Abstract
Objective
The primary aim of our study was to explore the mechanisms through which long non-coding RNA (lncRNA)-mediated sirtuin-1 (SIRT1) signaling regulates type II alveolar epithelial cell (AECII) senescence induced by a cigarette smoke-media suspension (CSM).
Methods
Pharmacological SIRT1 activation was induced using SRT2104 and senescence-associated lncRNA 1 (SAL-RNA1) was overexpressed. The expression of SIRT1, FOXO3a, p53, p21, MMP-9, and TIMP-1 in different groups was detected by qRT-PCR and Western blotting; the activity of SA-β gal was detected by staining; the binding of SIRT1 to FOXO3a and p53 gene transcription promoters was detected by Chip.
Results
We found that CSM increased AECII senescence, while SAL-RNA1 overexpression and SIRT1 activation significantly decreased levels of AECII senescence induced by CSM. Using chromatin immunoprecipitation, we found that SIRT1 bound differentially to transcriptional complexes on the FOXO3a and p53 promoters.
Conclusion
Our results suggested that lncRNA-SAL1-mediated SIRT1 signaling reduces senescence of AECIIs induced by CSM. These findings suggest a new therapeutic target to limit the irreversible apoptosis of lung epithelial cells in COPD patients.
Keywords: Chronic obstructive pulmonary disease, alveolar epithelial cells, sirtuin-1, cellular senescence, long non-coding RNA, cigarette smoke
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by degradation of pulmonary elastic tissue, destruction of alveolar structure, and enlargement of the air cavity. These factors play key roles in the progressive decline of lung function.1 A recent study reported that the prevalence of COPD in Chinese individuals older than 20 years was 8.6%.2 Current therapies have minor effects on maintenance of lung cells and preventing long-term decline of pulmonary function in patients with COPD. Senescence and apoptosis of alveolar epithelial cells in lung tissue are important pathogenic characteristics of COPD.3–5 As progenitors of type I alveolar epithelial cells, type II alveolar epithelial cells (AECIIs) help maintain the normal structure and function of alveoli.6 Prevention and treatment of alveolar injury is fundamental for COPD therapy.
Sirtuin-1 (SIRT1) is expressed in various cells and acts as an NAD+-dependent type III histone/protein deacetylase.7 SIRT1 can deacetylate p53, forkhead box O (FOXO) and other nonhistone proteins, playing an important role in gene transcription, cell senescence and energy metabolism.8 SIRT1 was reported to interact with the p53 gene promoter, inhibiting p53 activity and reducing cellular senescence.9 Previous work by us and other groups10,11 has shown that SIRT1 levels were decreased in the lung tissues of patients with COPD, suggesting that SIRT1-mediated cell senescence may play a key role in the development of COPD. Both FOXO3 and p53 are transcription factors that have been shown to modulate cellular senescence,12,13 and expression of FOXO3a in the airway tissues of COPD patients is correlated with the level of cellular senescence.14 Indeed, FOXO3a and p53 share regulatory mechanisms and target genes in pancreatic cancer cells,15 while their levels and activities showed an inverse pattern in tissues from patients with COPD.10
Long non-coding RNA (lncRNA) is a type of RNA found in eukaryotes.16 LncRNAs are involved in regulating gene expression at multiple levels and play important roles in cellular senescence, embryonic development and maintenance of genomic stability.17,18 Our previous study showed that the expression level of senescence-associated lncRNA 1 (SAL-RNA1) was low in senescent cells.10 However, the effects of SAL-RNA1 on AECII senescence have not been investigated. AECII senescence promotes the development of COPD; however, the mechanisms underlying cigarette smoke (CS)-induced cellular senescence and the role of SIRT1 and/or lncRNAs in preventing AECII senescence remain unclear. We therefore hypothesized that SIRT1 protects against CS-induced cellular senescence through regulation of FOXO3 and p53, and that this signaling network regulates senescence of AECIIs and development of COPD. In the present study, we isolated AECIIs in vitro and used these cells to study the mechanisms of cell senescence underlying COPD.
Materials and methods
Ethics statement
This study was approved by the Ethics Committee of Zhejiang Provincial People's Hospital (Hangzhou, China). Informed consent was obtained from all patients.
Cell culture
The human lung tissues used for primary human AECII isolation were collected from patients who underwent lung surgery at Zhejiang Provincial People's Hospital (Hangzhou, China) as previously described.19,20 Lung tissue sections were perfused with sterile saline solution. Sections were treated with 25–35 mL of tissue digestion solution (containing 2–3 mL of 100 kU/mL trypsin and 300 μL of elastase) for 50 minutes and then shaken for 5 minutes with DNase I (Sigma, St. Louis, MO, USA) at 25°C. The sections were perfused with an equal volume of Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F-12; Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS; HyClone, Logan, UT, USA) and DNase I (10 kU/mL) to terminate digestion. Tissue debris and nonadherent AECIIs were removed by filtration. The cells were resuspended in DMEM/F-12. Separation solution was added and anti-CD14 magnetic beads (Miltenyi, Bergisch Gladbach, Germany) were used to separate macrophages. AECIIs were maintained in DCCM‑1 medium (Thermo Fisher Scientific, Waltham, MA, USA) containing 10% FBS and 1% penicillin–streptomycin–glutamine (Thermo Fisher Scientific) at 37°C under a humidified atmosphere containing 5% CO2.
Electron microscopy
After fixing AECIIs for at least 4 hours, the cells were rinsed with phosphate-buffered saline (PBS) three times and dehydrated in a graded series of alcohol (50%, 70%, 80%, 90%, 95%, 100%, and 100% again; 15 minutes each). After drying, the coated samples were placed into the scanning electron microscope for observation and the images were collected.
Treatment with CS-media suspension (CSM)
CS was dissolved in 50 mL of DMEM (Gibco, Thermo Fisher Scientific) and sterilized using a 0.22-µm pinhole filter. The resulting CS-media suspension (CSM) was used within 30 minutes. The remaining samples were stored in a −80°C freezer. This solution was considered to represent 100% CS extract (CSE) and diluted to the desired concentration for each experiment in DMEM containing 10% FBS. AECIIs were plated in a six-well plate and cultured at 37°C under a humidified atmosphere containing 5% CO2. After cells had attached to plates, the culture medium was discarded. CSMs were washed three times with PBS to eliminate the influence of serum.
Lentivirus production and transduction
Recombinant lentiviral vectors encoding green fluorescent protein (GFP) and lncRNAs were constructed by Hanheng Biotech Co., Ltd. (Shanghai, China). Lentiviral vectors encoding GFP alone (LV-GFP) were prepared using the same method as controls. AECIIs were seeded in six-well plates. When confluency reached between 70% and 80% after approximately 24 hours, AECIIs were transduced with lentiviruses at a multiplicity of infection (MOI) of 20. Transduction efficiency was quantified via the percentage of GFP-positive cells in immunofluorescence assays. The effects of LV-SAL-RNA1 on the expression of SAL-RNA1 were assess using reverse transcription quantitative polymerase chain reaction (RT-qPCR) analysis. The primers used for SAL-RNA1 RT-qPCR are shown in Table 1.
Table 1.
