Abstract
Autism spectrum disorder is an early-onset neurodevelopmental condition. This study aimed to investigate the progressive structural alterations in the autistic brain during early childhood. Structural magnetic resonance imaging scans were examined in a cross-sectional sample of 67 autistic children and 63 demographically matched typically developing (TD) children, aged 2–7 years. Voxel-based morphometry and a general linear model were used to ascertain the effects of diagnosis, age, and a diagnosis-by-age interaction on the gray matter volume. Causal structural covariance network analysis was performed to map the interregional influences of brain structural alterations with increasing age. The autism group showed spatially distributed increases in gray matter volume when controlling for age-related effects, compared with TD children. A significant diagnosis-by-age interaction effect was observed in the fusiform face area (FFA, Fpeak = 13.57) and cerebellum/vermis (Fpeak = 12.73). Compared with TD children, the gray matter development of the FFA in autism displayed altered influences on that of the social brain network regions (false discovery rate corrected, P < 0.05). Our findings indicate the atypical neurodevelopment of the FFA in the autistic brain during early childhood and highlight altered developmental effects of this region on the social brain network.
Keywords: autism spectrum disorder, Granger causality, gray matter volume, neurodevelopment, structural magnetic resonance imaging
Introduction
Autism spectrum disorder (ASD) is an early-onset neurodevelopmental condition characterized by impairments in social interaction and communication and restricted/repetitive behaviors (American Psychiatric Association 2013). Across these domains, social impairments are considered a hallmark feature of autism. Findings from neuroanatomical studies suggest that autism is characterized by early overgrowth of the brain at the early period of life (Courchesne et al. 2011; Ecker et al. 2015), though primarily comes from studies on head circumference and total or compartmental brain volume (Courchesne et al. 2007; Schumann et al. 2010; Ecker et al. 2015). Early brain overgrowth in autism is reported to occur as early as during the second year of life (Hazlett et al. 2017). In addition, the social developmental model of the amygdala and fusiform face area (FFA) interactions of autism proposed that the congenital developmental alterations of the amygdala in autism are hypothesized to lead to differences in emotional salience; this phenomenon may then affect the FFA engagement in the formation of expert skills for faces; it may further lead to developmental differences of social skills regulated by social brain regions across childhood (Schultz 2005).
Early childhood is widely recognized as an important period for brain development including the formation and fine-tuning of neural circuits that make brain functional networks possible in typical development (Courchesne et al. 2007; Gilmore et al. 2018). It affects the lifelong cognitive behaviors and the risk for psychiatric disorders (Nelson 3rd et al. 2007; Gilmore et al. 2018). Although behavioral signs of autism may be present in the first two years of life, the first reliable diagnosis of autism clinically based on these core behavioral symptoms is usually made at 2–4 years of age. Delineating the neuroanatomical development of the autistic brain during early childhood may help understand the anatomical foundations of the altered large-scale, long-distance, functional connections in autism (Guo et al. 2019; Guo et al. 2020). It may also have implications for detecting the endogenous and exogenous risk factors that lead to structural and functional alterations in the autistic brain in other stages of life, and uncovering a promising method for early diagnosis and intervention of autism. However, the progressive structural alterations with increasing age in the autistic brain during early childhood remain essentially unknown.
Accumulating evidence has also characterized autism as a disorder of connectivity (Belmonte et al. 2004; Minshew and Williams 2007; Ecker et al. 2015). Conventional structural covariance network analysis provides a means to probe co-varying patterns in gray matter morphology among distributed brain regions (Zielinski et al. 2010; Alexander-Bloch et al. 2013; Liu et al. 2019; Han et al. 2020). These structural covariance networks are complementary to functional connectivity and white matter connectivity networks and possibly reflect interregional morphological covariance during maturation (Alexander-Bloch et al. 2013). There have been a considerable number of structural magnetic resonance imaging (MRI) studies attempting to delineate the potential alterations in neurodevelopmental coordination using structural covariance analysis in autism (Zielinski et al. 2012; Valk et al. 2015; Long et al. 2016; Sharda et al. 2016). However, such studies characterize the morphological correlation among regions ignoring the temporal progression of the morphological changes.
Causal structural covariance network (CaSCN) analysis, which integrates the Granger causality analysis (GCA) with a structural covariance approach, is emerging as a promising method for quantifying progressive profiles of structural network organization (Zhang et al. 2017). By incorporating temporal information, GCA can be utilized to identify the directional effects of anatomical changes among brain areas through development or during the course of diseases. A significant causal covariance coefficient from the seed region to the target region indicates that the structural development in the target region lags behind that in the seed region. These causal structural networks are assumed to reflect the chronological ordering of regional structural alterations under pathological conditions or during maturation. The CaSCN approach has been successfully applied to identify the origin regions of structural alterations and assess the progressive changes in gray matter with increasing disease duration in epilepsy, generalized anxiety disorder, and schizophrenia (Zhang et al. 2017; Jiang et al. 2018; Chen et al. 2020). The CaSCN analysis in patients with epilepsy showed progressive gray matter alterations from the hippocampus to brain regions including the frontal lobe, temporal lobe, subcortical regions, and cerebellum as the epilepsy duration and seizure times increase (Zhang et al. 2017). Another CaSCN study in schizophrenia demonstrated that gray matter damages originated from the thalamus, which exerted progressive influences on the frontal regions and further affected the temporal and occipital cortices and cerebellum with increased illness duration (Jiang et al. 2018). In generalized anxiety disorder, gray matter damages were showed to spread from the subgenual anterior cingulate cortex to the insula, frontal, and temporal cortices (Chen et al. 2020).
This study utilized a cross-sectional sample of 67 autistic children and 63 demographically matched typically developing (TD) children ranging from 2 to 7 years of age, aimed to assess progressive gray matter volume changes in early childhood autistic children compared with TD children. We sought to answer two questions: 1) Does gray matter development in the autistic brain differ from typical development during early childhood? and 2) What are the temporal influences of these structural development alterations on the development of other brain regions along with increasing age in autism? To this end, we first compared the age-related gray matter volume changes between autism and TD group using a general linear model and set the brain regions showing significant diagnosis-by-age interaction effects as the region of interest (ROI). Subsequently, we conducted a CaSCN analysis to explore the progressive influences of gray matter alterations of the ROI on that of other brain regions. Based on the social development model of the amygdala-FFA system in autism (Schultz 2005), autistic children are hypothesized to show disrupted gray matter development during early childhood, especially in the social brain network regions. These gray matter development changes in autism might lead to altered temporal influences on the gray matter changes of other social brain network regions.
