Abstract
BACKGROUND:
The Antimicrobial Use (AU) Option of the Centers for Disease Control and Prevention’s National Healthcare Safety Network (NHSN) is a surveillance resource that can provide actionable data for antibiotic stewardship programs. Such data are used to enable measurements of AU across hospitals and before, during, and after stewardship interventions.
METHODS:
We used monthly AU data and annual facility survey data submitted to the NHSN to describe hospitals and neonatal patient care locations reporting to the AU Option in 2017, examine frequencies of most commonly reported agents, and analyze variability in AU rates across hospitals and levels of care. We used results from these analyses in a collaborative project with Vermont Oxford Network to develop neonatal-specific Standardized Antimicrobial Administration Ratio (SAAR) agent categories and neonatal-specific NHSN Annual Hospital Survey questions.
RESULTS:
As of April 1, 2018, 351 US hospitals had submitted data to the AU Option from at least 1 neonatal unit. In 2017, ampicillin and gentamicin were the most frequently reported antimicrobial agents. On average, total rates of AU were highest in level III NICUs, followed by special care nurseries, level II-III NICUs, and well newborn nurseries. Seven antimicrobial categories for neonatal SAARs were created, and 6 annual hospital survey questions were developed.
CONCLUSIONS:
A small but growing percentage of US hospitals have submitted AU data from neonatal patient care locations to NHSN, enabling the use of AU data aggregated by NHSN as benchmarks for neonatal antimicrobial stewardship programs and further development of the SAAR summary measure for neonatal AU.
Neonates represent a challenging but high-priority population for targeted hospital antibiotic stewardship efforts. Maternal and infant risk factors for neonatal sepsis are common.1,2 These risks, coupled with the diagnostic difficulties that confront neonatal practitioners, often lead to initiation and continuation of antibiotics in the absence of a clear indication.1,3 Because mortality associated with neonatal sepsis is high, especially among preterm and very low birth weight infants,3–5 it is difficult to delay or avoid antibiotic use when documented risk factors are present. However, neonatal antibiotic exposure is associated with increased risk of various adverse events, including disruption to the developing microbiome, bronchopulmonary dysplasia, necrotizing enterocolitis, late-onset sepsis, invasive candidiasis, and mortality.1,6,7 Despite this challenge of weighing infection risk against adverse antibiotic effects, opportunities exist for reducing the number of unnecessary antibiotic starts, decreasing duration of therapy, and improving overall judiciousness of antimicrobial use (AU) in neonates.
Antibiotic stewardship programs (ASPs) aim to improve patient outcomes while minimizing unintended consequences associated with AU.8 To achieve these goals, ASPs are focused on decreasing inappropriate use, and the Centers for Disease Control and Prevention (CDC) developed the Core Elements of Hospital ASPs for facilities to implement to aid in this process.9,10 Determining antibiotic appropriateness is challenging in any patient population, even more so in neonates, whose signs and symptoms of infection are often subtle and nonspecific.3 Adding further complexity, rates of AU in NICUs are highly variable and often unexplained by burden of proven infection.6,11 In situations in which appropriate AU is not clearly defined, “an initial first step could be to educate the NICU team on their own prescribing practices to engender a discussion on best practices.”12 Electronic AU surveillance systems can contribute substantially to ASP efforts, because they enable hospitals to streamline data collection and reporting, provide timely, comparative AU benchmarks, and make available additional data and analytic options that can help guide medication use evaluations and prompt changes in prescribing practices when those changes are indicated.
The CDC’s National Healthcare Safety Network (NHSN) is a public health surveillance system used to collect, analyze, report, and make available data for monitoring, measuring, and responding to health care–associated infections, AU and antimicrobial resistance, blood transfusion safety events, and adherence to infection prevention practices and antibiotic stewardship. The NHSN’s Antimicrobial Use and Resistance (AUR) Module provides the infrastructure and support necessary for hospitals to electronically submit and monitor their AUR. The AU Option of the AUR Module enables hospitals to report, track, compare, and interpret AU to improve ASP decision-making. Here, we describe hospitals and units reporting neonatal data to the NHSN’s AU Option, summarize AU data reported in 2017, present plans to develop neonatal AU benchmark metrics through collaboration with the Vermont Oxford Network (VON), and discuss the integral role electronic surveillance plays in neonatal antimicrobial stewardship.
METHODS
Data Sources
We aggregated monthly hospital AU data for our analyses of AU in neonatal units. The AU Option enables hospitals to submit data from 4 NHSN-defined neonatal location types: level I well newborn nurseries, level II special care nurseries, level II-III NICUs, and level III NICUs.13 In the NHSN, levels I and II are defined in accordance with the American Academy of Pediatrics.14 The NHSN defines level II-III units as those housing both level II and level III newborns and infants, analogous to a mixed acuity unit for neonatal critical care patients.13 Level III and IV NICUs, as defined by the Academy of Pediatrics, are both considered level III in the NHSN.
