Abstract
Listeria monocytogenes‐caused primary infected abdominal aortic aneurysm is a very rare disease. Aortic wall tissue sampling is essential for confirmation of diagnosis. Surgical repair and long‐term antibacterial treatment are crucial for management.
Keywords: cardiovascular disorders, infectious diseases, vascular surgery
Listeria monocytogenes‐caused primary infected abdominal aortic aneurysm is a very rare disease. Aortic wall tissue sampling is essential for confirmation of diagnosis. Surgical repair and long‐term antibacterial treatment are crucial for management.
1. INTRODUCTION
Mycotic or primary infected aortic aneurysm (MAA) is a rare but dangerous and potentially life‐threatening condition. An intact and healthy intima layer usually makes aortic wall extremely resistant to infections. Hence, pre‐existing endothelial dysfunction (caused by atherosclerosis, degenerative aneurysm, noninfectious aortitis, congenital abnormalities etc) weakens this barrier in favor of bacterial contamination. MAA is mainly caused by hematogenous spread during bacteremia, by septic emboli to the vasa vasorum of the aortic wall, or by direct expansion of an adjacent infection, leading either to infectious degeneration of the arterial wall and aneurysm formation or suppuration of the already existing aortic aneurysm. 1
The incidence of MAA is up to 1.3% of all aortic aneurysms in the Western countries and even higher in East Asia. 1 , 2 Most patients are male and tend to be younger (mean age 69‐70 years) than those with a degenerative noninfected aneurysm (74‐78 years). 1 In about one third of MAA cases, the pathogen may remain undiscovered, although most of MAA aortites or periaortites are caused by common microorganisms including Gram‐positive (Staphylococcus and Enterococcus species, Streptococcus pneumoniae and Clostridium species) and Gram‐negative bacteria (Salmonella species). 1 Listeria monocytogenes seems to be a quite uncommon pathogen for vascular infections, occurring more frequently on the native artery than on the prosthesis (68% vs 32%). 3 According to literature data of 18 infectious aortitis cases (primary or graft infections), only nine were reported as MAA in the abdominal aortic area. 4 However, as not all relevant articles specify either the exact location of the involved area (thoracic or abdominal aorta) or the fact whether a case is primary or graft infection, the real number of MAA involving the abdominal aorta is probably higher (see Table 1). We present a case of a MAA caused by Listeria monocytogenes and provide a review of the literature.
TABLE 1.
Cases of the Listeria monocytogenes‐caused MAA involving the abdominal aorta
| Author | Year | Patient/case | Location | Treatment Graft type | Antibacterial treatment Intravenous | Per os | Outcome | ||
|---|---|---|---|---|---|---|---|---|---|
| Heikkinen,L.et al 15 | 1999 | M | 77 | EVAR infection | Open surgery | PTFE bifurcated graft | Vancomycin, imipenem, netilmicin, ampicillin | Doxycyclin | 10 – months symptom free |
| Brossier, J et al 16 | 2010 | M | 64 | Infrarenal AAA | Open surgery | Allograft (aortic tube) | Mainly penicillin with aminoglycoside | 30 – day alive | |
| M | 64 | Infrarenal AAA | Open surgery | Allograft (aortic tube) | 30 – day alive | ||||
| M | 65 | Infrarenal AAA | Open surgery | Silver + rifampicin‐soaked graft aorto‐biiliacal | 30 – day alive | ||||
| Haroon,Y. et al 7 | 2011 | M | 74 | Infrarenal AAA (degenerative) | Open surgery | 20 mm silver graft | Amoxicillin, gentamicin, vancomycin | Amoxicillin | Full recovery, followed up |
| Murphy, K. et al 17 | 2013 | M | 74 | Infrarenal AAA | Open surgery | Silver tube graft | Rifampicin, vancomycin, gentamicin, amoxicillin | Discharged alive | |
| M | 74 | Infrarenal AAA | Open surgery | Rifampicin‐soaked tube graft | Vancomycin, trimethoprim/sulfamethoxazole, penicillin V | Discharged alive | |||
| Molacek,J. et al 6 | 2014 | M | 63 | Perforated aortitis (abdominal aorta) | Open surgery | Linear silver graft | Gentamicin, ampicillin | N/A | Alive 2 y after surgery |
