There is a Blood Commentary on this article in this issue.
Key Points
The phase 2 CAVALLI study assessed efficacy and safety of venetoclax + R-CHOP in patients with DLBCL, including Bcl-2+ subpopulations.
Venetoclax + R-CHOP showed potential for improved efficacy vs R-CHOP alone, supporting further investigation of venetoclax in Bcl-2+ DLBCL.
Abstract
The phase 2 CAVALLI (NCT02055820) study assessed efficacy and safety of venetoclax, a selective B-cell lymphoma-2 (Bcl-2) inhibitor, with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in first-line (1L) diffuse large B-cell lymphoma (DLBCL), including patients demonstrating Bcl-2 protein overexpression by immunohistochemistry (Bcl-2 IHC+). Eligible patients were ≥18 years of age and had previously untreated DLBCL, Eastern Cooperative Oncology Group performance status ≤2, and International Prognostic Index 2 to 5. Venetoclax 800 mg (days 4-10, cycle 1; days 1-10, cycles 2-8) was administered with rituximab (8 cycles) and cyclophosphamide, doxorubicin, vincristine, and prednisone (6-8 cycles) in 21-day cycles. Primary end points were safety, tolerability, and complete response (CR) at end of treatment (EOT). Secondary end points were progression-free survival (PFS) and overall survival. Comparative analyses used covariate-adjusted R-CHOP controls from the GOYA/BO21005 study, an appropriate contemporary benchmark for safety and efficacy. Safety and efficacy analyses included 206 patients. CR rate at EOT was 69% in the overall population and was maintained across Bcl-2 IHC+ subgroups. With a median follow-up of 32.2 months, trends were observed for improved investigator-assessed PFS for venetoclax plus R-CHOP in the overall population (hazard ratio [HR], 0.61; 95% confidence interval [CI], 0.43-0.87) and Bcl-2 IHC+ subgroups (HR, 0.55; 95% CI, 0.34-0.89) vs R-CHOP. Despite a higher incidence of grade 3/4 hematologic adverse events (86%), related mortality was not increased (2%). Chemotherapy dose intensity was similar in CAVALLI vs GOYA. The addition of venetoclax to R-CHOP in 1L DLBCL demonstrates increased, but manageable, myelosuppression and the potential of improved efficacy, particularly in high-risk Bcl-2 IHC+ patient subgroups.
Visual Abstract
Introduction
The prognosis of patients with diffuse large B-cell lymphoma (DLBCL) has improved considerably with the addition of rituximab to cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) chemotherapy.1-6 Beyond cell-of-origin (COO) effects and adjustment for clinicopathologic risk factors, DLBCL subgroups defined by molecular biomarkers provide independent prognostic value.7-11 Specifically, overexpression of B-cell lymphoma-2 (Bcl-2), an antiapoptotic regulator linked to tumor aggressiveness, confers resistance to the proapoptotic activities of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) chemotherapy in the first-line (1L) setting and is associated with inferior outcome, identifying a patient population with unmet needs.7,12-17 Concurrent overexpression of Bcl-2 and Myc proteins (double-expressor lymphoma [DEL]; 20-30% of DLBCL) is a feature associated with adverse outcome. Additionally, patients with rearrangements of MYC and BCL-2 (high-grade B-cell lymphoma, formerly “double-hit” lymphoma [DHL]) have a particularly poor prognosis with R-CHOP.18-21
Venetoclax, a highly selective potent oral inhibitor of Bcl-2, has shown promising clinical activity in a range of non-Hodgkin lymphoma (NHL) subtypes.22,23 Results from the CAVALLI Phase 1b study (NCT02055820) support the potential of venetoclax as a rational targeted inhibitor and chemosensitizing agent.24 During CAVALLI phase 1b, the maximum tolerated dose of venetoclax plus R-CHOP was not reached, and the recommended phase 2 dose (RP2D) for the combination (supported by exposure-efficacy and exposure-safety analyses) was a noncontinuous dosing schedule of venetoclax, 800 mg on days 4 to 10 of cycle 1 and days 1 to 10 of cycles 2 to 8.
CAVALLI phase 1b reported increased rates of grade 3/4 hematologic adverse events (AEs) consistent with other studies using novel targeted agents combined with chemotherapy.25,26 In this small patient population (N = 24), the myelosuppressive effects of venetoclax plus R-CHOP were manageable with granulocyte colony-stimulating factor (G-CSF) prophylaxis, supportive measures, and dose modifications or delays (applied first to venetoclax). Subsequently, the phase 2 expansion further assessed myelosuppression, as well as the clinical efficacy of this regimen, in the 1L DLBCL setting.
Here, we report efficacy, safety, and biomarker analyses from the phase 2 portion of the CAVALLI study, using the RP2D of venetoclax plus R-CHOP in an expanded population of patients with previously untreated DLBCL.
Methods
Study design and participants
CAVALLI (NCT02055820; European Union Clinical Trials Register identifier: 2013-003749-40) is a multicenter open-label phase 1b/2 study assessing venetoclax in combination with standard R-CHOP or obinutuzumab (G)-CHOP in patients with B-cell NHL (dose-finding phase 1b stage) and with R-CHOP in previously untreated DLBCL (phase 2 expansion stage). The phase 2 part of CAVALLI was conducted at 50 sites across North America, Europe, and Australia.
After the first 20 patients completed the initial 2 treatment cycles, data were reviewed by the Internal Monitoring Committee and Scientific Oversight Committee to confirm safety and tolerability of the phase 2 dose, whereas ongoing enrollment continued. Additionally, Internal Monitoring Committee and Scientific Oversight Committee safety data reviews were conducted periodically throughout.
Eligible patients were ≥18 years of age, with previously untreated CD20+ DLBCL, an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 to 2, an International Prognostic Index (IPI) score of 2 to 5, ≥1 bidimensionally measurable lymphoma lesion >1.5 cm in its longest dimension, and adequate hematologic function. Patients with transformed lymphoma were considered for inclusion after discussion with the Medical Monitor. Key exclusion criteria included prior therapy for NHL, contraindication to rituximab or any of the components of CHOP, prior anthracycline therapy, central nervous system lymphoma or primary mediastinal DLBCL, and evidence of significant uncontrolled concomitant diseases.
The study protocol was approved by the institutional review board or ethics committees at participating institutions in accordance with the International Conference on Harmonization guidelines, including Good Clinical Practice and the ethical principles originating from the Declaration of Helsinki. Informed consent was obtained from all patients. All authors had access to study data.
Treatment
Patients received venetoclax, 800 mg orally on a noncontinuous dosing schedule of days 4 to 10 of cycle 1 and days 1 to 10 of cycles 2 to 8 (21-day cycles), as determined during the dose-finding phase 1b stage. Rituximab, 375 mg/m2, was given IV on day 1 of cycles 1 to 8. Standard CHOP, consisting of IV cyclophosphamide 750 mg/m2, doxorubicin 50 mg/m2, and vincristine 1.4 mg/m2 (with a 2.0 mg cap) on day 1 and prednisone 100 mg/d orally on days 1 to 5, was given for 6 cycles. Investigators were able to prescribe 2 additional cycles if they felt it was necessary and in the patient’s best interest.
Tumor lysis syndrome (TLS) prophylaxis was mandatory, including hydration and a uric acid–lowering agent (eg, allopurinol) administered daily ≥72 hours before the first venetoclax dose. Patients with bulky disease (ie, any lymph node or mass ≥10 cm on computed tomography scan) at screening and/or lymphocytosis due to circulating lymphoma cells were considered to be at higher risk for TLS and, therefore, required hospitalization for prophylaxis (eg, with rasburicase) and close monitoring during the initiation of venetoclax treatment. Supportive measures as per local standards of care were permitted. Further details relating to TLS mitigation used in CAVALLI have been published previously.24
The use of prophylactic G-CSF was mandated in later protocol versions for all cycles involving CHOP chemotherapy. Anti-infective medications to prevent recurrent viral, bacterial, or fungal infections were permitted but not mandated.
