Abstract
Age-related differences in dorsolateral prefrontal cortex (DLPFC) structure and function have each been linked to working memory. However, few studies have integrated multimodal imaging to simultaneously investigate relationships among structure, function, and cognition. We aimed to clarify how specifically DLPFC structure and function contribute to working memory in healthy older adults. In total, 138 participants aged 65–88 underwent 3 T neuroimaging and were divided into higher and lower groups based on a median split of in-scanner n-back task performance. Three a priori spherical DLPFC regions of interest (ROIs) were used to quantify blood-oxygen-level-dependent (BOLD) signal and FreeSurfer-derived surface area, cortical thickness, and white matter volume. Binary logistic regressions adjusting for age, sex, education, and scanner type revealed that greater left and right DLPFC BOLD signal predicted the probability of higher performing group membership (P values<.05). Binary logistic regressions also adjusting for total intracranial volume revealed left DLPFC surface area that significantly predicted the probability of being in the higher performing group (P = 0.017). The left DLPFC BOLD signal and surface area were not significantly associated and did not significantly interact to predict group membership (P values>.05). Importantly, this suggests BOLD signal and surface area may independently contribute to working memory performance in healthy older adults.
Keywords: cognitive aging, dorsolateral prefrontal cortex, multimodal neuroimaging, structural and functional magnetic resonance imaging, working memory
Introduction
The number of older adults in the general population is rapidly increasing, representing the fastest growing cohort in the United States of America. Specifically, the number of adults aged 65 and older is expected to almost double by the year 2050, generating an increased interest in promoting healthy aging (Roberts et al. 2018). Cognition has been shown to decline in advancing age, even in the absence of pathology. Working memory is a cognitive domain particularly vulnerable to the cognitive aging process (Baddeley 1992; Bizon et al. 2012; Glisky 2007; Goldman-Rakic 1995; Heinzel et al., 2014; Park et al. 2002; Schulze et al. 2011; Wang et al. 2011). Working memory is a limited-capacity system that allows for the manipulation and short-term storage of information while performing complex tasks (Baddeley 1992; Goldman-Rakic 1995). Age-related deficits in working memory result in poorer attention, planning, and reasoning abilities important for performing activities of daily living (i.e., taking medication, handling personal finances, and organization and planning of daily routines and appointments) (Stern et al. 1990; Yam and Marsiske 2013; Nissim et al. 2017).
The dorsolateral prefrontal cortex (DLPFC) is a functional neuroanatomical region central to working memory processes and has been shown to undergo structural declines that may contribute to working memory deficits in older age (Barbey et al. 2013; Dotson et al. 2016; Funahashi 2017; Goldman-Rakic 2011; Nissim et al. 2017; Salat et al. 1999). In advancing age, the DLPFC demonstrates marked reductions in gray matter surface area and cortical thickness (Lemaitre et al. 2012; Salat 2004). Cortical surface area is a measure of structural integrity that is susceptible to age-related atrophic changes (Dotson et al. 2016; Fischl et al. 1999a; Lemaitre et al. 2012; Nissim et al. 2017; Salat 2004), while cortical thickness is an indirect measure of neuronal density and may reflect neuronal loss due to neurodegenerative disease (Fischl and Dale 2000; Shefer 1973). Cortical surface area may be more sensitive in capturing brain changes due to non-pathological, healthy aging compared to cortical thickness. Nissim et al. (2017) demonstrated this pattern of frontal structural differences in association with working memory performance in older adults. Low performers had lower frontal cortical surface area compared to high performers, but there were no significant group differences in cortical thickness (Nissim et al. 2017). This further suggests surface area may be a more sensitive neural correlate to detect working memory deficits in healthy aging.
Age-related reductions in white matter volume may also occur and may decline at a faster rate within the frontal lobes, including regions like the DLPFC (Brickman et al. 2006; Good et al. 2001; Salat et al. 1999, 2001). Guttmann et al. (1998) demonstrated that age-related cortical white matter volume reductions can occur even in the absence of decreases in total gray matter. However, decreased white matter volume could lead to disrupted neuronal communication and subsequent cognitive difficulties. While emerging evidence demonstrates age-related white matter reductions in frontal regions are associated with working memory deficits (Golestani et al. 2014; Liu et al. 2017; Salat et al. 1999, 2001), few studies have investigated how differences in white matter underlying the DLPFC contribute to working memory difficulties in advancing age. Therefore, it is crucial to better characterize how white matter volumetric differences underlying the DLPFC influence working memory in the context of healthy, non-pathological aging.
Functional magnetic resonance imaging (fMRI) studies have demonstrated that the DLPFC plays a critical role in working memory. Numerous fMRI studies have shown alterations in blood-oxygen-level-dependent (BOLD) activation within the DLPFC during working memory task performance (Owen et al. 2005; Spreng et al. 2010; Barbey et al. 2013; Funahashi 2017; Finn et al. 2019). Cabeza et al. (2002) found that young adults with higher working memory task performance demonstrated a right lateralized pattern of BOLD activation in frontal brain regions, including the DLPFC. However, higher performing older adults exhibited a bilateral pattern of BOLD activation in the same frontal regions (Cabeza et al. 2002), suggesting that older adults potentially employ a compensatory mechanism. In this case, inefficient neural activation in response to increasing cognitive demands may necessitate recruitment of additional brain regions (Cabeza 2002; Cabeza et al. 2002).
The Scaffolding Theory of Aging and Cognition (STAC) further provides a possible explanation for differential BOLD patterns in young and older adults (Park and Reuter-Lorenz 2009; Reuter-Lorenz and Park 2014). STAC proposes older adults engage in “compensatory scaffolding,” a compensation mechanism for age-related structural and functional declines that results in bilateral activation patterns and allows for maintenance of higher cognitive performance. Older adults, relative to younger adults, demonstrate activation patterns including the declining region and associated compensatory regions recruited to meet the task demands (Park and Reuter-Lorenz 2009; Reuter-Lorenz and Park 2014).
However, few studies have investigated the direct effects of structural deficits on functional activation and associated cognitive performance. Structural and functional measures have typically been treated separately when investigating their associations with age-related cognitive decline. While many studies have examined the biological underpinnings of cognitive deficits in non-pathological older adults, there remains a dearth in knowledge integrating multimodal approaches to simultaneously investigate relationships among brain structure, function and cognition. There is a need to leverage the methodological strengths of these separate modalities by using multimodal neuroimaging to concurrently examine the contributions of brain structure and function to cognitive aging, and how differences in structure may affect the influence of brain function on cognition.
The objective of the present study was to investigate how individual differences in brain structure and function contribute to working memory performance in a large sample of older adults without clinically evident neurodegenerative disease. Our first overall aim was to determine the independent contributions of DLPFC function and structure to working memory performance. Specifically, we aimed to examine the relationship between working memory and DLPFC structure (i.e., cortical thickness, cortical surface area, and white matter volume). Given frontal structural patterns demonstrated by prior studies (Nisism et al., 2017; Salat et al. 1999, 2001), we hypothesized that greater DLPFC surface area and white matter volume, but not cortical thickness, would significantly predict the probability of higher working memory performance. We also aimed to examine the relationship between working memory and DLPFC function as measured by BOLD signal during a working memory task. Considering the compensatory strategies proposed by the STAC model, we hypothesized that greater BOLD signal in the bilateral DLPFC would significantly predict higher working memory performance. The second overall aim was to determine whether DLPFC structure moderates the relationship between DLPFC function (i.e., BOLD signal) and working memory performance. We hypothesized structure would moderate this relationship, such that those with greater DLPFC BOLD signal and greater DLPFC surface area or white matter volume would have significantly greater probability of higher working memory performance. Importantly, such results would provide novel insights into potential markers of successful, healthy aging, elucidating whether DLPFC structure and function interact or independently contribute to working memory.
