Abstract
Optimal sized balloon atrial septostomy improves hemodynamics in advanced pulmonary arterial hypertension. Occlutech Atrial Flow Regulator is designed to provide an atrial septal fenestration diameter titrated according to the age and right atrial pressures. This observational study analyzed symptoms, exercise distance, oxygen saturations, hemodynamics and echocardiographic parameters after Atrial Flow Regulator implantation in patients with syncope or right-heart failure. Patients with high-risk predictors of mortality during septostomy were scrutinized. Thirty-nine patients (9 children) with syncope (34/39) or right-heart failure (27/39) underwent Atrial Flow Regulator implantation without procedural complications. Six-minute walk distance increased from 310 ± 158.2 to 376.4 ± 182.6 m, none developed syncope. Oxygen saturations reduced from 96.4 ± 6.4% to 92 ± 4.9% at rest and further to 80.3 ± 5.9% on exercise. Right atrial pressures reduced from 9.4 ± 5 (2–27) mmHg to 6.9 ± 2.6 (1–12) mmHg, while cardiac index increased from 2.4 ± 0.8 (0.98–4.3) to 3 ± 1 (1.1–5.3) L/min/m2 and systemic oxygen transport increased from 546.1 ± 157.9 (256.2–910.5) to 637.2 ± 191.1 (301.3–1020.2) ml/min. Echocardiographic improvement included significant reduction of pericardial effusion and inferior caval congestion at a median follow-up of 37 months. Overall survival improved except two early and one late deaths in high-risk patients. Five of seven patients with advanced disease and key hemodynamic predictors of mortality survived. Acute hemodynamic benefits in pulmonary arterial hypertension after Atrial Flow Regulator were improved cardiac output, systemic oxygen transport, and reduced right atrial pressures. Improvement of symptoms especially syncope, exercise duration, and right ventricular systolic function as well as device patency were sustained on mid-term follow-up. Implantation was safe in all including young children without procedural complications. Mortality was noted only in patients who had high-risk predictors and patients at advanced stage of the disease.
Keywords: balloon atrial septostomy, cardiac index, six-minute walk distance, systemic oxygen transport, right ventricle
Key questions
What is already known?
Creation of a right-to-left interatrial shunt in advanced pulmonary arterial hypertension (PAH) provides relief from syncope and improves exercise tolerance due to increase in cardiac output and reduction in right atrial pressures.
What does this study add?
Implantation of Atrial Flow Regulator (AFR), a new device with a fixed diameter orifice to provide a controlled predictable atrial septal fenestration, titrated to patient size and hemodynamics is safe, easy, and devoid of device-related adverse effects.
How might this impact on clinical practice?
Utility of implantation of this device early in the course of the disease and its impact on improvement in survival in the current era of pathway-targeted pulmonary vasodilator therapy should be studied in future.
PAH is an irreversible disease with fatal outcome characterized by progressive obliteration of pulmonary vasculature causing right ventricular (RV) failure.1 Registry to EValuate EArly and Long-term PAH (REVEAL) has shown improvement of one-year survival to 91% with recent pathway-targeted therapy.1 Lung transplantation has a current median survival of 6.7 years, but carries a higher mortality in PAH compared to other conditions.2 It is unavailable for majority of patients in many parts of the world. PAH accounts for < 3% of the lung transplant recipients in large registries.2
Atrial septostomy relieves symptoms in patients with syncope and heart failure and serves as a bridge to transplant.3 Reviews and meta-analysis show its beneficial hemodynamic effects and improved short-term survival.4 Increasing experience has reduced the procedural mortality to < 2% in recent times.5 It improves cardiac index and systemic oxygen transport despite hypoxemia.6,7 The off-loaded RV shows improved function on echocardiography.8 Neurohumoral modifications after septostomy include reduction in sympathetic overactivity and pro-brain natriuretic peptide (proBNP) levels.9,10
Early spontaneous closure of septostomy in one-fourth of patients warrants repeat procedures.4 Large septostomy circumvents late closures, but life-threatening hypoxia may warrant emergency device occlusion.11 Fenestrated Amplatzer devices fail in long-term patency.12 An interatrial shunt fraction of 10–15% of cardiac output improves cardiac index and tissue oxygen delivery in severe PAH.13 AFRs (Occlutech, Istanbul, Turkey) are new devices that provide different atrial septal fenestration diameters, titrated to the hemodynamic needs and size of the patient.14 Animal studies show minimal inflammation, non-thrombogenicity, and retained patency.15 The clinical use of AFR is shown in heterogenous indications including PAH from multiple centers.16 We performed an observational study on utility of AFR on symptoms, hemodynamics, echocardiographic parameters, and survival with mid-term follow-up in patients with advanced PAH.
Methods
This retrospective observational single-center study analyzed the patient symptoms and mortality following AFR implantation for PAH and studied the changes in exercise tolerance, echocardiographic indices, hemodynamic parameters, and proBNP levels during their mid-term follow-up. The device patency and adverse effects were analyzed. Institutional review board and ethical committee approved compassionate use of AFR instead of balloon atrial septostomy. No sample size was calculated for this observational study. Study complied with Helsinki declaration.
Patient informed consent
After receiving optimal pathway-targeted therapy, patients with advanced symptoms such as syncope and right heart failure were explained about the need for additional interventions such as balloon atrial septostomy. Being a relatively new device, patients were educated about the controlled fenestration diameter orifice provided by AFR in comparison to the conventional balloon atrial septostomy. Each patient participated in getting the approvals for import of the device from the regulatory authorities. Patients provided an informed consent for AFR implantation and publication of results.
