Abstract
Background
Transthyretin cardiac amyloidosis (ATTR-CA) is an under-recognized cause of heart failure with preserved ejection fraction. In the United States, the valine-to-isoleucine substitution (Val122Ile) is the most common inherited variant. Data on sex differences in presentation and outcomes of Val122Ile ATTR-CA are lacking.
Methods and Results
In a retrospective, single-center study of 73 patients diagnosed with Val122Ile ATTR-CA between 2001–2018, sex differences in clinical and echocardiographic data at the time of diagnosis were evaluated. Pressure-volume analysis using non-invasive single beat techniques was used to compare chamber performance.
Compared to men (n=46), women (n=27) were significantly older at diagnosis (76 years vs 69 years, p<0.001). End-systolic pressure-volume relationship (5.1mmHg*m2/mL vs 4.3 mmHg*m2/mL, p = 0.27), arterial elastance (5.5mmHg*m2/mL vs 5.7mmHg*m2/mL, p = 0.62), and left ventricular capacitance were similar between sexes as was pressure-volume areas indexed to a left ventricular end-diastolic pressure of 30 mmHg, a measure of overall pump function. Three-year mortality rates were also similar (34% vs 43%, p = 0.64).
Conclusions
Despite being significantly older at time of diagnosis with Val122Ile ATTR-CA, women have similar overall cardiac chamber function and rates of mortality to men, suggesting a less aggressive disease trajectory. These findings should be confirmed with longitudinal studies.
Keywords: cardiac amyloidosis, sex differences, pressure-volume loops, echocardiography
BACKGROUND
Across many cardiovascular conditions, including ischemic heart disease, heart failure, valvular disease, and hypertension, differences in presentation, progression, and outcomes between the sexes are well-recognized.1,2 These differences are driven by physiologic differences as well as various social, economic, and cultural factors.3 Within the heart failure population specifically, the incidence of disease is relatively similar between sexes. However, disease epidemiology is markedly different: women are more likely to develop heart failure with preserved ejection fraction as compared to men who have higher rates of heart failure with reduced ejection fraction.4–6 Further, in response to high afterload conditions such as aortic stenosis, women have less pathophysiologic hypertrophy and fibrosis, while men demonstrate increased burden of concentric and eccentric hypertrophy.7 Understanding the influence of sex on cardiovascular disease is important because it can lead to insights to improve outcomes for both women and men.
Transthyretin cardiac amyloidosis (ATTR-CA) is increasingly recognized as the most common cause of restrictive cardiomyopathy in older adults and is characterized by the deposition of misfolded transthyretin (TTR) protein in the myocardium.8,9 Destabilization of the protein may either be caused by age-related changes in wild-type disease (wtATTR) or inherited mutations in the TTR gene (hATTR). In the United States, the most common mutation observed is the valine-to-isoleucine substitution (Val122Ile).8,9 This mutation is primarily seen in individuals of Afro-Caribbean descent, and in the United States is estimated to be present in 3.4% of African Americans.10,11 WtATTR-CA is almost exclusively a disease of older men. ATTR-CA due to the Val122Ile variant, although inherited in an autosomal dominant fashion, also appears to disproportionally affect men, with women comprising 20–30% of population-based studies.11,12 It is unclear to what extent these differences in prevalence reflect biases in diagnosis as opposed to sex-specific differences in disease expression or penetrance.
While sex differences in the prevalence of Val122Ile associated ATTR-CA are well-established, little is known about whether the phenotype of the cardiomyopathy at presentation or long-term outcomes differ by sex. The purpose of this study was to investigate sex differences at diagnosis of Val122Ile ATTR-CA in clinical features and cardiac performance using non-invasive pressure volume analysis, and to compare long-term outcomes. It was hypothesized that women would have less severe disease with more preserved cardiac function at the time of diagnosis.
METHODS
Study Design, Variables, and Definitions
Patients who presented to the Center for Advanced Cardiac Care at Columbia University Irving Medical Center between 2001 and 2018 with clinically significant cardiac amyloidosis due to Val122Ile mutations were included in this analysis. The diagnosis of hATTR-CA was made by at least one of the following in the presence of the Val122Ile mutation: (1) endomyocardial biopsy-proven congophilic deposits with confirmation of TTR as the precursor protein, (2) histologic documentation of congophilic deposition in at least one noncardiac organ and echocardiographic evidence of infiltrative cardiomyopathy without another cause of left ventricular hypertrophy, (3) positive Technetium-99m pyrophosphate (99mTc-PYP) cardiac scintigraphy defined by grade 2 or 3 myocardial uptake or a heart to contralateral ratio of >1.5 without evidence of a monoclonal protein in serum or urine, and (4) cardiac magnetic resonance or echocardiographic imaging consistent with ATTR-CA without another cause of increased wall thickness.13 Evidence for TTR as the precursor protein causing amyloid was confirmed by either immune-histochemistry or mass spectroscopy.14,15 This study was approved by the Institutional Review Board at Columbia University.