| Palacios,A. et al 8 | 2017 | M | 76 | Infrarenal AAA | Open surgery | 20 mm silver graft | Ampicillin, gentamicin | Amoxicillin | Alive, symptom free 1 y after surgery |
| Foulex, A. et al 3 | 2019 | M | 76 | EVAR periaortitis | Surgical revision, drainage of periaortal abscess (?) | Amoxicillin | Ciprofloxacin, doxycycline | Alive, asymptomatic at 6 mo follow‐up | |
| Shoai‐Tehrani, M. et al 4 | 2019 | 18 cases | Infrarenal Aorta | Open surgery | N/A | Amoxicillin + aminoglycoside (most frequent combination) | N/A | ||
| 7 cases | Prosthetic/descending aorta | ||||||||
| Our case | M | 79 | Infrarenal AAA (degenerative) | Open surgery | 18 mm antibiotic and silver‐soaked graft | Amoxicillin/clavulanic acid, amoxicillin sulbactam, meropenem, ampicillin | – | Alive, symptom free 8 mo later | |
2. CASE REPORT
A 79‐year‐old male patient with a history of hypertension, previous myocardial infarction, and sick sinus syndrome (pacemaker controlled) was admitted to the hospital for severe abdominal pain, lasting 2 days, and syncope. At the emergency department, the patient was hemodynamically stable, and he had no complaints about significant intestinal dysfunction nor any signs of peritonitis at clinical examination. However, there was a painful pulsatile mass in the abdomen and his body temperature was 37.3°C.
Blood tests confirmed inflammation with an elevated C‐reactive protein value of 210 mg/L (<5 mg/L), white blood cells 21.0 × E9/L (3.5‐8.8 × E9/L), and a slightly higher procalcitonin level of 0.15 µg/L (<0.05 µg/L). A normal hemoglobin value of 136 g/L (134‐170 g/L) could indicate a lack of critical blood loss or hemorrhage. The tests for kidney, liver, and pancreatic function and the indicators for myocardial infarction were within the normal range. One day before admission, a mild gallbladder wall thickening and gallstones had been found at ultrasound and cholecystitis had been suspected. An abdominal aortic aneurysm (AAA), 5 cm in diameter, had been found but without any signs of rupture or other emergency conditions. In‐hospital computed tomography angiography (CTA) confirmed an infrarenal AAA (diameter 5.5 cm) with a thickened wall and concomitant reactive changes in the periaortal soft tissue, but without other relevant acute intra‐abdominal pathology (Figure 1). This finding was interpreted as a possible covered rupture with an intramural hematoma by a radiologist. Considering all clinical signs, and laboratory and CTA findings, the rupture of the AAA was suspected by the vascular surgeon and the patient underwent an emergency open surgical repair (OSR). During the operation, inflammation and a thickening of the AAA wall without signs of rupture were visually detected. A resection of the aneurysm, debridement of the infected area, and aortic replacement with a linear 18 mm antibiotic‐soaked Dacron prosthesis were performed. A tissue biopsy from the AAA wall for microbiological examination showed growth of Listeria monocytogenes. However, additional blood cultures were negative for any pathogen.
FIGURE 1.
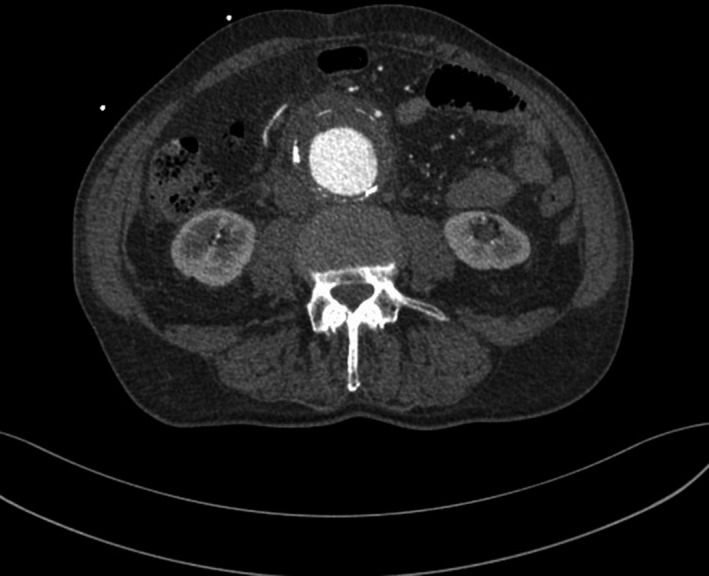
Preoperative computed tomography angiography revealing a thickened aortic wall with a periaortic soft tissue mass
Postoperatively, the patient had complications within redo surgery (right aortoiliac and left aortofemoral bypasses) due the acute leg ischemia, and he developed hospital‐acquired pneumonia later on. Antibiotic treatment was started with amoxicillin/clavulanic acid immediately after surgery, continued with amoxicillin/sulbactam after the confirmed diagnosis of Listeria monocytogenes‐caused aortic infection, and was changed to meropenem due to concomitant pneumonia; further prolonged treatment for listeriosis continued with intravenous ampicillin during 6 weeks. At a follow‐up visit 8 months after the surgical procedures, the patient was in a good condition and had no signs of infection (Figure 2); he still needs to be carefully followed up for late graft infection.