End points and assessments
The primary efficacy end point was positron emission tomography (PET)-complete response (CR), assessed by an independent review committee (IRC), according to modified Lugano 2014 criteria,27 for the intention-to-treat (ITT) and prespecified biomarker-driven populations. Prespecified biomarker analyses included investigator assessment of efficacy in the following patient subgroups: Bcl-2 protein overexpression by immunohistochemistry (Bcl-2 IHC+), Bcl-2 IHC−, DEL, DHL, and DLBCL COO subtypes (activated B cell [ABC] and germinal center B cell [GCB]). Bone marrow examination was required to confirm CR; if previously positive, not performed, or indeterminate at screening, bone marrow examination was repeated. Secondary efficacy end points included investigator-assessed objective response rate (ORR), CR, progression-free survival (PFS), overall survival (OS), and relative dose intensity (RDI). Safety end points included frequency and severity of AEs, grade 3/4 AEs, and serious AEs (SAEs). Graded physical examinations, vital sign assessments, and laboratory tests were also performed.
Assessments of disease response were conducted 15 to 21 days after completion of cycle 4 and 6 to 8 weeks after day 1 of the last cycle (end of treatment [EOT] response visit). Patients were followed for disease response every 3 months for up to 2 years until progression or study termination, whichever occurred first. Survival follow-up continued until death, loss to follow-up, consent withdrawal, or study termination.
Bcl-2 protein expression was assessed by immunohistochemistry (IHC) using the anti–Bcl-2 (124) mouse monoclonal antibody,28 whereas Myc IHC was assessed using the clone Y69 Epitomics antibody.29 Bcl-2 IHC scoring incorporated the percentage of positively stained tumor cells (≥50% of tumor cells, as previously defined18,30) and the intensity of tumor cell staining. Tumor cells were assigned an intensity of “weak” or “strong,” depending on whether the signal was substantially weaker or stronger than in mantle zone B cells and paracortical T cells in normal tonsils, which were used as references for “moderate” Bcl-2 IHC staining intensity. Based on ≥50% of tumor cells being assigned to the no-expression, weak-expression, moderate-expression, or strong-expression groups, tumors were allocated 1 of 4 possible IHC scores (0, 1+, 2+, or 3+). Samples scoring 0 or 1+ were designated Bcl-2 IHC−, and samples scoring 2+ or 3+ were called Bcl-2 IHC+. Similarly, tumors were classified as Myc IHC+ if ≥40% of cells showed Myc nuclear staining above background intensity.18,30
The DEL population was defined as all patients with DLBCL coexpressing Bcl-2 and Myc proteins, according to IHC. BCL-2 and c-MYC translocations were assessed by fluorescence in situ hybridization (FISH) using Vyvis LSI Bcl-2 and MYC Dual Color Break Apart Probes. COO subtype was assessed by RNA expression using a NanoString Lymph 2Cx assay.
Statistical analysis
The sample size in CAVALLI phase 2 was calculated with the intention of obtaining sufficient patients for estimation of PET-CR rates in the DLBCL biomarker subgroups (supplemental Materials, available on the Blood Web site). The planned enrollment was ∼160 to 200 patients, aiming to recruit ∼50 patients with DEL, 80 to 100 patients with Bcl-2 IHC+ DLBCL, and 40 to 50 patients with Bcl-2 IHC+ GCB or ABC DLBCL. With a subgroup size of 50 enrolled patients, the 95% confidence intervals (CIs) for estimation of PET-CR would have a margin of error not exceeding 16%. No formal hypothesis testing was planned.
Efficacy and safety analyses were based on all enrolled patients who received any venetoclax and/or R-CHOP treatment. To fully assess the safety and efficacy of venetoclax plus R-CHOP, the R-CHOP control arm (patients with IPI scores of 2-5 only) from the GOYA/BO21005 study (NCT01287741)31 was selected (January 2017, prior to primary analysis) as a statistically robust and clinically meaningful contemporary comparator for exploratory analyses (supplemental Materials).
The Bcl-2 biomarker-evaluable population (BEP) in GOYA included 370 of 703 patients. To ensure that this subset was representative of the overall ITT GOYA R-CHOP population, baseline characteristics (including age, ECOG PS, lactate dehydrogenase [LDH] levels, IPI, and COO) and PFS outcome were compared between the BEP and ITT populations and shown to be similar (supplemental Figure 1; supplemental Table 1). Additionally, no differences between baseline characteristics and PFS outcome for the IPI 2 to 5 subset of patients in the BEP (n = 308) and ITT population (n = 564) were identified, allowing meaningful analysis (supplemental Figure 1; supplemental Table 1). To ensure an analogous duration of follow-up between CAVALLI and GOYA patients, data from an interim analysis of GOYA were used.
Response rates were compared using a double-robust method adjusting for baseline covariates (age, sex, ECOG PS [≥2 vs <2], bone marrow involvement, IPI score [high vs nonhigh], bulky disease [>7.5 cm, as specified in the GOYA study], disease stage [IV vs I-III], LDH [low/normal vs high], and COO subtype [ABC, GCB; determined using NanoString Lymph 2Cx assay in both studies]).32 Adjusted Cox regression was used to compare PFS and OS, adjusted for baseline covariates. Safety analyses of CAVALLI vs GOYA were summarized descriptively.
Results
Patient population
In total, 211 patients were enrolled in CAVALLI phase 2; 206 (98%) patients were included in the efficacy and safety analyses (3 patients did not receive any treatment and 2 other patients with an IPI score of 1 were excluded from analyses). An overview of patient disposition is shown in supplemental Figure 2.
Patient demographics and baseline characteristics for CAVALLI phase 2 and GOYA IPI 2 to 5 are shown in Table 1. Overall baseline characteristics were similar between the 2 populations. The median age in CAVALLI phase 2 was 65 years (range, 18-85); 55% (113/206) of patients were male, and most had advanced-stage disease. The CAVALLI study had a higher proportion of patients with an IPI score of 4 or 5 compared with GOYA (25% vs 19%, respectively). Bcl-2 IHC data were available for 179 patients enrolled in CAVALLI; of those, 58% (n = 104) were Bcl-2 IHC+ compared with 49% (n = 151) in GOYA (where 308 patients with IPI 2-5 were evaluable for Bcl-2 IHC; Table 1). IHC+ Myc protein expression was reported for 74% (n = 133) of patients in CAVALLI compared with 82% (n = 248) in GOYA, whereas 45% (n = 80) of patients were categorized as DEL in CAVALLI compared with 41% (n = 124) in GOYA (Table 1). In CAVALLI, FISH analysis for BCL-2 and MYC was available for 139 patients; of those, 7 (5%) were categorized as DHL (vs 8/230, 3% categorized as DHL for GOYA; Table 1). Of the 161 enrolled patients with Bcl-2 and COO data available in CAVALLI, 44 (27%) had Bcl-2 IHC+/ABC DLBCL and 46 (29%) had Bcl-2 IHC+/GCB DLBCL.
Table 1.
