Abstract
Background:
Professional soccer players are often evaluated with asymptomatic lesions of the ankle and foot, and such abnormalities may eventually become clinically relevant.
Purpose:
To ascertain the prevalence of foot and ankle abnormalities in elite professional adult soccer players.
Study Design:
Case series; Level of evidence, 4.
Methods:
Professional adult male elite soccer players (n = 37) underwent magnetic resonance imaging (MRI) scans of both their feet and ankles. All competed for their respective national junior or adult soccer teams. MRI scans were performed with 1.5-T scanners and analyzed independently by 2 experienced radiologists.
Results:
The MRI scans of 86.5% of the players showed degenerative joint disease (DJD) in at least 1 of the joints of the foot and ankle. Articular cartilage lesions in the joints of the foot and ankle were evident in 42% of the scans. Of all lesions, 17% were grade 3 or 4 (Noyes and Stabler classification) cartilage lesions and accompanied by subchondral bone marrow edema. The greater the age, weight, and height of the players, the greater was the odds ratio of DJD of the ankle joint. Synovitis in at least 1 of the joints of the foot was detected in 64% of the MRI scans. Leg dominance significantly correlated with bone marrow edema of the talus.
Conclusion:
Elite professional soccer players are often evaluated with a high prevalence of asymptomatic osteochondral lesions with subchondral bone marrow edema in the foot and ankle. These osteochondral lesions may remain asymptomatic or, with the continuing high-intensity stresses that modern professional soccer demands of its athletes, may evolve and cause foot and ankle pain. It is unclear whether and which interventions can be implemented to prevent the occurrence of these abnormalities in the first place.
Keywords: foot, ankle, degenerative joint disease, soccer, elite athletes
Worldwide, about 200,000 professional soccer athletes16,22 are exposed to a high risk of lower limb joint trauma during training and matches,3,18,29 including joint injuries of the hip, knee, and ankle.3,15,23 These joints are exposed to weightbearing and rotational motions, and as such, these athletes are at high risk of developing early degenerative joint disease (DJD).4,20,30,31,33 Professional soccer training and competition play a major role in both the cause and epidemiology of DJD of the lower limbs, given the synergy of overuse, high-impact physical activity, and the high frequency of joint trauma.3,20,30,31
Joint injuries are very common in professional soccer players,17,18 and 17% to 40% of these injuries affect the ankle.12,13,21 This might lead to a higher incidence of intra-articular abnormalities in the lower limb joints.5,28 However, the literature on asymptomatic ankle and foot abnormalities in these athletes is scarce, and the prevalence of DJD changes in professional soccer athletes is undefined. Therefore, we investigated a cohort of asymptomatic elite professional male soccer players for evidence of early signs of DJD.
Methods
This study was performed from December 2014 to January 2019. Approval to conduct the study was given by the local ethics committee, and all participating athletes provided signed informed consent. Eligible for the study were 52 professional male elite soccer players who, during the study period, underwent a thorough medical examination before signing contracts with top-tier soccer clubs of the Russian Premier League. Magnetic resonance imaging (MRI) scans of both the feet and the ankles of each player (n = 74) were taken before the players started to play for their team. Via a questionnaire, leg dominance was determined from the self-reported preferred kicking leg.
The players were included in the study if they fulfilled the following criteria: (1) age ≥ 18 years; (2) no foot and ankle complaints at the time of clinical examination; (3) no history of ankle and foot surgery; (4) no foot and ankle surgery during the 12 months after the clinical and MRI examination performed for the purposes of the present investigation; (5) a signed valid professional contract with their soccer club after successfully passing a presigning medical examination; (6) began soccer training by the age of 6 years; (7) competed for their respective national junior or adult soccer team; and (8) participated in at least 80 (80-140) matches in the professional leagues of their countries of origin.
Exclusion criteria were as follows: (1) a history of foot and ankle surgery or any other lower limb joint surgery; (2) professional soccer training starting after the age of 7; (3) participation in soccer matches during the 5 days before the MRI; (4) history of injury of the foot and ankle that caused absence from training for more than 7 days; and (5) history of peri-articular and/or intra-articular injections into the joints of the foot and ankle.
