Abstract
Background:
Anatomical site is strongly associated with head and neck cancer etiology, and etiology and patient sociodemographic characteristics are prognostic factors for survival. It is not known whether the effects of these predictors persist over the post-diagnosis period or are strongest proximal to the time of diagnosis.
Methods:
Using survival time and cause of death for 180,434 head and neck cancer patients in the SEER Cancer Registry (1973–2015), we calculated empirical cumulative incidence of cancer-specific and other-cause death using a competing risks framework and estimated the time-dependent effects (hazard ratios) of anatomical tumor site (oropharynx, oral cavity, hypopharynx/larynx), age, sex, race, and year of diagnosis on cancer-specific and other-cause death, stratifying by tumor stage.
Results:
All effects were significantly time-varying (p <0.001). Non-oropharyngeal cancer patients had a higher hazard of cancer-specific death but a similar cumulative fraction of deaths due to higher rate of death from other causes. Cancer-specific survival has not changed for non-oropharyngeal cancer patients over the past decades but has improved since 2000 for oropharyngeal cancer patients. The effect of age and sex on cancer survival were strongest proximal to diagnosis, while the effect of race persisted over time.
Conclusions:
Recent improvements in survival for oropharyngeal cancer patients are may be due more to an increasing fraction of cancers attributable to HPV than to increasing treatment effectiveness. The prognostic strength of anatomical site and other predictors change over the post-diagnosis period.
Keywords: Head and neck cancer, oropharynx cancer, human papillomavirus, survival analysis, hazard model
Lay summary:
We generally assume that the effects of tumor and personal characteristics on a head and neck cancer patient’s survival are fixed over time, but here we show that many factors are most important only in the first few years after diagnosis. We also find that recent improvements in head and neck cancer patient survival appear to only benefit patients with cancers of the oropharynx. It may be that the improvement may be more due to an increasing fraction of cancers caused by the human papillomavirus (which generally have better outcomes) than to improvements in head and neck cancer treatment overall.
Precis:
The prognostic strength of tumor and patient characteristics for predicting patient survival changes over the post-diagnosis period. Recent improvements in survival for oropharyngeal cancer patients are may be due more to an increasing fraction of cancers attributable to HPV than to increasing treatment effectiveness.
Introduction
Cancers of the head and neck are primarily squamous cell carcinomas originating in the oral cavity, oropharynx, larynx, hypopharynx, nasopharynx, and sinonasal tract [1]. There are two primary etiologies for these cancers: i) tobacco and alcohol use and ii) the human papillomavirus (HPV) [2, 3], each with their own distinct presentation, outcomes, and molecular markers [4]. Most cancers caused by HPV are in the oropharynx, due to HPV’s preference (tropism) for those sites [5]. In the United States, it is estimated that the fraction of oropharynx cancers attributable to HPV is 70% or more and increasing [6–8], while HPV-attributable cancer in the oral cavity, hypopharynx, and larynx is relatively rare [9]. Globally, it is estimated that about 45% of oropharynx cancers are HPV-attributable, compared to 20–25% for other head and neck sites [10].
HPV status and related biomarkers are strongly associated with improved cancer-specific and overall survival times [1, 11–13]. HPV-negative tumors are less responsive to treatment, especially if the patient continues smoking [14, 15], and many patients with HPV-negative tumors have smoking-related comorbidities. Because it is strongly predictive of disease outcomes, HPV status has become a major factor in clinical care [16, 17]. In addition to HPV status, tumor stage at the time of diagnosis is strongly associated with future disease outcomes. Demographic factors, such as age and gender, are also likely associated with survival. As with most cancers, socioeconomic disparities in head and neck cancer survival persist [18].
While most analyses treat such factors as having a constant effect on survival, it is not well-understood whether this is an appropriate assumption or whether some of these effects might attenuate or otherwise change over the post-diagnosis period. For head and neck cancer, disease progression after diagnosis has a well understood time frame. Locoregional disease recurrence happens in 15–50% of head and neck cancer patients, depending on subsite, stage, treatment and other factors, and, if the disease is going to recur, it does so in less than 2 years in about 80% of patients [19]. Survival following recurrence is usually poor, with a median survival time of about 2 years [19, 20], though outcomes have improved in the past decades [21]. Thus it is likely that the magnitude of the importance of tumor characteristics, such as tumor site and stage, will change over the post-diagnosis period. Similarly, it is likely that the influence of demographic and socioeconomic disparities, as well as other covariates such as age, will change over the post-diagnosis period, necessitating the estimating of time-dependent effects (non-proportional hazards). Any temporal effects of these factors on survival could have implications for risk prediction, treatment, and care.
Our focus in this analysis is death due to head and neck cancer, rather than overall survival. It is clear that some factors, such as tumor stage, are likely to be more strongly associated with death from cancer than with death from other causes. Other factors, such as age, are likely to be more strongly associated with death from other causes than with death from head and neck cancer. Death due to head and neck cancer is directly affected by the quality of the treatment both for the initial primary occurrence and at the time of any recurrence, as well as the quality and effectiveness of the post diagnosis monitoring for recurrence. Thus, we might expect the date of diagnosis, a proxy for temporal changes in treatment and surveillance, to also be associated with cancer specific death rates, especially over the period considered by this study (1973–2015).
In this analysis, we consider cause-specific cancer death data for head and neck squamous cell carcinomas (OSCCs) from the U.S. Surveillance, Epidemiology, and End Results (SEER) cancer registry, grouped into three sites: oropharyngeal, oral cavity, and hypopharynx/larynx. We use a competing risks framework to observe the empirical evolution of cancer-specific and other-cause mortality and a Cox model framework with time-dependent coefficients to estimate how the effects of baseline covariates change over a patient’s time since diagnosis. While previous studies have used Cox proportional hazard models to explore head and neck cancer survival in SEER (e.g, [22]), none, to our knowledge, has looked at time-dependent effects.