Materials and Methods
Participants
The current study utilized baseline data from participants recruited for the Stimulated Brain (K01AG050707, n = 25) and the Augmenting Cognitive Training in Older Adults (ACT, R01AG054077, n = 113) studies with identical inclusion criteria (Woods et al. 2018). In total, 167 healthy older adults between ages 65 and 88 years old were recruited from the University of Florida (n = 117) and the University of Arizona (n = 50). All participants were right-handed, had no prior history of neurological disease (i.e., mild cognitive impairment, dementia, history of stroke, severe closed-head injury, neurodegenerative disease), and had no magnetic resonance imaging (MRI) contraindications. The Institutional Review Boards at the University of Florida and at the University of Arizona approved all study protocols, and all participants signed informed consent forms prior to completing any study procedures.
At an in-person screening visit, participants completed the National Alzheimer’s Coordinating Center (NACC) Unified Data Set (UDS), a comprehensive neuropsychological battery assessing various cognitive domains (Weintraub et al. 2009). The UDS serves as a screening measure for dementia and mild cognitive impairment (MCI), as defined by age, sex, and education normed scores > −1.5 SD below the mean in any one of the following five cognitive domains: general cognition, executive function/working memory, episodic memory, visuospatial, and language (Woods et al. 2018). Following the in-person screening visit, participants were also excluded if they were color blind, or had notable vision impairment (uncorrected vision worse than 20/80) or hearing loss (inability to hear a target at 20 dB or louder within background noise). Participants were also excluded if they had any abnormal findings on their baseline MRI brain scan, including tumors or cysts.
Of the 167 participants originally included in the present study sample, 8 participants did not complete the N-back task and 13 participants did not have usable functional or structural imaging data due to poor data quality (e.g., excess movement and resulting incomplete surface parcellation). An additional 8 participants were excluded from subsequent statistical analyses following examination of outliers across behavioral and neuroimaging variables (i.e., values greater than 3 standard deviations from the mean). In total, 138 participants were included in subsequent analyses (age: M = 71.41, SD = 5.19; 79 females; education: M = 16.16; SD = 2.36).
Working Memory Performance: N-Back Task
The N-Back task is an established working memory paradigm that requires participants to continuously monitor a series of stimuli. Generally, the participant is asked to respond when the presented stimulus is the same as the one presented n-trials previously. The N-back task used in the present study was created with E-Prime version 2.0 (Psychology Software Tools, Inc., Pittsburgh, PA, USA) and included both 2-back and 0-back versions of the task. Participants completed practice sessions of the N-back task prior to the MR scan to ensure a complete understanding of the instructions and ability to perform the task. Images of a series of letters were presented onto a screen outside the scanner and viewed through a mirror mounted on the head coil. Four blocks of the 0-back task and four blocks of the 2-back task were presented, with fifteen letters in each block. For both the 0-back and 2-back versions of the task, each letter stimulus was identical in font, size, and color and was presented for 1000 ms, followed by a fixation cross hair presented for 3000 ms. The ordering of 0-back and 2-back blocks were randomized within the task.
The participant responded via an MRI-compatible button box. During the 0-back version of the task, they were asked to press a button with their index finger when the letter presented was an “X” and to press a different button with their middle finger when the presented stimulus was any letter other than “X.” During the 2-back task, the participant was asked to press a button with their index finger when the letter was the same as the one presented two trials previously (i.e., targets) and to press a different button with their middle finger for letters that did not match the one presented two trials previously (i.e., distractors).
MRI Acquisition
All participants underwent magnetic resonance imaging (MRI) in a 3-Tesla Siemens Magnetom Prisma scanner with a 64-channel head coil at the University of Florida and in a 3-Tesla Siemens Magnetom Skyra scanner with a 32-channel head coil at the University of Arizona. T1-weighted MPRAGE structural scans were collected prior to BOLD fMRI for all participants using the following parameters at both sites: repetition time (TR) = 1800 ms; echo time (TE) = 2.26 ms; flip angle = 8o; field of view = 256 × 256 × 176 mm; voxel = 1 × 1 × 1 mm. A task-based BOLD fMRI sequence was collected using the following parameters: repetition time (TR) = 3000 ms; echo time (TE) = 30.0 ms; flip angle = 70o; field of view = 240 × 240 × 132 mm; voxel = 3 × 3 × 3 mm.
Regions of Interest (ROI) Selection
Owen et al. (2005) identified 14 regions of interest (ROIs) in a quantitative meta-analysis of 24 studies utilizing N-back tasks as measures of working memory. Three ROIs were selected a priori from this meta-analysis (Owen et al. 2005) to represent dorsolateral prefrontal cortical regions within the working memory network in our analyses (Table 1). Across the literature, there has been considerable variability in defining the precise location of the DLPFC. Anatomically, the DLPFC theoretically corresponds to Brodmann areas (BA) 9 and 46 (Cieslik et al. 2013). Given the DLPFC is a functional neuroanatomical region, we defined the spherical ROIs using functionally derived coordinates reported by Owen et al. (2005). Spherical ROIs were generated using the WFU PickAtlas GUI in SPM12 (Maldjian et al. 2003, 2004). The spherical ROIs were centered at corresponding Montreal Neurological Institute (MNI) coordinates, and ROI radius was calculated using volume (mm3), as reported by Owen et al. (2005). Upon visual inspection, each of the three spherical ROIs appears to fall within BA 9 and 46. Additionally, each of the three spherical ROIs was encompassed within the whole brain 2-back >0-back BOLD activation pattern across the sample (n = 138) (Fig. 1). None of the three generated spherical ROIs overlapped and all three were utilized in both functional and structural MR data extraction.
Table 1.
DLPFC regions of interest (ROIs), Montreal Neurological Institute (MNI) coordinates, volume and radius for spherical ROIs
| ROI | x | y | z | Volume (mm3) | Radius (mm) |
|---|---|---|---|---|---|
| Left DLPFC | −37.75 | 50.19 | 13.6 | 992 | 6.2 |
| −46.26 | 22.71 | 18.6 | 12 024 | 14.2 | |
| Right DLPFC | 44.53 | 38.76 | 24.43 | 8120 | 12.5 |
Figure 1.
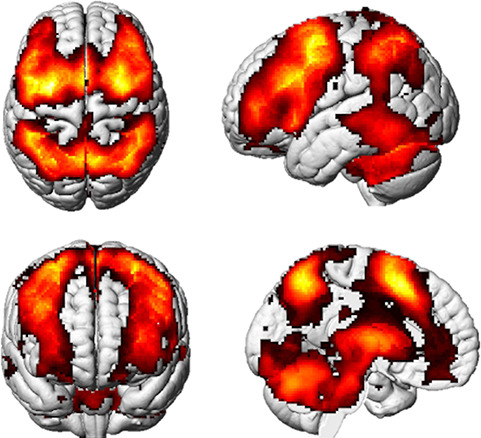
2-back>0-back subtraction contrast depicting whole-brain BOLD activation patterns in regions within the working memory network, including the DLPFC (n = 138, family wise error; FWE < 0.05).