Primers used for reverse transcription and quantitative polymerase chain reaction in this study.
| Genes | Primers | |
|---|---|---|
| RT-PCR | SIRT1 | Forward: 5′-CGTCTTATCCTCTAGTTCTTGTG-3′ |
| Reverse: 5′-ATCTCCATCAGTCCCAAATCC-3′ | ||
| FOXO3a | Forward: 5′-GAACGTGGGGAACTTCACTGGTGCTA-3′ | |
| Reverse: 5′-GGTCTGCTTTGCCCACTTCCCCTT-3′ | ||
| p53 | Forward: 5′-ATGAGCCGCCTGAGGTTGG-3′ | |
| Reverse: 5′-CAGCCTGGGCATCCTTGAGT-3′ | ||
| p21 | Forward: 5′-TGGCACCTCACCTGCTCTG-3′ | |
| Reverse: 5′-GTTTGGAGTGGTAGAAATCTGTCAT-3′ | ||
| 18SrRNA | Forward: 5′-CAGCCACCCGAGATTGAGCA-3′ | |
| Reverse: 5′-TAGTAGCGACGGGCGGTGTG-3′ | ||
| MMP-9 | Forward: 5′-GAGTGGACGCGACCGTAGTTG-3′ | |
| Reverse: 5ʹ-CGTCTTGTACTGCTTGCCCAGGAAGA-3′ | ||
| TIMP-1 | Forward: 5′-CTTGGTTCCCTGGCGTACTC-3′ | |
| Reverse: 5′-ACCTGATCCGTCCACAAACAG-3′ | ||
| SAL-RNA1 | Forward: 5ʹ-GGATCTATTTCCGGTGAATTCGCCACCAAGTAGAAATATGGTAAGGG-3ʹ | |
| Reverse 5ʹ-TCTAGAACTAGTCTCGAGAGTGGGGGGTTGGTGGTGG-3ʹ | ||
| qPCR | p53 (promoter) | Forward: 5ʹ-CATTGTTGTATTCCTGAGTGCCTAT-3ʹ |
| Reverse: 5ʹ-AGGTCTTGCACCTCTTCTGCAT-3ʹ | ||
| FOXO3a (promoter) | Forward: 5ʹ-GGATCTATTTCCGGTGAATTCGCCACCAAGTAGAAATATGGTAAGGG-3ʹ | |
| Reverse 5ʹ-TCTAGAACTAGTCTCGAGAGTGGGGGGTTGGTGGTGG-3ʹ |
qPCR, quantitative polymerase chain reaction; RT-PCR, reverse transcription polymerase chain reaction; SIRT1, sirtuin-1; FOX3a, forkhead box O3a; MMP-9, matrix metalloprotein 9; SAL-RNA1, senescence-associated lncRNA 1; TIMP-1, metallopeptidase inhibitor 1.
Administration of SRT2104
SRT2104 was administered as described previously.21 Cells were cultured in DMEM supplemented with 10% bovine serum albumin and penicillin-streptomycin. The cells were treated with vehicle (0.1% dimethyl sulfoxide) or 3 µM SRT2104 for 24 hours and collected for subsequent assays.
Cell groups and treatments
Cells were randomly assigned to four groups: group A (AECII control group), group B (AECII senescence group), group C (SIRT1 selective agonist SRT2104 group), and group D (LV-SAL-RNA1 transduction group). Cells in groups B (AECII senescence group) and C (SRT2104 group) were stimulated with 10% CSM. Cells in group C (SRT2104 group) were first incubated with SRT2104 and then stimulated with CSM to stimulate AECIIs. Cells in group D (LV-SAL-RNA1 transduction group) were first treated with LV-SAL-RNA1 and then stimulated with CSM.
Senescence‑associated β‑galactosidase (SA‑β‑gal) activity assay
CSM-treated AECIIs were fixed in 4% paraformaldehyde (Beyotime Institute of Biotechnology, Shanghai, China) for 20 minutes and washed twice with PBS. SA-β-gal activity was assessed using an in situ β-galactosidase staining kit (Beyotime Institute of Biotechnology) according to the manufacturer's protocol. Five fields of view from each of three sections from each AECII sample were examined using a light microscope (Olympus Corporation, Tokyo, Japan). SA-β-gal activity was measured by the rate of conversion of 4-methylumbelliferyl-β-D-galactopyranoside (MUG) to the fluorescent hydrolysis product 4-methylumbellifer-one (4-MU) at pH 6.0. MUG, 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-gal), and other chemicals were obtained from Sigma–Aldrich. The assay was performed as described previously.22
RT-qPCR
Cells were harvested for and RNA was isolated using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. The final RNA purity and concentration were determined using a spectrophotometer. cDNA was synthesized from the total RNA using PrimeScript™ RT with gDNA Eraser (TaKaRa Bio, Otsu, Japan) according to the manufacturer's protocol. The qPCR was performed using a SYBR-Green qPCR kit (TaKaRa Bio) and the Agilent MX3000P qPCR system (Agilent Technologies, Santa Clara, CA, USA). The thermocycling conditions were as follows: initial denaturation at 95°C for 15 minutes, followed by 45 cycles of denaturation at 95°C for 15 s, annealing at 58°C for 15 s, and elongation at 72°C for 1 minute. The primer sequences are shown in Table 1. All analyses were performed in triplicate, and 18S RNA was used as a reference. Relative RNA abundance was quantified by the comparative 2−ΔΔCq method.23
Western blotting
Proteins were extracted from AECIIs using radioimmunoprecipitation assay buffer (Beyotime Institute of Biotechnology). Protein concentrations were measured using a micro bicinchoninic acid protein assay kit (Thermo Fisher Scientific) according to the manufacturer's protocol. The protein lysates were separated by SDS-PAGE and electrotransferred to polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA, USA). The membranes were blocked with Tris-buffered saline containing 0.1% Tween-20 (TBS-T) and 5% bovine serum albumin (Sigma-Aldrich) for 2 hours at room temperature and then incubated with primary antibodies against p53 (#9282; 1:1000 in TBS-T; Cell Signaling Technology, Danvers, MA, USA), p21 (sc-397; 1:500 in TBS-T; Santa Cruz Biotechnology, Dallas, TX, USA), FoxO3a (#12829; 1:1000 in TBS-T; Cell Signaling Technology), SIRT1 (#2493; 1:1000 in TBS-T; Cell Signaling Technology) and β-actin (ab8227; 1:1000 in TBS-T; Abcam, Cambridge, UK) overnight at 4°C. The membranes were subsequently incubated with horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (#35552; 1:5000 in TBS‑T; Thermo Fisher Scientific) secondary antibody for 1 hour at room temperature on an orbital shaker. The blots were developed using an electrochemiluminescence solution (Thermo Fisher Scientific) and imaged using an UVP-GDS8000 gel analysis system (UltraViolet Products, Ltd., Cambridge, UK). Western blotting experiments were repeated three times.
Chromatin immunoprecipitation (ChIP)
ChIP assays were performed using an EMD ChIP chromatin immunoprecipitation kit (Millipore, Bedford, MA, USA) following the manufacturer’s instructions. Cells were fixed with 1% formaldehyde at 20°C for 10 minutes and resuspended in 1 mL of sodium dodecyl sulfate lysis buffer to obtain chromatin. The samples were homogenized using a desktop sonicator at low settings (amplitude 35%). The chromatin solution was incubated with primary antibodies and protein A/G agarose immunoprecipitation reagent overnight at 4°C with rotation. Human IgG was used as the negative control, and DNA (input) was used as the positive control. The samples were washed with LiCl and Tris-ethylenediaminetetraacetic acid buffers. Reverse crosslinking was performed at 65°C overnight. DNA was purified using a DNA purification kit (Qiagen, Valencia, CA, USA). DNA fragments were eluted from the protein A/G agarose reagent and precipitated. Finally, the purified DNA was used for RT-PCR amplification with specific primers (Table 1).