Methods
Data
We use cancer-specific cancer survival data in the SEER 18 Cancer Registry (1973–2015, varying by subregistry) [23] for malignant head and neck squamous cell carcinomas (HNSCCs). This analysis includes only patients whose first cancer was a HNSCC as cancer-specific survival in SEER is only defined for first cancers [24]. Similarly to previous analyses [25, 26], we group anatomic sites of carcinomas by their International Classification of Diseases (ICD-10) codes. The following sites are considered to by oropharyngeal, with possibly HPV etiology: base of tongue (C01), lingual tonsil (C2.4), palate excluding hard palate (C5.1–5.9), tonsil (C9.0–C9.9), oropharynx (C10.0–C10.9), pharynx not otherwise specified (C14.0), and Waldeyer ring (C14.2). The following sites are considered to be oral cavity and to likely not have an HPV etiology: oral tongue (C2.0–2.3, C2.8–C2.9), gum (C3.0–C3.9), floor of mouth (C4.0–C4.9), hard palate (C5.0), and other and unspecified parts of the mouth (C6.0–C6.9). The following sites are considered to be hypopharyngeal/laryngeal and to likely not have an HPV etiology: pyriform sinus (C12), hypopharynx (C13), and larynx and glottis (C32). Other head and neck sites, including lip (C00), salivary gland (C07–C08), and nasopharynx (C11), were not included in this analysis. We only included cancers with squamous cell histology (ICD-O-3 histology type codes: 8050–8076, 8078, 8083, 8084, and 8094).
Because cause-specific death is vulnerable to misclassification of death on death certificates, the SEER registry takes into account the tumor sequence, site of the original tumor, and comorbidities when determining which deaths are attributable to the cancer diagnosis [24]. Specifically, if a patient died with only one cancer diagnosis, then their death was attributed to cancer if the cause of death was given as cancer at that site, cancer within the same organ system, any malignant cancer, or AIDS with cancer. If a patient had more than one cancer diagnosis, then cancer-specific survival was only calculated for the first cancer diagnosed, and the cancer-death variable was similarly derived, with the exception that cause of death from other malignant cancers was not included as death from the primary cancer. Three event types were derived from the SEER cause-specific death variables: censored (alive), dead from cancer, dead from other causes. Only events with event times of at least one month were considered (i.e., we removed diagnosis that occurred at the time of death). This data set includes 180,434 individuals, with events spanning from one month to 41 years. We provide the number of individuals at risk (i.e., not dead or censored) by covariate at 0, 5, 10, and 15 years after diagnosis in Table 1. The median length of follow-up (i.e., time to censoring) was 5.4 years (range 0.1 to 42.9 years); there was no substantial difference in the length of follow-up for the different anatomical subsites (oropharyngeal: 4.9 years, oral cavity: 5.6 years, hypopharynx & larynx: 5.9 years).
Table 1:
Number of individuals diagnosed with oropharyngeal, oral cavity, or hypopharyngeal/laryngeal squamous cell carcinomas in the SEER Cancer Registry (1973–2015) by covariate and number of people at risk (i.e., not dead or censored) 5, 10, and 15 years after diagnosis.
| Covariate | At diagnosis | 5 years | 10 years | 15 years | ||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | |
| All | 180,434 | 69,469 | 33,066 | 13,722 | ||||
| Age | ||||||||
| <50 | 24,386 | 13.5% | 12,309 | 17.7% | 7,317 | 22.1% | 3,661 | 26.7% |
| 50–59 | 52,689 | 29.2% | 22,372 | 32.2% | 11,406 | 34.5% | 5,027 | 37.0% |
| 60–69 | 55,881 | 31.0% | 21,348 | 30.7% | 9,746 | 29.5% | 3,873 | 28.2% |
| 70+ | 47,478 | 26.3% | 13,440 | 19.3% | 4,597 | 13.6% | 1,161 | 8.5% |
| Sex | ||||||||
| Male | 135,416 | 75.1% | 51,986 | 74.8% | 24,503 | 74.1% | 9,964 | 72.6% |
| Female | 45,018 | 24.9% | 17,483 | 25.2% | 8,563 | 25.9% | 3,758 | 27.4% |
| Race | ||||||||
| White | 150,160 | 83.2% | 59,845 | 86.1% | 28,565 | 86.4% | 11,870 | 86.5% |
| Black | 21,563 | 12.0% | 6,263 | 9.0% | 2,818 | 8.5% | 1,107 | 8.1% |
| Other/unknown | 8,711 | 4.8% | 3,361 | 4.8% | 1,683 | 5.1% | 745 | 5.4% |
| Anatomical site | ||||||||
| Oropharynx | 60,859 | 33.7% | 21,579 | 31.1% | 9,110 | 27.6% | 2,943 | 21.4% |
| Oral cavity | 48,834 | 27.1% | 18,444 | 26.5% | 8,592 | 26.0% | 3,881 | 28.3% |
| Hypopharynx & larynx | 70,741 | 39.2% | 29,446 | 42.4% | 15,004 | 45.4% | 6,898 | 50.3% |
| Tumor stage | ||||||||
| Localized | 47,790 | 26.5% | 27,098 | 39.0% | 15,829 | 47.9% | 7,854 | 57.2% |
| Regional | 80,653 | 44.7% | 28,422 | 40.9% | 13,237 | 40.0% | 4,853 | 35.4% |
| Distant | 19,212 | 10.6% | 3,356 | 4.8% | 1,230 | 3.7% | 524 | 3.8% |
| Unstaged | 32,779 | 18.2% | 10,593 | 15.2% | 2,770 | 8.4% | 599 | 4.4% |
| Year of diagnosis | ||||||||
| 1973–1984 | 24,863 | 13.8% | 10,998 | 15.8% | 7,093 | 21.5% | 4,482 | 32.7% |
| 1985–1999 | 39,461 | 21.9% | 18,504 | 26.6% | 12,042 | 36.4% | 7,840 | 57.1% |
| 2000–2015* | 116,110 | 64.4% | 39,967 | 57.5% | 13,931 | 42.1% | 1,400 | 10.2% |
More recent diagnoses have not yet made it to 5, 10, or 15 years after diagnosis.
