Abstract
Objective:
Race is a significant prognostic factor in various cancers, including the breast. Its prognostic association with spinal meningioma has not been established, although the incidence of spinal meningioma varies by race. In this retrospective cohort study, we aimed to investigate the association of race with the incidence and survival of spinal meningioma among a large population sample.
Materials and Methods:
A comprehensive search was done in the surveillance, epidemiology, and end results database between 2000 and 2016 to identify patients with spinal meningioma. Overall and race-specific incidence were calculated. The effect of race on overall survival among these patients was determined with Kaplan–Meier curve and Cox proportional hazard models.
Results:
Of 3502 spinal meningioma patients, 82.6% were Caucasian, 7.7% were African-American, 8.8% were Asian/Pacific Islander and 0.7% were American Indian/Alaska Native. The overall age-adjusted incidence was 0.239/100,000; it was highest among Caucasian (0.249) and lowest among American Indian/Alaska Native patients (0.137). There was a race effect in overall survival in the unadjusted analysis with the worst overall survival reported for Caucasian patients, and the best reported for American Indian/Alaska Native patients. However, this relationship was insignificant in the adjusted analysis.
Conclusions:
Race was not significantly associated with overall survival among these patients. Future studies should use spinal-meningioma-specific survival as outcome to see whether there is a racial difference in survival.
Keywords: Incidence, race, spinal meningioma, survival
Introduction
Spinal meningioma is a slow-growing benign tumor of the spinal cord.[1] It generally occurs outside the cord but within its dural covering. It accounts for 7.5%–12.7% of all meningiomas (cranial and spinal) and 25%–46% of spinal neoplasms.[2] Spinal meningioma typically occurs among older individuals (age: 50–70 years),[3] but in rare instances, affects children.[4] It is more prevalent among females than males (ratio 3–4.2:1).[5] Spinal meningioma can be fully surgically resected without causing any neurological damage, and patients generally have an excellent prognosis.[2]
Several factors have been identified with the prognosis of spinal meningioma. The outcomes studied include postsurgical neurological functional status, recurrence, and survival.[3,6] A recurrent tumor status, higher Ki-67 index, and poor preoperative neurological functions were associated with poor postsurgical neurological functional status.[6] On the other hand, male gender, higher tumor grade, recurrent tumor status, and a Simpson resection III were significantly associated with meningioma recurrence.[6] Although spinal meningioma is more prevalent among females, mortality from it is higher among males.[3]
Race is a significant prognostic factor in various types of cancer.[7] In the US, African–Americans have a higher incidence and lesser survival of all malignancies combined compared to other races.[8] Race differences in susceptibility to cancers are partially due to the genomic diversity among the races. Studies have shown more genetic diversity and fewer levels of linkage disequilibrium in Africans relative to all other populations.[9] Furthermore, there are multiple single-nucleotide polymorphisms and copy number variations associated with racial diversity. In addition, a difference in DNA methylation between Europeans and Africans, which is an early step before cancer development, showed that there was an epigenetic difference by race.[10]
All this evidence suggests that race might have a prognostic value in the case of spinal meningioma, but data are lacking in this regard. A large-scale epidemiological study indicated that the incidence was higher among the Asian Pacific Islanders, Caucasians, and Hispanics.[11] Other small-scale studies also reported a racial difference in spinal meningioma.[12]
The current study used information contained in the surveillance, epidemiology, and end results (SEER) database and aimed to assess the association between race and survival in patients with spinal meningioma.
Materials and Methods
We obtained patients' data from the SEER database 18 registries of the US National Cancer Institute.[13] The data were extracted using SEER*Stat software version 8.3.5.
Ethical consideration
This was a secondary analysis of publicly available data from the SEER database. No additional ethical approval was required.
Data collection
All patients diagnosed with spinal meningioma between 2000 and 2016 were eligible. The primary tumor sites that were considered included spinal meninges (C70.1), spinal cord (C72.0), and cauda equina (C72.1). Patients were excluded if: (1) the diagnosis of spinal meningioma was not microscopically confirmed or was made from an autopsy, (2) the primary tumor site was unknown, and (3) they did not have an active follow-up.
