Abstract
Although the occurrence of cerebral aneurysms in pediatric age group describes as rare, giant ones are more commonly be found than in adults. Insufficient epidemiological information, their association with other medical comorbidities, diagnostic pitfalls, complex surgical anatomy, and issues should be considered during surgery to make them difficult to diagnose and manage. We report a 6-year-old boy with presenting complaint of acute-onset headache without any other symptoms and a small area of intracerebral hemorrhage detected on initial computed tomography (CT) scan. Primary evaluations failed to result in a definite diagnosis, and delayed vascular studies suggested vascular malformation or an aneurysm as the causative factor of hemorrhage. Surgical exploration led to the diagnosis of a giant partially thrombosed aneurysm at the A2 segment of the left anterior cerebral artery and successful clipping. One of our findings on preoperative CT angiography, “fountain sign,” may be useful for the diagnosis of partially thrombosed aneurysms when active bleeding from the aneurysm has been ruled out. Fountain sign can be a useful finding in the diagnosis of partially thrombosed aneurysms. Vascular lesions should always be considered as the primary cause of intracranial hemorrhage in pediatrics despite negative initial studies. Therefore, close follow-up and using delayed and multimodality vascular evaluations are crucial for successful management.
Keywords: Anterior cerebral artery, fountain sign, giant aneurysm, partially thrombosed aneurysm
Introduction
Intracranial aneurysms in pediatric age group are not common nor well defined. However, its rarity was evaluated approximately between 1% and 5% of all intracranial aneurysms by some studies.[1] In contrast, giant aneurysms (>25 mm) have a higher frequency among pediatric age group than in adults.[2] Differential diagnosis for primary causes of pediatric intracranial aneurysms varies from infectious diseases such as HIV and mycotic infections to inflammatory (Kawasaki, Behçet's, Takayasu arteritis, etc.) and genetic noninflammatory diseases including polycystic kidney disease, Ehlers–Danlos syndrome, neurofibromatosis, and tuberous sclerosis, all of which may present with symptoms related to cerebral aneurysms.[3] Although subarachnoid hemorrhage is the most common presenting symptom of intracranial aneurysms and usually leads to diagnosis, sometimes, cerebral aneurysms are regarded as one of the most complicated neurosurgical problems in the process of diagnosis after a hemorrhagic cerebral accident and may be found even incidentally on computed tomography (CT) or magnetic resonance imaging (MRI) in an asymptomatic patient.[4,5] In this report, we present a pediatric case of a giant partially thrombosed intracranial aneurysm (PTIA) in a rare location which could not be diagnosed definitely on preoperative evaluations.
Case Report
A 6-year-old boy, with no history of previous illness or trauma, referred to our center complaining of a sudden-onset headache without any accompanying symptoms including seizure, loss of consciousness, weakness, or visual disturbance. Upon physical examination, his vital signs were stable, and the patient was fully conscious without any neurological deficit. Admission CT scan showed a small area of intracerebral hemorrhage (ICH) in the inferomedial part of the left frontal lobe without intraventricular or subarachnoid hemorrhage, and a small calcified spot was also evident in the superior part of the hemorrhage [Figure 1]. CT angiography was negative for any vascular lesion, and on cerebral four-vessel digital subtraction angiography (DSA), the A1 segment of the left anterior cerebral artery was narrowed in a string-like pattern suggesting arterial dissection [Figure 2]. Hence, it was decided to manage the ICH conservatively, and he was discharged from hospital for delayed vascular evaluations.
Figure 1.
