Abstract
Introduction
Tremor is a disabling symptom of Multiple Sclerosis (MS). The development of objective methods of tremor characterisation to assess intervention efficacy and disease progression is therefore important. The possibility of using a Fast Fourier Transform (FFT) method for tremor detection was explored.
Methods
Acceleration from a wrist-worn device was analysed using FFTs to identify and characterise tremor magnitude and frequency. Processing parameters were explored to provide insight into the optimal algorithm. Participants wore a wrist tri-axial accelerometer during 9 tasks. The FAHN clinical assessment of tremor was used as the reference standard.
Results
Five people with MS and tremor (57.6 ± 15.3 years, 3 F/2M) and ten disease-free controls (42.4 ± 10.9 years, 5 M/5F) took part. Using specific algorithm settings tremor identification was possible (peak frequency 3–15Hz; magnitude greater than 0.06 g; 2 s windows with 50% overlap; using 2 of 3 axes of acceleration), giving sensitivity 0.974 and specificity 0.971 (38 tremor occurrences out of 108 tasks, 1 false positive, 2 false negatives). Tremor had frequency 3.5–13.0 Hz and amplitude 0.07–2.60g.
Conclusions
Upper limb tremor in people with MS can be detected using a FFT approach based on acceleration recorded at the wrist, demonstrating the possibility of using this minimally encumbering technique within clinical practice.
Keywords: Multiple sclerosis, tremor, wrist accelerometer sensor, Fast Fourier Transform analysis, algorithm development
Introduction
Multiple Sclerosis (MS) is one of the most common inflammatory chronic diseases of the central nervous system in young adults,1 affecting an estimated 2.3 million people worldwide. The main disease mechanism is the demyelination of axons leading to neuronal death. Symptoms can include visual disturbances, problems with coordination and balance, muscular weakness, impaired cognition, memory problems and tremor. The development of uncontrolled tremor can have a significant impact on the performance of everyday tasks2,3 and is one of the most disabling features in MS.3,4 This can lead to the loss of independence and can therefore pose an increasing burden on carers and health services. There is limited knowledge as to the exact prevalence of tremor in people with MS, with estimates ranging from 25 – 58% based on self-report.5–7
Gaining accurate figures on the prevalence of tremor is confounded by several factors. Firstly, MS is often of a relapsing remitting nature with symptoms occurring intermittently.3 Secondly, tremor is not routinely assessed in clinical practice. Typically the severity of general disability in people with MS is assessed with the Expanded Disability Status Scale (EDSS) which does not assess tremor,3 or the Kurtzke’s functional systems scale for cerebellar function,8 which also does not assess tremor directly.
Tremor may occur as rest tremor or action tremor.9 Action tremor can occur as postural or kinetic tremor. Kinetic tremor is further subdivided into simple (non-targeted), intention (targeted) and isometric (against a non-moving object). Tremor may therefore occur as an isolated movement or superimposed on other primary movement patterns.
To describe tremor it is necessary to know type, location, dominant frequency and amplitude of movement.9 Clinical rating scales for tremor include the FAHN tremor rating scale10 which has high intra (rs = 0.87-1.00) and inter rater (rs = 0.69-0.99) reliability11 based on visual assessment. However, this does not provide objective measurements of tremor magnitude and frequency. Objective measures have been reported using for example electromyography (EMG), accelerometry and inertial measurement units.12–14 However, these have often used multiple sensor units,14 or assessment sites that interfere with activities (e.g. on the index finger12,15,16) Due to the cumbersome nature of these measurement systems they have not been suitable for routine implementation in clinical practice. A less encumbering system is needed to allow practical, routine clinical use for the detection of tremor in people with MS.
The routine objective assessment of tremor would be invaluable in establishing prevalence and exploring intervention efficacy for both pharmacological and non-pharmacological therapies (e.g. limb cooling,17 physiotherapy, weight bracelets,18 and orthoses2,19) Drug therapies for tremor are currently seen as controversial and inconclusive.2,3,20 There is a need for a minimally encumbering objective measurement system for the detection of tremor in people with MS.
To address this requirement, an analysis algorithm was developed based on Fast Fourier Transform (FFT) techniques to detect tremor from accelerometer measurements recorded at the wrist. The hypothesis was that tremor could be isolated from other movements based on the frequency and amplitude of the tremor related components of movement. Such a device would allow routine objective assessment of tremor occurrence in people with MS.