Electrocardiographic and Echocardiographic Parameters
We reviewed electrocardiograms at the time of diagnosis for the presence of arrhythmias, conduction abnormalities, low QRS voltage, and pseudo-infarct (anterior or lateral) patterns. Standard measurements of cardiac size and function, and classification of left ventricular geometry per American Society of Echocardiography guidelines were obtained by Doppler transthoracic echocardiography at the time of diagnosis.16 Left ventricular volume and mass measurements were calculated using a formula described by Devereux et al.17 Left ventricular end-diastolic (LVEDV) and end-systolic volumes (LVESV) were extrapolated from linear end-diastolic and end-systolic chamber dimensions, respectively, using formulas from de Simone et al.18 Stroke volume was calculated as the difference between LVEDV and LVESV. Transmitral doppler recordings in the apical four-chamber view were used to calculate the mitral peak velocity of early filling (E). Early diastolic mitral annular velocity (e’) was determined from Tissue doppler imaging in the apical four-chamber view. The myocardial contraction fraction (the ratio of stroke volume to myocardial volume) was measured from linear dimensions in the parasternal long axis view as previously described.19
Non-Invasive Pressure-Volume Loop Analysis
In a subset of 56 patients with complete echocardiographic data available at the time of diagnosis, chamber performance was assessed using non-invasive, single-beat techniques to estimate pressure-volume indices.20 Patients that were not included in this analysis had echocardiograms performed outside of this institution by local providers, which were not available for review. Pressure-volume loops were constructed from three points on the pressure-volume plane, estimated from the brachial systolic blood pressure (SBP) and diastolic blood pressure (DBP), left ventricular end diastolic (LVEDV) and systolic (LVESV) volumes, and Doppler based estimates of the left ventricular end diastolic pressure (LVEDP). To account for the scaling relationship between cardiac chamber and body size, chamber volumes were indexed to body surface area (BSA).21,22 This was done by dividing both the LVEDV and LVESV by BSA. The end-systolic pressure (Pes) was estimated as 0.9*SBP. The LVEDP was estimated by 11.96 + 0.596*(E/e’).23 Cardiac chamber contractility (Res), represented by the end-systolic pressure volume relationship (ESPVR) in this case, was estimated by the ratio of end-systolic pressure to end-systolic volume in which V0 was assumed to be 0 ml.24 The end-diastolic pressure volume relationship (EDPVR) was characterized using a non-invasive single beat estimation described by Klotz et al, and was used to estimate end-diastolic volume at a pressure of 30 mmHg (V30), an index of ventricular capacitance.23 Arterial elastance (Ea), a measure of the hydraulic load of the arterial system imposed on the ventricle, was measured by dividing the Pes by stroke volume. The ratio of Ea/Res, a measure of ventricular-vascular coupling, was also calculated.24,25 Finally, to quantify and compare overall pump function between women and men, the areas between their respective EDPVR and ESPVR curves as a function of EDP were calculated. This area, termed the isovolumic pressure-volume area, indexes left ventricular performance by integrating both diastolic and systolic function, independent of afterload.26 A schematic demonstrating how the PV loops were constructed is provided in Supplemental Figure 1.
Outcomes
The outcome of interest was all-cause mortality. This was ascertained through review of the electronic medical record. For patients diagnosed after May 1, 2016, outcomes were censored at May 1, 2019. For all other patients, outcomes were censored at 3 years after the date of diagnosis.
Statistical Methods
Baseline characteristics, including clinical, electrocardiographic, and 2-dimensional echocardiographic data were compared between women and men. Variables were reported as means with standard deviations or medians with interquartile ranges if continuous and numbers with percentages if categorical. Differences between groups were tested for statistical significance using Wilcoxon rank-sum tests for continuous variables and for categorical variables, chi-squared tests or Fisher’s exact tests for expected values <10. Cochran-Armitage trend testing was used to evaluate trends in diagnosis patterns over the study period.
Survival curves for the primary end point of survival within 3 years after diagnosis were estimated by the Kaplan–Meier method. Mortality estimates with 95% confidence intervals (CIs) were derived from the life table. Difference in survival between groups was analyzed using logrank testing.
All tests were 2-tailed with a critical P value of 0.05. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
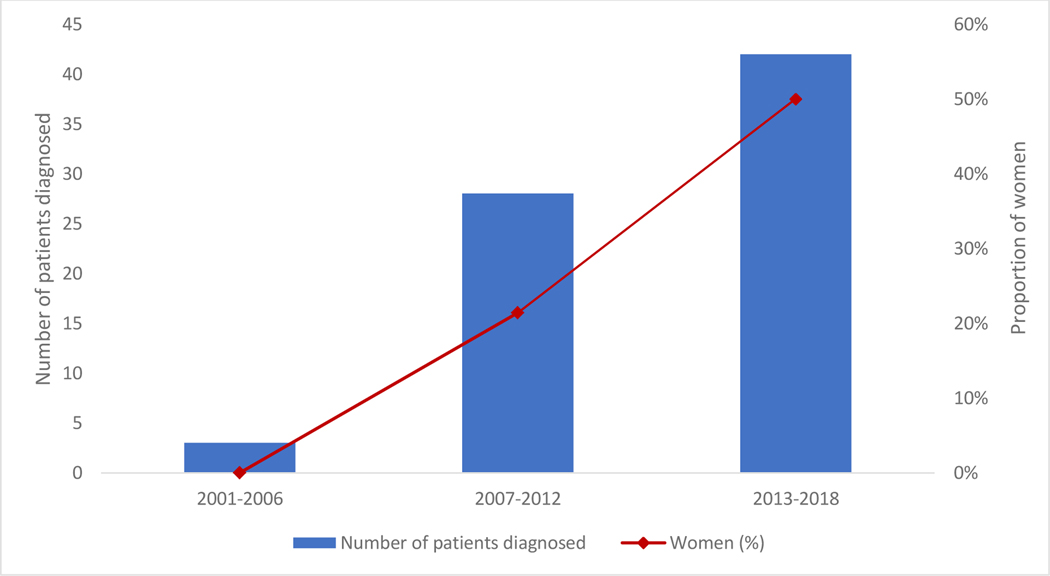
Over the study period, 73 patients (27 women and 46 men) presented with Val122Ile associated ATTR-CA. Baseline characteristics are summarized in Table 1. More than 90% (n = 68) of patients were diagnosed by biopsy or 99mTc-PYP cardiac scintigraphy. Overall, diagnosis of Val122Ile associated ATTR-CA increased over time, with approximately 60% (n = 42) of the study cohort diagnosed in the last 6 years of the 18-year study period. The proportion of women diagnosed also increased considerably over the study period, from 0% throughout the first third of the study period to 50% from 2013 to 2018 (p = 0.021, Figure 1).