We also extracted data on the following variables: race (Caucasian, African-American, American Indian/Alaska Native, and Asian or Pacific islander), gender (male, female), age at diagnosis into quartiles (<52, 52–63, 64–73, and >73), marital status (divorced/separated, married or domestic partner, single, and widowed), therapy including radiation and surgery (received and did not receive), vital status, tumor type (benign, borderline malignancy, and malignant), and size (<1 cm and ≥1 cm). There were no restrictions on any of the aforementioned variables except for race; patients with unknown race were excluded from the study.
Incidence statistics
The age-adjusted incidence rate (per 100,000) of spinal meningioma between 2000 and 2016 was calculated using the SEER*Stat software version 8.3.5. Its frequency, percentage, and rate of incidence were also determined for each race.
Statistical analyses
Statistical analyses were performed using R Studio version 3.2.5 software. Since variables related to patients' characteristics were all categorical, they were presented as frequencies and percentages. Comparison of these variables across the four racial groups was made using Chi-square or Fisher's exact test, whichever was more appropriate. A Kaplan–Meier curve was used to demonstrate the overall survival probability of the different racial groups, and a log-rank test was used to compare between them. Univariable and multivariable Cox regression models were used to compare the overall survival between the different races. The Caucasian race was used as a reference in the regression models, and adjustment was performed for the following covariates: age, gender, marital status, tumor site, size, behavior, radiation, and surgery. A two-sided P < 0.05 was considered statistically significant.
Results
Patient population and baseline characteristics
A total of 3502 patients with spinal meningioma were identified between 2000 and 2016 according to eligibility criteria. Of those, 2895 (82.7%) were Caucasian, 272 (7.8%) were African-American, 310 (8.8%) were Asian or Pacific Islanders, and 25 (0.7%) were American Indian/Alaska Native. The distribution of patients by age was <52 (23.5%), 52–63 (27.4%), 64–73 (24.3%), and >73 years (24.8%). Females were the majority overall (81%) as well as in each racial group. Almost all patients (96%) received surgical resection of their tumors, and a tiny minority (1.7%) received radiation. At last follow-up, 80.2% of the African-American patients were alive; the corresponding percentages were 85.5% for Caucasian, 89.0% for Asian or Pacific Islanders, and 96.0% for American Indian/Alaska Natives. The distribution of age, gender, marital status, surgery, and vital status were significantly different across the four racial groups [Table 1].
Table 1.
Characteristics of the included patients stratified by race
| Predictors | Caucasian (n=2895) | African-American (n=272) | Asian or pacific islander (n=310) | American Indian/Alaska native (n=25) | P |
|---|---|---|---|---|---|
| Age at diagnosis (%) | |||||
| <52 | 647 (22.3) | 100 (36.8) | 69 (22.3) | 8 (32.0) | <0.001 |
| 52-63 | 774 (26.7) | 82 (30.1) | 91 (29.4) | 12 (48.0) | |
| 64-73 | 698 (24.1) | 59 (21.7) | 89 (28.7) | 4 (16.0) | |
| >73 | 776 (26.8) | 31 (11.4) | 61 (19.7) | 1 (4.0) | |
| Gender (%) | |||||
| Female | 2329 (80.4) | 222 (81.6) | 260 (83.9) | 14 (56.0) | 0.007 |