Methods
People with MS and tremor and people without MS or tremor were recruited and observed whilst performing standardised tasks within a laboratory environment. A wrist worn accelerometer was used to develop a method of isolating tremor occurrence from other movements performed. The reference standard for the occurrence of tremor was based on observation against a standard scale (modified Fahn–Tolosa–Marin tremor rating scale). Clinical aspects of this work have been previously reported.21
Participant recruitment
Participants with MS-related upper limb tremor were identified by clinic staff and recruited through clinics in two health boards in Scotland as well as online through the UK MS Society. All participants had to be a minimum of 18 years old and diagnosed with MS according to the McDonald criteria.22 To be included, participants had to experience tremor in one or both upper limbs. Participants who were recruited online had to self-report tremor, while in those recruited through the National Health Service of the UK (NHS), tremor was clinically identified. Potential participants with a history of tremor prior to the diagnosis of MS, those who experienced other neurological conditions in addition to MS and those who had experienced a relapse within 30 days of taking part were excluded from participation. A control population was recruited from a Scottish university. The healthy controls had to be older than 18 years, with no functional impairment of the upper limbs or any other self-reported neurological conditions. Controls were not matched in age to the participants with MS.
Ethical approval was granted by the West of Scotland Research Ethics Committee for recruitment within NHS Scotland and the University Ethics Committee for recruitment outside of the NHS. All participants gave written and informed consent.
Data collection
To observe and identify tremor, participants were asked to complete a number of tasks based on assessments within the modified Fahn–Tolosa–Marin tremor rating scale (FAHN)10,11 and the Action Research Arm Test (ARAT).22,23 These tasks were selected from these assessments to allow the inclusion of a range of different types of actions including: rest poses, active support of the arms against gravity and targeted and non-targeted actions. A Health and Care Professions Council registered Occupational Therapist, with over 30 years of experience working with people with MS-related tremor, observed and rated tremor according to the standardised rating scale (0 – no tremor, 1 – mild tremor (<1cm), 2 – moderate tremor (1-5cm), 3 – marked tremor (5-10cm), 4 – severe tremor (>10cm)).10 The following tasks were all performed under formal observation once (to avoid fatigue) in a seated position:
Task 1 (FAHN): Maintaining posture against gravity – both arms outstretched in front of the body, shoulders in 90° flexion.
Task 2 (FAHN): Movement against gravity – arm stretched to the lateral side, shoulder in 90° abduction, then the finger was brought to the nose three times by flexing the elbow.
Task 3 (FAHN): Pouring water – from one firm plastic cup (8 cm tall, 7 cm in diameter) to another, not resting them on each other.
Task 4 (ARAT): Pick up a wooden cube, edge length 10 cm, and place it on the shelf of the ARAT table (37 cm above the table surface).
Task 5 (ARAT): A repetition of Task 3.
Task 6 (ARAT): Move two hollow metal tubes (diameters 2.5 cm and 1 cm) and place them over stick-holders at the back of the table (30 cm to the back, 5 cm raised from the table top).
Task 7 (ARAT): Pick up a ball bearing (diameter 6 mm) between the thumb and ring finger and place it in a petri dish on the shelf of the ARAT table.
Task 8 (ARAT): Bring the hand from a resting position on the thigh to the back of the head.
Task 9: Self-selected daily activity, reported to be affected by tremor. The control population was asked to colour in a rectangle (20x10cm) as quickly as possible as this was regarded as a tremor mimicking movement.
Tasks were performed in the above order by all participants. A practice of each task was allowed before data collection. Participants were allowed to rest between tasks to minimise any effects of fatigue on results.
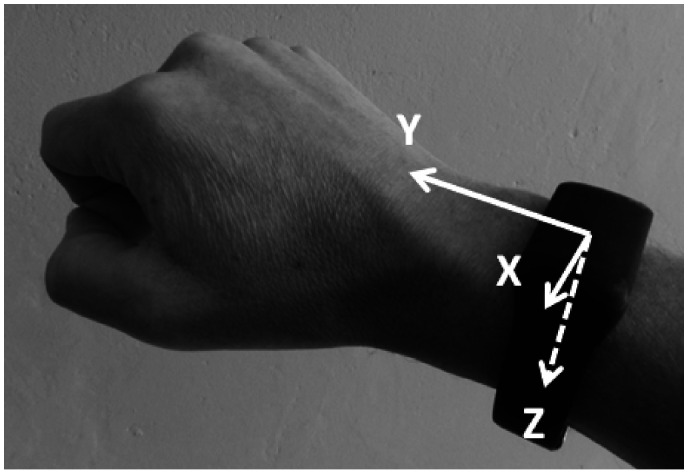
To record upper limb movement, participants were asked to wear a tri-axial accelerometer, AX3 (Axivity Sensor, Axiviy, Newcastle, UK, weight = 9 gram, 35x24x9mm), mounted to the wrist of the more affected arm in a plastic casing (13 gram). The accelerometer was attached dorsally on the forearm approximately 4 cm proximal to the wrist joint. The sensitive axes of the sensor were aligned with the longitudinal axis of the forearm, at right angles to the dorsal surface of the wrist and with the third axis perpendicular to these two (Figure 1). Data was collected at a nominal sampling frequency of 100 Hz and a range of ±8 g. The signal was synchronised to a video record of the movements by manually creating a spike in the signal by tapping the sensor. This synchronisation was used to identify the start and stop times of each activity in the sensor output.