Table 1.
Baseline characteristics of patients with Val122Ile ATTR-CA, by sex
| Women (n = 27) | Men (n = 46) | p value | |
|---|---|---|---|
| Clinical characteristics | |||
| Age at registry entry, y, mean (sd) | 76.4 (5.6) | 68.9 (7.77) | <0.0001 |
| Ethnicity, n (%) | |||
| Black | 26 (96.3) | 44 (95.6) | 0.89 |
| Other | 1 (3.7) | 2 (4.4) | |
| Systolic blood pressure, mmHg, mean (sd) | 115.6 (14.7) | 111.3 (18.1) | 0.13 |
| Diastolic blood pressure, mmHg, mean (sd) | 71.1 (10.6) | 70.1 (.1) | 0.77 |
| Mean arterial blood pressure, mean (sd) | 85.1 (10.8) | 83.0 (10.7) | 0.33 |
| Pulse pressure, mmHg, mean (sd) | 44.4 (11.2) | 41.2 (14.6) | 0.29 |
| Albumin, g/dL, mean (sd) | 3.9 (0.3) | 4.0 (0.4) | 0.38 |
| BMI, kg/m2, mean (sd) | 27.2 (6.2) | 26.2 (4.0) | 0.36 |
| Modified BMI, mean (sd) | 1,067 (248) | 1,058 (209) | 0.97 |
| BSA, m2, mean (sd) | 1.78 (0.22) | 1.99 (0.22) | <0.0001 |
| eGFR, mL/min/1.73m2, mean (sd) | 43.4 (12.6) | 46.9 (15.9) | 0.37 |
| NYHA class, n (%) | |||
| I | 0 (0) | 3 (6.5) | 0.20 |
| II | 9 (33.3) | 14 (30.4) | |
| III | 18 (66.7) | 25 (54.4) | |
| IV | 0 (0) | 4 (8.7) | |
| Year of diagnosis, n (%) | |||
| 2001–2006 | 0 (0) | 3 (6.5) | 0.0211 |
| 2007–2012 | 6 (22.2) | 22 (47.8) | |
| 2013–2018 | 21 (77.8) | 21 (45.6) | |
| Means of diagnosis, n (%) | |||
| EMB | 12 (44.4) | 32 (69.6) | 0.055 |
| Extra-cardiac biopsy | 0 (0) | 2 (4.4) | |
| 99mTc-PYP | 13 (48.2) | 9 (19.6) | |
| Imaging (MRI or TTE) | 2 (7.4) | 3 (6.5) | |
| History of carpal tunnel, n (%) | 13 (48.2) | 29 (63.0) | 0.021 |
| History of lumbar spinal stenosis, n (%) | 6 (22.2) | 9 (19.5) | 0.79 |
| Medications | |||
| Daily furosemide dose, mg, median [IQR]* | 40 [40–80] | 80 [40–100] | 0.022 |
| ACEi/ARB, n (%) | 14 (51.9) | 21 (45.7) | 0.81 |
| Beta-blocker, n (%) | 10 (37.0) | 24 (51.2) | 0.23 |
| Biomarkers† | |||
| BNP, pg/mL, median [IQR] | 740 [428–1092] | 816 [500–1652] | 0.60 |
| NT-proBNP, pg/mL, median [IQR] | 3,819 [2,227–9,406] | 2,098 [1,331–3,446] | 0.059 |
| Troponin T, ng/mL, median [IQR] | 0.06 [0.04–0.10] | 0.05 [0.01–0.11] | 0.45 |
| Troponin I, ng/mL, median [IQR] | 0.1 [0.05–0.17] | 0.2 [0.10–0.35] | 0.059 |
99mTc-PYP, Technetium-99m pyrophosphate cardiac scintigraphy; ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; BNP, brain natriuretic peptide; BSA, body surface area; dL, deciliter; eGFR, estimated glomerular filtration rate; EMB, endomyocardial biopsy; g, grams; IQR, interquartile range; min, minute; mL, milliliters; mmHg, millimeters of mercury; NT-proBNP, N-terminal-pro hormone BNP; NYHA, New York Heart Association; SD, standard deviation
Includes furosemide and equivalent diuretic dosages
For each biomarker, the number of patients with data available to calculate statistics are as follows: BNP = 41 patients, NT-proBNP = 45 patients, Troponin T = 38 patients, Troponin I = 54 patients
Figure 1.