FIGURE 2.
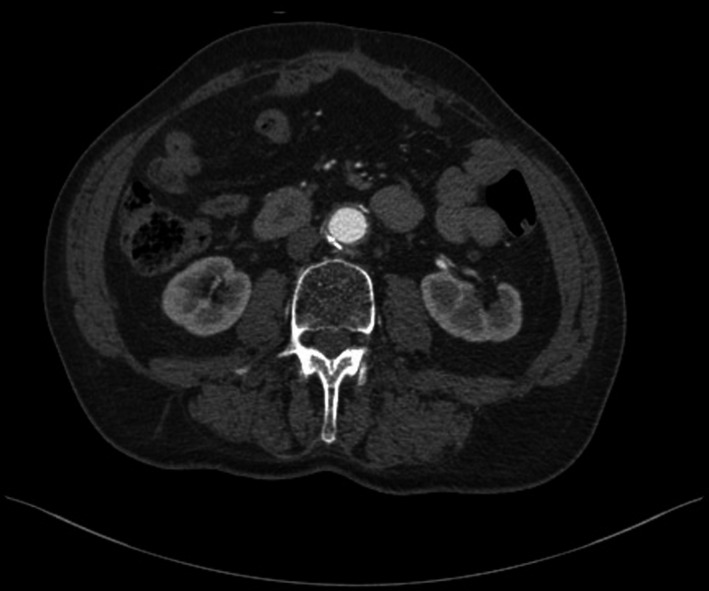
Postoperative computed tomography angiography 8 mo after resection of the aneurysm and aortic replacement with a tube graft, displaying no signs of reinfection
3. DISCUSSION
The main manifestations of listeriosis are gastroenteritis and infections of the central nervous system in severe cases, with sepsis and endocarditis being also reported. The vascular system or the arterial wall can become infected hematogenously in the case of previous aortic wall damage by atherosclerosis, aneurysmatic changes, aortitis, or congenital lesions. Besides bacteremia, which is the most likely route of infection, septic embolization to the vasa vasorum (as in infectious endocarditis), expansion from a neighboring infectious focus, as well as contamination in the setting of intravenous drug use, trauma, and invasive intravascular procedures have been described. 4 , 5 Usually, Listeria monocytogenes causes serious illness only in immunocompromised patients and can be a harmless saprophytic microorganism for some healthy persons. However, the main source of the infection is contaminated food—fish, meat, milk etc In our case, even without specific symptoms of gastroenteritis, we also considered food to be the possible primary source of contamination. Recently, there were several serious cases of Listeria monocytogenes infection in Estonia caused by contamination of domestic fish industry products.
The diagnosis of MAA is based on a combination of clinical presentation, laboratory test, and radiological imaging. The preferred imaging method to confirm the radiologic diagnosis of MAA is CTA, followed by much less used magnetic resonance computed tomography (MRTA). 1 , 3 , 6 , 7 , 8 Usually, CTA gives sufficient information about changes in the aortic wall (thickened, ruptured etc), as well as about reactive changes and about extra fluid or gas bubbles in the surrounding soft tissues. Although hybrid methods (positron emission tomography‐CT) can provide extra information about higher metabolic activity in the infected area, they are less available and take more time in emergency situations, such as MRTA, which is better applicable in chronic noninfectious aortitis. 4 , 5 , 6 A crucial step in the diagnosis is identification of the pathogen. Listeria monocytogenes‐caused vascular infection is confirmed either by positive blood culture (37%), or more often by positive tissue samples (98%) from the inflammatory aortic wall. 3 However, the source of infection was not identified in one third of the patients, nor was the causative organism identified in 21%‐40% of the cases. 1 In the present case, the diagnosis was only made on the basis of surgical sampling (blood cultures were all negative, despite the nonuse of any antimicrobial therapy). We strongly emphasize the need to establish an etiologic bacterial diagnosis to avoid empiric and precipitated use of antimicrobial agents.