Figure 1.
The location of the accelerometer with orientation of sensor axes: y-pointing distally, z-palmar directed, x-perpendicular to y and z.
Data analysis
First the raw signals were conditioned. Then, settings within a FFT analysis algorithm were explored to establish the optimal design for tremor detection. While there are numerous methods of analysing time frequency series, the FFT method of data analysis was chosen within the current study. This method was chosen as it allowed the identification of frequency components and related signal magnitude.
The raw output was downloaded from the accelerometer in binary format and converted to csv-format using the AX3 software package (Open Movement, V1.0.0.29) for analysis in Matlab (Matlab R2013a, The MathWorks Inc., USA). Whilst data collection was set to 100 Hz the devices actually recorded at 97.44 ± 1.64 Hz. The data was therefore interpolated (‘interp1’ Matlab function) to 100.00 Hz for further analysis.
Limited evidence from the literature about the characteristic of tremor in people with MS suggests a frequency range of 3-8Hz.5,6 However, in other conditions tremor has been detected up to 12 Hz.24 Therefore, to remove high frequency noise whilst preserving the tremor signal, a low-pass Butterworth filter of ninth order with a cut off frequency of 15 Hz was implemented (zero phase shift ‘filtfilt’ Matlab function).
Fast Fourier Transforms were used to characterise the frequency spectrum of the signals (‘fft’ Matlab function applied to each axis and resultant signal). To implement this analysis several parameters had to be set. These included the length of window for analysis and the overlap between window samples. Two different window lengths were trialled; 2 second windows, resulting in a frequency resolution of 0.394 Hz, and 3 second windows, resulting in an increased frequency resolution, of 0.196 Hz. In consideration of the temporal resolution two different settings were explored. One setting was without any overlap between the windows, the other with 50% overlap. The maximum frequency component of the signal was determined and used in further analysis.
A minimum frequency tremor threshold of 3 Hz was set, effectively identifying frequencies above as potential tremor. Minimum amplitude for the identification of tremor was imposed to reduce the likelihood of false detection. For 2 second windows a value of 0.06 g and for 3 second windows a value of 0.05 g was set. These thresholds were not directly optimised, but set based on observations of movement during tremor. Total displacement of movements with this amplitude at a frequency of 3 Hz (tremor threshold) would have been below approximately 2.5 mm (estimated based on an assumption of sinusoidal movement). Tremor was identified as occurring during windows with peak frequency between 3-15Hz with amplitude above the threshold for that window length. Any occurrence of tremor during a Task was considered a positive result for that task.
It was anticipated that tremor of the upper limb would occur in more than one axis of the accelerometer. Therefore, to optimise detection of tremor and to minimise false detection, combinations of axes outcomes were used. Two methods of achieving this were implemented. In the first, tremor was only considered to occur if it was detected in at least two out of three axes of the accelerometer, i.e. both the frequency and amplitude criteria had to be met in a minimum of two out of three axes. In the second, tremor was considered to occur if a suitable signal was detected in the resultant of the acceleration signal, i.e. the analysis was performed on the resultant acceleration signal.
The test accuracy for the tremor recognition algorithm (index test) was established against the reference standard of clinically assessing tremor (the target condition) with the use of the FAHN tremor rating scale. Tremor was considered to occur if a FAHN rating of 1 or greater was judged to be appropriate. The following definitions were used to determine the test accuracy: true positive outcomes were correctly identified tremor instances; false negatives were non-identified tremor instances by the index test that were identified as tremor by the reference standard; false positives were identified instances of tremor by the index test where there was no tremor identified by the reference standard; true negatives correctly classified non-tremor cases. The settings described above were explored to determine the occurrence, frequency and amplitude of tremor. Sensitivity, specificity and prediction rates were calculated. The optimum settings for the tremor detection algorithm were chosen based on the best test accuracy.
Data of the control population was used to test the chosen algorithm for falsely identified tremor (false-positive outcomes).