Trends in diagnosis of Val122Ile ATTR-CA from 2001–2018
Almost all patients self-identified as black. Women were significantly older at the time of diagnosis compared to men (76 years versus 69 years, p < 0.0001). New York Heart Association (NYHA) Functional Classification scores were similar between sexes with approximately two-thirds of patients presenting with NYHA class III or IV symptoms. Body mass index (BMI), modified BMI (calculated as the product of BMI and serum albumin concentration), estimated glomerular filtration rate (eGFR), and biomarkers, including serum brain natriuretic peptide and troponin, did not differ significantly between sexes. The body surface areas (BSA) of men were significant larger than that of women (1.78 m2 versus 1.99 m2, p <0.0001). Regarding extracardiac manifestations of ATTR-CA, women had significantly lower rates of carpal tunnel (48% versus 63%, p = 0.021).
Review of electrocardiographic (ECG) data, including conduction abnormalities and the presence of low voltages, did not yield significant differences between sexes (Table 2). Overall, 20% (n = 15) of the study population was in atrial fibrillation or flutter at the time of initial ECG.
Table 2.
Electrocardiographic and echocardiographic parameters of patients with Val122Ile ATTR-CA, by sex
| Women (n = 27) | Men (n = 46) | p value | |
|---|---|---|---|
| Electrocardiogram | |||
| Atrial fibrillation/flutter, n (%) | 7 (28.0) | 8 (18.1) | 0.34 |
| Conduction abnormalities, n (%)* | 11 (40.7) | 24 (52.2) | 0.35 |
| Low voltage, n (%) | 10 (37.0) | 21 (45.6) | 0.47 |
| tQRS/LVPW, mean (sd)† | 58.8 (21.5) | 61.5 (29.9) | 0.92 |
| Echocardiogram | |||
| IVS, mm, mean (sd) | 1.7 (0.4) | 1.7 (0.4) | 0.34 |
| LVPW, mm, mean (sd) | 1.7 (0.3) | 1.7 (0.4) | 0.85 |
| LVEDD, cm, mean (sd) | 4.2 (0.7) | 4.5 (0.7) | 0.11 |
| LVESD, cm, mean (sd) | 3.3 (0.7) | 3.7 (0.7) | 0.028 |
| LVEDV, mL, mean (sd) | 82.9 (26.6) | 93.8 (29.9) | 0.11 |
| LVEDVI, mL/m2, mean (sd) | 47.3 (16.7) | 47.3 (14.1) | 0.56 |
| LVESV, mL, mean (sd) | 41.8 (16.8) | 51.8 (19.8) | 0.028 |
| LVESVI, mL/m2, mean (sd) | 23.4 (10.1) | 28.2 (9.2) | 0.050 |
| SV, mL, mean (sd) | 40.8 (16.2) | 38.9 (13.8) | 0.86 |
| SVI, mL/m2, mean (sd) | 23.5 (10.2) | 20.1 (8.1) | 0.32 |
| E, cm/s, mean (sd) | 73.3 (16.9) | 71.9 (21.4) | 0.99 |
| e’, cm/s, mean (sd) | 4.9 (1.5) | 4.6 (1.7) | 0.16 |
| E/e’, mean (sd) | 16.5 (7.2) | 17.2 (6.7) | 0.47 |
| LV mass, g, mean (sd) | 307.0 (116.2) | 345.2 (134.2) | 0.23 |
| LV mass index, g/ m2, mean (sd) | 175.2 (75.7) | 172.9 (59.3) | 0.76 |
| Relative wall thickness, mean (sd) | 0.8 (0.2) | 0.8 (0.2) | 0.51 |
| LVEF, %, mean (sd) | 49.5 (12.0) | 43.2 (10.8) | 0.046 |
| Myocardial contraction fraction %, mean (sd) | 15.5 (8.3) | 13.8 (7.2) | 0.40 |
cm, centimeters; IVS, interventricular septum; g, grams; LVEDD, left ventricular end-diastolic diameter; LVEDV, left ventricular end-diastolic volume; LVEDVI, left ventricular end-diastolic volume indexed; LVESD, left ventricular end-systolic diameter; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; LVESVI, left ventricular end-systolic volume indexed; LVPW, left ventricular posterior wall; mm, millimeters; s, second; SD, standard deviation; SV, stroke volume; SVI, stroke volume indexed; tQRS, total QRS
Conduction abnormalities are intraventricular conduction abnormalities, including bundle branch blocks and fascicular blocks, and atrioventricular blocks
tQRS/LVPW: summation of the height of all QRS complexes divided by left ventricular wall thickness
Both women and men had relative wall thicknesses consistent with severe concentric ventricular hypertrophy (Table 2). Left ventricular ejection fraction was significantly higher in women (49.5% versus 43.2%, p = 0.046), driven more by smaller left ventricular end-diastolic volumes (82.9 mL versus 93.8 mL, p = 0.11) than by differences in stroke volume (40.8 mL versus 38.9 mL, p = 0.86) (Table 2). Myocardial contraction fraction did not differ significantly between groups (15.5% versus 13.8%, p = 0.40).
Fifty-six patients (77% of the study cohort) had complete echocardiographic data from the time of diagnosis available for review. Twenty-two women and 34 men were included. Baseline characteristics for this subgroup are provided in Supplemental Table 1 and did not differ significantly from characteristics of the overall study cohort.