Statistical analysis
Statistical analysis was performed using SPSS version 21.0 (SPSS, Inc., Chicago, IL, USA). Data were expressed as means ± standard deviations. Differences among multiple groups were assessed using one-way analysis of variance and differences between two groups were assessed using the Student’s t-test. Values of p < 0.05 were considered statistically significant.
Results
Isolation and characterization of AECIIs
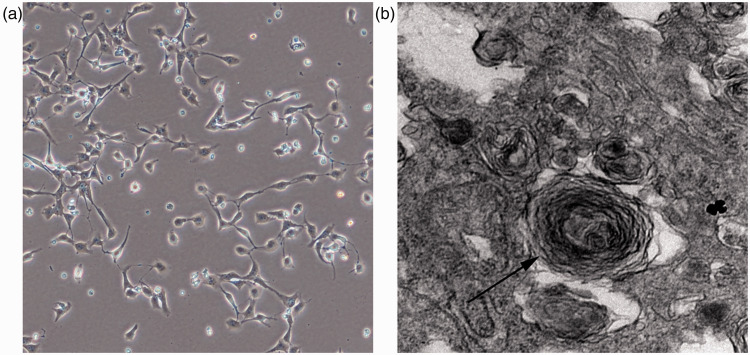
We successfully isolated and characterized primary AECIIs from human lung tissue as shown by electron microscopy (Figure 1).
Figure 1.
Type II alveolar epithelial cells (AECIIs) were successfully isolated and cultured from human lung tissue, then characterized using the Public Test platform of Medical College of Zhejiang University under transmission electron microscopy (a) AECII cells were cultured for 24 hours and then adhered to the well. (b) The structures of concentric round lamellar bodies were observed by electron microscopy.
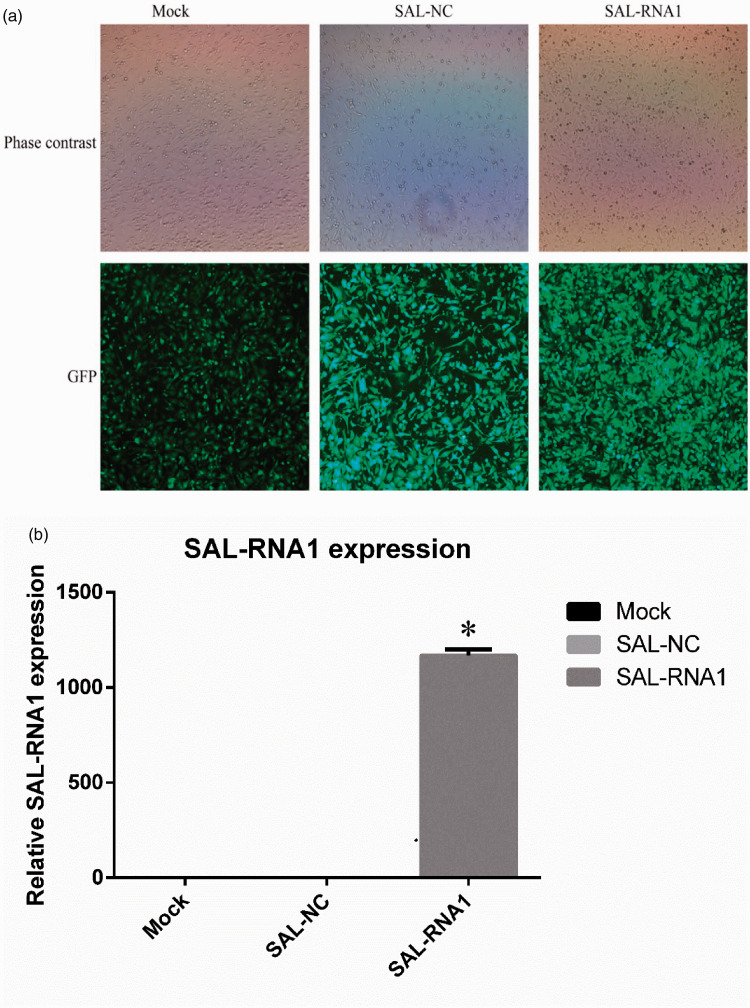
Lentiviral transduction with SAL-RNA1 constructs upregulated the expression of SAL-RNA1 in AECIIs
Lentiviral transfection efficiency was determined by fluorescence microscopy. Green-colored cells indicated successful transduction, and immunofluorescence assays were used to measure GFP expression percentage (Figure 2). The effects of LV-SAL-RNA1 transduction (MOI 20) on expression of SAL-RNA1 were assessed by qRT-PCR. Forty-eight hours after transfection with LV-SAL-RNA1, the mRNA abundance of SAL-RNA1 was significantly increased compared with control cells (p < 0.05) (Figure 2). These results demonstrated that LV-SAL-RNA1 transduction resulted in overexpression of SAL-RNA1 in AECIIs.
Figure 2.
Lentiviral transfection of type II alveolar epithelial cells (AECIIs) with a senescence-associated lncRNA 1 (SAL-RNA1) expression construct. A: Fluorescence images of AECIIs transduced with LV-SAL-RNA1 at a multiplicity of infection (MOI) of 20 are shown. B: The effects of LV-SAL-RNA1 (MOI 20) on the expression of SAL-RNA1 were assessed using quantitative real-time PCR. Data represent the means ± standard deviations of three samples from a single experiment representative of three independent experiments. *p < 0.05 vs. the SAL-NC (negative control) group. Mock refers to blank control. GFP, green fluorescent protein.