Functional MRI Processing and Regional BOLD Response Extraction
Pre-processing of functional images was performed using CONN Toolbox 17a and SPM12 running in MATLAB 2015b (Penny et al. 2007; Whitfield-Gabrieli and Nieto-Castanon 2012). Pre-processing steps included slice-timing correction, realignment, normalization, and smoothing using a Gaussian smoothing kernel of 8 mm. Additionally, T1-weighted structural volumes were segmented into gray matter, white matter, and cerebrospinal fluid. Following segmentation, structural volumes underwent normalization and registration to MNI space. The mean functional image was co-registered to the registered T1-weighted structural image. The registration matrix produced in this step was then used to register the pre-processed functional volumes to MNI space. Motion outliers or artifacts were detected using the artifact detection toolbox (ART) 97th percentile settings, with the mean global-signal deviation threshold set at z = +/− 5 and the subject-motion threshold set at 0.9 mm. Motion information and frame-wise outliers were later included as covariates in first-level analyses. Denoising of the BOLD fMRI signal was also performed to reduce noise due to physiological fluctuations.
Model contrasts were created utilizing the timing files generated in E-Prime for the 2-back and 0-back blocks of the N-back task. The task instructions were included in the model as a contrast of no interest. Subtraction contrasts were modeled for 2-back task periods over 0-back task periods for each participant (2-Back>0-Back; Fig. 1). A visual quality check was conducted to confirm full overlay of the spherical ROIs on the task-based BOLD activation maps. Employing REX software in SPM12, the 2-Back>0-Back subtraction contrast was used to extract individual subjects’ average beta values for each of the three DLPFC ROIs to represent the hemodynamic response during the 2-back task.
Structural MRI: T1 Pre-Processing
Structural T1 images underwent cortical reconstruction and volumetric segmentation using FreeSurfer version 6.0 image analysis suite. Technical details of these procedures are described elsewhere (Dale et al. 1999; Dale & Sereno 1993; Fischl 2004; Fischl et al. 1999a, 1999b, 2001; Fischl & Dale 2000; Fischl et al. 2002, 2004; Han et al. 2006; Jovicich et al. 2006; Reuter et al. 2010, 2012; Ségonne et al. 2007). Briefly, this FreeSurfer processing pipeline involves intensity correction and normalization, automated Tailarach affine transformation, removal of non-brain tissue, segmentation of white and gray matter structures, tessellation of the surface, surface smoothing and deformation, and automated topology correction. Manual edits were performed as necessary to remove non-brain tissue and correct any segmentation errors. Specifically, manual edits included removal of non-cortical matter included in the gray matter surface, removal of gray or non-cortical matter included in the white matter surface, and extension of the white matter surface. Whole brain intracranial volume (ICV) was then extracted and later included as a covariate in analyses to account for potential individual differences in head or brain size.
Cortical Thickness and Surface Area Data Extraction
Cortical thickness and surface area values were obtained for each of the three DLPFC ROIs. FreeSurfer version 6.0 image analysis suite was used to generate surface labels from the spherical volume-defined ROIs (see Fig. 2). The WFU PickAtlas T1 image was linearly registered to the FreeSurfer fsaverage subject template T1 structural volume using the Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB) Linear Registration Tool (FLIRT) in the FMRIB’s software library (FSL) (Smith et al. 2004; Jenkinson et al. 2012). The FreeSurfer fsaverage subject is a template in MNI305 standard space, created by using the spherical average of 40 subjects (Fischl 2012). The volume files for each volumetric (spherical) ROI were linearly registered to the fsaverage subject surface using FLIRT in FSL to create an fsaverage-ROI surface overlay. Each subject’s thickness and area files were linearly registered using FLIRT in FSL to the fsaverage brain to make sure each subject’s files were in the same standard space (MNI305). FreeSurfer was then used to extract cortical thickness and surface area values from each fsaverage-ROI mask for each subject.
Figure 2.
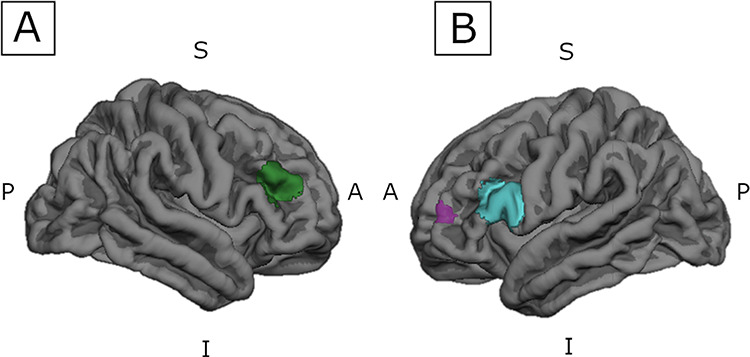
DLPFC surface created in FreeSurfer Analysis Suite v6.0. The spherical DLPFC ROIs were linearly registered to subject surfaces to extract cortical thickness and surface area values. S = Superior, I = Inferior, A = Anterior, P = Posterior. (A) Right DLPFC centered at MNI coordinates (44.53, 38.76, 24.43); (B) Left DLPFC; purple: centered at MNI coordinates (−37.75, 50.19, 13.6); blue: centered at MNI coordinates (−46.26, 22.71, 18.6).
White Matter Volume Data Extraction
White matter volume was obtained from each of the three DLPFC spherical ROIs originally generated in SPM12. The WFU PickAtlas T1 image was linearly registered to each subject’s T1 structural volumes using FLIRT in FSL, generating a registration matrix that was then applied to each of the three spherical volume-defined ROIs. FreeSurfer was then used to extract subject white matter volume for each ROI.
Statistical Analyses
SPSS version 25 was used for all statistical analyses. The total percent of targets correctly identified during the 2-back task was not normally distributed and therefore does not meet the general linear model normality assumptions. Given the distribution was negatively skewed even following power transformations, two groups were created using a median split in 2-back task performance to evaluate relative higher and lower performance. Participants were divided into two groups based on a median split in the total percent of correctly identified targets during the 2-back task (higher performers n = 76; lower performers n = 62). Notably, 23 participants correctly identified the median percent of 2-back targets (87.5%) and were included in the “higher” group following the median split, resulting in an uneven number of participants in each performance group.
Chi-squared tests were conducted to examine group differences in sex and scanner location. Independent samples t-tests were performed to examine group differences in 2-back task accuracy (percent targets correctly identified), age, and education. Separate binary logistic regressions adjusting for age, sex, education, and scanner type were conducted to evaluate whether DLPFC BOLD signal, cortical thickness, surface area, or white matter volume each predicted 2-back performance group membership (higher vs. lower). In the regression models containing cortical surface area or white matter volume, total ICV was included as an additional covariate to control for individual differences in total brain volume. Variation in brain volume due to differences in head size is strongly associated with surface area and white matter volume, but not cortical thickness or BOLD signal measures (Barnes et al. 2010). Thus, ICV was included as a covariate for structural measures that have been shown to scale with head size (i.e., surface area and white matter volume) and not thickness or BOLD indices. For structural measures (i.e., cortical thickness, surface area, or white matter volume) that significantly predicted 2-back performance group, variables were mean-centered and multiplied to create a structure by function (BOLD) interaction term (Aiken et al. 1991). Follow-up binary logistic regressions adjusting for age, sex, education, scanner type, and total ICV were then conducted to evaluate whether brain structure and function interact to predict working memory performance group.
Results
2-Back Task Group Characteristics
Independent samples t-tests confirmed the higher performing group had significantly greater performance on the 2-back task relative to the lower performing group (t(70.99) = −12.829; P < 0.001). Chi-squared analyses revealed that there were no significant group differences in sex or scanner location (sex: χ2(1) = 1.472, P = 0.225; scanner: χ2(1) = 0.151, P = 0.698). Independent samples t-tests revealed there were also no significant group differences in age, total number of years of completed education, and intracranial volume (age: t(136) = 0.390, P = 0.697; education: t(136) = −.353, P = 0.725, ICV: t(136) = −1.672, p = -.097). See Table 2 for complete group characteristics.
Table 2.