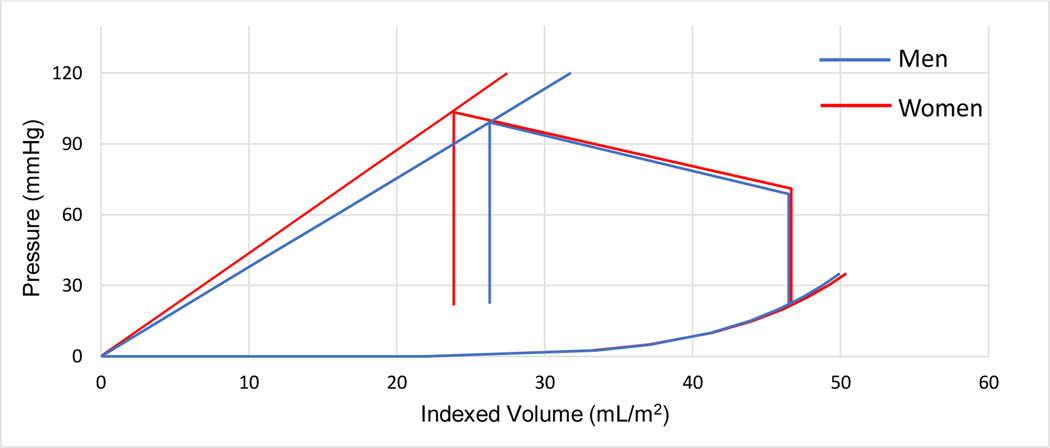
For these patients, left ventricular mechanical properties indexed to BSA were estimated using non-invasive pressure volume loop analysis. These parameters are summarized in Table 3 and graphically represented in the Figure 2a. Parameters without adjustment for BSA are included in the Supplemental File. Chamber contractility, reflected by Res (5.12 mmHg*m2 /mL versus 4.34 mmHg*m2/mL, p = 0.27) and afterload conditions, reflected by Ea (5.52 mmHg*m2/mL versus 5.69 mmHg*m2/mL, p = 0.62), were not significantly different between sexes. Chamber capacitance, reflected in predicted end-diastolic volume at a left end-diastolic pressure of 30 mmHg, was also similar between sexes (53.1 mL/m2 versus 48.8 mL/m2, p = 0.93).
Table 3.
Non-invasive pressure-volume loop indices indexed to body-surface area in patients with Val122Ile ATTR-CA, by sex
| Women (n = 22) | Men (n = 34) | p value | |
|---|---|---|---|
| Pes, mmHg, mean (SD) | 103.5 (14.0) | 99.2 (16.4) | 0.14 |
| Estimated LVEDP, mmHg, mean (SD) | 21.6 (4.26) | 22.2 (4.00) | 0.36 |
| Res, mmHg*m2/mL, mean (SD) | 5.12 (2.74) | 4.34 (1.91) | 0.27 |
| Ea, mmHg*m2/mL, mean (SD) | 5.52 (2.62) | 5.69 (2.64) | 0.62 |
| Ea/Res, mean (SD) | 1.23 (0.65) | 1.46 (0.66) | 0.22 |
| V30, mL/*m2, mean (SD) | 53.1 (22.0) | 48.8 (15.8) | 0.93 |
| PVAiso30, mmHg*mL/*m2, median [IQR] | 4,711 [3,622–6,461] | 4,223 [3,173–5,025] | 0.20 |
IQR, interquartile range; mL, milliliters; mmHg, millimeters of mercury; SD, standard deviation
Figure 2a.
Non-invasive pressure-volume loops in Val122Ile ATTR-CA by sex, indexed to body surface area
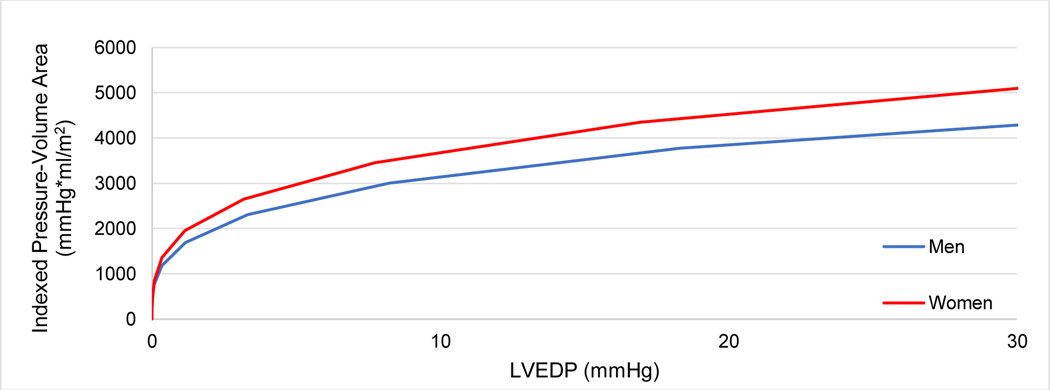
Isovolumic pressure-volume area indexed to BSA as a function of EDP is shown in Figure 2b. Overall, pressure-volume areas at a left ventricular end diastolic pressure of 30 mmHg (PVAiso30), were not significantly different in women compared to men (4,711 versus 4,223 mmHg*mL/*m2 for women and men, respectively, p = 0.20), indicating that after integration of both systolic and diastolic function, overall pump function was comparable (Table 3).
Figure 2b.