Hospitals submit AU as days of therapy (DOTs) for 90 antimicrobial agents across 4 routes of administration: digestive, intramuscular, intravenous, and respiratory. Electronic medication administration (eMAR) and/or barcoded medication administration (BCMA) systems serve as data sources for DOTs. Admission discharge transfer systems, used by hospitals to track patients throughout their hospital stays, serve as data sources for days present, the denominator in rate calculations.15 Days present are defined as the time period during which a patient is at risk for antimicrobial exposure for a given patient care location, calculated as the number of patients present in a particular location for any portion of a calendar day.15 DOTs and days present are aggregated monthly to the patient care location-level before submission; no patient-level information is reported. Participation is voluntary, and all reporting is electronic, with no manual data entry.15
AU Option Participation
We used neonatal AU data and hospital characteristics self-reported in the 2017 NHSN Annual Hospital Survey for a descriptive analysis of hospitals reporting neonatal data to the AU Option (2011–2018). We used 2017 NHSN central line–associated bloodstream infection data, a reporting requirement for hospitals participating in Centers for Medicare and Medicaid Services’ Hospital Inpatient Quality Reporting program, to estimate the number of hospitals in each state providing neonatal intensive care. With hospitals reporting central line–associated bloodstream infection data to the NHSN from an NICU as our denominator, we estimated the proportion of eligible facilities reporting NICU data to the AU Option in 2017, by state. We approximated the number of US hospitals providing well newborn care using an American Hospital Association report of hospitals with at least 1 birth in 2015.16 Using this estimate as our denominator, we calculated the proportion of eligible US hospitals reporting well newborn data to the AU Option in 2017.
AU Data Validation and Analyses
We reviewed all 2017 neonatal AU data submitted to NHSN and excluded records from analyses if days present were reported to be 0 or if large changes in location-level DOTs or days present (>70% change in value, where 1 value was >100) were observed from 1 month to the next. Using validated data pooled across months and facilities, we identified the 5 antimicrobial agents in each level of patient care with the greatest number of DOTs. Next, for each level of patient care within a facility, we calculated drug-specific pooled rates by dividing pooled DOTs by pooled days present and multiplying by 1000. SAS version 9.3 software (SAS Institute, Inc, Cary, NC) was used for all analyses.
Preparation for Development of Neonatal AU Benchmark Metrics
The AU Option offers numerous analysis tools to help in the interpretation and tracking of AU by ASPs. One option provides Standardized Antimicrobial Administration Ratios (SAARs) for select groups of antimicrobial agents and select locations. The SAAR is a National Quality Forum–endorsed metric used to compare observed AU (ie, DOTs reported by a hospital for a specified set of patient care locations and a defined time period) to predicted AU (ie, predicted DOTs, calculated by using nationally aggregated data that are risk adjusted for location and hospital characteristics).17 Because neonatal AU differs substantially from adult and pediatric AU in terms of clinical indications and choice of agents, the Division of Healthcare Quality Promotion at the CDC is collaborating with the VON to develop neonatal-specific SAARs.
The VON is a nonprofit organization with a global reach that works collaboratively with health care professionals to improve the quality and safety of health care for newborn infants. With VON’s guidance, the Division of Healthcare Quality Promotion organized a group of US subject matter experts to create materials necessary for the development of neonatal SAARs. This group, known as the Antibiotic Stewardship Program Special Interest Group (ASP-SIG), met monthly from 2017 to 2018. Through these meetings, neonatal-specific NHSN Annual Hospital Survey questions were developed to gather hospital-level patient care and admission information associated with neonatal AU for inclusion in predictive models. In addition, neonatal ASP targets were identified, and SAAR agent categories were created.
RESULTS
As of April 1, 2018, 351 acute care hospitals reported at least 1 month of neonatal AU data to the AU Option. Hospitals were primarily composed of large (interquartile range [IQR]: 170–395 beds) general acute care (90%) academic teaching hospitals (72%) (Table 1). More hospitals reported from a special care nursery or NICU but not a well newborn nursery (42%) than from a well newborn nursery alone (17%). These 351 hospitals reported from 544 unique neonatal units: 224 well newborn nurseries, 64 special care nurseries, 154 combined level II-III NICUs, and 102 level III NICUs. The majority of participating hospitals reported from 1 (56%) or 2 (38%) unique neonatal units (range: 1–8).
TABLE 1.
Characteristics of Hospitals Submitting At Least 1 Month of Data to the NHSN AU Option From 1 or More Neonatal Units, 2011–2018 (n = 351)
| Characteristic | Facilities, n (%) |
|---|---|
| Facility type | |
| General acute care | 316 (90.0) |
| Military | 13 (3.7) |
| Children’s | 10 (2.8) |
| Critical access | 6 (1.7) |
| Women’s | 3 (0.9) |
| Women’s and children’s | 3 (0.9) |
| Teaching statusa | |
| Nonteaching | 97 (27.6) |
| Undergraduate teaching | 54 (15.4) |
| Graduate teaching | 79 (22.5) |
| Major teaching | 121 (34.5) |
| Bed size | |
| >200 beds | 241 (68.7) |
| 51–200 beds | 91 (25.9) |
| ≤50 beds | 19 (5.4) |
| Location types reported | |
| Special care nursery or NICU only | 147 (41.9) |
| Well newborn nursery only | 59 (16.8) |
| Both special care nursery or NICU and well newborn nursery | 145 (41.3) |
Teaching status as defined in and reported through the NHSN Patient Safety Component Annual Hospital Survey. Undergraduate teaching refers to a facility that has a program for medical or nursing students only. Graduate teaching refers to a facility that has a program for postgraduate medical training (ie, residency and/or fellowships). Major teaching refers to a facility that has a program for medical students and postgraduate medical training.