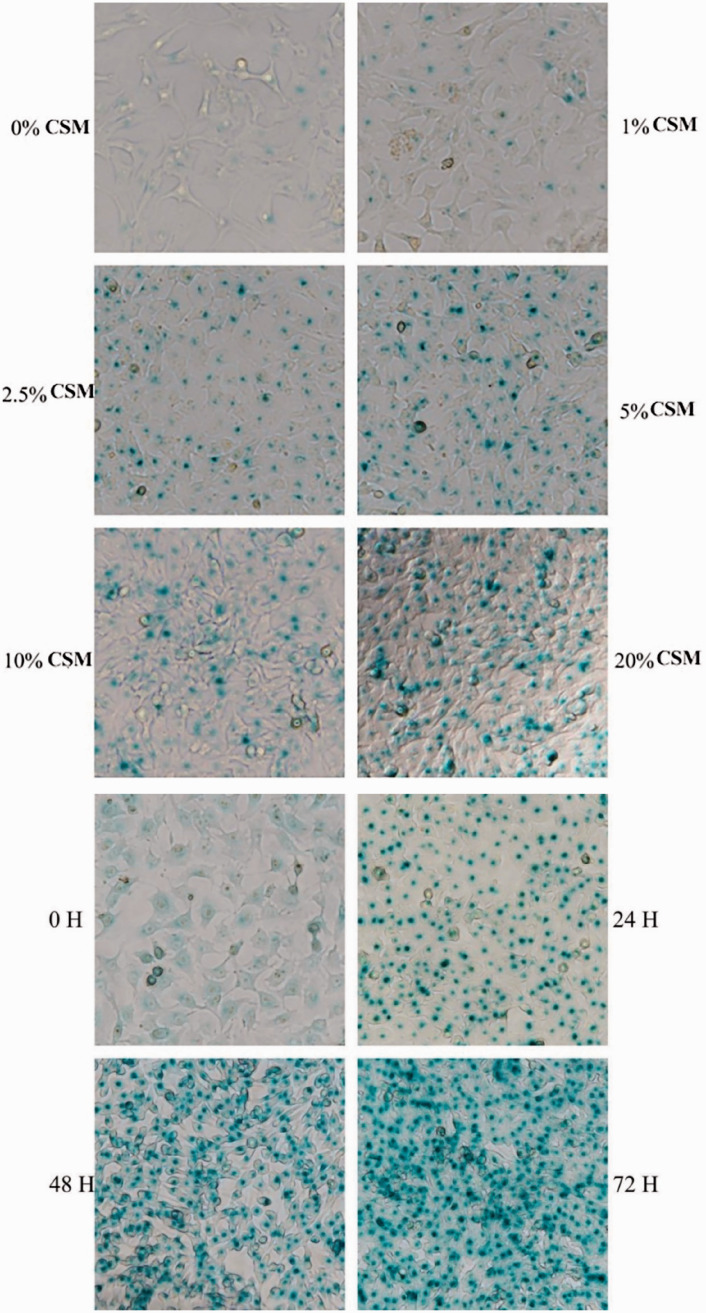
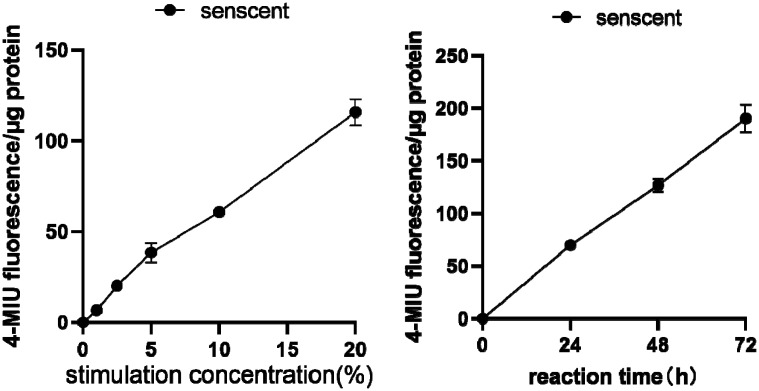
Effects of different concentrations of CSM on senescence in AECIIs
To study the effects of different CSM concentrations and stimulation times on AECIIs, we treated AECIIs with CSM (0%, 1%, 2.5%, 5%, 10%, or 20%) for 24, 48 or 72 hours. SA-β-gal activity was assayed in AECIIs to determine whether cellular senescence occurred in a dose-dependent and time-dependent manner. AECIIs showing blue color were considered SA-β-gal positive and senescent. As concentration of CSM increased and stimulation time was prolonged, SA-β-gal activity in AECIIs significantly increased (Figure 3). These results showed that CSM promoted senescence of AECIIs in a time- and dose-dependent manner.
Figure 3.
Effects of different cigarette smoke-media suspension (CSM) concentrations and stimulation times on type II alveolar epithelial cells (AECIIs). AECIIs were treated with different CSM concentrations (0%–20%) or treated with 10% CSE for different durations (0–48 hours). Senescence‑associated β‑galactosidase (SA-β-gal) activity was detected by staining. The cells were incubated with 4-methylumbelliferyl-β-D-galactopyranoside fluorogenic substrate at 37°C. Aliquots were removed from the reaction mixture over time (0–48 hours) and the fluorescence intensity of the hydrolysis product 4-methylumbellifer-one was measured. Readings were divided by the total protein per assay to correct for differences in extract concentration. SA-β-gal activity was increased in AECIIs with increasing CSM concentration and prolonged incubation time. Cells with blue-green staining indicated SA-β‑gal‑positive cells. Original magnification, ×200.
SIRT1 expression was decreased in CSM-treated AECIIs
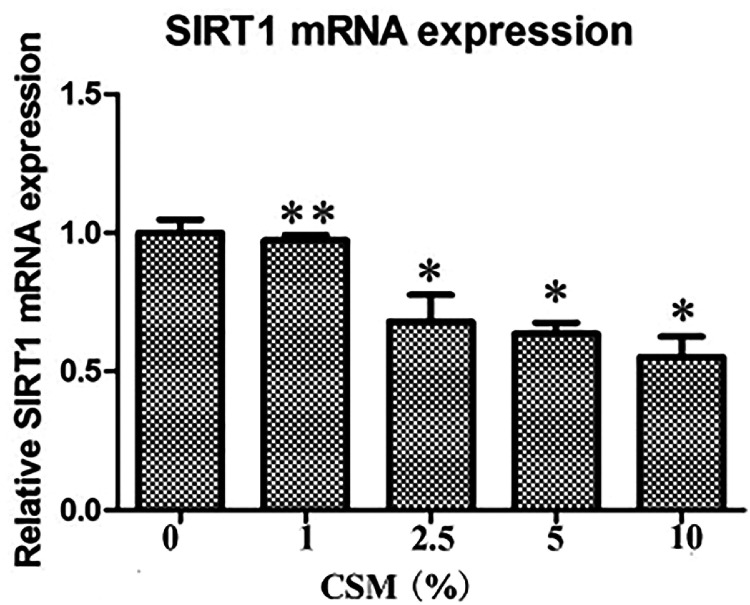
RT-qPCR was used to assess the effect of CSM stimulation on SIRT1 mRNA levels in AECIIs. Treatment of AECIIs with 0% to 10% CSM significantly decreased the mRNA abundance of SIRT1 in AECIIs compared with control cells in a dose-dependent manner (Figure 4).
Figure 4.
Sirtuin-1 (SIRT1) expression is decreased in cigarette smoke-media suspension (CSM)-treated type II alveolar epithelial cells (AECIIs). AECIIs were treated with CSM (0%–10%) for 24 hours. Total RNA was isolated, and SIRT1 mRNA levels were assessed by quantitative real-time PCR. Data represent the means ± standard deviations of three samples from a single experiment representative of three independent experiments. *p < 0.05 vs 0% CSE, **p > 0.05 vs 0% CSE.