Group characteristics
| Total sample n = 138 | High performers n = 76 | Low performers n = 62 | P | ||
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |||
| 2-back accuracy | 82.52% (16.44%) | 93.67% (4.84%) | 68.85% (15.29%) | <.001 * | |
| Age | 71.41 (5.19) | 71.25 (5.46) | 71.60 (4.87) | .697 | |
| Sex (M:F) | 59:79 | 36:40 | 23:39 | .233 | |
| Education | 16.16 (2.36) | 16.22 (2.25). | 16.08 (2.51) | .725 | |
| Scanner location (UF: UA) | 98:40 | 55:21 | 43:19 | .710 | |
| ICV (mm3) | 1474980.87 (133783.695) | 1492067.00 (137676.155) | 1454036.59 (125817.801) | .097 |
M = Male; F = Female; UF = University of Florida; UA = University of Arizona; ICV=Intracranial Volume
* P < 0.001
White Matter Volume
Binary logistic regressions adjusting for age, sex, education, and scanner type demonstrated DLPFC white matter volume did not significantly predict 2-back performance group for any of the 3 DLPFC ROIs. Specifically, those with greater white matter volume in either the left or the right DLPFC did not have a significantly greater probability of being in the higher performing group (P values>.05, see Table 3).
Table 3.
Binary logistic regressions: structure & function
| MNI coordinates [x, y, z] | B | Sig. | Exp(B) | 95% CI for Exp(B) [Lower, Upper] | ||
|---|---|---|---|---|---|---|
| BOLD signal | ||||||
| Left DLPFC | [−37.75, 50.19, 13.6] | .497 | .017 * | 1.644 | [1.094, 2.469] | |
| [−46.26, 22.71, 18.6] | .579 | .004 ** | 1.785 | [1.206, 2.640] | ||
| Right DLPFC | [44.53, 38.76, 24.43] | .488 | .009 ** | 1.629 | [1.129, 2.351] | |
| Cortical thickness | ||||||
| Left DLPFC | [−37.75, 50.19, 13.6] | .056 | .762 | 1.058 | [.736, 1.521] | |
| [−46.26, 22.71, 18.6] | −.024 | .900 | .976 | [.673, 1.417] | ||
| Right DLPFC | [44.53, 38.76, 24.43] | −.231 | .233 | .794 | [.543, 1.160] | |
| Surface area | ||||||
| Left DLPFC | [−37.75, 50.19, 13.6] | .477 | .017 * | 1.611 | [1.088, 2.386] | |
| [−46.26, 22.71, 18.6] | .315 | .112 | 1.370 | [.930, 2.019] | ||
| Right DLPFC | [44.53, 38.76, 24.43] | −.135 | .468 | .874 | [.607, 1.258] | |
| White matter volume | ||||||
| Left DLPFC | [−37.75, 50.19, 13.6] | −.046 | .812 | .955 | [.654, 1.395] | |
| [−46.26, 22.71, 18.6] | .069 | .772 | 1.071 | [.672, 1.708] | ||
| Right DLPFC | [44.53, 38.76, 24.43] | .306 | .140 | 1.358 | [.905, 2.039] | |
Note: Values standardized to z-scores
* P < 0.05
** P < 0.01
Cortical Thickness and Surface Area
Binary logistic regressions adjusting for age, sex, education, and scanner type revealed cortical thickness did not significantly predict 2-back performance group for any of the 3 DLPFC ROIs (P values>.05, see Table 3). Yet, cortical surface area did significantly predict 2-back performance group for the left DLPFC centered at MNI-coordinates (−37.75, 50.19, 13.6). Specifically, greater left DLPFC surface area significantly predicted the probability of being in the higher performing group (Exp(B) = 1.611, 95% CI = [1.088, 2.386], P = 0.017, see Table 3). For every 1 standardized unit increase in left DLPFC surface area, there was 1.611 times greater odds of being in the higher performing group.
Regional BOLD Response
Binary logistic regressions adjusting for age, sex, education, and scanner type revealed that the level of BOLD signal significantly predicted 2-back performance group for the left and right DLPFC. Greater BOLD signal in the left and right DLPFC each significantly predicted the probability of being in the higher 2-back performance group (P values< 0.05, see Table 3). Specifically, for every 1 standardized unit increase in DLPFC BOLD signal, there was 1.629 to 1.785 times greater odds of being in the higher performing group (Left DLPFC [−37.75, 50.19, 13.6]: Exp(B) = 1.644, 95% CI = [1.094, 2.469]; Left DLPFC [−46.26, 22.71, 18.6]: Exp(B) = 1.785, 95% CI = [1.206, 2.640]; Right DLPFC [44.53, 38.76, 24.43]: Exp(B) = 1.629, 95% CI = [1.129, 2.351]; see Table 3).
Brain Structure & Function Interaction
Since the surface area for the left DLPFC centered at MNI-coordinates (−37.75, 50.19, 13.6) was the only structural measure that significantly predicted 2-back performance group, follow-up analyses examined whether surface area and BOLD signal in this region interact to predict working memory group. However, results demonstrated that the left DLPFC BOLD signal and surface area did not interact to predict 2-back performance group. Specifically, those with greater left DLPFC BOLD signal and surface area did not have a significantly greater probability of being in the higher performing group (P values>.05, see Table 4, Fig. 3). Although they did not significantly interact, left DLPFC BOLD signal and surface area still each significantly predicted the probability of being in the higher performing group (P = 0.794, see Table 4). Given this, post hoc linear regression analyses were conducted for each group (higher and lower performers) adjusting for age, sex, education, scanner type, and total ICV to determine whether left DLPFC BOLD signal and surface area were significantly associated in higher and in lower performers. Collapsed across groups, left DLPFC BOLD signal and surface area were not significantly associated (β = 0.043, P = 0.639). Left DLPFC BOLD signal and surface area were also not significantly associated in either the higher performers (β = −.048, P = 0.707) or the lower performers (β = 0.049, P = 0.728), further suggesting BOLD signal and surface area independently contribute to 2-back performance.
Table 4.
Left DLPFC centered at (−37.75, 50.19, 13.6) surface area by BOLD interaction
| B | Sig. | Exp(B) | 95% CI for Exp(B) [Lower, Upper] | |
|---|---|---|---|---|
| Surface area | .491 | .017 * | 1.635 | [1.093, 2.444] |
| BOLD | .497 | .014 * | 1.644 | [1.105, 2.446] |
| Surface area × BOLD | −.053 | .794 | .948 | [.636, 1.414] |
* P < 0.05; values standardized to z-scores.
Figure 3.
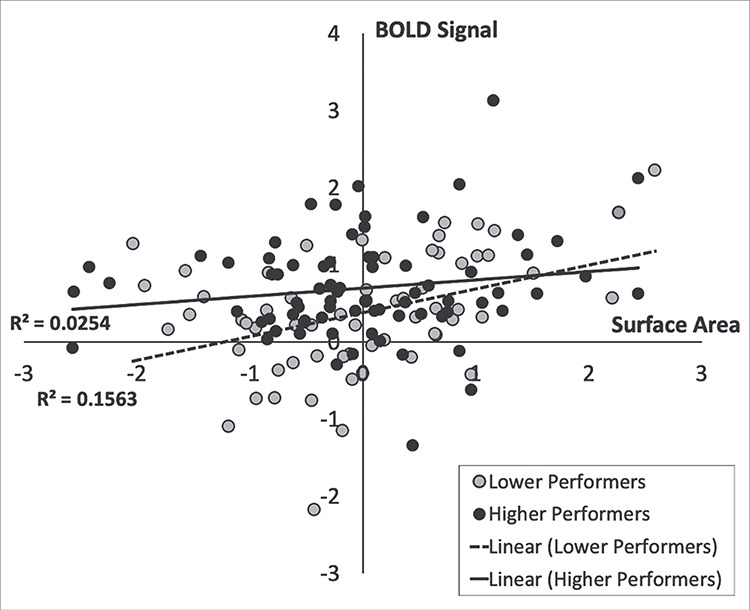
The left DLPFC surface area by BOLD interaction did not significantly predict 2-back performance group. Follow-up regression analyses revealed that BOLD signal and surface area in this region are not significantly associated in either the higher or lower performers.