Isovolumic pressure-volume area in Val122Ile ATTR-CA by sex, indexed to body surface area
At a median follow-up of 1.8 years from initial diagnosis, there was no difference in mortality between women and men using Kaplan-Meier estimates (34%, 95% confidence interval (CI) 13% - 70% versus 43%, 95% CI 28% - 63%, respectively, logrank p = 0.64).
DISCUSSION
This retrospective cohort study of 73 patients with Val122Ile associated ATTR-CA examined differences in the clinical presentation and echocardiographic measures of cardiac performance at diagnosis between sexes. We found that the number of patients diagnosed with ATTR-CA and specifically the proportion of women diagnosed, increased over the past two decades, an encouraging sign of both growing awareness of the disease among clinicians and advances in non-invasive diagnostic testing. Women presented at an older age than men. Based on non-invasive pressure-volume assessments, women and men demonstrated similar cardiac structural changes and similar overall cardiac pump function, suggesting a more indolent disease process in women. Mortality was similar between sexes.
Despite marked sex differences in disease prevalence both in wtATTR-CA and, to a lesser extent, hATTR-CA, few previous studies have investigated sex differences in the clinical presentation of ATTR-CA. In this study, women were significantly older than men at the time of diagnosis. Non-biological factors likely play a role, including known sex disparities in access to health care resources, a lack of clinical suspicion among physicians for the diagnosis of cardiac amyloidosis in women, and comparative delays in women seeking out health care resources. These mechanisms are well-documented in literature on sex disparities in ischemic heart disease and heart failure but to this point have not been studied in cardiac amyloidosis.3,4,27 The older age of women at the time of diagnosis may also reflect a relative delay in the development of clinical symptoms. This is supported by the fact that many markers of heart failure severity and overall morbidity were similar between sexes at the time of diagnosis despite the difference in age, including New York Heart Association functional class, myocardial contraction fraction, and PVAiso30. Longitudinal observations of women and men with hereditary TTR amyloidosis are needed to investigate this hypothesis. Sex-specific differences in time to disease onset are well-documented in other forms of ATTR amyloidosis, especially Val30Met familial amyloid polyneuropathy where several studies have demonstrated that women are significantly older at the time of diagnosis.28,29 This has not been studied to the same extent in Val122Ile ATTR-CA, which may in part be due to the male-predominance of the disease. In population-based studies of Val122Ile ATTR-CA, age at diagnosis ranges from 69–77 years, which is consistent with a median age of 73 years (IQR 68–77) at the time of diagnosis in this study.11,12
Additionally, women in this study cohort trended toward higher N-terminal pro-hormone brain natriuretic peptide (NT-proBNP) levels at the time of diagnosis compared to men although the difference did not meet a level of statistical significance. NT-proBNP is commonly used for staging and risk stratification in ATTR-CA. In this study, NT-proBNP levels were available for 45 of 73 patients in this study (62%), which in part is due to the fact that the study period spans 2001–2018, and routine measurement of NT-proBNP is a relatively recent practice. This limits interpretation of the difference in NT-proBNP levels by sex.
This study was the first, to our knowledge, to use the technique of pressure-volume analysis to compare overall cardiac chamber performance between women and men with Val122Ile associated ATTR-CA. When volumes were indexed to body surface area to account for known differences in chamber sizes between sexes21,22, women and men with Val122Ile associated ATTR-CA had structurally similar hearts at the time of diagnosis, in terms of both systolic and diastolic function, and similar overall cardiac chamber function.
These observations are important for several reasons. They reinforce that a diagnosis of ATTR-CA should be considered in all older patients with concentric hypertrophy and diastolic dysfunction, including women from whom clinical suspicion for cardiac amyloidosis may initially be lower. These findings also demonstrate that pressure-volume analysis is a useful technique to understand cardiac pathophysiology in ATTR-CA using parameters derived from peripheral blood pressure readings and transthoracic echocardiography.
Finally, the findings of this study may have important implications for the role of sex in the pathogenesis of Val122Ile associated ATTR-CA and disease trajectory. Overall pump function at the time of diagnosis, indexed by PVAiso30, was similar between women and men, as were mortality rates from that point forward. This is consistent with a large-scale study of patients in the UK with ATTR-CA which found no association between sex and survival.12 In contextualizing these findings, it is notable that at the time of diagnosis, women were on average more than 5 years older than men in this study. In contrast to the previously held conception of ATTR-CA as a slowly progressive condition, several recent studies suggest a more aggressive clinical trajectory, characterized by severe refractory heart failure, considerable morbidity and hospitalizations, and a median survival ranging from 20 to 30 month from the time of diagnosis.11,12,30 Using serial pressure-volume loop analyses over two years from the time of diagnosis, Bhuiyan et al. demonstrated the physiologic basis for this decline: worsening over a relatively short time-course of both ventricular diastolic and systolic function, ultimately resulting in progressive ventricular pump dysfunction.20 Given the rapidity of progressive cardiac dysfunction in hereditary ATTR-CA, the observation in this study cohort that at the time of presentation, women are older in age but have similar cardiac function to men, indicates that women may have a less aggressive disease phenotype. Women had a similar left ventricular wall thickness to men. Physiologically, women have lower normal values so this finding may indicate that at the time of diagnosis the degree of myocardial infiltration was relatively higher in women. However, relative wall thickness did not differ by sex. Further interpretation of these findings is limited by the fact that cardiac amyloid deposition was not directly measured in this study by, for example, quantification of extracellular volume on cardiovascular magnetic resonance imaging. However, even if women had a relatively higher degree of myocardial infiltration, perhaps due to longer exposure to disease, it did not appear to be driving poorer cardiac chamber performance.