Effects of CSM on FOXO3a, p53, p21, matrix metalloprotease (MMP)-9 and tissue inhibitor of metalloprotease (TIMP)-1 expression in AECIIs
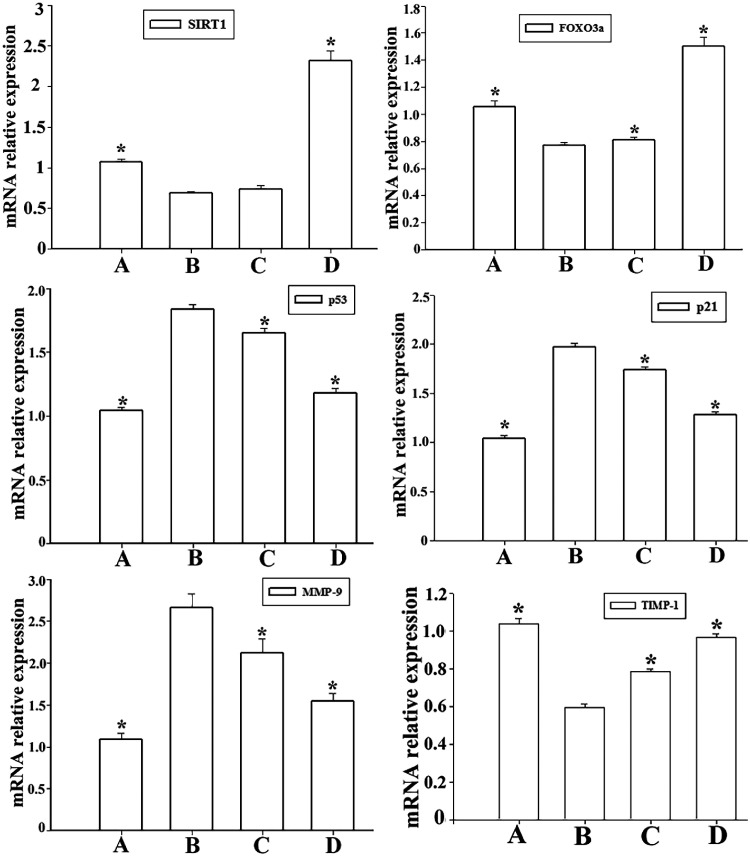
We also investigated the expression of FOXO3a, p53, p21, MMP-9 and TIMP-1 in CSM-treated AECIIs. Expression of FOXO3a, p53, p21, MMP-9 and TIMP-1 was assessed using western blotting and RT-qPCR (Figure 5 and Figure 6). Compared with control AECIIs, FOXO3a expression was downregulated by CSM at the protein and mRNA levels. However, p53 and p21 expression was increased significantly following CSM treatment. Accordingly, the MMP-9 mRNA and protein levels were significantly increased, while the expression of TIMP-1 was decreased (p < 0.05).
Figure 5.
The sirtuin-1 (SIRT1) activator SRT2104 suppresses p53 expression and rescues FOXO3a expression in cigarette smoke-media suspension (CSM)-treated type II alveolar epithelial cells (AECIIs). The AECIIs were treated with SRT2104 and then stimulated with 10% CSM for 24 hours. Total RNA was isolated, and the mRNA levels of SIRT1, forkhead box O3a (FoxO3a), p53, p21, matrix metalloprotease (MMP)-9, and tissue inhibitor of metalloprotease (TIMP)-1 were assessed by quantitative real-time PCR. Data represent the means ± standard deviations of three samples from a single experiment representative of three independent experiments. *p < 0.05 vs. senescent AECIIs. Groups: A (AECII control group), B (senescent AECII group), C (SIRT1 selective agonist SRT2104 group) and D (LV-SAL-RNA1 transduction group).
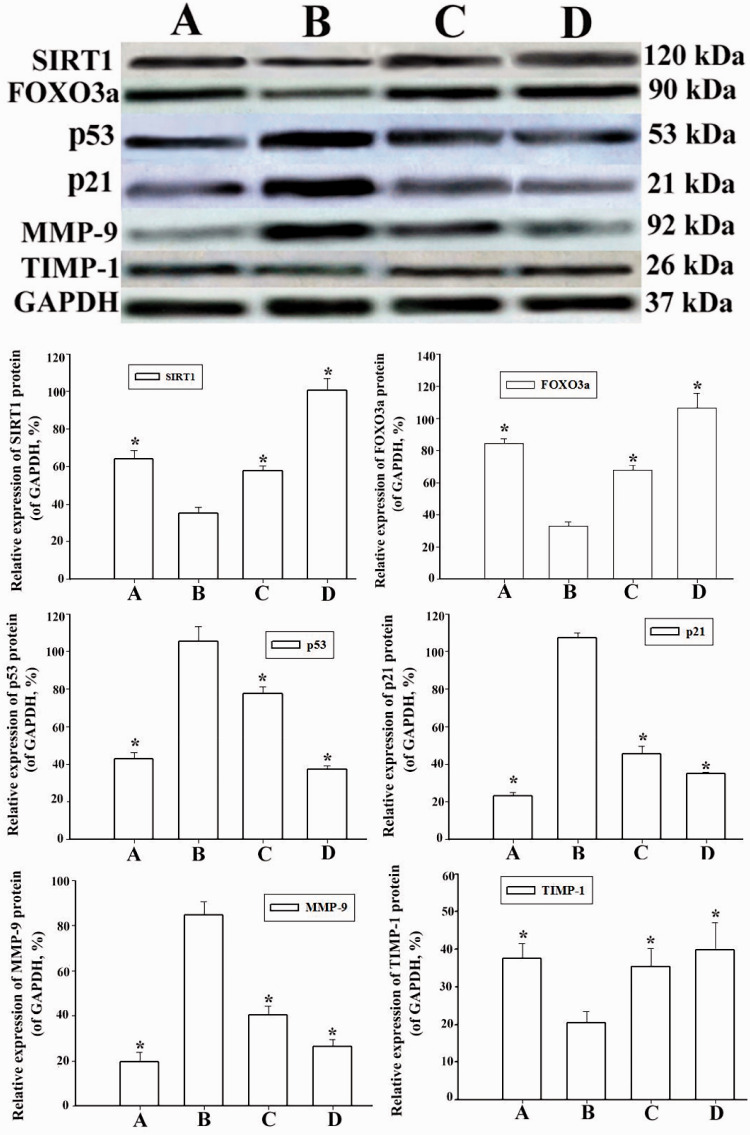
Figure 6.
Overexpression of senescence-associated lncRNA 1 (SAL-RNA1) upregulates sirtuin-1 (SIRT1) expression and suppresses p53 expression. AECIIs were transduced with the lentiviral vector LV-SAL-RNA1 and then treated with 10% cigarette smoke-media suspension (CSM) for 24 hours. Protein expression was assessed by western blotting. Bar graphs show densitometric analysis of blotting for SIRT1, forkhead box O3a (FoxO3a), p53, p21, matrix metalloprotease (MMP)-9, and tissue inhibitor of metalloprotease (TIMP)-1. Relative protein abundance was calculated using glyceraldehyde 3-phosphate (GAPDH) as the reference. Data represent the means ± standard deviations of three samples from a single experiment representative of three independent experiments. *p < 0.05 vs. senescent AECIIs. Groups: A (AECII control group), B (senescent AECII group), C (SIRT1 selective agonist SRT2104 group) and D (LV-SAL-RNA1 transduction group).
The SIRT1 activator 2104 suppresses p53 expression and rescues FOXO3a expression in CSM-treated AECIIs
RT-qPCR and western blotting were used to assess the effects of the SIRT1 selective activator 2104 on FOXO3a, p53, p21, MMP-9 and TIMP-1 expression. We pharmacologically induced SIRT1 activation using SRT2104 in CSM-treated AECIIs. As shown in Figure 5 and Figure 6, SIRT1 downregulated p53, p21 and MMP-9 expression in CSM-induced AECIIs compared with control AECIIs, reflecting the increased senescence of CSM-treated AECIIs. In contrast, the expression of FOXO3a and TIMP-1 in SRT2104-treated AECIIs was significantly upregulated, with no significant change in SIRT1 expression.