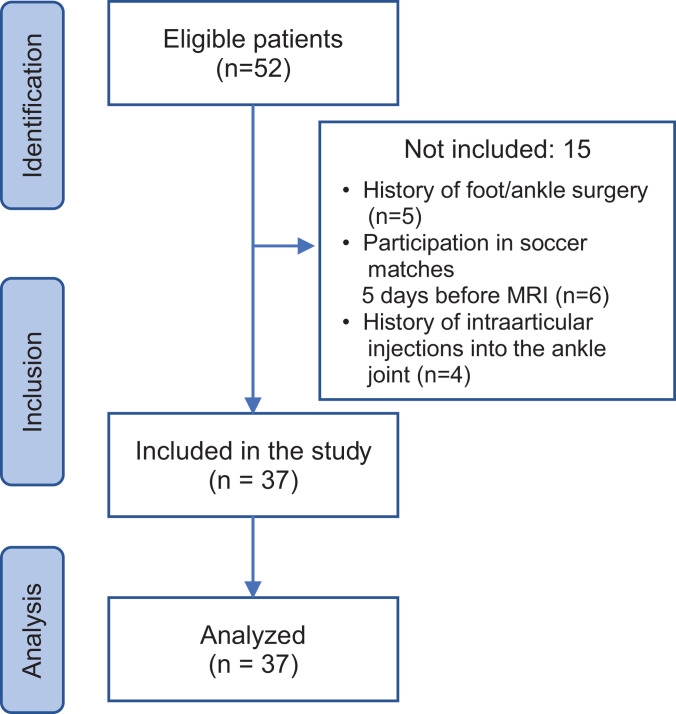
Of the 52 initial players, 37 were included in the study (mean age, 25.5 ± 4.3 years; range, 18-33 years; body mass index [BMI], 22.6 ± 1.2 kg/m2). Figure 1 shows the participant inclusion process.
Figure 1.
Flowchart for participant inclusion. MRI, magnetic resonance imaging.
Imaging
All MRI scans were performed with 1.5-T scanners (Philips Ingenia and Siemens Magnetom) with an extremity coil, using the sagittal, axial, and coronal planes with standard pulse sequences and proton density–weighted images with fat saturation (PD-WI FAT SAT), turbo inversion recovery magnitude (TIRM), T2-weighted and T1-weighted. The slice thickness was 3 mm. All images were obtained with the patient supine and nonweightbearing.
All MRI scans of the ankle and foot were analyzed to evaluate the presence of articular effusion severity, subchondral bone marrow edema (BME), DJD, Achilles tendon abnormalities, cartilage lesions, os trigonum, the Stieda process, Haglund deformity, and heel spurs. A heel spur was defined as spiking of the calcaneal attachment of the plantar fascia.
DJD was defined as consisting of joint and articular cartilage abnormalities. Two separate classifications were used to grade them. One, the Kellgren-Lawrence system was used to grade joint abnormalities19:
Grade 0: no radiographic features of DJD are present;
Grade 1: doubtful joint space narrowing (JSN) and possible osteophytic lipping;
Grade 2: definite osteophytes and possible JSN on anteroposterior weightbearing radiographs;
Grade 3: multiple osteophytes, definite JSN, sclerosis, and possible bony deformity;
Grade 4: large osteophytes, marked JSN, severe sclerosis, and definite bony deformity.
Two, the imaging appearance of the articular cartilage of the foot and ankle joints was assessed with a modified Noyes and Stabler scale,23 as used in our previous work6 and validated for our setting:
Grade 0: normal thickness and signal;
Grade 1: normal thickness with an altered signal;
Grade 2: superficial partial-thickness cartilage defect (<50% of the total cartilage thickness affected);
Grade 3: deep partial-thickness cartilage defect (>50% of the total cartilage thickness affected);
Grade 4: full-thickness chondral defect with exposure of subchondral bone
The appearance of the articular cartilage of the foot and ankle joints was evaluated independently of the Kellgren-Lawrence evaluation and is reported separately.
BME was defined as an area of low signal intensity on T1-weighted images and an area of high signal intensity on PD-WI FAT SAT and TIRM. The presence of synovitis was evaluated in 4 joints: ankle, subtalar, tarsometatarsal (Lisfranc joints), and transverse tarsal (Chopart) joints.
The Haglund deformity was measured using the angle of Fowler and Philipp, the angle of Steffensen and Evensen, and the parallel pitch line techniques.10
The presence of joint effusion was evaluated using sagittal MRI scans. Since only a thin layer of fluid was present between the adjacent articular bones, it was decided to calculate the volume of joint fluid using the formula of an ellipse (length × width × height × 0.52) in 4 recesses: (1) anterior recess of the ankle joint, (2) posterior recess of the ankle joint, (3) posterior recess of the true (posterior) subtalar joint, and (4) anterior recess of the talocalcaneal articulation of the talocalcaneonavicular joint. The fluid volume typically present in these joints is usually up to 3.5 to 4 mL. Synovitis in these joints was diagnosed if the calculated joint fluid volume was above these values.