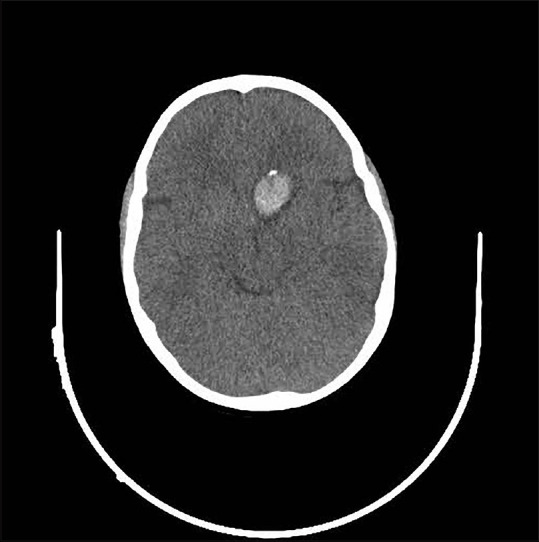
Axial brain computed tomography scan on admission showing a small calcification spot at the top of the intracerebral hemorrhage in the left frontal lobe
Figure 2.
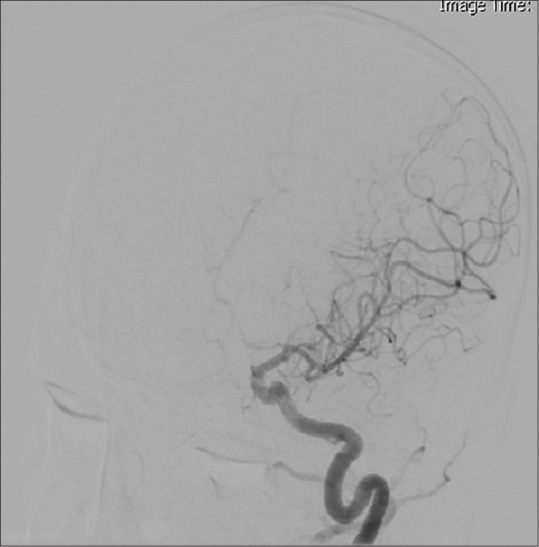
String-like narrowing of the A1 segment of the left anterior cerebral artery on the first digital subtraction angiography, suggesting arterial dissection
Three weeks later, a control CT scan and MRI were performed, and no new sign other than a resolving hematoma was found [Figure 3], but the second CT angiography and DSA at the same time revealed an 8-mm contrast-enhanced outpouching at the junction of left A1 and A2 segments projecting posterolaterally. In addition to this finding and persistence of the calcification spot, a small fountain-like vascular blush was visible at the tip of the aneurysm [Figure 4].
Figure 3.
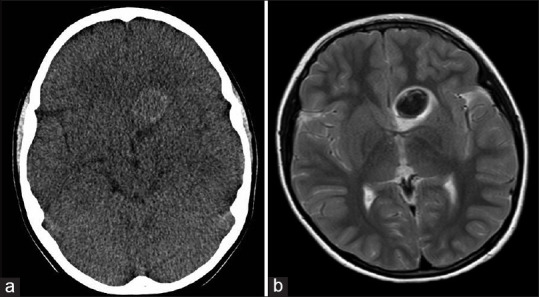
(a) Resolving hematoma within the aneurysm cavity on delayed axial brain computed tomography scan, about 3 weeks after hemorrhage; (b) Axial T2-weighted and coronal T1-weighted images showing blood product signals in the left frontobasal area in favor of hematoma in acute stage
Figure 4.
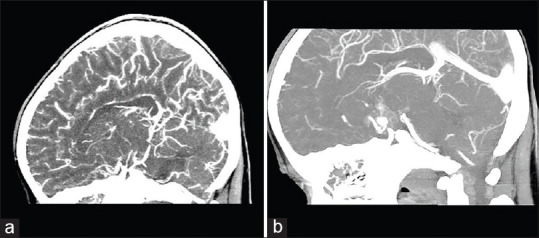
(a) Early brain computed tomography angiography during the first admission. (b) Fountain sign, a vascular blush from the tip of the aneurysmal outpouching on delayed brain computed tomography angiography
With preoperative impression of an arteriovenous malformation or a partially thrombosed aneurysm, the patient was scheduled for surgical exploration. We performed a left-sided pterional craniotomy and transsylvian in addition to anterior interhemispheric approaches to AcomA complex. After dissection of opticocarotid and chiasmatic cisterns and also rectus gyrus removal, it was found that we are facing a giant partially thrombosed aneurysm extending from suprasellar and chiasmatic cisterns upward deep into the left frontal lobe measuring more than 4 cm in maximum diameter [Figure 5]. The medial orbital gyrus was also removed, and the AcomA complex including bilateral A1, A2, and other small vascular branches was exposed. About 1 cm distal to the left A2 origin, an aneurysm neck with a diameter of about 1 cm was detected and clipped successfully. A temporary occlusion of the artery was performed before the final clipping for a few minutes under barbiturate burst suppression. Blood clots and firm thrombi were evacuated as much as possible to decompress normal neurovascular structures without any unnecessary effort to accomplish a complete removal of the aneurysm sac itself. This was due to a tough aneurysm wall and tight attachment to optic nerves and adjacent vessels.
