Abstract
No previous case of using fecal microbiota transplantation (FMT) to treat rheumatoid arthritis (RA) has been reported. We report a case of a patient with refractory RA successfully treated with FMT indicating that FMT may have a good therapeutic effect on RA.
Keywords: bacterial therapy, fecal microbiota transplantation, gut microbiota, rheumatoid arthritis
No previous case of using fecal microbiota transplantation (FMT) to treat rheumatoid arthritis (RA) has been reported. We report a case of a patient with refractory RA successfully treated with FMT indicating that FMT may have a good therapeutic effect on RA.
![]()
1. INTRODUCTION
Rheumatoid arthritis (RA) is an autoimmune disease. Some antirheumatic drugs may fail to treat RA and cause serious adverse reactions. Here, we present a case of a 20‐year‐old patient with 5 years’ history of RA, successfully treated with fecal microbiota transplantation (FMT).
Rheumatoid arthritis (RA) is a chronic, systemic, inflammatory autoimmune disease characterized by polyarthritis potentially leading to progressive joint destruction and disability. It affects up to 1% of adults worldwide. 1 , 2 Disease‐modifying antirheumatic drugs are commonly used to inhibit synovitis and other signs and symptoms of an active RA and prevent joint bone erosion and joint space narrowing or disappearing. Because some antirheumatic drugs can cause serious adverse reactions and have many common side effects, the pros and cons effects of these drugs must be carefully weighed.
There have been literatures supporting that both genetic and environmental factors are involved in the pathogenesis of RA. Up to date, about 100 genes have been described to be associated with susceptibility, protection, severity, activity, and treatment response of RA. 3 Smoking, hormones, microbiota, and infections may be critical environmental factors associated with the induction of RA. Antimicrobial drugs such as minocycline or salazosulfapyridine have been reported to be effective in some RA patients, from which it can be inferred that the gut microbiota may be correlated with this disease. Microbial exposures in gastrointestinal tract are key determinants of the overall immune tone at the mucosal barrier and represent a leading target for promising intervention strategies. Intestinal microbiota is a factor influencing metabolic homeostasis and the immune system; it is a site of remarkable interaction between microorganisms and human body. 4 Several studies have shown that the composition of the intestinal microbiota is altered in RA patients 5 , 6 indicating that the gut microbiota may contain potential therapeutic targets for RA. The above‐mentioned evidence suggests that gut microbiome dysbiosis plays an pivotal role in the etiopathogenesis of RA. Therefore, restoration of the gut microbiota to a homeostatic state appears to be of great importance.
An array of cases has been reported that fecal microbiota transplantation(FMT) successfully treated several diseases including clostridium difficile infection (CDI), inflammatory bowel disease (IBD), and autoimmune diseases like idiopathic thrombocytopenic purpura (ITP). 7 , 8 Here, we represent a case using FMT to treat RA.
2. CASE REPORT
A 20‐year‐old woman with no underlying disease was diagnosed with rheumatoid arthritis 5 years ago(inflammatory arthritis was present in 4 joints with supportive laboratory parameters and more than 6 weeks’ history). Symptoms got under control after initial treatment of methotrexate. Due to nausea and discomfort caused by methotrexate, she did not take medication regularly. 2 years ago, she presented severe RA flare and was treated with etanercept given by subcutaneous injection at the dose of 50 mg/week and 10 mg/day of oral leflunomide which significantly alleviated joint swelling and pain. So the dose of etanercept was reduced to 25mg/10 days last year. Nine months ago, she presented another severe RA flare. The dose of etanercept was increased to 25 mg/week, and leflunomide 20mg/day, along with diclofenac sodium at the dose of 75 mg /day, with which symptoms persisted.
Five months ago, the patient was admitted to our hospital for active RA flare with a DAS28 (Disease Activity Score28) at 6.6, and the titer of rheumatoid factor was 314IU/ml. After admission, she received methylprednisolone tablets 6mg/day and hydroxychloroquine sulfate tablets 0.2g twice/day Health assessment questionnaire disability Index(HAQ‐DI) dropped from 1.4 to 0.7, and symptoms and physical signs of joint swelling and pain were still noticeable. After being informed of the potential benefits, risks, and complications of FMT, the patient required to receive FMT treatment and signed the relevant informed consent.