To further evaluate potential compensatory mechanisms as proposed by the STAC model, exploratory follow-up analyses were conducted to determine relationships between contralateral DLPFC surface area and BOLD signal. Linear regression adjusting for age, sex, education, scanner location, and total ICV demonstrated that across the entire sample, surface area for the left DLPFC centered at MNI-coordinates (−37.75, 50.19, 13.6) did not significantly predict right DLPFC BOLD signal (β = 0.137, P = 0.128). Left DLPFC surface area also did not significantly predict right DLPFC BOLD signal in either the higher performers (β = 0.088, P = 0.483) or lower performers (β = 0.123, P = 0.392), suggesting greater right DLPFC BOLD signal is not necessarily a compensatory response to lower left DLPFC surface area. This provides further evidence that BOLD signal and surface area may independently contribute to 2-back performance.
Site-Specific Follow-Up Analyses
Although scanner location was included as a covariate, analyses were replicated within the sample recruited from UF (n = 98; the larger sample between the 2 study sites) to ensure findings were not driven by potential differences in MRI scanner. Consistent with results across the entire sample, binary logistic regressions adjusting for age, sex, education, and ICV revealed that cortical surface area significantly predicted 2-back performance group for the left DLPFC centered at MNI coordinates (−37.75, 50.19, 13.6). Specifically, greater left DLPFC surface area significantly predicted the probability of being in the higher performing group (Exp(B) = 1.966, 95% CI = [1.192, 3.244], P = 0.008). White matter volume and cortical thickness each did not significantly predict 2-back performance group for any of the 3 DLPFC ROIs (P values>.05). Additionally, binary logistic regressions adjusting for age, sex, education, and scanner type revealed that the level of BOLD signal significantly predicted 2-back performance group for the left DLPFC (−46.26, 22.71, 18.6) (Exp(B) = 1.612, 95% CI = [1.036, 2.507], P = 0.034). There was a trend for the left DLPFC centered at MNI-coordinates (−37.75, 50.19, 13.6) (Exp(B) = 1.533, 95% CI = [.986, 2.385], P = 0.058, which is likely due to the much smaller sample size relative to the entire sample. Furthermore, follow-up analyses demonstrated that left DLPFC BOLD signal and surface area did not interact to predict 2-back performance group (Exp(B) = 0.971, 95% CI = [.563, 1.677], P = 0.917). Altogether, these follow-up analyses within the UF sample resulted in patterns largely consistent with findings across the entire sample.
Discussion
Aging is associated with alterations in both brain structure and function that contribute to cognitive decline. Measures of brain structure and function have been investigated as independent modalities, yet little is known about how they may interact to contribute to the cognitive aging process. The present study leveraged multimodal neuroimaging to provide novel insight into the contributions of DLPFC surface area and BOLD signal to working memory in a large, well-characterized sample of healthy older adults. Here, we demonstrated that left DLPFC surface area and BOLD signal did not interact, but rather, independently predicted working memory performance in healthy, non-pathological aging. Importantly, these findings demonstrate that age-related differences in brain structure and function may not be directly related in their contributions to working memory in older adults. Altogether, results from the present study inform the identification of successful aging markers that can serve as potential targets for intervention strategies.
DLPFC Structural and Functional Correlates of Working Memory
As hypothesized, DLPFC cortical surface area, but not cortical thickness, significantly predicted the probability of higher working memory performance. Cortical thickness is considered an indirect measure of neuronal density, which is affected more by neurodegenerative processes than in non-pathological aging (Du et al., 2007; Fischl & Dale, 2000; Kabani et al., 2001; Shefer, 1973). Cortical surface area is representative of the integrity and complexity of gray matter and may therefore be more vulnerable to the aging process (Dotson et al., 2016; Fischl & Dale, 2000; Lemaitre et al., 2012; Nissim et al., 2017; Salat, 2004). Given the DLPFC is central to working memory, reduced DLPFC surface area, and thereby compromised structural integrity and complexity, may result in poorer working memory ability in older age.
Although a prior study demonstrated that less right DLPFC surface area is associated with poorer working memory (Nissim et al. 2017), the present study did not demonstrate significant associations between right DLPFC surface area and working memory performance. However, there is at least one notable difference that may be associated with discrepant findings between the prior and current study. While Nissim et al. (2017) excluded for mild cognitive impairment or neurodegenerative brain disease, this study relied only on performance on a cognitive screening measure (Montreal Cognitive Assessment; MoCA) to exclude for cognitive impairment (i.e., MoCA Total Score < 20). As such, discrepencies between left versus right lateralized findings may be a result of variability in cognitive criteria. In addition, the total sample size in Nissim et al. 2017, totaled 56 participants, versus 138 participants in the current study—representing significant differences in power between studies. The present study included a large, well-classified sample of healthy older adults without mild cognitive impairment, as defined by performance > − 1.5 SD below the mean in any one of five cognitive domains on a comprehensive cognitive battery. As such, our findings that the left DLPFC surface area is predicitive of working memory may be characteristic of a healthy aging sample.
Our results also indicated greater BOLD signal in both the left and right DLPFC is predictive of higher working memory performance in non-pathological aging. This confirms prior research that suggests greater BOLD signal is representative of more resources required in that particular brain region to achieve higher task performance (Cabeza et al. 2002; Owen et al. 2005; Dennis & Cabeza 2011). The STAC model supports the notion that greater BOLD signal (i.e., overactivation of the DLPFC) is a marker of the brain engaging in compensatory scaffolding in response to declines in neuronal structure and function (Park and Reuter-Lorenz 2009; Reuter-Lorenz and Park 2014). Greater DLPFC BOLD activation may therefore reflect older adults’ ability to compensate for structural declines to maintain higher working memory performance.
Further, Cabeza et al. (2002) theorized that bilateral activation in older adults, compared to younger adults with activation lateralized to the right hemisphere, is reflective of a compensatory mechanism. Cabeza et al. (2018) noted that increased BOLD activation in associated regions is linked to insufficiency in available neural resources relative to cognitive task demands. Park & Reuter-Lorenz (2009) proposed that older adults who have experienced regional structural declines may engage associated, more structurally intact regions, resulting in bilateral activation patterns (Park and Reuter-Lorenz 2009; Reuter-Lorenz and Park 2014). It is possible that higher performing older adults in the present study may have compensated for potential structural deficits in associated regions (e.g., right DLPFC) by recruiting cognitive resources from the structurally intact left DLPFC.
We demonstrated that greater surface area within only the left DLPFC, and not the right DLPFC, is predictive of higher working memory performance. Preserved structural integrity of the left DLPFC in older age may promote successful aging and the potential capacity for compensatory engagement. Conversely, older adults with less surface area across both the right and left DLPFC may have a decreased ability to compensate for such age-related structural deficits. Indeed, Cabeza et al. (2018) proposed that compensation would be greatest in those with significant structural declines, but advanced degeneration would result in reduced capacity for compensation. Future longitudinal studies should continue to study how preserved structural integrity in older age contributes to the ability to engage associated brain regions as a potential compensatory mechanism.