Baseline characteristics (safety-evaluable population)
| Parameter | CAVALLI phase 2 (N = 206) | GOYA IPI 2 to 5 (N = 564) |
|---|---|---|
| Age, median (range), y | 65 (18-85) | 62 (18-83) |
| Female | 93 (45) | 267 (47) |
| ECOG PS* | ||
| 0-1 | 172 (84) | 476 (84) |
| 2 | 34 (17) | 87 (15) |
| Stage III-IV | 177 (86) | 478 (85) |
| IPI | ||
| 2-3 | 155 (75) | 455 (81) |
| 4-5 | 51 (25) | 109 (19) |
| COO | n = 171 | n = 373 |
| ABC | 48 (28) | 104 (28) |
| GCB | 101 (59) | 213 (57) |
| Unclassified | 22 (13) | 56 (15) |
| Bcl-2 IHC | n = 179 | n = 308 |
| High† | 104 (58) | 151 (49) |
| Low | 75 (42) | 157 (51) |
| Myc IHC | n = 179 | n = 304 |
| Positive | 133 (74) | 248 (82) |
| Negative | 46 (26) | 56 (18) |
| Double expressor | n = 179 | n = 299 |
| Yes | 80 (45) | 124 (41) |
| No | 99 (55) | 175 (59) |
| Bcl-2† and Myc IHC | n = 179 | n = 299 |
| Bcl-2 IHC+/Myc IHC+ (DHL, DEL) | 80 (45) | 124 (41) |
| Bcl-2 IHC+/Myc IHC− | 24 (13) | 24 (8) |
| Bcl-2 IHC−/Myc IHC+ | 53 (30) | 119 (40) |
| BCL-2 FISH | n = 151 | n = 265 |
| Positive | 40 (26) | 59 (22) |
| Negative | 89 (59) | 145 (55) |
| Undetermined | 22 (15) | 61 (23) |
| MYC FISH | n = 142 | n = 232 |
| Positive | 12 (8) | 20 (9) |
| Negative | 110 (78) | 178 (77) |
| Undetermined | 20 (14) | 34 (15) |
| BCL-2 & MYC FISH | n = 139 | n = 230 |
| BCL-2 FISH+/MYC FISH+ (DHL) | 7 (5) | 8 (3) |
Unless noted otherwise, data are n (%).
One patient in the GOYA study had an ECOG PS of 3.
High is defined as an IHC score of 2+ or 3+.
At the time of analysis (data cutoff 28 June 2019), median follow-up in CAVALLI was 32.2 months. Data cutoff for GOYA was 29 April 2016; median follow-up was 29.6 months. Median diagnosis to treatment interval was well matched between the study populations: 26 days for CAVALLI vs 24 days for GOYA.
Treatment received
In total, 144 of 206 (70%) CAVALLI patients completed venetoclax treatment (supplemental Figure 2). Median number of treatment cycles was 8 (range, 1-8) for venetoclax and rituximab, and 6 (range, 1–8) for each component of CHOP. A total of 134 (65%) patients received 6 cycles of R-CHOP, whereas only 5 (2%) patients received 7 cycles and 15 (7%) patients received 8 cycles. The proportion of patients who received ≥90% overall dose intensity was 61% for venetoclax, 73% for rituximab, cyclophosphamide, and doxorubicin, 69% for vincristine, and 74% for prednisone (Table 2). Furthermore, the RDI of R-CHOP in CAVALLI was maintained at a level similar to that seen in GOYA (Table 2), with the exception of rituximab. Although there was little difference in the administered dose of rituximab, the RDI was lower in CAVALLI (73%) than in GOYA (83%) as a result of dose delays in several patients in the CAVALLI study (supplemental Figure 3).
Table 2.
Patients achieving >90% dose intensity (safety-evaluable population)
| CAVALLI phase 2 (N = 206) | GOYA IPI 2 to 5 (N = 564) | |
|---|---|---|
| Venetoclax | ||
| DI, median (range), % | 95 (4-103) | N/A |
| Patients with >90% DI, n (%) | 123 (61) | N/A |
| Rituximab | ||
| DI, median (range), % | 96 (9-106) | 97 (12-111) |
| Patients with >90% DI, n (%) | 148 (73) | 466 (83) |
| Cyclophosphamide | ||
| DI, median (range), % | 96 (12-128) | 96 (15-110) |
| Patients with >90% DI, n (%) | 148 (73) | 410 (73) |
| Doxorubicin | ||
| DI, median (range), % | 96 (12-128) | 95 (15-110) |
| Patients with >90% DI, n (%) | 148 (73) | 403 (72) |
| Vincristine | ||
| DI, median (range), % | 96 (17-101) | 95 (13-167) |
| Patients with >90% DI, n (%) | 141 (69) | 400 (71) |
| Prednisone | ||
| DI, median (range), % | 97 (10-141) | 96 (3-200) |
| Patients with >90% DI, n (%) | 152 (74) | 425 (75) |
DI, dose intensity.
Efficacy
PET-CR rates at EOT by IRC were 69% (143/206) overall, 64% (67/104) in the Bcl-2 IHC+ population, and 66% (53/80) in the DEL population (Table 3). Rates were similar to those in the GOYA cohort. PET-CR rates for patients in the Bcl-2 IHC+ subgroups are provided in supplemental Table 2.
Table 3.
IRC-assessed PET-CR rates (safety-evaluable population)
| CAVALLI phase 2 (N = 206) | GOYA IPI 2 to 5 (N = 564) | Adjusted delta CR (95% CI), % | |
|---|---|---|---|
| Lugano 2014 responses, n (%) | |||
| All | 143 (69) | 354 (63) | 6 (0-13) |
| Bcl-2 IHC+ | n = 104 | n = 151 | |
| 67 (64) | 91 (60) | 2 (0-12) | |
| DEL | n = 80 | n = 124 | |
| 53 (66) | 75 (61) | 2 (0-14) | |
IRC-based ORR assessments in CAVALLI were similar to the matched GOYA control in the overall population (83% vs 80%; supplemental Table 3). The IRC-assessed ORR was numerically higher in CAVALLI than in GOYA in patients with Bcl-2 IHC+ disease (83% vs 77%) and in the DEL subgroup (84% vs 77%; supplemental Table 3). Investigator-assessed ORR and CR were numerically superior in CAVALLI compared with GOYA in the overall population and in the Bcl-2 IHC+ and DEL subgroups (supplemental Table 4).
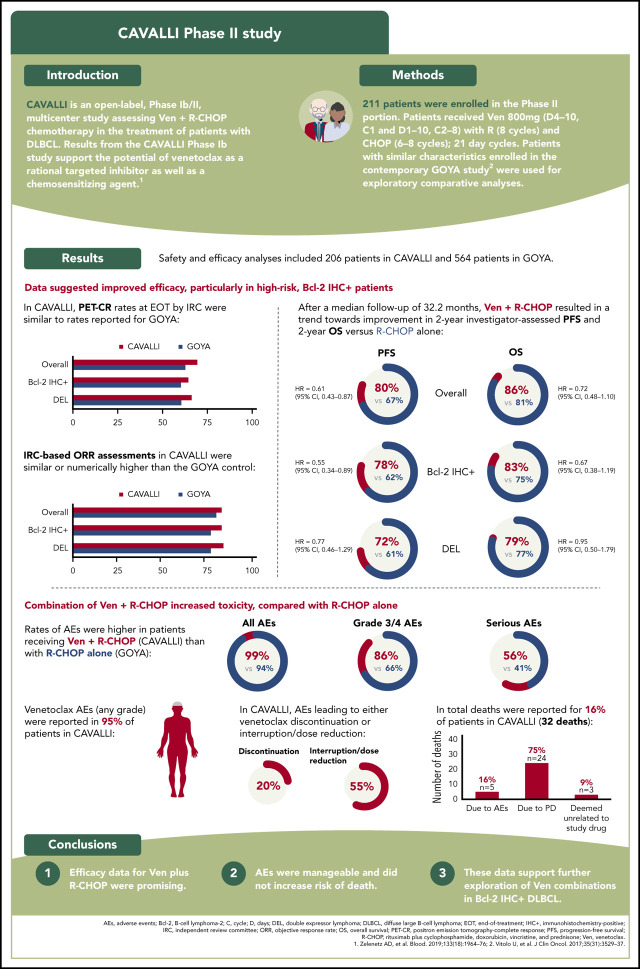
After a median follow-up of 32.2 months, investigator-assessed 2-year PFS for venetoclax plus R-CHOP vs the matched GOYA control was 80% vs 67% (hazard ratio [HR], 0.61; 95% CI, 0.43-0.87), 78% vs 62% (HR, 0.55; 95% CI, 0.34-0.89), 83% vs 75% (HR, 0.77; 95% CI, 0.39-1.50) and 72% vs 61% (HR, 0.77; 95% CI, 0.46-1.29) in the overall population and the Bcl-2 IHC+, Bcl-2 IHC−, and DEL subgroups, respectively (Figure 1; Table 4). IRC-assessed PFS results corroborated these findings (supplemental Figure 4; supplemental Table 5). Two-year PFS in CAVALLI vs GOYA for patients in the Bcl-2 IHC+ subgroups are provided in supplemental Figures 5 and 6, and supplemental Tables 6 and 7.
