Abstract
Eradication therapy of Helicobacter pylori may be safe if hemin has been intravenously administered in advance, even in patients with a history of recurrent acute porphyria attack.
Keywords: Helicobacter pylori, hemin, porphyria
Eradication therapy of Helicobacter pylori may be safe if hemin has been intravenously administered in advance, even in patients with a history of recurrent acute porphyria attack.

1. INTRODUCTION
Helicobacter pylori infection increases the risk of gastric cancer and should be treated in young patients. 1 However, some drugs used to treat H. pylori inhibit cytochrome P450 3A4 and appear to induce porphyria, an illness that is often diagnosed in young adults. Porphyria is a potentially life‐threatening condition that causes severe abdominal pain, nausea, constipation, confusion, and seizures. 2 Not all H. pylori therapies are contraindicated, 2 but the risk of H. pylori therapy‐induced porphyria attacks is not well‐understood. No statistical data have been reported regarding the safe use of these drugs. Thus, caution should be used when administering specific drugs because of their porphyria attack‐inducing effects. 3
2. CASE HISTORY
We herein report a case of successful treatment of H. pylori in a female patient with porphyria. The patient, who was in her 20s, suffered from alcohol‐induced whole body pain, constipation, hypertension, tachycardia, reversible cerebral vasoconstriction syndrome, posterior reversible encephalopathy syndrome, and epileptic seizures triggered. Elevated porphyrin precursors in the urine led to the diagnosis of acute intermittent porphyria. Hemin administration (the details of which we had previously reported) 4 promptly improved her symptoms. Thereafter, she experienced recurrent menstrual‐related porphyria attacks that presented as constipation, abdominal pain, and leg pain. Constipation and elevated urinary porphyrin precursor levels regularly coincided with the period after ovulation. We had previously reported that porphyria attacks might also be associated with vascular spasms due to deficiencies in nitric oxide synthase, which is a heme protein. 4 Because heme proteins are involved in progesterone metabolism, nitric oxide deficiency due to heme deficiency might induce intestinal ischemia and constipation; thus, we considered constipation as a symptom of mild porphyria. The patient developed hypertension and tachycardia on the day after the appearance of the pain; therefore, hemin needed to be administered early at the stage of constipation. Once a month, on the day before or after she was expected to ovulate, the patient was intravenously administered 0.4 mg/kg of hemin (Figure 1). This drug, which remains in the body for ~3 weeks, was safely administered without increasing the patient's ferritin levels. For 4 years after the start of hemin treatment, the patient maintained a stable course without severe attacks. Family history revealed that the patient's sister exhibited elevated urinary porphyrin precursor levels and was diagnosed with asymptomatic porphyria after the patient's diagnosis.
FIGURE 1.
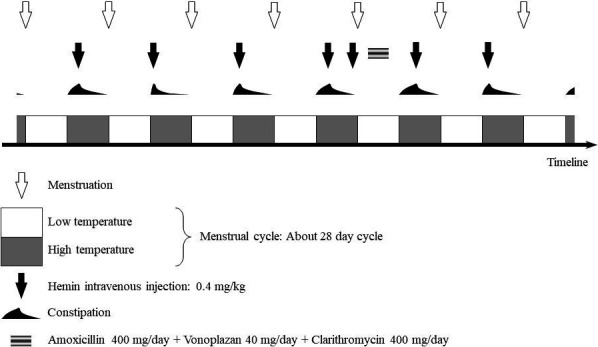
Treatment flowchart. Hemin was intermittently administered according to the menstrual cycle
The patient complained of upper abdominal pain unrelated to porphyria and was evaluated by upper gastrointestinal endoscopy. Atrophic changes in the patient's gastric mucosa were observed, and the results of the rapid urease test were positive; thus, she was diagnosed with H. pylori‐related chronic gastritis. The patient's medication regimen was modified such that hemin was administered at the onset of constipation; and clarithromycin, vonoprazan, and amoxicillin were administered for 1 week after the onset of menstruation (Figure 1). It is estimated that sufficient hemin remains in the body during this period. Negative results were obtained in a rapid urease test 5 weeks after the completion of eradication therapy. This protocol eliminated H. pylori without triggering porphyria symptoms such as constipation.
3. DISCUSSION
Clarithromycin is a first‐line drug for H. pylori eradication. It is reportedly a cytochrome P450 3A4 inhibitor, but that is not in itself a contraindication for porphyria. 5 , 6 Clarithromycin has been classified as “Possibly porphyrinogenic” based on the classification proposed in the Drug Database for Acute Porphyria in NAPOS. There have been no reports on the safety of metronidazole monotherapy or combination therapy for H. pylori in patients with porphyria, and although it is believed that amoxicillin and vonoprazan do not to trigger porphyria attacks, evidence remains insufficient. We previously described how porphyria attacks can be triggered by the accumulation of porphyrin precursors or the lack of heme, 4 indicating that porphyria attacks may be prevented by supplying the appropriate amount of hemin. Here, we present a protocol that can suppress medication‐induced porphyria, even in patients with a history of recurrent acute attacks. Specifically, we believe that this report might serve as a future reference for eradicating H. pylori in patients with porphyria.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
TT: involved in writing—original draft, data curation, and investigation. YK, KK, TY, MK, HK, and KD: involved in investigation. AM: involved in data curation and investigation. TT, HM, and TM: supervised the study.
ACKNOWLEDGMENTS
We gratefully acknowledge the work of past and present members of our hospital. Published with written consent of the patient.
Takata T, Kokudo Y, Morishita A, et al. Premedication of hemin for eradication therapy of Helicobacter pylori in patients with porphyria. Clin Case Rep.2021;9:944–946. 10.1002/ccr3.3715
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, Takata, T., upon reasonable request.
REFERENCES
- 1. Fukase K, Kato M, Kikuchi S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open‐label, randomised controlled trial. Lancet. 2008;372(9636):392‐397. [DOI] [PubMed] [Google Scholar]
- 2. Karim Z, Lyoumi S, Nicolas G, Deybach J‐C, Gouya L, Puy H. Porphyrias: a 2015 update. Clin Res Hepatol Gastroenterol. 2015;39(4):412‐425. [DOI] [PubMed] [Google Scholar]
- 3. Spiritos Z, Salvador S, Mosquera D, Wilder J. Acute intermittent porphyria: current perspectives and case presentation. Ther Clin Risk Manag. 2019;15:1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Takata T, Kume K, Kokudo Y, et al. Acute intermittent porphyria presenting with posterior reversible encephalopathy syndrome. Accompanied by prolonged vasoconstriction. Int Med. 2017;56(6):713‐717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Asaka M, Satoh K, Sugano K, et al. Guidelines in the management of Helicobacter pylori infection in Japan. Helicobacter. 2001;6(3):177‐186. [DOI] [PubMed] [Google Scholar]
- 6. Zhou S, Chan SY, Goh BC, et al. Mechanism‐based inhibition of cytochrome P450 3A4 by therapeutic drugs. Clin Pharmacokinet. 2005;44(3):279‐304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, Takata, T., upon reasonable request.