Left DLPFC Surface Area by BOLD Interaction
While considerable evidence suggests greater surface area and greater BOLD signal are each associated with better working memory performance (Courtney et al. 1998; Cabeza et al. 2002; Barbey et al. 2013; Nissim et al. 2017; Suzuki et al. 2018), previous studies have not evaluated their interactive effect on cognition. Here, we show that cortical surface area and BOLD signal in the left DLPFC do not significantly interact to predict higher working memory performance. In both higher and lower performers, left DLPFC BOLD signal and surface area were not significantly related, suggesting differences in surface area do not directly affect the level of the BOLD signal within that region. However, BOLD and surface area were each still significant predictors of higher performance above and beyond the interaction term. This further supports that greater left DLPFC BOLD signal and surface area, or other underlying factors influencing these metrics, are distinct contributors to higher working memory performance.
Furthermore, the STAC model seemingly assumes structural declines directly influence bilateral activation patterns observed in higher performing older adults via compensatory scaffolding (Park and Reuter-Lorenz 2009; Reuter-Lorenz and Park 2014). We present evidence that age-related structural deficits are not necessarily directly related to greater BOLD activation in either the higher or lower performers. Yet, these structural and functional measures were still independently predictive of higher working memory performance in healthy older adults. One possible explanation is that the STAC model represents an inverted U-shaped function between brain structure and functional activation, such that higher performing adults with intact structure may not require compensation and thus may not demonstrate a heightened BOLD response. Additionally, the older adults in the present study may have engaged in compensatory scaffolding to maintain higher working memory performance, but there may be an underlying mechanism that mediates such compensation.
Limitations and Future Directions
While findings from the present study provide novel insight into the independent structural and functional contributions of the DLPFC to higher working memory performance, the following limitations should be considered. First, our sample of older adults was highly educated, as 68.1% of participants obtained a Bachelor’s degree or higher. While we covaried for education in our analyses and there were no significant group differences in education, future work should include older adults representing various levels of education.
Notably, the present study examined associations within a healthy non-pathological aging sample, with individuals excluded for presence of mild cognitive impairment or dementia, as determined by performance on the UDS (Weintraub et al. 2009; Woods et al. 2018). Given the higher performing nature of this healthy aging sample resulted in a negatively skewed distribution even following power transformations, participants were divided into two groups based on a median split in 2-back task performance. Results from the present study elucidate how measures of DLPFC structure and function relate to higher and lower working memory performance in a healthy older adult cohort (i.e., successful aging). However, we cannot comment on how these structural and functional measures contribute to declines in working memory observed in MCI or pathological aging. Additionally, this was a cross-sectional study. Future work should include longitudinal studies evaluating associations between brain structure, function, and cognition, and how these associations may predict progression to MCI or neurodegenerative disease (i.e., Alzheimer’s Disease).
Furthermore, the present study did not evaluate differences in DLPFC structure or function with parametric manipulation of working memory load, as working memory was characterized based one 2-back task performance alone. As proposed by Cabeza et al. (2018), compensation occurs via recruitment of additional neural resources to meet cognitive demand. Prior studies have shown that older adults reach maximum BOLD activation at lower working memory load relative to younger adults (Schneider-Garces et al. 2010; Heinzel et al. 2014; Iordan et al. 2020). Such a ceiling for BOLD activation occurs at the limit of available neural resources and is followed by a decline in activation and performance once the limit of resources has been exceeded (Cabeza et al. 2018). Given this, higher BOLD activation with greater working memory load has been thought to represent high neural capacity, which may be supportive of successful aging and preserved cognitive performance. Future studies including parametric manipulation of working memory load would be beneficial to further characterize relationships between BOLD activation and working memory performance that support successful aging.
Contrary to our hypothesis, DLPFC white matter volume did not significantly predict the probability of being in the higher performing working memory group. It is possible white matter volume may not be a sensitive enough metric to detect differences in neuronal communication and associated cognitive processes that occur in healthy, non-pathological aging. Rather, differences in the integrity of white matter tracts underlying such cognitive processes may have a greater impact on cognition in non-pathological aging. Specifically, differences in the integrity of white matter pathways within a working memory network, specifically those connecting the left and right DLPFC, may underlie observed bilateral BOLD activation patterns and mediate associations between structural deficits (i.e., surface area), BOLD signal, and working memory performance. However, white matter tract integrity data were not collected in the parent study. Future studies should incorporate additional modalities measuring white matter integrity (e.g., diffusion weighted imaging metrics of fractional anisotropy, etc.) in addition to white matter volume to further elucidate how white matter alterations contribute to the cognitive aging process.
In addition, the current study did not assess magnetic resonance spectroscopy to evaluate proton or phosphorus nuclei-based markers of neural metabolism. Age-related regional declines in cerebral adenosine triphosphate (ATP), creatine, or other markers of energy metabolism may also help explain structural and functional contributions to working memory within the DLPFC (Bachelard 1979; Schmitz et al. 2018). Future studies expanding multimodal assessment of the DLPFC to include both microstructural and neurometabolic markers will help to further elucidate the neural processes underlying working memory deficits in healthy older adults.
Conclusion
This study leverages multimodal structural and functional neuroimaging to provide important new insights into the independent contributions of DLPFC structure and function to working memory in cognitive aging. Out of all structural measures examined, only DLPFC surface area was predictive of the probability of higher performance. Our findings suggest that preserved structural integrity of the left DLPFC in older age may promote successful aging and the potential capacity for compensatory engagement, while older adults with less bilateral surface area may have a decreased ability for compensation. However, we present evidence that greater DLPFC BOLD signal and surface area are not directly related or interact to influence working memory. Rather, our results suggest these measures of structure and function independently predict higher working memory performance. There may be underlying microstructural or neurometabolic mechanisms that mediate the structural and functional differences and associated working memory performance demonstrated in the present study. Expanded multimodal study of the DLPFC will serve to determine whether alterations in DLPFC structure and function relate to working memory are truly independent or whether the present findings are representative of a more complex model that remains undefined.
Funding
The National Institute on Aging (NIA R01AG054077; NIA K01AG050707, T32AG020499); the National Heart, Lung, and Blood Institute (NHLBI)/National Institute of Health (T32HL134621); the State of Arizona and Arizona Department of Health Services (ADHS); the McKnight Brain Research Foundation.
Contributor Information
Nicole D Evangelista, Center for Cognitive Aging and Memory Clinical Translational Research, McKnight Brain Institute, University of Florida, Gainesville, FL, 32611, USA; Department of Clinical and Health Psychology, College of Public Health and Health Professions, University of Florida, Gainesville, FL, 32611, USA.
Andrew O’Shea, Center for Cognitive Aging and Memory Clinical Translational Research, McKnight Brain Institute, University of Florida, Gainesville, FL, 32611, USA; Department of Clinical and Health Psychology, College of Public Health and Health Professions, University of Florida, Gainesville, FL, 32611, USA.
Jessica N Kraft, Center for Cognitive Aging and Memory Clinical Translational Research, McKnight Brain Institute, University of Florida, Gainesville, FL, 32611, USA; Department of Neuroscience, College of Medicine, University of Florida.
Hanna K Hausman, Center for Cognitive Aging and Memory Clinical Translational Research, McKnight Brain Institute, University of Florida, Gainesville, FL, 32611, USA; Department of Clinical and Health Psychology, College of Public Health and Health Professions, University of Florida, Gainesville, FL, 32611, USA.
Emanuel M Boutzoukas, Center for Cognitive Aging and Memory Clinical Translational Research, McKnight Brain Institute, University of Florida, Gainesville, FL, 32611, USA; Department of Clinical and Health Psychology, College of Public Health and Health Professions, University of Florida, Gainesville, FL, 32611, USA.
Nicole R Nissim, Department of Neurology, University of Pennsylvania, Philadelphia, PA, 19104, USA.