Materials and Methods
Participants: HMU Cohort
A total of 42 autistic children and 57 TD children participated in this study. Autistic children were recruited through the Children Development and Behavior Research Center of Harbin Medical University (Harbin, Heilongjiang Province, China). TD participants were recruited from local kindergartens. All participants were native Chinese speakers. ASD was clinically diagnosed on the basis of the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5) (American Psychiatric Association 2013) and confirmed with the Autism Diagnostic Interview-Revised (ADI-R) (Lord et al. 1994, 2000) and the Autism Diagnostic Observation Schedule (ADOS) (Lord et al. 2000), administered by two or more pediatric or psychiatry associate chief physicians. ADI-R and ADOS assessments were conducted using the Traditional Mandarin research translation versions for the ADI-R and ADOS protocols, approved and authorized by Western Psychological Services (https://www.wpspublish.com/). These assessments were performed by professionally trained physicians and certified to reliably administer ADI-R and ADOS. The exclusion criteria for the autism group included known psychiatric, neurological (e.g., epilepsy, Tourette’s syndrome) or genetic (e.g., fragile X, Rett syndrome) disorders, a history of a loss of consciousness for more than 5 min, and those currently taking psychoactive medication. None of the TD children were on psychotropic medications or had a reported history of any severe medical problems or any neurological or psychiatric conditions. TD and autistic children also underwent the Peabody Picture Vocabulary Test (PPVT) in Chinese. Written informed consent was obtained from the guardians of each participant after fully explaining the purpose of the study. This study was approved by the ethics review committee of Harbin Medical University (HMU).
This study was limited to male children for the following reasons. Sexual dimorphism has been frequently associated with the neuroanatomical heterogeneity of autism (Lai et al. 2013; Duerden et al. 2020). However, the number of autistic females in our sample is limited (HMU cohort: N = 7; UESTC cohort: N = 8) partly due to the 3:1 male-to-female ratio in the prevalence of autism (Loomes et al. 2017). Small sample studies could reduce the statistical power and increase failures to find true effects, resulting in low reliability and reproducibility of results (Button et al. 2013; Lombardo et al. 2019). Therefore, in order to eliminating the potential effects introduced by sex, the few female participants were excluded from this study.
Children with apparent motion artifacts based on the visual inspection of the structural images were excluded. Given the mismatch between autism and TD groups in terms of age and handedness, we further used a random matching algorithm (Supplementary Materials) to optimize the group differences on age (using two-sample t-tests) and handedness (using χ2 tests) and create a well-matched dataset as in previous studies (Nomi and Uddin 2015). These criteria identified a well-matched dataset of 35 autistic boys and 35 TD boys (Table 1).
Table 1.
Demographics and clinical characteristics of the participants
| HMU cohort | UESTC cohort | ASD versus TD | |||||
|---|---|---|---|---|---|---|---|
| ASD (n = 35) | TD (n = 35) | P value | ASD (n = 32) | TD (n = 28) | P value | P value | |
| Age (years) | 4.97 ± 1.21 | 4.99 ± 0.90 | 0.93a | 5.13 ± 1.12 | 5.54 ± 0.96 | 0.14a | 0.32a |
| Age range (years) | 3.47–7.94 | 3.18–6.89 | — | 3.61–7.57 | 2.78–6.78 | — | — |
| Handedness (right/left/mixed) | 28/7/0 | 32/3/0 | 0.17b | 24/2/0 | 25/1/1 | 0.51b | 0.21b |
| ADOS communication | 5.90 ± 1.81 | — | — | 6.05 ± 1.93 | — | — | — |
| ADOS social | 9.23 ± 2.40 | — | — | 10.00 ± 3.48 | — | — | — |
| ADOS RRB | 1.87 ± 1.25 | — | — | 1.60 ± 1.31 | — | — | — |
| ADI-R social | 22.03 ± 3.55 | — | — | 23.61 ± 4.19 | — | — | — |
| ADI-R communication | 15.13 ± 4.18 | — | — | 14.33 ± 3.63 | — | — | — |
| ADI-R RRB | 7.07 ± 2.55 | — | — | 3.33 ± 2.03 | — | — | — |
Notes: Demographic information for each variable is presented as mean ± SD. ASD: autism spectrum disorder; TD: Typically developing controls; ADOS: Autism Diagnostic Observation Schedule; RRB: restricted and repetitive behaviors; ADI-R: Autism Diagnostic Interview-Revised. Handedness was available for 26 ASD subjects and 27 TD subjects in UESTC cohort. ADOS scores were available for 30 ASD subjects in HMU cohort and 20 ASD subjects in UESTC cohort; ADI-R scores were available for 30 ASD subjects in HMU cohort and 18 ASD subjects in UESTC cohort.
aIndicates P values for two sample t-test.
bIndicates P values for χ2 test.
Participants: UESTC Cohort
A total of 40 autistic children and 38 TD children were included in this study. Autistic children were recruited through the Sichuan Bayi Rehabilitation Center (Chengdu, Sichuan Province, China). TD participants were recruited from local kindergartens. All participants were native Chinese speakers. Autistic children received a clinical diagnosis of ASD based on the DSM-5 and confirmed with the ADOS and/or ADI-R. ADI-R and ADOS assessments were conducted using the Traditional Mandarin research translation versions for the ADI-R and ADOS protocols and performed by professionally trained physicians and certified to reliably administer ADI-R and ADOS. The exclusion criteria for autism and TD children were identical to those of the HMU cohort. Written informed consent was obtained from the guardians of each participant after fully explaining the purpose of this study. This study was approved by the research ethical committee of the University of Electronic Science and Technology of China (UESTC). After excluding the female children and low-quality structural MRI, the resulting 32 autistic boys and 28 TD boys were well-matched for age and handedness (Table 1).
This study (HMU and UESTC cohorts) was registered at ClinicalTrials.gov (Name: Brain Plasticity of Autism in Response to Early Behavioral Intervention: A Multimodal MRI Study; URL: https://clinicaltrials.gov/ct2/show/NCT02807766; Identifier: NCT02807766).
Data Acquisition: HMU Cohort
MRI was acquired at the Department of MR Diagnosis of the Second Hospital affiliated of Harbin Medical University by using a 3.0 Tesla Achieva Magnetic Resonance System (Philips). Anatomical T1 images of the whole brain were obtained via a volumetric 3D spoiled gradient recall sequence: repetition time (TR) = 8.5 ms; echo time (TE) = 3.9 ms; matrix size = 256 × 256; flip angle (FA) = 9°; FOV = 256 × 256 mm2; voxel size = 1 × 1 × 1 mm3; and 176 axial slices. Resting-state functional MRI and diffusion tensor imaging data were also acquired but not used in the current study. Each participant received 50 mg/kg chloral hydrate by a nurse trained and certified to administer sedation. Sedation was conducted by following the guidelines and protocols established by the radiology sedation committee at the hospital. A caregiver for each child was present throughout the duration of the scan.