Overexpression of SAL-RNA1 upregulates SIRT1 expression and downregulates p53 expression
To investigate the effect of SAL-RNA1 overexpression on CSM-induced AECII apoptosis, we lentivirally transduced AECIIs with a SAL-RNA1 expression construct. After 24 hours, we treated the cells with CSM. After an additional 24 hours, we measured expression of SIRT1, FOXO3a, p53, p21, MMP-9 and TIMP-1 in AECIIs by western blotting and RT-qPCR. As shown in Figure 5 and Figure 6, expression of SIRT1 and FOXO3a was significantly increased in LV-SAL-RNA1-transduced AECIIs compared with control cells, whereas p53 and p21 expression was significantly decreased. These data showed that overexpression of SAL-RNA1 downregulated p53 and p21 expression in CSM-treated AECIIs. In addition, the antiapoptotic effect of SIRT1 was substantially enhanced in SAL-RNA1-overexpressing cells (p < 0.05).
SIRT1 binds transcriptional complexes on the FOXO3a and p53 promoters of AECIIs
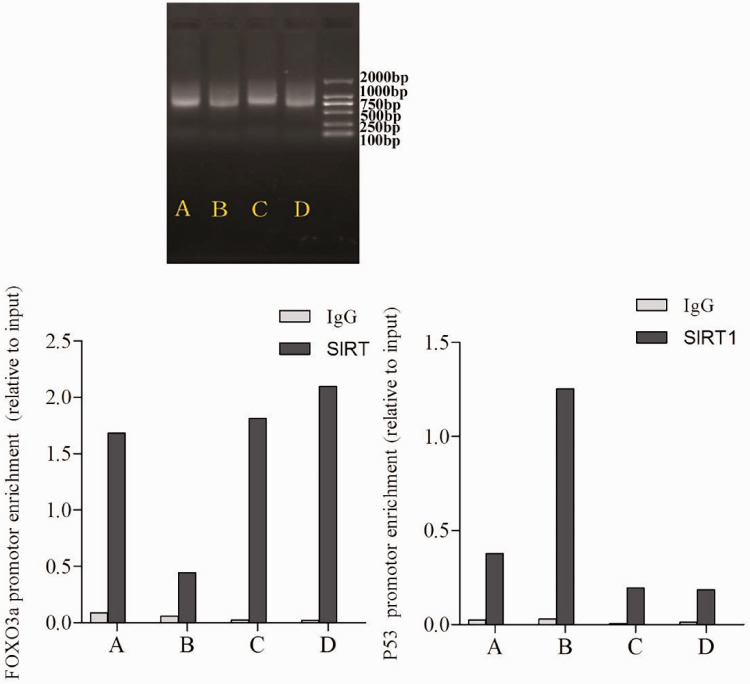
To explore whether SIRT1 was involved in binding to transcriptional complexes on the FOXO3a/p53 promoter and to study the effects of CSM on SIRT1, FOX3a and p53 promoter binding to histones, we assessed SIRT1 binding to the FOXO3a/p53 promoter regions using ChIP. As shown in Figure 7, the precipitated DNA bands were mainly around 750 bp. Binding of SIRT1 to the FOXO3a promoter was higher in control cells, SRT2104-treated cells, and LV-SAL-RNA1 transduced cells, but lower in senescent AECIIs. We further found that binding of SIRT1 to p53 was higher in senescent AECIIs compared with control cells, SRT2104-treated cells and LV-SAL-RNA1-transduced cells (Figure 7). These data indicated that SIRT1 is involved in binding transcriptional complexes on the FOXO3a and p53 promoters of AECIIs. Overexpression of SIRT1 and SAL-RNA1 increased SIRT1 binding to the FOXO3a promoter region. In contrast, CSM treatment increased SIRT1 binding to the p53 promoter region.
Figure 7.
Sirtuin-1 (SIRT1) is involved in transcription complex binding on the forkhead box O3a (FOXO3a) and p53 promoters. Chromatin immunoprecipitation (ChIP) assays were performed with chromatin prepared from type II alveolar epithelial cells (AECIIs). Chromatin was immunoprecipitated with normal rabbit IgG or antibody against SIRT1, and precipitated genomic DNA was analyzed by reverse transcription-quantitative polymerase chain reaction for the FOXO3a and p53 promoter regions. Groups: A (AECII control group), B (senescent AECII group), C (SIRT1 selective agonist SRT2104 group) and D (LV-SAL-RNA1 transduction group).
Discussion
COPD is characterized by persistent airflow limitations caused by massive inhalation of harmful particles or gases.1 Cigarette smoking is a major risk factor for development of COPD.24 CS exposure also induces cellular senescence and apoptosis of alveolar cells.25 Aging-associated SA-β-gal activity is considered an important biomarker of cellular senescence.22 Primary AECIIs are notoriously difficult to isolate. In this study, we successfully isolated and characterized primary AECIIs by electron microscopy. We found that the activity of SA-β-gal in CSM-treated AECIIs was significantly increased in a dose- and time-dependent manner. Taken together, our data showed that AECII senescence increased with increasing CSM concentration and stimulation time. Further studies are needed to understand the relationship between COPD and AECII senescence.
SIRT1 is known as a longevity gene because it is involved in deacetylation, delaying apoptosis and improving metabolism. The expression levels of SIRT1 and FOXO3a in the lung tissues of patients with COPD were significantly downregulated.11 Downregulation of SIRT1 expression promoted dysfunction and cellular senescence of endothelial progenitor cells in the lung tissues of patients with COPD.26 Thus, SIRT1-mediated cellular senescence is involved in the progression of COPD, but it remains unclear whether SIRT1 protects against CS-induced alveolar epithelial cell senescence. The current study showed that SIRT1 was substantially decreased in CSM-treated AECIIs. Furthermore, pharmacological activation of SIRT1 by SRT2104 significantly reduced AECII senescence induced by CSM. Another study27 found that SIRT1 expression was decreased in the lung tissues of patients with COPD, leading to an increase in the acetylation of TIMP-1. Nakamaru et al.28 found that SIRT1 was a negative regulator of MMP-9, and that SIRT1 activity contributed to an imbalance of TIMP-1/MMP-9 and promoted lung tissue damage. To clarify the effects of SIRT1 on AECII senescence during COPD, we used RT-qPCR and western blotting to assess SIRT1 and TIMP-1/MMP-9 mRNA and protein expression. Our findings indicated that SIRT1 is an important regulator of AECII senescence induced by CS, in agreement with a previous study.29 Thus, SIRT1 may protect against CS-induced alveolar epithelial cell senescence. However, our study did not consider the number of passages, which may be critical to our experimental results. The human lung tissues used in this study were mainly collected from the elderly; in future studies, we hope to examine tissues from young healthy donors as a comparison.