Alejandro Albizu, Center for Cognitive Aging and Memory Clinical Translational Research, McKnight Brain Institute, University of Florida, Gainesville, FL, 32611, USA; Department of Neuroscience, College of Medicine, University of Florida.
Cheshire Hardcastle, Center for Cognitive Aging and Memory Clinical Translational Research, McKnight Brain Institute, University of Florida, Gainesville, FL, 32611, USA; Department of Clinical and Health Psychology, College of Public Health and Health Professions, University of Florida, Gainesville, FL, 32611, USA.
Emily J Van Etten, Department of Psychology and McKnight Brain Institute, University of Arizona, Tucson, AZ, 85721, USA.
Pradyumna K Bharadwaj, Department of Psychology and McKnight Brain Institute, University of Arizona, Tucson, AZ, 85721, USA.
Samantha G Smith, Department of Psychology and McKnight Brain Institute, University of Arizona, Tucson, AZ, 85721, USA.
Hyun Song, Department of Psychology and McKnight Brain Institute, University of Arizona, Tucson, AZ, 85721, USA.
Georg A Hishaw, Department of Psychiatry, Department of Neurology, College of Medicine, University of Arizona, Tucson, AZ, 85721, USA.
Steven DeKosky, Center for Cognitive Aging and Memory Clinical Translational Research, McKnight Brain Institute, University of Florida, Gainesville, FL, 32611, USA; Department of Neurology, College of Medicine, University of Florida.
Samuel Wu, Department of Biostatistics, College of Public Health and Health Professions, College of Medicine, University of Florida, Gainesville, FL, 32611, USA.
Eric Porges, Center for Cognitive Aging and Memory Clinical Translational Research, McKnight Brain Institute, University of Florida, Gainesville, FL, 32611, USA; Department of Clinical and Health Psychology, College of Public Health and Health Professions, University of Florida, Gainesville, FL, 32611, USA.
Gene E Alexander, Department of Psychology and McKnight Brain Institute, University of Arizona, Tucson, AZ, 85721, USA; Brain Imaging, Behavior and Aging Laboratory, Departments of Psychology and Psychiatry, Neuroscience and Physiological Sciences Graduate Interdisciplinary Programs, BIO5 Institute and McKnight Brain Institute, University of Arizona, Tucson, AZ, 85721, USA.
Michael Marsiske, Center for Cognitive Aging and Memory Clinical Translational Research, McKnight Brain Institute, University of Florida, Gainesville, FL, 32611, USA; Department of Clinical and Health Psychology, College of Public Health and Health Professions, University of Florida, Gainesville, FL, 32611, USA.
Ronald Cohen, Center for Cognitive Aging and Memory Clinical Translational Research, McKnight Brain Institute, University of Florida, Gainesville, FL, 32611, USA; Department of Clinical and Health Psychology, College of Public Health and Health Professions, University of Florida, Gainesville, FL, 32611, USA.
Adam J Woods, Center for Cognitive Aging and Memory Clinical Translational Research, McKnight Brain Institute, University of Florida, Gainesville, FL, 32611, USA; Department of Clinical and Health Psychology, College of Public Health and Health Professions, University of Florida, Gainesville, FL, 32611, USA; Department of Neuroscience, College of Medicine, University of Florida.
References
- Aiken LS, West SG, Reno RR. 1991. Multiple regression: testing and interpreting interactions. Nachdr. ed. Newbury Park, California: SAGE. [Google Scholar]
- Bachelard HS 1979. Brain energy metabolism. Biochem Soc Trans. 7:264–264. [Google Scholar]
- Baddeley A 1992. Working memory. Science. 255:556–559. [DOI] [PubMed] [Google Scholar]
- Barbey AK, Koenigs M, Grafman J. 2013. Dorsolateral prefrontal contributions to human working memory. Cortex. 49:1195–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes J, Ridgway GR, Bartlett J, Henley SMD, Lehmann M, Hobbs N, Clarkson MJ, MacManus DG, Ourselin S, Fox NC. 2010. Head size, age and gender adjustment in MRI studies: a necessary nuisance? NeuroImage. 53:1244–1255. [DOI] [PubMed] [Google Scholar]
- Bizon JL, Foster TC, Alexander GE, Glisky EL. 2012. Characterizing cognitive aging of working memory and executive function in animal models. Front Aging Neurosci. 4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Zimmerman ME, Paul RH, Grieve SM, Tate DF, Cohen RA, Williams LM, Clark CR, Gordon E. 2006. Regional white matter and neuropsychological functioning across the adult lifespan. Biol Psychiatry. 60:444–453. [DOI] [PubMed] [Google Scholar]
- Cabeza R 2002. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 17:85–100. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. 2002. Aging gracefully: compensatory brain activity in high-performing older adults. NeuroImage. 17:1394–1402. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Albert M, Belleville S, Craik FIM, Duarte A, Grady CL, Lindenberger U, Nyberg L, Park DC, Reuter-Lorenz PA et al. 2018. Maintenance, reserve and compensation: the cognitive neuroscience of healthy ageing. Nat Rev Neurosci. 19:701–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslik EC, Zilles K, Caspers S, Roski C, Kellermann TS, Jakobs O, Langner R, Laird AR, Fox PT, Eickhoff SB. 2013. Is there “one” DLPFC in cognitive action control? Evidence for heterogeneity from co-activation-based parcellation. Cereb Cortex. 23:2677–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney SM, Petit L, Haxby JV, Ungerleider LG. 1998. The role of prefrontal cortex in working memory: examining the contents of consciousness. Philos Trans R Soc B Biol Sci. 353:1819–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. 1999. Cortical surface-based analysis.I. segmentation and surface reconstruction. NeuroImage. 9:179–194. [DOI] [PubMed] [Google Scholar]
- Dale AM, Sereno MI. 1993. Improved localizadon of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: a linear approach. J Cogn Neurosci. 5:162–176. [DOI] [PubMed] [Google Scholar]
- Dennis NA, Cabeza R. 2011. Neuroimaging of healthy cognitive aging In: The Handbook of Aging and Cognition. Routledge. [Google Scholar]
- Dotson VM, Szymkowicz SM, Sozda CN, Kirton JW, Green ML, O’Shea A, McLaren ME, Anton SD, Manini TM, Woods AJ. 2016. Age differences in prefrontal surface area and thickness in middle aged to older adults. Front Aging Neurosci. 7:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du A-T, Schuff N, Kramer JH, Rosen HJ, Gorno-Tempini ML, Rankin K, Miller BL, Weiner MW. 2007. Different regional patterns of cortical thinning in Alzheimer’s disease and frontotemporal dementia. Brain J Neurol. 130:1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn ES, Huber L, Jangraw DC, Molfese PJ, Bandettini PA. 2019. Layer-dependent activity in human prefrontal cortex during working memory. Nat Neurosci. 22:1687–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B 2004. Automatically parcellating the human cerebral cortex. Cereb Cortex. 14:11–22. [DOI] [PubMed] [Google Scholar]
- Fischl B 2012. FreeSurfer. NeuroImage. 62:774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM. 2000. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci. 97:11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM. 2001. Automated manifold surgery: constructing geometrically accurate topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 20:70–80. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S et al. 2002. Whole brain segmentation. Neuron. 33:341–355. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJW, Makris N, Ségonne F, Quinn BT, Dale AM. 2004. Sequence-independent segmentation of magnetic resonance images. NeuroImage. 23:S69–S84. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. 1999a. Cortical surface-based analysis. NeuroImage. 9:195–207. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. 1999b. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 8:272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi S 2017. Working memory in the prefrontal cortex. Brain Sci. 7:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisky E 2007. Changes in cognitive function in human aging In: Riddle D, editor. Brain aging. Boca Raton, FL: CRC Press/Taylor & Francis, pp. 3–20. [PubMed] [Google Scholar]