Statistical analysis
We consider six covariates available in the SEER registry: tumor stage at diagnosis, age at diagnosis, sex, race, anatomical site (oropharynx, oral cavity, hypopharynx/larynx), and year of diagnosis. We use SEER historic stage (localized, regional, distant) to be consistent across the 1973–2015 time period. Because we consider long-term survival, we use a competing risks framework [27–29] to acknowledge that the underlying at-risk population is changing due to mortality from causes other than the disease. This framework can be simply expressed with a multistate model (Figure 1a). In the presence of competing risks, the Kaplan-Meier estimator for standard survival analyses is not appropriate because censoring is no longer uninformative [27, 28]. Instead, we estimate the cumulative incidence in each state with an Aalen–Johansen estimator [30] (Figure 1b).
Figure 1:

a). A multistate model for a competing risks framework acknowledging two mutually exclusive (i.e., competing) endpoints. We use cubic B-splines to estimate the time-dependent effects of baseline covariates on the hazard of cause-specific and other mortality. b) Empirical fraction (cumulative incidence) of head and neck cancer patients who are alive, have died of their cancer, or have died of other causes, as a function of time since diagnosis. c) Empirical fraction (cumulative incidence) of patients diagnosed with head and neck cancer who have died from that cancer, stratified by tumor stage. Those who have not died of their cancer may have died from other causes or are alive.
While these multistate cumulative incidence estimates are empirical, we also implement cause-specific Cox regression to estimate the impact of covariates on the hazard. Because hazard models estimate instantaneous rates, we do not need to account for competing risks unless we want to use the hazard model to estimate overall survival (both the cancer and other-cause hazard models would need to be simultaneously integrated to model cumulative incidence) [31]. Time-dependent effects have been used to relax the proportional hazards assumption of Cox regression [32], allowing us to detect if certain covariates are important immediately after detection or across the entire postdiagnosis period. The hazard λ(t) is given as a function of the baseline hazard λ0(t) and time-varying coefficient β(t) and fixed covariate x, λ(t) = λ0(t)eβ(t)x. Stratification by another covariate allows separate baseline hazards to be computed for each strata j, λj(t) = λ0,j(t)eβ(t)x, while the estimate values of β are constant across strata. Here, we stratify by tumor stage because there are substantial differences in the baseline hazards by tumor stage (Figure 1c). As in the traditional Cox model, a non-parametric Breslow estimator is used to calculate the Cox partial likelihood.
Previous work with time-dependent coefficients has been largely limited to basic functions, incorporating linear effects or step functions. Here we implement a natural cubic spline estimator. Splines are piece-wise polynomials that are smoothly joined: two n-degree polynomials are smoothly joined at a point known as a knot if the functions and their n−1 derivatives are continuous at that point. Here we use cubic B-splines with an intercept [33, 34]. Knots were chosen by quintiles of the times of cancer death, namely 6, 12, 22, and 50 months, with boundary knots at 1 and 477 months, but we constrain presentation of results to a 15-year period of interest. All Cox regression models are stratified by tumor stage (localized/regional/distant/unstaged). The other five cancer survival factors are model variables: anatomical site, age at diagnosis, sex, race, and year of diagnosis. We also run the models separately for oropharyngeal and non-oropharyngeal cancer patients, dropping anatomical site as a variable. We implement the multivariable, cause-specific Cox regression using a stochastic gradient ascent method [35, 36] in R (v3.6.1). Confidence intervals for the spline effects are calculated as in [37].
Results
In the following plots (Figures 2–4), we show the fraction of patients diagnosed with head and neck cancer who have died of the disease as a function of time since diagnosis (i.e., cumulative incidence); these are empirical results using the Aalen–Johanson estimator. Below these plots, we also show the corresponding estimated time-dependent hazard ratio—i.e., how much more likely someone is to die of cancer at that time than someone in the reference group; these are results from the Cox models with time-varying effects. All effects on cancer death were significantly time-varying (p <0.001).