Results
Forty-nine potential participants expressed interest in taking part in the study. Eleven were not suitable as they did not meet the inclusion/exclusion criteria. Unfortunately, a further 26 declined to take part in the study once they had been fully informed of the requirements (3 no reason given, 7 not willing to travel, 5 requested payment for participation, 1 personal reasons, 5 medical reasons (worsening of symptoms), 5 were only looking for a treatment for their tremor or MS).
Initially a total of 12 people were accepted into the study. However, of these 12 only 5 exhibited tremor during data collection. Those who self-reported tremor, but did not exhibit tremor were all diagnosed with relapsing remitting MS (age 47.1 ± 12.5 years. 4 female, 3 male. 14.1 ± 11.6 years since diagnosis). All five participants who exhibited tremor were diagnosed with secondary progressive MS (age 57.6 ± 15.3 years. 3 female, 2 male. 13.5 ± 3.1 years since diagnosis). Three participants experienced both postural and action tremors (simple kinetic and intention tremor); the other two experienced action tremors only.
A further ten participants were recruited as control population. Their mean age was 42.4 ± 10.92 years (5 female, 5 male).
Out of a total of 108 tasks the therapist identified 38 cases of tremor. For detection of these tremor episodes using the wrist worn accelerometer, using 2 second windows was favourable over 3 second windows due to a higher sensitivity to tremor (Table 1). Furthermore, higher test accuracy was achieved in all cases with the 2 out of 3 axes condition in comparison to using the resultant acceleration signal. An overlap between analysis windows increased the test accuracy further. Between all different settings, the highest tremor recognition rate was achieved with the 2 out of 3 axes criteria when using 2-second windows with 50% overlap (37 true positives = 97.4% tremor recognition rate) (Table 1). Only one instance of tremor remained unidentified while tremor was falsely identified in two cases. One of these was a voluntary shaking of the hand which the therapist (correctly) classified as no tremor. In the second instance tremor could not be identified due to other, more prominent frequency contents. Generally, using the resultant signal produced the lowest positive prediction values (0.880-0.937) and the highest rate of false positive outcomes (up to 7 instances).
Table 1.
The outcomes of tremor recognition algorithm run with different parameters; 2 and 3 second windows, with and without 50% overlap. The results are shown for the use of the resultant signal and the 2 out of 3 axes condition.
| True positives | False negatives | False positives | True negatives | Sensitivity/Specificity | ||
|---|---|---|---|---|---|---|
| 2 secondsno overlap | 2 out of 3 | 35 | 3 | 1 | 69 | .921/.971 |
| resultant | 35 | 3 | 3 | 67 | .921/.943 | |
| 3 secondsno overlap | 2 out of 3 | 34 | 4 | 0 | 70 | .868/1 |
| resultant | 28 | 10 | 0 | 70 | .684/1 | |
| 2 seconds50% overlap | 2 out of 3 | 37 | 1 | 2 | 68 | .974/.971 |
| resultant | 37 | 1 | 7 | 63 | .974/.900 | |
| 3 seconds50% overlap | 2 out of 3 | 35 | 3 | 0 | 70 | .921/1 |
| resultant | 31 | 7 | 3 | 67 | .789/.971 | |
Using the algorithm on the data of the control population, there were no false positive outcomes for tremor recognition during Tasks 1-8 (sensitivity = 1, specificity = 1, prediction value = 1). ‘Tremor’ was only detected during the tremor simulation (Task 9) when participants were asked to colour in a rectangle as fast as possible. The peak frequencies for this task ranged from 6.1-13.9 Hz with amplitude 0.13–1.08g.
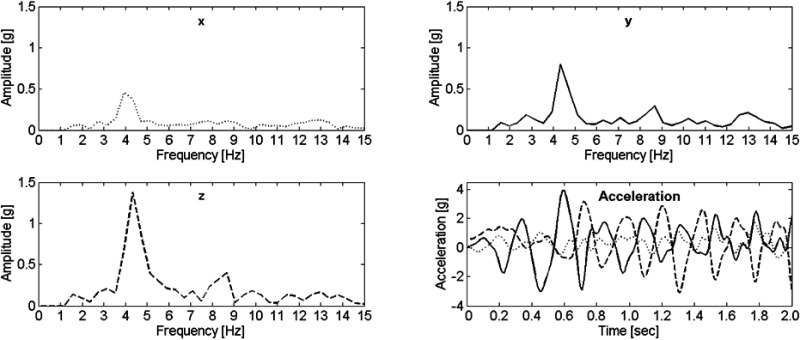
An automatically identified instance of tremor is shown in Figure 2. Tremor was identified on all three axes and the amplitude spectra show clear peaks at 4.3 Hz (y and z) and 3.9 Hz (x).