Inclusions and exclusions
After targeted therapy with at least two drugs and maximal tolerated diuretics, patients were included if they had (i) syncope due to acute pulmonary hypertensive episodes after excluding other reasons and/or (ii) right heart failure; six-minute walk distance (6MWD) < 420 m; hospitalization within one year for PAH. Age under three years, active sepsis, coagulation disease, intolerance to antiplatelets, nickel allergy, pregnancy, thrombosed femoral veins, intracardiac thrombus, and previously implanted atrial septal occluders were exclusions.
Echocardiography
Echocardiography identified inferior caval vein congestion (>21 mm), pericardial effusion (>3 mm), RV fractional area change (FAC), RV peak systolic strain, and tricuspid annular plane systolic excursion (TAPSE). The other parameters analyzed were pulmonary acceleration time (PAT) and right atrial size. Echocardiography also helped to identify the subgroup of PAH.
Choosing the fenestration diameter
AFR (Fig. 1), a double-disc nitinol device with 4, 6, 8, or 10 mm central fenestration, allowing a right-to-left interatrial shunt when the right atrial pressure markedly increases during acute RV failure, has been described earlier.14 In patients < 8 years or weighing < 20 kg, 6-mm device was chosen. In older patients, right atrial pressures guided the fenestration size; 8 mm AFR was chosen in patients with right atrial pressures >12 mmHg and 10 mm AFR in patients with right atrial pressures <12 mmHg. This decision was modified on case-to-case basis as there were no previous definite guidelines.
Fig. 1.
Occlutech Atrial Flow Regulator.
A self-expanding double disc nitinol wire mesh device with a central fenestration.
Hemodynamics
After femoral vascular access, heparinization maintained activated clotting time above 250 s. Procedure was performed in fully awake state in adults and conscious sedation with spontaneous breathing in children, avoiding general anesthesia except very young children and sick patients. As AFR created a right-to-left shunt precluding thermodilution, indirect Fick method was used to compare cardiac output before and after the procedure, with assumed oxygen consumption. Pulmonary vascular resistance (PVR) was calculated in resting state and after acute vasodilator testing using nitric oxide. Responders received a trial of calcium channel blocker instead of AFR. Left atrial pressures after septal puncture were used for calculating PVR. The REVEAL risk score was calculated for each patient.17
AFR implantation
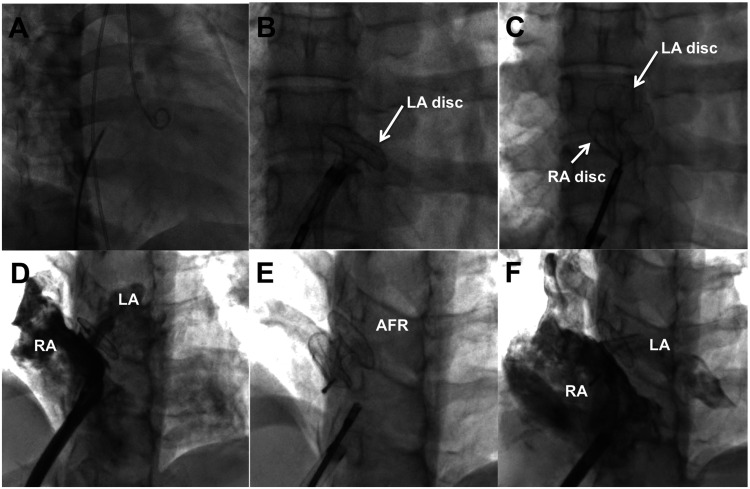
The thin part of oval fossa of the atrial septum is punctured with Brockenbrough needle using fluoroscopic landmarks, to advance an appropriate-sized Mullins sheath (Cook Medical, Bloomington, IN) into the left atrium over the wire.18 A thick and tough septum is pre-dilated with a 10-mm balloon before placing the Mullins sheath. The size of the sheath required for deploying a 6, 8, 10 mm AFR are 10 F, 12 F, and 14 F, respectively (Fig. 2, Video 1). An appropriate-sized AFR is advanced through the Mullins sheath, left atrial disc deployed in the left atrium before pulling the sheath against the atrial septum to deploy the right atrial disc. Saline contrast echocardiography through the side-arm of the delivery system confirmed patency before release (Video 2). Antiplatelet therapy for six months included aspirin and clopidogrel.
Fig. 2.
Implantation of AFR device.
After interatrial septal puncture with Brockenbrough needle (a), an appropriate Mullins sheath is advanced into the left atrium through which the left atrial disc of the atrial flow regulator (AFR) is opened in the left atrium (b) followed by release of right atrial disc in right atrium (c). After confirmation of right-to-left shunt of contrast through the central fenestration (d), the AFR is released (e). Right atrial contrast spill-over to left atrium (f) through the fenestration is seen after release of the AFR.
Morbidity and mortality
Patients were monitored after AFR implantation three-monthly for change of symptoms, features of heart failure, all-cause hospitalizations, uptitration of medications, and adverse effects of drugs. Patients who did not come for follow-up appointments were contacted telephonically and data were collected from their primary care cardiologists. All interim out-of-hospital mortality was interviewed. After REVEAL score risk stratifications, patients with key hemodynamic indicators predicting mortality during septostomy were scrutinized.3 In addition, high-risk decompensated patients on inotropes were also scrutinized.7
Data collection
Symptoms of syncope and right heart failure, NewYork Heart Association (NYHA) class, resting and exercise oxygen saturations and 6MWD, echocardiographic indices, and hemodynamic readings were recorded before and after AFR implantation. Subgroup and severity of PAH were quantified. The systemic oxygen transport was calculated by multiplying cardiac output and arterial oxygen content. The radiation dose, fluoroscopic duration, and procedural complications were noted. Three-monthly follow-up identified change in symptoms, 6MWD, proBNP levels, and echocardiographic parameters. AFR patency was checked by pulse oximetry, agitated saline contrast echocardiography, and color Doppler study.