Figure 1.
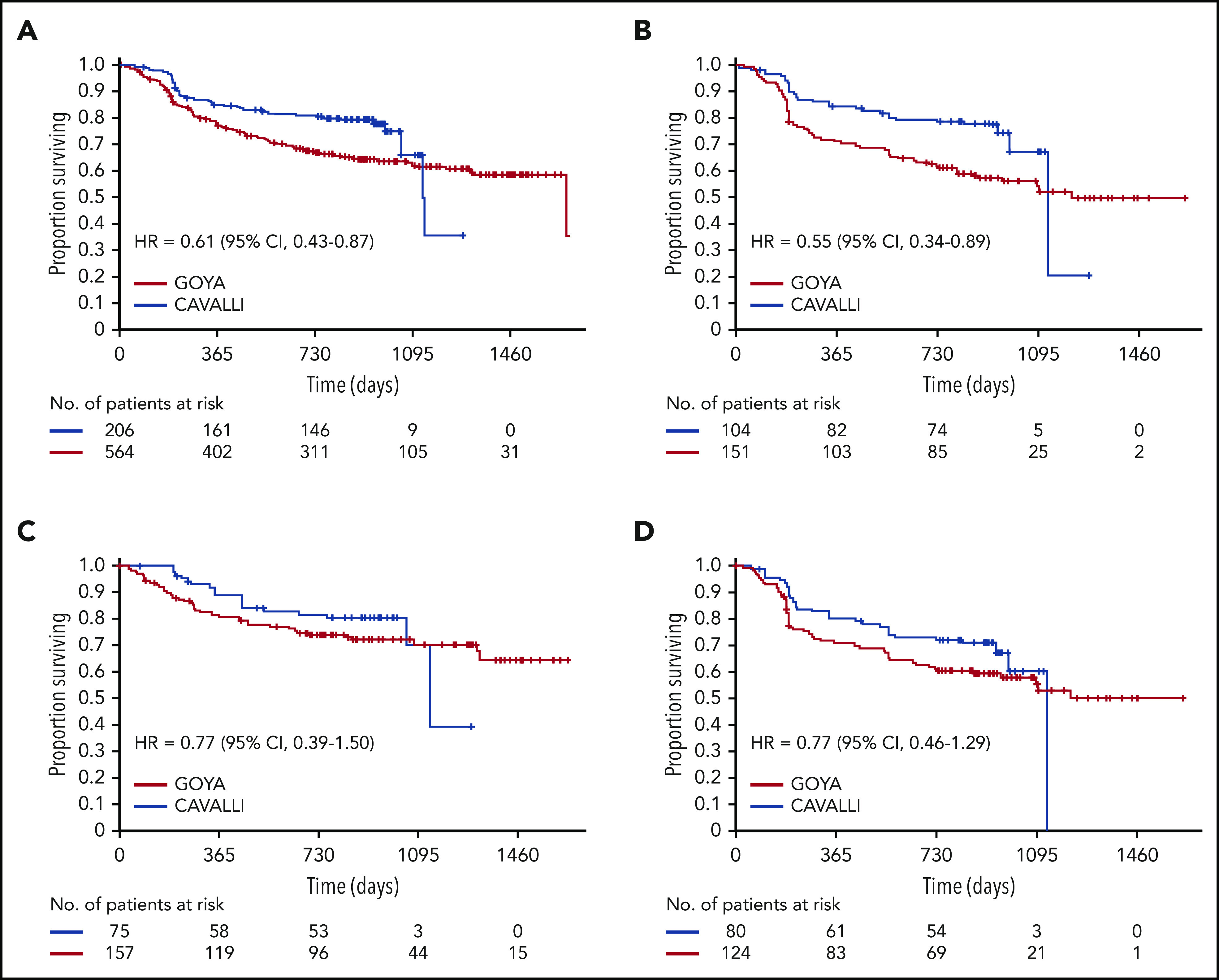
Investigator-assessed PFS in CAVALLI vs GOYA IPI 2 to 5. Kaplan-Meier curves for the overall population (A) and the Bcl-2 IHC+ (B), Bcl-2 IHC− (C), and DEL (D) subgroups. The following covariates were adjusted: age, sex, ECOG PS, bone marrow involvement, IPI (high vs nonhigh), bulky disease (>7.5 cm), disease stage (IV vs I-III), LDH, and COO.
Table 4.
Investigator-assessed PFS and OS (safety-evaluable population)
| Population | CAVALLI phase 2 | GOYA IPI 2 to 5 | PFS HR (95% CI) adjusted* | OS HR (95% CI) adjusted* | ||||
|---|---|---|---|---|---|---|---|---|
| N | PFS (24 mo) | OS (24 mo) | N | PFS (24 mo) | OS (24 mo) | |||
| All | 206 | 79.6 | 85.6 | 564 | 67.2 | 80.9 | 0.61 (0.43-0.87) | 0.72 (0.48-1.10) |
| Bcl-2 IHC+ | 104 | 77.6 | 82.8 | 151 | 61.7 | 75.4 | 0.55 (0.34-0.89) | 0.67 (0.38-1.19) |
| Bcl-2 IHC− | 75 | 83.4 | 89.9 | 157 | 74.6 | 83.2 | 0.77 (0.39-1.50) | 0.90 (0.41-1.98) |
| DEL | 80 | 72.3 | 79.0 | 124 | 60.6 | 77.4 | 0.77 (0.46-1.29) | 0.95 (0.50-1.79) |
Covariates include age, sex, ECOG PS, body mass index, IPI (high vs nonhigh), bulky disease (>7.5 cm), disease stage (IV vs I-III), LDH, and COO subtype.
Two-year OS for venetoclax plus R-CHOP vs the matched GOYA control (after a median follow-up of 32.2 months) was 86% vs 81% (HR, 0.72; 95% CI, 0.48-1.10), 83% vs 75% (HR, 0.67; 95% CI, 0.38-1.19), 79% vs 74% (HR, 0.95; 95% CI, 0.50-1.79), and 90% vs 83% (HR, 0.90; 95% CI, 0.41-1.98) in the overall population and the Bcl-2 IHC+, Bcl-2 IHC−, and DEL subgroups, respectively (Figure 2; Table 4). OS for patients in the Bcl-2 IHC+ subgroups in CAVALLI vs GOYA are provided in supplemental Figure 7 and supplemental Table 6.
Figure 2.