In 2017, 311 facilities reported at least 1 month of data to the AU Option, 76% reported ≥6 months, and 57% reported ≥11 months. In 2017, 1041 hospitals reported to NHSN from any level II-III or level III NICU, 213 (20.5%) of which submitted to the AU Option from an NICU. Submission of neonatal data to the AU Option varied greatly by state, as did the number of hospitals with NICUs eligible for participation (Fig 1). At least half of eligible hospitals in Vermont, Utah, Colorado, Missouri, and Oregon reported 2017 NICU data to the AU Option. Eligible hospitals in 10 states, Washington, District of Columbia, and Puerto Rico have not yet reported. Approximately 3062 US hospitals reported at least 1 birth in 2015. In 2017, 177 (5.8%) hospitals reported data to the AU Option from a well newborn nursery.
FIGURE 1.
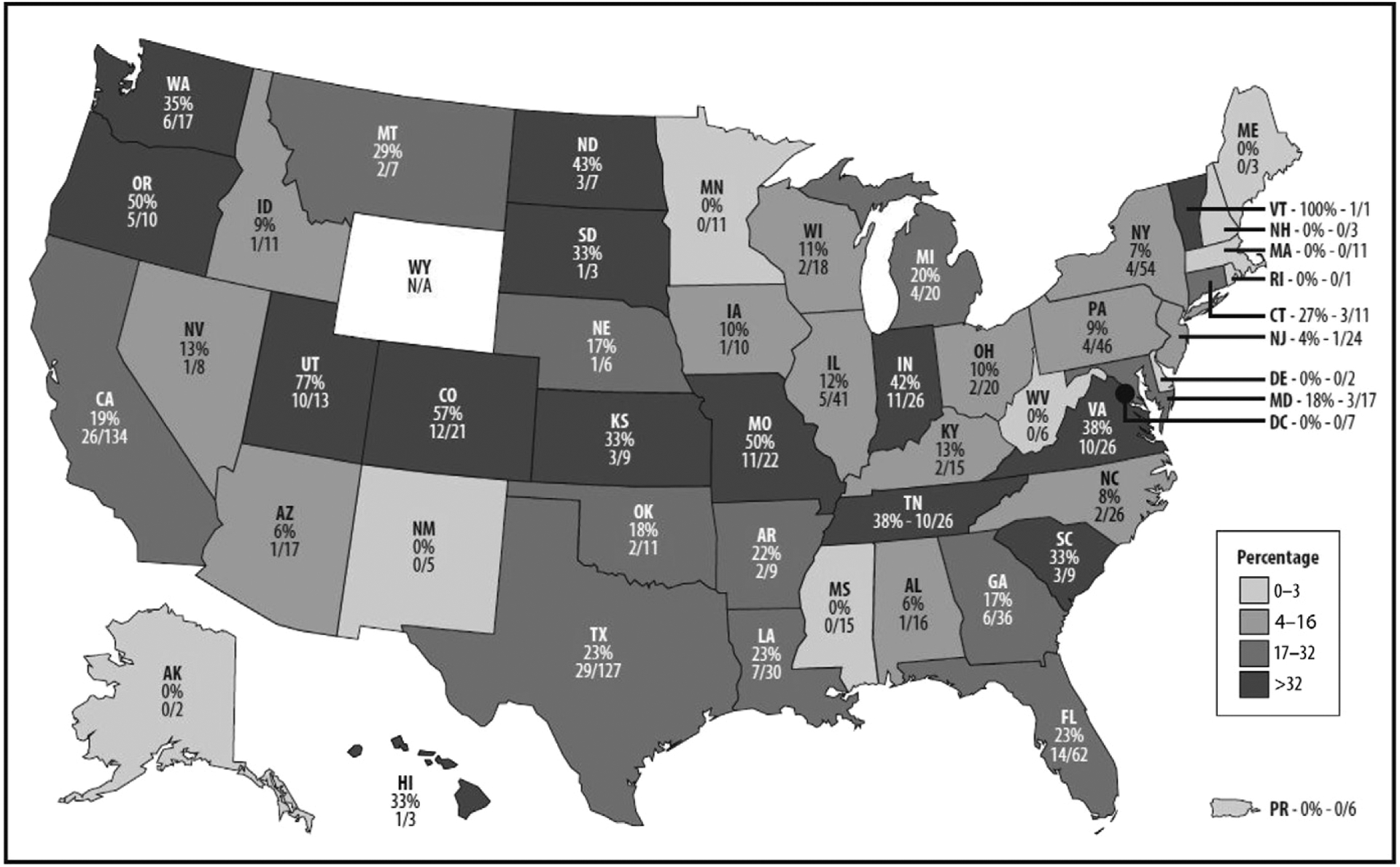
Number and percentage of US general acute care hospitals with a level II-III or level III NICU reporting to the NHSN AU Option in 2017, by state.
Internal validation of 2017 neonatal AU data submitted to the NHSN led to the exclusion of 1.7% of records. Validated 2017 data included 309 hospitals reporting from 456 unique neonatal units. Among these 309 hospitals, ampicillin and gentamicin were the 2 most commonly used antimicrobial agents in all 4 levels of neonatal care, and the majority of total AU in each level of care (71%–97%) was composed of 4 agents (Fig 2). A larger variety of antimicrobial drugs was used in NICUs compared to special care and well newborn nurseries, with 50 different antimicrobial agents used at least once in a level III NICU, 45 in a level II-III NICU, 31 in a special care nursery, and 22 in a well newborn nursery. Among hospitals reporting ≥4 months of 2017 neonatal AU data, the median number of unique antimicrobial agents used was 10 (IQR: 6–18) among hospitals reporting only from a special care nursery or NICU and 3 (IQR: 2–4) among hospitals reporting only from a well newborn nursery.