Figure 2.
The amplitude spectra of an automatically detected instance of tremor for all three axes and the corresponding acceleration signal. y-pointing distally (solid line), z-palmar directed (dashed line), x-perpendicular to y and z (dotted line).
The tremor characteristics identified using this algorithm for those participants experiencing tremor during the assessment are detailed in Table 2. The frequency range of which tremor was observed to occur was 3.5–13.0 Hz with amplitudes ranging from 0.07–2.60g. For four out of the five participants who exhibited tremor the range of mean frequency was 4.3–5.2 Hz, with a single participant at a much higher mean frequency of 10.5 Hz. The two participants exhibiting the highest mean amplitude of tremor also demonstrated wide amplitude ranges.
Table 2.
Results of participants with tremor when analysed with the 2 out of 3 axis condition and a 2 second window (50% overlap). The top row gives the expert FAHN tremor rating. The outcomes are given for the 2 axes with overall mean and standard deviation values. Frequencies are given in Hz, while the amplitudes are given in g (multiples of 9.81 ms−2) beneath the corresponding frequencies. Blank entries indicate that tremor was not observed during these tasks.
| Task 1 | Task 2 | Task 3 | Task 4 | Task 5 | Task 6 | Task 7 | Task 8 | Task 9 | Mean (SD) | |
|---|---|---|---|---|---|---|---|---|---|---|
| P003 | ||||||||||
| FAHN rating | 2 | 2 | 3 | 2 | 3 | 2 | 2 | 3 | 0 | |
| Frequency | 10.6/10.6 | 13.0/11.5 | 10.6/10.6 | 13.0/7.9 | 10.6/10.6 | 11.0/9.5 | 11.4/9.1 | 9.4/8.7 | – | 10.5 (1.4) |
| Amplitude | 0.24/0.22 | 2.60/1.5 | 0.94/0.75 | 0.84/0.23 | 0.94/0.75 | 1.30/0.45 | 0.48/0.30 | 1.54/1.38 | – | 0.90 (0.64) |
| P007 | ||||||||||
| FAHN rating | 1 | 4 | 3 | 3 | 3 | 3 | 3 | 3 | 4 | |
| Frequency | 8.3/3.9 | 4.3/4.3 | 5.9/3.6 | 6.3/4.3 | 5.9/3.6 | 4.3/4.3 | 7.5/4.3 | 8.3/4.7 | 4.7/4.3 | 5.2 (1.5) |
| Amplitude | 0.52/0.29 | 1.52/0.65 | 0.46/0.30 | 1.96/0.40 | 0.46/0.30 | 1.01/0.69 | 0.87/0.33 | 0.43/0.20 | 1.65/0.71 | 0.71 (0.51) |
| P009 | ||||||||||
| FAHN rating | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | |
| Frequency | 4.3/4.3 | 4.7/4.7 | 4.7/4.3 | 4.3/4.3 | 4.7/4.3 | 4.7/4.3 | 4.7/4.3 | 4.7/3.1 | 7.1/5.1 | 4.6 (0.7) |
| Amplitude | 0.10/0.07 | 0.19/0.10 | 0.16/0.11 | 0.14/0.10 | 0.19/0.11 | 0.31/0.15 | 0.15/0.13 | 0.19/0.16 | 0.13/0.12 | 0.15 (0.05) |
| P010 | ||||||||||
| FAHN rating | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | |
| Frequency | – | 6.3/3.9 | 3.9/3.9 | 4.7/4.3 | 5.9/3.5 | 4.3/3.9 | 3.9/3.5 | – | 3.9/3.6 | 4.3 (0.9) |
| Amplitude | – | 0.23/0.18 | 0.10/0.09 | 0.30/0.30 | 0.29/0.18 | 0.13/0.12 | 0.16/0.13 | – | 0.35/0.18 | 0.20 (0.08) |
| P011 | ||||||||||
| FAHN rating | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | |
| Frequency | – | – | 4.7/4.7 | 4.7/4.7 | 4.7/4.7 | 9.1/4.7 | – | – | 4.7/4.7 | 5.1 (1.4) |
| Amplitude | – | – | 0.40/0.25 | 0.13/0.13 | 0.25/0.13 | 0.24/0.22 | – | – | 0.29/0.18 | 0.22 (0.09) |
Discussion
A wrist worn sensor was used to record outcomes from an accelerometer in those with MS who reported upper limb tremor. Direct observation of standard activities was used to determine the reference standard of tremor occurrence against the outcomes of the FFT signal analysis algorithm. The frequency content of the accelerometer signal was examined and the best combination of algorithm settings determined for the detection of tremor during a set of clinically relevant activities. A 2 s window length, 50% overlap using a ‘2 out of 3 axes’ occurrence within a frequency range of 3–15Hz and an amplitude above 0.06 g gave optimal results. A sensitivity of 0.974 and a specificity of 0.971 were achieved.