| Male | 566 (19.6) | 50 (18.4) | 50 (16.1) | 11 (44.0) | |
| Marital status (%) | |||||
| Divorced/separated | 301 (10.4) | 35 (12.9) | 19 (6.1) | 3 (12.0) | <0.001 |
| Married or domestic partner | 1600 (55.3) | 105 (38.6) | 187 (60.3) | 11 (44.0) | |
| Single (never married) | 410 (14.2) | 85 (31.2) | 51 (16.5) | 5 (20.0) | |
| Unknown | 172 (5.9) | 17 (6.2) | 14 (4.5) | 4 (16.0) | |
| Widowed | 412 (14.2) | 30 (11.0) | 39 (12.6) | 2 (8.0) | |
| Site (%) | |||||
| C70.1-spinal meninges | 2819 (97.4) | 265 (97.4) | 303 (97.7) | 23 (92.0) | 0.746 |
| C72.0-spinal cord | 74 (2.6) | 7 (2.6) | 7 (2.3) | 2 (8.0) | |
| C72.1-cauda equina | 2 (0.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Size (%) | 0.88 | ||||
| <1 cm | 1500 (51.8) | 139 (51.1) | 156 (50.3) | 10 (40.0) | |
| ≥1 cm | 5 (0.2) | 1 (0.4) | 1 (0.3) | 0 (0.0) | |
| Unknown | 1390 (48.0) | 132 (48.5) | 153 (49.4) | 15 (60.0) | |
| Behavior (%) | |||||
| Benign | 2784 (96.2) | 253 (93.0) | 298 (96.1) | 25 (100.0) | 0.232 |
| Borderline malignancy | 75 (2.6) | 14 (5.1) | 9 (2.9) | 0 (0.0) | |
| Malignant | 36 (1.2) | 5 (1.8) | 3 (1.0) | 0 (0.0) | |
| Surgery (%) | |||||
| Not received | 115 (4.0) | 8 (2.9) | 7 (2.3) | 5 (20.0) | <0.001 |
| Received | 2780 (96.0) | 264 (97.1) | 303 (97.7) | 20 (80.0) | |
| Radiation (%) | |||||
| Not received/unknown | 2846 (98.3) | 265 (97.4) | 305 (98.4) | 25 (100.0) | 0.656 |
| Received | 49 (1.7) | 7 (2.6) | 5 (1.6) | 0 (0.0) | |
| Vital status (%) | |||||
| Alive | 2445 (84.5) | 240 (88.2) | 276 (89.0) | 24 (96.0) | 0.027 |
| Dead | 450 (15.5) | 32 (11.8) | 34 (11.0) | 1 (4.0) |
Surveillance, epidemiology, and end results population-based incidence statistics
The age-adjusted rate of spinal meningioma was 0.239/100,000. The rate was highest in Caucasian patients (0.249), followed by Asian or Pacific Islander (0.23), African-American (0.173), and American Indian/Alaska Native (0.137) [Table 2].
Table 2.
Population-based incidence statistics for spinal meningioma stratified by race
| Count (%) | Rate | SE | |
|---|---|---|---|
| Total | 3502 (100) | 0.239 | 0.004 |
| Race | |||
| Caucasian | 2895 (82.667) | 0.249 | 0.005 |
| African-American | 272 (7.767) | 0.173 | 0.011 |
| American Indian/Alaska native | 25 (0.714) | 0.137 | 0.029 |
| Asian or Pacific islander | 310 (8.852) | 0.23 | 0.013 |
Percent is for total cases. Rates are per 100,000 and age-adjusted to the 2000 US standard population. SE – Standard error
Race-specific survival analysis for spinal meningioma patients
The Kaplan–Meier curve showed that Caucasian patients had the worst survival rate, American Indian/Alaska Native patients had the best survival rate, and Asian or Pacific Islanders and African-American patients had the intermediate survival rate; the log-rank test showed that this difference was statistically significant (P = 0.03) [Figure 1]. Compared to Caucasians, the overall mortality was 33% lower for African-Americans (hazard ratio [HR] = 0.67, 95% confidence interval [CI] = 0.46, 0.98), 31% lower for Asian or Pacific Islanders (HR = 0.69, 95% CI = 0.48, 0.99), and 80% lower for American Indian/Alaska Natives (HR = 0.20, 95% CI = 0.03, 1.46); the results were significant for all the races except American Indian/Alaska Native [unadjusted model; Table 3]. The mortality, however, was not statistically different among the racial groups after adjustment with covariates [Table 3].
Figure 1.