Sex hormones may be important modulators of the degree of amyloid deposition and disease phenotype. In a study of patients with both hereditary and wildtype ATTR-CA, Rapezzi et al. found that women, compared to men, had lower levels of myocardial involvement on echocardiographic review. Those women with higher degrees of myocardial involvement were more likely to be post-menopausal, implicating female sex hormones as a potential protective factor.31 Additionally, Gonçalves et al. showed using mouse models that while both estrogen and androgen upregulate liver production of TTR, androgens are a relatively stronger inducer.32 Longitudinal study of the progression of ATTR-CA in women and men is needed to understand the relationship between age, sex, and disease progression.
Limitations
This study has several limitations. First, the study sample size was small and represents the experience of a single clinical center. Second, linear ventricular dimensions measured using two-dimensional transthoracic echocardiography were used to estimate ventricular volumes. While three-dimensional cardiac imaging modalities provide more accurate estimates, logistical and technical challenges make these studies more difficult to obtain. Data on biomarkers at the time of diagnosis, including troponin and pro-BNP which are important prognostic markers in cardiac amyloidosis, were not consistently available. Not all patients in the study cohort had echocardiograms available for review. The baseline characteristics of the patients with available imaging did not differ significantly from the characteristics of the study cohort. Nevertheless, the missingness of the dataset may introduce biases that cannot be adjusted for. Additionally, comparisons were made between men and women, not between sex- and age-matched controls. Because under normal conditions men have larger hearts than women even after accounting for body size, it could be that men with ATTR-CA actually exhibit a greater degree of ventricular size reduction than women. This would render our conclusion of a more indolent form of disease in women conservative. Finally, estimates of cardiac performance were derived from non-invasively measured pressure-volume loop relationships using single-beat techniques. While direct catheter-based measurements of EDPVR and ESPVR are the gold standard for this analysis, their necessarily invasive nature makes it difficult to gather data on large patient cohorts and restricts use to a small number of technically capable centers. Despite these limitations, this study is the first to describe and compare clinical and ventricular properties of women and men with Val122Ile ATTR-CA.
Conclusions
This retrospective pressure-volume based analysis demonstrates that despite being significantly older at time of diagnosis of Val122Ile ATTR-CA, women have similar overall cardiac pump function and 3-year mortality rates compared to men. Given the rapidity of progressive cardiac dysfunction in hereditary ATTR-CA, these findings suggest that sex is an important modifier of illness trajectory.
Supplementary Material
Acknowledgments
DISCLOSURES
Dr. Rosenblum reports grant support from NIH T32 HL007854. Dr. Maurer reports the following disclosures: Grant support from NIH HL HL139671–01, AG R21AG058348 and AG K24AG036778. Dr. Maurer’s institution received funding for clinical trials for Pfizer, Prothena, Eidos and Alnylam. He has received consulting income from Pfizer, EIdos, Prothena, Akcea and Alnylam.
Footnotes
All authors made substantial contributions to all of the following: (1) the conception and design of the study, or acquisition of data, or analysis and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, (3) final approval of the version to be submitted.
All authors have approved the final article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Wenger NK, Speroff L, Packard B. Cardiovascular health and disease in women. New England Journal of Medicine. 1993;329(4):247–256. [DOI] [PubMed] [Google Scholar]
- 2.EUGenMed T, Group CCS, Regitz-Zagrosek V, et al. Gender in cardiovascular diseases: impact on clinical manifestations, management, and outcomes. European Heart Journal. 2015;37(1):24–34. [DOI] [PubMed] [Google Scholar]
- 3.Mosca L, Barrett-Connor E, Wenger NK. Sex/gender differences in cardiovascular disease prevention: what a difference a decade makes. Circulation. 2011;124(19):2145–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lam CSP, Arnott C, Beale AL, et al. Sex differences in heart failure. European Heart Journal. 2019;40(47):3859–3868c. [DOI] [PubMed] [Google Scholar]
- 5.Gori M, Lam CSP, Gupta DK, et al. Sex-specific cardiovascular structure and function in heart failure with preserved ejection fraction. European Journal of Heart Failure. 2014;16(5):535–542. [DOI] [PubMed] [Google Scholar]
- 6.Piro M, Della Bona R, Abbate A, Biasucci LM, Crea F. Sex-related differences in myocardial remodeling. Journal of the American College of Cardiology. 2010;55(11):1057–1065. [DOI] [PubMed] [Google Scholar]