Data Acquisition: UESTC Cohort
MRI scans were acquired at the University of Electronic Science and Technology of China by using a 3.0 Tesla GE DISCOVERY MR750 scanner (General Electric). Anatomical T1 images of the whole brain were acquired using a 3D T1 sequence with the following parameters: TR = 6.02 ms; TE = 1.96 ms; matrix size = 256 × 256, FA = 9°; FOV = 256 × 256 mm2; voxel size = 1 × 1 × 1 mm3; and 156 axial slices. All TD children were instructed to watch cartoons while keeping as still as possible throughout the entire scan. Autistic participants were under sedation of 50 mg/kg chloral hydrate performed by a trained and certified nurse/doctor. Sedation was conducted by following the guidelines and protocols established by the radiology sedation committee at the Sichuan Bayi Rehabilitation Center. MRI acquisition was implemented in the presence of the caregiver for each child.
Voxel-Based Morphometry Analysis
Anatomical images were processed using the Computational Anatomy Toolbox 12 (CAT12, http://dbm.neuro.uni-jena.de/cat/) for Statistical Parametric Mapping 12 (SPM12, https://www.fil.ion.ucl.ac.uk/spm/) to obtain voxel-wise gray matter volumes for each child. Each image was manually reoriented to set the image origin at the anterior commissure for better co-registration. These images were then segmented into gray matter, white matter, and cerebrospinal fluid by using the customized pediatric tissue probability maps created with the Template-O-Matic 8 toolbox (TOM8, https://irc.cchmc.org/software/tom.php). The Diffeomorphic Anatomical Registration through Exponentiated Lie (DARTEL) algebra algorithm was applied to the gray matter images to create customized gray matter template through iterative registration (Ashburner 2007). After an affine spatial normalization of the gray matter template to the MNI space, the resulting deformations were applied to the gray matter images for normalization to the MNI space. Jacobian modulation was performed on the image intensities to preserve the gray matter volume at each voxel. Finally, the images were smoothed with an isotropic Gaussian kernel (full width at half maximum = 8 mm). Total intracranial volumes (TIVs) were calculated for each child by summing the volume values of the gray matter, white matter, and cerebrospinal fluid.
Quality Control Analysis
Image quality checks included the following steps: 1) each structural image was visually inspected for obvious artifacts due to head motion or incomplete coverage of the entire brain; 2) segmentation quality of the voxel-based morphometry (VBM) analysis was visually checked; and 3) homogeneity of the VBM maps between any pair of participants was assessed using correlation coefficients in CAT 12. Images with mean homogeneity lower than 2 standard deviations (SDs) from the sample mean were visually rechecked. No participant was excluded during the quality control analysis.
Statistical Analysis of VBM
Between-group differences at each voxel for gray matter volume and its age-related changes were identified with a general linear model by using SPM 12
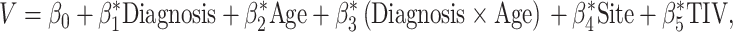 |
where V denotes the gray matter volume estimated by VBM analysis, Diagnosis is autism or TD children, Age is the continuous age factor, Diagnosis × Age is the diagnosis-by-age interaction, Site is the covariate for cohort effects (using a dummy coding scheme), and TIV is the covariate for TIV. Given that TIV is not orthogonal to VBM maps, age, and site, mean-centered TIV was included as a nuisance parameter for each group separately.
Corrections for multiple comparisons for the effects of diagnosis, age, and diagnosis-by-age interaction were conducted using the Monte Carlo Simulation implemented in the Data Processing and Analysis for (Resting-State) Brain Imaging toolbox (DPABI v4.0, http://rfmri.org/dpabi) (Yan et al. 2016). Statistical significance was determined with a combination of cluster-level P < 0.05 and voxel-level P < 0.005. For clusters with a significant interaction effect between diagnosis and age, Pearson correlation analysis was performed between VBM and age with site and TIV as covariates for each group. Post hoc analysis using quadratic growth curves was also applied to brain regions showing significant interaction effect to exclude the potential artifact introduced by the linear model (Supplementary Fig. 2). Age-related analysis of the white matter volume is presented in Supplementary Figures 5 and 6.
Between-group differences in the age-related changes of global brain measures were examined using the following model:
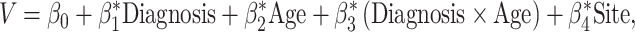 |
where V denotes the total volume of brain, gray matter, or white matter.
Seed-Based CaSCN Analysis
Seed-based CaSCN analysis was performed following the procedures described in previous studies to further investigate the progressive effects of anatomical alterations on structural brain networks throughout development (Zhang et al. 2017; Jiang et al. 2018). In light of the well-recognized role of the FFA in social perceptual impairments of autism (Waiter et al. 2004; Dalton et al. 2005; Dziobek et al. 2010), and autistic children showed the greatest age-related anatomical alterations in the FFA compared with TD children in the above VBM analysis (see Results), the FFA region was denoted as the seed region for CaSCN analysis. Specifically, all voxels showing significant diagnosis-by-age interaction effects in the FFA were constructed as the ROI and then used as the seed region of CaSCN analysis. For each group, the gray matter volume images were age ranked in ascending sequence to create artificial temporal information to the cross-sectional dataset. Signed-path coefficient GCA was applied to the pseudo-time series of gray matter volume images to map the progressive influence of anatomical alterations to the FFA on the whole brain using the Resting State fMRI Data Analysis Toolkit (REST, http://www.restfmri.net) (Zang et al. 2012; Fan et al. 2019). Site, TIV, and time interval of sequenced age were added as covariates. Finally, Z score transformation computed by subtracting the global mean value and then divided by the SD was performed on the voxel-wise Granger causality map to assess statistical significance. Between-group comparisons were performed on Z maps to explore the group differences of gray matter volume covariance, within a predefined union mask of CaSCN of autism and TD groups (P < 0.05, uncorrected). False discovery rate (FDR) was corrected for multiple comparisons with a significance threshold of P < 0.05.
Correlations between Altered Gray Matter Volume and Autism Symptoms
Spearman correlation analysis was performed to explore whether the gray matter volume differences identified by significant effects of diagnosis and a diagnosis-by-age interaction were related to the measures of symptom severity among autistic individuals. Spearman correlation coefficients can be used to assess the monotonic relationships between two variables whether they change linearly or nonlinearly. Age, site, and TIV were added as covariates. Autism symptom severity was assessed by social, communication, and restricted and repetitive behavior scores in ADOS. Bonferroni correction was performed for multiple comparisons with statistical significance set at P < 0.05.
Results
Global Brain Measures
Although autistic children showed a large total volume of the brain (1567 ± 157 cm3), gray matter (777 ± 60 cm3), and white matter (449 ± 48 cm3) than TD children (TIV: 1475 ± 126 cm3; gray matter: 741 ± 56 cm3; white matter: 431 ± 35 cm3), the main effect of diagnosis or diagnosis-by-age interaction effect in any of three global brain measures during early childhood was not significant. A significant main effect of age was observed in the total volume of the white matter, which significantly increased with age (T(125) = 2.39, P = 0.018). However, the age-related changes in the total volume of the brain (T(125) = −0.02, P = 0.98) or gray matter (T(125) = −0.049, P = 0.96) were not significant.