Figure 2:
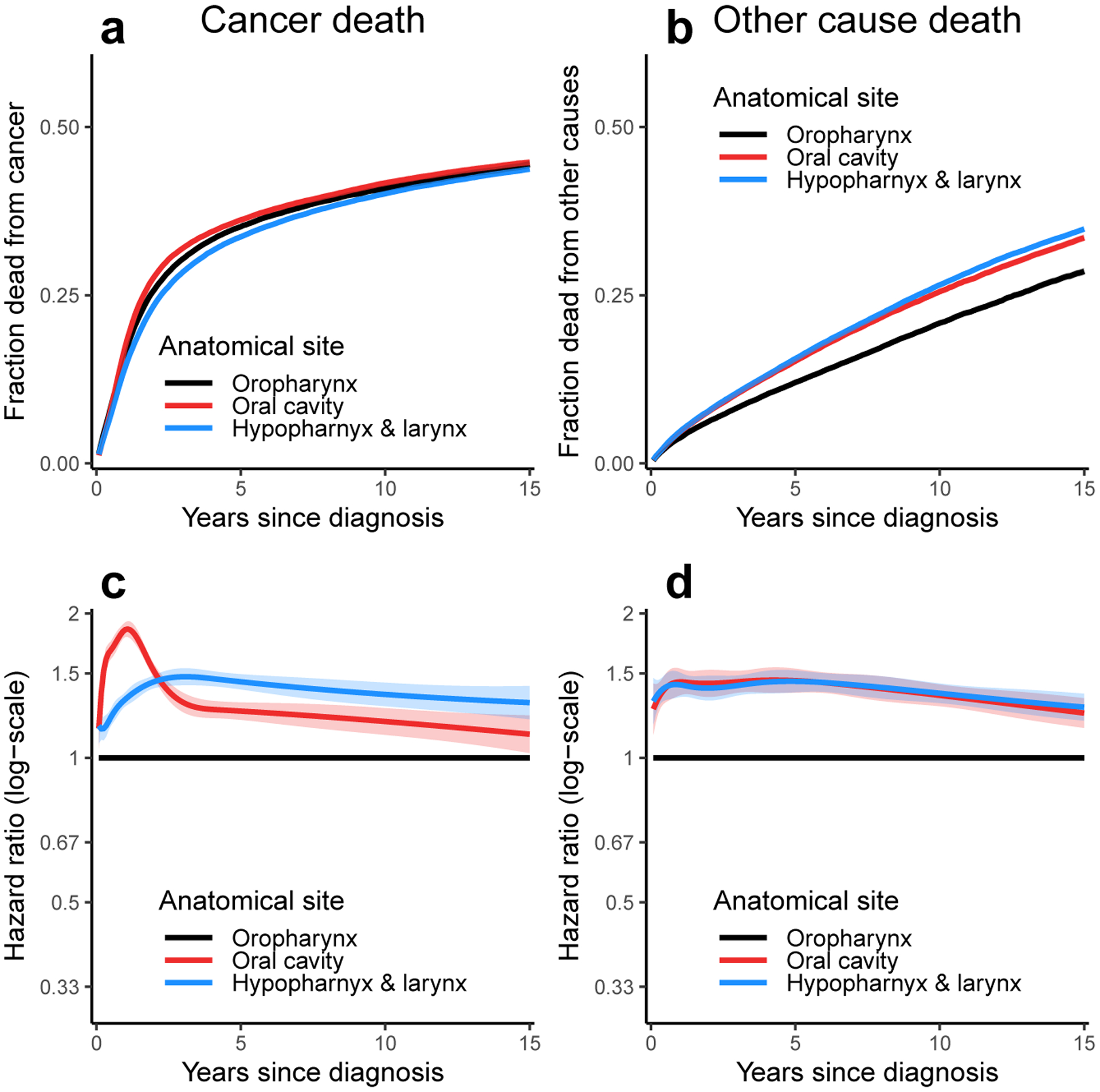
Fraction of patients (i.e., cumulative incidence) diagnosed with head and neck cancer who have died from a) that cancer and b) other causes as a function of time since diagnosis for each anatomical site. Time-dependent hazard ratios (eβ(t)x) for c) cancer death and d) other cause death for each anatomical site in multivariable models stratified by tumor stage. The ribbons in all plots represent 95% confidence intervals for the estimate; confidence intervals for the cumulative incidence plots may be obscured by line thickness.
Figure 4:

Fraction of patients (i.e., cumulative incidence) diagnosed with head and neck cancer who have died from that cancer as a function of time since diagnosis for a) all head and neck cancers, b) oropharyngeal cancers, and c) oral cavity and hypopharyngeal/laryngeal cancers. Time-dependent hazard ratios (eβ(t)x) for cancer death for year of diagnosis for d) all head and neck cancers, e) oropharyngeal cancers and f) oral cavity and hypopharyngeal/laryngeal cancers in multivariable models (adjusting for sex, race, year of diagnosis, and anatomical site) stratified by tumor stage. The ribbons in all plots represent 95% confidence intervals for the estimate; confidence intervals for the cumulative incidence plots may be obscured by line thickness.
We see that the empirical fraction of patients who die from their cancers does not differ dramatically by anatomical sites (Figure 2a). In part, this result reflects the improved survival of oropharyngeal cancer patients despite their tumors being more advanced overall (among oropharyngeal cancer patients with staged cancers 13% were localized, 69% were regional, and 17% were distant compared to 44% localized, 45% regional, and 10% distant for non-oropharyngeal cancer patients). However, we shall also see that because of the competing risk of other death, important underlying differences are masked when considering cancer-specific survival on its own. Patients with cancer of the oral cavity or of the hypopharynx/larynx are more likely to die of other causes than patients with oropharyngeal cancer (Figure 2b). When we examine the time-dependent hazard ratios (which take tumor stage into account through baseline hazard stratification) for cancer death (Figure 2c) and other-cause death (Figure 2d), we see that patients with oral cavity and hypopharynx/larynx cancers have a higher hazard of both cancer death and death from other causes. The relative hazard of death due to oral cavity cancer in particular appears to peak in the 1–2 years following diagnosis, possibly due to recurrence. The relative hazard of death due to other causes is similarly high for oral cavity and hypopharyngeal/laryngeal cancer patients; a larger fraction of these cancers may be caused by tobacco exposure, which can cause myriad other morbidities and result in higher overall mortality. The higher hazards of both death due to cancer and to other causes together result in the empirical fraction seen in Figure 2a; that is, while the hazard of cancer death is higher in the non-oropharyngeal head and neck cancer groups, there are also fewer patients alive who can die of cancer.
We similarly consider the time-varying effect of age of diagnosis, sex, race, and year of diagnosis on cancer survival (Figure 3). As expected, survival decreases with age at diagnosis (Figure 3a, d). Although the effect of age is substantial at the time of diagnosis, it appears to attenuate dramatically over the first 1–2 years before stabilizing or slowly increasing in magnitude again. This means that younger patients have an initially lower likelihood of death from cancer but that the rate of cancer death accelerates after the first year. When considering sex, the hazard of cancer death is lower for men than women in the first 1–2 years but is the same after (Figure 3b, e). A more substantial difference is seen by race, with higher hazard rates in black than white patients (Figure 3c, f). This disparity is initially high and attenuates slightly over subsequent decades. Corresponding results for other cause mortality are shown in the supplemental material.