Figure 5.
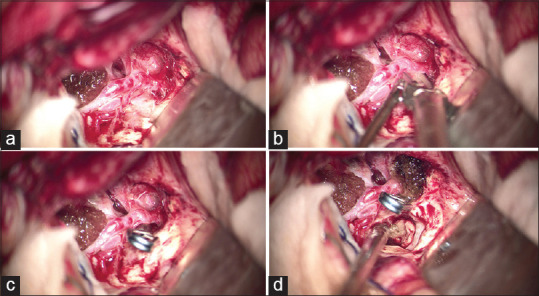
Intraoperative surgical view to the anterior communicating artery complex, aneurysm neck, and inferior extension of the aneurysm into chiasmatic and suprasellar cisterns, (a) before, (b) during, and (c) after clipping. (d) Evacuation of intra-aneurysmal clots following clip placement
Postoperative days in the intensive care unit and pediatric ward were uneventful, and the patient was discharged without any neurological deficit 10 days after surgery. The patient had no sign in favor of postoperative complications on the DSA 2 months after surgery [Figure 6], and studies for evaluation of any underlying connective tissue or rheumatologic diseases were negative.
Figure 6.
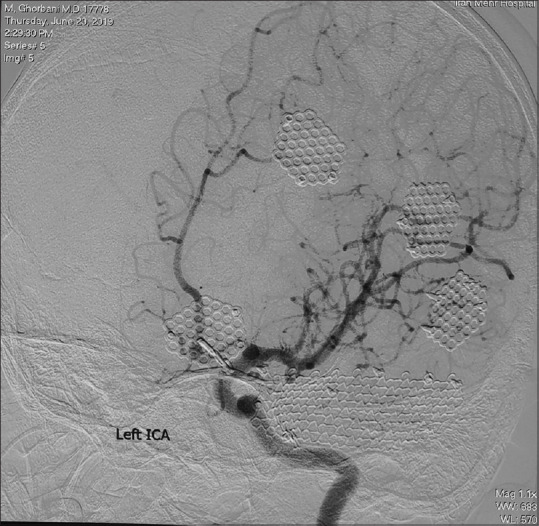
Left anterior oblique view of left internal carotid artery digital subtraction angiography revealing complete occlusion of the aneurysm and preserving the A2 segment of the anterior cerebral artery
Literature of review
To get a better understanding of the characteristics of PTIA, located in the anterior cerebral artery, we have collected the total available reported cases in the literature till 2019. Based on our review, 14 cases out of 12 studies in the literature have met the criteria for inclusion in this review [Table 1]. Some factors such as sex distribution, age, segment of the involvement, early symptoms, diagnosis, and ultimate outcome were assessed in the review. There were 5 women and 9 men, with a mean age of 43.6 ± 26. Aneurysms were measured to be larger than 25 mm (giant) in four cases, and A1 segment was the most common portion for the formation of PTIA. It has been perceived that symptoms related to mass effect, including headache and progressive visual loss, are the most common presenting symptoms of the patients at emergencies. However, two patients were reported to be asymptomatic during diagnosis and were diagnosed by this vascular defect incidentally.[8,9] Moreover, there are some scarce symptoms that have been reported in the literature that are impossible to judge about their origin definitely. Although the occurrence of such symptoms in patients with partially thrombosed aneurysm is extremely uncommon and being reported rarely, the possibility of compressive effect of the aneurysmal mass, especially in cases with giant aneurysm, cannot be ignored. In fact, some rare symptoms like gait disturbance might be seen due to a persistent compressive effect of aneurysm on motor structures of the brain,[13] or blurred vision might occur due to interruption of the optic nerve system.[15] In our review, we observed five reported cases which the contrast MRI modality was not decisive in their diagnosis and was only suggestive of an abnormal formation.[7,10,13,14,15,17] Although PTIA seems to be challenging in management and sometimes needs initiative approaches through the treatment, the literature indicated that more than 78% of the patients have had a favorable outcome after the treatment.