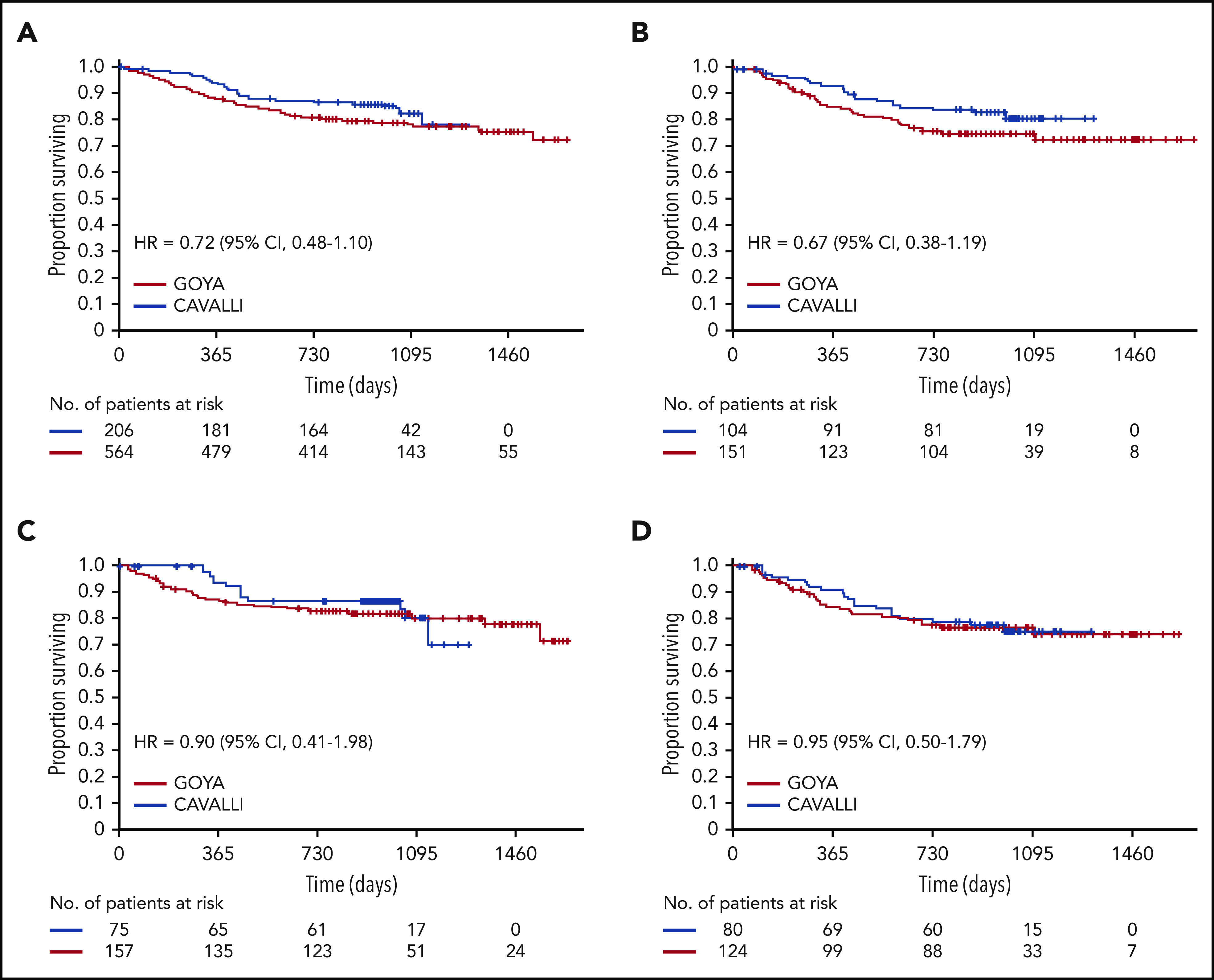
OS in CAVALLI vs GOYA IPI 2-5. Kaplan-Meier curvesfor the overall population (A) and the Bcl-2 IHC+ (B), Bcl-2 IHC− (C), and DEL (D) subgroups. The following covariates were adjusted: age, sex, ECOG PS, bone marrow involvement, IPI (high vs nonhigh), bulky disease (>7.5 cm), disease stage (IV vs I-III), LDH, and COO.
Safety
An overview of safety in CAVALLI vs GOYA is shown in Table 5. Rates of AEs were higher in patients receiving venetoclax with R-CHOP in CAVALLI than with R-CHOP alone in GOYA for all AEs (99% vs 94%, respectively), grade 3/4 AEs (86% vs 66%, respectively), and SAEs (56% vs 41%, respectively; Table 5). The most common any-grade AEs in CAVALLI vs GOYA were neutropenia (68% vs 41%, respectively), infections and infestations (63% vs 46%, respectively), nausea (52% vs 27%, respectively), fatigue (40% vs 18%, respectively), and diarrhea (39% vs 14%, respectively).
Table 5.
Overview of safety and AEs reported at grade 3/4 severity in ≥5 patients (2%) with venetoclax plus R-CHOP (safety-evaluable population)
| CAVALLI phase 2 (N = 206) | GOYA IPI 2 to 5 (N = 564) | |||
|---|---|---|---|---|
| Safety overview | ||||
| Any AE | 204 (99) | 528 (94) | ||
| Total number of AEs, n | 3345 | 4926 | ||
| AE with fatal outcome (grade 5) | 5 (2) | 30 (5) | ||
| SAE | 116 (56) | 230 (41) | ||
| Grade 3/4 AE | 178 (86) | 373 (66) | ||
| AE leading to withdrawal from any treatment | 50 (24) | 56 (10) | ||
| AE (MedDRA preferred term), n (%) | All grades | Grade 3/4 | All grades | Grade 3/4 |
| Neutropenia | 141 (68) | 141 (68) | 232 (41) | 219 (39) |
| Infections and infestations* | 130 (63) | 47 (23) | 260 (46) | 90 (16) |
| Nausea | 107 (52) | 7 (3) | 155 (27) | 2 (< 1) |
| Fatigue | 83 (40) | 6 (3) | 100 (18) | 11 (2) |
| Diarrhea | 80 (39) | 9 (4) | 79 (14) | 9 (2) |
| Anemia | 76 (37) | 50 (24) | 91 (16) | 50 (9) |
| Vomiting | 68 (33) | 7 (3) | 64 (11) | 2 (< 1) |
| Febrile neutropenia | 63 (31) | 63 (31) | 93 (16) | 92 (16) |
| Thrombocytopenia | 53 (26) | 45 (22) | 17 (3) | 9 (2) |
| Hypokalemia | 35 (17) | 8 (4) | 44 (8) | 14 (2) |
| Asthenia | 33 (16) | 5 (2) | 61 (11) | 7 (1) |
| Leucopenia | 24 (12) | 21 (10) | 65 (12) | 54 (10) |
| Pneumonia | 13 (6) | 9 (4) | 52 (9) | 28 (5) |
| Hyperglycemia | 8 (4) | 6 (3) | 20 (4) | 7 (1) |
| Sepsis | 6 (3) | 5 (2) | 10 (2) | 6 (1) |
| Hyponatremia | 5 (2) | 5 (2) | 10 (2) | 9 (2) |
Unless otherwise indicated, data are n (%).
MedDRA, Medical Dictionary for Regulatory Activities.
System organ class (all others are preferred terms).
In CAVALLI, rates of grade 3/4 cytopenias and grade 3 to 5 infections were similarly distributed across cycles 1 to 6, with a reduction in cycles 7 and 8 (supplemental Figure 8). Incidence of grade 3/4 thrombocytopenia appeared to be cumulative across cycles 1 to 6. In total, 33% received red blood cells, and 15% received platelet concentrate. Higher rates of grade 3/4 cytopenias (including neutropenia, anemia, and thrombocytopenia), febrile neutropenia, and infections occurred in CAVALLI phase 2 vs GOYA (neutropenia: 68% vs 39%, respectively; anemia: 24% vs 9%, respectively; thrombocytopenia: 22% vs 2%, respectively; febrile neutropenia: 31% vs 16%, respectively; and infections and infestations: 23% vs 16%, respectively; Table 5). Furthermore, 1 patient in CAVALLI developed nonserious grade 3 laboratory TLS before venetoclax administration (abnormal electrolytes were phosphorus, potassium, and uric acid). This AE occurred before the study protocol was amended with updated TLS prophylaxis recommendations.
Venetoclax-related AEs (any grade) were reported in 95% of patients in CAVALLI. The majority of these were (Medical Dictionary for Regulatory Activities preferred terms) blood and lymphatic system disorders (74% of patients), gastrointestinal disorders (65% of patients), and general disorders and administration site conditions (45% of patients). Rates of venetoclax-related neutropenia and febrile neutropenia were 61% and 27% respectively, whereas nausea, fatigue, diarrhea, thrombocytopenia, vomiting, and asthenia occurred in 40%, 29%, 27%, 21%, 20%, and 9% of patients, respectively. AEs leading to venetoclax discontinuation or interruption/dose reduction occurred in 41 (20%) and 114 (55%) patients, respectively (supplemental Table 8).