While the participants with MS were all either self-reported or were clinically assessed to have tremor, only five out of the twelve actually exhibited recognisable tremor during the activities studied. This perhaps highlights the variable nature of the disease with symptoms being inconsistent over time. This is reinforced by the fact that all those exhibiting tremor had a diagnosis of secondary progressive MS whereas those who did not had a diagnosis of relapsing-remitting MS. In those that did exhibit tremor there was a range of severity according to the FAHN rating scale from 1 to 4 across the participants. This clinically assessed tremor severity range was mirrored by a wide range in both frequency (3.5–13.0 Hz) and magnitude (0.07–2.60g) of objectively measured tremor.
For the reference standard for tremor detection the clinical FAHN tremor rating scale was used. This required an expert health professional to judge if tremor was occurring and how severe this tremor was. For the current study the health professional assessing the movements had many years of experience of examining patients with MS and using this clinical rating scale, providing reassurance that outcomes were reliable. Use of multiple experienced raters, with documented processes to reach agreement, would have enhanced confidence in the observational outcomes. Additionally, detection of tremor might have been enhanced by examining muscle activity. However, by using the FAHN scale a typically used clinical assessment tool was employed.
The use of an accelerometer to detect the movements associated with tremor was considered to be the optimal approach for tremor detection for use in a routinely applicable sensor. The sensor could be worn easily (mounted using a wrist strap) and required no expert set up (as would EMG for example). This compares favourably with multisensory systems14 and more cumbersome sets ups.12,15,16 For example, Pulliam et al16 used a sensor strapped to the back of the base of the index finger and Heldman et al12 used a sensor on the index finger tip. Both these configurations potentially interfere with natural movements. These movement-sensor-based systems, did allow monitoring of tremor during daily activities, but it is unclear how the monitor configurations affected behaviour. The nature of tremor as an abnormal cyclical movement of a body part with a frequency different from usual movements during the tasks being performed presented the opportunity to identify the relevant signal from an accelerometer’s output.
Analysis of signals using FFTs is only one of the possible options for isolating specific movement components. An alternative to the detection of individual frequency components would be to attempt to detect specific patterns through such techniques as wavelet decomposition25 or empirical mode decomposition.26 These techniques would have enhanced the analysis in allowing the detection of more complex movement consisting of different frequency sub-components. These analyses should be explored in further work.
There was evidence that tremor movements are complex and often not aligned with a particular axis of the sensor. This was evidenced by the best outcomes which required frequency content to be identified in at least 2 out of 3 axes. Further, the poor performance of analysis of the resultant signal indicated that tremor may manifest itself as a circular motion at the wrist. This would explain why the resultant signal did not contain frequency components in the required range, but separate axes of the signal did.
Using different window lengths changes the effective frequency resolution of the analysis. Shorter windows would reduce frequency resolution. This appeared to enhance the performance of the algorithm as 2 s windows were better than 3 s windows. This may indicate that for tremor there is a spread of frequency content. For the 2 s window a resolution of only 0.394 Hz was available, perhaps indicating that a peak representing a ∼0.4 Hz frequency range is suitable for detecting tremor. At this lower frequency resolution the frequency contents of the tremulous movement are binned into broad frequency categories. For higher frequency resolution analysis neighbouring frequency contents would be spread across multiple frequency points close to each other, resulting in lower individual amplitude components. However, it is also possible that when window length increases to 3 s, additional movement content dominates the tremor component, preventing detection. The enhanced performance of the 2 s windows may therefore be a reflection of enhanced temporal resolution rather than altered frequency resolution. The increased sensitivity of using 2 s windows also came with a slightly increased risk of falsely identifying tremor. However, overall the prediction values for 2 second windows were highest.
Both the 2 as well as the 3 second windows had an increased sensitivity and higher rates of false positives when overlapping windows were used to further increase the temporal resolution. Due to the increase of the prediction values in both cases it was apparent that overlapping windows were favourable compared to non-overlapping windows. While not directly addressed in the current study, if overlapping windows were to be used in a larger data set this may lead to unacceptable increases in computer processing time. If processing time were a concern then non-overlapping windows could be used, but with a risk of more false negative outcomes (Table 1).