Table 1.
Characteristics of partially thrombosed aneurysms that occur on ACA recorded in the literature
| Reference | Age/Sex | Type | Location | Symptom | Diagnosis | Treatment | Outcome |
|---|---|---|---|---|---|---|---|
| Tajima et al., 1993[6] | 42/female | Saccular | A1 (right) | Monocular blindness | Right carotid angiogram | Outflow occlusion | Favorable |
| Mori et al., 2004[7] | 73/female | Irregular shaped | A1-A2 junction (right) | Loss of consciousness | Conventional angiography | Clipping | No improvement and death |
| Huang et al., 2005[8] | 12/male | Saccular | A1 (right) | Asymptomatic | NR | Clipping | Favorable |
| 7/male | Giant | A1 (left) | Headache | NR | Clipping | Favorable | |
| Cho et al., 2009[9] | 71/female | NR | NR | Asymptomatic | MR angiography | Coiling | No improvement |
| Gelfenbeyn et al., 2009[10] | 69/female | Fusiform dissected | A3 (left) | Headache | DSA | Excision and bypass | Favorable |
| Roccatagliata et al., 2010[11] | 31/male | NR | A2 | Seizure | MRI | Conservative | Favorable |
| 29/male | NR | A2 | Headache | MRI | Conservative | Favorable | |
| Moon et al., 2012[12] | 49/female | Serpentine (giant) | A2 (right) | Loss of consciousness | MRI | Outflow occlusion and bypass | Favorable |
| Castro et al., 2013[13] | 67/male | Fusiform | A1 (left) | gait disturbance, incontinence and progressive visual loss | MR angiography | Ventriculoperitoneal shunt | Favorable |
| Anil et al., 2016[14] | 56/male | Saccular | A1-A2 junction (left) | Delirium | Conventional angiography | WEB | Equivocal |
| Ito et al., 2016[15] | 79/male | Doughnut shaped (giant) | A2 (right) | Blurred vision | CT angiography | Outflow occlusion and anastomosis | Favorable |
| Agarwal et al., 2018[16] | 11/male | Pseudo-aneurysm | A1 (right) | SAH | MRI | Clipping | Favorable |
| Ikeuchi et al., 2019[17] | 15/male | Fusiform (giant) | A1-A2 junction (left) | Headache, SAH | Surgical exploration | Clipping | Favorable |
NR – Not reported; MRI – Magnetic resonance imaging; DSA – Digital subtraction angiography; WEB – Woven EndoBridge; CT – Computed tomography; SAH – Subarachnoid hemorrhage; ACA – Anterior cerebral artery
Discussion
Intracranial aneurysms in pediatric age group are rare and consisting <5% of the population who are diagnosed with aneurysms. According to a study, conducted by Garg et al. on the entire 2726 patients with intracranial aneurysm, treated in their center between 2001 and 2013, only 62 cases (2.7%) were <18 years of age.[18] For the first time, PTIA was described by Handa et al.[19] and it was provided with precise information concerning the CT view details and its characteristics in 1978. Since then, several studies have been conducted on its details, especially on diagnosis and various treatment techniques. The incidence of partially thrombosed aneurysm is estimated between 9% and 13%, and it has been perceived to be more common among patients with giant aneurysm.[20] Despite several studies on different features of these aneurysms, still, some aspects of this disorder including presentation, diagnosis, and association with other diseases have remained untold.[21]