Achilles tendon abnormalities were defined as an increased intratendinous signal on PD-WI FAT SAT, TIRM, and T1-weighted images, and tendon enlargement, with or without edema in the Kager fat pad. Cartilage lesions were evaluated in the tibiotalar, fibulotalar, talonavicular, calcaneocuboid, subtalar, and tarsometatarsal joints. The tibia, fibula, talus, and calcaneus were evaluated for BME. All images were randomized utilizing the eFim Workstation software (IBM Watson Health) and saved for subsequent analysis. Two independent radiologists with at least 7 years of experience in musculoskeletal sports medicine imaging (A.B. and K.K.) analyzed the images. The radiologists were blinded as to patient age, fitness level, and whether the scans of the right and the left foot and ankle were from the same individual.
Statistical Analysis
The data were stored in a Microsoft Excel spreadsheet and analyzed using SPSS Statistical Package Version 23.0 (IBM). The results were considered statistically significant at P < .05. Distribution normality was assessed using the Kolmogorov-Smirnov test. Logistic regression was used to analyze the association between osteochondral lesions and anthropometric parameters. The correlation between age, BMI, and cartilage lesions was assessed using Spearman rank correlation coefficient. The Mann-Whitney test was used to compare cartilage lesions in the dominant and nondominant leg. Descriptive statistics (mean ± SD) were calculated. The Spearman rank correlation coefficient, logistic regression, chi-square test, Fisher test, Kolmogorov-Smirnov test, and Mann-Whitney U-test were used for the analysis. The relevant statistical methods are listed in the tables.
Results
Of the 37 study participants, 30 (81%) were right-leg dominant, 5 (14%) were left-leg dominant, and 2 (6%) were ambidextrous. A total of 64 ankle joints (86.5%) were evaluated with evidence of DJD of various severity in at least 1 of the joints examined. DJD was observed in 3 or 4 joints in 26 players (35.1%; 26/74), most commonly in the talonavicular, tibiotalar, and subtalar joints (Table 1 and Figures 2 and 3).
Table 1.
DJD Prevalence by Localization and Severity Grade (n = 74)a
| Kellgren-Lawrence Classification | ||||
|---|---|---|---|---|
| Joint | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
| Tibiotalar | 16 (21.6) | 1 (1.3) | 0 (0) | 0 (0) |
| Fibulotalar | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Talonavicular | 26 (35.1) | 23 (31) | 2 (2.7) | 0 (0) |
| Subtalar | 15 (20.3) | 1 (1.3) | 0 (0) | 0 (0) |
| Calcaneocuboid | 9 (12.2) | 4 (5.4) | 0 (0) | 0 (0) |
| Tarsometatarsal | 0 (0) | 4 (5.4) | 0 (0) | 1 (1.3) |
aData are reported as n (%).
Figure 2.
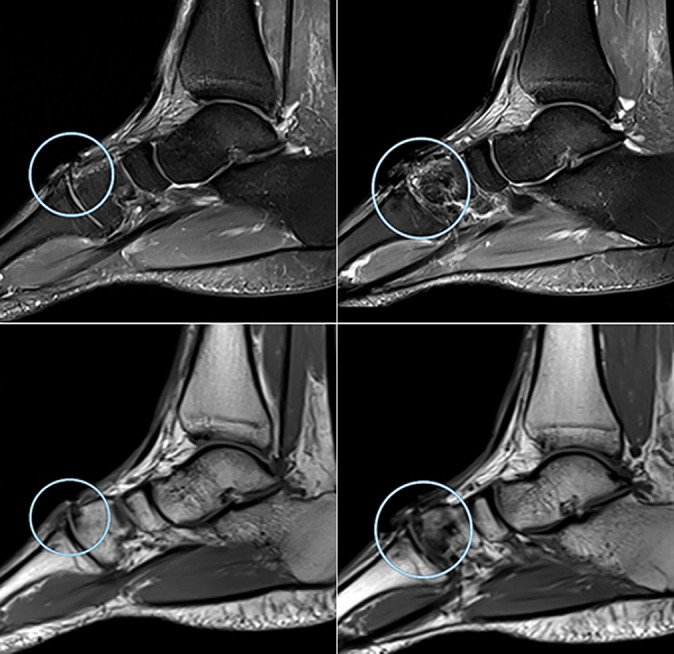
Degenerative joint disease in a 28-year-old professional soccer athlete. Sagittal and axial magnetic resonance imaging projections show signs of Kellgren-Lawrence grade 4 degenerative joint disease at the level of the tarsometatarsal joint (Lisfranc) (in circle) in the form of thinning of the articular cartilage with exposure of the articular surfaces, uneven narrowing of the joint space, areas of dystrophic bone marrow edema, and small cystic restructuring in adjacent bones and marginal osteophytes.
Figure 3.
Degenerative joint disease in a professional soccer athlete (in circle).