During cycle 1 of CAVALLI, the majority of patients (188/206; 91%) received G-CSF prophylaxis. However, the timing of administration with respect to antilymphoma treatment, the formulation (pegylated [pegfilgrastim] or nonpegylated [filgrastim] G-CSF), and the number of daily filgrastim doses were left to the investigators’ clinical judgment and local practice. This resulted in considerable variation in prescribing patterns across sites, with a median of 5 days (range, 1-10) of consecutive prophylactic filgrastim administration during cycle 1. Pegfilgrastim prophylaxis was uniform in terms of cycle timing (between day +2 and +3) and resulted in a significant reduction in the rates of grade 3/4 neutropenia (16/75; 21%) and febrile neutropenia (2/75; 3%) in patients who received an appropriately timed dose in cycle 1 vs patients who did not receive any G-CSF prophylaxis (10/18; 56% and 1/18; 6%, respectively). This trend cannot be confirmed for subsequent treatment cycles because more patients received G-CSF and there were treatment delays, making interpretation difficult (supplemental Table 9).
Data regarding prophylactic antibiotic use were collected per patient across the entire treatment course and appeared to be lower in patients without any grade 3 to 5 infections than in those with ≥1 reported episode (99/158; 63% vs 41/48; 85%), most likely reflecting the more stringent use of antimicrobial prophylaxis following a significant episode of infection, rather than prior to the event.
In total, there were 32 deaths in CAVALLI; 24 were attributed to progressive disease (investigator assessed), and 5 were due to AEs (1 case each of neutropenia [cause of death was sepsis on day 12], pseudomonal sepsis [on day 523], and sudden cardiac death [on day 347], and 2 cases of acute myeloid leukemia [on days 729 and 1137]). An additional nonfatal case of acute myeloid leukemia was reported in CAVALLI (3/206 patients) compared with 2 of 564 patients (1 fatal, 1 nonfatal) in GOYA. The remaining 3 deaths in CAVALLI were due to events deemed unrelated to study drugs (acute respiratory failure, multiple comorbidities, bilateral pneumonia) outside of the safety reporting period (>1 year after day 1, cycle 1).
Discussion
The phase 2 (expansion) CAVALLI study in 1L DLBCL included high-risk patient subgroups identified by Bcl-2 protein expression status, and distributed across ABC and GCB COO subtypes, to support a prespecified hypothesis that Bcl-2 IHC+ patients would gain greater benefit than Bcl-2 IHC− patients from the addition of venetoclax to R-CHOP.
The GOYA study was considered to be a relevant contemporary benchmark for safety and efficacy for this single-arm study, based on the fact that it was conducted at similar sites, used a suitable control-arm regimen (R-CHOP for 6-8 cycles),31 had comparable enrollment criteria with similar per-protocol patient management, and an appropriate follow-up duration. Furthermore, the Bcl-2 IHC (Ventana; investigational-use only) assay used in both trials enables direct comparison of Bcl-2 protein expression status. Proportions of DLBCL patients demonstrating Bcl-2 and Myc protein overexpression were similar in the GOYA and CAVALLI studies and, in turn, were consistent with Bcl-2 positivity rates (IHC and FISH) from the published literature.16,17,31
Efficacy data for venetoclax plus R-CHOP were encouraging. In CAVALLI, PET-CR rates were similar to GOYA for the overall patient population, the Bcl-2 IHC+ population, and patients with DEL disease. Importantly, a signal for improved investigator-assessed PFS for venetoclax plus R-CHOP compared with matched R-CHOP GOYA controls was suggested in the CAVALLI population overall, mainly driven by the Bcl-2 IHC+ subpopulation. This signal was less clear in the Bcl-2 IHC− and DEL subpopulations. Notably, a randomized trial (NCT03984448) exploring the use of venetoclax plus rituximab chemotherapy in high-risk DLBCL is ongoing.
Rates of AEs, grade 3/4 AEs, and SAEs were higher in CAVALLI vs GOYA, and the majority of patients in CAVALLI experienced venetoclax-related AEs, highlighting the increased toxicity of the venetoclax + R-CHOP combination. CAVALLI reported higher rates of hematologic toxicity and infection (grade 3/4 AEs) than in R-CHOP controls.31 Although primary prophylaxis with G-CSF was mandated in the phase 2 protocol, the number and timing of the doses administered were not specified, leading to variable practices between countries and across sites. Such prescribing inconsistencies, along with the mechanism of action of venetoclax and the expected toxicity profile for R-CHOP, might have contributed to the higher rate of grade 3/4 neutropenia and febrile neutropenia. Crucially, these additional events were manageable and did not translate into an increased risk for death in CAVALLI.
Other nonhematological AEs were consistent with the predictable safety profile of venetoclax. TLS mitigation strategies33 implemented during CAVALLI phase 1b, including the initiation of venetoclax on cycle 1 day 4 to reduce the potential risk of rapid tumor debulking at first treatment administration (when patients are likely to have the highest tumor burden), proved adequate in phase 2.
Published data highlight a correlation between maintaining R-CHOP dose intensity and improved long-term outcome in patients with treatment-naive DLBCL.34-36 The RDI of R-CHOP in CAVALLI was maintained at a level similar to that in GOYA. However, 61% of patients received ≥90% of the full dose intensity for venetoclax, lower than for each individual component of the R-CHOP backbone. Although this was largely anticipated because of the study protocol recommending modification of venetoclax first to preserve R-CHOP delivery, it may also reflect caution exercised by investigators using a new drug combination.
The starting dose of venetoclax in CAVALLI phase 1b (200/d for 21 days) was poorly tolerated, and dose-limiting toxicities were observed. Following the reduction of venetoclax administration to 10 of 21 days, the 200-mg dose was tolerated, and it was possible to increase the dose to 800 mg/d for 10 days (RP2D regimen), allowing a greater overall dose intensity. Furthermore, reducing the dose duration led to improved safety compared with decreasing the dose. Future clinical development of venetoclax in DLBCL should use the RP2D regimen from treatment start and mandate primary prophylaxis with G-CSF (including timing and duration) as part of the schedule of assessments. In line with the above observations, recurrent febrile neutropenia or prolonged grade 3/4 myelosuppression requiring clinical intervention might initially be mitigated using shorter treatment duration (eg, reduction from 10 days to 5 days), followed by venetoclax dose reductions in subsequent cycles.
In conclusion, the combination of venetoclax with R-CHOP demonstrated increased, but manageable, myelosuppression and potential improved outcomes in high-risk Bcl-2 IHC+ subgroups compared with matched covariate-adjusted R-CHOP control data from the GOYA study. CAVALLI phase 2 data provide a rationale to further explore this combination in previously untreated DLBCL Bcl-2 IHC+ patients, for whom the unmet medical need is greatest.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
The authors thank the patients and study investigators who participated in the CAVALLI study. Special thanks are also given to Jeremy Ross (AbbVie), who supported the CAVALLI study from concept (biological rationale, biomarker hypotheses, and study design) to input on data interpretation at study readout; Joe Paulson (Genentech; Principal Statistical Scientist Primary Health Care, Early Development Oncology Biostatistics group), who provided statistical analysis and data interpretation at study readout; and Jill Ray (Genentech; Senior Companion Diagnostic Project Leader), who supported the biomarker hypothesis and analysis of data.
Venetoclax is being developed as a collaboration between Genentech Inc. and AbbVie. Genentech Inc. and AbbVie provided financial support for the CAVALLI phase 2 study and participated in the design, study conduct, analysis, and interpretation of data, as well as the writing, review, and approval of the manuscript. A.D.Z. was supported by National Institutes of Health National Cancer Institute Core Grant P30 CA008748 and Memorial Sloan Kettering Cancer Center Specialized Program of Research Excellence (SPORE) in Lymphoma Grant P50 CA192937. Under the direction of the authors, third-party medical writing assistance was provided by Rachel Dobb (Gardiner-Caldwell Communications), which was funded by F. Hoffmann-La Roche Ltd.
