ± 2.1 | 0.01 | 1.7 ± 2.2 | 1.7 ± 2.7 | 0.00 |
| BUN, mg/dL | 647 | 30.3 ± 15.8 | 29.8 ± 16.6 | 0.03 | 30.0 ± 16.1 | 31.2 ± 19.9 | − 0.07 |
| Sodium, mg/dL | 668 | 138.4 ± 5.3 | 139.6 ± 5.7 | − 0.23 | 138.2 ± 5.1 | 139.4 ± 6.1 | − 0.21 |
| Potassium, mg/dL | 668 | 4.0 ± 0.6 | 3.9 ± 0.6 | 0.10 | 3.9 ± 0.6 | 4.0 ± 0.6 | − 0.08 |
| Platelet, 1000/μL | 662 | 182.9 ± 76.3 | 182.5 ± 81.0 | 0.01 | 179.9 ± 75.3 | 195.5 ± 87.0 | − 0.19 |
| Hematocrit, % | 664 | 36.4 ± 7.3 | 35.9 ± 6.7 | 0.07 | 36.5 ± 7.4 | 35.9 ± 6.6 | 0.08 |
| WBC, 1000/μL | 663 | 10.2 ± 5.3 | 10.6 ± 5.1 | − 0.07 | 9.7 ± 5.0 | 10.9 ± 4.8 | − 0.25 |
| Albumin, mg/dL | 456 | 3.4 ± 0.5 | 3.3 ± 0.5 | 0.07 | 3.4 ± 0.5 | 3.2 ± 0.5 | 0.33 |
| Lactate, mg/dL | 263 | 28.6 ± 35.4 | 26.8 ± 20.5 | 0.07 | 27.7 ± 38.2 | 25.7 ± 20.5 | 0.07 |
| INR | 506 | 1.3 ± 0.4 | 1.4 ± 0.4 | − 0.22 | 1.3 ± 0.4 | 1.3 ± 0.4 | − 0.07 |
PSM propensity score matching, STD standard difference, LVEF left ventricular ejection fraction, eGFR estimated glomerular filtration rate, AMI acute myocardial infarction, PCI percutaneous coronary intervention, IABP intra-aortic balloon pumping, ICU intensive care unit, SOFA Sequential Organ Failure Assessment, APACHE Acute Physiology and Chronic Health Evaluation, ACEi angiotensin- converting enzyme inhibitor, ARBs angiotensin II receptor blockers, MRA mineralocorticoid receptor antagonist, ALT alanine aminotransferase, BNP B-type natriuretic peptide, BUN blood urea nitrogen, NA not available, WBC white blood count, INR international normalized ratio
‡Data were presented as number (%), mean ± standard deviation or median [25th, 75th percentile];
*Included in the calculation of propensity score
Table 2.
Baseline characteristics of patients with eGFR < 30 or dialysis who received levosimendan versus dobutamine alone
| Variable | Before PSM‡ | After PSM‡ | |||||
|---|---|---|---|---|---|---|---|
| Valid N | Levosimendan (n = 52) |
Dobutamine (n = 374) |
STD | Levosimendan (n = 43) |
Dobutamine (n = 81) |
STD | |
| Age, year* | 426 | 72.6 ± 12.1 | 70.4 ± 11.9 | 0.18 | 72.6 ± 11.6 | 71.3 ± 11.3 | 0.11 |
| Male* | 426 | 31 (59.6) | 237 (63.4) | − 0.08 | 23 (53.5) | 51 (63.0) | − 0.19 |
| LVEF, %* | 426 | 25.9 ± 7.4 | 29.6 ± 7.1 | − 0.51 | 26.4 ± 7.5 | 25.6 ± 8.1 | 0.10 |
| eGFR, mL/min/1.73m2* | 279 | 18.8 ± 6.7 | 19.1 ± 6.7 | 0.08 | 18.3 ± 6.6 | 21.1 ± 6.1 | − 0.43 |
| Prior dialysis* | 426 | 18 (34.6) | 129 (34.5) | 0.00 | 15 (34.9) | 30 (37.0) | − 0.04 |
| Inotropic agents during the index admission | |||||||
| Dopamine* | 426 | 14 (26.9) | 51 (13.6) | 0.34 | 10 (23.3) | 19 (23.5) | < 0.01 |