Only 2 false positives occurred when using the 2 second window with 50% overlap. The first was identified as a voluntary shaking of the hand that a participant (P002) used during Task 7, which the therapist did not rate as tremor. For the second false positive the cause of the peak in frequency components was not clearly identifiable. No rhythmic movement was observable as the participant placed a tube on one of the sockets (Task 6) before quickly pulling back the arm. This was recorded as the only true false positive when using 2 second windows.
The control participants performed identical tasks to the participants with MS. ‘Tremor’ was only detected in the control participants during Task 9 where movements similar to tremor were mimicked. This task involved rapid movement of a pencil backwards and forward whilst colouring in a rectangular shape. No false positive tremor instances were recorded for the control participants.
The range of frequency of tremor detected within the current study (3.5–13.0 Hz) is broader than previously reported.5,6 While only one participant exhibited tremor in the high frequency range, this may suggest that the range of tremor frequency in those with MS is wider than previously reported, or that the one participant had tremor with a different underlying neurological cause compared to the majority of participants.
The tasks used within this analysis were based on those used within typical clinical assessment of functional capability. They all involved the participant being in a seated position and, therefore, did not include secondary bodily movements like walking. It is not possible to say from the results of the current analysis if the algorithm would be able to accurately isolate tremor instances from free-living movement patterns. Future developments of this work would be targeted at isolation of tremor occurrence during free-living daily activities to allow long term characterisation of tremor. This would require the collection of a multi-hour video based data set of free-living activity with synchronous sensor recording. Importantly, this would require a large number of tremor occurrences with concurrent activity such as ambulation and self-care.
Conclusion
Upper limb tremor in people with MS was detected using FFT analysis with high sensitivity and specificity during a series of tasks based on clinical assessment of functional capability. The best settings for the algorithm were to explore a frequency range of 3–15Hz, a magnitude greater than 0.06 g, using 2 out of 3 axes of an accelerometer signal with 2 s windows with 50% overlap. Detected tremor had peak frequency content from 3.5–13.0 Hz with amplitudes ranging from 0.07–2.60g.
This work has demonstrated that it is possible to detect and objectively quantify the occurrence of upper limb tremor in people with MS using FFTs applied to wrist acceleration. This technique provides the opportunity for routine quantification of characterisation of tremor with a minimally encumbering monitoring device, which could be implemented in studies examining the effectiveness of interventions aimed at reducing MS-related upper limb tremor.
Acknowledgements
The authors thank the MS Society for their support of this work. We would like to acknowledge the grant team for their support in securing the funding for this research: Dr Ben Stansfield, Dr Jenny Preston, Prof Frederike van Wijck, Dr Paul Mattison, Lynn Lamont, Linda Miller, Dr Sivaramkumar Shanmugam and Dr Helen Gallagher.
Footnotes
Ethical approval: Ethical approval was gained from the West of Scotland Research Ethics Committee (13–WS-0253) for recruitment within the UK National Health Service (NHS) and the School of Health and Life Sciences’ Ethics Committee of Glasgow Caledonian University for recruitment out-with the NHS (HLS 12/50).
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This research was funded as part of a research grant from the MS Society, UK (Registered charity nos 1139257/SC041990; Reference: 968/12).
Guarantor: Dr Ben Stansfield.
Contributorship: All authors contributed to the conception of the study and protocol development. ST gained ethical approval. ST and JP recruited participants. ST performed the data analysis with BS. ST wrote the first draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
ORCID iDs
Frederike van Wijck https://orcid.org/0000-0003-0855-799X
Ben Stansfield https://orcid.org/0000-0003-1581-2928
References
- 1.Multiple Sclerosis International Federation, 2014. Atlas of MS 2013, London, www.msif.org/wp-content/uploads/2014/09/Atlas-of-MS (accessed December 2019).