FIGURE 2.
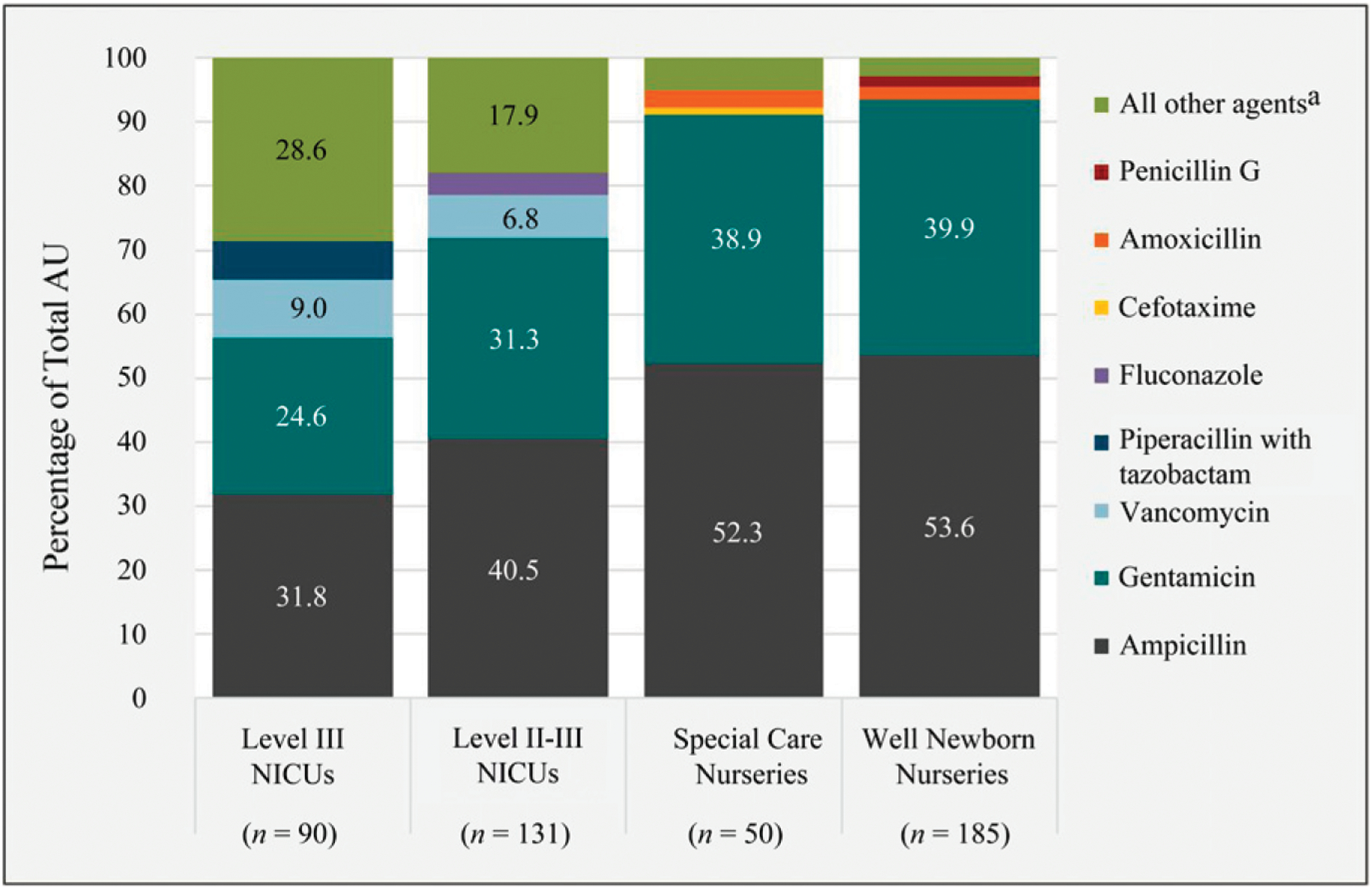
Percentage of total AU contributed by the 4 most commonly used agents in each level of neonatal patient care. a All other agents as follows: level III NICUs: amikacin, amoxicillin, amoxicillin and/or clavulanate, amphotericin B, amphotericin B liposomal, ampicillin and/or sulbactam, anidulafungin, azithromycin, aztreonam, cefazolin, cefdinir, cefepime, cefixime, cefotaxime, cefotetan, cefoxitin, ceftaroline, ceftazidime, ceftriaxone, cefuroxime, cephalexin, ciprofloxacin, clindamycin, colistimethate, daptomycin, doxycycline, ertapenem, erythromycin, fidaxomicin, fluconazole, levofloxacin, linezolid, meropenem, metronidazole, micafungin, nafcillin, nitrofurantoin, oseltamivir, oxacillin, penicillin G, penicillin V, posaconazole, rifampin, sulfamethoxazole and/or trimethoprim, tobramycin, voriconazole; level II-III NICUs: amikacin, amoxicillin, amoxicillin and/or clavulanate, amphotericin B, amphotericin B liposomal, ampicillin and/or sulbactam, azithromycin, aztreonam, caspofungin, cefazolin, cefdinir, cefepime, cefotaxime, cefotetan, cefoxitin, ceftaroline, ceftazidime, ceftriaxone, cefuroxime, cephalexin, ciprofloxacin, clindamycin, daptomycin, doxycycline, erythromycin, imipenem and/or cilastatin, levofloxacin, linezolid, meropenem, metronidazole, micafungin, nafcillin, nitrofurantoin, oseltamivir, oxacillin, penicillin G, penicillin V, piperacillin and/or tazobactam, rifampin, sulfamethoxazole and/or trimethoprim, tobramycin; level II special care nurseries: amoxicillin and/or clavulanate, azithromycin, cefazolin, cefdinir, cefepime, cefixime, ceftaroline, ceftazidime, ceftriaxone, cefuroxime, cephalexin, clindamycin, erythromycin, fluconazole, linezolid, meropenem, metronidazole, nafcillin, nitrofurantoin, oseltamivir, oxacillin, penicillin G, penicillin V, piperacillin and/or tazobactam, rifampin, sulfamethoxazole/trimethoprim, vancomycin; level I well newborn nurseries: azithromycin, cefazolin, cefepime, cefotaxime, cefoxitin, ceftazidime, ceftriaxone, cephalexin, clindamycin, erythromycin, fluconazole, levofloxacin, metronidazole, nafcillin, oseltamivir, oxacillin, piperacillin and/or tazobactam, vancomycin.
Although special care nurseries reported the highest median rates of ampicillin and gentamicin, total AU was highest in level III NICUs, followed by special care nurseries, level II-III NICUs, and well newborn nurseries (Table 2). On average, level III NICUs had more days present per month (IQR: 327–1233) compared to level II-III NICUs (IQR: 168–533), well newborn nurseries (IQR: 152–523), and special care nurseries (IQR: 51–215).
TABLE 2.