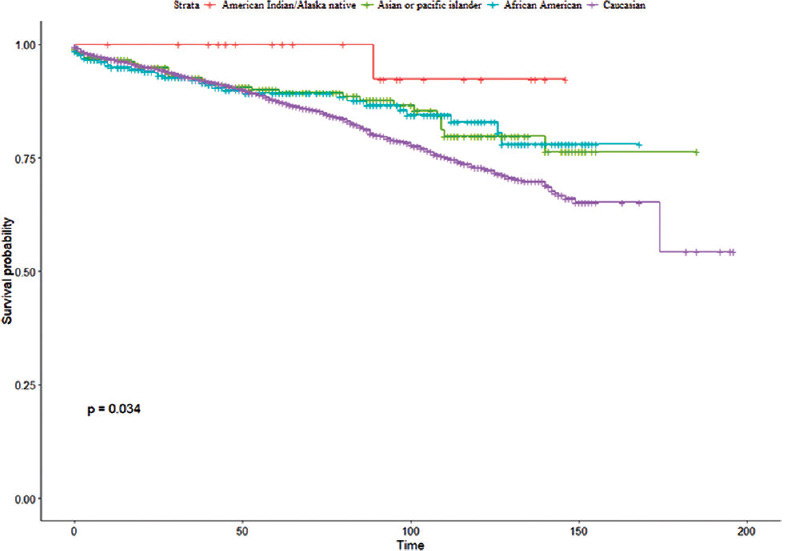
Kaplan–Meier survival curve showing the overall survival for spinal meningioma patients based on race
Table 3.
Uni- and multi-variable Cox proportional hazard model for the effect of race on overall survival of spinal meningioma
| Overall survival | ||
|---|---|---|
| HR (95% CI) | P | |
| Uni-variable analysis | ||
| Caucasian | Reference | Reference |
| American Indian/Alaska native | 0.20 (0.03, 1.46) | 0.113 |
| Asian or pacific islander | 0.69 (0.48, 0.99) | 0.044 |
| African-American | 0.67 (0.46, 0.98) | 0.04 |
| Multivariable analysis* | ||
| Caucasian | Reference | Reference |
| American Indian/Alaska native | 0.38 (0.05, 2.69) | 0.329 |
| Asian or pacific islander | 0.90 (0.63, 1.30) | 0.581 |
| African-American | 0.97 (0.66, 1.43) | 0.88 |
*Covariates included in the adjusted model were age, site, size, gender, marital status, behavior, radiation, and surgery. HR – Hazard ratio; CI – Confidence interval
Discussion
In various types of cancer, for example, breast, colon, and leukemia, survival varies by race.[14,15,16] The racial effect in survival among spinal meningioma patients has been unclear. A number of factors may have contributed to this lack of clarity, such as (1) small-sized studies, (2) single-center studies, and (3) studies that included patients who received a specific type of treatment.[17,18,19,20] This study contributes to the knowledge by assessing the race effect on survival by drawing from a large spectrum of spinal meningioma patients in a population-based database (SEER).
In this study, Caucasian patients had the worst survival rate, American Indian/Alaska Native patients had the best survival rate, and Asian/Pacific Islanders and African-American patients had an intermediate survival rate. Nevertheless, there was no race effect when the model was adjusted for covariates. There are two potential explanations behind a null finding. The mortality from spinal meningioma is very low; therefore, there were not enough outcome events to find a difference by race. This study used all-cause mortality and not cancer-specific mortality (spinal meningioma) as outcome. Due to competing causes of death, it is difficult to find an effect when all-cause mortality is used as outcome unless the disease in question is a leading cause of death,[21] which spinal meningioma may not be.
Most available studies on spinal meningioma, except one, were descriptive in nature and pertained to the incidence rates in different racial groups.[11,22] The sole study that reported prognostic estimates by race was by Maiti et al.[12] It enrolled 38 patients who underwent surgical resection and found no significant difference between Caucasians and African–Americans in terms of recurrence (P = 0.745) or functional improvement (P = 0.606) at 1-year follow-up. Although it was a small-sized study, had a short follow-up, and did not include a broad spectrum of patients, its findings were supportive of this current study.
This study's findings on spinal meningioma incidence by race were consistent with earlier published studies. The study by Kshettry et al. showed that Caucasian patients and Asian Pacific Islanders had the highest incidence of spinal meningioma, and African–American and American Indian/Alaskan Native individuals had a significantly lower incidence.[11] A second study found that nonHispanic Caucasians had a significantly higher incidence rate than nonHispanic African-Americans.[22] The observed racial difference in incidence might be attributed to the disparities in genetic determinants (for example, polymorphisms, mutations), lifestyle, socioeconomic status, and health care access.[23]
This study had several strengths and limitations. It was a population-based study with robust sample size. The diagnosis of spinal meningioma and the outcome (survival) were ascertained by validated means. Although SEER is an important database for clinical research, the information contained in it might be subject to error (underreporting, misclassification, etc.,).[24] In addition, patients might have migrated in and out of the registry areas, and the database might not have all the necessary variables. For example, the multivariate model of this study was not adjusted for several critical variables, such as surgical complications, treatment compliance, and other medical comorbidities as they were not available in the SEER database. Meningiomas have a long asymptomatic phase and are frequently underreported tumors, and therefore, potential variations in reporting are to be expected. This might have affected the incidence estimates of spinal meningioma.