- Goldman-Rakic PS 1995. Cellular basis of working memory. Neuron. 14:477–485. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS 2011. Circuitry of primate prefrontal cortex and regulation of behavior by representational memory In: Terjung R, editor. Comprehensive physiology. Hoboken. NJ, USA: John Wiley & Sons, Inc., cp010509. [Google Scholar]
- Golestani AM, Miles L, Babb J, Castellanos FX, Malaspina D, Lazar M. 2014. Constrained by our connections: white matter’s key role in interindividual variability in visual working memory capacity. J Neurosci. 34:14913–14918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RNA, Friston KJ, Frackowiak RSJ. 2001. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage. 14:21–36. [DOI] [PubMed] [Google Scholar]
- Guttmann CRG, Jolesz FA, Kikinis R, Killiany RJ, Moss MB, Sandor T, Albert MS. 1998. White matter changes with normal aging. Neurology. 50:972–978. [DOI] [PubMed] [Google Scholar]
- Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, Busa E, Pacheco J, Albert M, Killiany R et al. 2006. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. NeuroImage. 32:180–194. [DOI] [PubMed] [Google Scholar]
- Heinzel S, Lorenz RC, Brockhaus W-R, Wustenberg T, Kathmann N, Heinz A, Rapp MA. 2014. Working memory load-dependent brain response predicts behavioral training gains in older adults. J Neurosci. 34:1224–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordan AD, Cooke KA, Moored KD, Katz B, Buschkuehl M, Jaeggi SM, Polk TA, Peltier SJ, Jonides J, Reuter-Lorenz PA. 2020. Neural correlates of working memory training: evidence for plasticity in older adults. NeuroImage. 217:116887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. 2012. FSL. NeuroImage. 62:782–790. [DOI] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R, Kennedy D, Schmitt F, Brown G, Macfall J et al. 2006. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. NeuroImage. 30:436–443. [DOI] [PubMed] [Google Scholar]
- Kabani N, Le Goualher G, MacDonald D, Evans AC. 2001. Measurement of cortical thickness using an automated 3-D algorithm: a validation study. NeuroImage. 13:375–380. [DOI] [PubMed] [Google Scholar]
- Lemaitre H, Goldman AL, Sambataro F, Verchinski BA, Meyer-Lindenberg A, Weinberger DR, Mattay VS. 2012. Normal age-related brain morphometric changes: nonuniformity across cortical thickness, surface area and gray matter volume? Neurobiol Aging. 33:617.e1–617.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Yang Y, Xia Y, Zhu W, Leak RK, Wei Z, Wang J, Hu X. 2017. Aging of cerebral white matter. Ageing Res Rev. 34:64–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JH. 2004. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. NeuroImage. 21:450–455. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. 2003. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 19:1233–1239. [DOI] [PubMed] [Google Scholar]
- Nissim NR, O’Shea AM, Bryant V, Porges EC, Cohen R, Woods AJ. 2017. Frontal structural neural correlates of working memory performance in older adults. Front Aging Neurosci. 08:328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. 2005. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 25:46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. 2002. Models of visuospatial and verbal memory across the adult life span. Psychol Aging. 17:299–320. [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. 2009. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol. 60:173–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny W, Friston K, Ashburner J, Kiebel S, Nichols T, editors. 2007. Statistical parametric mapping: the analysis of funtional brain images. 1st ed. Amsterdam; Boston: Elsevier/Academic Press. [Google Scholar]
- Reuter M, Rosas HD, Fischl B. 2010. Highly accurate inverse consistent registration: a robust approach. NeuroImage. 53:1181–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Schmansky NJ, Rosas HD, Fischl B. 2012. Within-subject template estimation for unbiased longitudinal image analysis. NeuroImage. 61:1402–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Park DC. 2014. How does it STAC up? Revisiting the scaffolding theory of aging and cognition. Neuropsychol Rev. 24:355–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AW, Ogunwole SU, Blakeslee L, Rabe MA. 2018. The population 65 years and older in the United States. 2016:25 Retrieved from https://www.census.gov/content/dam/Census/library/publications/2018/acs/ACS-38.pdf. [Google Scholar]
- Salat DH 2004. Thinning of the cerebral cortex in aging. Cereb Cortex. 14:721–730. [DOI] [PubMed] [Google Scholar]
- Salat DH, Kaye JA, Janowsky JS. 1999. Prefrontal gray and white matter volumes in healthy aging and Alzheimer disease. Arch Neurol. 56:338. [DOI] [PubMed] [Google Scholar]
- Salat DH, Kaye JA, Janowsky JS. 2001. Selective preservation and degeneration within the prefrontal cortex in aging and Alzheimer disease. Arch Neurol. 58:1403. [DOI] [PubMed] [Google Scholar]
- Schmitz B, Wang X, Barker PB, Pilatus U, Bronzlik P, Dadak M, Kahl KG, Lanfermann H, Ding X-Q. 2018. Effects of aging on the human brain: a proton and phosphorus MR spectroscopy study at 3T: H- and P-MRS study of aging effects. J Neuroimaging. 28:416–421. [DOI] [PubMed] [Google Scholar]
- Schneider-Garces NJ, Gordon BA, Brumback-Peltz CR, Shin E, Lee Y, Sutton BP, Maclin EL, Gratton G, Fabiani M. 2010. Span, CRUNCH, and beyond: working memory capacity and the aging brain. J Cogn Neurosci. 22:655–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze ET, Geary EK, Susmaras TM, Paliga JT, Maki PM, Little DM. 2011. Anatomical correlates of age-related working memory declines. J Aging Res. 2011:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ségonne F, Pacheco J, Fischl B. 2007. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans Med Imaging. 26:518–529. [DOI] [PubMed] [Google Scholar]
- Shefer VF 1973. Absolute number of neurons and thickness of the cerebral cortex during aging, senile and vascular dementia, and Pick’s and Alzheimer’s diseases. Neurosci Behav Physiol. 6:319–324. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE et al. 2004. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 23(Suppl 1):S208–S219. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Wojtowicz M, Grady CL. 2010. Reliable differences in brain activity between young and old adults: a quantitative meta-analysis across multiple cognitive domains. Neurosci Biobehav Rev. 34:1178–1194. [DOI] [PubMed] [Google Scholar]
- Stern Y, Hesdorffer D, Sano M, Mayeux R. 1990. Measurement and prediction of functional capacity in Alzheimer’s disease. Neurology. 40:8–8. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Kawagoe T, Nishiguchi S, Abe N, Otsuka Y, Nakai R, Asano K, Yamada M, Yoshikawa S, Sekiyama K. 2018. Neural correlates of working memory maintenance in advanced aging: evidence from fMRI. Front Aging Neurosci. 10:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Gamo NJ, Yang Y, Jin LE, Wang X-J, Laubach M, Mazer JA, Lee D, Arnsten AFT. 2011. Neuronal basis of age-related working memory decline. Nature. 476:210–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, Cummings J, DeCarli C, Foster NL, Galasko D et al. 2009. The Alzheimer’s disease Centers’ uniform data set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 23:91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A. 2012. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2:125–141. [DOI] [PubMed] [Google Scholar]
- Woods AJ, Cohen R, Marsiske M, Alexander GE, Czaja SJ, Wu S. 2018. Augmenting cognitive training in older adults (the ACT study): design and methods of a phase III tDCS and cognitive training trial. Contemp Clin Trials. 65:19–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam A, Marsiske M. 2013. Cognitive longitudinal predictors of older adults’ self-reported IADL function. J Aging Health. 25:163S–185S. [DOI] [PMC free article] [PubMed] [Google Scholar]