Group Differences in Gray Matter Volume
We identified a significant main effect of diagnosis in gray matter volume across the brain when controlling for the effects of age, diagnosis-by-age interaction, site, and TIV, including the bilateral rectus, right inferior occipital gyrus, right inferior temporal gyrus, left lingual gyrus/cerebellum, right calcarine, right superior temporal gyrus, and left cuneus (Fig. 1, Table 2). Relative to the TD group, the autistic group showed significantly increased gray matter volume in all those regions during early childhood. No brain regions showed reductions in gray matter volume among autistic individuals.
Figure 1.

Significant main effect of diagnosis in the voxel-based morphometry analysis. Autistic children showed larger gray matter volume than TD children in the bilateral rectus (F(1, 124) = 13.99, P < 0.001), right inferior occipital gyrus (F(1, 124) = 12.73, P = 0.001), right inferior temporal gyrus (F(1, 124) = 14.88, P < 0.001), left lingual gyrus/cerebellum (F(1, 124) = 17.66, P < 0.001), right calcarine (F(1, 124) = 16.39, P < 0.001), right superior temporal gyrus (F(1, 124) = 16.36, P < 0.001), and left cuneus (F(1, 124) = 14.28, P < 0.001). *P < 0.05, **P < 0.01, ***P < 0.001. ASD, autism spectrum disorder; TD, typically developing children.
Table 2.
Significant main effect of diagnosis and diagnosis-by-age interaction effect in the voxel-based morphometry analysis
| Brain regions | Hemi | Voxels | BA | MNI coordinates | F value | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Main effect of diagnosis | |||||||
| Rectus | L/R | 336 | 11 | −1.5 | 48 | −25.5 | 13.99 |
| Inferior occipital gyrus | R | 496 | 18/19 | 39 | −90 | −18 | 12.73 |
| Inferior temporal gyrus | R | 428 | 20/37 | 63 | −63 | −9 | 14.88 |
| Lingual gyrus/Cerebellum | L | 1207 | 18/19/30 | −12 | −43.5 | −9 | 17.66 |
| Calcarine | R | 1123 | 29/30/31 | 27 | −66 | 12 | 16.39 |
| Superior temporal gyrus | R | 303 | 22 | 70.5 | −6 | 9 | 16.36 |
| Cuneus | L | 284 | 19 | −6 | −88.5 | 28.5 | 14.28 |
| Interaction effect between diagnosis and age | |||||||
| Cerebellum/Vermis | L/R | 886 | — | −6 | −58.5 | −16.5 | 12.73 |
| FFA | R | 148 | 37 | 40.5 | −40.5 | −12 | 13.57 |
Note: Hemi: hemisphere; L: left; R: right; BA: Brodmann Area.
Diagnosis-by-Age Interaction Effects on Gray Matter Volume
Significant interaction effects between diagnosis and age on gray matter volume were observed in two clusters, including the cerebellum/vermis and FFA (Fig. 2, Table 2). In the cerebellum/vermis, gray matter volume remained relatively constant with the increasing age in the TD group (r = 0.19, P = 0.15), whereas gray matter volume was negatively correlated with the age in the autism group (r = −0.49, P < 0.001). Moreover, gray matter volume in the FFA showed a negative relationship with age in the TD group (r = −0.32, P = 0.01). In contrast, the gray matter volume in this region linearly increased with the increasing age in the autism group (r = 0.25, P = 0.04). Clusters with significant main effects of age are presented in Supplementary Figure 1.
Figure 2.

Significant interaction effects between diagnosis and age identified in the voxel-based morphometry analysis. Clusters in the cerebellum/vermis (F(1, 124) = 12.73, P = 0.001) (A) and FFA (F(1, 124) = 13.57, P < 0.001) (B) showed significant diagnosis-by-age interaction effects. Group differences on gray matter changes with increasing age were compared between ASD and TD groups using Z-tests. The color bar represents F values of the diagnosis-by-age interaction effect in the cerebellum/vermis (A) and FFA (B). ASD, autism spectrum disorder; TD, typically developing children; L, left; R, right.
Age-associated gray matter volume analyses of the social brain regions were also performed and provided in Supplementary Figure 3.
Between-Group Differences in CaSCN of the FFA
In the TD group, the gray matter morphometry in the FFA had significant temporal influences on a broad set of brain regions (Fig. 3B). The positive influences from the FFA were observed primarily in the frontal cortex, posterior temporal cortex, temporal pole, and parietal cortex, while the negative influences were primarily located in the sensorimotor cortex, visual cortex, medial temporal cortex, thalamus, and cerebellum. Compared with the TD group, the autism group had significantly altered temporal influences related to the FFA in the left inferior frontal gyrus (IFG)/insula, bilateral posterior superior temporal sulcus (pSTS), right intraparietal sulcus, right middle temporal pole, left IFG, left medial prefrontal cortex (mPFC)/ACC, and left PCC/precuneus (Fig. 3C, Table 3); such brain structures are implicated in social behavior (Gotts et al. 2012). Significant temporal influences of morphometric alterations of the FFA on that of these brain regions present in the TD group were absent or even opposite in the autism group. Although Granger causal values in the peak coordinates of mPFC/ACC cluster were not significant in the TD group (Table 3), this cluster revealed by between-group comparisons spatially overlapped with a cluster of positive influences in the TD group. Furthermore, the gray matter volume in the FFA in the autism group showed altered temporal influences on that of several clusters located in the fusiform gyrus, cerebellum, middle and superior temporal gyrus, hippocampus/thalamus, calcarine, cuneus/superior occipital gyrus, middle occipital gyrus, middle and IFG, dorsolateral prefrontal cortex, and precuneus (Fig. 3C, Table 3).
Figure 3.

CaSCN of the FFA. CaSCN of the FFA in ASD (A) and TD (B) groups (P < 0.05, uncorrected). (C) Brain regions with significant differences in CaSCN of the FFA between ASD and TD groups (FDR corrected, P < 0.05). ASD, autism spectrum disorder; TD, typically developing children; L, left; R, right.
Table 3.