Conclusions
The incidence of spinal meningioma was highest in Caucasians, who experienced the worst survival rate, as compared to other racial groups. This racial difference in survival lost its statistical significance when the model was adjusted for covariates. Future studies should use spinal-meningioma-specific mortality as outcome to see whether there is a racial difference in survival.
Financial support and sponsorship
This work was supported by Sulaiman Al Rajhi University, Saudi Arabia, but it had no role in the study design, the collection, analysis, or interpretation of data, in the writing of the report, or in the decision to submit the article for publication.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to thank Ms. Erin Strotheide for her editorial contributions to this manuscript.
References
- 1.Maiuri F, de Caro ML, de Divitiis O, Vergara P, Mariniello G. Spinal meningiomas: Age-related features. Clin Neurol Neurosurg. 2011;113:34–8. doi: 10.1016/j.clineuro.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 2.Alan N, Flickinger JC, Gerszten PC. Spinal meningioma. In: Chang EL, Brown PD, Lo SS, Sahgal A, Suh JH, editors. Adult CNS Radiation Oncology. Switzerland: Springer International Publishing AG; 2018. pp. 117–25. [Google Scholar]
- 3.Westwick HJ, Shamji MF. Effects of sex on the incidence and prognosis of spinal meningiomas: A surveillance, epidemiology, and end results study. J Neurosurg Spine. 2015;23:368–73. doi: 10.3171/2014.12.SPINE14974. [DOI] [PubMed] [Google Scholar]
- 4.Wu L, Yang C, Liu T, Fang J, Yang J, Xu Y. Clinical features and long-term outcomes of pediatric spinal meningiomas. J Neurooncol. 2017;133:347–55. doi: 10.1007/s11060-017-2441-9. [DOI] [PubMed] [Google Scholar]
- 5.Epstein NE. Nursing review of spinal meningiomas. Surg Neurol Int. 2018;9:41. doi: 10.4103/sni.sni_408_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hua L, Zhu H, Deng J, Tian M, Jiang X, Tang H, et al. Clinical and prognostic features of spinal meningioma: A thorough analysis from a single neurosurgical center. J Neurooncol. 2018;140:639–47. doi: 10.1007/s11060-018-2993-3. [DOI] [PubMed] [Google Scholar]
- 7.Aizer AA, Wilhite TJ, Chen MH, Graham PL, Choueiri TK, Hoffman KE, et al. Lack of reduction in racial disparities in cancer-specific mortality over a 20-year period. Cancer. 2014;120:1532–9. doi: 10.1002/cncr.28617. [DOI] [PubMed] [Google Scholar]
- 8.DeSantis CE, Siegel RL, Sauer AG, Miller KD, Fedewa SA, Alcaraz KI, et al. Cancer statistics for African Americans, 2016: Progress and opportunities in reducing racial disparities. CA Cancer J Clin. 2016;66:290–308. doi: 10.3322/caac.21340. [DOI] [PubMed] [Google Scholar]
- 9.Campbell MC, Tishkoff SA. African genetic diversity: Implications for human demographic history, modern human origins, and complex disease mapping. Annu Rev Genomics Hum Genet. 2008;9:403–33. doi: 10.1146/annurev.genom.9.081307.164258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song MA, Brasky TM, Marian C, Weng DY, Taslim C, Dumitrescu RG, et al. Racial differences in genome-wide methylation profiling and gene expression in breast tissues from healthy women. Epigenetics. 2015;10:1177–87. doi: 10.1080/15592294.2015.1121362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kshettry VR, Hsieh JK, Ostrom QT, Kruchko C, Benzel EC, Barnholtz-Sloan JS. Descriptive epidemiology of spinal meningiomas in the United States. Spine (Phila Pa 1976) 2015;40:E886–9. doi: 10.1097/BRS.0000000000000974. [DOI] [PubMed] [Google Scholar]
- 12.Maiti TK, Bir SC, Patra DP, Kalakoti P, Guthikonda B, Nanda A. Spinal meningiomas: Clinicoradiological factors predicting recurrence and functional outcome. Neurosurg Focus. 2016;41:E6. doi: 10.3171/2016.5.FOCUS16163. [DOI] [PubMed] [Google Scholar]
- 13.SEER*Stat Database: Incidence-SEER 18 Regs Research Data+Hurricane Katrina Impacted Louisiana Cases; Nov 2018 Sub (2000-2016) -Linked To County Attributes-Total U.S., 1969-2017 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Released April 2019, Based on the November 2018 Submission. Surveillance, Epidemiology, and End Results (SEER) Program. [Last accessed on 2019 Sep 01]. Available from: http://www.seer.cancer.gov .