- 7.Treibel TA, Kozor R, Fontana M, et al. Sex dimorphism in the myocardial response to aortic stenosis. JACC Cardiovascular Imaging. 2018;11(7):962–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruberg FL, Berk JL. Transthyretin (TTR) cardiac amyloidosis. Circulation. 2012;126(10):1286–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruberg FL, Grogan M, Hanna M, Kelly JW, Maurer MS. Transthyretin amyloid cardiomyopathy: JACC state-of-the-art review. Journal of the American College of Cardiology. 2019;73(22):2872–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quarta CC, Buxbaum JN, Shah AM, et al. The amyloidogenic V122I transthyretin variant in elderly black Americans. The New England Journal of Medicine. 2015;372(1):21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maurer MS, Hanna M, Grogan M, et al. Genotype and phenotype of transthyretin cardiac amyloidosis: THAOS (Transthyretin Amyloid Outcome Survey). Journal of the American College of Cardiology. 2016;68(2):161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lane T, Fontana M, Martinez-Naharro A, et al. Natural history, quality of life, and outcome in cardiac transthyretin amyloidosis. Circulation. 2019;140(1):16–26. [DOI] [PubMed] [Google Scholar]
- 13.Kittleson MM, Maurer MS, Ambardekar AV, et al. Cardiac amyloidosis: evolving diagnosis and management: a scientific statement from the American Heart Association. Circulation. 2020:Cir0000000000000792. [DOI] [PubMed] [Google Scholar]
- 14.Gillmore JD, Maurer MS, Falk RH, et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation. 2016;133(24):2404–2412. [DOI] [PubMed] [Google Scholar]
- 15.Castano A, Haq M, Narotsky DL, et al. Multicenter study of planar technetium 99m pyrophosphate cardiac imaging: predicting survival for patients with ATTR cardiac amyloidosis. JAMA Cardiology. 2016;1(8):880–889. [DOI] [PubMed] [Google Scholar]
- 16.Cheitlin MD, Armstrong WF, Aurigemma GP, et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography. Summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to update the 1997 guidelines for the clinical application of echocardiography). Journal of the American Society of Echocardiography. 2003;16(10):1091–1110. [DOI] [PubMed] [Google Scholar]
- 17.Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. The American Journal of Cardiology. 1986;57(6):450–458. [DOI] [PubMed] [Google Scholar]
- 18.de Simone G, Devereux RB, Ganau A, et al. Estimation of left ventricular chamber and stroke volume by limited M-mode echocardiography and validation by two-dimensional and Doppler echocardiography. The American Journal of Cardiology. 1996;78(7):801–807. [DOI] [PubMed] [Google Scholar]
- 19.Maurer MS, Koh WJ, Bartz TM, et al. Relation of the myocardial contraction fraction, as calculated from M-mode echocardiography, with incident heart failure, atherosclerotic cardiovascular disease and mortality (results from the Cardiovascular Health Study). The American Journal of Cardiology. 2017;119(6):923–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhuiyan T, Helmke S, Patel AR, et al. Pressure-volume relationships in patients with transthyretin (ATTR) cardiac amyloidosis secondary to V122I mutations and wild-type transthyretin: Transthyretin Cardiac Amyloid Study (TRACS). Circulation Heart Failure. 2011;4(2):121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfaffenberger S, Bartko P, Graf A, et al. Size matters! Impact of age, sex, height, and weight on the normal heart size. Circulation Cardiovascular Imaging. 2013;6(6):1073–1079. [DOI] [PubMed] [Google Scholar]
- 22.Maurer MS, Burkhoff D, Fried LP, Gottdiener J, King DL, Kitzman DW. Ventricular structure and function in hypertensive participants with heart failure and a normal ejection fraction: the Cardiovascular Health Study. Journal of the American College of Cardiology. 2007;49(9):972–981. [DOI] [PubMed] [Google Scholar]
- 23.Klotz S, Hay I, Dickstein ML, et al. Single-beat estimation of end-diastolic pressure-volume relationship: a novel method with potential for noninvasive application. American Journal of Physiology - Heart and Circulatory Physiology. 2006;291(1):H403–412. [DOI] [PubMed] [Google Scholar]
- 24.Chen CH, Fetics B, Nevo E, et al. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. Journal of the American College of Cardiology. 2001;38(7):2028–2034. [DOI] [PubMed] [Google Scholar]
- 25.Kelly RP, Ting CT, Yang TM, et al. Effective arterial elastance as index of arterial vascular load in humans. Circulation. 1992;86(2):513–521. [DOI] [PubMed] [Google Scholar]
- 26.Suga H, Goto Y, Futaki S, et al. Systolic pressure-volume area (PVA) as the energy of contraction in Starling’s law of the heart. Heart and Vessels. 1991;6(2):65–70. [DOI] [PubMed] [Google Scholar]
- 27.Healy B. The Yentl syndrome. New England Journal of Medicine. 1991;325(4):274–276. [DOI] [PubMed] [Google Scholar]
- 28.Fernandes A, Coelho T, Rodrigues A, et al. Clinicopathological correlations of sural nerve biopsies in TTR Val30Met familial amyloid polyneuropathy. Brain Communications. 2019;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sousa A, Coelho T, Barros J, Sequeiros J. Genetic epidemiology of familial amyloidotic polyneuropathy (FAP)-type I in Póvoa do Varzim and Vila do Conde (north of Portugal). American Journal of Medical Genetics. 1995;60:512–21. [DOI] [PubMed] [Google Scholar]
- 30.Connors LH, Prokaeva T, Lim A, et al. Cardiac amyloidosis in African Americans: comparison of clinical and laboratory features of transthyretin V122I amyloidosis and immunoglobulin light chain amyloidosis. American Heart Journal. 2009;158(4):607–614. [DOI] [PubMed] [Google Scholar]
- 31.Rapezzi C, Riva L, Quarta CC, et al. Gender-related risk of myocardial involvement in systemic amyloidosis. Amyloid. 2008;15(1):40–48. [DOI] [PubMed] [Google Scholar]
- 32.Goncalves I, Alves CH, Quintela T, et al. Transthyretin is up-regulated by sex hormones in mice liver. Molecular and Cellular Biochemistry. 2008;317(1–2):137–142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.