| Norepinephrine* | 426 | 10 (19.2) | 77 (20.6) | − 0.03 | 9 (20.9) | 18 (22.2) | − 0.03 |
| Epinephrine* | 426 | 5 (9.6) | 59 (15.8) | − 0.19 | 4 (9.3) | 9 (11.1) | − 0.06 |
| AMI during the index admission* | 426 | 16 (30.8) | 85 (22.7) | 0.18 | 14 (32.6) | 22 (27.2) | 0.12 |
| PCI during the index admission* | 426 | 11 (21.2) | 36 (9.6) | 0.32 | 9 (20.9) | 16 (19.8) | 0.03 |
| Myocarditis during the index admission* | 426 | 3 (5.8) | 2 (0.5) | 0.30 | 1 (2.3) | 1 (1.2) | 0.08 |
| Mechanical ventilator* | 426 | 36 (69.2) | 151 (40.4) | 0.61 | 27 (62.8) | 46 (56.8) | 0.12 |
| IABP* | 426 | 13 (25.0) | 26 (7.0) | 0.51 | 7 (16.3) | 15 (18.5) | − 0.06 |
| ICU days | 426 | 11 [4, 18] | 3 [0, 10] | NA | 11 [4, 21] | 5 [0, 13] | NA |
| Admission days | 426 | 29 [15.5, 49] | 24 [13, 42] | NA | 29 [16, 53] | 25 [13, 39] | NA |
| Risk score* | |||||||
| SOFA | 426 | 8.4 ± 2.1 | 8.9 ± 2.4 | − 0.21 | 8.6 ± 2.2 | 8.6 ± 2.0 | − 0.04 |
| APACHE III | 426 | 50.2 ± 13.3 | 49.8 ± 15.4 | 0.03 | 50.9 ± 13.8 | 49.4 ± 13.5 | 0.11 |
| Comorbidities | |||||||
| Atrial fibrillation | 426 | 16 (30.8) | 145 (38.8) | − 0.17 | 15 (34.9) | 24 (29.6) | 0.11 |
| Diabetes mellitus | 426 | 35 (67.3) | 249 (66.6) | 0.02 | 29 (67.4) | 52 (64.2) | 0.07 |
| Hypertension | 426 | 43 (82.7) | 311 (83.2) | − 0.01 | 35 (81.4) | 63 (77.8) | 0.09 |
| Dyslipidemia | 426 | 35 (67.3) | 223 (59.6) | 0.16 | 28 (65.1) | 57 (70.4) | − 0.11 |
| Baseline medications | |||||||
| Aspirin | 426 | 43 (82.7) | 250 (66.8) | 0.37 | 35 (81.4) | 61 (75.3) | 0.15 |
| Clopidogrel | 426 | 35 (67.3) | 214 (57.2) | 0.21 | 28 (65.1) | 44 (54.3) | 0.22 |
| Ticagrelor | 426 | 18 (34.6) | 72 (19.3) | 0.35 | 12 (27.9) | 22 (27.2) | 0.02 |
| Beta-blockers | 426 | 46 (88.5) | 325 (86.9) | 0.05 | 38 (88.4) | 67 (82.7) | 0.16 |
| ACE inhibitors | 426 | 30 (57.7) | 163 (43.6) | 0.29 | 23 (53.5) | 35 (43.2) | 0.21 |
| ARB | 426 | 37 (71.2) | 235 (62.8) | 0.18 | 29 (67.4) | 53 (65.4) | 0.04 |
| MRA | 426 | 34 (65.4) | 196 (52.4) | 0.27 | 31 (72.1) | 39 (48.1) | 0.50 |
| Digoxin | 426 | 20 (38.5) | 153 (40.9) | − 0.05 | 20 (46.5) | 29 (35.8) | 0.22 |
| Amiodarone | 426 | 30 (57.7) | 188 (50.3) | 0.15 | 27 (62.8) | 33 (40.7) | 0.45 |
| Ivabradine | 426 | 28 (53.8) | 84 (22.5) | 0.68 | 22 (51.2) | 23 (28.4) | 0.48 |
| Baseline laboratory data | |||||||
| Hemoglobin, g/dl | 425 | 11.3 ± 2.7 | 10.0 ± 2.0 | 0.55 | 11.1 ± 2.7 | 10.5 ± 2.2 | 0.27 |
| ALT, U/L | 376 | 28 [15, 130] | 23 [15, 45] | NA | 35 [16, 130] | 29 [16, 70] | NA |
| BNP, pg/mL | 271 |
4634 [1625, 5000] |
2412 [1231.9, 4700] |
NA |
4634 [1625, 5000] |
3030 [1580, 4700] |
NA |
| HCO3, mmol/L | 261 | 21.1 ± 6.0 | 23.0 ± 5.8 | − 0.31 | 21.2 ± 6.3 | 21.6 ± 5.0 | − 0.08 |