Articular cartilage lesions in the examined joints were evident in 31 MRI scans (42%). The talonavicular joint was affected in 26 cases (35%). Seventeen percent of all MRI scans had grade 3 or 4 cartilage lesions. These lesions were associated with BME in all cases (Table 2 and Figures 4 and 5).
Table 2.
Cartilage Lesion Prevalence by Location and Severity Grade (n = 74)a
| Modified Noyes and Stabler Classification | ||||
|---|---|---|---|---|
| Joint | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
| Tibiotalar | 3 (4) | 1 (1.3) | 3 (4) | 0 (0) |
| Fibulotalar | 1 (1.3) | 0 (0) | 0 (0) | 0 (0) |
| Talonavicular | 18 (24.3) | 3 (4) | 4 (5.4) | 1 (1.3) |
| Subtalar joint | 0 (0) | 0 (0) | 1 (1.3) | 0 (0) |
| Calcaneocuboid | 2 (2.7) | 0 (0) | 0 (0) | 0 (0) |
| Tarsometatarsal | 1 (1.3) | 0 (0) | 1 (1.3) | 3 (4) |
aData are reported as n (%).
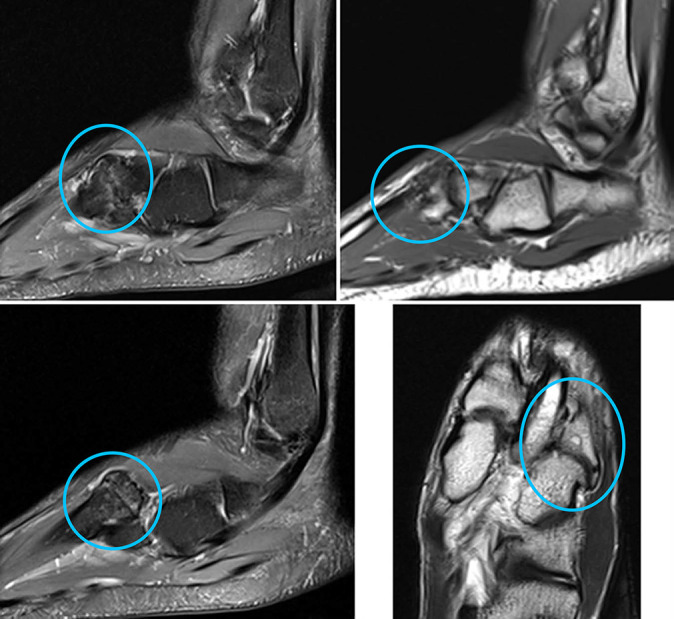
Figure 4.
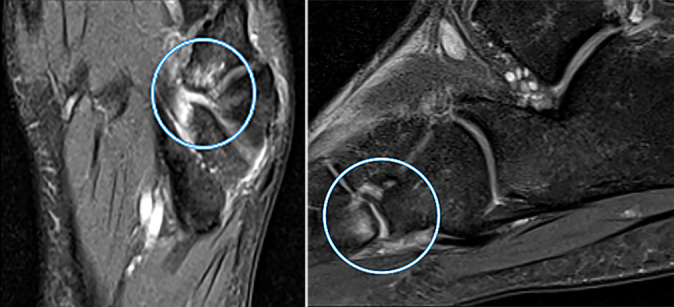
Sagittal and axial magnetic resonance imaging scans of a cartilage lesion of the tarsometatarsal joint in a 19-year-old professional soccer athlete. Signs of grade 3 (modified Noyes and Stabler classification) cartilage lesion at the level of the tarsometatarsal joint (Lisfranc joint) (in circle) in the form of thinning of the articular cartilage with exposure of the articular surfaces and signs of bone marrow edema in the subchondral divisions of the base of the fourth metatarsal bone.
Figure 5.
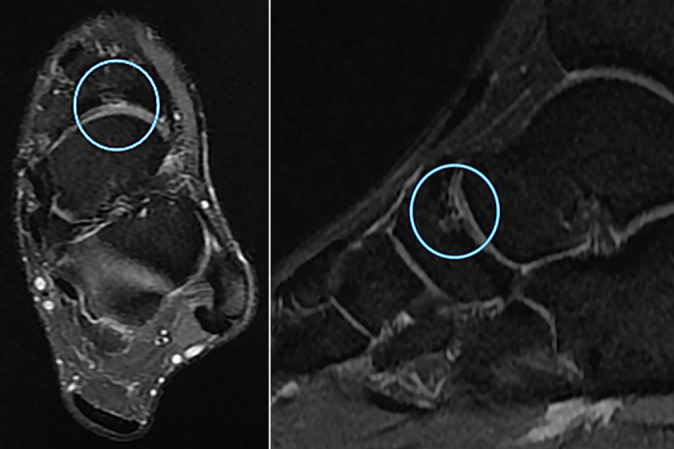
Sagittal and axial magnetic resonance imaging scans of a cartilage lesion of the transverse tarsal joint in a 21-year-old professional soccer athlete. Signs of grade 3 (modified Noyes and Stabler classification) cartilage lesion at the level of the transverse tarsal (Chopart) joint (in circle) in the form of thinning of the articular cartilage with exposure of the articular surfaces and signs of bone marrow edema in the subchondral navicular bone.