Footnotes
Qualified researchers may request access to individual patient-level data through the clinical study data request platform (www.clinicalstudydatarequest.com). Further details on Roche's criteria for eligible studies are available at https://clinicalstudydatarequest.com/study-sponsors/study-sponsors-roche.aspx. Further details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents can be found at https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.D.Z. and F. Morschhauser conceived and designed the study; A.B., F. Morschhauser, and A.D.Z. wrote the manuscript; and all authors were involved in the analysis and interpretation of the data, as well as the review and/or revision of the manuscript.
Conflict-of-interest disclosure: F. Morschhauser consults for or advises F. Hoffmann-La Roche Ltd.; has received honoraria from Bayer, Bristol Myers Squibb, Celgene, Epizyme, F. Hoffmann-La Roche Ltd., Gilead Sciences, and Janssen Pharmaceuticals; and is a member of advisory boards for F. Hoffmann-La Roche Ltd., Celgene, Bristol Myers Squibb, Gilead Sciences, Bayer. and Epizyme. I.W.F. consults or advises for AbbVie, AstraZeneca, BeiGene, F. Hoffmann-La Roche Ltd., Gilead Sciences, Janssen Pharmaceuticals, Juno Therapeutics, Kite Pharma, MorphoSys, Nurix Therapeutics, Seattle Genetics, Takeda, TG Therapeutics, Unum Therapeutics, Verastem, and Yingli Pharmaceutical and has received research funding from AbbVie, Acerta Pharma, Agios, ArQule, Astra Zeneca, BeiGene, Calithera Biosciences, Celgene, Constellation Pharmaceuticals, Curis, F. Hoffmann-La Roche Ltd., FORMA Therapeutics, Forty Seven, Genentech, Gilead Sciences, Incyte, Infinity Pharmaceuticals, Janssen Pharmaceuticals, Juno Therapeutics, Karyopharm Therapeutics, Kite Pharma, Merck, MorphoSys, Novartis, Pharmacyclics, Pfizer, Portola Pharmaceuticals, Seattle Genetics, Takeda, Teva, TG Therapeutics, Trillium Therapeutics, Unum Therapeutics, and Verastem. R. Gasiorowski has received honoraria from AbbVie, MSD, Novartis, and Takeda. R. Greil consults or advises for AbbVie, Amgen, AstraZeneca, Celgene, Daiichi Sankyo, F. Hoffmann-La Roche Ltd., Gilead Sciences, Merck, MSD, Novartis, and Takeda; has received honoraria from AbbVie, Amgen, AstraZeneca, Boehringer Ingelheim, Celgene, Daiichi Sankyo, Eisai, F. Hoffmann-La Roche Ltd., Genentech, Gilead Sciences, Janssen-Cilag Pharmaceuticals, Merck, MSD, Mundipharma, Novartis, Pfizer, Sandoz, Sanofi Aventis, and Takeda; has received research funding from AbbVie, Amgen, AstraZeneca, Celgene, F. Hoffmann-La Roche Ltd., Genentech, Gilead Sciences, GSK, Merck, MSD, Mundipharma, Novartis, Pfizer, Ratiopharm, and Takeda; and has received travel support from Amgen, AstraZeneca, Celgene, F. Hoffmann-La Roche Ltd., Gilead Sciences, MSD, and Novartis. Á.I. has received honoraria and is a member of advisory boards for Celgene, F. Hoffmann La-Roche Ltd., Janssen Pharmaceuticals, Novartis, Pfizer, and Takeda and has received research funding from Seattle Genetics and Takeda. N.A.J. is an employee of AbbVie, F. Hoffmann-La Roche Ltd-, and Lundbeck; consults or advises for AbbVie, Bristol Myers Squibb, F. Hoffmann-La Roche Ltd., and Merck; has received honoraria from AbbVie, Bristol Myers Squibb, F. Hoffmann La-Roche Ltd., Lundbeck, Merck, and Seattle Genetics; is a member of advisory boards for AbbVie, F. Hoffmann-La Roche Ltd., and Lundbeck; has received research funding from AbbVie, F. Hoffmann-La Roche Ltd., and Lundbeck; has received travel support from F. Hoffmann-La Roche Ltd. and Lundbeck; and has received a significant proportion of antibodies at no cost from BD Biosciences. J.-F.L. has received honoraria from Janssen Pharmaceuticals and research funding from Bayer, F. Hoffmann-La Roche Ltd., Gilead Sciences. and Merck. P.J.L. has received honoraria from Celgene, F. Hoffmann-La Roche Ltd., Genmab, Janssen-Cilag Pharmaceuticals, Servier, and Takeda; research funding from F. Hoffman-La Roche Ltd., Servier, and Takeda; and participates in the speakers bureau for F. Hoffmann-La Roche Ltd. G.A.S. consults or advises for AbbVie, Autolus, Celgene, Epizyme, F. Hoffmann-La Roche Ltd., Gilead Sciences, Janssen Pharmaceuticals, Karyopharm, Kite Pharma, Merck, MorphoSys, Novartis, Servier, and Takeda; has received honoraria from AbbVie, Amgen, Bristol Myers Squibb, Celgene, Epizyme, F. Hoffmann La Roche Ltd., Gilead Sciences, Janssen Pharmaceuticals, Karyopharm, Kite Pharma, Merck, MorphoSys, Novartis, Servier, and Takeda; is a member of advisory boards for AbbVie, Autolus, Celgene, F. Hoffmann-La Roche Ltd., Gilead Sciences, Janssen Pharmaceuticals, Karyopharm, Kite Pharma, Merck, MorphoSys, Novartis, Servier, and Takeda; and participates in educational events for AbbVie, Amgen, Celgene, F. Hoffmann-La Roche Ltd., Gilead Sciences, Janssen Pharmaceuticals, Karyopharm, Kite Pharma, Merck, MorphoSys, Novartis, Servier, and Takeda. M.T. consults or advises for AbbVie, Amgen, Bristol Myers Squibb, Celgene, Gilead Sciences, Incyte, Janssen Pharmaceuticals, MorphoSys, Takeda, and F. Hoffmann-La Roche Ltd. and has received honoraria from AbbVie, Amgen, Bristol Myers Squibb, Gilead Sciences, Incyte, Janssen Pharmaceuticals, MorphoSys, Takeda, and F. Hoffmann-La Roche Ltd. S.d.V. consults for or advises Verastem and Bayer and is a member of the advisory board for Portola Pharmaceuticals. F. Mir is a former employee of F. Hoffmann-La Roche Ltd. D.S., Y.J., and E.P. are employees of Genentech and hold Roche stock. S.Y.K. is an employee of AbbVie and holds stock in the company. A.S., E.C., N.S., K.H., and A.B. are employees of F. Hoffmann-La Roche Ltd. E.C. holds patents and receives royalties from F. Hoffmann-La Roche Ltd. A.B. holds stock in F. Hoffmann-La Roche Ltd. A.D.Z. consults or advises for AbbVie, Adaptive Biotechnologies, BeiGene, Biotech, Amgen, AstraZeneca, Celgene, F. Hoffmann-La Roche Ltd., Genentech, Gilead Sciences, Janssen Pharmaceuticals, MorphoSys, and Novartis; has received honoraria from AbbVie, Adaptive Biotech, Amgen, AstraZeneca, Celgene, F. Hoffmann-La Roche Ltd., Genentech, Gilead Sciences, Janssen Pharmaceuticals, MorphoSys, and Novartis; has received research funding from BeiGene, Gilead Sciences, MEI Pharma, and F. Hoffmann-La Roche Ltd., and is a chair for a BeiGene Data Monitoring Committee. The remaining authors declare no competing financial interests.
Correspondence: Franck Morschhauser, Univ. Lille, CHU Lille, ULR 7365 - GRITA - Groupe de Recherche sur les formes Injectables et les Technologies Associées, F-59000 Lille, France; e-mail: franck.morschhauser@chru-lille.fr.