OSR is regarded as the gold standard for definite treatment of MAA, followed by prolonged antibacterial therapy, which means that basic management principles should be the same as in vascular graft or endograft infection. 1 , 9 , 10 OSR includes resection of aneurysm, maximal local debridement, and revascularization by extra‐anatomical bypass or in situ reconstruction. However, OSR in the inflammatory area and the choice of the bypass material could be a challenge. Usage of standard prosthetic material can lead to ongoing infection and further complications. Infection related complications may occur in 0%‐20% of cases after in situ grafts. 1 Although the silver‐coated prosthesis and the prosthesis with an antimicrobial covering have also shown a reinfection rate of approximately 11%, several cases of successful recovery from Listeria monocytogenes‐caused MAA infection are reported in the literature. 7 , 8 , 11 Careful debridement of the infected area is mandatory in these cases. Biological material (autologous vein, allogenous vein or artery, xenogenous pericardial graft) can be the best option in situ, but it might not be available in emergency situations. Extra‐anatomical bypass with debridement of the infected area can also be considered. Earlier studies have found high complication rate after extra‐anatomical bypass, with late aortic stump rupture in up to 20% of cases. 11 , 12 In the present case, we used the antibiotic‐soaked Dacron prosthesis as the only available option in the emergency situation. 1
Endovascular aortic aneurysm repair (EVAR) with aggressive antibiotic treatment is gaining more attention. The main concern is ongoing infection after placement of prosthetic material in the infected area. However, there are already reports of cases of successful endovascular treatment, with overall outcomes poorer compared with those of open surgery but still better than the results of antimicrobial treatment alone (mortality rate up to 58%). EVAR could be an option for patients who are not eligible for OSR (aged, fragile, with many comorbidities etc). In emergency situations, EVAR may also serve as a bridge to later definitive surgery. 9 , 10 , 13 A recent study of OSR and EVAR for MAA, including 132 patients with 144 abdominal MAAs, showed significant early survival benefit for EVAR (up to four years) with no late disadvantages in terms of late infection rates or aneurysm related complications or survival, suggesting that endovascular repair is an acceptable alternative to OSR. 14
Antibacterial treatment with prolonged ampicillin or amoxicillin administration seems to be the first effective choice against Listeria monocytogenes infection in most cases; however, it can be combined with aminoglycoside. Erythromycin, vancomycin, rifampicin, and trimethoprim‐sulfamethoxazole are appropriate medications as well, but their choice always depends on the sensibility of the pathogen to antimicrobial agents. 4 , 8 The recommended length of antibacterial treatment ranges from mostly advised 4‐6 weeks to 4 months or even lifelong administration, to avoid both ongoing infection after the replacement of vascular graft and infection recurrence due to the pathogen's nature. 1 , 9 In the studied patient, we started antibiotic treatment immediately after surgery and it lasted 6 weeks.
We presented a case of successful treatment (OSR combined with antibiotics) of MAA caused by the rare pathogen Listeria monocytogenes. In this case, the diagnosis was made on the basis of clinical presentation, CTA, and aortic wall sampling. Early detection and OSR in combination with long‐term focused treatment with antibiotics are crucial in successful management of MAA.
CONFLICT OF INTEREST
None declared.
AUTHORS' CONTRIBUTIONS
HJ: contributed to case management, data collection and interpretation, and manuscript drafting; JK: contributed to diagnosis and management of the case, conception of the article, data interpretation, manuscript editing, and supervision.
ACKNOWLEDGEMENTS
The authors are indebted to Ms E. Jaigma for the linguistic revision of the text.
Järve H, Kals J. Listeria monocytogenes infectious abdominal aortic aneurysm: Case report and review of the literature. Clin Case Rep.2021;9:800–804. 10.1002/ccr3.3652
DATA AVAILABILITY STATEMENT
All data collected or analyzed during this case report are included in this published article.