Figure 3:
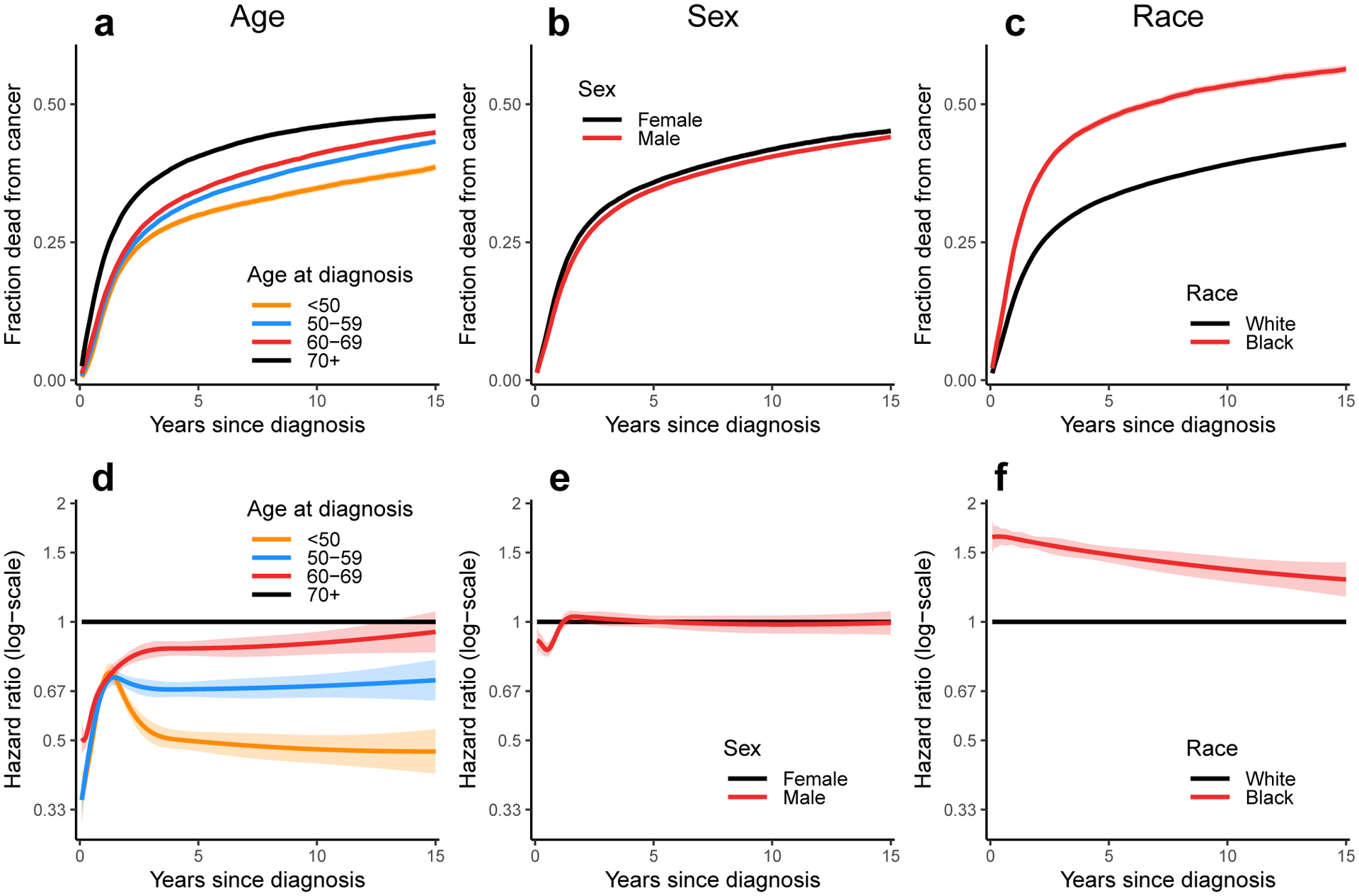
Fraction of patients (i.e., cumulative incidence) diagnosed with head and neck cancer who have died from that cancer as a function of time since diagnosis for a) age at diagnosis, b) sex, and c) race. Time-dependent hazard ratios (eβ(t)x) for cancer death for d) age at diagnosis, e) sex, and f) race in multivariable models (adjusting for sex, race, year of diagnosis, and anatomical site) stratified by tumor stage. The ribbons in all plots represent 95% confidence intervals for the estimate; confidence intervals for the cumulative incidence plots may be obscured by line thickness.
Finally, we see a moderate impact of year of diagnosis (Figure 4a), with head and neck tumors diagnosed after 2000 associated with lower rates of death due to cancer, particularly after the first year post-diagnosis. While the impact of age at diagnosis, sex, and race are largely qualitatively similar for oropharyngeal and non-oropharyngeal cancers (with covariates generally having a stronger effect on the hazard of cancer death for oropharyngeal cancer; see supplemental material), there is a dramatic difference between the two groups in survival by year of diagnosis (oropharyngeal in Figure 4b and non-oropharyngeal in 4c). For the oropharyngeal cancers, survival has improved dramatically over time, with hazard of cancer death for those diagnosed in 2000–15 up to less than half of that for those diagnosed in 1973–1984 (Figure 4e). For the non-oropharyngeal head and neck cancers, on the other hand, survival has been relatively constant over time (Figure 4f).
Discussion
Here, we assessed how the cause-specific hazard of death from head and neck cancer changes over the 15 years post diagnosis. Our results demonstrate that many factors in head and neck cancer survival do not have constant effect across a patient’s post-diagnosis trajectory. Indeed, we found that the effects of baseline age and sex attenuate in the first few years after diagnosis, while the effects of race and year of diagnosis attenuated more slowly over a 15-year time frame. These results indicate that the proportional hazards assumptions made in Cox models with constant effects are violated in many cases and may result in misleading estimates. However, the need to investigate time-varying effects in cancer survival is only recently being appreciated (e.g., [38, 39]). Because clinical decisions are often informed by survival prognosis, it is essential to accurately characterize both how and when prognostic factors are associated with survival. As individualized risk calculators continue to be developed and refined (e.g., [40, 41], time-dependent effects should be considered during model development. For example, risk-prediction models could integrate the time-dependent cancer-specific and other-cause mortality hazard rates specific to a patient’s sociodemographic and tumor characteristics to create predictions of the likelihood of survival, cancer death, and other-cause death over time.
We know that HPV status is predictive of cancer survival and that HPV is the etiological agent of a larger fraction of oropharyngeal than oral cavity or hypopharyngeal/laryngeal cancers [11, 13]. Our analysis is consistent with the prior literature: we found that patients with oropharyngeal cancers have a lower cancer-specific death rate (Figure 2c). There has been a modest improvement from 1973 to 2015 in the number who will die from any head and neck cancer within 15 years (Figure 4). But, as we showed, this improvement is predominantly due to the lower hazard for oropharyngeal cancers diagnosed in 2000–15 compared to those diagnosed in 1973–84. The improvement in survival, then, may be more a reflection of changing etiologies at the population level [6]—that is, an increase in the proportion of cancers caused by HPV than caused by tobacco, alcohol, or other risk factors—than improvements in care in general.
