Abstract
COVID‐19 has been associated with several neurological complications. We presented a case of Bell's palsy as a possible neurological complication of COVID‐19 infection. Further research should be conducted to clarify the association, correlation, or causality between COVID‐19 and neuroimmunological diseases.
Keywords: Bell’s palsy, COVID‐19, Facial nerve, Facial palsy, Neurological complication, SARS‐CoV‐2
COVID‐19 has been associated with several neurological complications. We presented a case of Bell's palsy as a possible neurological complication of COVID‐19 infection. Further research should be conducted to clarify the association, correlation, or causality between COVID‐19 and neuroimmunological diseases.

1. INTRODUCTION
The coronavirus disease (COVID‐19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), is a defining global health crisis of our time and the biggest challenge we have faced since World War II. 1 Since its emergence in Asia around the end of 2019, the virus has spread to every corner of the world, with cases increasing exponentially. 2 The wealthy countries in Europe and North America are affected the most; however, the poorest of the poor in Africa, Southeast Asia, and South America have not been left untouched. 3 Though COVID‐19 stricken population varies with geography and economy throughout the world, it has undoubtedly beaten the most resonant chords of every human being's physical, mental, and social realm, directly or indirectly. Despite the containing efforts, this minuscule creature is moving with all its might, smothering those who are least able to cope.
Many studies are being conducted on different aspects of COVID‐19, including disease diagnosis, management, and treatment to overcome this outbreak. It is now known that COVID‐19 is not merely a respiratory disease but also an infection that affects almost all human body organ systems. 4 With the increasing awareness of SARS‐CoV‐2 and its clinical manifestations, the literature shows that a large number of infected patients exhibit neurological symptoms. 5 Although headache, myalgia, headache, altered sensorium, hyposmia, and hypogeusia are commonly reported, ischemic stroke, cerebral hemorrhage, encephalitis, acute myelitis, and Guillain‐Barré syndrome are not uncommon. 5 However, in the medical literature, only a few cases of Bell's palsy related to COVID‐19 have been published. Here in this article, we report a case of a 48‐year‐old Nepalese man with COVID‐19 associated with Bell's palsy.
2. CASE REPORT
A forty‐eight‐year‐old nonsmoker, occasional alcohol consumer, migrant laborer man from Rautahat District, Nepal, returned to his home from Mumbai, India, on May 15 and quarantined locally for SARS‐CoV‐2 evaluation. On May 19, he tested positive for SARS‐CoV‐2 and was isolated. On May 27, he developed right‐sided deviation of mouth with incomplete closure of the left eye. It was gradual on onset over three days and was progressive.
He was found to have a mild dry cough on and off for the last four days with a decrease in smell sensation on detailed inquiry. There were no fever, runny nose, headache, chest pain, and shortness of breath. There was no history of weakness of limbs. There was a history of vesicular skin lesions, ear discharge, vision loss, trauma, recent exposure to the vaccine, or new medication. There was no history of weight loss, malignancy, and exposure to toxic substances. Her professional history was not significant. He is a known case of diabetes mellitus on antidiabetic medication for the last four years. There was no significant social or family history and no known surgical intervention. Before he was quarantined, his blood sugar was 140 and 186 mg/dL fasting and posted prandial, respectively.
He was referred to our center for further management of COVID‐19 and associated complication. On clinical examination, the patient was alert, and oriented to time, place, and person. Pallor, icterus, cyanosis, edema, clubbing, and lymphadenopathy were absent. His pulse was 78 beats per minute; the temperature was 97.2 degrees Fahrenheit; blood pressure was 130/70 mm of Hg, and saturation of 97% was maintained at room air.
All but seventh cranial nerve examinations were normal. There were asymmetric wrinkling of the forehead (loss of wrinkle in the left forehead), inability to raise the left eyebrow, failure to close the left eyelid, and right deviation of the angle of the mouth (left‐sided droop), suggesting left seventh cranial nerve palsy of lower motor neuron type (Figure 1). Signs of meningeal irritations were absent. Skull and spine examinations were regular. Muscle's bulk, tone, and power were within the standard limit. Superficial and deep reflexes were normal. There was no abnormal muscle movement. Planter reflex was downgoing. Cerebellar, sensory, and gate examinations were unremarkable. Careful inspection of the ear canal, tympanic membrane, and oropharynx and palpation of the parotid gland revealed no abnormality. There was no noticeable change in fundoscopy. Lung examination revealed coarse crackles in the right lower lobe. The cardiac examination was unremarkable.
Figure 1.