| Total bilirubin, mg/dL | 288 | 1.6 ± 2.0 | 1.5 ± 2.1 | 0.05 | 1.6 ± 2.1 | 1.5 ± 2.0 | 0.06 |
| BUN, mg/dL | 419 | 66.7 ± 37.0 | 70.3 ± 38.6 | − 0.10 | 70.6 ± 38.0 | 68.0 ± 36.7 | 0.07 |
| Sodium, mg/dL | 426 | 139.2 ± 6.5 | 139.2 ± 6.3 | 0.00 | 140.0 ± 6.8 | 139.4 ± 6.7 | 0.09 |
| Potassium, mg/dL | 426 | 4.2 ± 0.8 | 4.1 ± 0.7 | 0.06 | 4.3 ± 0.7 | 4.1 ± 0.7 | 0.17 |
| Platelet, 1000/μL | 424 | 163.7 ± 71.9 | 169.1 ± 85.4 | − 0.07 | 166.3 ± 75.3 | 183.3 ± 92.1 | − 0.20 |
| Hematocrit, % | 425 | 34.1 ± 7.6 | 30.7 ± 5.9 | 0.51 | 34.0 ± 7.6 | 32.1 ± 6.5 | 0.28 |
| WBC, 1000/μL | 424 | 9.9 ± 3.9 | 10.1 ± 5.5 | − 0.04 | 10.4 ± 3.9 | 10.6 ± 5.0 | − 0.05 |
| Albumin, mg/dL | 328 | 3.2 ± 0.5 | 3.1 ± 0.5 | 0.05 | 3.1 ± 0.5 | 3.2 ± 0.5 | − 0.11 |
| Lactate, mg/dL | 184 | 29.9 ± 34.1 | 32.9 ± 32.5 | − 0.09 | 30.5 ± 36.2 | 41.5 ± 39.9 | − 0.29 |
| INR | 319 | 1.7 ± 1.0 | 1.6 ± 0.8 | 0.17 | 1.8 ± 1.0 | 1.5 ± 0.8 | 0.34 |
PSM propensity score matching, STD standard difference, LVEF left ventricular ejection fraction, eGFR estimated glomerular filtration rate, AMI acute myocardial infarction, PCI percutaneous coronary intervention, IABP intra-aortic balloon pumping, ICU intensive care unit, SOFA Sequential Organ Failure Assessment, APACHE Acute Physiology and Chronic Health Evaluation, ACEi angiotensin-converting enzyme inhibitor, ARBs angiotensin II receptor blockers, MRA mineralocorticoid receptor antagonist, ALT alanine aminotransferase, BNP B-type natriuretic peptide, BUN blood urea nitrogen, NA not available, WBC white blood count, INR international normalized ratio
‡Data were presented as number (%), mean ± standard deviation or median [25th, 75th percentile];
*Included in the calculation of propensity score
Mortality rates in the eGFR ≥ 30 mL/min/1.73 m2 subgroup
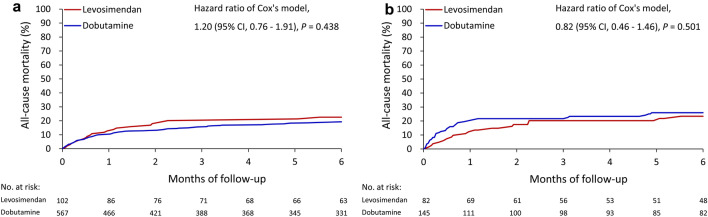
For the patients with an eGFR ≥ 30 mL/min/1.73 m2, the overall in-hospital mortality rates were 12.7% in the levosimendan group and 10.8% in the dobutamine group [hazard ratio (HR) 0.80, 95% confidence interval (CI) 0.43–1.51]. The 30-day mortality rates were 12.7 and 10.1% in the levosimendan and dobutamine groups, respectively (HR 1.23, 95% CI 0.68–2.25). After PSM, the overall in-hospital mortality rates were 11.0% and 17.2% in the levosimendan and dobutamine groups, respectively (HR 0.37, 95% CI 0.15–0.92). The 30-day mortality rates were 12.2 and 19.3% in the levosimendan and dobutamine groups, respectively (HR 0.59, 95% CI 0.8–1.23). The differences in the 90- and 180-day mortality rates were nonsignificant between groups before or after PSM (Table 3; Fig. 2a, b).
Table 3.