Despite the difference in cancer-specific death rates across anatomical sites, the overall probability of dying from cancer is similar across the anatomical sites (Figure 2a) because the other-cause mortality is also higher in the non-oropharyngeal head and neck cancers (Figure 2b). Our results emphasize the importance of the competing risks perspective in cancer survival, particularly for cancers with a comparatively low initial hazard rate. Cancer specialists may need to consider patients’ health more broadly—such as by encouraging smoking cessation [42, 43]—particularly when patients are not seeing a primary care provider for general preventive care.
We found a notable difference in the hazard rate of death from head and neck cancer in the first year after diagnosis for older patients compared to younger patients, which may be a result of the fact that older patients are less likely to be prescribed or able to complete the aggressive treatments (surgery or high-dose cisplatin chemotherapy) that are standard in head and neck cancer. This analysis expands on previous work that considered the prognostic significance of age in oropharyngeal cancer [44].
Further, we found that men are overall less likely to die from their head and neck cancer and that this difference is largely due to lower hazard in the first two years after diagnosis (and is not due to difference in other cause mortality). When considering the sites separately (see supplementary material), we see that this effect is only seen for oropharyngeal cancers. Previous work has also suggested that there appears to be no sex differences in survival for non-oropharyngeal sites [45]. However, the same previous analysis found female oropharyngeal cancer patients had better, rather than worse, survival [45]. A second study that did not distinguish between oropharyngeal and non-oropharyngeal sites found a non-significant advantage to being female [18]. More work is needed to determine the effect of sex on head and neck cancer survival. It possible that conflicting results may be due to different prevalence of HPV status by sex in the studied populations.
Racial disparities are well documented in head and neck cancer survival [18, 45–47]. In our analysis, the hazard ratio plots indicate an initial hazard ratio of more than 1.5 for blacks vs whites, which only slightly diminishes over time. Because this hazard ratio considers tumor stage and adjusts for age and other covariates, our interpretation is that this may be a genuine disparity, due to access to and quality of care. The differences in the hazard in the first few years after diagnosis may suggest that on average white patients tend to receive better treatment and care than black patients. The fact that the differences persist at longer times after treatment may also reflect that on average white patients tend to have better access to follow-up care and better follow-up care than black patients. Other work has suggested that racial disparities are attributable almost entirely to differential HPV status (with black patients less likely to have HPV+ tumors) [45, 46]. Although our work cannot directly address this point, we find that racial disparities are larger among oropharyngeal cancer patients but still present for the oral cavity and hypopharyngeal/laryngeal cancer patients (see supplementary material). These results are consistent with differences in HPV status for oropharyngeal cancer but suggest that HPV status is not sufficient to completely account for survival disparities.
In these data, fewer than 50% of patients diagnosed with head and neck cancer die of that cancer. Of those who do die of their cancer, 50% will die within the first two years. Head and neck cancer is generally considered to be curable in the sense that if the tumor does not recur within the first five years after treatment, it is unlikely to recur later [19]. This fact is reflected in the cumulative incidence plots which stop increasing sharply after about four years. It is also notable, and perhaps surprising, that in this data set there are many deaths due to head and neck cancer after 10 years, so the cumulative incidence curves never completely level off, even after 15 years. These later deaths may be caused by second primary malignancies but be attributed to the first primary [24]. Second primaries are relatively common in head and neck, possibly because of field cancerization [48] or continued smoking. Cumulative incidence of second head and neck primaries may be as high as 25% in 10 years [49].
There are two main limitations to this study. First, the available SEER data do not currently have important risk factor data, including HPV-status (except for a subset of patients in the SEER Head and Neck with HPV Status Database [13]) and smoking- and alcohol-use histories. While anatomical site is a first level proxy for head and neck cancer etiology, both HPV+ and HPV- tumors can be found at each head and neck site but with different prevalence. Hence, while this analysis is revealing and suggestive, any true effects of etiology are likely to be attenuated. Future analysis could investigate survival patterns for subgroups of the anatomical sites to possibly derive different, functional groupings of subsites. Next, while smoking is a strong risk factor for developing head and neck cancer, its impact on disease progression after diagnosis is less well defined. It is well known that smoking history and continued smoking is associated with worse cancer outcomes (both for all cancer and for head and neck specifically) [50–52]. Because of smoking’s association with lung cancer and other lung diseases, it will have a strong association with the hazard of death from other causes, but the strength of this association is less clear. Similarly, comorbidities would be expected to be strongly associated with the hazard of death from other causes. Comorbidities also impact the hazard of death from head and neck cancer because they can preclude candidacy for surgery or chemotherapy, including high-dose cisplatin. However, since SEER does not provide these data, these interesting questions cannot be addressed here.
The second limitation is that long-term survival is subject to improper cause-of-death ascertainment and coding. Although SEER has developed methodologies to classify the cause of death into cancer related and other cause [24], the classifications rely on physician cause-of-death coding, which is subject to human error, may have institutional or regional idiosyncrasies, and may be less likely to be linked to the cancer as time since diagnosis increases. In the case of multiple tumors at the same or different sites in the same organ system, a cancer cause of death is attributed to the primary tumor; this methodology may account for the continued cancer-specific deaths more than 10–15 years after diagnosis.
In summary, we find that although oropharyngeal and non-oropharyngeal cancer patients have similar probabilities of dying due to their cancer over time, this result belies the lower overall survival of non-oropharyngeal cancer patients. Moreover, cancer-specific survival has improved for oropharyngeal cancer patients but not non-oropharyngeal cancer patients since 2000, likely due to an increasing fraction of HPV-attributable cancers among the oropharyngeal cancer patients. We find that the effects of predictors of head and neck cancer-specific survival—including age, sex, and race—are not constant over the post-diagnosis period, suggesting that future cancer survival analyses and risk calculators should take time-dependent effects into account.
Supplementary Material
Acknowledgments
This work was supported by National Institutes for Health grants U01CA182915 (AB, ME, RM) and U54CA229974 (AB, RM) and by the University of Michigan Rogel Cancer Center P30CA046592 (AB, KH, SC, MB, JT). The study sponsors had no role in study design; collection, analysis, and interpretation of data; writing the report; nor the decision to submit the report for publication.
Footnotes
Conflict of interest: The authors declare no potential conflicts of interest.