Higher body mass and height and older age were associated with a higher incidence of DJD of the tibiotalar joint, while older age was associated with grade 3 or 4 cartilage lesions of the talonavicular joint (Table 3).
Table 3.
Association Between Osteochondral Lesions and Anthropometric Parametersa
| Age | Weight | Height | BMI | |
|---|---|---|---|---|
| Presence of DJD of the tibiotalar joint |
P = .03 OR = 1.162 95% CI = 1.015-1.33 |
P = .007 OR = 1.125 95% CI = 1.033-1.226 |
P = .022 OR = 1.113 95% CI = 1.016-1.22 |
P = .2 |
| Presence of cartilage lesions of the tibiotalar joint | P = .15 | P = .37 | P = .37 | P = .61 |
| Presence of DJD of the talonavicular joint | P = .07 | P = .67 | P = .47 | P = .81 |
| Presence of cartilage lesions of the talonavicular joint |
P = .021 OR = 1.392 95% CI = 1.051-1.844 |
P = .25 | P = .32 | P = .77 |
aStatistically significant associations (P < .05) include odds ratio (OR) and 95% CI. Polynomial logistic regression. DJD, degenerative joint disease.
A statistically significant positive association was found between age, weight, BMI, and DJD of the tibiotalar joint and between talonavicular joint cartilage lesions and age.
Synovitis in at least 1 of the joints of the foot was detected in the MRI scans of 50 feet (67.6%). Synovitis most commonly affected the ankle and subtalar joints, its incidence being 40.5% and 39% (30 and 29 cases), respectively.
Greater body mass was associated with a significantly higher prevalence of synovitis in the Chopart joint (calcaneocuboid and talonavicular joints). There was no statistically significant association between leg dominance and the presence of synovitis in any of the joints examined, cartilage lesions, or DJD. However, leg dominance was significantly associated with BME of the talus (Table 4)
Table 4.
Relationship Between Cartilage Lesions and Age, BMI, and Leg Dominancea
| Ageb | BMI | Leg Dominancec | |
|---|---|---|---|
| Stage of cartilage lesions of the tibiotalar joint |
r
S = 0.287 P = .013 |
P = .76 | P = .66 |
| Stage of cartilage lesions of the talonavicular joint |
r
S = 0.229 P = .05 |
P = .12 | P = .23 |
| BME of the talus | P = .08 | P = .3 | P = .043 |
aBolded P values indicate statistical significance (P < .05). BME, bone marrow edema; BMI, body mass index.
bSpearman correlation coefficient and P value.
cMann-Whitney test.
Cartilage lesions of the tibiotalar and talonavicular joints showed a significant association with player age (Table 4).
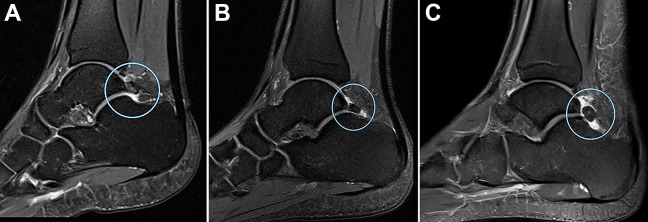
An os trigonum was found in 39% (n = 29) of the scans, and Haglund deformity was found in 12% (n = 9) of the scans (Figure 6). The presence of an os trigonum was associated with a prominent Stieda process (P = .01) (Figure 7). There was no evidence of a statistically significant association between the presence of an os trigonum and calcaneocuboid cartilage lesions, DJD of the talonavicular joint, and ankle joint synovitis.
Figure 6.
Sagittal magnetic resonance imaging scans showing os trigonum. (A) Os trigonum in a 22-year-old professional soccer athlete (in circle). (B) Os trigonum with coexistent synovitis in posterior synovial bursa of the ankle joint in a 24-year-old professional soccer athlete (in circle). (C) Os trigonum with coexistent bone marrow edema in a 21-year-old professional soccer athlete (in circle).
Figure 7.