Adverse events evaluation
Device-related complications included incorrect positioning, embolization, hypoxia due to oversized AFR, premature closure, infection, and problems with delivery system. Procedural risks included death, stroke, cardiac injury, groin vascular complications, complications of transseptal puncture, air embolism, arrhythmias, thrombus formation, and immediate surgical interventions. Events on follow-up monitoring included loss of patency, recurrence of syncope, worsening of heart failure, adverse drug reactions, transplantation, or death.
Statistical methods
Data were analyzed using SPSS version 23. Frequency counts and percentages were presented for categorical variables. Significance of change in variables before and after AFR implantation was analyzed using paired t test. Descriptive variables were analyzed using Chi Square Test. McNemar’s test was used to test the significance of change in NYHA class. Box and whisker plots presented interquartile range within the box and 5th and 95th centiles at ends.
Results
Thirty-nine patients including nine children with PAH had AFR implantation since June 2016 with a median follow-up of 33 months (9–53 months); 34/39 patients experienced syncope and 27/39 patients had features of right heart failure indicating an advanced WHO functional class status (Table 1). Seven patients with high-risk indicators that predict mortality during septostomy were optimized with inotropes and maximum tolerated diuretics before procedure; 35/39 patients had a REVEAL score above six that predicted one-year survival < 70% despite optimal pathway-targeted therapy.17
Table 1.
Patient clinical details.
| Total number of patients | : | 39 | |
| Male:Female ratio | : | 11:28 | (28%:72%) |
| Age | : | 24.9 ± 12.6 years | (3–62 years) |
| Patients under 12 years | : | 9/39 | |
| Weight | : | 48.4 ± 31.6 kg | (11–85 kg) |
| Height | : | 149.7 ± 40.2 cm | (91–184 cm) |
| Body surface area | : | 1.41 ± 0.64 m2 | (0.53–2.09 m2) |
| Body mass index | : | 20.8 ± 7.4 kg/m2 | (11.9–31.8 kg/m2) |
| Etiology | |||
| Idiopathic | : | 25 | |
| Familial | : | 7 | |
| Hereditary | : | 1 (associated with HHT) | |
| Operated CHD | : | 5 (atrial septal defect: 3; ventricular septal defect: 2) | |
| Collagen vascular disease associated: | 1 | ||
| Symptom status | NYHA class IV | : 4/39 (10%) | |
| NYHA class III | : 14/39 (36%) | ||
| NYHA class II | : 21/39 (54%) | ||
| Syncope/presyncope | : 34/39 (87%) | ||
| Right heart failure | : 27/39 (69%) | ||
| Six-minute walk distance | : 310 ± 158.2 m (0–420 m) | ||
| Pulse oximeter saturations at rest | : 96.4 ± 6.4% (87–99%) | ||
| Anemia (hemoglobin less than 11 g/dl) | : 7/39 (18%) | ||
| ProBNP | : 3542.9 ± 10,088.4 pg/ml (22–63,619 pg/ml) | ||
| Drug therapy | Phosphodiesterase-5 inhibitor | : 39/39 | |
| Endothelin receptor antagonist | : 39/39 | ||
| Diuretics | : 19/39 | ||
| Decompensated on inotropes | : 4/39 | ||
| Key predictive indicators of mortality: | 6/39 (3 decompensated on inotropes) | ||
| Right atrial pressure > 20 mmHg | : 1/39 | ||
| Left ventricular end-diastolic pressures > 18 mmHg | : 3/39 | ||
| Resting oxygen saturation < 90% | : 2/39 | ||
| Pulmonary vascular resistance index > 55 Wood units | : 0/30 | ||
| REVEAL-2.0 scores | Below 6 (one-year survival 93%) | : 4/39 | |
| 7–12 (one-year survival 70%) | : 29/39 | ||
| Above 12 (one-year survival 40%) | : 6/39 | ||
CHD: congenital heart disease; HHT: hereditary hemorrhagic telengectasia; ProBNP: pro-brain natriuretic peptide.
Echocardiographic parameters
Echocardiographic parameters varied depending on the disease severity (Table 2). Eighteen patients (46%) had inferior vena caval congestion and 10 patients (26%) had pericardial effusion. TAPSE below 18 mm was seen in 37 patients (95%). RV FAC below 35% was also seen in 37 patients (95%). PAT was < 80 ms in 24 patients (62%) and < 60 ms in 8 patients (21%). Right atrium was larger than 45 mm in 33 patients (85%); more than 55 mm in 29 patients. The tricuspid regurgitation was severe in 7 (18%), moderate in 11 (28%), and mild in 21 (54%) patients. Mean RV systolic pressures were 103.97 ± 42.4 mmHg (70–170 mmHg).
Table 2.