Figure shows left‐sided Bell's palsy in our patient. There were asymmetric wrinkling of the forehead (loss of wrinkle in the left forehead), inability to raise the left eyebrow, inability to close the left eyelid, and right deviation of the angle of the mouth (left‐sided droop), suggesting left seventh cranial nerve palsy of lower motor neuron type
Biochemistry and hematology panel showed normal renal and liver function, absence of leukocytosis with normal differential cell count, a normal hemoglobin level of 13.6 mg/dL, a normal coagulation profile, and a reduced platelet count of 85 000 cells/μL. His fasting blood sugar level was raised to 350 mg/dl. C‐reactive protein level was raised. Urinalysis, including urinary ketone bodies and head computed tomography (plain and contrast), revealed no abnormality. Radiological findings in the HRCT chest done on June 3 showed interlobular septal thickening with ground‐glass opacity in the right lower lobe (Figure 2).
Figure 2.
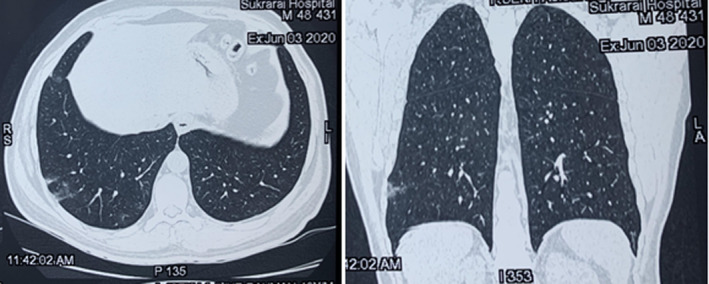
High‐resolution computed tomography chest showing interlobular septal thickening with peripheral ground‐glass opacity in right lower lobe
On hospital stay, he did not develop any severity of COVID 19. He was on regular treatment with glibenclamide for the last four years. As his glucose level was high, his antidiabetic regimen was changed. He received metformin, sitagliptin, glimepiride, and regular insulin subcutaneous for sugar control during his hospital stay. For dry eyes, he received tear plus drops (ingredients: carboxymethylcellulose, dextran, glycerin, hypromellose, polyethylene glycol). Prednisolone was administered considering its benefit for both Bell's palsy and COVID‐19. Facial palsy started improving after two weeks of admission, and throat swab polymerase chain reaction (PCR) for SARS‐CoV‐2 came negative by the 19th day of admission. He was discharged on the 20th day of admission with resolved COVID‐19. After 45 days, the treating team contacted the patient by phone and realized that he was in good condition, and his facial weakness had improved significantly. However, there are still some residual weaknesses.
3. DISCUSSION
Bell's palsy is the most common cranial nerve palsy, with obscure etiology and pathophysiology. Although Bell's palsy is by definition an idiopathic disease, mounting evidence shows that several viral infections are related to it. Most studies have shown that Bell's palsy is related to the immune response after a viral infection, and it usually occurs 1‐2 weeks after a viral infection. Herpes simplex virus, human immunodeficiency virus, herpes zoster virus, Epstein‐Barr virus, etc, are common viral infections associated with Bell's palsy. 6
Bell's palsy is thought to result from compression of the seventh cranial nerve at the geniculate ganglion due to significant nerve inflammation. The first portion of the facial canal, the labyrinthine segment, is the narrowest, and it is here that most cases of compression occur. Due to the narrow opening of the facial canal, inflammation causes the nerve's compression and ischemia. 7 We assume that patient in our report got Bell's palsy secondary to COVID‐19. The SARS‐CoV‐2 virus might have irritated the facial nerve, causing edema and compression against the facial canal. However, we have no direct evidence of central nervous system involvement. In this case, a lumbar puncture was not performed to rule out the central nervous system's involvement. Therefore, at this stage, we can only say that Bell's palsy in our case is associated with COVID‐19, but it is not a complication. A few case reports have been published showing its association with COVID‐19. 8 , 9 , 10 However, none have shown direct evidence; hence, more evidence in the form of positive cerebrospinal fluid SARS‐CoV‐2 PCR is needed to confirm whether Bell's palsy with COVID‐19 is causal or casual.
In contrast to the aforementioned reports, our patient only received a corticosteroid. Currently, available evidence suggests that antiviral agents' addition to steroids for Bell's palsy is not associated with more significant facial motor function recovery. There is no evidence that acyclovir is beneficial when administered alone or used in combination with prednisolone. 11 , 12
Apart from Bell's palsy, other immunological disorders of the nervous system have also been proposed to be associated with COVID‐19. A systematic review had analyzed 28 publications, including 37 patients with COVID‐19 who had Guillain‐Barré syndrome. Two patients presented with neurological symptoms before developing COVID‐19 symptoms. For the remainder, the meantime to the onset of neurological symptoms was 11 ± 6.5 days from the onset of COVID‐19, and a majority of patients (31/37, 84%) developed GBS while experiencing ongoing symptoms from COVID‐19. 13 Similarly, COVID‐19 has also been reported to be associated with acute transverse myelitis. 14 , 15 , 16 , 17 , 18
Nevertheless, it is still debatable whether these neuroimmunological disorders occur directly from the viral infection or as autoimmune sequelae. The pathogenesis of the disease behind this manifestation is not fully understood yet. Indeed, it is possible that our patient had Bell's palsy from an alternative etiology, yet this scenario is unlikely given the patient's viral prodrome.