References
- [1].Shanthi Marur, Gypsyamber D Souza, Westra William H, Forastiere Arlene a. HPV-associated head and neck cancer: a virus-related cancer epidemic. The Lancet Oncology. 2010;11:781–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Nadarajah Vigneswaran, Williams Michelle D.. Epidemiologic Trends in Head and Neck Cancer and Aids in Diagnosis Oral and Maxillofacial Surgery Clinics of North America. 2014;26:123–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rettig Eleni M., D’Souza Gypsyamber. Epidemiology of Head and Neck Cancer Surgical Oncology Clinics of North America. 2015;24:379–396. [DOI] [PubMed] [Google Scholar]
- [4].Leemans C Reń e, Snijders Peter J.F., Brakenhoff Ruud H.. The molecular landscape of head and neck cancer Nature Reviews Cancer. 2018;18:269–282. [DOI] [PubMed] [Google Scholar]
- [5].Nagayasu Egawa, Kiyofumi Egawa, Heather Griffin, John Doorbar. Human Papillomaviruses; Epithelial Tropisms, and the Development of Neoplasia Viruses. 2015;7:3863–3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chaturvedi Anil K., Engels Eric A., Pfeiffer Ruth M., et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. Journal of Clinical Oncology. 2011;29:4294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Walline Heather M., Komarck Chris, McHugh Jonathan B., et al. High-risk human papillomavirus detection in oropharyngeal, nasopharyngeal, and oral cavity cancers comparison of multiple methods JAMA Otolaryngology - Head and Neck Surgery. 2013;139:1320–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gillison Maura L., Chaturvedi Anil K., Anderson William F., Fakhry Carole. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma Journal of Clinical Oncology. 2015;33:3235–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Combes Jean Damien, Franceschi Silvia. Role of human papillomavirus in non-oropharyngeal head and neck cancers 2014. [DOI] [PubMed] [Google Scholar]
- [10].Cathy Ndiaye, Marisa Mena, Laia Alemany, et al. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: a systematic review and meta-analysis The Lancet Oncology. 2014;15:1319–1331. [DOI] [PubMed] [Google Scholar]
- [11].Gillison Maura L, Alemany Laia, Snijders Peter J F, et al. Human papillomavirus and diseases of the upper airway: head and neck cancer and respiratory papillomatosis. Vaccine. 2012;30 Suppl 5:F34–54. [DOI] [PubMed] [Google Scholar]
- [12].Salazar Christian R., Anayannis Nicole, Smith Richard V., et al. Combined P16 and human papillomavirus testing predicts head and neck cancer survival International Journal of Cancer. 2014;135:2404–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mahal Brandon A., Catalano Paul J., Haddad Robert I., et al. Incidence and Demographic Burden of HPV-Associated Oropharyngeal Head and Neck Cancers in the United States Cancer Epidemiology Biomarkers & Prevention. 2019:617–632. [DOI] [PubMed] [Google Scholar]
- [14].Cinciripini Paul M, Karam-Hage Maher, Kypriotakis George, et al. Association of a comprehensive smoking cessation program with smoking abstinence among patients with cancer JAMA Network Open. 2019;2:e1912251–e1912251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Justin Smith, Domenico Nastasi, Reece Tso, Venkat Vangaveti, Bronia Renison, Madhavi Chilkuri. The effects of continued smoking in head and neck cancer patients treated with radiotherapy: A systematic review and meta-analysis Radiotherapy and Oncology. 2019;135:51–57. [DOI] [PubMed] [Google Scholar]
- [16].O’Sullivan Brian, Huang Shao Hui, Jie Su, et al. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): a multicentre cohort study The Lancet Oncology. 2016;17:440–451. [DOI] [PubMed] [Google Scholar]
- [17].Lydiatt William M, Patel Snehal G, O’Sullivan Brian, et al. Head and neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual CA. 2017;67:122–137. [DOI] [PubMed] [Google Scholar]
- [18].Choi Seung Hee, Terrell Jeffrey E., Fowler Karen E., et al. Socioeconomic and other demographic disparities predicting survival among head and neck cancer patients PLOS One. 2016;11:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chang Jer Hwa., Wu Chia Che, Yuan Kevin Sheng Po, Wu Alexander T.H., Wu Szu Yuan. Locoregionally recurrent head and neck squamous cell carcinoma: Incidence, survival, prognostic factors, and treatment outcomes Oncotarget. 2017;8:55600–55612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jarrard Goodwin W.. Salvage Surgery for Patients With Recurrent Squamous Cell Carcinoma of the Upper Aerodigestive Tract: When Do the Ends Justify the Means? The Laryngoscope. 2000;110:1–18. [DOI] [PubMed] [Google Scholar]
- [21].Jayaram Sharan Chakkyath, Muzaffar Sayed Jameel, Ikhlaaq Ahmed, Jagtar Dhanda, Vinidh Paleri, Hisham Mehanna. Efficacy, outcomes, and complication rates of different surgical and nonsurgical treatment modalities for recurrent/residual oropharyngeal carcinoma: A systematic review and meta-analysis Head & Neck. 2016;38:1855–1861. [DOI] [PubMed] [Google Scholar]
- [22].Janz Tyler A., Graboyes Evan M., Nguyen Shaun A., et al. A Comparison of the NCDB and SEER Database for Research Involving Head and Neck Cancer Otolaryngology - Head and Neck Surgery. 2019;160:284–294. [DOI] [PubMed] [Google Scholar]
- [23].Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2017 Sub (1973–2015 varying) - Linked To County Attributes - Total U.S., 1969–2016 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2018, based on the November 2017 submission. 2018. [Google Scholar]
- [24].Nadia Howlader, Ries Lynn A G, Mariotto Angela B., Reichman Marsha E., Ruhl Jennifer, Cronin Kathleen A.. Improved estimates of cancer-specific survival rates from population-based data Journal of the National Cancer Institute. 2010;102:1584–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chaturvedi Anil K, Engels Eric A, Anderson William F, Gillison Maura L. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. Journal of Clinical Oncology. 2008;26:612–9. [DOI] [PubMed] [Google Scholar]
- [26].Chaturvedi Anil K., Anderson William F., Lortet-Tieulent Joannie, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers Journal of Clinical Oncology. 2013;31:4550–4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Satagopan JM, Ben-Porat L, Berwick M, Robson M, Kutler D, Auerbach AD. A note on competing risks in survival data analysis British Journal of Cancer. 2004;91:1229–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bernhard Haller, Georg Schmidt, Kurt Ulm. Applying competing risks regression models: An overview Lifetime Data Analysis. 2012;19:33–58. [DOI] [PubMed] [Google Scholar]
- [29].Nadia Howlader, Mariotto Angela B., Woloshin Steven, Schwartz Lisa M.. Providing clinicians and patients with actual prognosis: Cancer in the context of competing causes of death Journal of the National Cancer Institute Monographs. 2014;49:255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Terry Therneau, Cynthia Crowson, Elizabeth Atkinson. Multi-state models and competing risks https://cran.rproject.org/web/packages/survival/vignettes/compete.pdf 2017.