Change in echocardiographic parameters.
| Parameters | Preimplantation of AFR (N = 39) | Three-month follow-up (N = 37) | Six-month follow-up (N = 37) | One-year follow-up (N = 34) |
|---|---|---|---|---|
| Pericardial effusion | 10/39 (26%) | 5/37 (14%)P = 0.18a | 2/37 (5.5%)P = 0.016a | 0/34 (0%)P < 0.05a |
| IVC congestion | 18/39 (46%) | 8/37 (22%)P = 0.024a | 4/37 (11%)P < 0.001a | 2/34 (16%)P <0.001a |
| TAPSE (mm) | 12.7 ± 6.6 (7–20) | 15.5 ± 8.6 (8–26)P = 0.002b | 14.7 ± 6.8 (10–24)P = 0.007b | 14.8 ± 7.82 (9–22)P = 0.001b |
| FAC (%) | 23.2 ± 14.6 (7.7–50) | 24.2 ± 13.6 (14–39)P = 0.609b | 23.9 ± 12.7 (14–38)P = 0.486b | 22.4 ± 13.4 (11–39)P = 0.849b |
| PAT(msec) | 73.5 ± 35.3 (32–110) | 71.3 ± 33.4 (36–108)P = 0.812b | 70.6 ± 30.9 (34–104)P = 0.81b | 66.4 ± 29.5 (34–100)P = 0.469b |
| RVSP (mm Hg) | 103.9 ± 43 (70–170) | 99.4 ± 51.2 (55–175)P = 0.198b | 104.4 ± 50.3 (60–160)P = 0.77b | 103.7 ± 52.5 (65–166)P = 0.751b |
| RV Strain (%) | 9.4 ± 3.9 (2–20) | 11.6 ± 5.8 (3–25)P = 0.008b | 11.6 ± 9.6 (4–24)P = 0.012b | 11.5 ± 8.5 (4–25)P = 0.085b |
| Diameter of RA (mm) | 60 ± 23.2 (33–84) | 54.3 ± 28.4 (39–80)P = 0.814b | 53.6 ± 31.2 (35–77)P = 0.234b | 56.6 ± 18.6 (47–76)P = 0.652b |
Note: Bold fonts indicate significance.
aChi-square test.
bPaired t test.
AFR: Atrial Flow Regulator; FAC: fractional area change; IVC: inferior vena cava; PAT: pulmonary acceleration time in milliseconds; RA: right atrium; RV: right ventricle; RVSP: right ventricular systolic pressure in millimeters of mercury; TAPSE: tricuspid annular plane systolic excursion in millimeters; P: p value.
Procedural details
Procedure was successful in all patients including three patients with a surgically patched atrial septum. There were no procedural complications. General anesthesia was avoided except in three children. The mean fluoroscopic time was 52.2 ± 0.7 min and dose area product was 20.4 ± 19.5 Gray-square centimeters. A 10-mm AFR was implanted in 18 patients (46.2%), 8 mm in 17 patients (43.7%), and 6 mm in 4 patients (10.3%).
Change in hemodynamics
The pulmonary artery systolic pressures were suprasystemic in 14 patients, near-systemic in 9, and subsystemic in 16 patients. Two young patients who showed vasodilator response were excluded. None of the patients implanted with AFR showed a vasodilator response or a right-to-left shunt on oximetry. The mean superior caval vein saturation was below 70% in 33 patients (85%) and did not change significantly after the procedure (Supplemental Figure 1). Cardiac index and systemic oxygen transport improved after AFR implantation (Table 3). The other two changes were a reduction in right atrial pressures and increase of aortic systolic pressures (Supplemental Figure 2).
Table 3.
Hemodynamics—before and after AFR implantation.
| Parameter | Before AFR implantation | After AFR implantation | P valuesa |
|---|---|---|---|
| RA pressure (mmHg) | 9.4 ± 5 (2–27) | 6.9 ± 2.6 (1–12) | 0.021 |
| LA pressure (mmHg) | 6.6 ± 3.9 (1–20) | 6.1 ± 2.1 (1–10) | 0.907 |
| RVEDP (mmHg) | 14.3 ± 6.6 (4–40) | 13.9 ± 2.7 (10–20) | 0.33 |
| LVEDP (mmHg) | 12.3 ± 7.4 (5–48.6) | 12 ± 3.3 (5–18) | 0.479 |
| PA systolic (mmHg) | 107.5 ± 24.6 (66–180) | 116 ± 27.7 (70–160) | 0.2640 |
| PA mean (mmHg) | 68.9 ± 15.6 (42–98) | 75.3 ± 20.5 (45–118) | 0.319 |
| PA diastolic (mmHg) | 47.5 ± 13.7 (15–70) | 49.4 ± 17.9 (20–90) | 0.836 |
| Ao systolic (mmHg) | 110 ± 12.1 (88–160) | 116.7 ± 18.6 (85–150) | 0.003 |
| Ao mean (mmHg) | 89.7 ± 14.7 (55–142) | 85.3 ± 14.7 (62–110) | 0.898 |
| Ao diastolic (mmHg) | 68.9 ± 8.9 (56–98) | 68.1 ± 13.1 (42–90) | 0.299 |
| PVRI (wood units.m2) | 26.4 ± 10.9 (10–54.9) | 33.2 ± 13.5 (12.89–64.4) | 0.07 |
| SVRI (wood units.m2) | 35.3 ± 14.5 (13.44–96.0) | 32.3 ± 11.0 (12.7–55.9) | 0.745 |
| PVRI/SVRI (ratio) | 0.8 ± 0.3 (0.35–1.33) | 1.1 ± 0.6 (0.36–3.24 ) | 0.06 |
| Venous saturation (%) | 64.1 ± 13.2 (48.5–78.5) | 62.4 ± 15.6 (47.3–79.1) | 0.106 |
| Aortic saturation (%) | 93.9 ± 7.6 (85.5–99.0) | 88.5 ± 12.4 (75.4–98.2) | 0.001 |
| CI (l/min/m2) | 2.4 ± 0.8 (0.98–4.3) | 3 ± 1.0 (1.1–5.3) | <0.001 |
| SOT (ml/min) | 546.1±157.9 (256.2–910.5) | 637.2 ± 191.1 (301.3–1020.2) | <0.001 |
Notes: Values in means ± standard deviation (range in brackets). Bold fonts indicate significance.