Distribution of Data Reported to the AU Option (2017) by Level of Neonatal Care (Monthly Days Present and Pooled Rates) for the 5 Antimicrobial Agents With the Highest Mean Rates and for All Antimicrobial Agents Combined
| 5% | 10% | 25% | 50% | Mean | 75% | 90% | 95% | |
|---|---|---|---|---|---|---|---|---|
| Level III NICUs (n = 89) | ||||||||
| Days present | 126 | 173 | 327 | 606 | 815 | 1233 | 1751 | 1988 |
| Rate (DOTs per 1000 d present) | ||||||||
| Ampicillin | 16 | 33 | 75 | 100 | 107 | 148 | 174 | 184 |
| Gentamicin | 0.3 | 30 | 51 | 81 | 80 | 106 | 136 | 146 |
| Vancomycin | 0.0 | 2.2 | 8.3 | 19 | 24 | 34 | 51 | 59 |
| Piperacillin/tazobactam | 0.0 | 0.0 | 0.0 | 2.2 | 14 | 12 | 34 | 70 |
| Fluconazole | 0.0 | 0.0 | 0.0 | 7.8 | 13 | 21 | 31 | 41 |
| All antimicrobial agentsa | 74 | 115 | 200 | 285 | 294 | 376 | 456 | 505 |
| Level II and III NICUs (n = 130) | ||||||||
| Days present | 33 | 87 | 168 | 302 | 437 | 533 | 891 | 1110 |
| Rate (DOTs per 1000 d present) | ||||||||
| Ampicillin | 8.3 | 38 | 67 | 112 | 132 | 175 | 247 | 299 |
| Gentamicin | 6.0 | 20 | 45 | 83 | 95 | 124 | 169 | 222 |
| Vancomycin | 0.0 | 0.0 | 0.7 | 7.8 | 14 | 21 | 33 | 52 |
| Ceftazidime | 0.0 | 0.0 | 0.0 | 0.0 | 6.0 | 2.2 | 9.8 | 42 |
| Fluconazole | 0.0 | 0.0 | 0.0 | 0.3 | 5.6 | 6.7 | 21 | 27 |
| All antimicrobial agents | 43 | 85 | 161 | 249 | 278 | 369 | 474 | 588 |
| Special care nurseries (n = 50) | ||||||||
| Days present | 6 | 18 | 51 | 111 | 157 | 215 | 369 | 488 |
| Rate (DOTs per 1000 d present) | ||||||||
| Ampicillin | 0.0 | 22 | 66 | 132 | 153 | 227 | 316 | 371 |
| Gentamicin | 0.0 | 7.9 | 42 | 99 | 115 | 173 | 244 | 258 |
| Cefotaxime | 0.0 | 0.0 | 0.0 | 0.0 | 3.8 | 0.0 | 11 | 24 |
| Penicillin G | 0.0 | 0.0 | 0.0 | 0.0 | 3.6 | 0.0 | 5.5 | 18 |
| Ceftazidime | 0.0 | 0.0 | 0.0 | 0.0 | 2.7 | 0.0 | 1.5 | 21 |
| All antimicrobial agents | 0.0 | 38 | 131 | 263 | 289 | 406 | 605 | 657 |
| Well Newborn nurseries (n = 174) | ||||||||
| Days present | 31 | 73 | 152 | 303 | 399 | 523 | 808 | 1017 |
| Rate (DOTs per 1000 d present) | ||||||||
| Ampicillin | 0.0 | 0.0 | 0.3 | 3.8 | 14 | 16 | 30 | 73 |
| Gentamicin | 0.0 | 0.0 | 0.0 | 2.4 | 10 | 12 | 23 | 58 |
| Amoxicillin | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | 0.2 | 1.0 | 1.7 |
| Penicillin G | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | 0.0 | 0.7 | 1.3 |
| Cefotaxime | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | 0.0 | 0.3 | 1.2 |
| All antimicrobial agents | 0.0 | 0.0 | 1.5 | 7.8 | 25 | 29 | 61 | 133 |
The rate was calculated by DOTs per 1000 days present. N is the number of hospitals reporting at least 1 month of data from specified level of care in 2017.