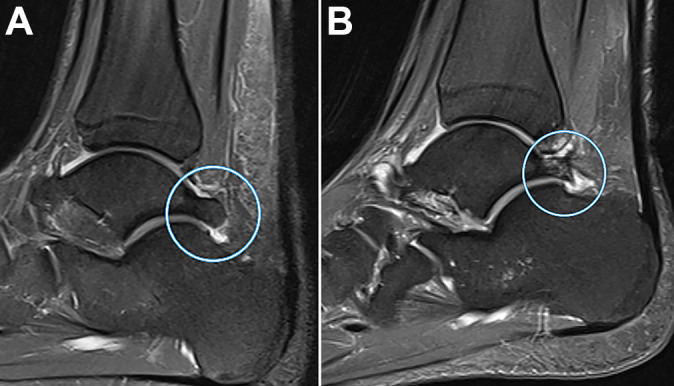
Sagittal magnetic resonance imaging scans showing a Stieda process (A) in a 24-year-old professional soccer athlete (in circle) and (B) with coexistent bone marrow edema (in circle).
Discussion
The current study demonstrated a high prevalence of asymptomatic changes in the ankle and hindfoot of elite professional male soccer players. The MRI scans of 86.5% of players showed DJD, and cartilage lesions of different severity were detected in 42% of the scans. Noyes and Stabler grade 3 or 4 cartilage lesions with findings of BME were observed in 17% of the scans. The greater age, weight, and height increase the probability of the presence of DJD of the ankle joint. Also, there was a high prevalence of synovitis and os trigonum, which was associated with synovitis in the posterior aspect of the ankle.32 There was a statistically significant positive association between leg dominance and BME of the talus.
The high prevalence of asymptomatic changes in the foot and ankle evidenced in the present study is in line with previous studies in athletes. There was a 100% prevalence of osteochondral and soft tissue damage in the ankles of asymptomatic professional snowboarders (mean age, 23.4 years)7 as well as a high prevalence of asymptomatic abnormalities in the Achilles and other tendons in the lower limb of elite fencers.14
Tenosynovitis, Achilles tendon abnormalities, synovitis, BME, and lateral malleolus ligament injuries are significantly more frequent among juvenile male soccer players than in control patients,16 and similar findings have been demonstrated in adult male soccer players.21 However, these studies did not evaluate cartilage lesions and did not ascertain the association between leg dominance and osteochondral lesions.16,22
In former professional soccer athletes, the prevalence rate of osteoarthritis (OA) of both the hip and the knee is significantly higher compared with that of age- and sex-matched controls. However, data on the OA or DJD of the ankle and foot are not available. The data provided by Kuijt et al20 showed that OA of the ankle joint is more prevalent than in the general population based on the prevalence of self-reported diagnosed ankle OA (between 12% and 17%). However, it should be considered that the incidence of radiographic DJD in the general population reaches 12%.11,20,28
Armenis et al3 showed that the radiographic evidence of DJD of the ankle and foot was present in 8.8% of soccer players, compared with 3.7% in the general population (P < .05). However, in this study, soccer players did not report more clinical symptoms of OA than the general population, which indicates that many such foot and ankle lesions are clinically asymptomatic.3
The higher incidence of cartilage lesions and BME of the talus in the dominant leg might be explained by the more frequent foot-to-ball contact of the dominant leg. Thus, leg dominance might be an additional risk factor for injuries in professional soccer players, as previously shown in soccer players sustaining anterior cruciate ligament injuries.8
The present study showed a high prevalence of os trigonum in elite professional players involved. It seems that professional soccer athletes have a higher prevalence of os trigonum than the general population. Given the repetitive resisted plantarflexion of the ankle required of soccer, an os trigonum syndrome (a type of posterior ankle impingement syndrome), flexor hallucis longus tenosynovitis, or entrapment can therefore be more frequent in soccer players than other athletes.2
The role of footwear in lower limb injuries is debatable. Soccer boots may increase the plantar pressures in elite male professional soccer athletes and lead to excessive foot loadings and subsequent DJD.9 We did not control for footwear, and this may be interpreted as a limitation of our investigation
The association between age, BMI, and osteochondral lesion incidence in the foot and ankle has been previously described.15,24 However, we could not identify any studies showing the effects of age and height on the incidence and severity of the asymptomatic osteochondral lesions of the foot in professional soccer players.