Mortality rates of patients with eGFR ≥ 30 who received levosimendan versus dobutamine alone
| Outcomes | Event (%) | Levosimendan vs. dobutamine | ||
|---|---|---|---|---|
| Levosimendan | Dobutamine | HR (95% CI) | P value | |
| Before PSM patient numbers | 102 | 567 | ||
| In-hospital mortality | 13 (12.7) | 61 (10.8) | 0.80 (0.43–1.51) | 0.491 |
| 5-day mortality | 3 (2.9) | 19 (3.4) | 0.87 (0.26–2.94) | 0.822 |
| 14-day mortality | 7 (6.9) | 38 (6.7) | 1.004 (0.45–2.25) | 0.992 |
| 30-day mortality | 13 (12.7) | 57 (10.1) | 1.23 (0.68–2.25) | 0.496 |
| 90-day mortality | 20 (19.6) | 83 (14.6) | 1.31 (0.80–2.13) | 0.285 |
| 180-day mortality | 22 (21.6) | 99 (17.5) | 1.20 (0.76–1.91) | 0.438 |
| After PSM patient numbers | 82 | 145 | ||
| In-hospital mortality | 9 (11.0) | 25 (17.2) | 0.37 (0.15–0.92) | 0.032 |
| 5-day mortality | 2 (2.4) | 10 (6.9) | 0.34 (0.07–1.60) | 0.173 |
| 14-day mortality | 5 (6.1) | 19 (13.1) | 0.44 (0.17–1.14) | 0.091 |
| 30-day mortality | 10 (12.2) | 28 (19.3) | 0.59 (0.28–1.23) | 0.160 |
| 90-day mortality | 16 (19.5) | 31 (21.4) | 0.85 (0.45–1.60) | 0.604 |
| 180-day mortality | 18 (22.0) | 36 (24.8) | 0.82 (0.46–1.46) | 0.501 |
HR hazard ratio, CI confidence interval, PSM propensity score matching
Fig. 2.
All-cause mortality of the patients with eGFR ≥ 30 mL/min/1.73 m2 who received levosimendan versus dobutamine alone before (a) and after (b) propensity score matching
Mortality rates in the eGFR < 30 mL/min/1.73m2 or dialysis subgroup
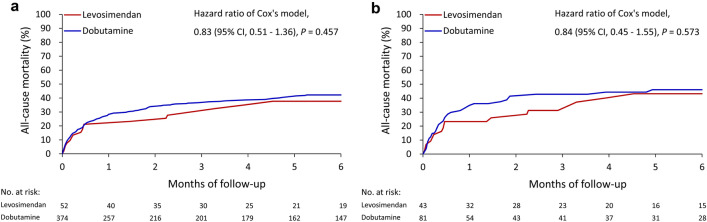
For the patients with an eGFR < 30 mL/min/1.73 m2 or on maintenance dialysis, the overall in-hospital mortality rates were 26.9 and 30.5% in the levosimendan and dobutamine groups, respectively (HR 0.76, 95% CI 0.44–1.32). The 30-day mortality rates were 21.2 and 27.8% in the levosimendan and dobutamine groups, respectively (HR 0.73, 95% CI 0.39–1.37). After PSM, the overall in-hospital mortality rates were 30.2 and 37.0% in the levosimendan and dobutamine groups, respectively (HR 0.67, 95% CI 0.36–1.26). The 30-day mortality rates were 23.3 and 34.6% in the levosimendan and dobutamine groups, respectively (HR 0.65, 95% CI 0.30–1.37). The differences in the 90- and 180-day mortality rates were nonsignificant between the two groups before or after PSM (Table 4; Fig. 3a, b).
Table 4.
Mortality rates of patients with eGFR < 30 or dialysis who received levosimendan versus dobutamine alone
| Outcomes | Event (%) | Levosimendan vs. dobutamine | ||
|---|---|---|---|---|
| Levosimendan | Dobutamine | HR (95% CI) | P value | |
| Before PSM patient numbers | 52 | 374 | ||
| In-hospital mortality | 14 (26.9) | 114 (30.5) | 0.76 (0.44–1.32) | 0.330 |
| 5-day mortality | 5 (9.6) | 45 (12.0) | 0.79 (0.31–1.98) | 0.611 |
| 14-day mortality | 11 (21.2) | 76 (20.3) | 1.01 (0.54–1.91) | 0.968 |
| 30-day mortality | 11 (21.2) | 104 (27.8) | 0.73 (0.39–1.37) | 0.330 |
| 90-day mortality | 14 (26.9) | 132 (35.3) | 0.72 (0.42–1.25) | 0.247 |
| 180-day mortality | 18 (34.6) | 148 (39.6) | 0.83 (0.51–1.36) | 0.457 |
| After PSM patient numbers | 43 | 81 | ||
| In-hospital mortality | 13 (30.2) | 30 (37.0) | 0.67 (0.36–1.26) | 0.212 |
| 5-day mortality | 4 (9.3) | 10 (12.3) | 0.76 (0.26–2.22) | 0.611 |
| 14-day mortality | 10 (23.3) | 21 (25.9) | 0.87 (0.42–1.80) | 0.707 |
| 30-day mortality | 10 (23.3) | 28 (34.6) | 0.65 (0.30–1.37) | 0.254 |
| 90-day mortality | 13 (30.2) | 34 (42.0) | 0.68 (0.35–1.31) | 0.248 |
| 180-day mortality | 17 (39.5) | 36 (44.4) | 0.84 (0.45–1.55) | 0.573 |
HR hazard ratio, CI confidence interval, PSM propensity score matching
Fig. 3.