REFERENCES
- 1.Coiffier B, Lepage E, Brière J, et al. . CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235-242. [DOI] [PubMed] [Google Scholar]
- 2.Feugier P, Van Hoof A, Sebban C, et al. . Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol. 2005;23(18):4117-4126. [DOI] [PubMed] [Google Scholar]
- 3.Coiffier B, Thieblemont C, Van Den Neste E, et al. . Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood. 2010;116(12):2040-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pfreundschuh M, Schubert J, Ziepert M, et al. ; German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL) . Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol. 2008;9(2):105-116. [DOI] [PubMed] [Google Scholar]
- 5.Pfreundschuh M, Trümper L, Osterborg A, et al. ; MabThera International Trial Group . CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7(5):379-391. [DOI] [PubMed] [Google Scholar]
- 6.Habermann TM, Weller EA, Morrison VA, et al. . Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24(19):3121-3127. [DOI] [PubMed] [Google Scholar]
- 7.Gascoyne RD, Adomat SA, Krajewski S, et al. . Prognostic significance of Bcl-2 protein expression and Bcl-2 gene rearrangement in diffuse aggressive non-Hodgkin’s lymphoma. Blood. 1997;90(1):244-251. [PubMed] [Google Scholar]
- 8.Salles G, de Jong D, Xie W, et al. . Prognostic significance of immunohistochemical biomarkers in diffuse large B-cell lymphoma: a study from the Lunenburg Lymphoma Biomarker Consortium. Blood. 2011;117(26):7070-7078. [DOI] [PubMed] [Google Scholar]
- 9.Reddy A, Zhang J, Davis NS, et al. . Genetic and functional drivers of diffuse large B cell lymphoma. Cell. 2017;171(2):481-494.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chapuy B, Stewart C, Dunford AJ, et al. . Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes [published correction appears in Nat Med. 2018;24(8):1290-1291]. Nat Med. 2018;24(5):679-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmitz R, Wright GW, Huang DW, et al. . Genetics and pathogenesis of diffuse large B-cell lymphoma. N Engl J Med. 2018;378(15):1396-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iqbal J, Meyer PN, Smith LM, et al. . BCL2 predicts survival in germinal center B-cell-like diffuse large B-cell lymphoma treated with CHOP-like therapy and rituximab. Clin Cancer Res. 2011;17(24):7785-7795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horn H, Ziepert M, Becher C, et al. ; German High-Grade Non-Hodgkin Lymphoma Study Group . MYC status in concert with BCL2 and BCL6 expression predicts outcome in diffuse large B-cell lymphoma. Blood. 2013;121(12):2253-2263. [DOI] [PubMed] [Google Scholar]
- 14.Valera A, López-Guillermo A, Cardesa-Salzmann T, et al. ; Grup per l’Estudi dels Limfomes de Catalunya i Balears (GELCAB) . MYC protein expression and genetic alterations have prognostic impact in patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Haematologica. 2013;98(10):1554-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petrella T, Copie-Bergman C, Brière J, et al. . BCL2 expression but not MYC and BCL2 coexpression predicts survival in elderly patients with diffuse large B-cell lymphoma independently of cell of origin in the phase 3 LNH03-6B trial. Ann Oncol. 2017;28(5):1042-1049. [DOI] [PubMed] [Google Scholar]
- 16.Szafer-Glusman E, Peale FV, Lei G, et al. . BCL2 expression identifies a population with unmet medical need in previously untreated (1L) patients with DLBCL [abstract]. Blood. 2017;130(suppl 1). Abstract 418. [Google Scholar]
- 17.Tsuyama N, Sakata S, Baba S, et al. . BCL2 expression in DLBCL: reappraisal of immunohistochemistry with new criteria for therapeutic biomarker evaluation. Blood. 2017;130(4):489-500. [DOI] [PubMed] [Google Scholar]
- 18.Johnson NA, Slack GW, Savage KJ, et al. . Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30(28):3452-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Staiger AM, Ziepert M, Horn H, et al. ; German High-Grade Lymphoma Study Group . Clinical impact of the cell-of-origin classification and the MYC/ BCL2 dual expresser status in diffuse large B-cell lymphoma treated within prospective clinical trials of the German High-Grade Non-Hodgkin’s Lymphoma Study Group. J Clin Oncol. 2017;35(22):2515-2526. [DOI] [PubMed] [Google Scholar]
- 20.Swerdlow SH, Campo E, Pileri SA, et al. . The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thieblemont C, Brière J. MYC, BCL2, BCL6 in DLBCL: impact for clinics in the future? Blood. 2013;121(12):2165-2166. [DOI] [PubMed] [Google Scholar]
- 22.Davids MS, Roberts AW, Seymour JF, et al. . Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-Hodgkin lymphoma. J Clin Oncol. 2017;35(8):826-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Vos S, Swinnen LJ, Wang D, et al. . Venetoclax, bendamustine, and rituximab in patients with relapsed or refractory NHL: a phase Ib dose-finding study. Ann Oncol. 2018;29(9):1932-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zelenetz AD, Salles G, Mason KD, et al. . Venetoclax plus R- or G-CHOP in non-Hodgkin lymphoma: results from the CAVALLI phase 1b trial. Blood. 2019;133(18):1964-1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Younes A, Thieblemont C, Morschhauser F, et al. . Combination of ibrutinib with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) for treatment-naive patients with CD20-positive B-cell non-Hodgkin lymphoma: a non-randomised, phase 1b study. Lancet Oncol. 2014;15(9):1019-1026. [DOI] [PubMed] [Google Scholar]
- 26.Nowakowski GS, LaPlant B, Macon WR, et al. . Lenalidomide combined with R-CHOP overcomes negative prognostic impact of non-germinal center B-cell phenotype in newly diagnosed diffuse large B-Cell lymphoma: a phase II study. J Clin Oncol. 2015;33(3):251-257. [DOI] [PubMed] [Google Scholar]
- 27.Cheson BD, Fisher RI, Barrington SF, et al. ; United Kingdom National Cancer Research Institute . Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pezzella F, Tse AG, Cordell JL, Pulford KA, Gatter KC, Mason DY. Expression of the bcl-2 oncogene protein is not specific for the 14;18 chromosomal translocation. Am J Pathol. 1990;137(2):225-232. [PMC free article] [PubMed] [Google Scholar]
- 29.Gurel B, Iwata T, Koh CM, et al. . Nuclear MYC protein overexpression is an early alteration in human prostate carcinogenesis. Mod Pathol. 2008;21(9):1156-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scott DW, Mottok A, Ennishi D, et al. . Prognostic significance of diffuse large B-cell lymphoma cell of origin determined by digital gene expression in formalin-fixed paraffin-embedded tissue biopsies. J Clin Oncol. 2015;33(26):2848-2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vitolo U, Trněný M, Belada D, et al. . Obinutuzumab or rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in previously untreated diffuse large B-cell lymphoma. J Clin Oncol. 2017;35(31):3529-3537. [DOI] [PubMed] [Google Scholar]
- 32.Cole SR, Hernán MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed. 2004;75(1):45-49. [DOI] [PubMed] [Google Scholar]
- 33.Kater AP, Seymour JF, Hillmen P, et al. . Fixed duration of venetoclax-rituximab in relapsed/refractory chronic lymphocytic leukemia eradicates minimal residual disease and prolongs survival: post-treatment follow-up of the MURANO phase III study. J Clin Oncol. 2019;37(4):269-277. [DOI] [PubMed] [Google Scholar]
- 34.Bosly A, Bron D, Van Hoof A, et al. . Achievement of optimal average relative dose intensity and correlation with survival in diffuse large B-cell lymphoma patients treated with CHOP. Ann Hematol. 2008;87(4):277-283. [DOI] [PubMed] [Google Scholar]
- 35.Pettengell R, Schwenkglenks M, Bosly A. Association of reduced relative dose intensity and survival in lymphoma patients receiving CHOP-21 chemotherapy. Ann Hematol. 2008;87(5):429-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Terada Y, Nakamae H, Aimoto R, et al. . Impact of relative dose intensity (RDI) in CHOP combined with rituximab (R-CHOP) on survival in diffuse large B-cell lymphoma. J Exp Clin Cancer Res. 2009;28(1):116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.