COVID‐19 has been associated with several neurological complications. 5 We presented a case of Bell's palsy as a possible neurological complication of COVID‐19 infection. Given the current pandemic, the authors of this study felt that the benefits of publishing a case report outweighed the present risks of inaccurate predictions. We believe that further research should be conducted to clarify the association, correlation, or causality between COVID‐19 and neuroimmunological diseases.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
AB: established the diagnosis. RS, GN, and BG: reviewed the literature and designed the manuscript. SKR, BSC, BS, RN, KM, KD, and AJRM: reviewed the literature and prepared the article. All authors read and approved the final version of the manuscript.
INFORMED CONSENT
Written informed consent was obtained from the patient in the Nepali language to publish his clinical details including his photograph. Later, the paper was explained to the patient on telephone conversion. The treating physician is responsible and guarantor of the patient consent.
ACKNOWLEDGMENTS
We thank the patient and his family members for providing written informed consent for publication of patient's photograph.
Bastola A, Sah R, Nepal G, et al. Bell’s palsy as a possible neurological complication of COVID‐19: A case report. Clin Case Rep.2021;9:747–750. 10.1002/ccr3.3631
Data Availability Statement
Not applicable.
REFERENCES
- 1. Hartley DM, Perencevich EN. Public Health Interventions for COVID‐19: Emerging Evidence and Implications for an Evolving Public Health Crisis. JAMA. 2020;323(19):1908‐1909. [DOI] [PubMed] [Google Scholar]
- 2. Guan W, Ni Z, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. WHO Coronavirus Disease (COVID‐19) Dashboard | WHO Coronavirus Disease (COVID‐19) Dashboard [Internet]. [cited 2020 Aug 4]. Available from: https://covid19.who.int/
- 4. Roberts CM, Levi M, McKee M, Schilling R, Lim WS, Grocott MPW. COVID‐19: a complex multisystem disorder. Br J Anaesth. 2020;125(3):238‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nepal G, Rehrig JH, Shrestha GS, et al. Neurological manifestations of COVID‐19: a systematic review. Crit Care. 2020;24(1):421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heckmann JG, Urban PP, Pitz S, Guntinas‐Lichius O, Gágyor I. The Diagnosis and Treatment of Idiopathic Facial Paresis (Bell’s Palsy). Dtsch Arztebl Int. 2019;116(41):692‐702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sadé J. Pathology of Bell’s Palsy. Arch Otolaryngol. 1972;95(5):406‐414. [PubMed] [Google Scholar]
- 8. Wan Y, Cao S, Fang Q, Wang M, Huang Y. Coronavirus disease 2019 complicated with Bell ’ s palsy : a case report. Res Sq. 2020. [Google Scholar]
- 9. Elkhouly A, Kaplan A. Noteworthy Neurological Manifestations Associated With COVID‐19 Infection. Cureus. 2020;12(7):3‐7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10. Goh Y, Beh DLL, Makmur A, Somani J, Chan ACY. Pearls & Oy‐sters: Facial nerve palsy in COVID‐19 infection. Neurology. 2020;95(8):364‐367. [DOI] [PubMed] [Google Scholar]
- 11. Sullivan FM, Swan IRC, Donnan PT, et al. Early treatment with prednisolone or acyclovir in Bell’s palsy. N Engl J Med. 2007;357(16):1598‐1607. [DOI] [PubMed] [Google Scholar]
- 12. Goudakos JK, Markou KD. Corticosteroids vs Corticosteroids Plus Antiviral Agents in the Treatment of Bell Palsy: A Systematic Review and Meta‐analysis. Arch Otolaryngol Neck Surg. 2009;135(6):558‐564. [DOI] [PubMed] [Google Scholar]
- 13. Caress JB, Castoro RJ, Simmons Z, et al. COVID‐19‐associated Guillain‐Barré syndrome: The early pandemic experience. Muscle Nerve. 2020;62(4):485‐491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Valiuddin H, Skwirsk B, Paz‐Arabo P. Acute transverse myelitis associated with SARS‐CoV‐2: A Case‐Report. Brain Behav Immun Health. 2020;5:100091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. AlKetbi R, AlNuaimi D, AlMulla M, et al. Acute myelitis as a neurological complication of Covid‐19: A case report and MRI findings. Radiol Case Rep. 2020;15:1591‐1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Munz M, Wessendorf S, Koretsis G, et al. Acute transverse myelitis after COVID‐19 pneumonia. J Neurol. 2020;267(8):2196‐2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sotoca J, Rodríguez‐Álvarez Y. COVID‐19‐associated acute necrotizing myelitis. Neurol ‐ Neuroimmunol Neuroinflammation. 2020;7(5):e803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sarma D, Bilello LA. a case report of acute transverse myelitis following novel coronavirus infection. Clin Pract Cases Emerg Med. 2020;4(3):321‐323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