All-cause mortality rates of the patients with eGFR < 30 mL/min/1.73 m2 or dialysis who received levosimendan versus dobutamine alone before (a) and after (b) propensity score matching
Discussion
To the best of our knowledge, this is the first study to comprehensively investigate the short- and long-term survival of critical patients with both AHF and severe renal dysfunction who received levosimendan. We found that the patients who received levosimendan had similar survival rates to the patients who received dobutamine. Moreover, the differences in mortality rate were consistently nonsignificant between the two groups up to 180 days of follow-up, regardless of whether their eGFR was > 30 or < 30 mL/min/1.73 m2 or they were on maintenance dialysis.
Previous studies have reported that the use of levosimendan in patients with heart failure can improve cardiac output, urine amount, and eGFR. These results have been demonstrated in patients with acute or chronic left ventricular systolic dysfunction [6, 7, 10, 11, 17–20]. However, few studies have established the efficacy and safety of levosimendan in patients with severe renal dysfunction. Despite a lack of convincing evidence, depressed eGFR was thought to reduce the renal excretion of levosimendan leading to fatal arrhythmia, and several studies about levosimendan even excluded patients with an eGFR of < 30 mL/min/1.73 m2 [11, 21]. The Taiwan Food and Drug Administration (FDA) also does not recommend levosimendan for such patients. However, patients who are admitted for AHF frequently have different severities of renal dysfunction, and therefore the depressed GFR at that time could adversely affect the rate of levosimendan use. Although dobutamine may temporarily improve cardiac output, it can also lead to serious arrhythmia and myocardial ischemia [22, 23]. In contrast, the neutral effect on myocardial diastolic function and arrhythmogenic potential may suggest that levosimendan is a suitable alternative to dobutamine [24–26]. Levosimendan plays an important role in treating patients with severe renal dysfunction in our clinical practice.
In our CCUs, patients with acutely deteriorating heart failure are treated using diuretic agents, nitroglycerin, and ACEis (or angiotensin II antagonists) first, and we only prescribe inotropic agents for those with signs of systemic tissue hypoperfusion, including oliguria or a rapidly worsening plasma creatinine level. Because low cardiac output and systemic hypoperfusion in patients with reduced left ventricular systolic function is a critical situation, our intensivists are not limited with regard to the choice of treatment. It depends on their clinical judgement, including whether or not they combine inotropic agents with norepinephrine. The initial eGFR was ≥ 30 mL/min/1.73 m2 in most of our patients, but then became further depressed due to deteriorating cardiac function. We consider that renal dysfunction is reversible and not a contraindication for using levosimendan. For patients with an eGFR < 30 mL/min/1.73 m2, levosimendan is prescribed based on detailed discussions among the patients, their family members, and our critical medical teams in our clinical practice. The available medical evidence is discussed at these meetings [27–30], and the patients are carefully monitored to avoid adverse events. In this study, 53.9% of the patents in the levosimendan group received dopamine or dobutamine before the initiation of levosimendan in the index admission. This suggests that the patients were poor responders to dopamine or dobutamine. In our practice, some poor responders will receive mechanical cardiac support, such as veno-arterial ECMO or a temporary ventricular assist device (Centrimag), instead of levosimendan [34–36]. In the current study, none of the patients received a durable ventricular assist device or heart transplantation, and this situation is common in the medical environment in Taiwan.
The average LVEF of our study patients was < 30%. Before PSM, more patients in the levosimendan group had AMI and received PCI in the index admission, and dopamine and intra-aortic balloon pumps were more frequently used in this group. There was also a higher rate of myocarditis, however the difference was small. After PSM, the rates of AMI, PCI, mechanical ventilation, IABP, APACHE III, and SOFA scores between the two groups were similar. There were no significant differences in mortality rates up to 6 months between the two groups. In the subgroup with an eGFR < 30 mL/min/1.73 m2, the survival rate of the patients who received levosimendan was also equivalent to the patients who received dobutamine. Our results suggest that levosimendan did not increase mortality even in these critical AHF patients with severe renal dysfunction.