Between-group differences in CaSCN of the FFA
| Brain regions | Hemi | Voxels | BA | MNI coordinates | ASDa | TDa | ASD versus TDa | ||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| Fusiform gyrus | L | 147 | 20 | −31.5 | −6 | −34.5 | 2.70 | n.s. | 5.22 |
| Cerebellum | L/R | 281 | — | −6 | −49.5 | −10.5 | 2.65 | −2.31 | 4.95 |
| Middle temporal gyrus | R | 217 | 20 | 55.5 | −39 | −12 | 4.65 | n.s. | 6.44 |
| Superior temporal gyrus | L | 159 | 21/22 | −52.5 | −9 | −9 | 3.66 | n.s. | 4.24 |
| Hippocampus/Thalamus | L | 384 | — | −27 | −37.5 | −1.5 | 4.01 | n.s. | 6.06 |
| IFG/Insula* | L | 381 | 13 | −40.5 | 19.5 | 6 | 3.49 | −2.92 | 6.40 |
| Calcarine | R | 145 | 30 | 27 | −58.5 | 4.5 | n.s. | −3.33 | 4.91 |
| pSTS* | R | 233 | 13 | 48 | −42 | 15 | n.s. | −4.90 | 5.52 |
| pSTS* | L | 202 | 21 | −45 | −49.5 | 15 | n.s. | −5.43 | 5.96 |
| Intraparietal sulcus * | R | 398 | 40 | 33 | −45 | 45 | n.s. | −4.86 | 5.37 |
| Cerebellum | R | 757 | — | 10.5 | −82.5 | −30 | −5.16 | n.s. | −7.15 |
| Middle temporal pole * | R | 394 | 38 | 31.5 | 18 | −40.5 | −3.20 | 2.43 | −5.63 |
| IFG* | L | 693 | 47 | −42 | 22.5 | −13.5 | −2.68 | 3.15 | −5.83 |
| Cuneus/Superior occipital gyrus | R | 1780 | 18/19 | 16.5 | −90 | 21 | −4.07 | 3.12 | −7.18 |
| mPFC/Anterior cingulate cortex* | L | 503 | 10/32 | −7.5 | 51 | −1.5 | −3.70 | n.s. | −5.02 |
| Middle frontal gyrus | R | 383 | 10/46 | 36 | 45 | 3 | n.s. | 5.44 | −7.29 |
| IFG | L | 153 | 47 | −36 | 40.5 | 7.5 | n.s. | 4.02 | −6.53 |
| Middle temporal gyrus | L | 101 | 19 | −40.5 | −66 | 15 | n.s. | 3.25 | −4.91 |
| Middle occipital gyrus | R | 973 | 7 | 31.5 | −66 | 30 | −2.78 | 4.34 | −7.12 |
| Dorsolateral prefrontal cortex | R | 186 | 9 | 30 | 31.5 | 31.5 | −5.02 | n.s. | −7.29 |
| Dorsolateral prefrontal cortex | L | 151 | 9 | −37.5 | 25.5 | 33 | n.s. | 2.72 | −4.81 |
| Middle occipital gyrus | L | 212 | 7 | −24 | −64.5 | 37.5 | n.s. | 6.25 | −4.87 |
| Posterior cingulate cortex/Precuneus * | L | 221 | 7/31 | −6 | −40.5 | 34.5 | n.s. | 2.74 | −4.65 |
| Precuneus | L | 112 | 7 | −9 | −54 | 66 | −2.04 | 2.34 | −4.38 |
Notes: Hemi: hemisphere; L: left; R: right; BA: Brodmann Area;
aDenotes Z values; n.s.: non-significant. ; *Indicates social brain network regions.
Correlations with Autism Symptoms
No significant correlations were found between altered gray matter volume and ADOS subscores in autistic individuals.
Discussion
This study examined age-related differences in gray matter volume and CaSCN of the FFA in a cross-sectional, two-cohort sample of boys with and without autism spanning the age range of 2–7 years. Compared with TD boys, autistic boys showed a significantly altered gray matter volume changes with increasing age in the FFA and cerebellum/vermis. Further CaSCN analysis revealed changes to the temporal influences of the FFA on the neuroanatomy of the autistic brain through development; this phenomenon was especially observed in areas associated with the social brain network. Apart from the age-dependent differences in anatomical structures, the gray matter volume across the brain in the autism group increased prominently compared with the TD group. Overall, these observations suggest atypical brain growth and progressive structural network alterations associated with the FFA in autism during early childhood.
Increased Gray Matter Volume in Autistic Children
Our findings of enlarged gray matter volume converge with previous reports of early brain overgrowth in autistic individuals relative to TD children (Courchesne et al. 2007; Schumann et al. 2010; Ecker et al. 2015). Although not widely replicated, gray matter alterations in autistic children are mostly restricted to the frontal and temporal cortex (Courchesne et al. 2007; Liu et al. 2017). Similar to the regional specificity of structural brain changes in the current study (Fig. 1), brain morphometry findings reveal a consistent voxel-wise gray matter increase in the inferior temporal gyrus, inferior occipital gyrus, and prefrontal cortex in pediatric autism (Liu et al. 2017). Nevertheless, the etiology that drives these anatomical alterations of autistic brain remains elusive. An excessive number of neurons have been speculated as a prominent possible cause of the brain overgrowth in autistic children (Courchesne et al. 2007). Such an increase in neuron numbers could potentially disrupt the large-scale, long-distance cortical interactions mediating social cognitive functions in autism (Courchesne et al. 2007; Chen et al. 2018).
Our study also pointed out the altered anatomical morphometry in the cerebellum, after controlling for the effects of age, diagnosis-by-age interaction, site, and TIV (Fig. 1). Conflicting evidence for cerebellar alterations has been reported as enlarged, reduced or of similar volume in previous neuroimaging and postmortem studies in autism (Scott et al. 2009). Neuropathological studies have documented reduced numbers of cerebellar Purkinje cells in autism, but not all cases of autism display such atrophy patterns in the cerebellum (Whitney et al. 2008). The current lack of consensus on cerebellar alterations may be partly due to the developmental changes and heterogeneity of the autism phenotype. Age-related alterations in the cerebellum/vermis identified in the diagnosis-by-age interaction effects on anatomy further confirmed the on-going atypical processes in autistic brain (Fig. 2). Autistic individuals may exhibit enlarged cerebellum/vermis size in the first 2 years of life followed by a decline of growth. This speculation is in alignment with the notion that neurons in the deep cerebellar nuclei in autistic children are atypically large and greater in amount in comparison with those in the older autistic brains (Kemper and Bauman 2002). Decomposing the heterogeneity of autism within a developmental framework is imperative in future autism research (Lombardo et al. 2019).