AFR: atrial flow regulator; Ao: aortic; CI: cardiac index; LA: left atrium; LVEDP: left ventricular end diastolic pressure; PA: pulmonary artery; PVRI: indexed pulmonary vascular resistance; RA: right atrium; RVEDP: right ventricular end diastolic pressure; SOT: systemic oxygen transport; SVRI: indexed systemic vascular resistance
aP value—paired t test.
Early mortality
There were two early deaths within seven days. One 58-year-old female presented with heart failure, pericardial effusion, resting saturation of 88%, and received inotropes and optimal diuretics for seven days. The proBNP was 32,000 pg/ml and REVEAL-2.0 score was 18 points.17 The right atrial pressure was 16 mmHg, left ventricular end-diastolic pressure was 8 mmHg, and indexed pulmonary vascular resistance (PVRI) was 35 Wood units. She died six days later, despite a marginal transient improvement. The second patient aged 30 years with proBNP of 1000 pg/ml had advanced heart failure on home milrinone infusions. His right atrial pressure on diuretics was 10 mmHg, aortic saturation was 92%, indexed PVRI was 39 Wood units and REVEAL-2.0 score was 16 points.17 He died after 18 h following an episode of acute epigastric pain and hemodynamic collapse.
Late mortality
A 20-year-old male with familial PAH in NYHA class III, REVEAL score of 14 points remained stable for two years after AFR implantation on regular targeted therapy. He had a sudden death at home without any exercise, presumably due to an arrhythmia. Despite relief of syncope, he continued to have proBNP levels above 1300 pg/ml on follow-up with severe RV systolic dysfunction. REVEAL-2.0 score above 12 in these three deaths predicted an one-year survival < 40%.17
High-risk predictors of mortality
Right atrial pressure was 27 mmHg in a 38-year-old female with proBNP of 8000 pg/ml in NYHA class IV on multiple inotropes, despite maximum diuretics. A 6-mm AFR resulted in drop of aortic saturation from 94 to 77%. After 39 months, she improved to NYHA class II, 6MWD of 180 m, resting saturation was 78%, and proBNP was 3262 pg/ml. A second hemodynamic assessment showed right atrial pressure of 10 mmHg.
Two patients had resting saturations below 90%. One of them had an early mortality described above. The second patient aged 42 years with proBNP of 63,619 pg/ml was receiving inotrope and diuretic infusions. After intensive management, her right atrial pressure was 8 mmHg and left atrial pressure was 5 mmHg. Her saturation dropped from 87 to 72% after AFR. On 18-month follow-up, the proBNP reduced to 3534 pg/ml and 6MWD improved to 240 m.
Three patients with left ventricular end-diastolic pressures above 18 mmHg improved after procedure. No patients had PVRI above 55 wood units. Two of the four inotrope-dependent patients survived.
Post procedural symptom change
There was no recurrence of syncope (Table 4). The significant changes in NYHA class and 6MWD were sustained beyond 12 months (Supplemental Figure 3). The reduction in mean resting oxygen saturation and a more impressive reduction in post exercise saturations too were significant. Before AFR implantation, post exercise oxygen saturations could be measured only in 24 patients due to poor effort tolerance, as the sick patients could not complete six-minute walk. Even in patients who had pulse oximetry readings more than 95% in resting state after AFR implantation, the post exercise saturations dropped significantly indicating the dynamic interplay between systemic and pulmonary vascular resistance during exercise. The tolerance to hypoxemia was noted from improved functional class and 6MWD. ProBNP changes did not reach statistical significance.
Table 4.
Symptoms and ProBNP levels before and after AFR implantation.
| Parameters | Before AFR (N = 39) | Three month (N = 37) | Six month (N = 37) | One year (N = 34) |
|---|---|---|---|---|
| NYHA class | IV: 4 patientsIII: 14 patientsII: 21 patientsI: 0 patients | IV: 0 patientsIII: 10 patientsII: 23 patientsI: 4 patients | IV: 0 patientsIII: 2 patientsII: 29 patientsI: 6 patients | IV: 0 patientsIII: 1 patientII: 28 patientsI: 5 patients P = 0.003a |
| Syncope | 34/39 | NoneP = 0.000 | NoneP = 0.000 | NoneP = 0.000 |
| 6MWD (m) | 310 ± 158.2 | 376.4±182.6P < 0.001b | 378 ± 173.8P < 0.001b | 371 ± 155.2 P = 0.035b |
| Resting saturation (%) | 96.4 ± 6.4 | 92.3 ± 5.9P = 0.001a | 91.9 ± 6.5P = 0.002a | 92 ± 4.9P < 0.001b |
| Exercise saturation (%) | 96 ± 4.8 | 85.5 ± 7.2P = 0.003b | 81.7 ± 8.5P = 0.002b | 80.3 ± 5.9P < 0.001b |
| ProBNP (pg/ml) | 3542.9 ± 10,088.4 | 1370.5 ± 1428.7P = 0.203b | 1273.3 ± 1244.7P = 0.188b | 1866.3 ± 1557.9P = 0.324b |
Note: Bold fonts indicate significance.
aMcNemar test.
bPaired t test.
AFR: atrial flow regulator; NYHA: NewYork Heart Association; pg/ml: picograms/millilitres; ProBNP: pro-brain natriuretic peptide; 6MWD: six-minute walk distance.
Change in echocardiography
Inferior vena caval congestion and pericardial effusion reduced significantly after AFR (Supplemental Figure 4). While TAPSE and RV free wall strain significantly improved after AFR, PAT, FAC, RV systolic pressures and right atrial size did not change significantly (Table 2).