Limitations
There are several limitations to this study. First, because of the retrospective and real-practice design, we could not limit our intensivists in their choices of inotropic agents. The prescription of levosimendan was based on the clinical judgement of the intensivists. For example, the rate of dopamine use was higher in the levosimendan group at baseline, so we used PSM to minimize bias. In total, 53.9% of the patients who received levosimendan were prescribed dobutamine or dopamine before their index date, and the outcomes may have been affected if the clinicians decided to continue dobutamine or dopamine at that time. These results can be only confirmed by well-designed prospective studies; however, it would be ethically wrong if the patients are considered to be poor responders to initial inotropic agents and clinicians do not adjust their therapeutic policies. Second, we excluded patients with ECMO implantation from our study. Veno-arterial ECMO is considered to be an emergency life-saving method for patients with AHF complicated with refractory cardiogenic shock. However, refractory cardiogenic shock is a contraindication for levosimendan. Moreover, the disease severity in the patients who needed ECMO was highly heterogeneous, ranging from post-cardiotomy shock to ECMO-assisted cardiopulmonary resuscitation for prolonged cardiac arrest. Therefore, differences in mortality rate between those who received levosimendan and dobutamine may have been affected by the use ECMO. Third, we used ICD codes to identify patients with heart failure and other comorbidities. This is common in database studies, however it can lead to misestimations [31, 32]. To minimize possible bias, we only enrolled patients who received inotropic agents and had a reduced ejection fraction, thereby reinforcing the diagnosis of heart failure. Fourth, we used pre-dosing plasma creatinine to estimate GFR. Because creatinine is not an instant parameter reflecting renal function, the baseline GFR may have been overestimated in this study. Collecting urine for 24 h increases the accuracy of GFR values; however, this cannot always be done for such critical patients.
Conclusions
The critical AHF patients with or without severe renal dysfunction who received levosimendan had similar survival rates compared to the patients who received dobutamine while patients with veno-arterial ECMO were excluded. According to our results, an eGFR < 30 mL/min/1.73 m2 is not necessarily a contraindication for levosimendan.
Acknowledgements
This manuscript was edited by Wallace Academic Editing.
Abbreviations
- AHF
Acute heart failure
- AMI
Acute myocardial infarction
- CCU
Critical cardiac care unit
- CKD
Chronic kidney disease
- ECMO
Extracorporeal membrane oxygenation
- eGFR
Estimated glomerular filtration rate
- IABP
Intra-aortic balloon pump
- PSM
Propensity score matching
- LVEF
Left ventricular ejection fraction
- PCI
Percutaneous coronary intervention
Author contributions
CCC and KTL generated the ideas and completed the main writing works. WJH and YHC helped interpret the results. PHC performed the statistic works and summaries. All authors read and approved the final manuscript.
Funding
This study is granted by Chang-Gung Medical Research Project, CMRPG3K0191.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Ethics approval and consent to participate
This study has been approved by the Institutional Review Board of the Chang Gung Medical Foundation, 201901349B0.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Cze-Ci Chan and Kuang-Tso Lee contributed equally in this study.
References
- 1.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 2.Papp Z, Csapo K, Pollesello P, Haikala H, Edes I. Pharmacological mechanisms contributing to the clinical efficacy of levosimendan. Cardiovasc Drug Rev. 2005;23(1):71–98. doi: 10.1111/j.1527-3466.2005.tb00158.x. [DOI] [PubMed] [Google Scholar]
- 3.Yildiz O. Vasodilating mechanisms of levosimendan: involvement of K+ channels. J Pharmacol Sci. 2007;104(1):1–5. doi: 10.1254/jphs.CP0060010. [DOI] [PubMed] [Google Scholar]
- 4.Papp Z, Edes I, Fruhwald S, De Hert SG, Salmenpera M, Leppikangas H, Mebazaa A, Landoni G, Grossini E, Caimmi P, et al. Levosimendan: molecular mechanisms and clinical implications: consensus of experts on the mechanisms of action of levosimendan. Int J Cardiol. 2012;159(2):82–87. doi: 10.1016/j.ijcard.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 5.Bove T. Beneficial impact of levosimendan in critically ill patients with or at risk for acute renal failure: a meta-analysis of randomized clinical trials. Heart Lung Vessels. 2015;7(1):35–46. [PMC free article] [PubMed] [Google Scholar]