All antimicrobial agents for which data are reported to the AU Option.
We identified and prioritized 5 neonatal ASP targets and developed 7 SAAR antimicrobial categories to help hospitals monitor their AU to meet stewardship goals (Table 3). Once developed, neonatal SAARs will be submitted for National Quality Forum endorsement as a tool for public health surveillance and quality improvement; SAARs are not intended for public reporting and/or payment at this time.
TABLE 3.
Neonatal Antimicrobial Stewardship Targets and Proposed SAAR Antimicrobial Agent Categories
| Neonatal ASP Target | SAAR Agent Category | Antimicrobials Included | Routes of Administration | NHSN Units Included |
|---|---|---|---|---|
| Empiric treatment of late-onset sepsis | Vancomycin | Vancomycin | IV | II, II-III, III |
| Broad spectrum treatment of bacterial infections | Broad spectrum antibacterial agents | Piperacillin and/or tazobactam | IV | II, II-III, III |
| Cefepime | ||||
| Ertapenem | ||||
| Meropenem | ||||
| Imipenem and/or cilastatin | ||||
| Prophylaxis and treatment of invasive candidiasis | Fluconazole | Fluconazole | IV, oral | II, II-III, III |
| Global or total antibiotic use | All antibacterial agents | All antibacterial agents in AU option | IV, IM, oral, respiratory | II, II-III, III |
| Empiric treatment of early-onset sepsis | Ampicillin | Ampicillin | IV | All |
| Cefotaxime,Ceftazidime | Cefotaxime | IV | All | |
| Ceftazidime | ||||
| Empiric treatment of early-onset and late-onset sepsis | Aminoglycosides | Gentamicin | IV | All |
| Tobramycin | ||||
| Amikacin |
IM, intramuscular; IV, intravenous.
DISCUSSION
Only a small percentage of hospitals providing neonatal care in the United States reported AU data from neonatal patient care locations to the NHSN in 2017. However, the amount of data they submitted enabled this first-ever report of neonatal AU using data collected by hospitals from their point-of-care, antimicrobial administration record systems and submitted electronically to a national surveillance system. These data, in turn, enabled the VON, the CDC, and a group of neonatal AU experts to begin developmental work on new metrics for benchmarking neonatal AU.
The AU Option, which the NHSN launched with the primary goal of providing actionable data for hospital ASPs, is a work in progress, and the need for greater participation by neonatal units in AU surveillance is suggested from this study’s findings. Feedback from AU Option users emphasizes the utility of the SAAR in stewardship efforts and the desire for additional patient care locations in SAAR models.18,19 Many hospitals do not yet report neonatal data to the AU Option, and the CDC is working with the VON and other partner organizations to close that reporting gap. Some success has been achieved recently, reflected in an increase in neonatal AU reporting to the NHSN from 102 hospitals in April 2017 to 351 hospitals in April 2018. This upward trend in AU reporting and the inception or intensification of neonatal-specific ASPs in hospitals throughout the United States have created new opportunities for risk-adjusted AU summary measures, such as neonatal SAARs, to serve as benchmarks that can be used to help guide stewardship efforts.
In preparation for neonatal SAAR modeling, which the CDC plans to begin in summer 2019, the ASP-SIG group developed 6 survey questions (Supplemental Fig 3) to capture information thought to be important for risk adjustment. Beginning with the 2018 NHSN survey, hospitals providing neonatal care will be asked if they provide level III or higher neonatal intensive care, if they accept neonatal transfers for various complex procedures, and to which NHSN location infants residing in their mother’s room are mapped when administered antimicrobial agents. In addition, hospitals will report the total number of annual inborn and outborn admissions to special care nurseries and NICUs, in total and across 5 birth weight categories.
The CDC plans to use 2018 AU data as the baseline for neonatal SAAR models. To maximize the precision of SAAR model estimates, all acute care hospitals providing neonatal patient care are encouraged to submit AU data monthly for each of their neonatal units; locations reporting <9 months in 2018 will not be included in predictive models. Among the 351 hospitals described here, 42% report from a special care nursery or NICU but not a well newborn nursery. Although some hospitals only provide level II or higher care, this is not the majority and is unlikely to explain this result. In addition, some hospitals require that infants be transferred to a particular level of care before antimicrobial agents can be administered. However, even if AU is consistently 0 in lower levels of care as a result of this practice, we still ask that hospitals report data from those units.