Follow-up
AFR retained patency in all patients at a median follow-up was 33 months (9–53 months). No patient was lost to follow-up. There were no device-related complications or arrhythmias. Need for a diuretic reduced from 19 patients to 6 patients on follow-up. A repeat cardiac catheterization in 22 patients confirmed patency in all without significant differences in pulmonary pressures.
Discussion
Need for an AFR
Despite improved one-year survival to 91% in PAH after pathway-targeted therapy, sick patients still need a balloon septostomy as a bridge to transplant or destination therapy.1,19 Early septostomy is proposed to improve survival, but randomized trials are ethically not feasible.7,14,19,20 Careful patient selection and graded dilatation improves outcome after septostomy.5 Spontaneous closure after balloon septostomy ranges from 5 to 30%.19,20 Large septostomy causes severe hypoxia, left ventricular failure, and procedural mortality.21 Experimental cryoplasty, butterfly stents, and fenestrated occluders aim to retain patency.22–24 Occlutech AFR creates a predictable interatrial fenestration.14
Improvement in hemodynamics
Right-to-left interatrial shunt through the AFR at rest or at exercise improves left ventricular preload and increases cardiac output.14,16 Reduction in aortic saturation without fall in mixed venous saturation indicated maintenance of cardiac output in our study.14 Most studies on PAH used thermodilution for assessing cardiac output, but creation of interatrial shunt led to unreliability of thermodilution for calculating systemic cardiac output. So we used indirect Fick method for cardiac index calculations instead of thermodilution. A high correlation between thermodilution and indirect Fick method was shown in patients with PAH before and after balloon septostomy.20 Reduction in right atrial pressure indicated relief of systemic venous congestion. Right atrial pressure and cardiac index are indicators of survival in PAH.25
Is there an improved systemic oxygen transport?
Even though oxygen saturation falls after septostomy, improved systemic oxygen transport increases oxygen delivery to tissues.14 But this proposal is questioned by some authors as venous saturations do not increase following septostomy.26 They contend that polycythemia triggered by long-term hypoxemia increases the oxygen delivery.26 While eight patients had preprocedural anemia, none of them had significant anemia on follow-up and this indicated improvement of hemoglobin levels due to the right-to-left shunt. Unlike septostomy, Pott’s shunt may prevent cerebral hypoxemia but it deprives left ventricular preload, increases procedural risks, and has limited universal experience.
Sustained improvement on follow-up
A sustained clinical improvement after AFR was shown by maintained NYHA class and 6MWD on follow-up. When a dysfunctional RV is offloaded by a septostomy, a shift-to-left on the declining limb of the Frank–Starling curves improves the RV contractility.8,27,28 The improved RV systolic function maintains the exercise tolerance.26 Apart from improved RV function and increased tissue oxygen delivery due to hypoxia-induced polycythemia, complex interplay between cardiac output and tissue hypoxia as well as conditioning to hypoxia could be the additional factors for sustained symptomatic improvement.
Key role of right ventricle
Despite being a disease of pulmonary vascular bed, RV plays a major role in symptoms and survival. RV, which is designed as a volume pump, fails early due to increased afterload.26 Improved ventricular interactions from RV decompression improves exercise tolerance.26 Improved TAPSE and RV strain after AFR implantation in our study correlated with improved 6MWD and symptom status.
AFR in children
There were no deaths in the nine children, all of whom showed significant improvement. As children often present with epileptic seizures and dramatically improve after septostomy, an early septostomy is proposed in children.28 However septostomy may rarely lead to life-threatening hypoxia warranting urgent device closure of septostomy and this poses ethical issues in children.28 Such an uncontrolled hypoxia was avoided after AFR due to its predictable fenestration orifice.
High-risk patients
Right atrial pressures exceeding 20 mmHg, left ventricular end-diastolic pressures above 18 mmHg, PVRI above 55 Wood units, and resting saturations under 90% are known high-risk factors for septostomy.3 High-risk patients do not have any therapeutic option apart from emergent bilateral lung transplantation that is largely unavailable in many parts of the world. Five of six patients with these predictors improved in our study. Inotrope-dependent decompensated patients are also contraindicated for septostomy.7 Two of four of such sick patients with predicted high mortality survived.