- 6.Zorlu A, Yucel H, Yontar OC, Karahan O, Tandogan I, Katrancioglu N, Yilmaz MB. Effect of levosimendan in patients with severe systolic heart failure and worsening renal function. Arq Bras Cardiol. 2012;98(6):537–543. doi: 10.1590/S0066-782X2012005000048. [DOI] [PubMed] [Google Scholar]
- 7.Hou ZQ, Sun ZX, Su CY, Tan H, Zhong X, Hu B, Zhou Y, Shang DY. Effect of levosimendan on estimated glomerular filtration rate in hospitalized patients with decompensated heart failure and renal dysfunction. Cardiovasc Ther. 2013;31(2):108–114. doi: 10.1111/1755-5922.12001. [DOI] [PubMed] [Google Scholar]
- 8.Yilmaz MB, Grossini E, Silva Cardoso JC, Edes I, Fedele F, Pollesello P, Kivikko M, Harjola VP, Hasslacher J, Mebazaa A, et al. Renal effects of levosimendan: a consensus report. Cardiovasc Drugs Ther. 2013;27(6):581–590. doi: 10.1007/s10557-013-6485-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rafouli-Stergiou P, Parissis JT, Farmakis D, Bistola V, Frogoudaki A, Vasiliadis K, Ikonomidis I, Paraskevaidis I, Kremastinos D, Filippatos G, et al. Effects of levosimendan on markers of kidney function in patients with acutely decompensated heart failure and renal impairment. J Cardiovasc Med (Hagerstown) 2017;18(10):771–773. doi: 10.2459/JCM.0000000000000244. [DOI] [PubMed] [Google Scholar]
- 10.Ferreri C, Testa M, Feola M. The role of levosimendan unfusion in improving renal function in sorsening type II cardio-renal syndrome. Int J Biomed Sci. 2018;14(2):78–84. [Google Scholar]
- 11.Lannemyr L, Ricksten SE, Rundqvist B, Andersson B, Bartfay SE, Ljungman C, Dahlberg P, Bergh N, Hjalmarsson C, Gilljam T, et al. Differential effects of levosimendan and dobutamine on glomerular filtration rate in patients with heart failure and renal impairment: a randomized double-blind controlled trial. J Am Heart Assoc. 2018;7(16):e008455. doi: 10.1161/JAHA.117.008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou S, Zhang L, Li J. Effect of levosimendan in patients with acute decompensated heart failure: a meta-analysis. Herz. 2019;44(7):630–636. doi: 10.1007/s00059-018-4693-3. [DOI] [PubMed] [Google Scholar]
- 13.Taiwan Ministry of Health and Welfare—National Health Service, vol 2018. https://dep.mohw.gov.tw/DOS/lp-4487-113.html
- 14.Shao SC, Chan YY, Kao Yang YH, Lin SJ, Hung MJ, Chien RN, Lai CC, Lai EC. The Chang Gung Research Database-a multi-institutional electronic medical records database for real-world epidemiological studies in Taiwan. Pharmacoepidemiol Drug Saf. 2019;28(5):593–600. doi: 10.1002/pds.4713. [DOI] [PubMed] [Google Scholar]
- 15.Tsai MS, Lin MH, Lee CP, Yang YH, Chen WC, Chang GH, Tsai YT, Chen PC, Tsai YH. Chang Gung Research Database: a multi-institutional database consisting of original medical records. Biomed J. 2017;40(5):263–269. doi: 10.1016/j.bj.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, Van Lente F. Chronic Kidney Disease Epidemiology C: Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53(4):766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 17.Yilmaz MB, Yalta K, Yontar C, Karadas F, Erdem A, Turgut OO, Yilmaz A, Tandogan I. Levosimendan improves renal function in patients with acute decompensated heart failure: comparison with dobutamine. Cardiovasc Drugs Ther. 2007;21(6):431–435. doi: 10.1007/s10557-007-6066-7. [DOI] [PubMed] [Google Scholar]
- 18.Zemljic G, Bunc M, Yazdanbakhsh AP, Vrtovec B. Levosimendan improves renal function in patients with advanced chronic heart failure awaiting cardiac transplantation. J Card Fail. 2007;13(6):417–421. doi: 10.1016/j.cardfail.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Honore PM, Gutierrez LB, Redant S, Kaefer K, Gallerani A, De Bels D. How levosimendan can improve renal function? Crit Care. 2019;23(1):331. doi: 10.1186/s13054-019-2642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Najjar E, Stalhberg M, Hage C, Ottenblad E, Manouras A, Haugen Lofman I, Lund LH. Haemodynamic effects of levosimendan in advanced but stable chronic heart failure. ESC Heart Fail. 2018;5(3):302–308. doi: 10.1002/ehf2.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altenberger J, Parissis JT, Costard-Jaeckle A, Winter A, Ebner C, Karavidas A, Sihorsch K, Avgeropoulou E, Weber T, Dimopoulos L, et al. Efficacy and safety of the pulsed infusions of levosimendan in outpatients with advanced heart failure (LevoRep) study: a multicentre randomized trial. Eur J Heart Fail. 2014;16(8):898–906. doi: 10.1002/ejhf.118. [DOI] [PubMed] [Google Scholar]