p53 is a tumor suppressor protein that can induce cellular senescence by activating p21 expression. p53 is also deacetylated by SIRT1. Preliminary research by our group and others10,30 has found that alveolar epithelial cell senescence and hypoplasia occur in the lungs of patients with COPD, as well as in the lungs of rodents exposed to high-concentration oxygen. FOXO3a, a transcription factor, is also regarded as a nonhistone substrate of SIRT1. Recent evidence31–33 has shown that SIRT1 deacetylates FOXO3a and affects its activity, inhibiting ageing-associated gene expression and promoting cell survival. Moreover, Zhao et al.15 reported that FOXO3a and p53 interact to drive tumor cell senescence. Interestingly, the expression of FOXO3a in the airway tissues of COPD patients was associated with cellular senescence level.14 Thus, we investigated whether SIRT1/FOXO3a and SIRT1/p53 constituted a signaling network regulating AECII senescence and COPD development. We found that downregulation of SIRT1 and FOXO3a expression increased AECII senescence following CSM exposure. The selective SIRT1 activator SRT2104 reduced levels of p53, p21, and MMP-9 in human AECIIs but did not affect SIRT1 expression. Additionally, SRT2104 treatment enhanced the expression of anti-aging genes including FOXO3 and TIMP1. In addition, we further demonstrated that SIRT1 binds to the FOXO3 promoter region, regulating SIRT1 transcription. These findings demonstrate that FOXO3 mediates the protective effect of SIRT1 on CSM-induced senescence and apoptosis. However, further experiments are needed to understand how SIRT1 regulates the interactions of FOXO3-dependent transcriptional machinery with the promoters of anti-aging genes. Repeating our experiment with more donors would also be beneficial. In addition, the mechanism of interaction between the FOXO3a and p53 signaling pathways remains unclear and requires further research.
lncRNAs are involved in the regulation of various diseases including cancer, diabetes, immune diseases, and Alzheimer's disease.34–37 However, few studies have shown a specific relationship between lncRNAs and COPD. Whether lncRNAs were directly involved in the regulation of SIRT1 expression was not known. Abdelmohsen et al.38first showed that decreased SAL-RNA1 levels resulted in downregulation of SIRT1 and increased p21 and p53 protein expression. Cellular senescence was also exacerbated by decreasing SAL-RNA1 levels. Thus, we investigated whether SAL-RNA1 plays a key regulatory role in AECII senescence mediated by the SIRT1 signaling network. We showed for the first time that SAL-RNA1 overexpression significantly reduced cellular senescence induced by CSM in AECIIs. Surprisingly, we found that overexpression of SAL-RNA1 promoted high expression of SIRT1 and FOXO3a and reduced expression of aging-related genes. Lentivirus-mediated SAL-RNA1 transduction was involved in the regulation of the SIRT1/FoxO3a and SIRT1/p53 signaling pathways. However, we did not assess purity and growth kinetics of LV-SAL-RNA1, which needs to be verified by further experiments
In conclusion, the present study demonstrated that SIRT1 has a protective effect on CS-induced senescence in AECIIs and increases the expression of anti-aging genes such as FOXO3a. In addition, treatment with SRT2104 showed similar effects as CSM exposure on cellular senescence. Together, these findings suggest that SIRT1 reduces CS-induced senescence and apoptosis, which may ameliorate lung inflammation, inhibit alveolar epithelial cell senescence and delay the progression of COPD. Furthermore, we found that overexpression of SAL-RNA1 resulted in decreased cellular senescence, indicating that SAL-RNA1 plays a vital role in SIRT1 signaling -mediated cellular senescence. Functional acetylation assays may reveal the mechanisms through which SIRT1 agonism and SAL-RNA1 overexpression can affect progression of COPD. Therefore, activation of SIRT1 by SRT2104 and upregulation of SAL-RNA1 may be a potential therapeutic strategy for COPD patients.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_0300060520986049 for Senescence associated long non-coding RNA 1 regulates cigarette smoke-induced senescence of type II alveolar epithelial cells through sirtuin-1 signaling by Dong Yuan, Yuanshun Liu, Mengyu Li, Hongbin Zhou, Liming Cao, Xiaoqin Zhang and Yaqing Li in Journal of International Medical Research
Supplemental material, sj-pdf-2-imr-10.1177_0300060520986049 for Senescence associated long non-coding RNA 1 regulates cigarette smoke-induced senescence of type II alveolar epithelial cells through sirtuin-1 signaling by Dong Yuan, Yuanshun Liu, Mengyu Li, Hongbin Zhou, Liming Cao, Xiaoqin Zhang and Yaqing Li in Journal of International Medical Research
Supplemental material, sj-pdf-3-imr-10.1177_0300060520986049 for Senescence associated long non-coding RNA 1 regulates cigarette smoke-induced senescence of type II alveolar epithelial cells through sirtuin-1 signaling by Dong Yuan, Yuanshun Liu, Mengyu Li, Hongbin Zhou, Liming Cao, Xiaoqin Zhang and Yaqing Li in Journal of International Medical Research
Supplemental material, sj-pdf-4-imr-10.1177_0300060520986049 for Senescence associated long non-coding RNA 1 regulates cigarette smoke-induced senescence of type II alveolar epithelial cells through sirtuin-1 signaling by Dong Yuan, Yuanshun Liu, Mengyu Li, Hongbin Zhou, Liming Cao, Xiaoqin Zhang and Yaqing Li in Journal of International Medical Research
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the National Natural Science Foundation of China (No. 81870028, 81470241 and 81900029) and the Zhejiang Provincial Program for the Cultivation of High-Level Innovative Health Talents (No. A-2017-CXCR02).
ORCID iD: Dong Yuan https://orcid.org/0000-0002-8723-5077
References
- 1.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Arch Bronconeumol 2017; 53: 128–149. [DOI] [PubMed] [Google Scholar]
- 2.Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): A national cross-sectional study. Lancet 2018; 391: 1706–1717. [DOI] [PubMed] [Google Scholar]
- 3.Singh D, Roche N, Halpin D, et al. Current controversies in the pharmacological treatment of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2016; 194: 541–549. [DOI] [PubMed] [Google Scholar]
- 4.Farkas L, Farkas D, Warburton D, et al. Cigarette smoke exposure aggravates air space enlargement and alveolar cell apoptosis in Smad3 knockout mice. Am J Physiol Lung Cell Mol Physiol 2011; 301: L391–L401. [DOI] [PubMed] [Google Scholar]
- 5.Mimae T, Hagiyama M, Inoue T, et al. Increased ectodomain shedding of lung epithelial cell adhesion molecule 1 as a cause of increased alveolar cell apoptosis in emphysema. Thorax 2014; 69: 223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guillot L, Nathan N, Tabary O, et al. Alveolar epithelial cells: Master regulators of lung homeostasis. Int J Biochem Cell Biol 2013; 45: 2568–2573. [DOI] [PubMed] [Google Scholar]
- 7.Haigis MC, Sinclair DA. Mammalian sirtuins: Biological insights and disease relevance. Annu Rev Pathol 2010; 5: 253–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Satoh A, Stein L, Imai S. The role of mammalian sirtuins in the regulation of metabolism, aging, and longevity. Handb Exp Pharmacol 2011; 206: 125–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arunachalam G, Samuel SM, Marei I, et al. Metformin modulates hyperglycaemia-induced endothelial senescence and apoptosis through SIRT1. Br J Pharmacol 2014; 171: 523–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gu C, Li Y, Liu J, et al. LncRNA-mediated SIRT1/FoxO3a and SIRT1/p53 signaling pathways regulate type II alveolar epithelial cell senescence in patients with chronic obstructive pulmonary disease. Mol Med Rep 2017; 15: 3129–3134. [DOI] [PubMed] [Google Scholar]