Age-Associated Gray Matter Alterations in Autism
The FFA is classically considered to be selectively involved in face processing compared with perceiving nonface object as a critical part of social brain circuitry (Kanwisher et al. 1997). Neuroimaging studies of the FFA in autism are an area of interest in the light of prominent face perception differences among autistic individuals. One of the most consistent findings in neuroimaging studies of autism is the hypoactivation of the FFA during face processing (Dalton et al. 2005). These findings emphasize the critical role of FFA alterations in impaired face perception in autism. Our findings of atypical developmental changes in the FFA are consistent with previous observations of structural alterations in this area in the autistic brain (Waiter et al. 2004; Dziobek et al. 2010). Although not a longitudinal design, developmental disparity between autism and TD children in terms of gray matter volume was observed in the FFA (Fig. 2B). We predict that enlarged gray matter volume of the FFA will be observed in autism in late childhood, which would be worthy of replications in future longitudinal studies. This prediction coincides with the findings of increased gray matter volume of the FFA in autistic adolescents (Waiter et al. 2004). Age-related differences between autism and TD children in the FFA appear to be driven by the atypical development of the cortical thickness (Raznahan et al. 2010). Compared with controls, autistic individuals have thinned cortices in the fusiform gyrus during childhood and the opposite in later life, with similar trends observed in measures of cortical volume (Raznahan et al. 2010). Overall, our findings provide the anatomical evidence of altered growth processes in the FFA in early childhood autistic brain.
Progressive Structural Alterations Associated with the FFA in Autism
The features of social cognition in autism are commonly attributed to the so-called social brain network, which is specialized for social processing (Gotts et al. 2012). These structures include the FFA, amygdala, insula, IFG, mPFC, ACC, pSTS, PCC/precuneus, intraparietal sulcus, temporal pole, and temporoparietal junction. Efforts to carry out the structural covariance analysis of autism have suggested decreased structural covariance between the mPFC and PCC/precuneus (Valk et al. 2015) and between the fusiform gyrus and the amygdala (Dziobek et al. 2010). Although the consistency of these neuroanatomical alterations of the social brain regions in autism is encouraging, their chronological order during brain development remains unknown. Our findings provide additional support for the social development model of amygdala and FFA interactions of autism with the evidence that the FFA displayed altered temporal influences on other social brain regions in autism during early childhood. In typical development, the FFA is a critical hub for social brain network across childhood. Disrupted development of the FFA in autism appears to consequently affect the shaping of the brain regions mediating social processing that are impaired in this disorder (Patriquin et al. 2016). Premature development in these regions arising from early brain overgrowth possibly gives rise to such alterations in progressive covariance effects from FFA in autism. Future research examining CaSCN in autistic infants and toddlers can complement our understanding of the development of the social brain network. We expected to see an altered influence from the amygdala to the FFA in autism during early development, given the neuropathological change in the amygdala in infantile autism (Kemper and Bauman 2002).
Failures in the development of the FFA and social brain circuitry provide the neuroanatomical evidence for the second stage of amygdala-FFA model of autism (Schultz 2005). Age-related gray matter volume changes and CaSCN analyses of the FFA in disorders of social functioning, such as autism, schizophrenia, and major depressive disorder, may help understand the social brain network alterations underlying social cognition difficulties in these disorders (Dalton et al. 2005; Goulden et al. 2012; Maher et al. 2016). In addition, therapies for alterations in the FFA are expected to improve the development of the social brain network in autism (Schultz 2005). Early intervention targeting the FFA during early childhood may be more effective than in later life for autism, considering that this period is critical for the formation of brain functional architecture.
Limitations
This study has several limitations. First, only male children were included in this study due to the limited number of autistic females. However, biological sex is increasingly linked with the heterogeneity of autism (Lai et al. 2013). Sex/gender differences in the FFA-associated developmental changes and CaSCN should be clarified for autism in future studies with a large sample size. Second, age-related differences in the neuroanatomy were investigated using a cross-sectional design. Longitudinal studies spanning from early childhood to adulthood are needed to validate our findings. Third, given that autistic children enrolled in this study showed notable social communication impairments, we used PPVT scores to assess the verbal intelligence level of autistic and TD children and replicated our findings after excluding the potential effects of verbal intelligence (Supplementary Fig. 4). However, intelligence test tools designed for autism are needed in future research to better control the neuroanatomical heterogeneity of autism introduced by intelligence (Salmond et al. 2007). Fourth, since this study was limited to Chinese populations, it remains unclear whether these findings can be generalized to autism in other ethnic populations. Future studies should include autistic children from other ethnic groups to test the generalization of these findings to different ethnic populations.
Conclusion
This voxel-based morphometric study identified age-related neuroanatomical changes in the FFA and cerebellum in the autistic brain during early childhood. These findings support the idea of atypical brain development in autism. A disrupted development of the FFA could potentially alter the expected development of the social brain network, which mediates social perception and cognition. These findings provide neuroanatomical evidence for the developmental model of the amygdala–FFA system in autism and highlight the critical role of the FFA in the development of the social brain network of the autistic brain during early childhood.
Notes
Conflict of Interest: None declared.
Funding
Key Project of Research and Development of Ministry of Science and Technology (No. 2018AAA0100705); Natural Science Foundation of China (Nos 61533006, 61673089, 81871432, 81874270, U1808204); Fundamental Research Funds for the Central Universities (Nos 2672018ZYGX2018J079, ZYGX2019Z017); Sichuan Science and Technology Program (No. 2019YJ0180); National Institute for Health Research Cambridge Biomedical Research Centre to J.S.; Science and Technology Plan Project of Guizhou Province of China (No. [2018]5781 to H.C.); 2018 Talent Research Program of Guizhou University (No. 702570183301 to H.C.).
Supplementary Material
Trial registration number: This study was registered at ClinicalTrials.gov (Identifier: NCT02807766).
Contributor Information
Xiaonan Guo, Sichuan Provincial Center for Mental Health, The Center of Psychosomatic Medicine of Sichuan Provincial People's Hospital, University of Electronic Science and Technology of China, Chengdu 611731, China.
Xujun Duan, Sichuan Provincial Center for Mental Health, The Center of Psychosomatic Medicine of Sichuan Provincial People's Hospital, University of Electronic Science and Technology of China, Chengdu 611731, China; MOE Key Lab for Neuroinformation; High-Field Magnetic Resonance Brain Imaging Key Laboratory of Sichuan Province, School of Life Science and Technology, University of Electronic Science and Technology of China, Chengdu 610054, China.
John Suckling, Brain Mapping Unit, Department of Psychiatry, University of Cambridge, Cambridge CB2 0SZ, UK; Cambridgeshire and Peterborough NHS Foundation Trust, Cambridge CB21 5EF, UK.
Jia Wang, Department of Children's and Adolescent Health, Public Health College of Harbin Medical University, Harbin 150086, China.
Xiaodong Kang, Affiliated Sichuan Provincial Rehabilitation Hospital of Chengdu University of TCM, Sichuan Bayi Rehabilitation Center, Chengdu 611135, China.
Heng Chen, MOE Key Lab for Neuroinformation; High-Field Magnetic Resonance Brain Imaging Key Laboratory of Sichuan Province, School of Life Science and Technology, University of Electronic Science and Technology of China, Chengdu 610054, China; School of Medicine, Guizhou University, Guiyang 550025, China.