- [31].Prentice RL, Kalbfleisch JD, Peterson AV Jr, Flournoy N, Farewell VT, Breslow NE. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34:541–54 [PubMed] [Google Scholar]
- [32].Terry Therneau, Cynthia Crowson, Elizabeth Atkinson. Using Time Dependent Covariates and Time Dependent Coefficients in the Cox Model https://cran.r-project.org/web/packages/survival/vignettes/timedep.pdf 2017.
- [33].De Boor Carl. A practical guide to splines. Springer; 1978. [Google Scholar]
- [34].Wenjie Wang, Yan Jun. Package ‘splines2’ https://cran.r-project.org/web/packages/splines2/splines2.pdf 2018.
- [35].Kingma Diederik P, Ba Jimmy. Adam: A method for stochastic optimization arXiv preprint arXiv:1412.6980 2014. [Google Scholar]
- [36].He Kevin, Zhu Ji, Kang Jian, Li Yi. Minorization-Maximization-based Steepest Ascent for Large-scale Survival Analysis with Time-Varying Effects: Application to the National Kidney Transplant Dataset arXiv preprint arXiv:1912.12353 2019. [Google Scholar]
- [37].Sylvain Durrleman, Richard Simon. Flexible regression models with cubic splines Statistics in Medicine. 1989;8:551–561. [DOI] [PubMed] [Google Scholar]
- [38].Andreassen Bettina Kulle, Grimsrud Tom Kristian, Haug Erik Skaaheim. Bladder cancer survival: Women better off in the long run European Journal of Cancer. 2018;95:52–58. [DOI] [PubMed] [Google Scholar]
- [39].Mozumder Sarwar Islam, Rutherford Mark, Lambert Paul. Direct likelihood inference on the cause-specific cumulative incidence function: A flexible parametric regression modelling approach Statistics in Medicine. 2018;37:82–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wang Samuel J., Wissel Amanda R., Ord Celine B., et al. Individualized estimation of conditional survival for patients with head and neck cancer Otolaryngology - Head and Neck Surgery. 2011;145:71–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Emerick Kevin S., Leavitt Erica R., Michaelson James S., Diephuis Bradford, Clark John R., Deschler Daniel G.. Initial clinical findings of a mathematical model to predict survival of head and neck cancer Otolaryngology Head and Neck Surgery. 2013;149:572–578. [DOI] [PubMed] [Google Scholar]
- [42].Paul Cinciripini. Smoking Cessation in Patients With Cancer: Treatment Advances and the Oncologist’s Role Journal of the National Comprehensive Cancer Network. 2017;15:748–750. [DOI] [PubMed] [Google Scholar]
- [43].Jacek Jassem. Declaration from IASLC: Tobacco Cessation After Cancer Diagnosis https://www.iaslc.org/AboutIASLC/News-Detail/declaration-from-iaslc-tobacco-cessation-after-cancer-diagnosis. 2019.
- [44].Camilon PR, Stokes William A., Nguyen Shaun A., Lentsch Eric J.. The prognostic significance of age in oropharyngeal squamous cell carcinoma Oral Oncology. 2014;50:431–436. [DOI] [PubMed] [Google Scholar]
- [45].Carole Fakhry, Westra William H., Wang Steven J., et al. The prognostic role of sex, race, and human papillomavirus in oropharyngeal and nonoropharyngeal head and neck squamous cell cancer Cancer. 2017;123:1566–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kathleen Settle, Posner Marshall R., Schumaker Lisa M., et al. Racial survival disparity in head and neck cancer results from low prevalence of human papillomavirus infection in black oropharyngeal cancer patients Cancer Prevention Research. 2009;2:776–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ragin Camille C., Langevin Scott M., Marzouk Mark, Grandis Jennifer, Taioli Emanuela. Determinants of head and neck cancer survival by race Head & Neck. 2011;33:1092–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ryser Marc D., Lee Walter T., Ready Neal E., Leder Kevin Z., Foo Jasmine. Quantifying the dynamics of field cancerization in tobacco-related head and neck cancer: A multiscale modeling approach Cancer Research. 2016;76:7078–7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Lee Dong Hwan, Roh Jong Lyel, Baek Seunghee, et al. Second Cancer Incidence, Risk Factor, and Specific Mortality in Head and Neck Squamous Cell Carcinoma Otolaryngology - Head and Neck Surgery. 2013;149:579–586. [DOI] [PubMed] [Google Scholar]
- [50].Health US Department, Services Human, others. The health consequences of smoking50 years of progress: a report of the Surgeon General 2014. [Google Scholar]
- [51].Sharp Linda, McDevitt Joseph, Carsin Anne-Elie, Brown Christopher, Comber Harry. Smoking at diagnosis is an independent prognostic factor for cancer-specific survival in head and neck cancer: findings from a large, population-based study Cancer Epidemiology and Prevention Biomarkers. 2014;23:2579–2590. [DOI] [PubMed] [Google Scholar]
- [52].Peterson Lisa A., Bellile Emily L., Wolf Gregory T., et al. Cigarette use, comorbidities, and prognosis in a prospective head and neck squamous cell carcinoma population Head & Neck. 2016;38:1810–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