Although the AU Option provides an effective platform to collect and monitor AU, it has several limitations. Because reporting is voluntary, hospitals participating may differ from those not yet participating, and AU described in summary metrics, such as the SAAR, may not be representative of AU in all hospitals. In addition, no patient-level data are reported, and SAAR predictive models are limited to facility and location characteristics. Although some variability in neonatal AU is explained by these characteristics, there is little doubt that patient-level data would greatly improve the SAARs’ predictive abilities. In addition, levels of care are defined by using NHSN’s location mapping schema, and patient mix within a specific level of care may differ across hospitals or within hospitals across units. Also, as many hospitals emphasize family-centered care, well newborns are often roomed with their mother rather than a separate designated nursery, which may increase the complexity of accurately capturing AU in this population if hospitals inadvertently map infants in the NHSN to their mothers’ rooms rather than to a level I nursery.
The CDC works to mitigate these limitations in various ways. New survey questions are aimed to identify potential differences in patient population within facilities and are used to clarify how well newborns receiving antimicrobial agents are mapped in the NHSN. All survey responses will be considered as adjustments in SAAR models. In addition, the CDC plans to launch a neonatal component in the NHSN to provide hospitals with a platform to report patient-level neonatal late-onset sepsis and meningitis data. Once fully implemented, AU data can be analyzed in the presence of infection data, further informing us of factors important for improved risk adjustment in future SAAR models.
Despite these limitations, the AU Option has served as a valuable resource for hospitals seeking to better understand their AU and for the CDC to monitor AU across facilities, states, and throughout the nation. When looking at national rates of neonatal AU among hospitals reporting to the AU Option, significant variations across facilities and units are evident. Schulman et al6 found that among California NICUs, rates of AU were highly variable and independent of proven infection, necrotizing enterocolitis, surgical volume, and mortality and recommends working to identify warranted ranges of AU for each level of care. Data reported to the AU Option will continue to enable tracking of interfacility variation in neonatal AU.
As new stewardship interventions are introduced, AU data will enable the assessment of their impact. For example, rates of ampicillin and gentamicin can be compared before and after introduction of the early-onset sepsis calculator20 to determine if this tool is associated with decreased rates of antibiotics used empirically for early-onset sepsis. Or, as another example, many experts recommend oxacillin and gentamicin for suspected late-onset sepsis rather than vancomycin and gentamicin, unless infants are colonized with methicillin-resistant Staphylococcus aureus. According to data submitted to the AU Option, rates of oxacillin are low, and vancomycin remains the third most commonly used agent in NICUs, suggesting perhaps clinicians are unaware of current practice recommendations or hospitals have high rates of methicillin-resistant Staphylococcus aureus colonization.
These types of analyses will become even more meaningful as reporting to the AU Option increases. Hospitals interested in participating can learn more about system requirements and the implementation process on NHSN’s AUR Module Web site.21,22 Participation in the AU Option requires that facilities have either eMAR or BCMA systems in place for inpatient locations and that data are properly packaged using a standard Health Level Seven format. In a VON survey of 141 hospitals with NICUs, it was found that 92% of hospitals have an eMAR system and 94% have a BCMA system. The CDC provides current and prospective AU Option participants with resources and assistance to help guide implementation, submission, and validation efforts.13,15,21–23
As we look ahead to neonatal SAAR development, information provided here can serve as encouragement for hospitals not yet enrolled in NHSN’s AU Option to become involved and for current participants to ensure data submitted is complete and valid. While we plan to provide risk-adjusted AU benchmark metrics using patient-level information in the future, in the near term, SAARs adjusted for hospital- and location-level factors can serve as a starting place for hospitals to investigate at which points their neonatal AU may deviate from what is predicted.
CONCLUSIONS
Accurately monitoring AU in a timely manner is an important part of neonatal antimicrobial stewardship and patient care.
The NHSN’s AU Option provides hospitals with the surveillance tools necessary to electronically track and efficiently interpret neonatal AU, identify opportunities for improvement, and assess the effectiveness of interventions.
Supplementary Material
ACKNOWLEDGMENTS
We thank all members of our ASP-SIG for their subject matter expertise, ongoing contributions, and support toward improving antimicrobial stewardship in neonates and many hours dedicated to helping us develop neonatal-specific NHSN Annual Hospital Survey questions and SAAR agent categories.
FINANCIAL DISCLOSURE: Dr Buus-Frank is the executive vice president and director of Quality Improvement and Education at the Vermont Oxford Network (VON); Dr Horbar receives salary as the chief executive and scientific officer of the VON and is an unpaid member of the board of directors. He receives grant funding from the VON and the Centers for Disease Control and Prevention (CDC; Safety and Healthcare Epidemiology Prevention Research Development Program [SHEPheRD]; assessing the validity and reliability of the National Healthcare Safety Network [NHSN] neonatal late-onset sepsis and meningitis case criteria and case ascertainment process [contract 200-2016-91783 domain 5 task order]); Dr Edwards is an employee of the University of Vermont and receives grant funding from the VON and the CDC (SHEPheRD; assessing the validity and reliability of the NHSN neonatal late-onset sepsis and meningitis case criteria and case ascertainment process [contract 200-2016-91783 domain 5 task order]); Dr Guzman-Cottrill is a consultant with the Oregon Health Authority Healthcare-Associated Infections Program; Dr Soll is an employee of the University of Vermont and the VON. He receives grant funding from the CDC (SHEPheRD; assessing the validity and reliability of the NHSN neonatal late-onset sepsis and meningitis case criteria and case ascertainment process [contract 200-2016-91783 domain 5 task order)]) and the Gerber Foundation, for which he conducts neonatal systematic reviews regarding preterm infant nutrition; the other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: No external funding.