- 2.Koch M, Mostert J, Heersema D, et al. Tremor in multiple sclerosis. J Neurol 2007; 254: 133–145 (accessed December 2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Labiano-Fontcuberta A, Benito-León J. Understanding tremor in multiple sclerosis: prevalence, pathological anatomy, and pharmacological and surgical approaches to treatment. In: Louis E (ed.) Tremor and other hyperkinetic movements, 2(0):tre-02-109-765-2, 2012. [DOI] [PMC free article] [PubMed]
- 4.Feys P, Romberg A, Ruutiainen J, et al. Interference of upper limb tremor on daily life activities in people with multiple sclerosis. Occup Ther Health Care 2004; 17: 81–95. [DOI] [PubMed] [Google Scholar]
- 5.Alusi SH, Worthington J, Glickman S, et al. A study of tremor in multiple sclerosis. Brain 2001; 124: 720–730. [DOI] [PubMed] [Google Scholar]
- 6.Pittock SJ, McClelland RL, Mayr WT, et al. Prevalence of tremor in multiple sclerosis and associated disability in the Olmsted County population. Mov Disord 2004; 19: 1482–1485. [DOI] [PubMed] [Google Scholar]
- 7.Rinker JR, Salter AR, Walker H, et al. Prevalence and characteristics of tremor in the NARCOMS multiple sclerosis registry: a cross-sectional survey. BMJ Open 2015; 5: e006714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alusi SH, Glickman S, Aziz TZ, et al. Tremor in multiple sclerosis. J Neurol Neurosurg Psychiatry 1999; 66: 131–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deuschl G, Bain PG, Brin MF. Consensus statement of the movement disorder society on tremor. Ad hoc scientific committee. Mov Disord 1998; 13 Suppl 3: 2–23. [DOI] [PubMed] [Google Scholar]
- 10.Fahn S, Tolosa E, Marin C. Parkinson’s disease and movement disorders: Clinical rating scale for tremor. In: Jankovic A, Tolosa E. (eds) Clinical rating scale for tremor Munich: Urban und Schwarzenberg, 1988. [Google Scholar]
- 11.Hooper J, Taylor R, Pentland B, et al. Rater reliability of Fahn’s tremor rating scale in patients with multiple sclerosis. Arch Phys Med Rehabil 1998; 79: 1076–1079. [DOI] [PubMed] [Google Scholar]
- 12.Heldman DA, Jankovic J, Vaillancourt DE, et al. Essential tremor quantification during activities of daily living. Parkinsonism Relat Disord 2011; 17: 537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Breit S, Spieker S, Schulz JB, et al. Long-term EMG recordings differentiate between Parkinsonian and essential tremor. J Neurol 2008; 255: 103–111. [DOI] [PubMed] [Google Scholar]
- 14.Ketteringham L, Neild S, Hyde R, et al. Measuring intention tremor in multiple sclerosis using inertial measurement unit (IMU) devices. In: Proceedings of the international conference on biomedical electronics and devices (BIODEVICES-2011), Rome, Italy, 26-29 January 2011; 04–211.
- 15.Giuffrida JP, Riley DE, Maddux BN, et al. Clinically deployable kinesia technology for automated tremor assessment. Mov Disord 2009; 24: 723–730. [DOI] [PubMed] [Google Scholar]
- 16.Pulliam CL, Eichenseer SR, Goetz CG, et al. Continuous in-home monitoring of essential tremor. Parkinsonism Relat Disord 2014; 20: 37–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feys P, Helsen W, Liu X, et al. Effects of peripheral cooling on intention tremor in multiple sclerosis. J Neurol Neurosurg Psychiatry 2005; 76: 373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hewer RL, Cooper R, Morgan MH. An investigation into the value of treating intention tremor by weighting the affected limb. Brain 1972; 95: 579–590. [DOI] [PubMed] [Google Scholar]
- 19.O'Connor RJ, Kini MU. Non-pharmacological and non-surgical interventions for tremor: a systematic review. Parkinsonism Relat Disord 2011; 17: 509–515. [DOI] [PubMed] [Google Scholar]
- 20.Buijink AW, Contarino MF, Koelman JH, et al. How to tackle tremor – systematic review of the literature and diagnostic work-up. Front Neurol 2012; 3: 146–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teufl S, Preston J, van Wijck F, et al. Objective identification of upper limb tremor in multiple sclerosis using a wrist-worn motion sensor: establishing validity and reliability. Br J Occup Therapy 2017; 80: 596–602. [Google Scholar]
- 22.Lyle RC. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. Int J Rehabil Res 1981; 4: 483–492. [DOI] [PubMed] [Google Scholar]
- 23.Platz T, Pinkowski C, van Wijck F, et al. Reliability and validity of arm function assessment with standardized guidelines for the Fugl-Meyer test, action research arm test and box and block test: a multicentre study. Clin Rehabil 2005; 19: 404–411. [DOI] [PubMed] [Google Scholar]
- 24.Bain PG. Tremor. Parkinsonism & Related Disorders 2007; 13: S369–S374. [DOI] [PubMed] [Google Scholar]
- 25.Pang Y, Christenson J, Jiang F, et al. Assessment of tremor and bradykinesia in Parkinson’s disease. J Neurosci Methods 2020; 333: 108576. [DOI] [PubMed] [Google Scholar]
- 26.Ayache S-S, Al-Ani T, Farhat W-H, et al. Analysis of tremor in multiple sclerosis using Hilbert-Huang transform. Neurophysiol Clin 2015; 45: 475–484. [DOI] [PubMed] [Google Scholar]