Findings of asymptomatic foot and ankle abnormalities might be important to improve the diagnostic precision of clinical examination of patients with nonspecific foot pain.26,29 In addition to clinical examination and thorough medical history collection, MRI is routinely employed for accurate diagnosis. This enables evaluation of possible articular cartilage lesions, BME, and ligament injuries.1 Most studies on this topic are of a low level of evidence, and management lacks evidence-based support.25
Our study has several limitations. It lacks a control group, and we did not perform biomechanical assessment to analyze whether the players had lower limb malalignment. It also should be noted that the sample is small given the necessarily limited number of elite professional soccer players of that level: this obviously affects the statistical power of our investigation. Also, the Kellgren-Lawrence scale used in our study is widely used in similar research, but we are aware that there are other scales that may be used to assess the degree of DJD of the foot and ankle, such as the modified Takakura scale,27 which was specifically developed for OA of the ankle. We did not calculate inter- or intraobserver variation in the grading systems used in our study. This may be viewed as a significant limitation. In this investigation, we did not study the relationship between the position on the field, which could account for different physical demands during training and matches, and the imaging changes detected. Moreover, the possible relationship between the muscular balance of the lower extremities and the changes detected was not studied.
Conclusion
Elite professional soccer players are often evaluated with a high prevalence of asymptomatic osteochondral lesions with subchondral BME in the foot and ankle. This should be taken into account when interpreting MRI scans for diagnostic and presigning purposes. These osteochondral lesions may remain asymptomatic or, with the continuing high-intensity stresses that modern professional soccer demands of its athletes, evolve and cause foot and ankle pain. It is unclear whether and which interventions can be implemented to prevent the occurrence of these abnormalities in the first place. Further research should focus on studying the natural history of the osteochondral lesions evidenced at MRI and their possible association with increased foot and ankle injuries. Future research should aim to study the effects of various preventive training and treatment programs on the progression of the changes identified, along with the acute impact of professional soccer practice on articular cartilage using markers of cartilage degradation.
Footnotes
Final revision submitted July 14, 2020; accepted July 29, 2020.
The authors declared that they have no conflicts of interest in the authorship and publication of this contribution. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from Sechenov First Moscow State Medical University (Sechenov University).
References
- 1. Angioi M, Maffulli G, McCormack M, Morrissey D, Chan O, Maffulli N. Early signs of osteoarthritis in professional ballet dancers: a preliminary study. Clin J Sport Med. 2014;24(5):435–437. [DOI] [PubMed] [Google Scholar]
- 2. Aparisi Gómez MP, Aparisi F, Bartoloni A, et al. Anatomical variation in the ankle and foot: from incidental finding to inductor of pathology. Part I: ankle and hindfoot. Insights Imaging. 2019;10(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Armenis E, Pefanis N, Tsiganos G, Karagounis P, Baltopoulos P. Osteoarthritis of the ankle and foot complex in former Greek soccer players. Foot Ankle Spec. 2011;4(6):338–343. [DOI] [PubMed] [Google Scholar]
- 4. Badekas T, Takvorian M, Souras N. Treatment principles for osteochondral lesions in foot and ankle. Int Orthop. 2013;37(9):1697–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beals CT, Magnussen RA, Graham WC, Flanigan DC. The prevalence of meniscal pathology in asymptomatic athletes. Sports Med. 2016;46(10):1517–1524. [DOI] [PubMed] [Google Scholar]
- 6. Bezuglov EN, Lyubushkina AV, Khaitin VY, et al. Prevalence of asymptomatic intra-articular changes of the knee in adult professional soccer players. Orthop J Sports Med. 2019;7(11):2325967119885370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Briggs K, Ho C, Ryan W, Bower G, Hackett T, Clanton T. High prevalence of osteochondral and soft-tissue damage in the ankles of asymptomatic professional snowboarders: a prospective evaluation with 3 T MRI. Br J Sports Med. 2014;48:572. [Google Scholar]
- 8. Brophy R, Silvers HJ, Gonzales T, Mandelbaum BR. Gender influences: the role of leg dominance in ACL injury among soccer players. Br J Sports Med. 2010;44(10):694–697. [DOI] [PubMed] [Google Scholar]