REFERENCES
- 1. Wanhainen A, Verzini F, Van Herzeele I, et al. Editor's Choice ‐ European Society for Vascular Surgery (ESVS) 2019 Clinical practice guidelines on the management of abdominal aorto‐iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2019;57:8‐93. [DOI] [PubMed] [Google Scholar]
- 2. Sörelius K, Mani K, Björck M, et al. Endovascular treatment of mycotic aortic aneurysms: a European multicenter study. Circulation. 2014;130:2136‐2142. [DOI] [PubMed] [Google Scholar]
- 3. Shoai‐Tehrani M, Pilmis B, Maury MM, et al. Listeria monocytogenes‐associated endovascular infections: a study of 71 consecutive cases. J Infect. 2019;79:322‐331. [DOI] [PubMed] [Google Scholar]
- 4. Foulex A, Coen M, Cherkaoui A, et al. Listeria monocytogenes infectious periaortitis: a case report from the infectious disease standpoint. BMC Infect Dis. 2019;19:326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gornic HL, Creger MA. Aortitis. Circulation. 2008;117:3039‐3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Molacek J, Treska V, Baxa J, Ceric B, Houdek K. Acute conditions caused by infectious aortitis. Aorta (Stamford). 2014;3:93‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Haroon Y, Bhalla A, El‐Tahir A. Listeria monocytogenes: a rare case for an infected abdominal aortic aneurysm. Vasc Endovasc Surg. 2011;45:773‐774. [DOI] [PubMed] [Google Scholar]
- 8. Palacious A, Llorente AM, Ordónez O, de Aragón AM. Mycotic abdominal aortic aneurysm due the Listeria monocytogenes . Scientific letters/Enferm Infecc Microbiol Clin. 2017;35:264‐270. [Google Scholar]
- 9. Hsu RB, Chen RJ, Wang SS, Chu SH. Infected aortic aneurysms: Clinical outcome and risk factor analysis. J Vasc Surg. 2004;40:30‐35. [DOI] [PubMed] [Google Scholar]
- 10. Lin CH, Hsu RB. Primary infected aortic aneurysm: clinical presentation, pathogenesis, and outcome. Acta Cardiol Sin. 2014;30:514‐521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chakfé N, Diener H, Lejay A, et al. European Society for Vascular Surgery (ESVS) 2020 Clinical practice guidelines on the management of vascular graft and endograft infections. Eur J Vasc Endovasc Surg. 2020;59:339‐384. [DOI] [PubMed] [Google Scholar]
- 12. Bacourt F, Koskas F. Axillobifemoral bypass and aortic exclusion for vascular septic lesions: a multicenter retrospective study of 98 cases. Ann Vasc Surg. 1992;6:119‐126. [DOI] [PubMed] [Google Scholar]
- 13. Yu SY, Lee CH, Hsich HC, Chou AH, Ko PJ. Treatment of primary infected aortic aneurysm without aortic resection. J Vasc Surg. 2012;56:943‐950. [DOI] [PubMed] [Google Scholar]
- 14. Sorelius K, Wanhainen A, Furebring M, et al. Nationwide study of the treatment of mycotic abdominal aortic aneurysms comparing open and endovascular repair. Circulation. 2016;134:1822‐1832. [DOI] [PubMed] [Google Scholar]
- 15. Heikkinen L, Valtonen M, Lepäntalo M, Saimanen E, Järvinen A. Infrarenal endoluminal bifurcated stent graft infected with Listeria monocytogenes . J Vasc Surg. 1999;29:554‐556. [DOI] [PubMed] [Google Scholar]
- 16. Brossier J, Lesprit P, Marzelle J, Allaire E, Becquemin JP, Desgranges P. New bacteriological patterns in primary infected Aorto‐iliac aneurysms: a single‐Centre experience. Eur J Vasc Endovasc Surg. 2010;40:582‐588. [DOI] [PubMed] [Google Scholar]
- 17. Murphy K, Al‐Jundi W, Nawaz S. Mycotic aneurysms of the abdominal aorta due to Listeria monocytogenes . Int J Surg Case Rep. 2013;4:626‐628. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data collected or analyzed during this case report are included in this published article.