Some characteristics that may differentiate pediatric aneurysms from those in adults include higher incidence of unusual locations such as posterior or peripheral circulation, greater number of giant aneurysms, and also male predominance. According to a review conducted by Sorteberg and Dahlberg, giant aneurysms consist of 19% of all intracranial aneurysms in pediatrics which is more prevalent than in adults.[4] Giving their more intricacy in pediatrics, close follow-ups and delayed vascular studies seem to be necessary in cases of suspected vascular lesions despite negative primary evaluations. This high index of suspicion should be considered in younger age groups, cases of connective tissue diseases, family history of cerebrovascular disorders, any history of head trauma, especially penetrating injuries, and abnormal calcifications on imaging views.[3,18,22]
Partially thrombosed aneurysm is a challenging issue to diagnose because it can not necessarily be detected in the same way among different cases. Though, most of the cases of nonthrombosed aneurysms might be diagnosed by DSA and conventional angiography. However, partially thrombosed needs further evaluation because it might be misdiagnosed by other intracranial mass formations. Based on the literature, MRI seems to be a suitable technique to provide the specific views of “onion skin” on T1-weighted images and “flow void sign” appearance on both T1- and T2-weighted images for definite diagnosis.[23] However, some cases need more specific evaluation even further than contrast MRI. As we have seen in our presented case, contrast MRI was not decisive and the two mentioned definitive views of PTIA were absent. Finally, a giant partially thrombosed was detected based on suggestive findings of DSA, followed by contrast MRI, and later was proved by surgical exploration.
An interesting sign that may lead to diagnosis is the “fountain sign,” which is described classically as an active blood leakage from a ruptured aneurysm at the time of angiography.[24] In fact, “vascular blush from the tip of the aneurysmal outpouching on delayed brain CT angiography” is actually a vascular blush from the aneurysmal intra-dome site, which is completely filled with the contrast medium, toward the rest of the dome that is partially occupied by the thrombus. Whereas, we saw it in our case as blood scattering into the thrombosed part of the giant aneurysm in the absence of any active bleeding, and it could be a useful phenomenon for diagnosis [Figure 4]. In this regard and to the best of our knowledge, this is for the first time that the fountain sign is represented as a key finding in the diagnosis of a patient with nonruptured PTIA.
Since PTIAs are challenging in terms of treatment, they sometimes need other techniques further than direct clipping. Based on the aneurysm type and its location, some cases might need reconstructive surgery along with endovascular intervention. Shi et al. proposed a mixed technique of endovascular coil embolization after protective surgical bypass which had a favorable outcome in cases with giant PTIA.[25] In 2011, Miyamoto et al.[26] described a new technique through the management of partially thrombosed giant aneurysms by flow reduction along with vascular bypass surgery which had successful results in cases with inaccessible aneurysm. The latest technique that proved its effectiveness and could be useful in saccular aneurysm is Woven EndoBridge (WEB). However, it has been reported that solely treating with WEB might result in a fatal rupture of PTIA in patients.[14]
During surgery, wide dissection for defining distorted vascular anatomy seems to be essential for safe surgical clipping of giant aneurysms. After successful aneurysm securing, surgical decompression of neurovascular structures by clot removal is required. However, excessive effort to dissection and excising the aneurysm wall would be catastrophic in some cases.