- 22.Tarjan J, Nagy L, Liziczai I, Junger E. Arrhythmic effects of intermittent dobutamine therapy in chronic heart disease failure. The Working Group of Cardiology of the Academic Committee of Veszprem. Hungary. Am J Ther. 1998;5(6):405–411. doi: 10.1097/00045391-199811000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Stump GL, Wallace AA, Gilberto DB, Gehret JR, Lynch JJ., Jr Arrhythmogenic potential of positive inotropic agents. Basic Res Cardiol. 2000;95(3):186–198. doi: 10.1007/s003950050181. [DOI] [PubMed] [Google Scholar]
- 24.Gong B, Li Z, Yat Wong PC. Levosimendan treatment for heart failure: a systematic review and meta-analysis. J Cardiothorac Vasc Anesth. 2015;29(6):1415–1425. doi: 10.1053/j.jvca.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 25.Lilleberg J, Ylonen V, Lehtonen L, Toivonen L. The calcium sensitizer levosimendan and cardiac arrhythmias: an analysis of the safety database of heart failure treatment studies. Scand Cardiovasc J. 2004;38(2):80–84. doi: 10.1080/14017430410025783. [DOI] [PubMed] [Google Scholar]
- 26.Lilleberg J, Toivonen L. Effects of levosimendan on cardiac arrhythmia in patients with severe heart failure. Crit Care. 2002;6(Suppl 1):p139. doi: 10.1186/cc1595. [DOI] [Google Scholar]
- 27.Bouchez S, Fedele F, Giannakoulas G, et al. Levosimendan in acute and advanced heart failure: an expert perspective on posology and therapeutic application. Cardiovasc Drugs Ther. 2018;32(6):617–624. doi: 10.1007/s10557-018-6838-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puttonen J, Kantele S, Kivikko M, et al. Effect of severe renal failure and haemodialysis on the pharmacokinetics of levosimendan and its metabolites. Clin Pharmacokinet. 2007;46(3):235–246. doi: 10.2165/00003088-200746030-00004. [DOI] [PubMed] [Google Scholar]
- 29.Fedele F, Karason K, Matskeplishvili S. Pharmacological approaches to cardio-renal syndrome: a role for the inodilator levosimendan. Eur Heart J Suppl. 2017;19(Suppl C):C22–C28. doi: 10.1093/eurheartj/sux002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cholley B, Caruba T, Grosjean S, et al. Effect of levosimendan on low cardiac output syndrome in patients with low ejection fraction undergoing coronary artery bypass grafting with cardiopulmonary bypass: the LICORN randomized clinical trial. JAMA. 2017;318(6):548–556. doi: 10.1001/jama.2017.9973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campbell PG, Malone J, Yadla S, et al. Comparison of ICD-9-based, retrospective, and prospective assessments of perioperative complications: assessment of accuracy in reporting. J Neurosurg Spine. 2011;14(1):16–22. doi: 10.3171/2010.9.SPINE10151. [DOI] [PubMed] [Google Scholar]
- 32.Llorens P, Javaloyes P, Martín-Sánchez FJ, et al. Time trends in characteristics, clinical course, and outcomes of 13,791 patients with acute heart failure. Clin Res Cardiol. 2018;107(10):897–913. doi: 10.1007/s00392-018-1261-z. [DOI] [PubMed] [Google Scholar]
- 33.Zhou Z, Rahme E, Abrahamowicz M, Pilote L. Survival bias associated with time-to-treatment initiation in drug effectiveness evaluation: a comparison of methods. Am J Epidemiol. 2005;162(10):1016–1023. doi: 10.1093/aje/kwi307. [DOI] [PubMed] [Google Scholar]
- 34.Rihal CS, Naidu SS, Givertz MM, et al; Society for Cardiovascular Angiography and Interventions (SCAI), Heart Failure Society of America (HFSA), Society of Thoracic Surgeons (STS), American Heart Association (AHA), and American College of Cardiology (ACC). 2015 SCAI/ACC/HFSA/STS Clinical Expert Consensus Statement on the Use of Percutaneous Mechanical Circulatory Support Devices in Cardiovascular Care: Endorsed by the American Heart Association, the Cardiological Society of India, and Sociedad Latino Americana de Cardiología Intervencionista; Affirmation of Value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d'intervention. J Am Coll Cardiol. 2015; 65(19):2140–11 [DOI] [PubMed]
- 35.Tehrani BN, Truesdell AG, Psotka MA, et al. A Standardized and comprehensive approach to the management of cardiogenic shock. JACC Heart Fail. 2020;8(11):879–891. doi: 10.1016/j.jchf.2020.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Combes A, Price S, Slutsky AS, et al. Temporary circulatory support for cardiogenic shock. Lancet. 2020;396(10245):199–212. doi: 10.1016/S0140-6736(20)31047-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.