Laboratory investigations indicated no restrictions on treatment for the recipient. Donated stool for FMT was obtained from a 8‐year‐old healthy girl. The donor had not taken antibiotics or probiotics within the past 1 month. To prevent transmission of infectious diseases from the donor to the recipient, the donor underwent preliminary screening by a screening questionnaire for family history and individual disease history and then took a laboratory examination for common pathogens, including human immunodeficiency virus, hepatitis A, B, and C virus, syphilis, enteropathogenic Escherichia coli (EPEC), Shigella, Salmonella, C. difcile toxin, Epstein‐Barrvirus, and cytomegalovirus, as well as fungi, ova, cysts, and parasites. The screening process of donors was published in our previous studies. 8 , 9 Fecal samples from a donor were kept in an airtight container and were chilled and then were delivered to the laboratory preferably within 1 h of passage. A specimen of stool weighing 100 g was diluted with 500 mL preservative‐free normal saline. The mixture was suspended by using a blender and then filtered through different precision filters sequentially. Finally, the suspension was collected for use. 10 After the fecal microbiota suspension was prepared, FMT was then administered to the patient's colon with 300ml of fecal suspension via colonoscopy under anesthesia.
The patient did not complain of discomfort after FMT. On the seventh day after FMT, HAQ‐DI dramatically dropped to 0.05, which has been maintained to date for 4 months. The examination results on the 42th day after FMT(6th March 2020) and the 78th day after FMT(12th April 2020) showed that the DAS28 was 1.9 and 1.4, respectively, and the titer of rheumatoid factor was 158 IU/mL and 135.9 IU/mL, respectively (Figure 1). Since the 48th day after FMT, the dose of methylprednisolone tablets had been reduced to 4 mg/day, and the dose of hydroxychloroquine sulfate tablets maintained at 0.2 g twice/day. Since the 78th day after FMT (12th April 2020), methylprednisolone tablets were reduced to 2 mg/day and hydroxychloroquine sulfate tablets to 0.1 g twice/day. The dose of etanercept was reduced to 25 mg/10 days.
Figure 1 A.
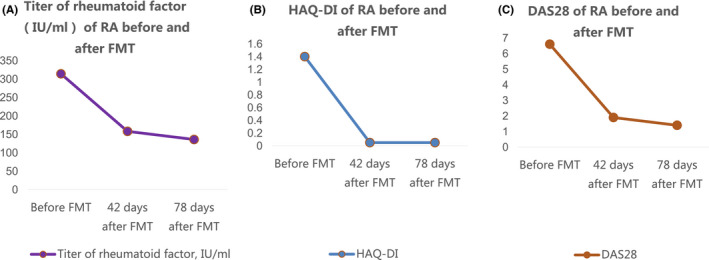
Titer of rheumatoid factor(IU/mL) of RA before and after FMT. B. HAQ‐DI of RA before and after FMT. C. DAS28 of RA before and after FMT
3. DISCUSSION
As far as we know, this is the first reported case that used FMT to treat RA successfully. This case indicates FMT may be an effective treatment for RA. To explore the underlying mechanisms, relevant literatures have been reviewed. While the precise etiology of RA remains unknown, emerging evidence supports the hypothesis that the local autoimmune processes leading to RA autoimmunity might originate from the mucosal immune system. 11 The importance of the intestinal microbiota in the pathogenesis of arthritis is emphasize via studies on animals and humans.
Studies have shown that the composition of the intestinal microbiota is altered in RA patients. The bacterial genera Bacteroides, Escherichia‐Shigella, and Prevotella species were more abundant in RA patients. In contrast, Lactobacillus, Alloprevotella, Enterobacter, and Odoribacter were less abundant in the RA group than in the control group. 12 Spearman's correlation analysis of blood physiological measures of RA showed that bacterial genera such as Dorea and Ruminococcus were positively correlated with RF‐IgA and anti‐CCP antibodies. Furthermore, Alloprevotella and Parabacteroides were positively correlated with the erythrocyte sedimentation rate, and Prevotella‐2 and Alloprevotella were positively correlated with C‐reactive protein, both biomarkers of inflammation. These findings suggest that the gut microbiota may contribute to RA development via interactions with the host immune system. 12 , 13
Maeda et al 5 identified that Prevotella had a high capacity to induce Th17 cell‐related cytokines, such as IL‐6 and IL‐23. Increased Prevotella abundance is associated with augmented T helper type 17‐mediated mucosal inflammation, which is in accordance with the marked capacity of Prevotella in driving Th17 immune responses in vitro. 14 Studies on mice have shown that disruptions in the intestinal microbiota could induce production of proinflammatory cytokines, interleukin‐17, and increased levels of Th17 cells, even in extraintestinal tissues. Th17 cells migrate into the peripheral lymphoid tissue and secrete IL‐17, which in turn, acts directly on B cells and induces systemic B‐cell differentiation and antibody production. This ultimately can drive the onset of arthritits via molecular pattern recognition from gut microbiota. 15 Thus, gut microbiota could induce Th17 cell differentiation, which was consistent with the reported elevated circulating Th17 cells populations in patients with RA. 16
These findings suggest that FMT may successfully treat the patient with RA through the reconstruction of a beneficial microbiota which then helps to alleviate or stop the process of inflammation. To confirm the hypothesis, more studies should be conducted.