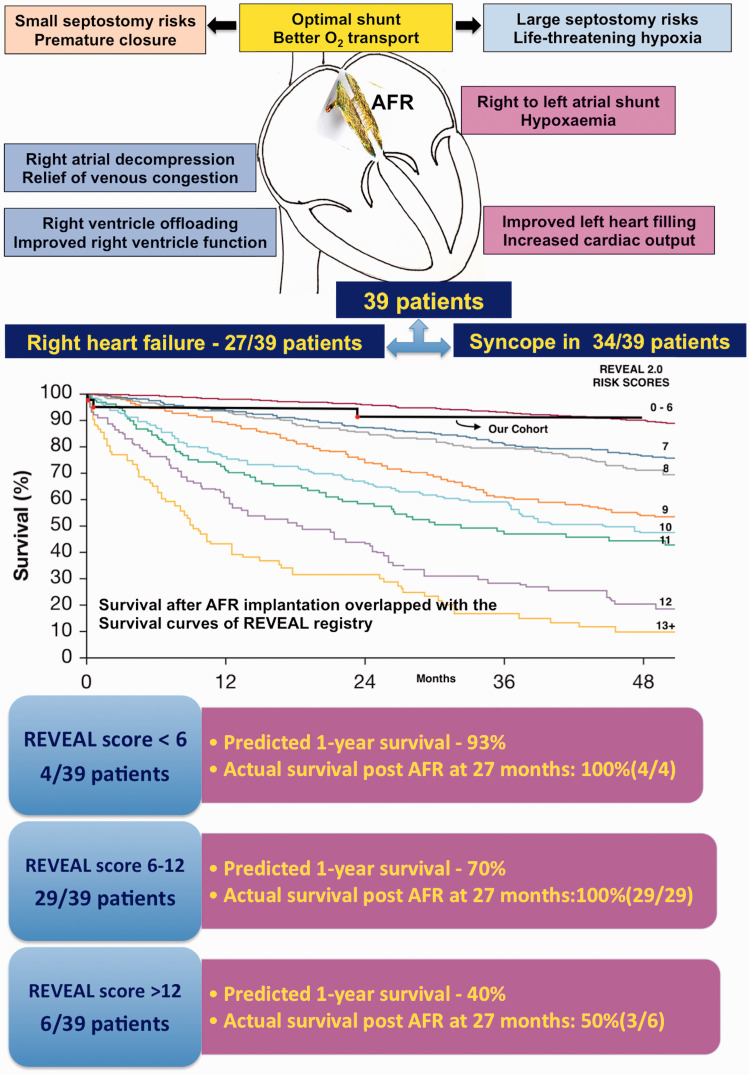
REVEAL-2.0 scores
Six patients had a REVEAL score above 12 that predicted one-year survival < 40%.17 Twenty-nine patients had scores between 7 and 12 predicting one-year survival of 70%. An overlap of the actual survival in our group after AFR with the predicted survival curves in REVEAL registry showed the advantage after AFR (Fig. 3). A large septostomy may be counterproductive in worsening hemodynamics.29,30 Ideal shunt fraction should be titrated to patient size and hemodynamics, like the one provided by AFR.13
Fig. 3.
Actual survival compared to predicted survival.
Kaplan–Meier survival estimate predicted by Registry to EValuate EArly and Long-term (REVEAL) PAH disease management 2.0 risk scores and actual survival in our study group shown by black line where mortality was plotted by red dots. Among the multiple colored lines, the top line represented survival if REVEAL-2.0 score was < 6 and the last line represented survival if the score was more than 12.
Source: reproduced with permission from Benza et al., 2019.17
Limitations
Being a rare orphan disease, the number of patients enrolled is small but is the largest study reporting the use of AFR. As 34/39 patients with syncope would be labeled as World Health Organization functional class IV, we used NYHA functional class instead. Inclusion of high-risk patients with no other options led to two early deaths. Survival curves based on REVEAL registry was overlapped with actual survival in our study group, but our small study sample is not powered to study this difference. While our median follow-up was 33 months and longest follow-up was 53 months, a longer follow-up was needed in diseases with high mortality. Indirect Fick oximetry and Lafarge derived oxygen consumption was applied before and after AFR. We did not compare outcomes in this cohort with other patients attending PAH clinic who did not get AFR.
Conclusions
AFR offers a safe and controlled septostomy in adults and children with a predictable fenestration titrated to patient size and right atrial pressures. Patients show acute benefits in cardiac output and right atrial pressures as well as sustained benefits on symptoms and RV systolic function. Lack of procedural and device-related complications as well as retained patency prove utility of AFR. Despite higher mortality, sick patients with advanced disease get symptom relief. Early implantation is better than high-risk procedures at a very advanced stage. A larger study should be powered to study survival advantage.
Acknowledgements
Occlutech donated the atrial flow regulator devices.
Footnotes
Conflict of interest: The author(s) declare that there is no conflict of interest.
Ethical approval: Obtained from ethical committee of Madras medical mission.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Kothandam Sivakumar https://orcid.org/0000-0001-8489-2322
Supplemental Material: Supplemental material for this article is available online.
Contributorship
Kothandam Sivakumar: conception, design, draft of manuscript revising it critically for important intellectual content and final approval manuscript submitted.
Gopalavilasam R. Rohitraj: analysis, interpretation of data and drafting of manuscript, and final approval manuscript submitted.
Monica Rajendran: data collection, analysis and interpretation of data, and final approval manuscript submitted.
Nithya Thivianathan: analysis and interpretation of data and final approval manuscript submitted.