Footnotes
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
REFERENCES
- 1.Bhat R, Custodio H, McCurley C, et al. Reducing antibiotic utilization rate in preterm infants: a quality improvement initiative. J Perinatol. 2018;38(4):421–429 [DOI] [PubMed] [Google Scholar]
- 2.Hornik CP, Fort P, Clark RH, et al. Early and late onset sepsis in very-low-birth-weight infants from a large group of neonatal intensive care units. Early Hum Dev. 2012;88(2 suppl 2):S69–S74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simonsen KA, Anderson-Berry AL, Delair SF, Davies HD. Early-onset neonatal sepsis. Clin Microbiol Rev. 2014;27(1): 21–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klinger G, Levy I, Sirota L, Boyko V, Lerner-Geva L, Reichman B; Israel Neonatal Network. Outcome of early-onset sepsis in a national cohort of very low birth weight infants. Pediatrics. 2010;125(4). Available at: www.pediatrics.org/cgi/content/full/125/4/e736 [DOI] [PubMed] [Google Scholar]
- 5.Schrag SJ, Farley MM, Petit S, et al. Epidemiology of invasive early-onset neonatal sepsis, 2005 to 2014. Pediatrics. 2016;138(6):e20162013. [DOI] [PubMed] [Google Scholar]
- 6.Schulman J, Dimand RJ, Lee HC, Duenas GV, Bennett MV, Gould JB. Neonatal intensive care unit antibiotic use. Pediatrics. 2015;135(5):826–833 [DOI] [PubMed] [Google Scholar]
- 7.Cantey JB, Huffman LW, Subramanian A, et al. Antibiotic exposure and risk for death or bronchopulmonary dysplasia in very low birth weight infants. J Pediatr. 2017;181:289–293.e1 [DOI] [PubMed] [Google Scholar]
- 8.Dellit TH, Owens RC, McGowan JE Jr, et al. ; Infectious Diseases Society of America; Society for Healthcare Epidemiology of America. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159–177 [DOI] [PubMed] [Google Scholar]
- 9.Fridkin S, Baggs J, Fagan R, et al. ; Centers for Disease Control and Prevention (CDC). Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63(9): 194–200 [PMC free article] [PubMed] [Google Scholar]
- 10.Ho T, Buus-Frank ME, Edwards EM, et al. Adherence of newborn-specific antibiotic stewardship programs to CDC recommendations. Pediatrics. 2018; 142(6):e20174322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cantey JB, Wozniak PS, Sánchez PJ. Prospective surveillance of antibiotic use in the neonatal intensive care unit: results from the SCOUT study. Pediatr Infect Dis J. 2015;34(3):267–272 [DOI] [PubMed] [Google Scholar]
- 12.Cantey JB, Patel SJ. Antimicrobial stewardship in the NICU. Infect Dis Clin North Am. 2014;28(2):247–261 [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. CDC locations and descriptions and instructions for mapping patient care locations. Available at: www.cdc.gov/nhsn/PDFs/pscManual/15LocationsDescriptions_current.pdf. Accessed April 16, 2018
- 14.American Academy of Pediatrics Committee on Fetus and Newborn. Levels of neonatal care. Pediatrics. 2012;130(3): 587–597 [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Antimicrobial Use and Resistance (AUR) Module Available at: www.cdc.gov/nhsn/pdfs/pscmanual/11pscaurcurrent.pdf. Accessed March 25, 2018
- 16.American Hospital Association. AHA annual survey database. Available at: https://www.ahadataviewer.com/additional-data-products/AHA-Survey/. Accessed August 9, 2017
- 17.van Santen KL, Edwards JR, Webb AK, et al. The Standardized Antimicrobial Administration Ratio: a new metric for measuring and comparing antibiotic use. Clin Infect Dis. 2018;67(2):179–185 [DOI] [PubMed] [Google Scholar]
- 18.Livorsi DJ, O’Leary E, Pierce T, et al. A novel metric to monitor the influence of antimicrobial stewardship activities. Infect Control Hosp Epidemiol. 2017; 38(6):721–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scheetz MH, Crew PE, Miglis C, et al. Investigating the extremes of antibiotic use with an epidemiologic framework. Antimicrob Agents Chemother. 2016; 60(6):3265–3269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warren S, Garcia M, Hankins C. Impact of neonatal early-onset sepsis calculator on antibiotic use within two tertiary healthcare centers. J Perinatol. 2017; 37(4):394–397 [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. National Healthcare Safety Network: surveillance for antimicrobial use and resistance options. Available at: www.cdc.gov/nhsn/acute-care-hospital/aur/index.html. Accessed March 28, 2018
- 22.Centers for Disease Control and Prevention. NHSN CDA submission support portal: getting started. Available at: www.cdc.gov/nhsn/cdaportal/gettingstarted.html. Accessed March 28, 2018
- 23.Centers for Disease Control and Prevention. AU Option implementation data validation. Available at: www.cdc.gov/nhsn/pdfs/ps-analysis-resources/aur/AU-Option-Implementation-Data-Validation-P.pdf. Accessed March 28, 2018
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