- 9. Carl HD, Pauser J, Swoboda B, Jendrissek A, Brem M. Soccer boots elevate plantar pressures in elite male soccer professionals. Clin J Sport Med. 2014;24(1):58–61. [DOI] [PubMed] [Google Scholar]
- 10. Chauveaux D, Liet P, Le Huec JC, Midy D. A new radiologic measurement for the diagnosis of Haglund’s deformity. Surg Radiol Anat. 1991;13(1):39–44. [DOI] [PubMed] [Google Scholar]
- 11. Drawer S, Fuller CW. Propensity for osteoarthritis and lower limb joint pain in retired professional soccer players. Br J Sports Med. 2001;35(6):402–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Elleuch MH, Guermazi M, Mezghanni M, et al. Knee osteoarthritis in 50 former top-level footballers: a comparative (control group) study. Ann Readapt Med Phys. 2008;51(3):174–178. [DOI] [PubMed] [Google Scholar]
- 13. Engström B, Johansson C, Tornkvist H. Soccer injuries among elite female players. Am J Sports Med. 1991;19(4):372–375. [DOI] [PubMed] [Google Scholar]
- 14. Giombini A, Dragoni S, Di Cesare A, Di Cesare M, Del Buono A, Maffulli N. Asymptomatic Achilles, patellar, and quadriceps tendinopathy: a longitudinal clinical and ultrasonographic study in elite fencers. Scand J Med Sci Sports. 2013;23(3):311–316. [DOI] [PubMed] [Google Scholar]
- 15. Heir T, Eide G. Age, body composition, aerobic fitness and health condition as risk factors for musculoskeletal injuries in conscripts. Scan J Med Sci Sports. 1996;6(4):222–227. [DOI] [PubMed] [Google Scholar]
- 16. Huang YB, Zhao YX, Xiao JJ, Li MW, Zhang R, Li SL. Comparative analysis of the ankle joints in juvenile male soccer players with imaging [in Chinese]. Zhonghua Yi Xue Za Zhi. 2016;96(25):1971–1975. [DOI] [PubMed] [Google Scholar]
- 17. Junge A, Dvorak J. Soccer injuries: a review on incidence and prevention. Sports Med. 2004;34(13):929–938. [DOI] [PubMed] [Google Scholar]
- 18. Keller CS, Noyes FR, Buncher CR. The medical aspects of soccer injury epidemiology. Am J Sports Med. 1987;15(3):230–237. [DOI] [PubMed] [Google Scholar]
- 19. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kuijt M-TK, Inklaar H, Gouttebarge V, Frings-Dresen MHW. Knee and ankle osteoarthritis in former elite soccer players: a systematic review of the recent literature. J Sci Med Sport. 2012;15(6):480–487. [DOI] [PubMed] [Google Scholar]
- 21. Lee HH, Chu CR. Clinical and basic science of cartilage injury and arthritis in the football (soccer) athlete. Cartilage. 2012;3(1)(suppl):63S–68S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li S, Zhao W, Hao S, Hu S, Zhang R, Zhang X. Imaging study of ankle injury in professional soccer player of males [in Chinese]. Zhonghua Yi Xue Za Zhi. 2015;95(17):1290–1294. [PubMed] [Google Scholar]
- 23. Noyes FR, Stabler CL. A system for grading articular cartilage lesions at arthroscopy. Am J Sports Med. 1989;17(4):505–513. [DOI] [PubMed] [Google Scholar]
- 24. Richmond SA, Nettel-Aguirre A, Doyle-Baker PK, Macpherson A, Emery CA. Examining measures of weight as risk factors for sport-related injury in adolescents. J Sports Med. 2016;2016:7316947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shimozono Y, Yasui Y, Ross AW, Kennedy JG. Osteochondral lesions of the talus in the athlete: up to date review. Curr Rev Musculoskelet Med. 2017;10(1):131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Soder RB, Simoes JD, Soder JB, Baldisserotto M. MRI of the knee joint in asymptomatic adolescent soccer players: a controlled study. AJR Am J Roentgenol. 2011;196:W61–W65. [DOI] [PubMed] [Google Scholar]
- 27. Tanaka Y, Takakura Y, Hayashi K, Taniguchi A, Kumai T, Sugimoto K. Low tibial osteotomy for varus-type osteoarthritis of the ankle. J Bone Joint Surg Br. 2006;88(7):909–913. [DOI] [PubMed] [Google Scholar]
- 28. Turner AP, Barlow JH, Heathcote-Elliott C. Long term impact of playing professional football in the United Kingdom. Br J Sports Med. 2000;34(5):332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Walczak BE, McCulloch PC, Kang RW, Zelazny A, Tedeschi F, Cole BJ. Abnormal findings on knee magnetic resonance imaging in asymptomatic NBA players. J Knee Surg. 2008;21:27–33. [DOI] [PubMed] [Google Scholar]
- 30. Walls RJ, Ross KA, Fraser EJ, et al. Football injuries of the ankle: a review of injury mechanisms, diagnosis and management. World J Orthop. 2016;7(1):8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yasui Y, Hannon CP, Hurley E, Kennedy JG. Posterior ankle impingement syndrome: a systematic four-stage approach. World J Orthop. 2016;7(10):657–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zwiers R, Baltes TPA, Opdam KTM, Wiegerinck JI, van Dijk CN. Prevalence of os trigonum on CT imaging. Foot Ankle Int. 2017;39(3):338–342. [DOI] [PubMed] [Google Scholar]
- 33. Zwiers R, Wiegerinck JI, Murawski CD, Smyth NA, Kennedy JG, van Dijk CN. Surgical treatment for posterior ankle impingement. Arthroscopy. 2013;29(7):1263–1270. [DOI] [PubMed] [Google Scholar]