- 11.Rajendrasozhan S, Yang SR, Kinnula VL, et al. SIRT1, an antiinflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008; 177: 861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yasuhara S, Kanakubo E, Perez ME, et al. The 1999 Moyer award. Burn injury induces skeletal muscle apoptosis and the activation of caspase pathways in rats. J Burn Care Rehabil 1999; 20: 462–470. [DOI] [PubMed] [Google Scholar]
- 13.Guterres FA, Martinez GR, Rocha ME, et al. Simvastatin rises reactive oxygen species levels and induces senescence in human melanoma cells by activation of p53/p21 pathway. Exp Cell Res 2013; 319: 2977–2988. [DOI] [PubMed] [Google Scholar]
- 14.Ganesan S, Unger BL, Comstock AT, et al. Aberrantly activated EGFR contributes to enhanced IL-8 expression in COPD airways epithelial cells via regulation of nuclear FoxO3A. Thorax 2013; 68: 131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao G, Cui J, Zhang JG, et al. SIRT1 RNAi knockdown induces apoptosis and senescence, inhibits invasion and enhances chemosensitivity in pancreatic cancer cells. Gene Ther 2011; 18: 920–928. [DOI] [PubMed] [Google Scholar]
- 16.Batista PJ, Chang HY. Long noncoding RNAs: Cellular address codes in development and disease. Cell 2013; 152: 1298–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohsawa R, Seol JH, Tyler JK. At the intersection of non-coding transcription, DNA repair, chromatin structure, and cellular senescence. Front Genet 2013; 4: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grammatikakis I, Panda AC, Abdelmohsen K, et al. Long noncoding RNAs (lncRNAs) and the molecular hallmarks of aging. Aging (Albany NY) 2014; 6: 992–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ehrhardt C, Kim KJ, Lehr CM. Isolation and culture of human alveolar epithelial cells. Methods Mol Med 2005; 107: 207–216. [DOI] [PubMed] [Google Scholar]
- 20.Elbert KJ, Schafer UF, Schafers HJ, et al. Monolayers of human alveolar epithelial cells in primary culture for pulmonary absorption and transport studies. Pharm Res 1999; 16: 601–608. [DOI] [PubMed] [Google Scholar]
- 21.Mercken EM, Mitchell SJ, Martin-Montalvo A, et al. SRT2104 extends survival of male mice on a standard diet and preserves bone and muscle mass. Aging Cell 2014; 13: 787–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gary RK, Kindell SM. Quantitative assay of senescence-associated beta-galactosidase activity in mammalian cell extracts. Anal Biochem 2005; 343: 329–334. [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 24.Scanlon PD, Connett JE, Waller LA, et al. Smoking cessation and lung function in mild-to-moderate chronic obstructive pulmonary disease. The Lung Health Study. Am J Respir Crit Care Med 2000; 161: 381–390. [DOI] [PubMed] [Google Scholar]
- 25.Pace E, Di Vincenzo S, Ferraro M, et al. Carbocysteine counteracts the effects of cigarette smoke on cell growth and on the SIRT1/FoxO3 axis in bronchial epithelial cells. Exp Gerontol 2016; 81: 119–128. [DOI] [PubMed] [Google Scholar]
- 26.Paschalaki KE, Starke RD, Hu Y, et al. Dysfunction of endothelial progenitor cells from smokers and chronic obstructive pulmonary disease patients due to increased DNA damage and senescence. Stem Cells 2013; 31: 2813–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao H, Hwang JW, Sundar IK, et al. SIRT1 redresses the imbalance of tissue inhibitor of matrix metalloproteinase-1 and matrix metalloproteinase-9 in the development of mouse emphysema and human COPD. Am J Physiol Lung Cell Mol Physiol 2013; 305: L615–L624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamaru Y, Vuppusetty C, Wada H, et al. A protein deacetylase SIRT1 is a negative regulator of metalloproteinase-9. FASEB J 2009; 23: 2810–2819. [DOI] [PubMed] [Google Scholar]
- 29.Chun P. Role of sirtuins in chronic obstructive pulmonary disease. Arch Pharm Res 2015; 38: 1–10. [DOI] [PubMed] [Google Scholar]
- 30.Londhe VA, Sundar IK, Lopez B, et al. Hyperoxia impairs alveolar formation and induces senescence through decreased histone deacetylase activity and up-regulation of p21 in neonatal mouse lung. Pediatr Res 2011; 69: 371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yao H, Chung S, Hwang JW, et al. SIRT1 protects against emphysema via FOXO3-mediated reduction of premature senescence in mice. J Clin Invest 2012; 122: 2032–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salminen A, Kaarniranta K. Insulin/IGF-1 paradox of aging: Regulation via AKT/IKK/NF-κB signaling. Cell Signal 2010; 22: 573–577. [DOI] [PubMed] [Google Scholar]
- 33.Brunet A, Sweeney LB, Sturgill JF, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 2004; 303: 2011–2015. [DOI] [PubMed] [Google Scholar]
- 34.Novikova IV, Hennelly SP, Sanbonmatsu KY. Sizing up long non-coding RNAs: Do lncRNAs have secondary and tertiary structure? Bioarchitecture 2012; 2: 189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee C, Kikyo N. Strategies to identify long noncoding RNAs involved in gene regulation. Cell Biosci 2012; 2: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knauss JL, Sun T. Regulatory mechanisms of long noncoding RNAs in vertebrate central nervous system development and function. Neuroscience 2013; 235: 200–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng X, Gralinski L, Armour CD, et al. Unique signatures of long noncoding RNA expression in response to virus infection and altered innate immune signaling. mBio 2010; 1: e00206–e00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abdelmohsen K, Panda A, Kang MJ, et al. Senescence-associated lncRNAs: Senescence-associated long noncoding RNAs. Aging Cell 2013; 12: 890–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_0300060520986049 for Senescence associated long non-coding RNA 1 regulates cigarette smoke-induced senescence of type II alveolar epithelial cells through sirtuin-1 signaling by Dong Yuan, Yuanshun Liu, Mengyu Li, Hongbin Zhou, Liming Cao, Xiaoqin Zhang and Yaqing Li in Journal of International Medical Research
Supplemental material, sj-pdf-2-imr-10.1177_0300060520986049 for Senescence associated long non-coding RNA 1 regulates cigarette smoke-induced senescence of type II alveolar epithelial cells through sirtuin-1 signaling by Dong Yuan, Yuanshun Liu, Mengyu Li, Hongbin Zhou, Liming Cao, Xiaoqin Zhang and Yaqing Li in Journal of International Medical Research
Supplemental material, sj-pdf-3-imr-10.1177_0300060520986049 for Senescence associated long non-coding RNA 1 regulates cigarette smoke-induced senescence of type II alveolar epithelial cells through sirtuin-1 signaling by Dong Yuan, Yuanshun Liu, Mengyu Li, Hongbin Zhou, Liming Cao, Xiaoqin Zhang and Yaqing Li in Journal of International Medical Research
Supplemental material, sj-pdf-4-imr-10.1177_0300060520986049 for Senescence associated long non-coding RNA 1 regulates cigarette smoke-induced senescence of type II alveolar epithelial cells through sirtuin-1 signaling by Dong Yuan, Yuanshun Liu, Mengyu Li, Hongbin Zhou, Liming Cao, Xiaoqin Zhang and Yaqing Li in Journal of International Medical Research