References
- 1.Benza RL, Miller DP, Gomberg-Maitland M, et al. Predicting survival in pulmonary arterial hypertension. Insights from the registry to evaluate early and long-term pulmonary arterial hypertension disease management (REVEAL). Circulation 2010; 122: 164–172. [DOI] [PubMed] [Google Scholar]
- 2.Chambers DC, Cherikh WS, Harhay MO, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty-sixth adult lung and heart-lung transplantation report-2019; focus theme: donor and recipient size match. J Heart Lung Transplant 2019; 38: 1042–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhamra-Ariza P, Keogh AM, Muller DW. Percutaneous interventional therapies for the treatment of patients with severe pulmonary hypertension. J Am Coll Cardiol 2014; 63: 611–618. [DOI] [PubMed] [Google Scholar]
- 4.Khan MS, Memon MM, Amin E, et al. Use of balloon atrial septostomy in patients with advanced pulmonary arterial hypertension. A systematic review and meta-analysis. Chest 2019; 156: 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sandoval J. Interventional therapies in pulmonary hypertension. Rev Esp Cardiol 2018; 71: 565–574. [DOI] [PubMed] [Google Scholar]
- 6.Keogh AM, Mayer E, Benza RL, et al. Interventional and surgical modalities of treatment in pulmonary hypertension. J Am Coll Cardiol 2009; 54: S67–S77. [DOI] [PubMed] [Google Scholar]
- 7.Law MA, Grifka RG, Mullins CE, et al. Atrial septostomy improves survival in select patients with pulmonary hypertension. Am Heart J 2007; 153: 779–784. [DOI] [PubMed] [Google Scholar]
- 8.Espinola-Zavaleta N, Vargas-Barrón J, Tazar JI, et al. Echocardiographic evaluation of patients with primary pulmonary hypertension before and after atrial septostomy. Echocardiography 1999; 16: 625–634. [DOI] [PubMed] [Google Scholar]
- 9.O’Byrne ML, Rosenzweig ES, Barst RJ. The effect of atrial septostomy on the concentration of brain-type natriuretic peptide in patients with idiopathic pulmonary arterial hypertension. Cardiol Young 2007; 17: 557–559. [DOI] [PubMed] [Google Scholar]
- 10.Ciarka A, Vachièry JL, Houssière A, et al. Atrial septostomy decreases sympathetic overactivity in pulmonary arterial hypertension. Chest 2007; 131: 1831–1837. [DOI] [PubMed] [Google Scholar]
- 11.Rich S, Lam W. Atrial septostomy as palliative therapy for refractory primary pulmonary hypertension. Am J Cardiol 1983; 51: 1560–1561. [DOI] [PubMed] [Google Scholar]
- 12.Lammers AE, Derrick G, Haworth SG, et al. Efficacy and long-term patency of fenestrated Amplatzer devices in children. Catheter Cardiovasc Interv 2007; 70: 578–584. [DOI] [PubMed] [Google Scholar]
- 13.Weimar T, Watanabe Y, Kazui T, et al. Impact of differential right-to-left shunting on systemic perfusion in pulmonary arterial hypertension. Catheter Cardiovasc Interv 2013; 81: 888–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajeshkumar R, Pavithran S, Sivakumar K, et al. Atrial septostomy with a predefined diameter using a novel Occlutech atrial flow regulator improves symptoms and cardiac index in patients with severe pulmonary arterial hypertension. Catheter Cardiovasc Interv 2017; 90: 1145–1153. [DOI] [PubMed] [Google Scholar]
- 15.McLennan D, Ivy D, Morgan GJ. Transvenous implantation of the Occlutech atrial flow regulator: preliminary results from swine models. Congenit Heart Dis 2019; 14: 819–831. [DOI] [PubMed] [Google Scholar]
- 16.Lehner A, Schulze-Neick I, Fischer M, et al. The creation of an interatrial right-to-left shunt in patients with severe irreversible pulmonary hypertension: rationale, devices, outcomes. Current Cardiol Rep 2019; 21: 31. [DOI] [PubMed] [Google Scholar]
- 17.Benza RL, Gomberg-Maitland M, Elliott CG, et al. Predicting survival in patients with pulmonary arterial hypertension. The REVEAL risk score calculator 2.0 and comparison with ESC/ERS-based risk assessment strategies. Chest 2019; 156: 323–337. [DOI] [PubMed] [Google Scholar]
- 18.Earley MJ. How to perform a transseptal puncture. Heart 2009; 95: 85–92. [DOI] [PubMed] [Google Scholar]
- 19.Kuhn BT, Javed U, Armstrong EJ, et al. Balloon dilatation atrial septostomy for advanced pulmonary hypertension in patients on prostanoids therapy. Catheter Cardiovasc Interv 2015; 85: 1066–1072. [DOI] [PubMed] [Google Scholar]
- 20.Reichenberger F, Pepke-Zaba J, McNeil K, et al. Atrial septostomy in the treatment of severe pulmonary arterial hypertension. Thorax 2003; 58: 797–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerstein D, Levy PS, Hsu DT, et al. Blade balloon atrial septostomy in patients with severe primary pulmonary hypertension. Circulation 1995; 91: 2028–2035. [DOI] [PubMed] [Google Scholar]
- 22.Girona J, Gran F, Garcia B, et al. Percutaneous double stent atrial septostomy. Catheter Cardiovasc Interv 2007; 69: 227–230. [DOI] [PubMed] [Google Scholar]
- 23.Althoff TF, Knebel F, Panda A, et al. Long-term follow-up of a fenestrated Amplatzer atrial septal occluder in pulmonary arterial hypertension. Chest 2008; 133: 283–285. [DOI] [PubMed] [Google Scholar]
- 24.Guerrero M, Cajigas H, Awdish R, et al. First-in-man experience with cryoplasty during graded balloon atrial septostomy to reduce spontaneous closure in a patient with severe pulmonary arterial hypertension. Eurointervention 2014; 9: 1235–1236. [DOI] [PubMed] [Google Scholar]
- 25.D’Alonso GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension: results of a national prospective study. Ann Intern Med 1991; 115: 343–349. [DOI] [PubMed] [Google Scholar]
- 26.Lammers AE, Haworth SG, Diller GP. Atrial septostomy in patients with pulmonary hypertension: should it be recommended? Expert Rev Respir Med 2011; 5: 363–376. [DOI] [PubMed] [Google Scholar]
- 27.Allcock RJ, O’Sullivan JJ, Corris PA. Atrial septostomy for pulmonary arterial hypertension. Heart 2003; 89: 1334–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Micheletti A, Hislop AA, Lammers A, et al. Role of atrial septostomy in the treatment of children with pulmonary arterial hypertension. Heart 2006; 92: 969–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sandoval J, Gaspar J, Pulido T, et al. Graded balloon dilatation atrial septostomy in severe primary pulmonary hypertension. J Am Coll Cardiol 1998; 32: 297–304. [DOI] [PubMed] [Google Scholar]
- 30.Zierer A, Melby SJ, Voeller RK, et al. Interatrial shunt for chronic pulmonary hypertension: differential impact of low-flow vs high-flow shunting. Am J Physiol Heart Circ Physiol 2009; 296: H639–H644. [DOI] [PMC free article] [PubMed] [Google Scholar]