Conclusion
Although intracranial aneurysms are rare in children, giant ones are more prevalent in younger patients than in adults. A high index of suspicion, close follow-ups, and delayed vascular evaluations could be helpful or even essential for preventing misdiagnoses in pediatric cases and other high-risk patients such as those with connective tissue diseases. In this regard, “fountain sign” may be a useful finding for making the diagnosis of giant partially thrombosed aneurysms in vascular studies. According to our review of literature, the symptoms that may relate to aneurysmal mass effect, including headache and blurry vision, are found as the most reported symptoms of the patients before definite diagnosis with PTIA. However, it should not be ignored that some patients might show other symptoms when they are brought to the emergencies.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the legal guardian has given his consent for images and other clinical information to be reported in the journal. The guardian understands that names and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kakarla UK, Beres EJ, Ponce FA, Chang SW, Deshmukh VR, Bambakidis NC, et al. Microsurgical treatment of pediatric intracranial aneurysms: Long-term angiographic and clinical outcomes. Neurosurgery. 2010;67:237–49. doi: 10.1227/01.NEU.0000371727.71991.64. [DOI] [PubMed] [Google Scholar]
- 2.Saraf R, Shrivastava M, Siddhartha W, Limaye U. Intracranial pediatric aneurysms: Endovascular treatment and its outcome. J Neurosurg Pediatr. 2012;10:230–40. doi: 10.3171/2012.5.PEDS1210. [DOI] [PubMed] [Google Scholar]
- 3.Weiss PF, Corao DA, Pollock AN, Finkel TH, Smith SE. Takayasu arteritis presenting as cerebral aneurysms in an 18 month old: A case report. Pediatr Rheumatol Online J. 2008;6:4. doi: 10.1186/1546-0096-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sorteberg A, Dahlberg D. Intracranial non-traumatic aneurysms in children and adolescents. Curr Pediatr Rev. 2013;9:343–52. doi: 10.2174/221155281120100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keedy A. An overview of intracranial aneurysms. Mcgill J Med. 2006;9:141–6. [PMC free article] [PubMed] [Google Scholar]
- 6.Tajima A, Ito M, Ishii M. Complete recovery from monocular blindness caused by aneurysmal compression to optic nerve-report of two cases. Neurol Med Chir (Tokyo) 1993;33:19–23. doi: 10.2176/nmc.33.19. [DOI] [PubMed] [Google Scholar]
- 7.Mori K, Kasuga C, Nakao Y, Yamamoto T, Maeda M. Intracranial pseudoaneurysm due to rupture of a saccular aneurysm mimicking a large partially thrombosed aneurysm (”ghost aneurysm”): Radiological findings and therapeutic implications in two cases. Neurosurg Rev. 2004;27:289–93. doi: 10.1007/s10143-004-0336-7. [DOI] [PubMed] [Google Scholar]
- 8.Huang J, McGirt MJ, Gailloud P, Tamargo RJ. Intracranial aneurysms in the pediatric population: Case series and literature review. Surg Neurol. 2005;63:424–32. doi: 10.1016/j.surneu.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 9.Cho YD, Park JC, Kwon BJ, Hee Han M. Endovascular treatment of largely thrombosed saccular aneurysms: Follow-up results in ten patients. Neuroradiology. 2010;52:751–8. doi: 10.1007/s00234-009-0622-8. [DOI] [PubMed] [Google Scholar]
- 10.Gelfenbeyn M, Natarajan SK, Sekhar LN. Large distal anterior cerebral artery aneurysm treated with resection and interposition graft: Case report. Neurosurgery. 2009;64:E1008–9. doi: 10.1227/01.NEU.0000339119.92564.29. [DOI] [PubMed] [Google Scholar]
- 11.Roccatagliata L, Guédin P, Condette-Auliac S, Gaillard S, Colas F, Boulin A, et al. Partially thrombosed intracranial aneurysms: Symptoms, evolution, and therapeutic management. Acta Neurochir (Wien) 2010;152:2133–42. doi: 10.1007/s00701-010-0772-9. [DOI] [PubMed] [Google Scholar]