In conclusion, this case indicates that FMT may have a good therapeutic effect on RA and deserve further investigation. There are limitations in our study. Questions including how long the therapeutic effects will last, what dose of FMT is optimal, how frequent FMT should be performed, and whether the response is similar remain to be answered. Therefore, use of FMT requires high‐quality, prospective, randomized, controlled trials with large samples for this disease.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
Jiaqi Zeng: prepared the fecal microbiota suspension, followed up the patient, collected the clinical data, and drafted the report. Wei Zheng: collected the clinical data. Lihua Peng: performed the FMT procedure. Nana Zhang: prepared the fecal microbiota suspension. Feng Huang and Di Wu: referred the patient and revised the report. Yunsheng Yang: designed, and implemented this study and revised the article.
ETHICS STATEMENT
The study obtained the approval of the ethics committee of Chinese PLA General Hospital (ethics number: S2020‐310‐01). The patient provided written informed consent.
ACKNOWLEDGMENT
The authors thank the patient for the support of this case report. Published with written consent of the patient.
Zeng J, Peng L, Zheng W, et al. Fecal microbiota transplantation for rheumatoid arthritis: A case report. Clin Case Rep.2021;9:906–909. 10.1002/ccr3.3677
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this article.
REFERENCES
- 1. Tanaka T, Narazaki M, Kishimoto T. IL‐6 in inflammation, immunity, and disease. Cold Spring Harbor Perspect Biol. 2014;6(10):a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nishimoto N, Amano K, Hirabayashi Y, et al. Drug free REmission/low disease activity after cessation of tocilizumab (Actemra) Monotherapy (DREAM) study. Modern Rheumatol. 2014;24(1):17‐25. [DOI] [PubMed] [Google Scholar]
- 3. Rodríguez‐Elías AK, Maldonado‐Murillo K, López‐Mendoza LF, Ramírez‐Bello J. Genetics and genomics in rheumatoid arthritis (RA): An update. Gaceta Méd México. 2016;152(2):218. [PubMed] [Google Scholar]
- 4. Zhang X, Zhang D, Jia H, et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat Med. 2015;21(8):895‐905. [DOI] [PubMed] [Google Scholar]
- 5. Maeda Y, Kurakawa T, Umemoto E, et al. Dysbiosis contributes to arthritis development via activation of autoreactive T cells in the intestine. Arthritis Rheumatol. 2016;68(11):2646‐2661. [DOI] [PubMed] [Google Scholar]
- 6. Scher JU, Sczesnak A, Longman RS, et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife. 2013;2:e01202 10.7554/eLife.01202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou Y, Xu H, Huang H, et al. Are There Potential Applications of Fecal Microbiota Transplantation beyond Intestinal Disorders? BioMed Res Int. 2019;2019:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ren R, Sun G, Yang Y, et al. A pilot study of treating ulcerative colitis with fecal microbiota transplantation. Zhonghua Nei Ke Za Zhi. 2015;54(5):411‐415. [PubMed] [Google Scholar]
- 9. Zhao H, Shi Y, Luo XI, Peng L, Yang Y, Zou L. The effect of fecal microbiota transplantation on a child with tourette syndrome. Case Rep Med. 2017;2017:1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang Y, Ren R, Sun G, et al. Pilot study of cytokine changes evaluation after fecal microbiota transplantation in patients with ulcerative colitis]. Int Immunopharmacol. 2020. [DOI] [PubMed] [Google Scholar]
- 11. Catrina AI, Joshua V, Klareskog L, et al. Mechanisms involved in triggering rheumatoid arthritis. Immunol Rev. 2016;269(1):162‐174. [DOI] [PubMed] [Google Scholar]
- 12. Sun Y, Chen Q, Lin P, et al. Characteristics of gut microbiota in patients with rheumatoid arthritis in Shanghai, China. Front Cell Infect Microbiol. 2019;9:369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maeda Y, Takeda K. Host–microbiota interactions in rheumatoid arthritis. Exp Mol Med. 2019;51(12):150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Larsen MJ. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology. 2017;151(4):363‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mukhopadhyay A, Singh H. Gut bacterial peptides with autoimmunity potential as environmental trigger for late onset complex diseases: In–silico study. PLoS One. 2017;12(7):e0180518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang Y, Li Y, Lv TT, et al. Elevated circulating Th17 and follicular helper CD4+ T cells in patients with rheumatoid arthritis. Apmis Acta Pathol Microbiol Immunol Scand. 2015;123(8):659‐666. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article.