Bharat B Biswal, MOE Key Lab for Neuroinformation; High-Field Magnetic Resonance Brain Imaging Key Laboratory of Sichuan Province, School of Life Science and Technology, University of Electronic Science and Technology of China, Chengdu 610054, China; Department of Biomedical Engineering, New Jersey Institute of Technology, Newark, NJ 07102, USA.
Jing Cao, Affiliated Sichuan Provincial Rehabilitation Hospital of Chengdu University of TCM, Sichuan Bayi Rehabilitation Center, Chengdu 611135, China.
Changchun He, MOE Key Lab for Neuroinformation; High-Field Magnetic Resonance Brain Imaging Key Laboratory of Sichuan Province, School of Life Science and Technology, University of Electronic Science and Technology of China, Chengdu 610054, China.
Jinming Xiao, MOE Key Lab for Neuroinformation; High-Field Magnetic Resonance Brain Imaging Key Laboratory of Sichuan Province, School of Life Science and Technology, University of Electronic Science and Technology of China, Chengdu 610054, China.
Xinyue Huang, MOE Key Lab for Neuroinformation; High-Field Magnetic Resonance Brain Imaging Key Laboratory of Sichuan Province, School of Life Science and Technology, University of Electronic Science and Technology of China, Chengdu 610054, China.
Runshi Wang, Sichuan Provincial Center for Mental Health, The Center of Psychosomatic Medicine of Sichuan Provincial People's Hospital, University of Electronic Science and Technology of China, Chengdu 611731, China.
Shaoqiang Han, MOE Key Lab for Neuroinformation; High-Field Magnetic Resonance Brain Imaging Key Laboratory of Sichuan Province, School of Life Science and Technology, University of Electronic Science and Technology of China, Chengdu 610054, China.
Yun-Shuang Fan, MOE Key Lab for Neuroinformation; High-Field Magnetic Resonance Brain Imaging Key Laboratory of Sichuan Province, School of Life Science and Technology, University of Electronic Science and Technology of China, Chengdu 610054, China.
Jing Guo, MOE Key Lab for Neuroinformation; High-Field Magnetic Resonance Brain Imaging Key Laboratory of Sichuan Province, School of Life Science and Technology, University of Electronic Science and Technology of China, Chengdu 610054, China.
Jingping Zhao, Department of Psychiatry, The Second Xiangya Hospital, Central South University, Changsha 410011, China.
Lijie Wu, Department of Children's and Adolescent Health, Public Health College of Harbin Medical University, Harbin 150086, China.
Huafu Chen, Sichuan Provincial Center for Mental Health, The Center of Psychosomatic Medicine of Sichuan Provincial People's Hospital, University of Electronic Science and Technology of China, Chengdu 611731, China; MOE Key Lab for Neuroinformation; High-Field Magnetic Resonance Brain Imaging Key Laboratory of Sichuan Province, School of Life Science and Technology, University of Electronic Science and Technology of China, Chengdu 610054, China.
References
- Alexander-Bloch A, Giedd JN, Bullmore ET. 2013. Imaging structural co-variance between human brain regions. Nat Rev Neurosci. 14:322–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association 2013. Diagnostic and statistical manual of mental disorders (DSM-5®). Arlington (VA): American Psychiatric Publication. [Google Scholar]
- Ashburner J 2007. A fast diffeomorphic image registration algorithm. NeuroImage. 38:95–113. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. 2004. Autism and abnormal development of brain connectivity. J Neurosci. 24:9228–9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, Munafo MR. 2013. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 14:365–376. [DOI] [PubMed] [Google Scholar]
- Chen H, Wang J, Uddin LQ, Wang XM, Guo XN, Lu FM, Duan XJ, Wu LJ, Chen HF. 2018. Aberrant functional connectivity of neural circuits associated with social and sensorimotor deficits in young children with autism Spectrum disorder. Autism Res. 11:1643–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Cui Q, Fan Y-S, Guo X, Tang Q, Sheng W, Lei T, Li D, Lu F, He Z et al. 2020. Progressive brain structural alterations assessed via causal analysis in patients with generalized anxiety disorder. Neuropsychopharmacology. 45:1689–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Pierce K, Schumann CM, Redcay E, Buckwalter JA, Kennedy DP, Morgan J. 2007. Mapping early brain development in autism. Neuron. 56:399–413. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Campbell K, Solso S. 2011. Brain growth across the life span in autism: age-specific changes in anatomical pathology. Brain Res. 1380:138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, Alexander AL, Davidson RJ. 2005. Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci. 8:519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerden EG, Chakravarty MM, Lerch JP, Taylor MJ. 2020. Sex-based differences in cortical and subcortical development in 436 individuals aged 4-54 years. Cereb Cortex. 30:2854–2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziobek I, Bahnemann M, Convit A, Heekeren HR. 2010. The role of the fusiform-amygdala system in the pathophysiology of autism. Arch Gen Psychiatry. 67:397–405. [DOI] [PubMed] [Google Scholar]
- Ecker C, Bookheimer SY, Murphy DGM. 2015. Neuroimaging in autism spectrum disorder: brain structure and function across the lifespan. Lancet Neurol. 14:1121–1134. [DOI] [PubMed] [Google Scholar]
- Fan Y-S, Li Z, Duan X, Xiao J, Guo X, Han S, Guo J, Yang S, Li J, Cui Q et al. 2019. Impaired interactions among white-matter functional networks in antipsychotic-naive first-episode schizophrenia. Hum Brain Mapp. 41:230–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore JH, Knickmeyer RC, Gao W. 2018. Imaging structural and functional brain development in early childhood. Nat Rev Neurosci. 19:123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotts SJ, Simmons WK, Milbury LA, Wallace GL, Cox RW, Martin A. 2012. Fractionation of social brain circuits in autism spectrum disorders. Brain. 135:2711–2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulden N, McKie S, Thomas EJ, Downey D, Juhasz G, Williams SR, Rowe JB, Deakin JF, Anderson IM, Elliott R. 2012. Reversed frontotemporal connectivity during emotional face processing in remitted depression. Biol Psychiatry. 72:604–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Duan X, Suckling J, Chen H, Liao W, Cui Q, Chen H. 2019. Partially impaired functional connectivity states between right anterior insula and default mode network in autism spectrum disorder. Hum Brain Mapp. 40:1264–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Duan X, Chen H, He C, Xiao J, Han S, Fan Y-S, Guo J, Chen H. 2020. Altered inter- and intrahemispheric functional connectivity dynamics in autistic children. Hum Brain Mapp. 41:419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Cui Q, Wang X, Chen Y, Li D, Li L, Guo X, Fan Y-S, Guo J, Sheng W et al. 2020. The anhedonia is differently modulated by structural covariance network of NAc in bipolar disorder and major depressive disorder. Prog Neuro-Psychopharmacol Biol Psychiatry. 