- 12.Moon HS, Kim TS, Joo SP. Surgical treatment of giant serpentine aneurysm of A2-a3 segment distal anterior cerebral artery: Technical case report. J Korean Neurosurg Soc. 2012;52:501–4. doi: 10.3340/jkns.2012.52.5.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castro JC, Agulleiro JD, Villa JF, Pinzón AM. Anterior cerebral artery aneurism presenting as a third ventricular mass and hydrocephalus Case report. Neurocirugia (Asturias, Spain) 2013;24(1):41–6. doi: 10.1016/j.neucir.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Anil G, Goddard AJ, Ross SM, Deniz K, Patankar T. WEB in partially thrombosed intracranial aneurysms: A word of caution. AJNR Am J Neuroradiol. 2016;37:892–6. doi: 10.3174/ajnr.A4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito H, Miyano R, Sase T, Wakui D, Matsumori T, Takasuna H, et al. Outflow occlusion with A3-A3 anastomosis for a doughnut-shaped partially thrombosed giant A2 aneurysm. Surg Neurol Int. 2016;7:S1069–71. doi: 10.4103/2152-7806.196379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agarwal V, Barrow DL. Management of a previously coiled anterior cerebral artery aneurysm in a child: 3-dimensional operative video. Oper Neurosurg. 2018;15:E90. doi: 10.1093/ons/opy041. [DOI] [PubMed] [Google Scholar]
- 17.Ikeuchi Y, Koyama J, Azumi M, Akutsu N, Kawamura A, Kohmura E. Case report: Anterior cerebral artery pediatric fusiform thrombosed giant aneurysm. Interdiscip Neurosurg. 2020;19:100561. [Google Scholar]
- 18.Garg K, Singh PK, Sharma BS, Chandra PS, Suri A, Singh M, et al. Pediatric intracranial aneurysms--our experience and review of literature. Childs Nerv Syst. 2014;30:873–83. doi: 10.1007/s00381-013-2336-9. [DOI] [PubMed] [Google Scholar]
- 19.Handa J, Nakano Y, Aii H, Handa H. Computed tomography with giant intracranial aneurysms. Surg Neurol. 1978;9:257–63. [PubMed] [Google Scholar]
- 20.Cho YD, Park JC, Kwon BJ, Hee Han M. Endovascular treatment of largely thrombosed saccular aneurysms: Follow-up results in ten patients. Neuroradiology. 2010;52:751–8. doi: 10.1007/s00234-009-0622-8. [DOI] [PubMed] [Google Scholar]
- 21.Vargas SA, Diaz C, Herrera DA, Dublin AB. Intracranial aneurysms in children: The role of stenting and flow-diversion. J Neuroimaging. 2016;26:41–5. doi: 10.1111/jon.12305. [DOI] [PubMed] [Google Scholar]
- 22.Proust F, Toussaint P, Garniéri J, Hannequin D, Legars D, Houtteville JP, et al. Pediatric cerebral aneurysms. J Neurosurg. 2001;94:733–9. doi: 10.3171/jns.2001.94.5.0733. [DOI] [PubMed] [Google Scholar]
- 23.Rashad S, Hassan T, Shimizu H, Ibrahim T, Sultan A, El-Sabaa B. De novo giant partially thrombosed aneurysm complicating STA-MCA bypass site in 3 years: Case report with review of the literature. Neurosurg Q. 2015;25:296–301. [Google Scholar]
- 24.Garge SS, Visana DR, Vyas PD, Modi PD, Poyam SN, Ghatge S. Actively leaking ruptured acom aneurysm: Fountain sign. Neurol India. 2014;62:234–5. doi: 10.4103/0028-3886.132454. [DOI] [PubMed] [Google Scholar]
- 25.Shi ZS, Ziegler J, Duckwiler GR, Jahan R, Frazee J, Ausman JI, et al. Management of giant middle cerebral artery aneurysms with incorporated branches: Partial endovascular coiling or combined extracranial-intracranial bypass – A team approach. Neurosurgery. 2009;65:121–9. doi: 10.1227/01.NEU.0000335173.80605.1D. [DOI] [PubMed] [Google Scholar]
- 26.Miyamoto S, Funaki T, Iihara K, Takahashi JC. Successful obliteration and shrinkage of giant partially thrombosed basilar artery aneurysms through a tailored flow reduction strategy with bypass surgery. J Neurosurg. 2011;114:1028–36. doi: 10.3171/2010.9.JNS10448. [DOI] [PubMed] [Google Scholar]