- 14.Clarke CA, Keegan TH, Yang J, Press DJ, Kurian AW, Patel AH, et al. Age-specific incidence of breast cancer subtypes: Understanding the African-American-white crossover. J Natl Cancer Inst. 2012;104:1094–101. doi: 10.1093/jnci/djs264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White A, Joseph D, Rim SH, Johnson CJ, Coleman MP, Allemani C. Colon cancer survival in the United States by race and stage (2001-2009): Findings from the CONCORD-2 study. Cancer. 2017;123(Suppl 24):5014–36. doi: 10.1002/cncr.31076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tai EW, Ward KC, Bonaventure A, Siegel DA, Coleman MP. Survival among children diagnosed with acute lymphoblastic leukemia in the United States, by race and age, 2001 to 2009: Findings from the CONCORD-2 study. Cancer. 2017;123(Suppl 24):5178–89. doi: 10.1002/cncr.30899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arima H, Takami T, Yamagata T, Naito K, Abe J, Shimokawa N, et al. Surgical management of spinal meningiomas: A retrospective case analysis based on preoperative surgical grade. Surg Neurol Int. 2014;5:S333–8. doi: 10.4103/2152-7806.139642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamura M, Tsuji O, Fujiyoshi K, Hosogane N, Watanabe K, Tsuji T, et al. Long-term surgical outcomes of spinal meningiomas. Spine (Phila Pa 1976) 2012;37:E617–23. doi: 10.1097/BRS.0b013e31824167f1. [DOI] [PubMed] [Google Scholar]
- 19.Sandalcioglu IE, Hunold A, Müller O, Bassiouni H, Stolke D, Asgari S. Spinal meningiomas: Critical review of 131 surgically treated patients. Eur Spine J. 2008;17:1035–41. doi: 10.1007/s00586-008-0685-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Postalci L, Tugcu B, Gungor A, Guclu G. Spinal meningiomas: Recurrence in ventrally located individuals on long-term follow-up; a review of 46 operated cases. Turk Neurosurg. 2011;21:449–53. [PubMed] [Google Scholar]
- 21.Tan KS, Eguchi T, Adusumilli PS. Competing risks and cancer-specific mortality: Why it matters. Oncotarget. 2018;9:7272–3. doi: 10.18632/oncotarget.23729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schellinger KA, Propp JM, Villano JL, McCarthy BJ. Descriptive epidemiology of primary spinal cord tumors. J Neurooncol. 2008;87:173–9. doi: 10.1007/s11060-007-9507-z. [DOI] [PubMed] [Google Scholar]
- 23.Özdemir BC, Dotto GP. Racial differences in cancer susceptibility and survival: more than the color of the skin? Trends Cancer. 2017;3:181–97. doi: 10.1016/j.trecan.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duggan MA, Anderson WF, Altekruse S, Penberthy L, Sherman ME. The Surveillance, Epidemiology, and End Results (SEER) Program and Pathology: Toward Strengthening the Critical Relationship. Am J Surg Pathol. 2016;40:e94–102. doi: 10.1097/PAS.0000000000000749. [DOI] [PMC free article] [PubMed] [Google Scholar]