99:109865. [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Gu H, Munsell BC, Kim SH, Styner M, Wolff JJ, Elison JT, Swanson MR, Zhu H, Botteron KN. 2017. Early brain development in infants at high risk for autism spectrum disorder. Nature. 542:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Luo C, Li X, Duan M, He H, Chen X, Yang H, Gong J, Chang X, Woelfer M et al. 2018. Progressive reduction in gray matter in patients with schizophrenia assessed with MR imaging by using causal network analysis. Radiology. 287:633–642. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. 1997. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 17:4302–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper TL, Bauman ML. 2002. Neuropathology of infantile autism. Mol Psychiatry. 7(Suppl 2):S12–S13. [DOI] [PubMed] [Google Scholar]
- Lai MC, Lombardo MV, Suckling J, Ruigrok AN, Chakrabarti B, Ecker C, Deoni SC, Craig MC, Murphy DG, Bullmore ET. 2013. Biological sex affects the neurobiology of autism. Brain. 136:2799–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yao L, Zhang W, Xiao Y, Liu L, Gao X, Shah C, Li S, Tao B, Gong Q et al. 2017. Gray matter abnormalities in pediatric autism spectrum disorder: a meta-analysis with signed differential mapping. Eur Child Adolesc Psychiatry. 26:933–945. [DOI] [PubMed] [Google Scholar]
- Liu F, Tian H, Li J, Li S, Zhuo C. 2019. Altered voxel-wise gray matter structural brain networks in schizophrenia: association with brain genetic expression pattern. Brain Imaging Behav. 13:493–502. [DOI] [PubMed] [Google Scholar]
- Lombardo MV, Lai MC, Baron-Cohen S. 2019. Big data approaches to decomposing heterogeneity across the autism spectrum. Mol Psychiatry. 24:1435–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Z, Duan X, Chen H, Zhang Y, Chen H. 2016. Structural covariance model reveals dynamic reconfiguration of triple networks in autism spectrum disorder. Applied Informatics. 3:1–7. [Google Scholar]
- Loomes R, Hull L, Mandy WPL. 2017. What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry. 56:466–474. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. 1994. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 24:659–685. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH Jr, Leventhal BL, DiLavore PC, Pickles A, Rutter M. 2000. The autism diagnostic observation schedule—generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 30:205–223. [PubMed] [Google Scholar]
- Maher S, Ekstrom T, Holt D, Ongur D, Chen Y. 2016. The core brain region for face processing in schizophrenia lacks face selectivity. Schizophr Bull. 42:666–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshew NJ, Williams DL. 2007. The new neurobiology of autism: cortex, connectivity, and neuronal organization. Arch Neurol. 64:945–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CA 3rd, Zeanah CH, Fox NA, Marshall PJ, Smyke AT, Guthrie D. 2007. Cognitive recovery in socially deprived young children: the Bucharest early intervention project. Science. 318:1937–1940. [DOI] [PubMed] [Google Scholar]
- Nomi JS, Uddin LQ. 2015. Developmental changes in large-scale network connectivity in autism. Neuroimage Clinical. 7:732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriquin MA, DeRamus T, Libero LE, Laird A, Kana RK. 2016. Neuroanatomical and neurofunctional markers of social cognition in autism spectrum disorder. Hum Brain Mapp. 37:3957–3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Toro R, Daly E, Robertson D, Murphy C, Deeley Q, Bolton PF, Paus T, Murphy DG. 2010. Cortical anatomy in autism spectrum disorder: an in vivo MRI study on the effect of age. Cereb Cortex. 20:1332–1340. [DOI] [PubMed] [Google Scholar]
- Salmond CH, Vargha-Khadem F, Gadian DG, de Haan M, Baldeweg T. 2007. Heterogeneity in the patterns of neural abnormality in autistic spectrum disorders: evidence from ERP and MRI. Cortex. 43:686–699. [DOI] [PubMed] [Google Scholar]
- Schultz RT 2005. Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. Int J Dev Neurosci. 23:125–141. [DOI] [PubMed] [Google Scholar]
- Schumann CM, Bloss CS, Barnes CC, Wideman GM, Carper RA, Akshoomoff N, Pierce K, Hagler D, Schork N, Lord C. 2010. Longitudinal magnetic resonance imaging study of cortical development through early childhood in autism. J Neurosci. 30:4419–4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JA, Schumann CM, Goodlin-Jones BL, Amaral DG. 2009. A comprehensive volumetric analysis of the cerebellum in children and adolescents with autism spectrum disorder. Autism Res. 2:246–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharda M, Khundrakpam BS, Evans AC, Singh NC. 2016. Disruption of structural covariance networks for language in autism is modulated by verbal ability. Brain Struct Funct. 221:1017–1032. [DOI] [PubMed] [Google Scholar]
- Valk SL, Martino AD, Milham MP, Bernhardt BC. 2015. Multicenter mapping of structural network alterations in autism. Hum Brain Mapp. 36:2364–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waiter GD, Williams JH, Murray AD, Gilchrist A, Perrett DI, Whiten A. 2004. A voxel-based investigation of brain structure in male adolescents with autistic spectrum disorder. NeuroImage. 22:619–625. [DOI] [PubMed] [Google Scholar]
- Whitney ER, Kemper TL, Bauman ML, Rosene DL, Blatt GJ. 2008. Cerebellar Purkinje cells are reduced in a subpopulation of autistic brains: a stereological experiment using calbindin-D28k. Cerebellum. 7:406–416. [DOI] [PubMed] [Google Scholar]
- Yan C-G, Wang X-D, Zuo X-N, Zang Y-F. 2016. DPABI: data processing & analysis for (resting-state) brain imaging. Neuroinformatics. 14:339–351. [DOI] [PubMed] [Google Scholar]
- Zang ZX, Yan CG, Dong ZY, Huang J, Zang YF. 2012. Granger causality analysis implementation on MATLAB: a graphic user interface toolkit for fMRI data processing. J Neurosci Methods. 203:418–426. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Liao W, Xu Q, Wei W, Zhou HJ, Sun K, Yang F, Mantini D, Ji X, Lu G. 2017. Hippocampus-associated causal network of structural covariance measuring structural damage progression in temporal lobe epilepsy. Hum Brain Mapp. 38:753–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski BA, Gennatas ED, Zhou JA, Seeley WW. 2010. Network-level structural covariance in the developing brain. Proc Natl Acad Sci U S A. 107:18191–18196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski BA, Anderson JS, Froehlich AL, Prigge MB, Nielsen JA, Cooperrider JR, Cariello AN, Fletcher PT, Alexander AL, Lange N et al. 2012. scMRI reveals large-scale brain network abnormalities in autism. PLoS One. 7:e49172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


